Summary
Purpose
Metastatic solid tumors to the oral cavity are rare, frequently indicative of an end-stage disease process, and associated with poor survival rates. We performed a 20-year retrospective clinical analysis of our institution’s cases of solid metastases to the oral cavity, and investigated these patients’ clinical outcomes.
Material and Methods
A retrospective study of patients with metastatic solid tumors to the oral cavity over a 20-year period (October 1996 to September 2015) was conducted at Memorial Sloan Kettering Cancer Center. Patients were selected if they had a histopathologically confirmed diagnosis. Demographic, pathologic, and clinical information were reviewed to identify patient outcomes.
Results
A total of 44 patients with metastatic non-melanocytic non-hematopoietic tumor to the oral cavity were identified: 24 males and 20 females (39 adults and 5 children) with a mean age of 54.3 years. In all, 24 cases involved the jaw and 20 cases involved the oral soft tissue. Eight patients (18.2%) had oral cavity metastases as the first indication of an occult malignancy. In adult patients, the common primary sites were the lungs (n = 9, 20%), kidney (n = 7, 16%), breast (n = 5, 11%), and colon (n = 4, 9%); and in pediatric patients the adrenal gland (3/5) was the most common site. Of the adult patients, 33 (84.6%) died of disease. From the time of metastasis diagnosis, patients with jaw metastases had a median and mean survival of 12 months and 27.7 months, respectively. In comparison, patients with oral soft tissue metastases had a median survival time of 5 months, and mean of 8 months. One pediatric patient (20%) died of disease 8 months after metastasis diagnosis.
Conclusion
Metastatic solid tumors to the oral cavity can be the first sign of a malignancy. Pediatric patients with oral cavity metastases have a better prognosis compared to adult patients. In this series, adults with oral soft tissue involvement had shorter survival times compared to patients with jaw involvement.
INTRODUCTION
Metastatic solid tumors to the oral cavity are rare. Involvement of the jaw can be considered more common than involvement of oral soft tissue (Hirshberg et al., 2008; Summerlin, 1994). Metastases to the oral cavity can arise from any part of the body, but tumors of epithelial origin (carcinoma) occur more frequently (D'Silva et al., 2006; Hirshberg et al., 2008; Bodner et al., 2006; Antunes and Antunes, 2008). The common primary sites of metastatic oral cavity tumors are the breast in females and lung in males (Hirshberg et al., 2008; Allon et al., 2014). Metastatic dissemination to the oral cavity is highly indicative of an end-stage disease process, with reported survival time after oral metastases diagnosis at 3.7 to 8.25 months (van der Waal et al., 2003; Hirshberg et al., 2008; Murillo et al., 2013; Allon et al., 2014). Most patients who present with metastases to the oral cavity have already been diagnosed with primary tumors; however, in 22% to 25% of cases, oral cavity metastasis is the first manifestation of the disease (Hirshberg et al., 2008; Zachariades, 1989).
Metastatic disease involving the mandible, particularly the posterior region, is more common than the maxilla, whereas the gingiva is the most frequently involved oral soft tissue (Hirshberg et al., 2008; Hirshberg et al., 1994; Allon et al., 2014; Hirshberg et al., 1993; Zachariades, 1989).
The clinical presentation of metastatic tumors to the oral cavity range from jaw pain, exophytic lesion (either as a swelling or mass that may be ulcerated), paresthesia, and numbness, as well as misleading presentations such as toothache, dentoalveolar swelling, and loose tooth. The latter signs and symptoms can lead clinicians to consider an odontogenic disease process (D'Silva et al., 2006; Murillo et al., 2013; McClure et al., 2013).
Due to the rarity of metastatic tumors to the oral cavity and their often innocuous presentation, clinical and histopathologic diagnosis may be challenging (D'Silva et al., 2006; Hirshberg et al., 2014; Sauerborn et al., 2011). In this retrospective study, we describe a series of patients with metastatic tumors to the oral cavity and investigate the clinical outcomes of these patients.
MATERIAL AND METHODS
The study was approved by the Memorial Sloan Kettering Cancer Center (MSKCC) Institutional Review Board. A retrospective patient record review during a 20-year period (October 1996 to September 2015) was conducted for the identification of patients with metastatic tumors to the oral cavity. Patients with histopathologically confirmed diagnosis were included in the study. The following keywords were searched from our pathology electronic records: metastatic; jaw; jaw bone; mandible; maxilla; gingiva; gingival mucosa; alveolar mucosa; buccal mucosa; cheek mucosa; labial mucosa; palate; palatal mucosa; tongue; floor of mouth. Patients with melanoma, myeloma, lymphoma, and leukemia involving the jaw were excluded from this study. Patients with a clinical diagnosis of a jaw metastasis without histopathologic diagnosis were also excluded. The following clinical information was reviewed: sex, age at diagnosis, site of primary tumor, site of metastatic disease, vital status, histopathologic diagnosis, time duration from oral cavity metastasis diagnosis to patient’s death, clinical presentation of metastases, list of positive immunohistochemical (IHC) stains in the biopsied specimen from the oral cavity, and therapy instituted.
