Abstract
We aimed to assess the therapeutic effect of reimplantation of biliary metal stents by percutaneous transhepatic cholangial drainage (PTCD) in patients with recurrent malignant obstructive jaundice (MOJ). Furthermore, we explored the prognostic value of inflammation-based markers in these patients.
We reviewed 33 cases of recurrent MOJ after implantation of biliary metal stents by PTCD, all of which underwent reimplantation of stents under digital subtraction angiography guidance. Levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and bilirubin were compared between before and after reimplantation (1 week, 1 month, and 3 months postoperatively). Preoperative clinical data were collected to calculate the inflammation-based markers, including systemic immune-inflammation index (SII, neutrophil × platelets/ lymphocyte), platelets-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), and monocyte-to-lymphocyte ratio (MLR). The primary outcome was overall survival (OS), which was estimated by the Kaplan–Meier method and Cox regression analysis.
The levels of ALT, AST, total bilirubin, and direct bilirubin significantly reduced after the reimplantation operation. During a median follow-up time of 10 months, 18 (54.5%) patients died. Gender, albumin, SII, PLR, NLR, and MLR were found to be associated with OS by the log-rank test and univariate analysis. Multivariate Cox analysis identified elevated levels of SII and PLR as significant factors for predicting poor OS.
Reimplantation is clinically feasible in patients with recurrent MOJ after implantation of biliary metal stents. SII and PLR are independent, useful inflammation-based prognostic models for predicting outcomes in these patients.
Keywords: inflammation-based markers, malignant obstructive jaundice, overall survival, percutaneous transhepatic cholangial drainage, platelets-to-lymphocyte ratio, reimplantation of biliary metal stents, systemic immune-inflammation index
1. Introduction
Malignant obstructive jaundice (MOJ) refers to an obstruction of intrahepatic or extrahepatic bile duct due to the growth of malignant tumors. MOJ may be caused by primary and secondary malignancies of liver, bile duct, gallbladder, pancreas, or periampullary area. As bile fails to enter into the digestive tract, obstructive jaundice may eventually lead to various pathophysiological disorders, such as liver damage, cardiovascular system injury, immune function decrease, intestinal barrier dysfunction, endotoxemia, coagulation disorders, malnutrition, or even death [1]
Surgical resection is considered as the optimal therapy for MOJ.[2] However, the majority of patients are unable to undergo radical surgery because of poor systemic or local tumor conditions. In contrast, percutaneous biliary metallic stent implantation is an effective, palliative treatment method in such patients.[3] Recent years, the survival time and the life quality of patients have been greatly improved by using this approach in our department.[4] However, biliary stents would become obstructed after the implantation in partial patients due to various reasons and it may eventually lead to MOJ recurrence.[5] In our department, as a large percentage of patients with recurrent MOJ had relatively good general health and could tolerate the stent reimplantation procedure, we successfully performed reimplantation of biliary metal stents by percutaneous transhepatic cholangial drainage (PTCD) under digital subtraction angiography (DSA) in these patients. To date, the outcomes and potential prognostic factors in patients with recurrent MOJ have never been investigated.
Recently, published evidence suggests that systemic inflammation is associated with survival in various types of malignancies.[6–8] In addition, several inflammatory response-related parameters, such as systemic immune-inflammation index (SII, neutrophil×platelets/lymphocyte), platelets-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), and monocyte-to-lymphocyte ratio (MLR), have been proposed as usefully prognostic biomarkers in several malignancies, such as biliary tract neoplasms and pancreatic carcinoma.[9,10] However, to our knowledge, no studies have reported the prognostic significance of the above inflammation-based markers in patients with recurrent MOJ. In this study, we reported our experience in treating recurrent MOJ and explored the prognostic values of the inflammation-based markers in these patients.
2. Materials and methods
2.1. Study population
The study included 33 patients who experienced MOJ recurrence after the implantation of biliary metal stents by PTCD in our department. All the patients were reimplanted with biliary stent by PTCD under DSA between June 2012 and September 2016. Patients also underwent routine biochemical testing, computerized tomography (CT), magnetic resonance cholangiopancreatography (MRCP), and cholangiography in order to confirm the obstruction of biliary stent. This study was approved by the Institutional Review Board of the First Affiliated Hospital of Bengbu Medical College. Informed consent was obtained from all patients.
2.2. Instruments
The Innova 3100 DSA fluoroscopy device (General Electric) was used during reimplantation surgery. The tubes used for PTCD were pigtail catheters with hydrophilic coating (Guangzhou Leadgem Medical Devices Co., Ltd., China). The biliary stents were self-expandable metal stents (Nanjing Micro-Tech Co., Ltd., China). Biliary needles, catheter sheaths, loach guide wires, ultra-smooth guide wires, ultra-slippery exchange guide wires, and catheters of various sizes (8×40 mm,8×60 mm, 8×80 mm, 8×100 mm, 10×40 mm, 10×60 mm, 10×80 mm, and 10×100 mm, as appropriate) were used. A Hitachi 2000 ultrasound device with a standard abdominal probe was used, with the frequency set to 3.5 MHz.
