Abstract
This nationwide population-based study investigated the risk of Parkinson disease (PD) in relation to diabetes mellitus (DM) through the National Health Insurance Research Database in Taiwan.
A retrospective study was conducted, consisting of 36,294 patients who were newly diagnosed with DM between January 1, 2000 and December 31, 2006 and 108,882 individuals without DM as healthy controls from insurance claims data from Taiwan's National Health Research Institutes Dataset. The subjects were followed up until December 31, 2011 or until the first manifestation of PD. The hazard ratio (HR) of DM for PD incidence was estimated by Cox proportional hazard regression model.
Compared with the non-DM cohort, the incidence density rate of PD was 1.36-fold higher in the DM cohort (1.53 vs 2.08 per 1000 person-years) with an adjusted HR of 1.19 (95% confidence interval = 1.08–1.32) after adjusting for age, sex, comorbidities, and medication use. The adjusted HR of PD for DM with a larger magnitude was observed in females (1.29, 1.12–1.49); individuals age 65 years and older (1.20, 1.06–1.35); those without schizophrenia (1.20, 1.08–1.33), bipolar disorder (1.20, 1.08–1.33), hypertension (1.18, 1.06–1.32), hyperlipidemia (1.21, 1.09–1.34), chronic obstructive pulmonary disease (1.19, 1.06–1.32), coronary artery disease (1.22, 1.09–1.36), stroke (1.23, 1.10–1.37), asthma (1.20, 1.08–1.34), flunarizine use (1.21, 1.08–1.35), zolpidem use (1.16, 1.04–1.30), Charlson comorbidity index score of 0 (1.23, 1.08–1.40), and those using metoclopramide (1.35, 1.14–1.60) and zolpidem (1.46, 1.12–1.90).
DM increased the risk of PD during a mean follow-up of 7.3 years. Further mechanistic research on the effect of DM on PD is needed.
Keywords: diabetes mellitus, medication use, Parkinson disease
1. Introduction
Changes in human behavior and lifestyle have globally increased the prevalence of diabetes mellitus (DM), and an estimated 220 million people who are affected by DM was reported in 2010.[1] The global figure of persons with DM is estimated to be 350 million in 2025.[1] Recent studies have demonstrated the mitochondria as the key regulator of glucose-stimulated insulin secretion in pancreatic β-cells.[2,3] Increasing evidence has shown that mitochondrial function is closely related to various facets of diabetes, including pancreatic β-cell dysfunction, insulin resistance, obesity, and vascular complications.[2,4]
Many diseases of mitochondrial dysfunction affect more than 1 system in the human body. They affect organs that require a lot of energy, including the heart, skeletal muscle, and brain. Parkinson disease (PD) is a progressive neurodegenerative disease. One study of specific gene mutations that cause PD has reinforced the relevance of oxidative stress and mitochondrial dysfunction in the familial and the sporadic forms of the disease.[5] The result of the study indicates that the PD associated proteins are either mitochondrial proteins or associated with mitochondria, and all interface with the pathways of oxidative stress and free radical damage.[5] Although the exact causes of neuronal damage are unknown, several lines of evidence suggested that mitochondrial dysfunction is one of the biochemical abnormalities described in the brains of PD patients.[5,6] The incidence rates of PD among DM patients increase with age.[7] Many studies in the literature indicate that PD and DM, both age-related chronic diseases, share remarkably similar pathways of mitochondrial dysfunction and suggest the association of DM with PD.[7–9] However, the relationship between DM and PD was inconsistent with several epidemiological reports, ranging from a positive[7,10,11] to null association[11] or even an inverse correlation.[12] Lu et al conducted a meta-analysis study from 14 reports and concluded that evidence from case–control studies suggested that diabetic individuals may have a decreased incidence of PD despite significant heterogeneity.[12] In the other meta-analysis exploring this line of question including cohort and case–control studies, the pooled results of 4 cohort studies with large sample size demonstrated that diabetes was associated with a significant 37% increased risk of PD[11] whereas no association between diabetes and PD was found in a meta-analysis of 7 case–control studies.[11] The major limitation of these case–control studies was their small sample size. Only 3 studies had sample size >1000.[13–15] As for prior cohort studies, 2 studies included subjects with type 1 and type 2 diabetes. In addition, most of these studies came from Western countries, and none could rule out the possible explanation of well-known medication that causes PD on such an association between type 2 DM and PD.
Many authors have used the National Health Insurance Research Database (NHIRD) in Taiwan to study PD and DM.[7,10] However, none of these studies simultaneously considered diabetes status, comorbidity, and medication use. To clarify the role of type 2 DM on the risk of PD incidence, we conducted a large cohort study of Chinese patients with and without type 2 DM in Taiwan by using the NHIRD. We also examined the interaction and joint association of type 2 diabetes with comorbidities, including schizophrenia, bipolar disorder, hypertension, and hyperlipidemia, as well as flunarizine use, metoclopramide use, and zolpidem use on PD incidence in individuals with and without type 2 DM. The subjects’ ages ≥20 years and were followed up for an average of 7.3 years. To acquire an adequate number of cases to provide a robust estimation of the potential role of type 2 DM in PD incidence, data from a large representative population followed up for a sufficient length of time are necessary, and the NHIRD fulfills this requirement. The high accuracy of the NHIRD has been reported, and this nationwide population-based dataset appears to be a valid resource for population research.[16,17] Furthermore, the NHIRD allows researchers to trace the medical service utilization history of all citizens in Taiwan and provides a unique opportunity to examine the possible association between DM and the risk of PD.
2. Methods
2.1. Data sources
Taiwan implemented the National Health Insurance (NHI) program, a universal insurance system established by the Bureau of National Health Insurance of the Department of Health in 1995. The NHI program covered 22.60 million out of the 22.96 million people in Taiwan by the end of 2007. The NHIRD, a dataset of claims from Taiwan's NHI program, was the data source of the present study. The NHIRD contains information on patients’ date of birth, sex, records of outpatient visits and hospitalizations, and details of prescriptions, such as prescribed drugs, dosages, and expenditure amounts, as well as diagnosed diseases coded in accordance with the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). Each individual has a unique personal identification number. To protect privacy, personal information of all insured patients has been scrambled cryptographically to ensure anonymity. Our study was approved by the Ethics Review Board of China Medical University (CMU-REC-101-012).
