Supplemental Digital Content is available in the text
Keywords: female, glycogen synthase kinase-3β (GSK3β), major depressive disorder (MDD), meta-analysis, single nucleotide polymorphism (SNP)
Abstract
Major depressive disorder (MDD) is one of the most prevalent psychiatric illnesses with a heritability ranging from 40% to 50%. The single nucleotide polymorphism (SNP) rs334558 on the glycogen synthase kinase-3β (GSK3β) gene has been identified as a genetic risk loci associated with schizophrenia and bipolar disorder. However, results from replication studies examining the association between rs334558 and MDD remain inconsistent.
In the present study, first, we conducted a meta-analysis of the association between rs334558 and MDD by combining 5 available case-control samples totaling 2311 cases and 2535 controls. Second, genotyping data from patients with MDD at our institution, after further stratification by gender, were analyzed to determine the association between rs334558 and MDD.
All studies retrieved and included in the meta-analysis were from Korea and China. The meta-analysis suggested that the functional polymorphism rs334558 within the GSK3β promoter region was associated with MDD risk (P < 0.05). The associations were observed both in the allelic and genetic models. Analysis of the genotyping data extracted from our hospital database revealed that rs334558 exhibited exclusive association with MDD in female patients (P=0.015).
Our findings suggest that GSK3β rs334558 polymorphisms might be a potential risk for MDD, and females with GSK3β rs334558 polymorphisms might have higher penetrance of MDD. If validated in larger scale samples and in different ethnic populations, these findings might be of value as diagnostic references for MDD.
1. Introduction
Major depressive disorder (MDD), with a lifetime prevalence of 12% to 17%,[1] has become one of the most frequently occurring mental illnesses worldwide. With the heritability of 40% to 50%, great efforts have been made to identify candidate genes that contribute to the pathogenesis of MDD. Recent genome-wide association studies (GWAS) have identified some genetic loci of MDD,[2] but few of these genetic variations including functional-related single nucleotide polymorphisms (SNPs) have been validated. Thus, the candidate gene approaches still widely used to elucidate the genetic basis of MDD.
The glycogen synthase kinase 3-beta (GSK3β) gene has been recognized as a candidate gene associated with schizophrenia (SZ) and bipolar disorder (BD).[3,4] GSK3β at 3q13.3 encodes a serine-threonine kinase that regulates gene expression, cell adhesion, and cell polarity. Recent evidence suggests a putative role of this gene in MDD. First, GSK3β is highly expressed in the brain, especially in the hippocampus.[5] Second, GSK3β is involved in signal transduction cascades of neuronal cell development[6] and neuroplasticity.[7] Third, mood stabilizers or antidepressants have been demonstrated to inhibit or reduce the GSK3β levels in vivo,[8] whereas inhibition of GSK3β has been shown to have effects similar to mood stabilizers or antidepressants.[9]
It has also been shown that a single nucleotide polymorphism within the GSK3β promoter region (nt-171 to +29), rs334558 (-50C/T) affects GSK3β expression, which is associated with the disease risk of Parkinson's disease.[10] However, till now, results on whether rs334558 is also a risk factor for MDD remain inconsistent. A previous study conducted by us failed to find possible association of rs334558 with MDD. This study, however, was limited by a relatively small size of samples (559 cases and 486 controls, all were of Chinese Han nationality).[11] Considering the heterogeneity of the cohort, this sample size might not be big enough to unveil the power of rs334558. Therefore, in the hope of finding valuable information that might be missed due to a small sample size in our previous study, we performed this meta-analysis by combining all available published data to assess the association between GSK3β rs334558 and MDD risk. Additionally, as gender is the most reported factor that could exert impact on the cohort heterogeneity, we re-analyzed our data after gender stratification.
2. Materials and methods
2.1. Meta-analysis
2.1.1. Identification of eligible studies
The PubMed, EMBASE, Web of Science, ScienceDirect, and China National Knowledge Infrastructure databases without language restriction (updated to May15, 2016) were searched for eligible studies using following terms: “depression or depressive or affective disorder,” “GSK3 or GSK-3 or GSK3,” and “variant or polymorphism or SNP.” The reference lists of retrieved publications were checked as well for further relevant studies.