RESULTS
Based on the inclusion criteria, 44 patients were histopathologically identified as having metastatic tumor to the oral cavity. There were 24 males and 20 females with a mean age of 54.3 years (range, 7 months to 85 years). A total of 39 (88.6%) adult patients and 5 (11.4%) pediatric patients made up this series. In all, 22 (50%) cases involved the mandible (20 cases in the posterior region, including 4 cases in the ramus and 2 in the anterior region); 2 cases were in the maxilla, both in the anterior region; 11 (25%) cases involved the gingiva; 5 cases were in the buccal mucosa; 3 cases involved the tongue; and 3 cases involved the palatal mucosa. Two patients had 2 oral soft tissue involvements each, in 1 patient to the gingiva and palatal mucosa and in another patient to the gingiva and tongue. In adult patients, the primary sites were the lung (n = 9), kidney (n = 7), breast (n = 5), colon (n = 4), prostate (n = 2), liver (n = 1), pleura (n = 1), thyroid (n = 1), testis (n = 1), soft tissue buttock (n = 1), stomach (n = 1), submandibular (n = 1), uterus (n = 1), adrenal gland (n = 1), ureter (n = 1), and pancreaticobilliary (n = 1), and in 1 patient the primary site was unknown. In the pediatric patients, the primary sites were the adrenal gland (n = 3), eye (n = 1), and mediastinum (n = 1). Eight (18.2%) patients (cases 1, 3, 5, 20, 22, 25, 28, and 37) in this study had oral metastases as the first indication of an occult malignancy (sites: uterus, lung [n = 2], pancreaticobillary, colorectal, ureter, kidney, and adrenal gland). In the adult patients, 33 (84.6%) of 39 patients died of their disease and 6 patients were alive at the time of this study. In the pediatric patients, 1 patient died of disease and 4 patients were alive at the time of this study. Table 1 is a summary of the analyzed patient information. Carcinomas made up the majority (33 of 44; 75%) of the histologic subtypes in the study group. The clinical presentation of the metastases to the jaw varied and included jaw pain, jaw swelling/mass, lower lip and chin numbness, toothache, tooth abscess, non-healing tooth infection, and tooth exfoliation. The radiographic presentations of the jaw metastases were that of an expansile lytic lesion with ill-defined margins and cortical disruption in most cases (Figures 1 and 2). However, the clinical presentation of the metastases to the oral soft tissue represented mainly masses and swellings of the gingiva, buccal mucosa, and tongue (Figure 3). Other clinical symptoms and signs for the oral cavity metastases were pain, restriction in mouth opening, and difficulty with mastication.
Table 1.
Demographic data of patients with metastatic solid tumors to the oral cavity
| Case no. Sex | Sex | Age (y) | Primary site | Metastatic site | Vital status (DOD/alive) |
|---|---|---|---|---|---|
| 1 | F | 50 | Uterus | Posterior mandible | DOD |
| 2 | M | 69 | Lung | Posterior mandible | DOD |
| 3 | M | 70 | Lung | Posterior mandible | DOD |
| 4 | F | 52 | Lung | Anterior maxilla | DOD |
| 5 | M | 58 | Lung | Posterior mandible | DOD |
| 6 | M | 69 | Pleura (lung) | Tongue | DOD |
| 7 | F | 54 | Lung | Buccal mucosa | DOD |
| 8 | M | 62 | Lung | Maxillary gingiva and palatal mucosa |
DOD |
| 9 | F | 65 | Lung | Mandibular and maxillary gingiva |
DOD |
| 10 | M | 40 | Lung | Palatal mucosa | DOD |
| 11 | F | 65 | Lung | Maxillary gingiva | DOD |
| 12 | F | 76 | Breast | Mandibular ramus | DOD |
| 13 | F | 68 | Breast | Posterior mandible | Alive |
| 14 | F | 73 | Breast | Mandibular ramus | DOD |
| 15 | F | 64 | Breast | Mandibular gingiva | Alive |
| 16 | F | 69 | Breast | Tongue | DOD |
| 17 | M | 79 | Prostate | Posterior mandible | DOD |
| 18 | M | 63 | Prostate | Posterior mandible | DOD |
| 19 | M | 71 | Liver | Posterior mandible | DOD |
| 20 | M | 85 | Pancreaticobilliary | Posterior mandible | DOD |
| 21 | F | 77 | Colon | Posterior mandible | DOD |
| 22 | M | 74 | Colorectal | Mandibular ramus | DOD |
| 23 | F | 43 | Colon | Anterior maxilla | DOD |
| 24 | M | 59 | Colon | Mandibular gingiva | Alive |
| 25 | F | 64 | Ureter (renal pelvis) | Posterior mandible | DOD |
| 26 | F | 61 | Kidney | Anterior mandible | DOD |
| 27 | F | 63 | Kidney | Anterior-posterior mandible | DOD |
| 28 | F | 18 | Kidney | Mandibular gingiva | Alive |
| 29 | M | 75 | Kidney | Buccal mucosa | DOD |
| 30 | M | 70 | Kidney | Buccal mucosa | DOD |
| 31 | M | 59 | Kidney | Mandibular gingiva | DOD |
| 32 | M | 66 | Kidney | Buccal mucosa | Alive |
| 33 | M | 21 | Adrenal gland | Posterior mandible | Alive |
| 34 | M | 5 | Eye | Posterior mandible | DOD |
| 35 | F | 9 | Adrenal gland | Mandibular ramus | Alive |
| 36 | F | 1.75 | Adrenal gland | Posterior mandible | Alive |
| 37 | M | 0.6 | Adrenal gland | Posterior mandible | Alive |
| 38 | F | 54 | Thyroid | Mandibular gingiva | DOD |
| 39 | M | 48 | Buttock | Mandibular gingiva and tongue |
DOD |
| 40 | M | 35 | Testis | Maxillary gingiva | DOD |
| 41 | M | 60 | Stomach | Maxillary gingiva | DOD |
| 42 | M | 76 | Submandibular gland | Palatal mucosa | DOD |
| 43 | M | 44 | Unknown | Buccal mucosa | DOD |
| 44 | F | 3 | Mediastinum | Anterior mandible | Alive |
Abbreviations: DOD, dead of disease; F, female; M, male.
