Abstract
Objective
To test differences in neural sensitivity to facial expressions, including expressions with open versus closed mouths, exhibited by (1) adults with autism spectrum disorder (ASD) compared to neurotypical adults, and by (2) short versus long serotonin transporter allele (SLC6A4) carriers.
Methods
Event related potentials (ERPs) to happy, fearful, and neutral expressions were collected from neurotypical adults (n = 25) and adults with ASD (n = 27)–of whom 32 had short and 13 had homozygous long SLC6A4 alleles.
Results
In the neurotypical group, we confirmed that the N170, VPP and EPN, but not the P1, were influenced by emotional expressions, and determined the EPN was the earliest component modulated by open mouth. Compared to the neurotypical group, individuals with ASD exhibited differences in EPN amplitude in response to open versus closed mouths and in hemispheric distribution. Across groups, short serotonin transporter allele carriers had reduced P1 amplitude compared to long allele carriers.
Conclusions
Individuals with ASD exhibited a different pattern of neural response when encoding and recognizing facial expressions at the EPN component. Across groups, SLC6A4 allele type modulated early sensory attention at the P1.
Significance
These results provide insight into the nature of early responses to emotional information according to genetic variation and clinical condition.
Keywords: Autism, Serotonin, Emotion, Event related potential, Electrophysiology, SLC6A4
1. Introduction
Processing of emotional stimuli involves early stage perception, the generation and awareness of physiological responses to emotional cues, and cognitive and semantic categorization. A number of factors influence emotional processing including genetic background and experiences. There is evidence that individual emotional responses are modulated by genotype (e.g., Bevilacqua and Goldman, 2011), which interacts with a lifetime of environmental experiences (e.g., Caspi et al., 2003). In this study we investigated the impact of two conditions: (1) autism spectrum disorder (ASD) and (2) length-based allelic variants of the serotonin transporter gene, SLC6A4, on early stage attention and perception of emotional information conveyed by faces. ASD is likely a condition in which both genetic background and experiences are impacted by a variety of factors, whereas SLC6A4 allele length likely confers a more specific genetic and environmental impact. We examined whether the profiles of these conditions could be distinguished by their neural responses during initial attention and perception of emotional cues on faces given that both ASD and SLC6A4 are associated with behavioral and neural differences in emotional responding. Understanding the early perceptual processing in adults with ASD and SLC6A4 allele variants using event related potentials (ERPs) adds to existing knowledge about neural function and provides more precise clinical understanding of how breakdowns in emotional processing might occur. Moreover, this study provides the foundation for examining the specific role of SLC6A4 allele variants within ASD, because converging evidence including apparent differences in transmission of SLC6A4 allele length as a function of ASD status (e.g., Devlin et al., 2005; Guhathakurta et al., 2008; Kistner-Griffin et al., 2011; Wassink et al., 2007; but see Huang and Santangalo, 2008) and higher observed whole blood serotonin levels (e.g., Schain and Freedman, 1961; see also Cook and Leventhal, 1996 for review) implicates serotonin in ASD.
1.1. Emotion processing and neural differentiation
Early-stage ERPs (see Table 1) are sensitive to differences in emotional expressions, particularly negative expressions. Investigating these components provides important clues about early differences in attention, perception and discrimination of faces and the emotions they convey. We examined early ERP components reported for a passive viewing task that used the MacBrain Face stimulus set (Tottenham et al., 2009): the P1, Vertex Positive Potential/N170, and Early Posterior Negativity (Smith et al., 2013). Each component represents an aspect of early attention or perception that may be modulated by viewing emotional cues, particularly negative emotions, with minimal task demands.
Table 1.
Early stage event-related potentials associated with emotion processing.
| Component | Window and leads | Role in processing |
|---|---|---|
| P1 | 60–130 ms; medial-occipital | Early selective attention and sensory processing; detecting configural face information |
| N170 | 150–180 ms; lateral-occipital and posterior temporal | Preliminary perceptual encoding of faces underlying categorization; possibly sensitive to negative expressions |
| VPP | 150–180 ms; central midline | Attention; detecting emotional versus neutral expressions |
| EPN | 200–350 ms; temporal occipital | Perceptual attention; encoding and recognizing positive and negative facial expressions |
The P1 is thought to reflect early selective attention and sensory processing of visual stimuli (Hillyard et al., 1973; Olofsson et al., 2008) and is sensitive to first order configural information contained in faces (Boutsen et al., 2006; Mercure et al., 2008). Recent work demonstrates that the P1 may be sensitive to negative emotional expressions (Luo et al., 2010; Rellecke et al., 2012), particularly when attended by the fovea (Eimer and Holmes, 2007; Wijers and Banis, 2012). This attentional modulation of the P1 component is thought to be due to extrastriate generators that are sensitive to threat-related, fearful stimuli (Wijers and Banis, 2012).
Next are the N170 and Vertex Positive Potential (VPP), which occur during the same time window and may share underlying neural mechanisms within the occipito-temporal cortex including the middle temporal gyrus and fusiform gyrus (Joyce and Rossion, 2005). The N170 is sensitive to faces and more negative fluctuations in N170 amplitude are observed over the right hemisphere for faces relative to other stimuli (Bentin et al., 1996). The N170 also appears sensitive to basic stimulus characteristics (Thierry et al., 2007; but see Bentin et al., 2007). There is some evidence that the N170 is enhanced for faces with emotional expressions relative to neutral faces (Blau et al., 2007; Righart and de Gelder, 2008), particularly negative expressions such as anger, fear and sadness (Batty and Taylor, 2003; Williams et al., 2006), but these effects have not been detected consistently (Eimer and Holmes, 2007; Eimer et al., 2003; Rellecke et al., 2013). The VPP is thought to reflect attention and the differences in attention to emotional versus neutral expressions (Luo et al., 2010). The VPP is included in the current investigation because more consistent effects of emotion have been detected for the VPP than the N170, perhaps due to greater sensitivity of the VPP to frontal contributors.
Finally, the Early Posterior Negativity (EPN), an enhanced negative-tending amplitude, appears to differ robustly in response to both negative and positive emotions relative to neutral expressions (Foti et al., 2009; Holmes et al., 2009; Rellecke et al., 2011; Schacht and Sommer, 2009; Schupp et al., 2004). The EPN is thought to reflect the perceptual attention underlying coding and recognition of facial expressions in the occipital and temporal cortex (Bradley et al., 2007; Rellecke et al., 2011; Schacht and Sommer, 2009; Schupp et al., 2003, 2004, 2006).
One aspect of neural responding to emotional faces that has not been systematically investigated is the impact of open versus closed mouths. Behaviorally, open mouths influence early processing of facial expressions. The ability to use information from particular features even when faces are shown for less than 150 ms suggests that holistic cues about expression may be available pre-attentively (Scheller et al., 2012). Additionally, open-mouthed faces enhance visual search (Horstmann et al., 2012), suggesting that teeth provide a salient perceptual cue. Varying degrees of teeth and gums are revealed in different facial expressions (Walter et al., 2014) and are linked to the naturalness of expressions (Korb et al., 2014; Van Der Geld et al., 2008), thus the appearance of teeth may be confounded with certain expressions. As well, critical information used to discriminate emotion varies by expression with more information about happiness conveyed by the mouth and more information about fear conveyed by the eyes as reflected in the scanning patterns of neurotypical adults (Eisenbarth and Alpers, 2011). In the context of early ERP components, the presence of open versus closed mouths may influence attention via different degrees of visual contrast between teeth and gums as well as perception of key facial information that underlies subsequent discrimination of emotions. Indeed, due to perceptual differences of “toothiness,” the MacBrain stimuli used in the current study have open versus closed mouth versions of each facial expression and validation revealed enhanced accuracy for identification of expressions with open mouths (Tottenham et al., 2009).