2.3. Surgical approach
PTCD, including localization of the puncture point, was performed under ultrasound guidance for patients with cholangitis or for whom preoperative imaging showed obvious intrahepatic biliary duct dilation. Lidocaine (2%) was used for local anesthesia, and an 18G needle was used for puncturing. After reaching the target, the needle was withdrawn and the guidewire was passed into the intrahepatic bile duct. The opening in the skin was expanded with a scalpel and the 7F-8.5F pigtail catheter was inserted over the guidewire. Then, the guidewire was subsequently removed, and the drain was connected to the drainage bag and sutured in place to the skin.
One week after the PTCD, biliary stent reimplantation was performed under DSA. Ultrasound or DSA fluoroscopy was used to guide the puncture and entry into the intrahepatic bile duct for patients without optimal preoperative biliary duct dilation. After cholangiography confirmed that PTCD was located within the intrahepatic bile duct, a guide wire was inserted and the PTCD tube was removed. Next, the catheter sheath was inserted, followed by the cholangiographic catheter over the guidewire. The direction of guidewire was repeatedly adjusted to allow the guidewire and the cholangiographic catheter entering into the primary stent at the upper end and completely passing through the stent until the distal end of the duct. Another picture was taken to measure the length and the diameter of the proximal end of the obstructed bile duct. The specifications of the stent that would be used were selected based on these measurements. The cholangiographic catheter was withdrawn and the deployed biliary metal stent was inserted over the guide wire. By DSA-guided adjustment, the stents were slowly placed and released so that the marks at the 2 ends of the stent passed the respective 2 ends of the primary stent by 1 cm. For the cases in which a single stent was not long enough, double stents were used. If stent expansion was poor due to the use of multiple stents, balloon dilation was performed by inserting a balloon over the guidewire. After removing the guide wire, the tube for PTCD was inserted through the original PTCD track and cholangiography was performed again. Entry of contrast material into the distal end of the obstructed biliary duct indicated that the placement of the stent was successful. Finally, the PTCD tube was repositioned (Fig. 1).
Figure 1.
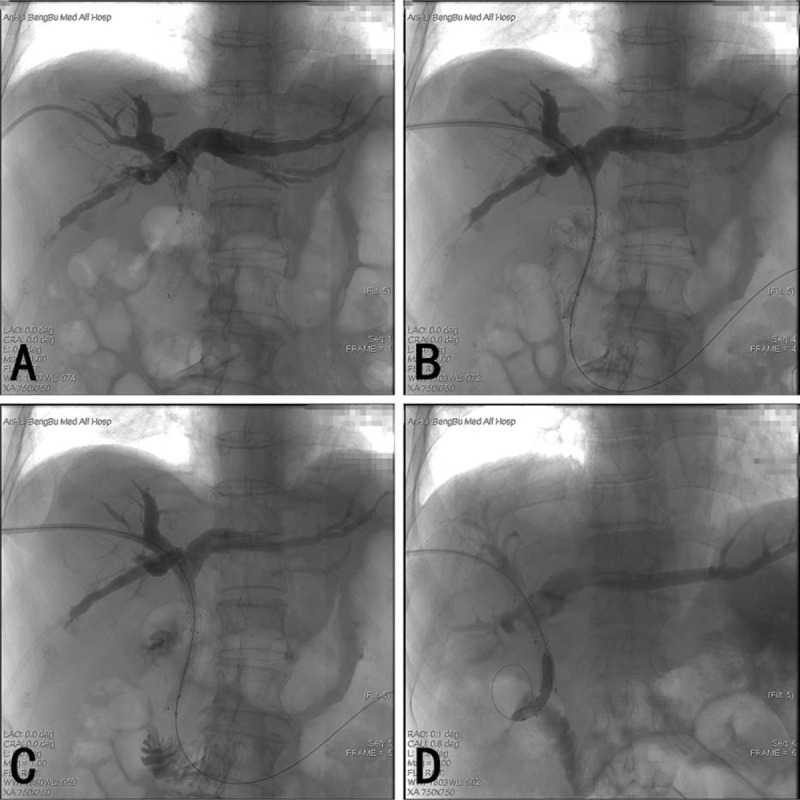
The operation progress of reimplantation of biliary metal stents by PTCD. (A) Cholangiography of PTCD indicating primary stent obstruction. (B) Cholangiographic catheter passes the stent into the intestine. (C) Stent re-implantation. (D) Dilated balloon expanding the implanted stent. PTCD = percutaneous transhepatic cholangial drainage.
2.4. Data collection
We used the electronic medical records to collect the relative data. The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total serum bilirubin (TBIL), and direct bilirubin (DBIL) were noted and compared before the procedure and at 1 week, 1 month, and 3 months after operation. We also collected the preoperative levels of platelet, neutrophil, lymphocyte, and monocyte counts to calculate the SII, PLR, NLR, and MLR.