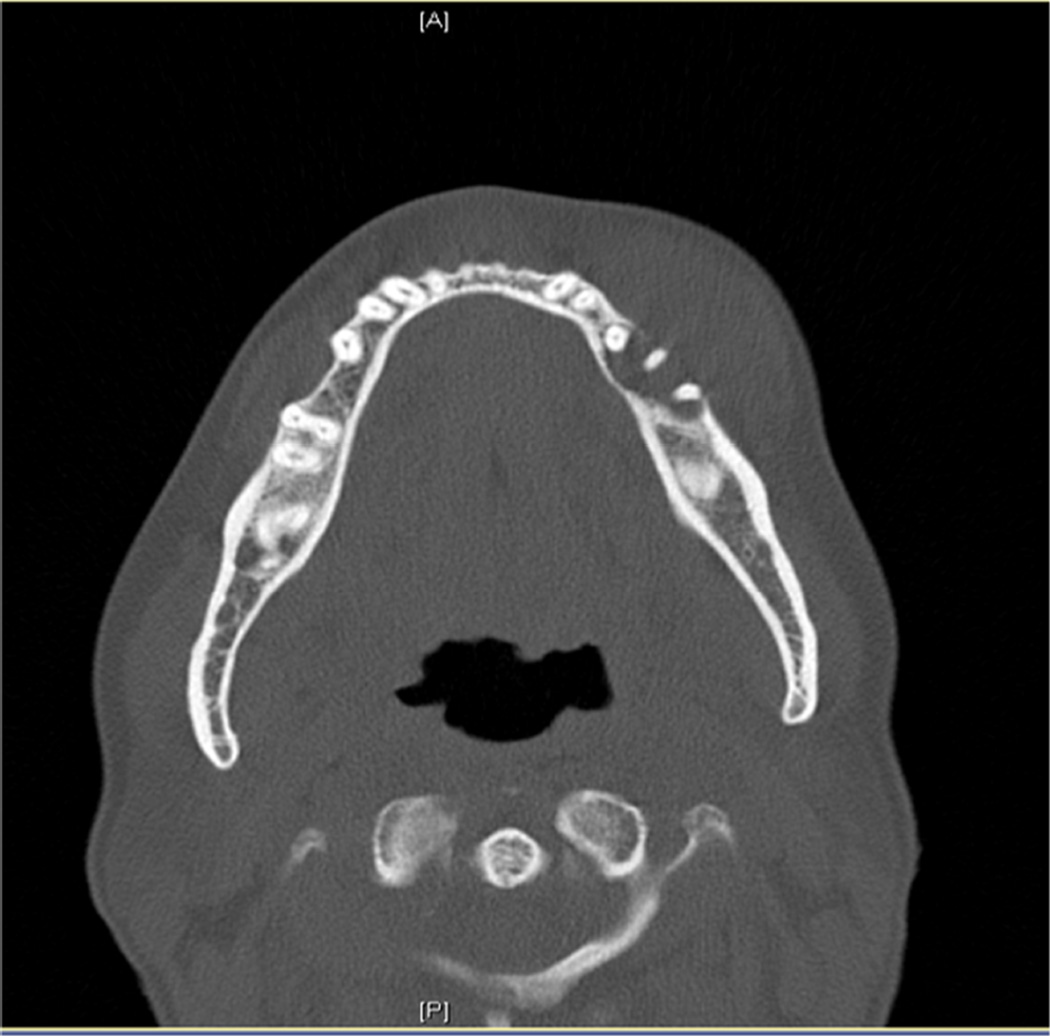
Figure 1.
Computed tomogram: axial view of a patient (case 1) with metastatic high-grade pleomorphic sarcoma to the mandible from the uterus first identified in the mandible, which demonstrates a destructive, erosive lytic lesion.