1.2. Emotion processing in ASD
Emotional processing, a core component of the social communication system is disrupted in a number of mental health disorders in adults, including autism spectrum disorders (ASD). One hallmark of ASD is reduced social and emotional reciprocity, including reduced or inappropriate responses to the expressions of others and inappropriate or diminished responses to emotional situations (American Psychiatric Association, 2013). However, experimental paradigms investigating the nature of these difficulties have yielded mixed results (e.g., Hobson, 1986; Humphreys et al., 2007; Pelphrey et al., 2002; but see Adolphs et al., 2001; Grossman et al., 2000). Intelligence and age may contribute to the variability in measurement, in part, due to the ability to use cognitive or compensatory strategies. Investigation of underlying neural function may provide insight about the use of compensatory strategies, which, in contrast to the early attention and perception of emotional cues examined in the current study, would occur at later processing stages and involve different neural systems.
Children with ASD have atypical early stage ERP responses to emotional faces with reduced amplitudes and slower latencies at the P1 and N170 (Batty et al., 2011; Tye et al., 2014) and weaker and slower dipoles underlying the ERP signal on the scalp (Wong et al., 2008). In young children with ASD, the pre-cursor N170 to fear is related to social-communication skills (Dawson et al., 2005). Basic early stage processing of neutral faces, as assessed via ERPs, suggests some improvement by adulthood in latency and differentiation of faces vs. other objects (Webb et al., 2012). Although group by emotion interactions were not detected, O’Connor et al. (2005) suggest some impairment in early stage processing of emotion faces in adults with ASD represented by delayed latencies at the P1 and N170 and reduced amplitudes at the N170.
Eye tracking studies find that adults with ASD may attend to the mouth region to a higher degree, on average, than individuals without ASD while discriminating emotional expressions (Rutherford and Towns, 2008; Spezio et al., 2007). Thus, stimulus selection (e.g., open versus closed mouths) for investigations of emotional response in ASD could impact the salience of information conveyed for individuals with ASD if their initial attention to the face differs. Early attention and perception of this information would also potentially modulate neural responses.
1.3. Emotion processing and the serotonin system
Serotonin transporter (SLC6A4) allele length is a specific genetic factor potentially related to neural responsiveness to emotional information (Canli and Lesch, 2007; Hariri et al., 2002; Heinz et al., 2005), particularly with differences in responding to negative emotional information and experiences (e.g., Caspi et al., 2003; Hariri et al., 2002). ERP responses to emotional stimuli by carriers of the short (s) allele are reduced at the N400 for faces (Battaglia et al., 2005) and increased at the EPN for affective pictures (Herrmann et al., 2007) suggesting differences in the contextual processing of emotions. As well, carriers of the short allele have more difficulty disengaging their attention from happy and fearful faces (Beevers et al., 2009) and have greater amygdala activation to angry faces (e.g., Hariri et al., 2002; von dem Hagen et al., 2011). Because allele length influences the transcription of serotonin (Lesch et al., 1996), carriers of the short allele have lower neurotransporter levels and decreased serotonin uptake at the synapse (see Wurtman, 2005 for review). These differences are thought to underlie the response to emotional information by carriers. By examining early neural responses related to emotion perception, we can potentially detect group differences and provide information about the point in the processing stream at which groups first diverge. In particular, examination of early neural markers of attention (P1) may capture potential differences in vigilance to threat-related or emotional stimuli or difficulty disengaging attention for previous emotional stimuli.
A recent eye tracking study of children with short versus homozygous long alleles found that short allele carriers looked preferentially to the mouth region and less to the eye region while viewing happy, angry and neutral faces, which the authors suggested may be an indicator of social anxiety and shyness (Christou et al., 2015). As with ASD, a different pattern of visual attention to mouths may modulate early neural markers of attention and perception depending on the mouth position and its relevance to emotion perception.
1.4. Goals of the current study
Our primary goal was to compare the pattern of neural responses during early stage attention and perception of emotional cues for (1) individuals with ASD versus neurotypical adults and (2) short versus homozygous long SLC6A4 carriers. SLC6A4 transmission may differ in ASD, so the direct comparison of these conditions is informative in providing a potential means of distinguishing subgroups within ASD. Given the broad set of genetic and environmental risk factors for ASD, we predicted that having ASD would have a broader impact (i.e., at more stages in the processing stream and across a wider range of emotional expressions) on early ERPs than the impact of serotonin transporter expression, which represents a single genetic factor that appears primarily linked with detecting negative emotions. Consistent with the previous investigation of emotion discrimination in adults with ASD, we predicted reduced amplitude at the N170 for faces with emotional cues. Given the sensitivity of the VPP and EPN to early perception of emotional information, we further predicted that these components would be most sensitive to differences in early emotional processing by individuals with ASD with the VPP being most sensitive to differences in fearful expressions and the EPN being sensitive to differences in perception and encoding of both fearful and happy faces. We also examined whether serotonin transporter (SLC6A4) allele length influenced very early ERP components. Although effects of being a short allele carrier are detected at later stages of processing (Battaglia et al., 2005; Herrmann et al., 2007), early stage responses to faces with emotional expressions have not been examined. Given evidence that SLC6A4 allele length impacts response to negative emotions, we predicted that fearful expressions would produce the greatest differences in responding and they would be most apparent at the P1, which would be sensitive to early attention differences and most influenced by negative emotions. Increased attention or vigilance by short SLC6A4 allele carriers would result in increased P1 amplitude whereas difficulty disengaging from previous stimuli may reduce the P1 amplitude.
Second, we examined the impact of open versus closed mouths in early perception of emotions for both conditions, given reports that both individuals with ASD and short SLC6A4 allele carriers demonstrate a bias toward the mouth and away from the eye region when viewing faces with emotional information. Being overly focused on mouths may modulate neural response: First, the salience of open-mouthed expressions in the fearful condition would differ by group based on the general importance of eye information for identifying fearful faces; and second, a more similar response across groups for happy faces would be expected given the general importance of the mouth for identifying happy faces. We are not aware of previous investigations of open versus closed mouths at early stage ERPs, but if differences emerge and are due to the influence of low-level visual properties (i.e., high contrast of teeth) driving early attention, they should be present at the P1. In contrast, if differences are a result of the information about emotional cues that are perceived and encoded, groups should exhibit a different pattern of happy relative to fearful neural response at the EPN.
Before proceeding with our primary goals, it was important to confirm the sensitivity of our paradigm, particularly the manipulation of open-versus closed-mouth versions of facial expressions, among a group of neurotypical adults without ASD. Viewing faces without a face-specific behavioral demand has been used less frequently in electrophysiological investigations of emotion processing but allows for wider use among clinical populations. Our task required only a response to a non-face target to ensure basic attention to the stimuli. We first confirmed that ERP components related to very early perceptual awareness, initial discrimination, and encoding of emotional information conveyed in faces were sensitive to emotional facial expressions when viewed by a group of neurotypical adults. We also systematically examined ERPs in the neurotypical group using a stimulus set that included both open- and closed-mouth versions of each expression.
2. Methods
2.1. Participants
Twenty-seven adults with autism spectrum disorders (25 male, 2 female) and 25 neurotypical adults (23 male, 2 female) were included in the current study and provided adequate, artifact free electrophysiological data. Subjects ranged in age from 18 to 45 years. Groups did not differ in sex distribution, X2 (1, N = 52) = 0.006, p = .94, or age, t(50) = −0.48, p = .64. Participants with ASD met research criteria based on the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1999) and had clinical diagnoses of Autistic Disorder, Asperger’s Disorder or Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS), determined by expert clinical diagnostic judgment using Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria (American Psychiatric Association, 2000). All individuals with ASD also met Autism Diagnostic Interview-Revised criteria for autism on the social and communication domains (ADI-R; Lord et al., 1994). Meeting full criteria on the ADI-R was not required because parents were not always available or were not always confident in their recollection of the onset of early symptoms. Exclusionary criteria for participants with ASD and the comparison group included known genetic disorders, seizures, significant sensory or motor impairments, major physical abnormalities, serious head injuries, or use of anticonvulsant or barbiturate medications. Exclusionary criteria for neurotypical adults also included birth or developmental abnormalities, psychotropic medication usage, or first-degree relatives with ASD. Participants were recruited from a variety of community sources including Autism Clinics, Autism Support Groups, and local Medical Center and Community College campuses. As a result, the neurotypical group represented a wider range of age, educational level and socioeconomic status than a traditional college student sample. The University of Washington Human Subjects Institutional Review Board approved the study procedures and all subjects consented.