The incidence of postoperative complications such as bile leak, biliary tract bleeding, amylase elevation, and biliary tract infection, was also observed. Finally, routine biochemical test, CT, MRCP, and cholangiography were used to evaluate stent patency.
2.5. Statistical analysis
SPSS version 21.0 (IBM Corp.) was used for data processing. Continuous variables were expressed as mean ± standard deviation for these with normal distribution and median (range) for the non-normally distributed variables. Comparisons between different groups were performed using the Wilcoxon test for continuous data. The receiver operating characteristic (ROC) curve was used to determine the optimal cut-off point (the highest value of specificity plus sensitivity) of the systemic inflammation models for discriminating between deceased and living patients.
The primary outcome observed was overall survival (OS), which was estimated by the Kaplan–Meier curve. The survival differences were analyzed by the log-rank test. All the variables that were significant (P < 0.05) in univariate analysis were entered into a multivariate analysis with Cox proportional hazard regression models. A P value < 0.05 was considered statistically significant.
3. Results
3.1. Patient characteristics and clinical indicators
Demographic data, serologic tests, and inflammatory response related parameters of the 33 patients are summarized in (Table 1). All patients were reimplanted with the biliary stents as the experience of MOJ recurrence after the initial implantation of stents by PTCD. There were 27 cases of clinically diagnosed cholangiocarcinoma, 4 cases of pancreatic cancer, 1 case of gallbladder cancer, and 1 case of periampullary carcinoma. The ages ranged from 49 to 89 years and 16 were male among these patients. After the reimplantation, clinical symptoms such as jaundice, chills, and fever significantly improved in all patients. At 1 week, 1 month, and 3 months postoperatively, the levels of ALT, AST, TBIL, and DBIL significantly reduced compared to the preoperative levels (Fig. 2, all P < 0.05).
Table 1.
Basic characteristics of the 33 patients with recurrent MOJ.
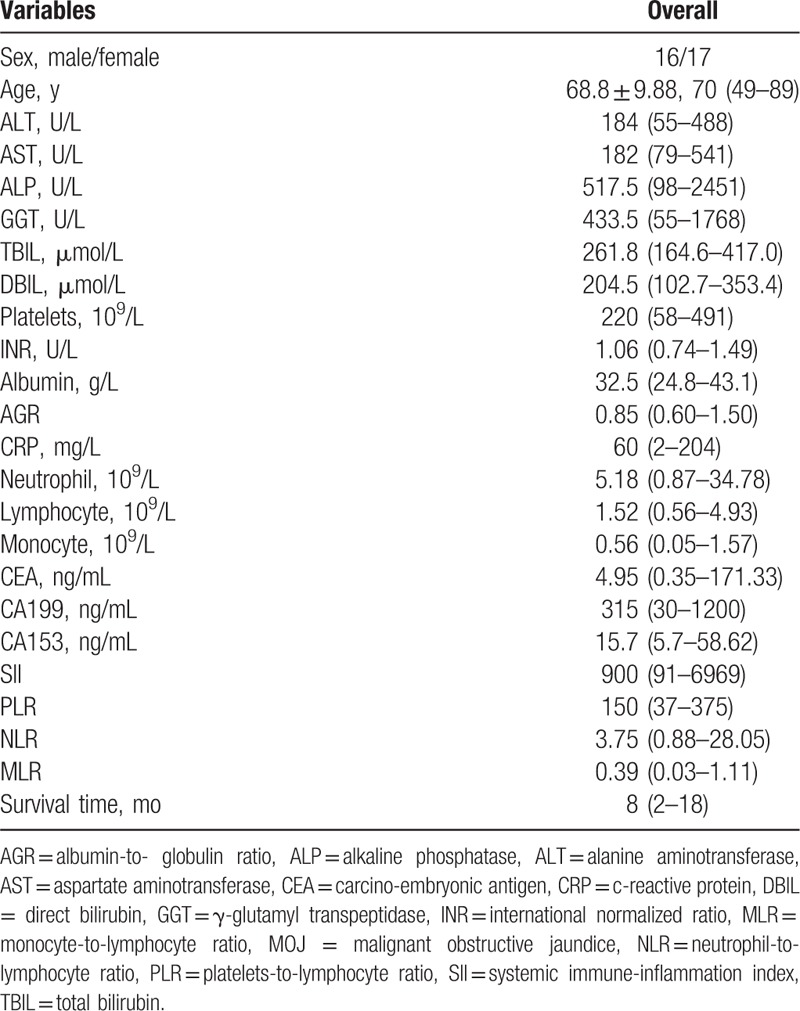
Figure 2.
Comparison of patients’ liver function between preoperation and postoperation. When compared to the preoperative, the levels of ALT (A), AST (B), TBIL (C), and DBIL (D) significantly improved at 1 week, 1 month, and 3 months postoperatively (∗P < 0.05). ALT = alanine aminotransferase, AST = aspartate aminotransferase, DBIL = direct bilirubin, TBIL total serum bilirubin.