The publications were considered suitable if they met the inclusion criteria:(1) studies investigated the association of GSK3β rs334558 with susceptibility of MDD; (2) they were case-control studies, including candidate gene association studies and GWAS with genotypic data and odds ratio (95%CI available); (3) the diagnosis of MDD was in accordance with Diagnostic and Statistical Manual of Mental Disorder Fourth Edition (DSM-IV) or International Classification of Diseases-10 criteria; and(4) the reported frequencies of rs334558 were in Hardy–Weinberg equilibrium (HWE) in the cohorts (P > 0.05). The publications from the same author or affiliation were carefully examined to ensure their independence.
2.1.2. Data extraction
Two reviewers, SL and WL, independently extracted the following data from each eligible study: last name of the first author, year of publication, ethnicity, country, sample size, gender ratio, diagnostic criteria, allelic and genotypic distribution of rs334558 in cases and controls, and so on.
2.1.3. Statistical analysis
For each study, HWE for cases and controls were re-assessed by χ2 test with an eligible threshold of P > 0.01. Crude ORs and their 95% CIs were used to measure the association strength of GSK3β rs334558 with MDD risk. Different ORs were calculated as follows: (1) T allele vs C allele (the allelic model), (2) TT genotype vs TC+CC genotypes (the recessive model), and (3) TT+TC genotypes vs CC genotype (the dominant model).
The Review Manager 5.3 (http://tech.cochrane.org/revman/) was used to conduct the meta-analysis of combined studies and the superiority test. Heterogeneity among individual studies was tested by χ2-based Q statistic. P < 0.10 and I2 > 50% indicated the evidence of heterogeneity. If there was statistically significant heterogeneity across studies, a random effect model (Dersimonian–Laird method) was used to merge data. Otherwise, a fixed effect model (Mantel–Haenszel method) was adopted. The superiority test was conducted to confirm the absence of association of rs334558 with MDD.
The Stata12.0 statistical software package (http://www.stata.com/) was used to assess the potential publication bias. Both the Egger regression test for a funnel plot and the Begg–Mazumdar test were applied for publication bias analysis and P < 0.05 was considered statistically significant. Power analysis was performed using the Power and Sample Size Program software (http://ps-power-and-sample-size-calculation.software.informer.com).
2.2. Stratified analysis
2.2.1. Subjects
A total of 581 MDD patients (264 males, 317 females, mean age 32.0 ± 9.8 years, range 18–65 years) were recruited from the Department of Psychiatry, First Hospital of Shanxi Medical University, Taiyuan, China. The diagnosis was made by at least 2 consultant psychiatrists according to the DSM-IV criteria for MDD. All patients were also diagnosed using the Chinese Version of the Modified Structured Clinical Interview for DSM-IV TR Axis I Disorders Patient Edition (SCID-I/P, 11/2002 revision). Pregnant patients and those with significant medical conditions, unstable psychiatric features, a history of alcoholism or drug abuse, neurological illness, or concomitant additional Axis I psychiatric disorders were excluded. Meanwhile, a total of 486 healthy volunteers (269 females, 217 males, mean age 32.8 ± 8.6 years, range 18–65 years) who did not have a history of neuropsychiatric disorders were recruited from the community or during regular health screening visits as the control group. All subjects were from the same geographical areas in Northern China and were of Chinese Han nationality. All participants provided written informed consent. This study was approved by the Ethical Committee for Medicine, the First Hospital of Shanxi Medical University, China.
2.2.2. Statistical analysis
Genotyping was performed as previously described.[11] The HWE for genotypic distributions of rs334558 was examined by the χ2 goodness-of-fit test with the significance level at P < 0.05. The analysis of allelic associations was performed by the UNPHASED program. To reduce the inflation of false positive rates due to multiple testing, 10,000 permutations were performed to obtain a global P-value corrected, and the significant level was set as a corrected P-value of 0.05.