2.2. Study population
We conducted a population-based retrospective cohort study that included 2 groups. We initially identified 37,616 newly diagnosed patients with DM (ICD-9-CM Code 250) age ≥20 years in outpatient and inpatient care from the NHIRD from January 1, 2000 to December 31, 2006. Individuals included in the DM group had at least 3 outpatient claims or at least 1 inpatient claim of ICD-9 Code 250 from 2000 to 2006, with the first diagnosis as the index date. In Taiwan, the diagnosis of DM in clinical practice was based on American Diabetes Association criteria (ICD-9-CM diagnosis code 250). Generally for diabetes diagnosis of a new patient, an individual who has fasting plasma glucose > 126 mg/dL (or 7.0 mmol/L) or plasma glucose ≥ 200 mg/dL (or 11.1 mmol/L) during an oral glucose tolerance test is asked to have a repeated test on a different day to confirm the diagnosis in order to increase the validity of diabetes diagnosis. Among these patients, a total of 272 who had previously been diagnosed with PD and 1050 who had withdrawn from the NHI program within a year of follow-up were excluded from the analysis. Up to 36,294 patients with newly diagnosed DM were included in the DM cohort. For each identified patient with DM, 3 insured people without a history of DM or PD were randomly selected and frequency-matched by sex, age (5 years), and index year. The non-DM group comprised 108,882 individuals.
2.3. Outcome ascertainment, comorbidity, and medication use
The main outcome was the occurrence of PD (ICD-9 Code 332.0), which was determined by linking records with ambulatory and inpatient care data in the NHIRD. Patients with PD were defined as those with a diagnostic code of PD (ICD-9-CM 332.0) with at least 3 or more consistent diagnoses in ambulatory care or 1 in inpatient care. To rule out the possible reverse causality, we excluded new PD cases within 1 year of follow-up. There is 1 major reason why we defined individuals with diagnoses in at least 3 ambulatory visits as cases. This operational definition enhances the validity of outcome ascertainment by excluding probable cases and by including cases really suffering from the disease or condition of interest. Usually an individual with symptoms will seek for outpatient care and physicians will evaluate his/her disease status with diagnostic tests. For the case of PD, a neurological history is taken including exclusion of other diseases that imitate PD, such as stroke or hydrocephalus, movement disorder assessments by specialists are made, and/or challenge test through medication is prescribed, such as levodopa and apomorphine. Then he/she will have the second outpatient visit to get his/her test results. If he/she is diagnosed as a case, he/she will have follow-up outpatient visits for treatments. Using this operational definition, we can rule out those individuals who had been suspected to have disease in the first visit but turn out not to have the disease in the second visit. We used this process in previous study.[18] All patients were observed from the index date to the date of PD diagnosis, withdrawal from the NHI program, or the end of 2011. Pre-existing comorbidities included schizophrenia (ICD-9 Code 295, V11.0), bipolar disorder (ICD-9 Code 296), hypertension (ICD-9 Codes 401–405), hyperlipidemia (ICD-9 Code 272.2), chronic obstructive pulmonary disease (COPD) (ICD-9 Codes 490–492, 494, 496), coronary artery disease (CAD) (ICD-9 Codes 410–413, 414.01–414.05, 414.8, and 414.9), stroke (ICD-9 Codes 430–438), and asthma (ICD-9-CM: 493, 494), which were identified from outpatient or inpatient claims within 2 years prior to the index date. The Charlson comorbidity index (CCI) was derived from chronic conditions identified from inpatient claims prior to the index date, including 10 conditions with a weight of 1 (myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular accident, dementia, COPD, connective tissue disease, peptic ulcer disease, mild liver disease, and DM), 6 conditions with a weight of 2 (moderate to severe chronic kidney disease, hemiplegia, DM with end-organ damage, leukemia, tumor of any type, and malignant lymphoma), 1 condition with a weight 3 (moderate to severe liver disease), and 2 conditions with a weight of 6 (acquired immune deficiency syndrome and metastatic solid tumor).
We considered 4 types of medication use, namely, insulin, flunarizine, metoclopramide, and zolpidem. Individuals who had filled at least 1 prescription for these medications within 2 years prior to the index date identified from outpatient medical orders were defined as users.
2.4. Statistical analysis
Age and comorbidities were compared in patients with and without DM by using Chi-squared test for categorical variables and t test for continuous variables. The cumulative incidence curves of PD in the DM and non-DM cohorts were assessed using Kaplan–Meier analysis, and the differences between the cohorts were compared using log-rank test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using multivariate Cox proportional hazards regression model to evaluate the association between DM and the incidence of PD. We also used the Cox model to estimate the interaction between DM and comorbidity or medication use. To explore the joint effect of DM and each chronic disease or medication use, 3 dummy variables were created. Using individuals without DM and without chronic disease or medication use as reference group, these 3 dummy variables measured only the effects of DM, chronic disease or medication use only, and combined DM and chronic disease or medication use. The interactions of DM with comorbidities, including schizophrenia, bipolar, hypertension, and hyperlipidemia, and medication use at baseline of flunarizine, metoclopramide, and zolpidem, were further examined by adding their product terms into the full model. Likelihood-ratio test was used to determine the significance of these interactions. The relative excess risk due to interaction (RERI), proportion attributable to interaction (AP), and synergy index (S index) were also calculated to assess whether the interaction was on an additive scale. The RERI, AP, and S index were interpreted as follows: RERI = 0, AP = 0, or S index = 1 indicates no interaction; PERI > 0, AP > 0, or S index > 1 shows positive interaction; and RERI < 0, AP < 0, or S index < 1 means negative interaction. All statistical analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC). Significance levels were set at a 2-tailed P < 0.05.