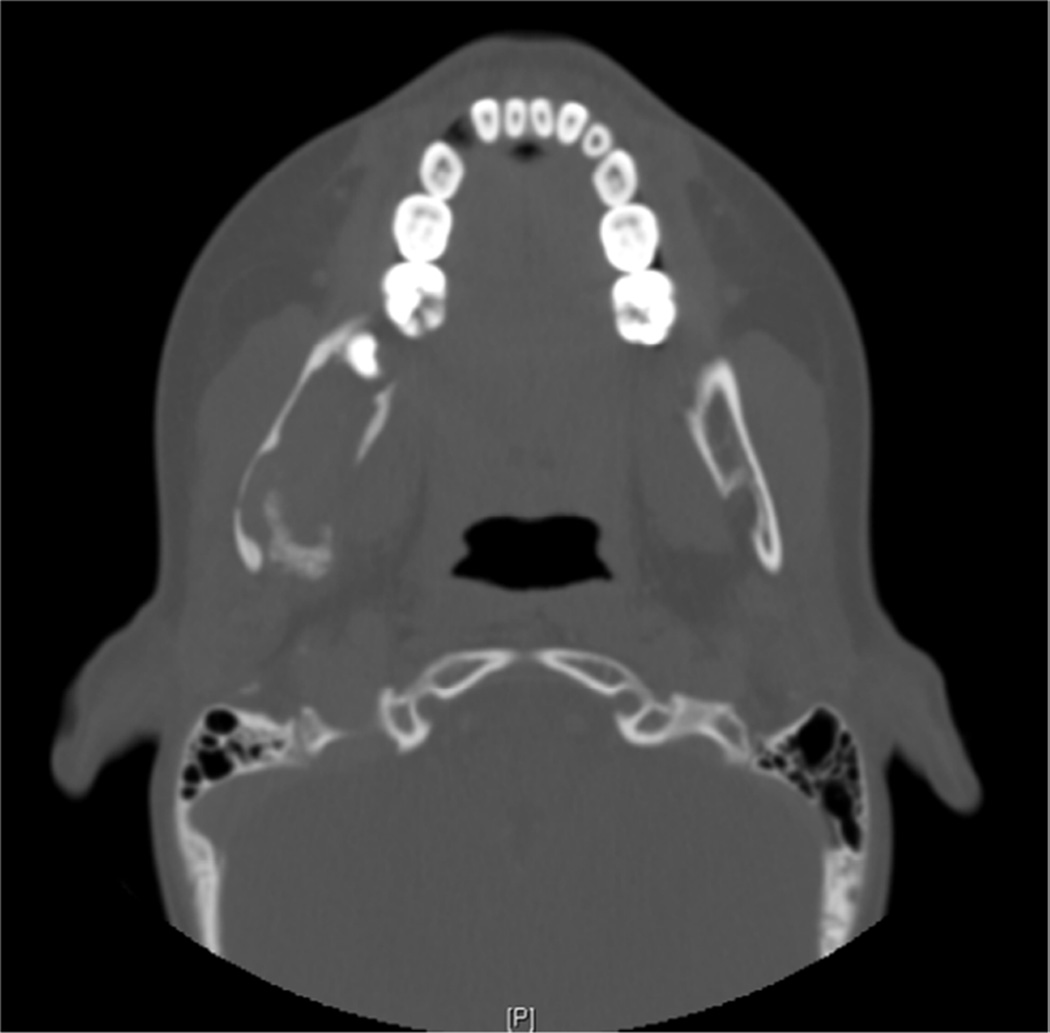
Figure 2.
Computed tomogram: axial view of a patient (case 37) with metastatic neuroblastoma to the mandibular ramus from the adrenal gland first identified in the mandible, which demonstrates a destructive, erosive lytic lesion.
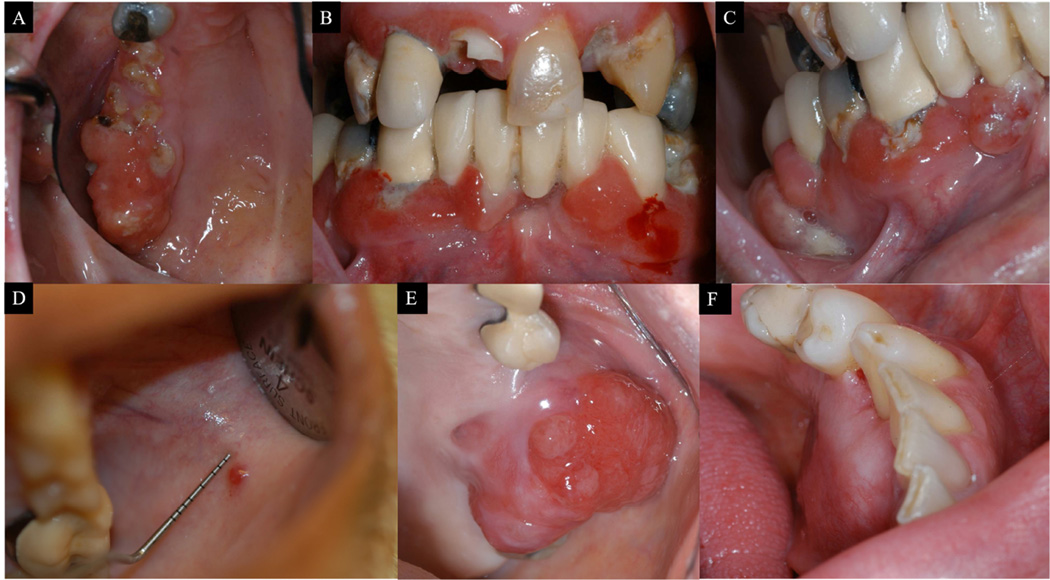
Figure 3.