3. Results
3.1. Characteristics of studies included for the meta-analysis
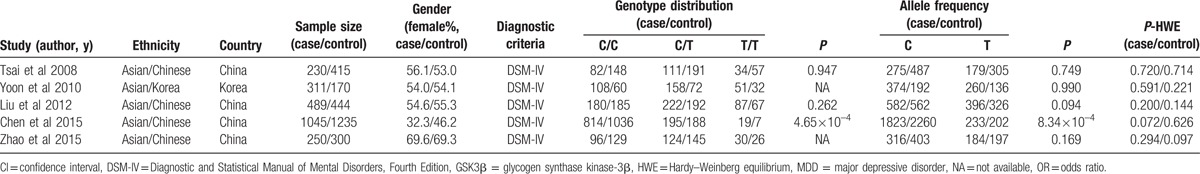
Based on our literature search strategy (a flow chart of the search process is detailed in Fig. 1), a total of 5 studies all involving populations from Asia finally entered the meta-analysis, of which, 1 was from Korea[12] and 4 were from China,[3,11,13,14] including a study of our own.[11] All the participants were recruited from local communities and there was no overlap among these samples. All the cases had 1 or more episodes of MDD meeting the DSM-IV criteria. In all 5 studies, the cases and the controls were age- and gender-matched. However, the information on other confounding factors was inconsistent with Liu et al[11] and Yoon and Kim[12] reporting that both the cases and the controls did not have family history of psychopathology, whereas the other 3 studies did not address confounding factors. Genotypic distributions of control and case cohorts of these 5 studies did not deviate from HWE (P > 0.05). Characteristics of these studies were summarized in Table 1.
Figure 1.
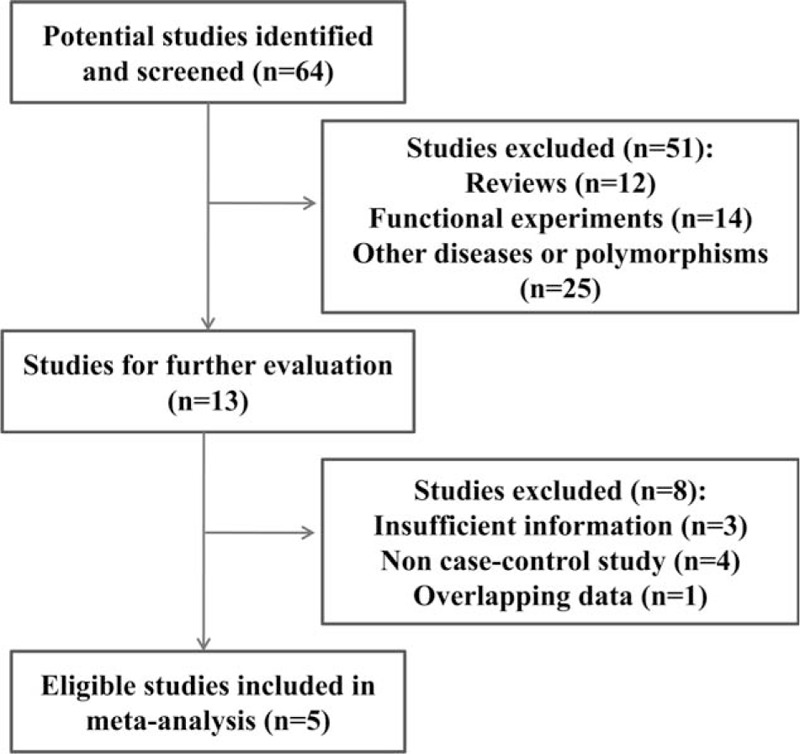
Flow chart of selection procedure for meta-analysis.
Table 1.
Characteristics of included studies on the association of GSK3β rs334558 with MDD.