3. Results
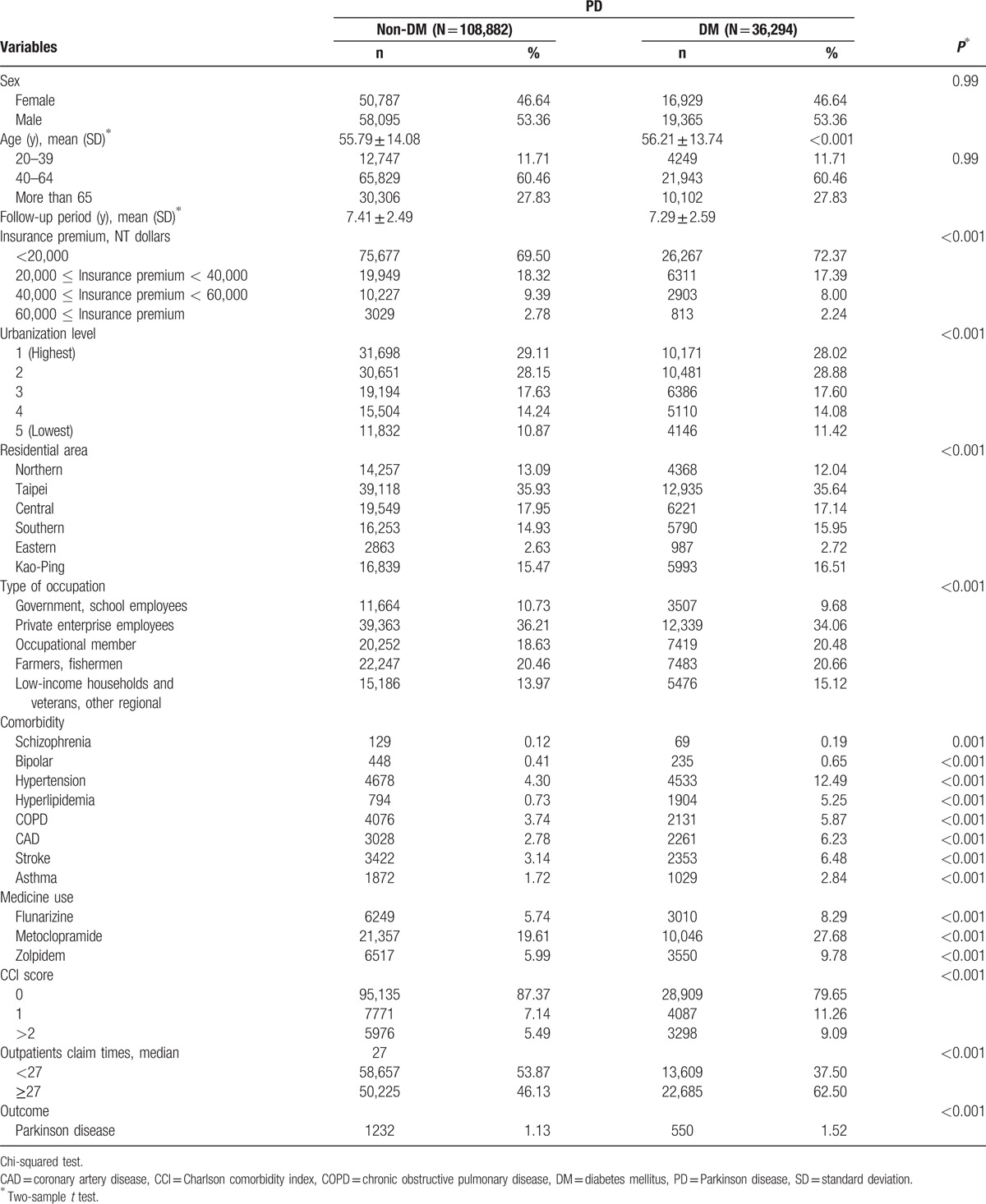
The sample comprised 36,294 DM patients and 108,882 non-DM patients who exhibited similar sex and age distributions (Table 1). The corresponding average ages of the DM and non-DM cohorts were 56.21 and 55.79 years, and the mean follow-up years were 7.41 and 7.29, respectively. Compared with the non-DM cohort, the DM cohort exhibited a higher prevalence of insurance premium <NT 20,000 dollars; hypertension (12.49% vs 4.30%); hyperlipidemia (5.25% vs 0.73%); COPD (5.87% vs 3.74%), CAD (6.23% vs 2.78%), stroke (6.48% vs 3.14%), asthma (2.84% vs 1.72%); flunarizine (8.29% vs 5.74), metoclopramide (27.68% vs 19.61%), and zolpidem (9.78% vs 5.99%); CCI score >2 (9.09% vs 5.49%); and PD incidence (1.52% vs 1.13%).
Table 1.
Demographic characteristics and comorbidity in patients with and without DM cohort groups.
Kaplan–Meier analysis demonstrated that the cumulative incidence curves of PD were significantly higher in the DM cohort than in the non-DM cohort (log-rank test P < 0.001) (Fig. 1). The mean follow-up periods for the DM and non-DM cohorts were 7.29 years (standard deviation [SD] 2.59) and 7.41 years (SD 2.49), and the incidence rates of PD (per 1000 person-years) were 2.08 and 1.53, respectively. Multivariate Cox proportional hazards regression analyses revealed a 1.19-fold higher risk of PD in DM patients than in non-DM patients after age and comorbidities were adjusted (95% CI = 1.08–1.32) (Table 2). Moreover, we observed age (age group of 40–64 years: 11.78, 5.58–24.88; ≥65 years: 61.72, 29.27–130.13 compared with the age group of 20–39 years), insurance premium (individuals with insurance premium 40,000–60,000 NT dollars: 0.74, 0.58–0.95; those with insurance premium ≥60,000 NT dollars: 0.43, 0.24–0.79 compared with individuals with <20,000 NT dollars), schizophrenia (4.21, 2.16–8.17), bipolar disorder (2.18, 1.52–3.13), stroke (1.31, 1.12–1.53), flunarizine use (1.36, 1.19–1.56), zolpidem use (1.41, 1.22–1.63), CCI score (score 1: 1.83, 1.61–2.07; and score ≥2: 1.48, 1.27–1.73), and number of outpatients visits ≥27 (1.45, 1.29–1.63), which were significant factors for PD incidence. We further explored the association between DM and PD by stratifying age, sex, comorbidity, and medication use (Table 3). The adjusted HR of PD for DM with a larger magnitude was observed in females (1.29, 1.12–1.49); individuals age 65 years and older (1.20, 1.06–1.35); those without schizophrenia (1.20, 1.08–1.33), bipolar disorder (1.20, 1.08–1.33), hypertension (1.18, 1.06–1.32), hyperlipidemia (1.21, 1.09–1.34), COPD (1.19, 1.06–1.32), CAD (1.22, 1.09–1.36), stroke (1.23, 1.10–1.37), asthma (1.20, 1.08–1.34), flunarizine use (1.21, 1.08–1.35), zolpidem use (1.16, 1.04–1.30), CCI score of 0 (1.23, 1.08–1.40), and number of outpatients visits ≥27 (1.20, 1.07–1.35) and those with metoclopramide (1.35, 1.14–1.60) and zolpidem use (1.46, 1.12–1.90). We did not detect any significant interaction between DM and any comorbidity or medication use (P > 0.05).