3.2. Complications
No complications were found in patients with ultrasound-guided puncture. However, all the 4 patients with direct puncture experienced complications after the reimplantation. One case of biliary tract bleeding was treated by applying hemostatic agents intravenously. Another case of bile leakage was treated by the peritoneal drainage. In addition, 2 cases of biliary tract infection were treated with antibiotic therapy.
3.3. Determination of the cut-off value for the SII, PLR, NLR, and MLR
The ROC curve of SII, PLR, NLR, and MLR indicated that 644, 139, 2.47, and 0.45 were optimal cut-off values with 88.9% sensitivity and 83.3% specificity, 77.8% sensitivity and 75.0% specificity, 83.3% sensitivity and 69.2% specificity, 55.6% sensitivity and 92.3% specificity, respectively. Of the 4 models, the SII and PLR were significant indicators for determining the deceased from the living patients, with the area under the curve (AUC) values 0.843 (95% CI: 0.673–1.000, P = 0.002) and 0.838 (95% CI: 0.696–0.980, P = 0.002), respectively.
3.4. Predictors of survival
During a median follow-up time of 10 months, 18 (54.5%) patients died. The median survival time of all patients was 8 months and Fig. 3 showed the Kaplan–Meier cumulative OS curve. The log-rank analysis demonstrated that the OS varied significantly with different levels of the SII, PLR, NLR, and MLR (Fig. 4A–D, all P < 0.05).
Figure 3.
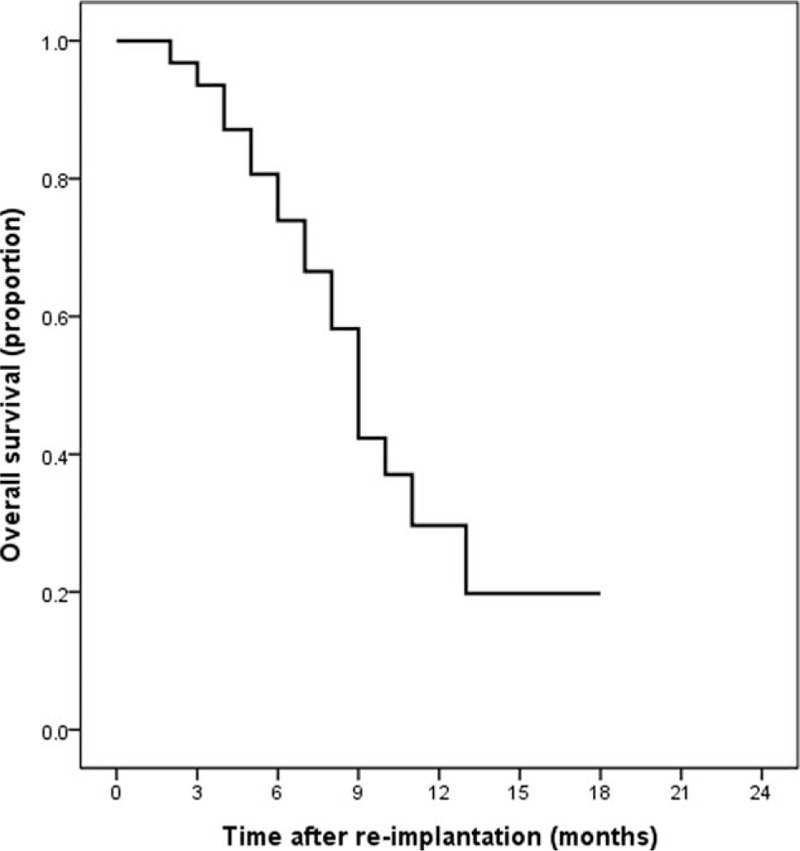
The survival curve of patients after reimplantation of biliary metal stents.
Figure 4.
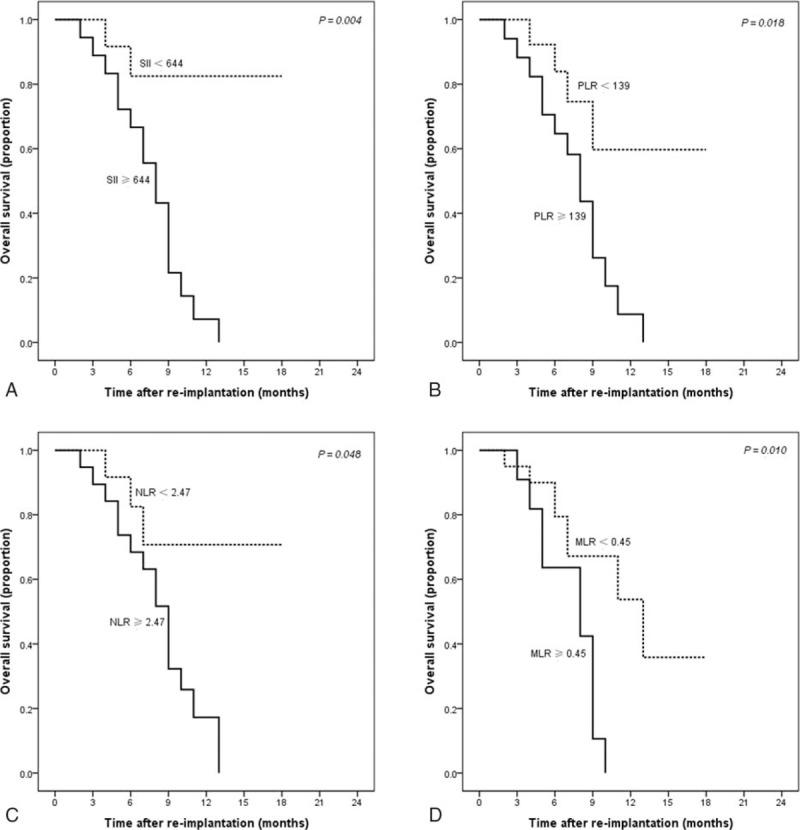
Cumulative survival curves of patients stratified according to the SII (A), PLR (B), NLR (C), and MLR (D). MLR = monocyte-to-lymphocyte ratio, NLR = neutrophil-to-lymphocyte ratio, PLR = platelets-to-lymphocyte ratio, SII = systemic immune-inflammation index.