Clinical pictures of metastatic lung undifferentiated carcinoma to multiple mandibular and maxillary gingival sites (A—C). Case 9, metastatic renal cell carcinoma involving buccal mucosa (D); case 32, metastatic lung adenocarcinoma involving maxillary gingiva (E); case 11, metastatic colon adenocarcinoma involving mandibular gingiva (F); case 24
All cases were histopathologically evaluated, and 1 or more ancillary/IHC stains was performed in 29 cases in order to arrive at an accurate diagnosis: e.g., cytokeratins, thyroid transcription factor–1, estrogen receptor, progesterone receptor, prostate-specific antigen, vimentin, smooth muscle actin, epithelial membrane antigen, CDX2, HepPar-1, and neuroendocrine markers (chromogranin and synaptophysin) (Figure 4). Oral cavity metastases were managed with radiotherapy, surgical resection, chemotherapy, or a combination of treatment modalities. In the adult patients, the median and mean time from the diagnoses of jaw metastases to the patient’s death were 12 months, and 27.7 months, respectively (range, 2 to 142 months), whereas the median and mean time from the diagnoses of oral soft tissue metastases to the patient’s death were 5 months and 8 months, respectively (range: 5 days to 34 months). At the time of the review, 6 adult patients were alive (2 to 93 months) post-metastasis diagnosis. One pediatric patient died 8 months after the jaw metastasis diagnosis. The remaining 4 pediatric patients were alive (85 to 233 months) after the metastasis diagnosis. The summary of the histologic subtype, duration of oral cavity metastasis diagnosis to patient’s death, clinical presentations, positive immunohistochemical stains, and therapy instituted in the management of oral cavity metastases are presented in Table 2.
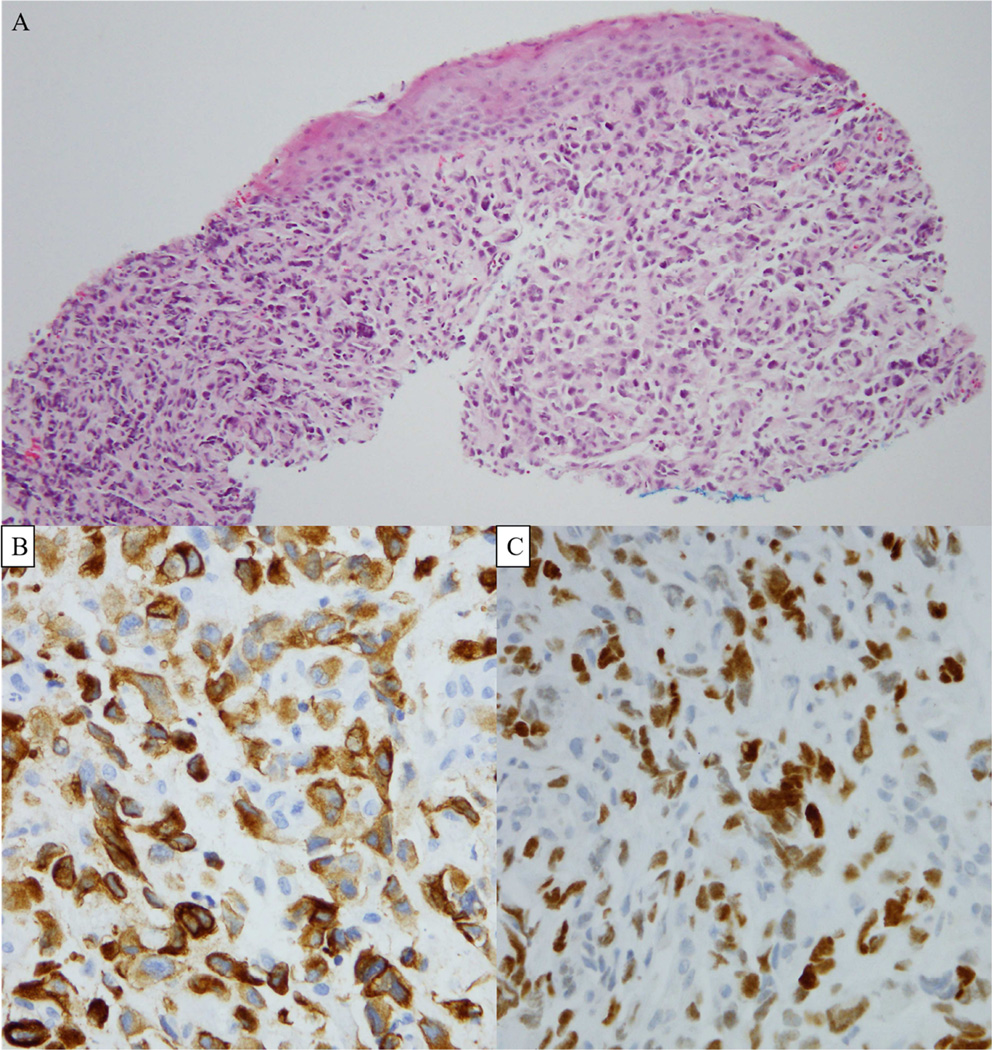
Figure 4.