Cognitive ability was assessed using an abbreviated version of the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III; Wechsler, 1997a) consisting of the Vocabulary, Comprehension, Object Assembly, and Block Design subtests. Diagnostic groups did not differ in Full Scale, Verbal or Performance IQ. Basic memory for faces and objects was measured using the Wechsler Memory Scales Facial Memory subtest (WMS; Wechsler, 1997b) and Woodcock Johnson-Revised Picture Recognition subtest (WJ-R; Woodcock and Johnson, 1989), respectively. Relative to the neurotypical group, the group with ASD had worse recognition of both faces (immediate and delayed) and objects. See Table 1 for descriptive statistics.
2.2. DNA extraction and genotyping
Analyses of SLC6A4 allele status were limited to white participants (N = 48) because race potentially influences expression and function of SLC6A4 alleles (Gelernter et al., 1997; Munafò et al., 2008). Blood samples were obtained from 45 of the 48 participants. Genotypes for SLC6A4 loci were determined via DNA extracted from blood following the protocol of Gelernter et al. (1997). Of the 45 subjects, 32 were short-allele carriers (i.e., s/s homozygotes or s/l heterozygotes; 13 ASD and 19 Non-ASD) and were compared to the 13 long-allele homozygotes (l/l, 9 ASD, 4 Non-ASD). Diagnostic groups did not differ in the proportion of individuals homozygous for the long allele, X2 (1 df) = 3.03, p = .08. The allele groups (s/s and s/l vs. l/l) also did not differ on age, Full Scale IQ, face or object memory.
2.3. EEG recording procedure
The EEG was recorded continuously from a 128-channel Geodesic Sensor Net (GSN, Electrical Geodesics, Inc.) soaked in potassiumchloride electrolyte solution and fitted according to the manufacturer’s specifications. Impedances were below 50 kΩ. The signals were recorded online using the vertex electrode as reference, amplified by 1000, and digitized by a NetAmps200 EGI amplifier (Eugene, OR). Hardware filters were set at 0.1 Hz high-pass and 200 Hz elliptical low-pass, with a sampling rate of 500 Hz. Data were re-filtered off-line using a low-pass filter to remove electrical noise (Kaisertype FIR filter, 30 Hz cutoff with 2 Hz rolloff).
2.4. Stimuli and experimental procedure
Stimuli consisted of gray-scale digital images of faces from the Macbrain stimulus set (Tottenham et al., 2009) presented on a computer monitor with a light gray background. Twenty-six actors (13 female) posed happy, fearful and neutral expressions with open and closed mouths. Images were standardized so that the center of the eyes was presented at the center of the screen. There were 64 fearful trials, 64 neutral face trials and 64 happy trials; per expression, half had open and half had closed mouths. Emotions and mouth positions were presented in random order. To monitor attention to the task, participants were asked to press a button to 40 target trials, which were sine wave gratings. Each trial consisted of 100 ms baseline, 300 ms stimulus presentation, and 1000–1400 ms random inter trial interval.
2.5. Data editing and analysis
EEG data were segmented with a 200 ms baseline period immediately preceding stimulus onset and 500 ms after the onset of the stimulus. Epochs were time-locked to stimulus onset using a photocell. Trials with artifacts were excluded from the averages using the following criteria: (1) presence of an eye blink using the Netstation Eye Blink algorithm set at 140 μV with an 80 ms moving average and confirmed by visual inspection, (2) presence of an eye movement using the Netstation Eye Movement algorithm set at 55 μV with an 80 ms moving average, or (3) fluctuations exceeding 70 μV with 100 ms moving average in 10 or more channels. Subjects for whom more than 50% of non-target trials were rejected were excluded from analyses. As a result, an additional 4 individuals with ASD and 1 individual in the neurotypical group who provided data during this task were excluded from the current study. The proportion of excluded subjects did not significantly differ by group, X2 (1 df, N = 57) = 1.45, p = .23. Excluded subjects are not included in Table 2 and did not differ from included subjects on age or IQ. Among the subjects with adequate data who were included in the study (i.e., ASD = 27, Neurotypical = 25), 82.3% of trials were included for the group with ASD and 85.9% were included in the neurotypical group. Groups did not differ in the overall number of trials included in analyses, nor number of good trials per condition, p values >.42. Data were then baseline corrected using the full 200 ms baseline period, averaged for each condition at the individual level, and re-referenced offline to the average of all electrodes minus the four eye channels.
Table 2.
Participant characteristics.
| Variable | Autism spectrum N = 27 (2 female) Mean (SD), range | Neurotypical N = 25 (2 female) Mean (SD), range | Significance t (p) |
|---|---|---|---|
| Age in years | 23.3 (7.7), 18–44 | 24.36 (7.8), 18–45 | −0.48 (ns) |
| SLC6A4 allele ratio | 13 s: 9 l/l | 19 s: 4 l/l | |
| Wechsler intelligence quotient | |||
| Full Scale | 112.7 (15.0), 86–137 | 113.9 (11.8), 91–139 | −0.30 (ns) |
| Verbal | 111.6 (15.5), 79–140 | 112.9 (11.1), 92–132 | −0.34 (ns) |
| Performance | 111.0 (16.0), 83–139 | 111.5 (13.9), 80–136 | −0.12 (ns) |
| Wechsler memory scales | |||
| Faces immediate | 7.8 (2.0), 4–12 | 9.6 (2.6), 5–18 | −3.1 (.003) |
| Faces delayed | 7.8 (1.9), 4–12 | 9.3 (2.2), 5–14 | −2.5 (.015) |
| Woodcock Johnson-revised | |||
| Picture recognition | 19.4 (4.1), 10–27 | 22.5 (3.7), 16–29 | −2.8 (.008) |
Note: Standard scores are reported for the Wechsler Adult Intelligence Scales, with M = 100, SD = 15. Scaled scores are reported for the Wechsler memory scales, with M = 10, SD = 3. Raw Woodcock Johnson scores are reported.
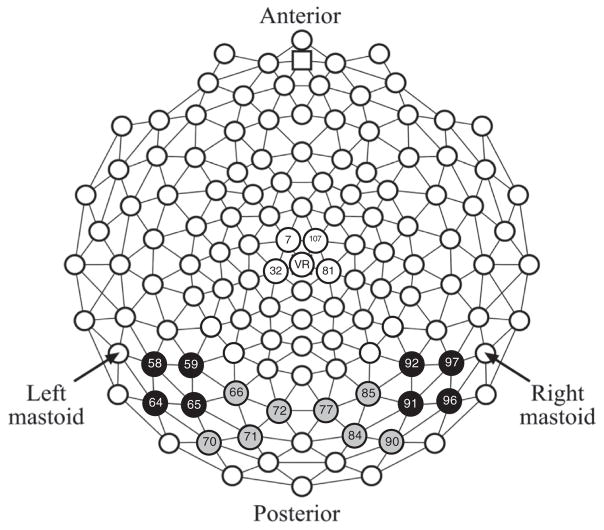
Amplitude for each component was measured as the mean voltage in a given measurement window. The P1 (60–130 ms) was measured from posterior medial occipital leads (left: 66, 70, 71, 72, and right: 77, 84, 85, 90); the N170 (120–180 ms) and EPN (200–350 ms) from posterior lateral occipital leads (left: 58, 59, 64, 65, and right: 91, 92, 96, 97); and the VPP (120–180 ms) from central parietal leads (60, 61, 62, 79, 86). See Fig. 1.
Fig. 1.