Univariate analysis showed that gender, ALB, monocyte count, SII, PLR, NLR, and MLR were significant prognostic factors that affected OS (Table 2). Furthermore, multivariate analysis revealed that SII and PLR were independent predictors for OS (Fig. 5).
Table 2.
Univariate and multivariate analyses of factors associated with overall survival of recurrent MOJ patients.
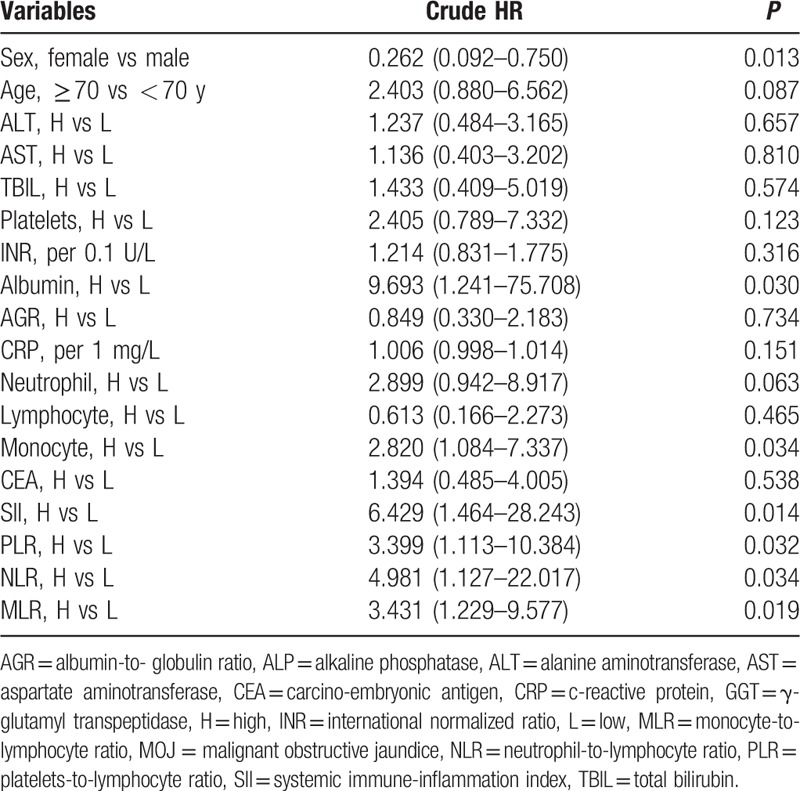
Figure 5.
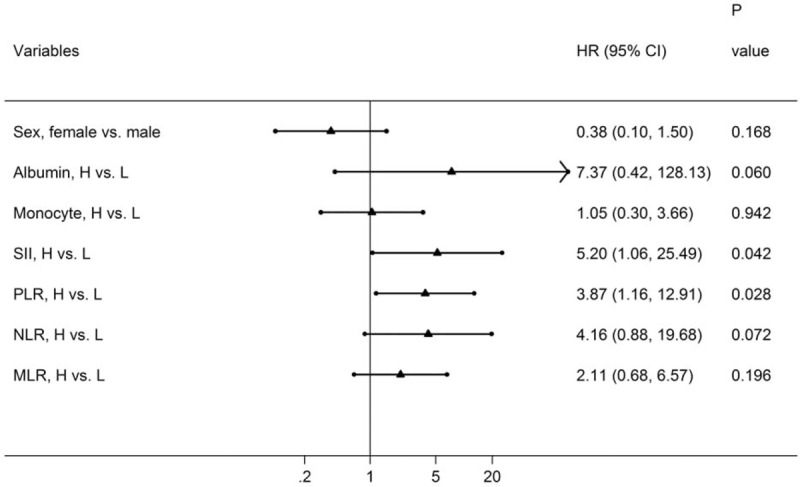
Forest plot based on the results of multivariate analysis of the factors associated with overall survival of recurrent MOJ patients. MOJ = malignant obstructive jaundice.