Photomicrographs of the biopsied metastatic lung undifferentiated carcinoma (hematoxylin and eosin, ×200) (A). Cytokeratin 7 demonstrating a cytoplasmic staining pattern (X400) (B), and TTF-1 demonstrating a nuclear staining pattern (X400) (C); case 9.
Table 2.
Summary of the histologic subtype, duration from oral cavity metastasis diagnosis to patient’s death, clinical presentations, therapy instituted in the management of oral cavity metastases, and positive immunohistochemical stains
| Case no. |
Histopathologic diagnosis |
Duration from jaw/soft tissue metastases to DOD (mo) |
Clinical presentation of jaw/soft tissue metastases |
Management of metastatic jaw/soft tissue disease |
Positive IHC stains |
|---|---|---|---|---|---|
| 1 | High-grade pleomorphic sarcoma |
13 | Buccogingival mass preventing chewing |
RT | SMA, Vimentin |
| 2 | Non-small cell carcinoma |
2 | Expansile lesion | RT | Cytokeratin |
| 3 | Small cell carcinoma | 9 | Non-healing tooth infection |
CT | ND |
| 4 | Poorly differentiated carcinoma |
3 | Lesion on the hard palate increased in size non- responsive to antibiotic |
RT | TTF-1, CK7, AE1/3 |
| 5 | Adenocarcinoma | 11 | Jaw pain attributed to toothache, tooth extracted and swelling developed |
RT | CK7 |
| 6 | Mesothelioma | 34 | Submucosal mass | CT | CK5/6, Calretinin |
| 7 | Poorly differentiated adenocarcinoma |
0.16 | Submucosal mass | CT | CK7, TTF-1, Napsin-A, |
| 8 | Undifferentiated carcinoma |
2 | Gingival swellings | CT | ND |
| 9 | Undifferentiated carcinoma |
7 | Swollen gingivae | CT | CK7, TTF-1 |
| 10 | Poorly differentiated adenocarcinoma |
14 | Palatal mass after an extraction |
CT | ND |
| 11 | Adenocarcinoma | 5 | Gingival mass | CT | CK7 |
| 12 | Infiltrating ductal carcinoma |
142 | Numbness of lower lip, jaw pain, trismus |
- | CK7, ER, PR |
| 13 | Adenocarcinoma | Alive, 93 mo after jaw metastases |
Numbness of lower lip, trismus |
RT | ER |
| 14 | Adenocarcinoma | 21 | Expansile lesion | CT | ND |
| 15 | Adenocarcinoma | Alive 35 mo after oral metastasis |
Gingival mass | CT | CK7, ER |
| 16 | Poorly differentiated adenocarcinoma |
8 | Submucosal mass | RT | CK7 |
| 17 | Adenocarcinoma | 65 | Numbness of lower lip, expansile lesion |
S/RT | ND |
| 18 | Adenocarcinoma | 10 | Numbness of lower lip, lesion around tooth |
- | PSA |
| 19 | Hepatocellular carcinoma |
10 | Tooth abscess, jaw pain | - | HepPar-1 |
| 20 | Mucinous adenocarcinoma |
11 | Jaw numbness | RT | CK7, CK19, CA19-9 |
| 21 | Adenocarcinoma | 5 | Chin numbness | RT | ND |
| 22 | Adenocarcinoma with signet-ring features |
21 | Jaw mass | RT | AE1/3, CK20, CDX2 |
| 23 | Adenocarcinoma | 21 | Facial pain and nasal congestion |
S/RT | CK20, CDX2 |
| 24 | Adenocarcinoma | Alive 2 mo after oral metastasis |
Gingival swelling | CT | ND |
| 25 | Transitional cell carcinoma |
19 | Teeth sensitivity, abscess, lip numbness, jaw pain and swelling |
RT | CK, CEA |
| 26 | Renal cell carcinoma | 12 | Lip numbness and jaw mass |
S | ND |
| 27 | Renal cell carcinoma | 96 | Jaw pain and jaw mass | RT | ND |
| 28 | Renal cell carcinoma | Alive 44 mo after oral metastasis |
Oral pain and gingival swelling |
S/CT | CK, Vimentin, CD10, EMA, PAX2, PAX8 |
| 29 | Renal cell carcinoma | 2 | Submucosal mass | CT | ND |
| 30 | Renal cell carcinoma | 19 | Submucosal mass | S/RT | ND |
| 31 | Renal cell carcinoma | 8 | Bilobed mass extending from buccal to lingual gingiva |
RT | ND |
| 32 | Renal cell carcinoma | Alive 4 mo after oral metastasis |
Pink raised lesion | S/CT | PAX8, CD10, CA IX |
| 33 | Neuroblastoma | Alive, 21 mo after jaw metastases |