Geodesic sensor map with electrodes included in analyses highlighted as follows: posterior medial occipital in gray (P1); posterior lateral occipital in black (N170 and EPN); and central parietal in white (VPP).
Repeated measures analysis of variance (ANOVA) was used to account for the multivariate nature of the data, using the Green-house–Geisser epsilon correction for nonsphericity (Jennings and Wood, 1976). Post hoc analyses employed the Bonferroni correction to account for multiple comparisons. We included three types of analyses. (1) We first examined within subjects effects among the neurotypical group in order to examine our general manipulation and confirm expected effects. Within-subjects factors of emotion (fearful, neutral, happy), mouth (open, closed) and, where appropriate, hemisphere (left, right) were examined. (2) Subsequent analyses included a between-subjects factor of group (ASD vs. neurotypical). (3) Finally, the role of genotype was examined wherein individuals who had at least one short allele (i.e., s/l, s/s) were compared with individuals who were homozygous for the long allele (i.e., l/l) (e.g., Hariri et al., 2002; Lesch et al., 1996). Prior to comparing ASD vs. neurotypical and short vs. homozygous long allele carriers, we conducted analyses to explore possible interactions between allele type and diagnosis. No significant interactions were detected (i.e., allele × diagnosis or allele × diagnosis × emotion), so diagnostic groups were examined independent of allele status and allele status was analyzed collapsed across diagnosis.
3. Results
3.1. The P1
3.1.1. Neurotypical group
There were no significant main effects or interactions related to emotion, mouth position or hemisphere in P1 amplitude (F values < .73, p values > .47).
3.1.2. ASD
When the group with ASD was included, there were no main effects or interactions related to diagnostic group at the P1 component (F values < .81, p values > .44). Analyzed separately, the group with ASD did not have a significant main effects or interactions related to emotion, mouth position, or hemisphere (F values < 2.70, p values > .09).
3.1.3. Serotonin
When the effect of allele was examined, the interaction between mouth position and allele type approached significance, F(1, 43) = 3.25, p = .08, , and all other interactions related to allele were non-significant (F values < 2.40, p values > .11). However, an overall effect of allele type was detected, F(1, 43) = 5.89, p = .02, , due to greater P1 mean amplitude for the for the l/l group (M = 2.1 μV) relative to the group with at least one short allele (M = 0.2 μV) across fearful, happy and neutral expressions.4
3.2. The N170
3.2.1. Neurotypical group
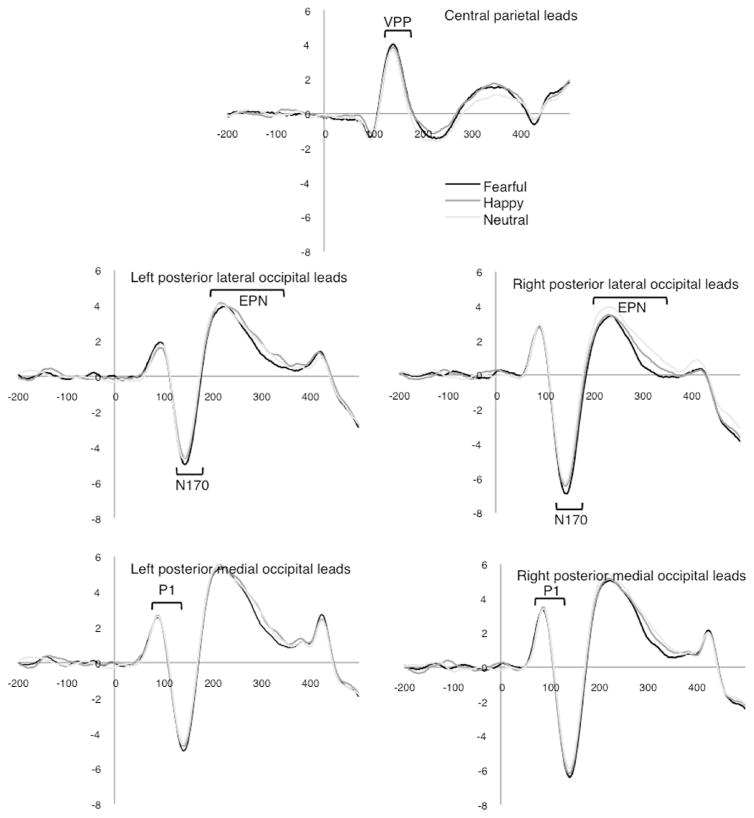
ERP amplitudes differed by emotional expression at the N170 component, F(1.9, 46.1) = 10.98, p < .001, . Bonferroni corrected post hoc tests revealed this was due to significant differences between fearful faces (M = −3.9 μV) and both neutral (M = −3.3 μV) and happy (M = −3.5 μV) faces, while the happy and neutral conditions did not differ. This is consistent with previous work suggesting the N170 is enhanced for negative emotions. There was also a significant effect of hemisphere, F(1, 24) = 9.9, p < .004, , with greater amplitude over the right leads (M = −4.2 μV) than the left (M = −2.9 μV). Finally, an emotion by hemisphere interaction was detected, F(1.7, 39.8) = 3.49, p < .05, . There was no effect of mouth (p = .10). Waveforms for the non-ASD group are shown in Fig. 2.
Fig. 2.
Waveforms for the neurotypical group.
3.2.2. ASD
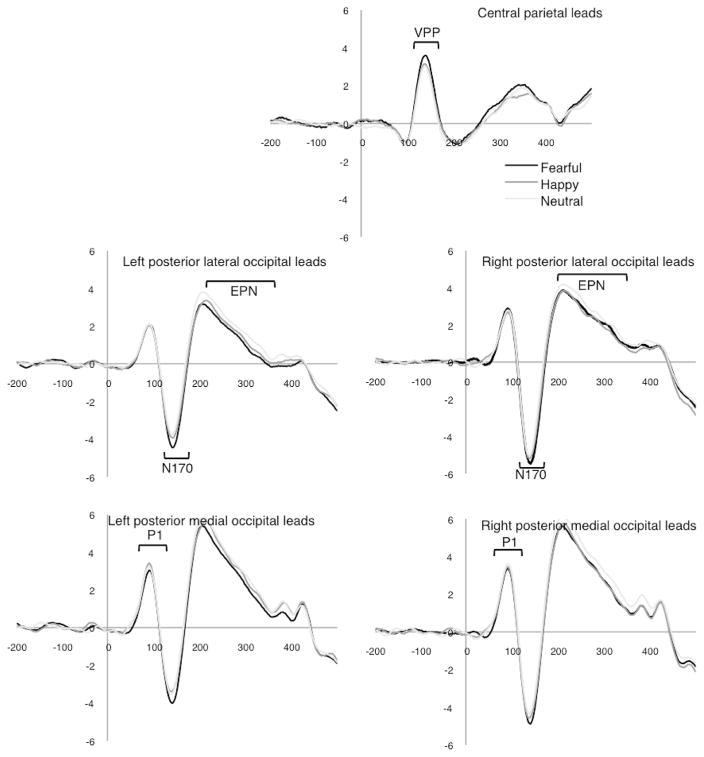
When diagnostic groups were compared, there were no main effects or interactions related to diagnostic group, though there was a trend for group × hemisphere, F(1, 50) = 3.43, p = .07, , with other F values < 2.99, p values > .09. Examined separately, individuals with ASD also had amplitude differences at the N170 for different emotional expressions, F(1.9, 48.5) = 6.53, p < .004, ; this was due to a significant difference between fearful faces (M = −2.6 μV) and neutral (M = −2.0 μV) but not happy (M = −2.3 μV) faces. Waveforms for the group with ASD are shown in Fig. 3.
Fig. 3.
Waveforms for the group with ASD.
3.2.3. Serotonin
No main effects or interactions related to allele group were detected for emotional expressions or mouth position, F values < 1.90, p values > .18.