3.2. Meta-analysis
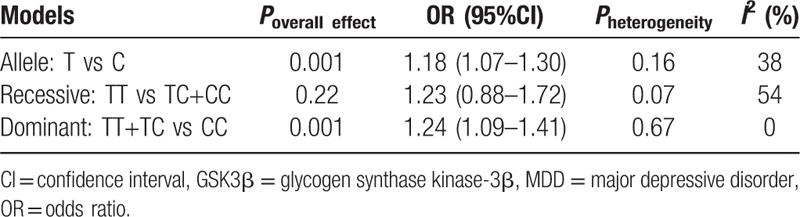
Five studies with a total of 2311 cases and 2535 controls were pooled to explore the association between GSK3β rs334558 and the susceptibility of MDD. The forest plot of the meta-analysis is shown in Fig. 2. Overall, we found significant statistical association of GSK3β rs334558 with MDD risk both in the allelic model and the dominant model, in which fixed-effect models were applied to estimate the ORs as there was no significant heterogeneity (T/C: OR = 1.18, 95%CI: 1.07–1.30, I2 = 38%, P = 0.001; TT+TC vs CC: OR = 1.24, 95%CI: 1.09–1.41, I2 = 0, P = 0.001). A random effect model was adopted since a heterogeneity was observed only in the recessive model (χ2 = 8.70, df = 4, P = 0.07, I2 = 54%), and there was no significant association observed in this model (TT vs TC+CC: OR = 1.23, 95%CI: 0.88–1.72, P = 0.22). Results in detail are summarized in Table 2.
Figure 2.
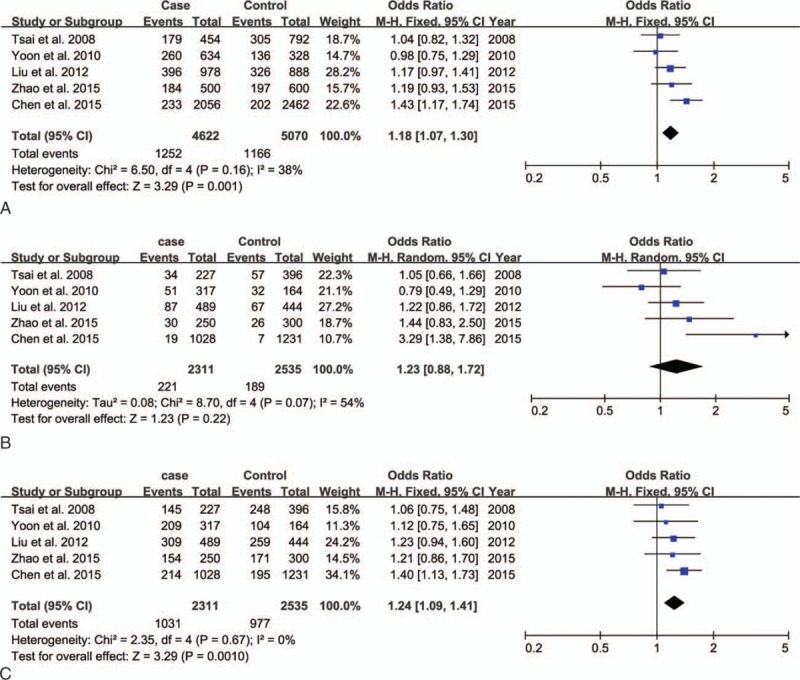
Forest plot of meta-analysis for the overall association between GSK3β rs334558 and MDD in all models. (A, T/C allelic model; B, TT/TC+CC recessive model; C, TT+TC/CC dominant model). GSK3β = glycogen synthase kinase-3β, MDD = major depressive disorder.
Table 2.
Meta-analysis for the association between GSK3β rs334558 and MDD.
3.3. Quality evaluation
Sensitive analyses were conducted to assess the influence of each study on the pooled OR. As shown in Table S1, it drew a different conclusion after omitting the study by Chen et al (2015). The instability of results might be because this study contributed much more to the pooled OR, eliminating this study would lead to insufficient power for the remaining studies.
Power analysis on the combined sample size was conducted using the following assumptions: 2311 MDD patients and 2535 controls (4622 patients and 5070 controls in allele), type I error probability (0.05), the frequency of rs334558 T-allele in Asian population (0.302), and the commonly observed OR (1.18). The present sample size thus had a 96.7% power in the detection of a significant association of rs334558 with MDD.
Publication bias was examined by Begg's funnel plot and Egger's test. Funnel plots about MDD risk indicated that there was no evidence of publication bias in all the genetic models (Fig. S1), and this was further confirmed by Egger's test (T vs C: PEgger's test = 0.241; TT vs TC+CC: PEgger's test = 0.285; TT+TC vs CC: PEgger's test = 0.050).