Figure 1.
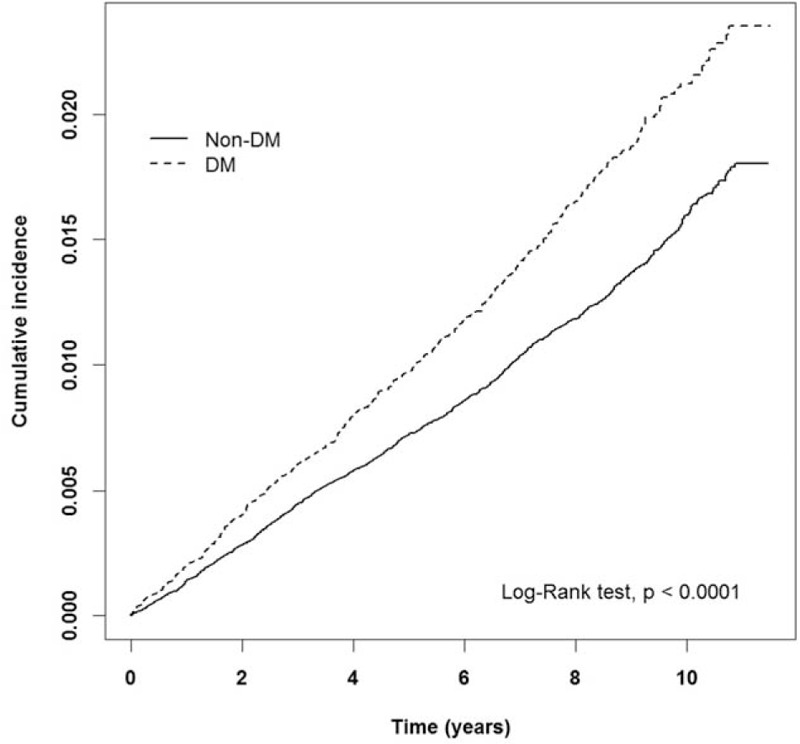
Cumulative incidence of Parkinson disease estimated by Kaplan–Meier method for patients with and without diabetes mellitus cohort.
Table 2.
Cox model measured hazard ratio and 95% confidence intervals of Parkinson disease associated with patients with DM.
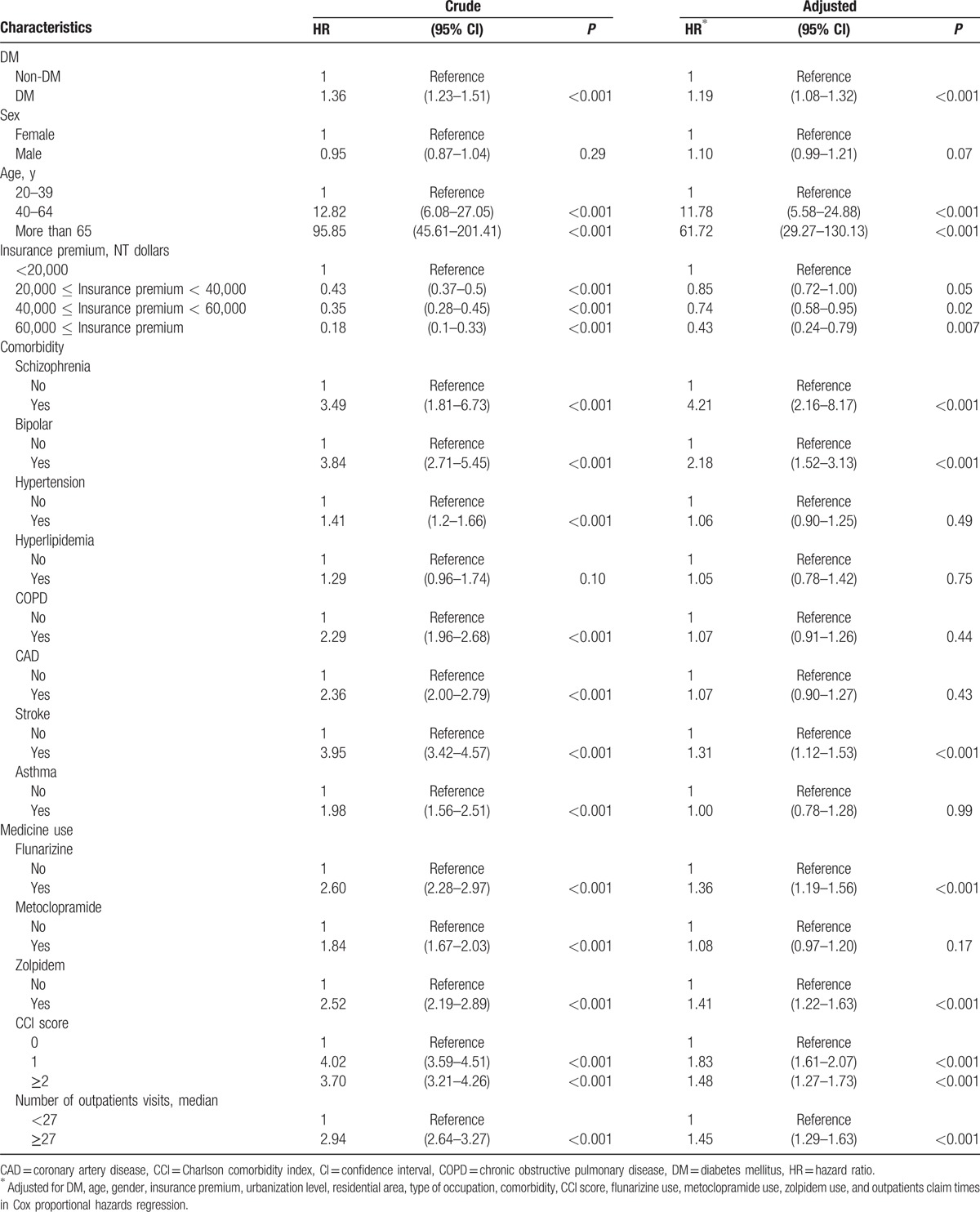
Table 3.