3.3. The Vertex Positive Potential (VPP)
3.3.1. Neurotypical group
VPP mean amplitude differed by emotion, F(1.7, 41.0) = 3.82, p = .04, , due to differences between neutral (M = 1.94 μV) and both happy (M = 2.28 μV) and fearful (M = 2.43 μV) faces, which did not survive Bonferroni correction. The effect of mouth was not significant (p = .64).
3.3.2. ASD
When diagnostic groups were compared, there were no overall group effects or interactions, F values < 2.40, p values > .10. The group with ASD also had the greatest VPP amplitude for fearful (M = 1.84 μV) faces, then happy (M = 1.58 μV) and neutral (M = 1.41 μV), yet the effect of emotion was non-significant when the ASD group was examined separately, F(1.9, 49.9) = 2.22, p = .12, . The effect of mouth was also non-significant (p = .76).
3.3.3. Serotonin
Examination of the short allele vs. the l/l allele group did not detect group differences or interactions (F values < .97, p values > .38) with respect to the VPP.
3.4. The Early Posterior Negativity (EPN)
3.4.1. Neurotypical group
A significant main effect of emotion was again detected, F(1.9, 44.5) = 7.62, p = .002, . Post hoc comparisons revealed this was due to a significant amplitude difference between fearful faces (M = 2.0 μV) and neutral (M = 2.6 μV) faces and a trend (p = .07) for fearful and happy (M = 2.4 μV) faces, while the happy and neutral conditions did not differ. The EPN was relatively most negative in the fearful condition. Additionally, there was an interaction of emotion × mouth, F(1.55, 37.2) = 6.43, p = .003, , and emotion × hemisphere, F(1.62, 38.8) = 5.11, p = .01, .
3.4.2. ASD
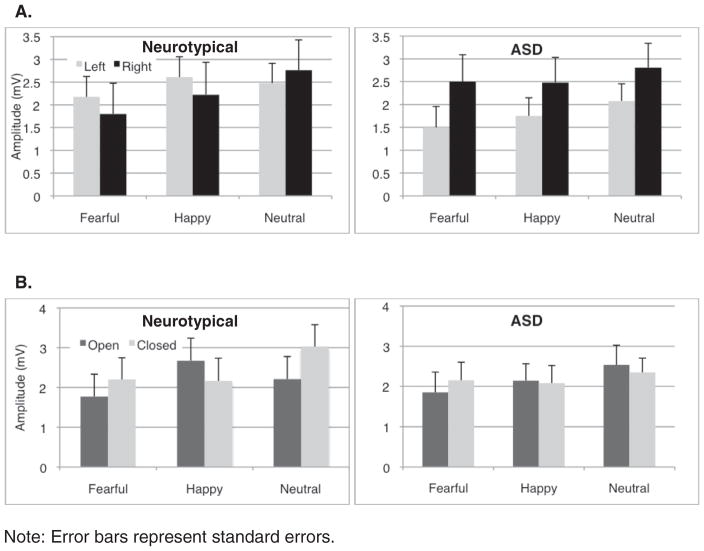
The group with ASD exhibited a different pattern of EPN responses relative to the comparison group. The groups differed in the scalp distribution of responses to specific emotions (group × emotion × hemisphere interaction), F(1.8, 88.4) = 5.0, p = .01, (in Fig. 4a). As well, a significant group × emotion × mouth interaction, F(1.8, 92.3) = 4.02, p = .02, , suggests that groups differed in their perception of key facial features involved in encoding and discriminating emotions (in Fig. 4b). Within the ASD group alone, there was a main effect of emotion, F(2, 50.2) = 4.10, p = .02, , such that fearful faces (M = 2.0 μV) differed from neutral (M = 2.4 μV) but not happy (M = 2.1 μV) faces. Critically, the effect of mouth, F(1, 26) = 0.02, p = .89, , and emotion × mouth were not significant, F (1.93, 50.2) = 1.37, p = .26, , suggesting the group interaction was due to EPN amplitude differences in neurotypical group.
Fig. 4.
Interaction effects between the group with ASD and neurotypical group at the EPN. (A) Significant group by emotion by hemisphere interaction effect was detected. (B) Significant group by emotion by mouth interaction effect was detected.
3.4.3. Serotonin
No differences emerged upon examination of the short allele vs. the l/l allele group (F values < 1.31, p values > .26).
4. Discussion
4.1. Neurotypical ERPs to emotion and mouth position
Before proceeding to our two main questions, we first confirmed that our task was sensitive to differences in emotional expression of faces at early components in a neurotypical adult sample. The P1 was not sensitive to emotional information (also see Foti et al., 2009; Weinberg and Hajcak, 2010). By the posterior N170, fearful expressions were processed differently than both neutral and happy expressions, suggesting that the negative valence was a critical differentiating factor. A slightly different pattern emerged at the frontal VPP. Faces with expressions were distinguished from neutral faces, suggesting that emotional intensity may be more influential for the VPP. Finally, the valence of the fearful faces was distinguished at the EPN component relative neutral expressions. Our task did not provide evidence that the EPN is differentially sensitive to pleasant expressions as has been previously suggested (Foti et al., 2009; Holmes et al., 2009; Rellecke et al., 2011; Schacht and Sommer, 2009; Schupp et al., 2004), but it was consistent with previous work demonstrating the discrimination of emotional expressions from neutral ones (Bradley et al., 2007; Rellecke et al., 2011; Schacht and Sommer, 2009; Schupp et al., 2004).
We also examined the impact of open versus closed mouths and found a significant interaction between mouth and emotional expressions at the EPN among the neurotypical group. This suggests that the presence of visible teeth did not influence early neural responses during basic sensory processing of the faces, but began to emerge at stages when emotional cues are recognized and encoded. Specifically interaction between emotion and mouth position for this group is consistent with mouths receiving more attention when processing happy faces than other expressions (Eisenbarth and Alpers, 2011), as the EPN amplitude was larger for open, happy mouths but not for fearful or neutral expressions.
4.2. Processing differences related to ASD and SLC6A4 allele type
We then turned to the main goal of investigating the pattern of early neural responses to emotional cues for (1) individuals with ASD versus neurotypical adults and (2) short versus homozygous long SLC6A4 carriers with the prediction that different profiles would distinguish these groups. This prediction was confirmed as detailed below. We also predicted that differences would be more widespread across components and emotional expression for individuals with ASD relative to short SLC6A4 carriers, which was not confirmed. Both conditions produced differences at primarily one component, but across multiple expressions.
4.2.1. ASD
The group with ASD differed from the neurotypical group at the EPN in terms of sensitivity to emotional information. Prior to the EPN, groups did not significantly differ in their responses at the P1, N170 or VPP. The failure to detect differences in overall N170 amplitudes while viewing faces with emotional expressions differed from O’Connor et al. (2005) but not from Webb et al. (2012). This is not surprising because the task used by O’Connor and colleagues involved active discrimination and because the participants in the current report substantially overlapped with those of Webb and colleagues. Our results suggest that the N170, which is associated with perception of information needed to distinguish faces from other categories (Bentin et al., 1996), is not modulated by emotional expressions in adults with ASD differently than in neurotypical adults.
In contrast to our prediction, the VPP also did not distinguish groups. Given the close correspondence between the N170 and VPP, thismay reflect the lack of findings at the N170. However, when groups were examined separately, the neurotypical group demonstrated a significant effect of emotion at the VPP whereas the group with ASD did not, suggesting subtle differences may emerge at this stage, perhaps due to frontal contributions to the VPP.
By the EPN, the group with ASD had different EPN scalp distribution. Specifically, the group with ASD exhibited a more right lateralized EPN than the comparison group. Our neurotypical group exhibited a similar scalp distribution to prior investigations of the EPN and emotional expression (e.g., Calvo and Beltrán, 2013; Rellecke et al., 2012), which did not find lateralization among typically developing adults for this component. Lateralized responses in the group with ASD suggest a possible reliance on different neural systems for perceiving and encoding emotional information. Previous work has also demonstrated differences in N170 scalp distribution in response to faces without emotions among adults with ASD (Webb et al., 2012) and first-degree relatives (Dawson et al., 2005). The current study extends that finding to perception of emotions.