3.4. Gender stratification
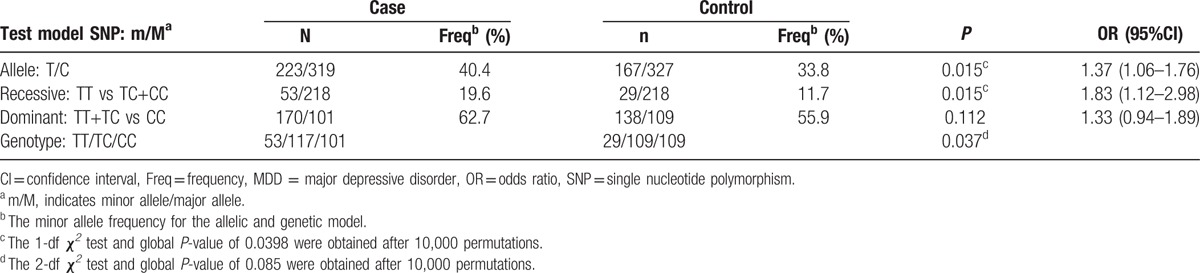
Within our own genotyping data, the genotypic and allelic distributions of rs334558 for the MDD group and controls by gender stratification were proved to be in the HWE (P > 0.05). There was no difference in age (t = –1.357, P = 0.175) or sex (χ2 = 0.066, P = 0.797) between patients and controls. After stratification by gender, both of the allelic and genotypic association were significant for rs334558 only in female patients with MDD (allele: χ2 = 5.943, P = 0.015; recessive model:χ2 = 5.324, P = 0.015; genotype: χ2 = 6.604, P = 0.037), but not in male patients (P = 0.843, Table S2). The frequency of the rs334558 minor allele (T) was significantly higher in female patients than female controls (OR = 1.369, 95%CI = 1.063–1.763). The association in allele and recessive model survived the 10,000 permutations with a global P-value of 0.0398, yet the genotypic association did not survive (P = 0.085, Table 3).
Table 3.
The allelic and genotypic associations of rs334558 with MDD in female.
4. Discussion
In the meta-analysis, we found that the minor allele (T) of rs334558 might convey risk for MDD, and there was no significant heterogeneity in both the allelic model and the dominant model, indicating that the magnitude of association between rs334558 variant and MDD is consistent among the 5 studies. Furthermore, analysis of the genotyping data extracted from our institution after gender stratification revealed significant association between rs334558 and MDD in female patients.
MDD is highly polygenic. Due to the phenotypic heterogeneity of the disorder, the low effect size of genetic variants and probably the small sample size that many studies investigated, so far, only a few susceptibility genes have been identified. Gender is one of the most reported factors that could exert impact on the cohort heterogeneity of MDD. It is known that there is about 45% of the genetic liability not shared between sexes.[15] Women are twice as likely to suffer from depression as men,[16] and female patients more likely have a chronic course, longer episodes, earlier onsets, and more severe symptoms than male patients.[17] GWAS previously failed to find any SNP of genome-wide significance until recently 2 loci were identified for MDD in 5303 Chinese women,[2] which indicates that the gender-specific effect may exert on the MDD development and the gender stratification is a promising method to resolve the verification inconsistencies. Indeed, in this study after stratified by gender, we observed significant association between rs334558 and MDD in female patients using our own genotyping data.
To date, hundreds of candidate SNPs have been submitted including GWAS,[2] but only a few genotypes have been reported functionally significant. As a functional polymorphism within the GSK3β promoter region, rs334558 has been systematically evaluated for the association with both SZ and BD by Chen et al[18] and Tang et al.[19] Both groups have found that this SNP is associated with SZ but not BD, suggesting that rs334558 might be useful for the differentiation of these 2 disorders.[19] Patients with either bipolar disorder or schizophrenia may experience depressive episodes; therefore, it is important to differentiate depressive episodes in these 2 disorders from MDD. The diagnosis of MDD in all 5 studies was made by experienced psychiatrists according to the DSM-IV criteria for MDD. Additionally, DSM-IV Axis I diagnoses codes were followed to exclude schizophrenia and bipolar disorder. Therefore, we believe it is highly unlikely that patients with bipolar disorder or schizophrenia were mistakenly recruited in studies.