4. Discussion
As the lack of aggressive and immediate treatment, patients with MOJ generally have a range of homeostatic disorders and a poor survival.[11,12] Although radical resection is the first treatment choice, it is often not feasible as the difficulties in early diagnosis of this malignancy and the poor condition of patients.[13] The advancements in imaging technologies allow obtaining accurate information and it can be used for a comprehensive preoperative evaluation of tumor resectability. For cases where radical resection is not feasible, we may choose laparotomy and internal drainage. However, surgery and anesthesia can cause physical and psychological trauma, whereas there was no significant improvement in the prognosis of patients.[14,15] Additionally, geriatric cases and patients with poor systemic conditions are unable to tolerate laparotomy. Thus, we recommended the minimally invasive biliary stenting procedure for drainage in some patients.
Biliary stents can be placed by endoscopy or by percutaneous transhepatic route. The endoscopic procedure requires a complex endoscopic system and has more stringent requirements on the patient's general health condition. Therefore, biliary stent placement by the percutaneous transhepatic route is more widely used in our department. However, biliary stent implantation is limited to inhibit tumor growth. Stent obstruction gradually emerges from tumor ingrowth through the stent meshor, biliary sludge deposition, or biofilm [16], and finally leads to the reoccurrence of MOJ.
In our department, some patients with recurrent MOJ sill have good overall health and have no distal metastases. However, impaired liver function is unavoidable in these patients. The laparotomy for recurrent MOJ is less likely and endoscopic treatment is also hard to implement. Thus, PTCD is the only feasible treatment method.[17] However, a simple PTCD has several drawbacks, such as drainage tube dislodgement, water and electrolyte imbalance, and gastrointestinal dysfunctions. It also increases the susceptibility to infections due to the long-term indwelling drain. Therefore, we performed biliary stent reimplantation by PTCD in patients with recurrent MOJ. In this study, we collected their pre- and postoperative clinical data and survival time. We found that symptoms and liver function significantly improved after stent reimplantation in patients with recurrent MOJ. Our results regarding postoperative survival time were very promising and were consistent with the 6- to 9-month median survival time reported in previous studies.[18–20] Therefore, we conclude that biliary stent reimplantation improves the quality of life and increases survival time in patients with recurrent MOJ. Moreover, PTCD does not require complex equipment and patients are free from the high risk of surgery and general anesthesia. Therefore, our results further suggest that reimplantation by PTCD has a potential value of clinical application in recurrent MOJ.
It has been indicated that, for patients with MOJ who are expected to live longer than 4 months, placement of a biliary stent is the recommended treatment.[21] Recurrent MOJ patients, who are accompanied with cachexia, may have relatively short expected survival time and may be unable to tolerate stent reimplantation. Thus, patients can be recommend for biliary stent reimplantation if they fulfill the following criteria: no dysfunction of a vital organ (heart, lung, kidney, etc.); no serious hypoalbuminemia, ascites, water, or electrolyte imbalance; overall systemic condition capable of tolerating stent implantation; tumors without signs of distant metastases, or with distant metastasis but acceptable general health condition; no intrahepatic bile duct tumor thrombus on preoperative CT, MRI, and cholangiography; large expected drainage range after stent insertion to enable effective jaundice reversal.
Percutaneous biliary stent reimplantation is more difficult than the initial stent placement operation. With PTCD cholangiography, the guidewire is inserted and the PTCD tube is removed. After the sheath is inserted, the cholangiographic catheter is also inserted and subsequently passed through the obstruction with the aid of the guide wire. It is important to note that the guidewire must be passed from the end of the stent and not through the mesh of the previous stent, or else the stent will not self-expand and it will diminish the efficiency of the treatment. Therefore, due to the influence of the initial stent, passing the guidewire is more difficult to achieve during the reimplantation than the first implantation. If necessary, ultrafine cholangioscopy can be used to direct the guidewire. Among the cases included in our study, there were 2 patients for whom the wire was unable to pass through the obstruction. It is important to keep in mind that the reimplanted stent must bypass the original stent. When necessary, multiple stents can be placed, and if stents self-expand poorly, balloon dilation can be used.
Biliary stents can only relieve the obstruction, but have no therapeutic effect on the tumor. In order to prevent stent obstruction, comprehensive treatments, such as the placement of radioactive seeds into the bile duct, radiofrequency ablation of intraductal tumors, intraductal chemotherapy infusion, and systemic chemotherapy, should be implemented.[22] For patients with biliary stent reimplantation, provided the overall condition permits, comprehensive treatment should also be implemented to prolong stent patency time and survival time. Furthermore, to improve prognosis, it is necessary to explore the potential factors that affect survival.