Incidence rates, hazard ratio, and confidence intervals of Parkinson disease for patients with and without DM stratified by demographic, comorbidity, and drug used.
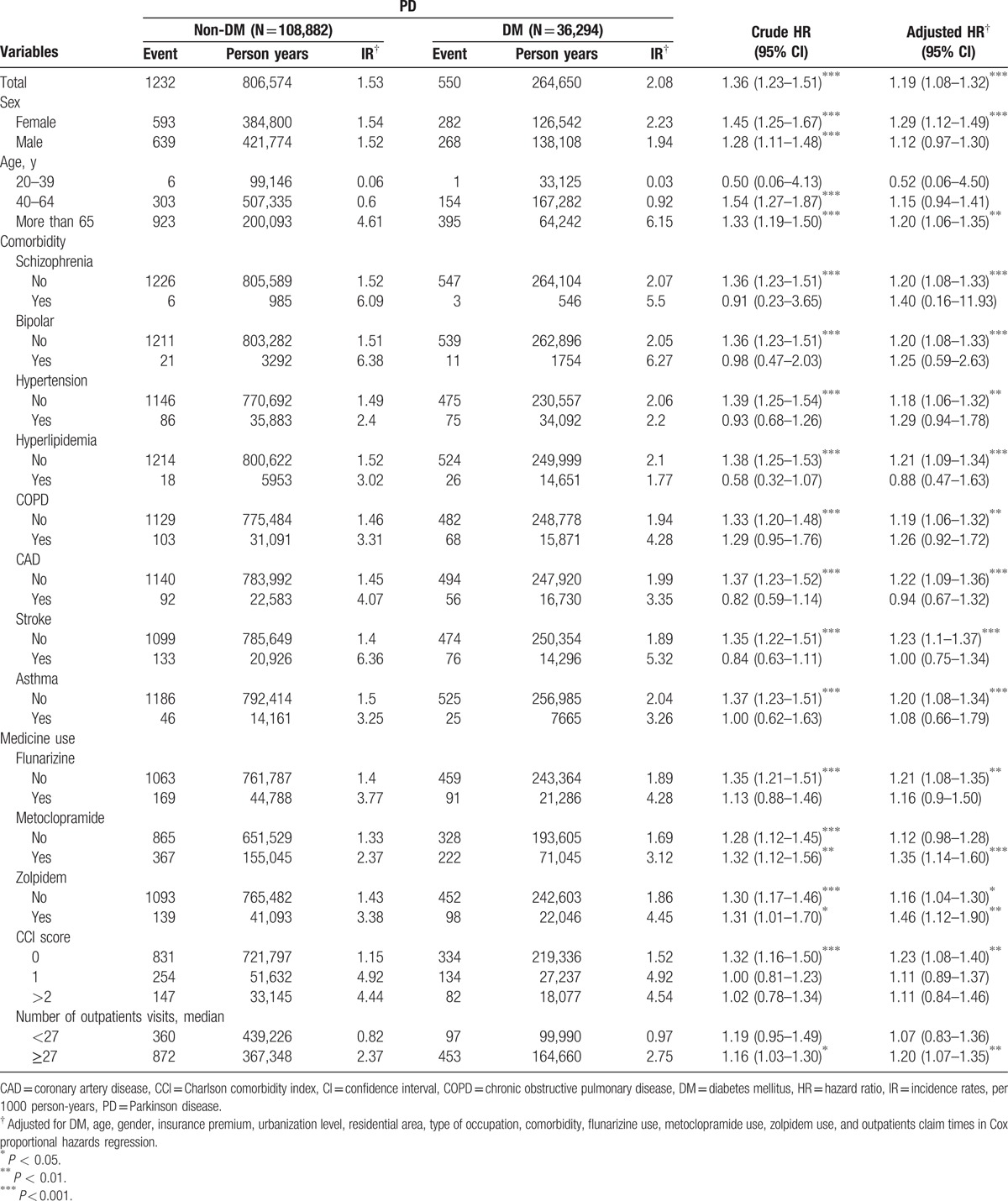
Figure 2 shows the adjusted HR of PD for the joint effects of DM and schizophrenia, bipolar disorder, hypertension, hyperlipidemia, COPD, CAD, stroke, asthma, flunarizine use, metoclopramide use, and zolpidem use. We observed significant HRs of PD for patients with diabetes along with schizophrenia, bipolar disorder, hypertension, COPD, stroke, flunarizine use, metoclopramide use, and zolpidem use compared with individuals without DM and no counterpart comorbidity or medication use (HR = 5.24, 1.67–16.46; 2.41, 1.32–4.41; 1.33, 1.05–1.68; 1.36, 1.06–1.75; 1.46, 1.15–1.86; 1.57, 1.26–1.96; 1.36, 1.16–1.59; and 1.81, 1.46–2.24, respectively). The primary effects of DM were all statistically significant with narrow 95% CIs and remained similar. The other risk factors exerting a significant primary effect were schizophrenia, bipolar disorder, CAD, stroke, flunarizine use, and zolpidem use. Although PERI, AP, and S-index all indicated a positive interaction between DM and medication use of metoclopramide and zolpidem, the tests were not significant because of limited power.
Figure 2.