4.2.2. Serotonin
A different pattern emerged when examining the discrimination of emotions between a group with at least one SLC6A4 short allele and a group homozygous for long alleles. We found evidence of differential processing by serotonin transporter genotype at the P1, but did not detect differences based on genotype for the N170, VPP, or EPN. This difference reflected larger amplitude P1 responses to fearful, neutral, and happy expressions by the group homozygous for long alleles. Battaglia et al. (2005) also report reduced P400s for short allele carriers. The P1 reflects early selective attention and sensory processing of basic information about the face (Boutsen et al., 2006; Hillyard et al., 1973; Mercure et al., 2008; Olofsson et al., 2008), and our findings indicate a reduction of neural resources allocated to early attention. This is inconsistent with the pattern expected for a high level of vigilance to fearful faces, which should increase P1 amplitude to fearful faces, and more consistent with difficulty disengaging attention from the previous stimulus. The majority of trials in our paradigm either follow happy or fearful faces. To the extent that short allele carriers have more difficulty disengaging their attention from happy and fearful faces (Beevers et al., 2009), this may lead to reduced allocation of neural resources for initial processing of the next face being presented. It is possible that the effect of genotype is more closely related to lingering arousal from late processing stages (i.e., beyond the early stage components examined in the current study) that are maintained until the P1 when another face is detected. This is consistent with recent work suggesting that low expressing 5-HTTLPR genotypes showed less habituation in neural response to negative emotional information (Wiggins et al., 2014).
4.3. Processing differences related to open versus closed mouths
Our second major goal was investigating the influence of open versus closed mouths on early attention and perception of emotions. We predicted that ASD and short SLC6A4 allele carriers would differ relative to comparison groups in their responses to open versus closed mouths, given eye tracking evidence of increased attention allocation to mouths with emotional expressions (Christou et al., 2015; Rutherford and Towns, 2008; Spezio et al., 2007). An overfocus on mouths should distinguish response patterns between happy and fearful, given the difference in the importance of mouth versus eye information for processing these expressions. As well, differences at the P1 would reflect the influence of low-level visual properties (i.e., high contrast of teeth) driving early attention and differences at the EPN would reflect differences in information about emotional cues that are perceived and encoded.
4.3.1. EPN
Among neurotypical adults, the interaction of emotion and mouth condition was observed at the EPN, consistent with the relative importance of encoding mouth information in the happy condition relative to other expressions (e.g., Scheller et al., 2012). In contrast, the group with ASD significantly differed from the neurotypical group in their response to open versus closed mouths at the EPN and, when examined separately, failed to differentiate open and closed mouths at the EPN. Thus, at the EPN, reduced neural response emerged for the group with ASD compared with neurotypical adults in the sensitivity to expressions as modulated by the presence of teeth. The different pattern of EPN responses to emotional expressions with open versus closed mouths exhibited by the group with ASD relative to the neurotypical group suggested that adults with ASD relied on different featural cues for recognizing and encoding emotional information. Additionally, adults with ASD may have different attention strategies for encoding emotions, which may result in reduced ability to encode more nuanced information about the intensity or naturalness of emotional expressions. During validation of the MacBrain stimulus set, open mouths enhanced the recognition accuracy of happy and fearful expressions, highlighting the disadvantage of failing to modulate neural responses underlying encoding of emotional cues.
4.3.2. P1
At the P1, significant group differences were not detected for the ASD group or short SLC6A4 allele carriers; however, a trend emerged at the P1 for SLC6A4 allele group by mouth condition, consistent with group differences in early selective attention to the mouth, which may be differentially influenced by low-level visual properties of the stimuli.
4.4. Synthesis, limitations and future directions
Our study replicated previous evidence that viewing faces with emotional cues affects the N170, VPP and EPN neural responses of neurotypical adults and, consistent with the relative importance of information conveyed by the mouth in discriminating happy expressions, demonstrated that open mouths enhance these EPN effects more than closed mouths for happy relative to fearful and neutral expressions-. This pattern of responding provided a foundation by which to examine the clinical condition of ASD and the influence of the SLC6A4 genotype on early emotion processing. Our central question of whether these conditions resulted in different profiles of early neural response to emotional cues revealed that the SLC6A4 genotype has an impact limited to early sensory processing and attention to faces with emotional information (the P1), with reduced allocation of neural resources to new faces. In contrast, the group with ASD was primarily distinguished at the EPN, when attention is focused on perceiving and encoding key information for discriminating emotions. Prior to examining these groups separately, we confirmed that there were not significant interaction effects between ASD and SLC6A4 genotype in our sample; however, we cannot rule out this intriguing possibility given our current sample size. Given the reported differences in serotonin levels in some cases of ASD and potential differences in expression of SLC6A4, this will be an important next step. Our study provides a starting point by identifying unique early stage neural responses to emotion by these conditions. As our results demonstrate, the P1 and EPN may be particularly important in understanding the potential interaction of having both the short SLC6A4 allele and ASD.
The current investigation provides a unique perspective but is not without limitations. First, we used static, grayscale faces as stimuli, which may have limited our ability to detect emotional responses (Hajcak et al., 2012). For example, we did not detect increased amplitude at the EPN for faces as has been reported using potent affective pictures among short allele carriers (Herrmann et al., 2007). Our investigation of open versus closed mouths highlights the importance of stimulus characteristics, particularly for investigations of individuals with ASD. Given the difficulty of recruiting and characterizing participants with ASD, systematic research related to stimulus-driven and task-related effects is limited, but these effects likely contribute to mixed findings. Second, our sample size is relatively small, particularly the number of participants homozygous for SLC6A4 long-alleles. Indeed, the number of neurotypical individuals who were homozygous for the long-allele (i.e., 4 l/l versus 19 s carriers) was smaller than expected. Yet, when groups were collapsed across diagnostic status, the power for comparing carriers of short vs. homozygous long-alleles was comparable to previous reports that detected effects at the N400 for facial expressions (N = 49; Battaglia et al., 2005) and at the EPN for affective pictures (N = 47; Herrmann et al., 2007). Replication with a larger sample and a more representative distribution of homozygous long-allele carriers in the neurotypical groups will build on this initial study by affording the power necessary to detect possible interaction effects between diagnostic status and allele length. Finally, we did not record simultaneous eye tracking data. Direct comparison could confirm the correspondence between the attention pattern to eyes and mouths and the pattern of neural responses to open versus closed mouths.
4.5. Clinical implications
Understanding the neural representation of early electrophysiological responses to emotional information provides important clues for developing interventions and tools for measuring symptoms and symptom change. The contrast between autism and SLC6A4 allele status highlights the potential role of ERPs in understanding early processing. The group with ASD was distinguished from the neurotypical group by the lateralization of EPN responses to different emotions and by mouth conditions at the EPN. This finding suggests that accounting for open versus closed mouths in clinical assessments of faces may be more sensitive to detecting emotion processing difficulties and highlights a potential avenue for intervention by training individuals with ASD to focus on the most relevant aspects of the facial expression when discriminating emotions. In contrast, the serotonin transporter allele type contributed to reduced initial sensory processing and attention at the P1, suggesting effects later in the processing stream may linger until a new face is detected. This provides clues to the potential processing stage at which serotonin in the synapse may have its strongest effect. Clinically, this indicates that later cognitive strategies may be useful in mediating the effect of this genotype and training short allele carriers to deliberately shift their attention may better prepare them for subsequent emotional experiences.
5. Conclusions
The current study provides information about early stage processing for faces with fearful and happy expressions, with differences related to negative emotion detected as early as the N170 and continuing through the EPN among neurotypical adults. Additionally, neurotypical adults were more sensitive than adults with ASD to expressions with open-versus closed-mouths at the EPN. The group with ASD was also distinguished from the neurotypical group by the lateralization pattern of EPN responses to different emotions. Serotonin transporter allele type contributed to reduced initial sensory processing and attention at the P1 in our investigation, suggesting effects later in the processing stream may linger until a new face is detected.