There have been several lines of clinical and genetic evidence showing that GSK3β might be linked to MDD risk, especially in females. It is reported that GSK3β can participate in the molecular mechanisms of the circadian clock in mammal.[20] Transgenic mice over expressing GSK3β have an increase in the nonrapid eye movement sleep episodes and a decrease in the mean episode duration, which exhibit a severe fragmentation of sleep-wake cycle, a similar manifestation also found in MDD patients.[21] Meanwhile, the suppression of GSK3β in forebrain has anxiolytic effects in mice.[5] In addition, rs334558 has been reported to be associated with onset age and the antidepressant response to total sleep deprivation in bipolar depression,[22] and the GSK3β interaction with 5-HTTLPR was also observed.[23] Furthermore, it has been proposed that the ovarian hormones estrogen and progesterone contribute to the higher incidence of depression.[24] By genetic analysis, GSK3β has been shown to be associated with increased frequency of polycystic ovary syndrome which requires the presence of hyperandrogenism,[25] suggesting a causal relationship between GSK3β and sex hormones.
Several limitations should be acknowledged. First, the populations of 5 eligible studies included in our meta-analysis were all from Asian region, limiting the generalization of our findings. Second, this meta-analysis cannot completely exclude potential publication bias due to the limited number of samples (n = 5), though both the Egger regression test and the Begg–Mazumdar test revealed no publication bias. Third, the results of meta-analysis were based on unadjusted estimates and a more precise evaluation stratified by the gender should be performed if individual data were available. Finally, the association analysis based on gender stratification was conducted in a relatively small sample size.
In conclusion, our findings suggest that GSK3β rs334558 polymorphisms might be a potential risk for MDD, and females with GSK3β rs334558 polymorphisms might have higher penetrance of MDD. If validated in larger scale samples and in different ethnic populations, these findings might be of value as diagnostic references for MDD.
Supplementary Material
Acknowledgments
The authors sincerely thank all the subjects for their support and participation and all the medical staff involved in collecting blood samples.
Footnotes
Abbreviations: BD = bipolar disorder, DSM-IV = Diagnostic and Statistical Manual of Mental Disorder Fourth Edition, GSK3β = glycogen synthase kinase-3β, HWE = Hardy–Weinberg equilibrium, MDD = major depressive disorder, SNP = single nucleotide polymorphism, SZ = schizophrenia.
Authorship—conceived and designed the experiments: KZ; collected and Analyzed the data: SL and LW; contributed reagents/materials/analysis tools: NS, CY, ZL, XL, and XC; wrote the first draft of the manuscript: SL and LW; critically revised and finally approved the manuscript: KZ.
Funding: This work was supported by the National Key Basic Research Program (No.2013CB531305), NSFC (81171290&81471379), Natural Science Foundation of Shanxi (201601D021150), and the Doctoral Fund of Shanxi Medical University (03201314).
SL and LW authors contributed equally to this work.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Wittchen HU, Essau CA, von Zerssen D, et al. Lifetime and six-month prevalence of mental disorders in the Munich Follow-Up Study. Eur Arch Psychiatry Clin Neurosci 1992;241:247–58. [DOI] [PubMed] [Google Scholar]
- [2].CONVERGE Consortium. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature 2015;523:588–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen J, Wang M, Waheed Khan RA, et al. The GSK3B gene confers risk for both major depressive disorder and schizophrenia in the Han Chinese population. J Affect Disord 2015;185:149–55. [DOI] [PubMed] [Google Scholar]