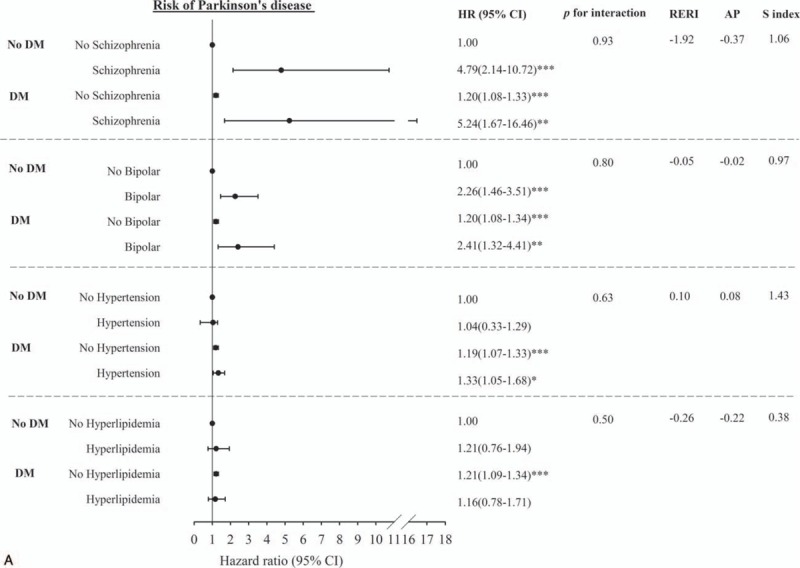
Multivariate-adjusted hazard ratios for the risk of Parkinson disease–Joint effects of diabetes with comorbidity including schizophrenia, bipolar, hypertension, hyperlipidemia (A); chronic obstructive pulmonary disease, coronary artery disease, stroke, asthma (B); and with medication use including flunarizine, metoclopramide, and zolpidem use (C).
Figure 2 (Continued).
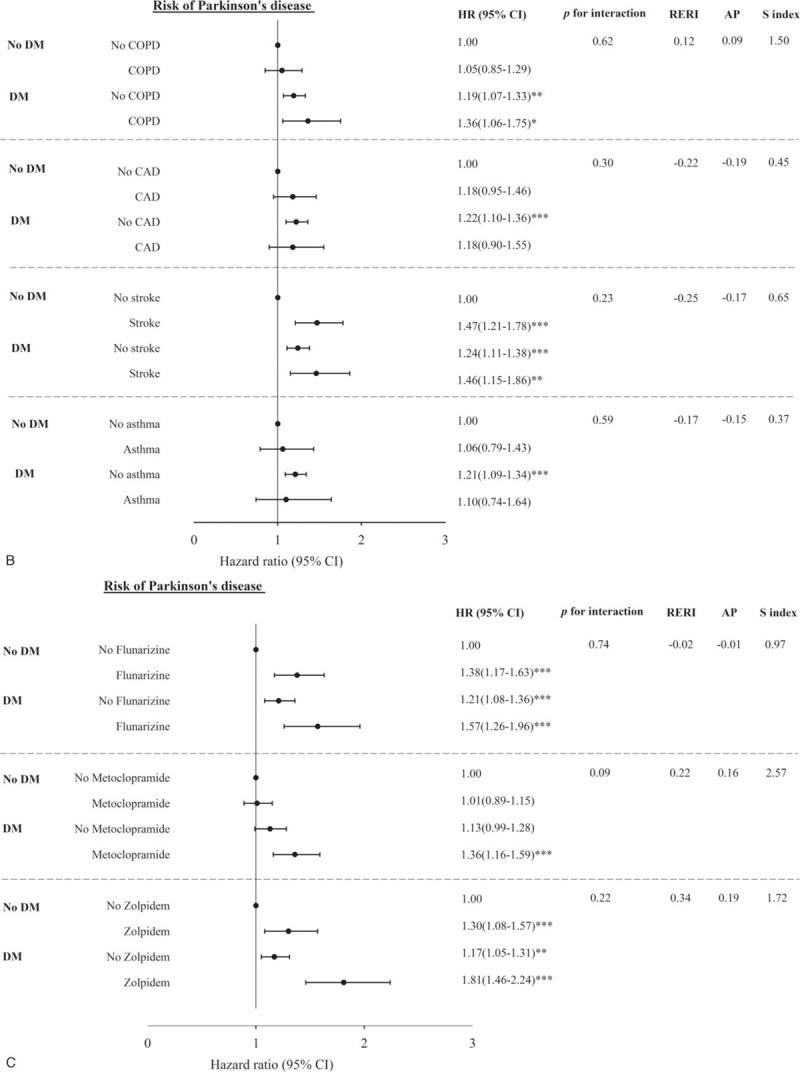
Multivariate-adjusted hazard ratios for the risk of Parkinson disease–Joint effects of diabetes with comorbidity including schizophrenia, bipolar, hypertension, hyperlipidemia (A); chronic obstructive pulmonary disease, coronary artery disease, stroke, asthma (B); and with medication use including flunarizine, metoclopramide, and zolpidem use (C).
Sensitivity analyses were conducted to investigate the validity of PD diagnosis by including PD cases diagnosed based on ICD-9 codes as well as taking PD medication. The PD medication considered in the study included trihexyphenidyl, rotigotine, amantadine, biperiden, bromocriptine, carbidopa, entacapone, levodopa, pergolide, ropinirole, pramipexole, selegiline, stalevo, rasagiline, and entacapone. Among 1782 PD cases diagnosed based on ICD-9 only, 1644 PD cases (92.26%) were confirmed by diagnosis of ICD-9 and PD medication. Similar significant associations were found and the HRs for patients with type 2 DM were 1.17 (1.05–1.31) when only diagnosed PD cases with PD medication were included in the analysis.
4. Discussion
This nationwide population-based study adds to the existing literature on the increasing 23% risk of PD in all DM patients after adjusting for age, gender, insurance premium, residential area, type of occupation, CCI scores, comorbidity of schizophrenia and bipolar disorder, flunarizine use, metoclopramide use, and zolpidem use. In DM patients, the PD risk is higher in females (HR: 1.29, 95% CI: 1.12–1.49) than in males (HR: 1.12, 95% CI: 0.97–1.30). The findings provide information for clinicians on the detection of PD cases during their clinical practice, specifically encountering female DM patients with medication use of flunarizine, metoclopramide, and zolpidem.
Age is another risk factor for developing PD that has been reported in many studies.[19–21] In the present research, only DM patients age older than 65 years were more likely to exhibit increased risk of PD (HR: 1.20, 1.06–1.35). In patients age <65 years, DM may not be a risk factor of developing PD. Further mechanistic research is needed to address any molecule-specific effects of age and DM on the risk of PD.
Recent evidence discloses that PD and DM share similar dysregulated pathways, such as mitochondrial dysfunction, endoplasmic reticulum stress, inflammation, and alterations in metabolism.[7–9] The current hypothesis suggests that exposure to environmental factors and genetic susceptibility play a role in the etiology and progression of both diseases. Our study demonstrated that DM increases PD risk in patients age older than 65 years, which may be ascribed to the duration of exposure to environmental factors. Although we cannot identify these environmental factors in this study, the results addressed an epidemiological evidence of the relationship among PD, DM, and age.