HIGHLIGHTS.
Adults with autism spectrum disorder exhibit a different pattern of neural activation (EPN) when encoding and recognizing facial expressions than neurotypical adults.
Short serotonin transporter allele (SLC6A4) carriers have reduced neural responses (P1) during early sensory attention to facial expressions compared to long allele carriers.
The N170, VPP and EPN, but not the P1, are influenced by emotional expressions and the EPN is the earliest component modulated by open mouths in neurotypical adults.
Acknowledgments
This work was supported by the National Institutes of Health NIMH STAART Centers Program U54 MH066399 Project 4: Electrophysiology and fMRI studies of social cognition in autism, P50 HD 055782, and R01 MH094293. The MacBrain Face Stimulus Set used for this project was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. A poster based on these data was presented at the International Meeting for Autism Research, Toronto, Canada, May, 2012. Special acknowledgement is given to the research participants.
Conflict of interest: None of the authors have potential conflicts of interest to be disclosed.
Footnotes
The effects of allele length were also examined within the ASD group alone. At the P1, a trend was detected for Allele, F(1, 20) = 3.34, p = .08, ηp2 = .14, whereas Emotion x Allele was non-significant with an effect size, ηp2 = .08. Allele was non-significant for other components with effect sizes at the N1: ηp2 < .04; VPP: ηp2 < .01; and EPN: ηp2 < .08. Allele × emotion effects were also non-significant, ηp2 all <.005.
References
- Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cogn Neurosci. 2001;13:232–40. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual-text revision (DSM-IV-TRim) 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: Author; 2013. [Google Scholar]
- Battaglia M, Ogliari A, Zanoni A, Citterio A, Pozzoli U, Giorda R, et al. Influence of the serotonin transporter promoter gene and shyness on children’s cerebral responses to facial expressions. Arch Gen Psychiatry. 2005;62:85–94. doi: 10.1001/archpsyc.62.1.85. [DOI] [PubMed] [Google Scholar]
- Batty M, Meaux E, Wittemeyer K, Roge B, Taylor MJ. Early processing of emotional faces in children with autism: An event-related potential study. J Exp Child Psychol. 2011;109:430–44. doi: 10.1016/j.jecp.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Batty M, Taylor MJ. Early processing of the six basic facial emotional expressions. Brain Res Cogn Brain Res. 2003;17:613–20. doi: 10.1016/s0926-6410(03)00174-5. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Wells TT, Ellis AJ, McGeary JE. Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. J Abnorm Psychol. 2009;118:670–81. doi: 10.1037/a0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cogn Neurol. 1996;8:551–65. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Taylor MJ, Rousselet GA, Itier RJ, Caldara R, Schyns PG, et al. Controlling interstimulus perceptual variance does not abolish N170 face sensitivity. Nat Neurosci. 2007;10:801–2. doi: 10.1038/nn0707-801. [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, Goldman D. Genetics of emotion. Trends Cogn Sci. 2011;15:401–8. doi: 10.1016/j.tics.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau VC, Maurer U, Tottenham N, McCandliss BD. The face-specific N170 component is modulated by emotional facial expression. Behav Brain Funct. 2007;3:7. doi: 10.1186/1744-9081-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutsen L, Humphreys GW, Praamstra P, Warbrick T. Comparing neural correlates of configural processing in faces and objects: an ERP study of the Thatcher illusion. Neuroimage. 2006;32:352–67. doi: 10.1016/j.neuroimage.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Hamby S, Low A, Lang PJ. Brain potentials in perception: picture complexity and emotional arousal. Psychophysiology. 2007;44:364–73. doi: 10.1111/j.1469-8986.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- Calvo MG, Beltrán D. Recognition advantage of happy faces: tracing the neurocognitive processes. Neuropsychologia. 2013;51:2051–61. doi: 10.1016/j.neuropsychologia.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch K-P. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10:1103–9. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Leventhal BL. The serotonin system in autism. Curr Opin Pediatr. 1996;8:384–454. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Christou AI, Wallis Y, Bair H, Crawford H, Frisson S, Zeegers MP, et al. BDNF Val66Met and 5-HTTLPR genotype are each associated with visual scanning patterns in faces in young children. Front Behav Neurosci. 2015;9:1–12. doi: 10.3389/fnbeh.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev Neuropsychol. 2005;27:403–24. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Devlin B, Cook EH, Jr, Coon H, Dawson G, Grigorenko EL, McMahon W, et al. Autism and the serotonin transporter: the long and short of it. Mol Psychiatry. 2005;10:1110–6. doi: 10.1038/sj.mp.4001724. [DOI] [PubMed] [Google Scholar]
- Eimer M, Holmes A. Event-related brain potential correlates of emotional face processing. Neuropsychologia. 2007;45:15–31. doi: 10.1016/j.neuropsychologia.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M, Holmes A, McGlone FP. The role of spatial attention in the processing of facial expression: an ERP study of rapid brain responses to six basic emotions. Cogn Affect Behav Neurosci. 2003;3:97–110. doi: 10.3758/cabn.3.2.97. [DOI] [PubMed] [Google Scholar]
- Eisenbarth H, Alpers GW. Happy mouth and sad eyes: scanning emotional facial expressions. Emotion. 2011;11:860–5. doi: 10.1037/a0022758. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: evidence from temporal-spatial PCA. Psychophysiology. 2009;46:521–30. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet. 1997;101:243–6. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Grossman JB, Klin A, Carter AS, Volkmar FR. Verbal bias in recognition of facial emotions in children with Asperger syndrome. J Child Psychol Psychiatry. 2000;41:369–79. [PubMed] [Google Scholar]
- Guhathakurta S, Sinha S, Ghosh S, Chatterjee A, Ahmed S, Gangopadhyay PK, et al. Population-based association study and contrasting linkage disequilibrium pattern reveal genetic association of SLC6A4 with autism in the Indian population from West Bengal. Brain Res. 2008;1240:12–21. doi: 10.1016/j.brainres.2008.08.063. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, Foti D. ERPs and the study of emotion. In: Luck SJ, Kappenman ES, editors. The oxford handbook of event-related potential components. New York: Oxford University Press; 2012. pp. 441–74. [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–3. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–1. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Huter T, Müller F, Mühlberger A, Pauli P, Reif A, et al. Additive effects of serotonin transporter and tryptophan hydroxylase-2 gene variation on emotional processing. Cereb Cortex. 2007;17:1160–3. doi: 10.1093/cercor/bhl026. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182:177–80. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hobson RP. The autistic child’s appraisal of expressions of emotion. J Child Psychol Psychiatry. 1986;27:321–42. doi: 10.1111/j.1469-7610.1986.tb01836.x. [DOI] [PubMed] [Google Scholar]
- Holmes A, Bradley BP, Krach Nielsen M, Mogg K. Attentional selectivity for emotional faces: evidence from human electrophysiology. Psychophysiology. 2009;46:62–8. doi: 10.1111/j.1469-8986.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- Horstmann G, Lipp OV, Becker SI. Of toothy grins and angry snarls—open mouth displays contribute to efficiency gains in search for emotional faces. J Vis. 2012;12:1–15. doi: 10.1167/12.5.7. [DOI] [PubMed] [Google Scholar]