Accumulating evidence indicates that systemic inflammatory responses play important roles in various stages of tumor development, such as initiation, promotion, invasion, and metastasis.[8–23] The tumor increases the process of inflammatory, which in turn predisposes to tumor progress via inhibiting apoptosis, promoting angiogenesis, and DNA damage.[24] Recently, numerous research has confirmed that, several hematological markers of inflammatory responses, such as SII, PLR, NLR, and MLR, are significant prognostic models in various types of cancers, such as prostate cancer,[25] lung cancer,[26] hepatocellular carcinoma,[27] and biliary tract cancers.[9]
MOJ is mainly caused by cholangiocarcinoma, pancreatic cancer, or gallbladder carcinoma, all of which are closely correlated with the underlying inflammation. In our cohort, none of the single parameters (platelets, neutrophil, and lymphocyte count) alone could significantly predict survival. However, the simple combinations of these parameters, such as inflammatory-related SII, PLR, and NLR, were powerful predictors in survival. Furthermore, multivariate analysis identified the SII and PLR as independent factors for predicting OS in patients with recurrent MOJ.
The mechanism of increased SII/PLR and poor prognosis is still not clear. The associations of the 2 indices with inflammation might be significant. In addition, all the parameters in SII and PLR were associated with tumor formation and progression. In vitro, platelets could accelerate tumor growth and invasion via releasing several platelet-derived mediators.[28,29] Recently, platelets have also been reported to significantly increase the risk of mortality from various cancers.[30] Furthermore, lymphocyte is a crucial component of the immune system and reflects immune response against tumor. The lymphocyte count is found to be positively correlated with tumor stage.[31] In addition, tumor-infiltrating leukocytes, including neutrophils and monocytes, may also play a crucial role in tumor development and progression.[32] Studies indicate that the increased preoperative monocyte count is negatively correlated with survival in malignant patients.[33,34]
Several limitations were noted in the current study. First, this study analyzed a single institution experience and was carried out with a retrospective cohort design. The study quality could not be effectively controlled and the confounding might be inevitably. Second, the sample size was small and it was unreliable to perform further stratified analysis. Various location of tumor may be a crucial potential confounder in our study and subgrouped analysis should be performed. However, among the 33 patients the study included, 27 cases were cholangiocarcinoma, 6 were other malignancies, and the results of subgrouped analysis were meaningless. Third, our results may only provide guiding significance in patients with recurrent MOJ. The prognostic values of these inflammatory-related markers in initial MOJ patients that treated by the PTCD or other approaches should be investigated in the future study.
In conclusion, in patients with MOJ recurrence after implantation of biliary metal stents by PTCD, reimplantation is clinically feasible as it improves the quality of life and increases the survival time. In addition, several inflammatory response-related markers, particularly SII and PLR, are independent, useful models for predicting outcomes.
Acknowledgments
The authors would like to thank Editage [www.editage.cn] for English language editing.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, DBIL = direct bilirubin, DSA = digital subtraction angiography, MLR = monocyte-to-lymphocyte ratio, MOJ = malignant obstructive jaundice, MRCP = magnetic resonance cholangiopancreatography, NLR = neutrophil-to-lymphocyte ratio, OS = overall survival, PLR = platelets-to-lymphocyte ratio, PTCD = percutaneous transhepatic cholangial drainage, SII = systemic immune-inflammation index, TBIL total serum bilirubin.
HJ and QP contributed equally to this work.
Funding: The study was supported by the Science and Technology Innovation Fund project of Anhui Province (grant no. 1501041155) and the Science and Technology Development Fund project of Bengbu Medical Collage (grant no. BYKF1404).
The authors have no conflicts of interest to disclose.
References
- [1].Wang L, Yu WF. Obstructive jaundice and perioperative management. Acta Anaesthesiol Taiwan 2014;52:22–9. [DOI] [PubMed] [Google Scholar]
- [2].Hucl T. Malignant biliary obstruction. Cas Lek Cesk 2016;155:30–7. [PubMed] [Google Scholar]
- [3].Chen Y, Wang XL, Yan ZP, et al. The use of (1)(2)(5)I seed strands for intraluminal brachytherapy of malignant obstructive jaundice. Cancer Biother Radiopharm 2012;27:317–23. [DOI] [PubMed] [Google Scholar]
- [4].Fei SX, Liu HC, Sun Z, et al. Evaluation of the curative effect of biliary stents combined with 125I particles for intracavitary treatment of malignant jaundice in cholangiocarcinoma. Chin J Clin Oncol 2015;45:564–9. [Google Scholar]
- [5].Isayama H, Nakai Y, Kogure H, et al. Biliary self-expandable metallic stent for unresectable malignant distal biliary obstruction: which is better: covered or uncovered? Dig Endosc 2013;25(suppl 2):71–4. [DOI] [PubMed] [Google Scholar]