Rapidly developing jaw mass |
RT | ND |
| 34 | Retinoblastoma | 8 | Tooth exfoliated, Jaw pain and mass |
CRT | NSE, p53, SYN, CD56 |
| 35 | Neuroblastoma | Alive, 170 mo after jaw metastases |
Jaw mass | S | ND |
| 36 | Neuroblastoma | Alive, 93 mo after jaw metastases |
Rapidly developing jaw mass in 1 week |
S | ND |
| 37 | Neuroblastoma | Alive, 85 mo after jaw metastases |
Patient irritable attributed to teething, rapidly developing jaw mass |
CT | SYN |
| 38 | Anaplastic thyroid carcinoma |
0.5 | Gingival mass | S | 34BE12, A4A, focal Desmin |
| 39 | Leiomyosarcoma | 1 | Painless submucosal mass | CT | SMA, Vimentin |
| 40 | Germ cell tumor | 3 | Gingival swelling | S/RT | CK, Beta-HCG |
| 41 | Adenocarcinoma | 5 | Ulcerated lesion | CT | |
| 42 | Angiosarcoma | 15 | Palatal mass | RT | CD31, CD34 |
| 43 | Adenocarcinoma with papillary features |
2 | Submucosal mass | CT | CK7, Mucincarmine |
| 44 | Neuroblastoma | Alive, 233 mo after jaw metastases |
Jaw mass | CT | Chromogranin, NSE |
Abbreviations: SMA, smooth muscle actin; CK, cytokeratins; TTF-1, thyroid transcription factor—1; ER, estrogen receptor; PR, progesterone receptor; PSA, prostate-specific antigen; NSE, neuron-specific enolase; EMA, epithelial membrane antigen; CA, carbonic anhydrase; ND, not done; DOD, dead of disease; RT, radiotherapy; S, surgical resection; CT, chemotherapy; CRT, chemoradiation therapy.
DISCUSSION
In this study, we describe a series of patients with metastaticc tumors to the oral cavity and investigate their clinical outcomes. The gender distribution (male predilection) and site predilection (the majority of jaw cases involved the mandibular posterior region and soft tissue cases involved the gingiva) are similar to previous literature reviews (Hirshberg et al., 2008; Allon et al., 2014). In the adult population, the most common primary sites were the lung, kidney, breast, and colon. Similar primary site distributions have been reported in the literature (Hirshberg et al., 2008; D'Silva et al., 2006; Allon et al., 2014; Zachariades, 1989). In the pediatric patients, the most common primary site was the adrenal gland. Certain primary tumor sites have a predilection for either the jaw or oral soft tissue; for example the lung is the most common primary tumor site to metastasize to the oral soft tissue, whereas the breast has a predilection for the jaw. Other primary tumor sites such as adrenal, prostate, and eye have a predilection for the jaw and are rarely found in the oral soft tissue (Mirra, 1989). Of the adult patients, 84.6% died of disease, and of the pediatric patients, 80% are still alive at the time of this study.
Although most oral cavity metastases are found in the presence of a widespread disease process, oral cavity metastasis might be the first manifestation of the disease in 22% to 25% of patients (Hirshberg et al., 2008; Pesis et al., 2014; Zachariades, 1989). In our study, 8 (18.2%) patients first presented with metastatic oral symptoms. The pathogenesis of oral metastases is unclear. Metastatic deposit can arise from secondary site, such as the lungs, or directly from the primary organ site, bypassing the lungs, via the valveless vertebral venous plexus (Batson, 1940; Cumming et al., 1990). Also, the role of the presence of teeth and chronic inflammation in gingival metastasis as a contributing factor to the draw of metastatic tumor cells has been suggested. In this study, 10 of 11 patients with gingival metastases had teeth or implants at the gingival site (Allon et al., 2014).