- Huang CH, Santangalo SL. Autism and serotonin transporter gene polymorphisms: a systematic review and meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:903–13. doi: 10.1002/ajmg.b.30720. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Minshew N, Leonard GL, Behrmann M. A fine-grained analysis of facial expression processing in high-functioning adults with autism. Neuropsychologia. 2007;45:685–95. doi: 10.1016/j.neuropsychologia.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Wood CC. The e-adjustment procedure for repeated-measures analyses of variance. Psychophysiology. 1976;13(3):277–8. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Joyce C, Rossion B. The face-sensitive N170 and VPP components manifest the same brain processes: the effect of reference electrode site. Clin Neurophysiol. 2005;116:2613–31. doi: 10.1016/j.clinph.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Kistner-Griffin E, Brune CW, Davis LK, Sutcliffe JS, Cox NJ, Cook EH., Jr Parent-of-origin effects of the serotonin transporter gene associated with autism. Am J Med Genet B Neuropsychiatr Genet. 2011;156:139–44. doi: 10.1002/ajmg.b.31146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korb S, With S, Niedenthal P, Kaiser S, Grandjean D. The perception and mimicry of facial movements predict judgments of smile authenticity. PLoS ONE. 2014;9:e99194. doi: 10.1371/journal.pone.0099194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule-WPS edition. Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Luo W, Feng W, He W, Wang N, Luo Y. Three stages of facial expression processing: ERP study with rapid serial visual presentation. Neuroimage. 2010;49:1857–67. doi: 10.1016/j.neuroimage.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercure E, Dick F, Johnson MH. Featural and configural face processing differentially modulate ERP components. Brain Res. 2008;1239:162–70. doi: 10.1016/j.brainres.2008.07.098. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol Psychiatry. 2008;63:852–7. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor K, Hamm JP, Kirk IJ. The neurophysiological correlates of face processing in adults and children with Asperger’s syndrome. Brain Cogn. 2005;59:82–95. doi: 10.1016/j.bandc.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: an integrative review of ERP findings. Biol Psychiatry. 2008;77:247–65. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. J Autism Dev Disord. 2002;32:249–61. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Rellecke J, Palazova M, Sommer W, Schacht A. On the automaticity of emotion processing in words and faces: event-related brain potentials evidence from a superficial task. Brain Cogn. 2011;77:23–32. doi: 10.1016/j.bandc.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Rellecke J, Sommer W, Schacht A. Does processing of emotional facial expressions depend on intention? Time-resolved evidence from event-related brain potentials. Biol Psychiatry. 2012;90:23–32. doi: 10.1016/j.biopsycho.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Rellecke J, Sommer W, Schacht A. Emotion effects on the N170: a question of reference? Brain Topogr. 2013;26:62–71. doi: 10.1007/s10548-012-0261-y. [DOI] [PubMed] [Google Scholar]
- Righart R, de Gelder B. Rapid influence of emotional scenes on encoding of facial expressions: an ERP study. Soc Cogn Affect Neurosci. 2008;3:270–8. doi: 10.1093/scan/nsn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford MD, Towns AM. Scan path differences and similarities during emotion perception in those with and without autism spectrum disorders. J Autism Dev Disord. 2008;38:1371–81. doi: 10.1007/s10803-007-0525-7. [DOI] [PubMed] [Google Scholar]
- Schacht A, Sommer W. Emotions in word and face processing: early and late cortical responses. Brain Cogn. 2009;69:538–50. doi: 10.1016/j.bandc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Schain RJ, Freedman DX. Studies on 5-hydroxindole metabolism in autistic and other mentally retarded children. J Pediatr. 1961;58:315–20. doi: 10.1016/s0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- Scheller E, Buchel C, Gamer M. Diagnostic features of emotional expressions are processed preferentially. PLoS ONE. 2012;7:e41792. doi: 10.1371/journal.pone.0041792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. Attention and emotion: an ERP analysis of facilitated emotional stimulus processing. Neuroreport. 2003;14(8):1107–10. doi: 10.1097/00001756-200306110-00002. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Ohman A, Junghofer M, Weike AI, Stockburger J, Hamm AO. The facilitated processing of threatening faces: an ERP analysis. Emotion. 2004;4:189–200. doi: 10.1037/1528-3542.4.2.189. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Flaisch T, Stockburger J, Junghöfer M. Emotion and attention: event-related brain potential studies. Prog Brain Res. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- Smith E, Weinberg A, Moran T, Hajcak G. Electrocortical responses to NIMSTIM facial expressions of emotion. Int J Psychophysiol. 2013;88:17–25. doi: 10.1016/j.ijpsycho.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Adolphs R, Hurley RSE, Piven J. Abnormal use of facial information in high-functioning autism. J Autism Dev Disord. 2007;37:929–39. doi: 10.1007/s10803-006-0232-9. [DOI] [PubMed] [Google Scholar]
- Thierry G, Martin CD, Downing P, Pegna AJ. Controlling for insterstimulus perceptual variance abolishes N170 face selectivity. Nat Neurosci. 2007;10:505–11. doi: 10.1038/nn1864. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka J, Leon AC, McCarry T, Nurse M, Hare TA, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye C, Battaglia M, Bertoletti E, Ashwood KL, Azadi B, Asherson P, et al. Altered neurophysiological responses to emotional faces discriminate children with ASD, ADHD and ASD+ADHD. Biol Psychol. 2014;103:125–34. doi: 10.1016/j.biopsycho.2014.08.013. [DOI] [PubMed] [Google Scholar]
- Van Der Geld P, Oosterveld P, Berge SJ, Kuijpers-Jagtman AM. Tooth display and lip position during spontaneous and posed smiling in adults. Acta Odontol Scand. 2008;66:207–13. doi: 10.1080/00016350802060617. [DOI] [PubMed] [Google Scholar]
- von dem Hagen EA, Passamonti L, Nutland S, Sambrook J, Calder AJ. The serotonin transporter gene polymorphism and the effect of baseline on amygdala response to emotional faces. Neuropsychologia. 2011;49:674–80. doi: 10.1016/j.neuropsychologia.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter RD, Goodacre BJ, Goodacre CJ, Naylor WP, Oyoyo U. A comparison of gingival display with a requested smile, Duchenne smile, grimace of disgust, and funnel-shaped expression. Prosthet Dent. 2014;112:220–7. doi: 10.1016/j.prosdent.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Wassink TH, Hazlett HC, Epping EA, Arndt S, Dager SR, Schellenberg GD, et al. Cerebral cortical gray matter overgrowth and functional variation of the serotonin transporter gene in autism. Arch Gen Psychiatry. 2007;64:709–17. doi: 10.1001/archpsyc.64.6.709. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Merkle K, Murias M, Richards T, Aylward E, Dawson G. ERP responses differentiate inverted but not upright face processing in adults with ASD. Soc Cogn Affect Neurosci. 2012;7(5):578–87. doi: 10.1093/scan/nsp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 3. San Antonio, TX: The Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler memory scale. 3. San Antonio, TX: The Psychological Corporation; 1997b. [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion. 2010;10:767–82. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- Wiggins JL, Swartz JR, Martin DM, Lord C, Monk CS. Serotonin transporter genotype impacts amygdala habituation in youth with autism spectrum disorders. Soc Cogn Affect Neurosci. 2014;9:832–8. doi: 10.1093/scan/nst039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijers AA, Banis S. Foveal and parafoveal spatial attention and its impact on the processing of facial expression: an ERP study. Clin Neurophys. 2012;123:513–26. doi: 10.1016/j.clinph.2011.07.040. [DOI] [PubMed] [Google Scholar]
- Williams LM, Palmer D, Liddell BJ, Song L, Gordon E. The ‘when’ and ‘where’ of perceiving signals of threat versus non-threat. Neuroimage. 2006;31:458–67. doi: 10.1016/j.neuroimage.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Wong TK, Fung PC, Chua SE, McAlonan GM. Abnormal spatiotemporal processing of emotional facial expressions in childhood autism: dipole source analysis of event-related potentials. Eur J Neurosci. 2008;28:407–16. doi: 10.1111/j.1460-9568.2008.06328.x. [DOI] [PubMed] [Google Scholar]
- Woodcock RW, Johnson MB. Woodcock-Johnson psycho-educational battery-revised (WJ-R) Itasca, IL: Riverside Publishing; 1989. [Google Scholar]
- Wurtman RJ. Genes, stress, and depression. Metabolism. 2005;54:16–9. doi: 10.1016/j.metabol.2005.01.007. [DOI] [PubMed] [Google Scholar]