- [4].Ronai Z, Kovacs-Nagy R, Szantai E, et al. Glycogen synthase kinase 3 beta gene structural variants as possible risk factors of bipolar depression. Am J Med Genet B Neuropsychiatr Genet 2014;165B:217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Latapy C, Rioux V, Guitton MJ, et al. Selective deletion of forebrain glycogen synthase kinase 3beta reveals a central role in serotonin-sensitive anxiety and social behaviour. Philos Trans R Soc Lond B Biol Sci 2012;367:2460–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Muyllaert D, Kremer A, Jaworski T, et al. Glycogen synthase kinase-3beta, or a link between amyloid and tau pathology? Genes Brain Behav 2008;7(suppl 1):57–66. [DOI] [PubMed] [Google Scholar]
- [7].Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol 2001;65:391–426. [DOI] [PubMed] [Google Scholar]
- [8].Mendes CT, Mury FB, de Sa Moreira E, et al. Lithium reduces Gsk3b mRNA levels: implications for Alzheimer disease. Eur Arch Psychiatry Clin Neurosci 2009;259:16–22. [DOI] [PubMed] [Google Scholar]
- [9].Higgins GA, Allyn-Feuer A, Barbour E, et al. A glutamatergic network mediates lithium response in bipolar disorder as defined by epigenome pathway analysis. Pharmacogenomics 2015;16:1547–63. [DOI] [PubMed] [Google Scholar]
- [10].Kwok JB, Hallupp M, Loy CT, et al. GSK3B polymorphisms alter transcription and splicing in Parkinson's disease. Ann Neurol 2005;58:829–39. [DOI] [PubMed] [Google Scholar]
- [11].Liu S, Sun N, Xu Y, et al. Possible association of the GSK3beta gene with the anxiety symptoms of major depressive disorder and P300 waveform. Genet Test Mol Biomarkers 2012;16:1382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yoon HK, Kim YK. Association between glycogen synthase kinase-3beta gene polymorphisms and major depression and suicidal behavior in a Korean population. Prog Neuropsychopharmacol Biol Psychiatry 2010;34:331–4. [DOI] [PubMed] [Google Scholar]
- [13].Tsai SJ, Liou YJ, Hong CJ, et al. Glycogen synthase kinase-3beta gene is associated with antidepressant treatment response in Chinese major depressive disorder. Pharmacogenomics J 2008;8:384–90. [DOI] [PubMed] [Google Scholar]
- [14].Zhao Y, Qiu Y, Liu Y, et al. Association study of the GSK3β gene polymorphism with major depressive disorder. Chin J Behav Med Brain Sci 2015;24:315–8. [Google Scholar]
- [15].Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000;157:1552–62. [DOI] [PubMed] [Google Scholar]
- [16].Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA 2003;289:3095–105. [DOI] [PubMed] [Google Scholar]
- [17].Lopez Molina MA, Jansen K, Drews C, et al. Major depressive disorder symptoms in male and female young adults. Psychol Health Med 2014;19:136–45. [DOI] [PubMed] [Google Scholar]
- [18].Chen G, Tang J, Yu G, et al. Meta-analysis demonstrates lack of association of the GSK3B -50C/T polymorphism with risk of bipolar disorder. Mol Biol Rep 2014;41:5711–8. [DOI] [PubMed] [Google Scholar]
- [19].Tang H, Shen N, Jin H, et al. GSK-3beta polymorphism discriminates bipolar disorder and schizophrenia: a systematic meta-analysis. Mol Neurobiol 2013;48:404–11. [DOI] [PubMed] [Google Scholar]
- [20].Leloup JC, Goldbeter A. Modelling the dual role of Per phosphorylation and its effect on the period and phase of the mammalian circadian clock. IET Syst Biol 2011;5:44. [DOI] [PubMed] [Google Scholar]
- [21].Ahnaou A, Drinkenburg WH. Disruption of glycogen synthase kinase-3-beta activity leads to abnormalities in physiological measures in mice. Behav Brain Res 2011;221:246–52. [DOI] [PubMed] [Google Scholar]
- [22].Benedetti F, Serretti A, Colombo C, et al. A glycogen synthase kinase 3-beta promoter gene single nucleotide polymorphism is associated with age at onset and response to total sleep deprivation in bipolar depression. Neurosci Lett 2004;368:123–6. [DOI] [PubMed] [Google Scholar]
- [23].Benedetti F, Dallaspezia S, Lorenzi C, et al. Gene–gene interaction of glycogen synthase kinase 3-beta and serotonin transporter on human antidepressant response to sleep deprivation. J Affect Disord 2012;136:514–9. [DOI] [PubMed] [Google Scholar]
- [24].Shors TJ, Leuner B. Estrogen-mediated effects on depression and memory formation in females. J Affect Disord 2003;74:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Goodarzi MO, Antoine HJ, Pall M, et al. Preliminary evidence of glycogen synthase kinase 3 beta as a genetic determinant of polycystic ovary syndrome. Fertil Steril 2007;87:1473–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