- [6].Tan DW, Fu Y, Su Q, et al. Prognostic significance of neutrophil to lymphocyte ratio in oncologic outcomes of cholangiocarcinoma: a meta-analysis. Sci Rep 2016;6:33789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Guthrie GJ, Roxburgh CS, Farhan-Alanie OM, et al. Comparison of the prognostic value of longitudinal measurements of systemic inflammation in patients undergoing curative resection of colorectal cancer. Br J Cancer 2013;109:24–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ha H, Nam AR, Bang JH, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget 2016;7:76605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- [11].Sutter CM, Ryu RK. Percutaneous management of malignant biliary obstruction. Tech Vasc Interv Radiol 2015;18:218–26. [DOI] [PubMed] [Google Scholar]
- [12].Scott-Conner CE, Grogan JB. The pathophysiology of biliary obstruction and its effect on phagocytic and immune function. J Surg Res 1994;57:316–36. [DOI] [PubMed] [Google Scholar]
- [13].Yao D, Kunam VK, Li X. A review of the clinical diagnosis and therapy of cholangiocarcinoma. J Int Med Res 2014;42:3–16. [DOI] [PubMed] [Google Scholar]
- [14].Wu WG, Gu J, Dong P, et al. Duct-to-duct biliary reconstruction after radical resection of Bismuth IIIa hilar cholangiocarcinoma. World J Gastroenterol 2013;19:2441–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shah SR. Issues in surgery for hilar cholangiocarcinoma. Indian J Surg 2012;74:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Krokidis M, Fanelli F, Orgera G, et al. Percutaneous palliation of pancreatic head cancer: randomized comparison of ePTFE/FEP-covered versus uncovered nitinol biliary stents. Cardiovasc Intervent Radiol 2011;34:352–61. [DOI] [PubMed] [Google Scholar]
- [17].Lawson AJ, Beningfield SJ, Krige JE, et al. Percutaneous transhepatic self-expanding metal stents for palliation of malignant biliary obstruction. S Afr J Surg 2012;50:54–8. [DOI] [PubMed] [Google Scholar]
- [18].Choi JM, Kim JH, Kim SS, et al. A comparative study on the efficacy of covered metal stent and plastic stent in unresectable malignant biliary obstruction. Clin Endosc 2012;45:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Maluf-Filho F, Retes FA, Neves CZ, et al. Transduodenal endosonography-guided biliary drainage and duodenal stenting for palliation of malignant obstructive jaundice and duodenal obstruction. JOP 2012;13:210–4. [PubMed] [Google Scholar]
- [20].Pan H, Liang Z, Yin TS, et al. Hepato-biliary-enteric stent drainage as palliative treatment for proximal malignant obstructive jaundice. Med Oncol 2014;31:853. [DOI] [PubMed] [Google Scholar]
- [21].Dumonceau JM, Tringali A, Blero D, et al. Biliary stenting: indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 2012;44:277–98. [DOI] [PubMed] [Google Scholar]
- [22].Soares KC, Kamel I, Cosgrove DP, et al. Hilar cholangiocarcinoma: diagnosis, treatment options, and management. Hepatobiliary Surg Nutr 2014;3:18–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45. [DOI] [PubMed] [Google Scholar]
- [25].Lolli C, Caffo O, Scarpi E, et al. Systemic immune-inflammation index predicts the clinical outcome in patients with mCRPC treated with abiraterone. Front Pharmacol 2016;7:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yang HB, Xing M, Ma LN, et al. Prognostic significance of neutrophil-lymphocyteratio/platelet-lymphocyteratioin lung cancers: a meta-analysis. Oncotarget 2016;7:76769–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xu X, Chen W, Zhang L, et al. Prognostic significance of neutrophil to lymphocyte ratio in patients with hepatocellular carcinoma after transcatheter arterial chemoembolization. Chin Med J (Engl) 2014;127:4204–9. [PubMed] [Google Scholar]
- [28].Carr BI, Cavallini A, D’Alessandro R, et al. Platelet extracts induce growth, migration and invasion in human hepatocellular carcinoma in vitro. BMC Cancer 2014;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sierko E, Wojtukiewicz MZ. Platelets and angiogenesis in malignancy. Semin Thromb Hemost 2004;30:95–108. [DOI] [PubMed] [Google Scholar]
- [30].Buergy D, Wenz F, Groden C, et al. Tumor-platelet interaction in solid tumors. Int J Cancer 2012;130:2747–60. [DOI] [PubMed] [Google Scholar]
- [31].Iwase R, Shiba H, Haruki K, et al. Post-operative lymphocyte count may predict the outcome of radical resection for gallbladder carcinoma. Anticancer Res 2013;33:3439–44. [PubMed] [Google Scholar]
- [32].Fidler IJ, Schroit AJ. Recognition and destruction of neoplastic cells by activated macrophages: discrimination of altered self. Biochim Biophys Acta 1988;948:151–73. [DOI] [PubMed] [Google Scholar]
- [33].Ren QQ, Fu SJ, Zhao Q, et al. Prognostic value of preoperative peripheral monocyte count in patients with hepatocellular carcinoma after liver transplantation. Tumour Biol 2016;37:8973–8. [DOI] [PubMed] [Google Scholar]
- [34].Sasaki A, Iwashita Y, Shibata K, et al. Prognostic value of preoperative peripheral blood monocyte count in patients with hepatocellular carcinoma. Surgery 2006;139:755–64. [DOI] [PubMed] [Google Scholar]