Another possible mechanism to explain the correlation between DM and PD we proposed is the insulin regulation pathway. Many in vitro and in vivo studies have demonstrated that the role of insulin in the regulation of brain dopaminergic activity and insulin dysregulation contributes to PD through disease-specific or general mechanisms is concluded.[8] Furthermore, recent functional brain image study also disclosed that insulin resistance was increased in the brain tissue of PD patients compared with the normal control group and suggested that a potential relationship between insulin resistance and brain structure in PD patients.[22]
Previous studies have investigated the association between diabetes and the risk of developing PD.[7,12,23] However, many antipsychotic drugs, such as flunarizine and zolpidem, and antiemetic drugs, such as metoclopramide, commonly cause PD[18]; in most of these studies, the authors did not include the potentially Parkinsonism-causing medicines in the adjusted variables. In the present research, DM patients took these medicines more frequently than non-DM patients (flunarizine, 8.29%:5.74%; metoclopramide, 27.68%:19.61%; and zolpidem, 9.78%:5.99%; P < 0.0001). If the adjusted variable did not include these drugs, the results may be misleading. The present study also provided the association between DM and PD in subgroups with and without medication use of flunarizine, metoclopramide, and zolpidem. Furthermore, DM patients who used flunarizine, metoclopramide, or zolpidem showed larger magnitude strength of association than individuals with DM or each medication use alone, hence indicating significant joint effects of DM with flunarizine, metoclopramide, and zolpidem use. DM patients with metoclopramide or zolpidem use also addressed the increased risk of PD. The adjusted HRs of DM patients in developing PD were 1.35 (95% CI, 1.14–1.60) or 1.46 (95% CI, 1.12–1.90) for the use of metoclopramide or zolpidem, respectively, compared with non-DM patients. These findings should be emphasized when physicians prescribe these medications in DM patients.
Possible explanations that can be accounted for the reasons why these medications increase the risk of PD are available. Metoclopramide is widely used to treat nausea and vomiting, help with emptying of the stomach in people with delayed stomach emptying because of either diabetes or following surgery, and cease migraine attack.[11,24,25] This drug belongs to the group of medications known as dopamine receptor antagonists.[25] Dopamine loss is the key PD pathological feature, and dopamine receptor agonists are the most effective symptomatic PD medication.[26] In our study, metoclopramide increasing PD risk is possible because of dopamine receptor blockade. Zolpidem is another drug that can increase PD risk, which has been demonstrated in many studies.[18,27] Although its mechanism is unclear, the risk of zolpidem use and PD is also demonstrated in the present study.
The strengths of this study include its nationwide, population-based, cohort design, with nearly complete follow-up information because the NHIRD dataset covered more than 99% of the 23.74 million residents of Taiwan and the nation-run NHI Bureau has contracted with 97% of all hospitals and 92% of all clinics nationwide. The dataset is also routinely monitored for diagnostic accuracy. The validity of these claim data is scrutinized by medical reimbursement specialists and peer review. The NHI Bureau performs quarterly expert reviews on random samples of every 50 to 100 outpatient and inpatient claims in each hospital and clinic to ensure the accuracy of the claim data. False diagnosis reports entail a severe penalty. The high accuracy of the NHIRD has been validated.[16,17] Given the considerable differences in age, gender, and comorbidities between patients with and without DM, matching with age, gender, and index year was applied to select the controls. The matching method was applied to enhance the comparability of these factors between patients with and without DM.
Several limitations are noted in this study. First, the diagnoses of PD and other comorbidities are completely dependent on ICD-9-CM codes. Nonetheless, the NHI Bureau randomly reviews the charts and interviews patients to verify the accuracy of the diagnoses. Hospitals with outlier chargers or practice may undergo an audit, with subsequent heavy penalties for malpractice or discrepancies. These processes enhance the validity of NHIRD. Second, the severity of PD and DM cannot be precisely extracted from the ICD-9-CM codes, thereby preventing further subgroup analysis. For example, NHIRD does not contain information of HbA1c, the core of hyperglycemia clinical management marker for diabetic patients. Thus, the association between poor glycemic control status and PD incidence cannot be explored. In addition, the database does not contain information on education, smoking, leisure-time physical activity, dietary habits, body mass index, which may also be risk factors for PD. Future studies linking administrative data and primary hospitalization information, such as severity of DM and detailed risk factors, are warranted. Generally, considering the magnitude and statistical significance of the observed effects in this study, these limitations are unlikely to compromise the results. Third, potential detection bias may exist because the mean number of outpatient visits in DM patients are higher than that in non-DM patients. Fortunately, the likelihood of this detection bias is low. In Taiwan, DM patients are cared in primary care unit, endocrinological division, or internal medicine department,[28] and the PD diagnosis is not often made by these physicians. PD diagnosis is usually made by neurologist in Taiwan. Moreover, when individuals are in the early stage or have prediagnostic features of PD, the probability of being detected by non-neurologist physician was very low because the prediagnostic features of PD are usually nonspecific symptoms and are not easy to be aware of by patients or family physicians. Due to the low detection rate, the detection bias is less likely even the fact that patients with DM had higher mean number of outpatient visits. When patients experienced classical motor symptom of PD, which are the clinical diagnostic criteria, both patients with and without DM are very likely to be referred to neurologic department for diagnosis when they seek for care for their classical motor symptom due to high accessibility of health care in Taiwan and high number of outpatient visits for subjects without DM (mean value was 32.3 with an SD of 31.9 within 2-year period). Thus, the likelihood of detecting their PD development in subjects without DM should be close to those with DM. In addition, PD is not a well-known complication of DM, it is less likely that the detection rate of PD is much higher in patients with DM than those without DM. Under the above circumstances, the likelihood of having detection bias is low. In order to rule out the possibility of detection bias, we additionally adjusted for the number of outpatient visits during 2-year period at baseline in the multivariate analysis and the results remain similar, indicating that the impact of the potential detection bias was small. Last, our study findings may not be generalized to other populations of different settings. However, our study findings may be generalized to other populations similar to our study population.
5. Conclusions
The present study indicates that DM increased the risk of developing PD in this Chinese population age 20 years and older over 7.3 years of follow-up. The magnitude of association is higher among women; individuals age 65 years and older; those without schizophrenia, bipolar disorder, hypertension, hyperlipidemia, COPD, CAD, stroke, asthma, flunarizine use, CCI score of 0, and number of outpatients visits ≥27; and those using metoclopramide and zolpidem. Further mechanistic investigations are warranted to validate the results.
Footnotes
Abbreviations: AP = proportion attributable to interaction, CAD = coronary artery disease, CCI = Charlson comorbidity index, CI = confidence interval, COPD = chronic obstructive pulmonary disease, DM = diabetes mellitus, HR = hazard ratio, ICD-9-CM = International Classification of Disease, 9th Revision, Clinical Modification, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, PD = Parkinson disease, RERI = relative excess risk due to interaction, SD = standard deviation, S index = synergy index.
Y-WY and T-FH have equally contributed as first author.
This study was supported in part by the Ministry of Science and Technology of Taiwan (NSC 101-2314-B-039-017-MY3, NSC 102-2314-B-039-005-MY2, MOST 104-2314-B-039-016, MOST 105-2314-B-039-021-MY3, and MOST 105-2314-B-039-025-MY3), the China Medical University (CMU104-S-17), and the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019).
The authors have no conflicts of interest to disclose.
References
- [1].Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001;414:782–7. [DOI] [PubMed] [Google Scholar]
- [2].Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol 2012;8:92–103. [DOI] [PubMed] [Google Scholar]
- [3].Wiederkehr A, Wollheim CB. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology 2006;147:2643–9. [DOI] [PubMed] [Google Scholar]
- [4].Kwak SH, Park KS, Lee KU, et al. Mitochondrial metabolism and diabetes. J Diabetes Investig 2010;1:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol 2008;7:97–109. [DOI] [PubMed] [Google Scholar]
- [6].Jin H, Kanthasamy A, Ghosh A, et al. Mitochondria-targeted antioxidants for treatment of Parkinson's disease: preclinical and clinical outcomes. Biochim Biophys Acta 2014;1842:1282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sun Y, Chang YH, Chen HF, et al. Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diabetes Care 2012;35:1047–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol 2004;3:169–78. [DOI] [PubMed] [Google Scholar]
- [9].Santiago JA, Potashkin JA. Shared dysregulated pathways lead to Parkinson's disease and diabetes. Trends Mol Med 2013;19:176–86. [DOI] [PubMed] [Google Scholar]
- [10].Wahlqvist ML, Lee MS, Hsu CC, et al. Metformin-inclusive sulfonylurea therapy reduces the risk of Parkinson's disease occurring with type 2 diabetes in a Taiwanese population cohort. Parkinsonism Relat Disord 2012;18:753–8. [DOI] [PubMed] [Google Scholar]
- [11].Saguil A, Lax JW. Acute migraine treatment in emergency settings. Am Fam Physician 2014;89:742–4. [PubMed] [Google Scholar]
- [12].Lu L, Fu DL, Li HQ, et al. Diabetes and risk of Parkinson's disease: an updated meta-analysis of case-control studies. PLoS ONE 2014;9:e85781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schernhammer E, Hansen J, Rugbjerg K, et al. Diabetes and the risk of developing Parkinson's disease in Denmark. Diabetes Care 2011;34:1102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rugbjerg K, Friis S, Ritz B, et al. Autoimmune disease and risk for Parkinson disease: a population-based case-control study. Neurology 2009;73:1462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Becker C, Brobert GP, Johansson S, et al. Diabetes in patients with idiopathic Parkinson's disease. Diabetes Care 2008;31:1808–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen Y-C, Yeh H-Y, Wu J-C, et al. Taiwan's National Health Insurance Research Database: administrative health care database as study object in bibliometrics. Scientometrics 2011;86:365–80. [Google Scholar]
- [17].Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Safety 2011;20:236–42. [DOI] [PubMed] [Google Scholar]
- [18].Yang YW, Hsieh TF, Yu CH, et al. Zolpidem and the risk of Parkinson's disease: a nationwide population-based study. J Psychiatr Res 2014;58:84–8. [DOI] [PubMed] [Google Scholar]
- [19].Caslake R, Taylor K, Scott N, et al. Age-, gender-, and socioeconomic status-specific incidence of Parkinson's disease and Parkinsonism in northeast Scotland: the PINE study. Parkinsonism Relat Disord 2013;19:515–21. [DOI] [PubMed] [Google Scholar]
- [20].Kaasinen V, Vahlberg T, Suominen S. Increasing age-adjusted male-to-female incidence ratio of Parkinson's disease. Mov Disord 2015;30:286–8. [DOI] [PubMed] [Google Scholar]
- [21].Yeh NC, Tien KJ, Yang CM, et al. Increased risk of Parkinson's disease in patients with obstructive sleep apnea: a population-based, propensity score-matched, longitudinal follow-up study. Medicine 2016;95:e2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Morris JK, Vidoni ED, Perea RD, et al. Insulin resistance and gray matter volume in neurodegenerative disease. Neuroscience 2014;270:139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cereda E, Barichella M, Pedrolli C, et al. Diabetes and risk of Parkinson's disease: a systematic review and meta-analysis. Diabetes Care 2011;34:2614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Eken C. Critical reappraisal of intravenous metoclopramide in migraine attack: a systematic review and meta-analysis. Am J Emerg Med 2014;33:331–7. [DOI] [PubMed] [Google Scholar]
- [25].Rao AS, Camilleri M. Review article: metoclopramide and tardive dyskinesia. Aliment Pharmacol Ther 2010;31:11–9. [DOI] [PubMed] [Google Scholar]
- [26].Smith Y, Wichmann T, Factor SA, et al. Parkinson's disease therapeutics: new developments and challenges since the introduction of levodopa. Neuropsychopharmacology 2012;37:213–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Huang HC, Tsai CH, Muo CH, et al. Risk of Parkinson's disease following zolpidem use: a retrospective, population-based cohort study. J Clin Psychiatry 2015;76:e104–10. [DOI] [PubMed] [Google Scholar]
- [28].Liao PJ, Lin ZY, Huang JC, et al. The relationship between type 2 diabetic patients’ early medical care-seeking consistency to the same clinician and health care system and their clinical outcomes. Medicine 2015;94:e554. [DOI] [PMC free article] [PubMed] [Google Scholar]