Due to the rarity and sometimes ambiguous presentation of these lesions, they can be a diagnostic challenge, with most soft tissue cases occurring on the gingiva (Hirshberg et al., 1993; Sauerborn et al., 2011). The clinical differential diagnosis could include pyogenic granuloma, peripheral giant cell granuloma, peripheral ossify fibroma, epulis, and vascular anomaly. The importance of histopathologic evaluation cannot be overstated. A treatment-resistant dental pathosis or an obvious jaw pathosis should be biopsied for histopathologic evaluation. The pathologist must determine the lineage and tumor type of the biopsied tissue to identify potentially curable lesions, for example, hormone-sensitive and chemo-sensitive tumors. A history of known primary tumor can be helpful in this process by morphologically comparing the histopathologic slides. If there is no cancer history, the use of ancillary/IHC stains is highly recommended to determine the lineage and subtype of the tumor. The following stains are very useful but not exhaustive: cytokeratins 7 and 20, thyroid transcription factor–1, estrogen receptor, progesterone receptor, mammaglobin, CDX2, renal cell carcinoma, carbonic anhydrase IX, CD10, prostate-specific antigen, and HepPar-1. In our study, all of the reported metastases to the oral cavity were biopsied and histopathologically evaluated, and IHC stains were applied when warranted. After a diagnosis is rendered, body scans/imaging should be performed to evaluate for the primary tumor site and other metastatic site(s). This study focused on patients histologically evaluated. The number of patients who presented with oral metastases is suspected to be more than what we reported in this study, as patients radiographically or clinically diagnosed but not biopsied before they died of disease were excluded.
Although management of this condition varies, the use of surgical resections alone in cases of jaw-only metastasis has been found to improve prognosis (Nakamura et al., 2001). In cases with multiple metastatic recurrent prostate cancers, the use of surgical resection and radiotherapy show promise (Ost et al., 2015). Meanwhile, some cases of metastatic neuroblastoma improve with chemotherapy (Bhattacharyya et al., 1999). In our series, 2 patients diagnosed with metastatic neuroblastoma to the jaw were successfully managed with chemotherapy.
The hallmark of malignancy is metastasis. Epithelial–mesenchymal transition (EMT) has been proposed as an essential mechanism by which solid cancer cells undergo invasion and metastasis (Thiery, 2002). The transition of epithelial to mesenchymal cell phenotype allow for cell motility, loss of adherens junctions, loss of lineage, and dedifferentiation (Thiery, 2002). Pro-EMT transcription factors are ZEB1 (zinc finger E-box binding homeobox 1), SLUG, SNAIL, and TWIST (Boutet et al., 2007; Peinado et al., 2007; Yang et al., 2004). These transcription factors cause a reduction in epithelial cadherin, a transmembrane protein that maintains epithelial integrity responsible for anchorage of cells to one another (Cano et al., 2000; Herranz et al., 2008). These factors also regulate angiogenesis, which facilitates tumor invasion and metastasis.
The use of anti-angiogenic agents that target angiogenesis holds significant promise in the management of malignancies. Anti-angiogenic medications such as bevacizumab and sunitinib have been approved by the U.S. Food and Drug Administration. Bevacizumab, an anti-vascular endothelial growth factor (anti-VEGF) has been approved for the management of metastatic colorectal cancer, non–small cell lung cancer, and metastatic renal cell carcinoma. Sunitinib (anti-VEGF receptor) is a multi-targeted receptor tyrosine kinase inhibitor that has been also approved for the management of renal cell carcinoma, Imatinib-resistant gastrointestinal stromal tumor, and pancreatic neuroendocrine tumors. These medications work by stimulation of endothelial cell apoptosis, inhibition of neovascularization, prevention of tumor-mediated vasodilation, and prevention of recruitment of endothelial progenitor cells (Ellis and Hicklin, 2008).
Recently, the use of personalized therapy in the management of cancer patients is now gaining ground by the use of genome sequencing. Next-generation sequencing allows hundreds of genetic abnormalities to be analyzed very quickly and with great precision. This new technology allows the oncologist to determine whether the patient’s cancer has a clinically useful mutation that makes the cancer susceptible to certain drugs, clinical trials, or individualistic targeted therapy. However, its role in clinical practice is still limited (Damodaran et al., 2015; Hyman et al., 2015).
CONCLUSION
Metastatic non-melanocytic solid tumors to the oral cavity are rare and can be the first clinical sign of a late-stage malignancy. Patient outcome after the diagnosis of metastasis to the oral cavity is poor and may be attributed to widespread disease at the time of diagnosis. Pediatric patients with oral cavity metastases have a better prognosis compared to adult patients. Metastases to the oral soft tissue (mean survival time of 8 months after metastasis diagnosis) carries a graver consequence compared to metastases to the jaw. This observation is supported by the existing literature; the average survival time after metastases to the oral soft tissue has been placed at 3.7 months, whereas the average survival time after metastases to the oral cavity is 7 months (Allon et al., 2014; Hirshberg et al., 2008).
Acknowledgments
This study was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: All authors declare that there are no financial conflicts associated with this study and that the funding source has no role in conceiving and performing the study.
REFERENCES
- Allon I, Pessing A, Kaplan I, Allon DM, Hirshberg A. Metastatic tumors to the gingiva and the presence of teeth as a contributing factor: a literature analysis. J Periodontol. 2014;85(1):132–139. doi: 10.1902/jop.2013.130118. [DOI] [PubMed] [Google Scholar]
- Antunes AA, Antunes AP. Gnathic bone metastasis: a retrospective study of 10 cases. Braz J Otorhinolaryngol. 2008;74(4):561–565. doi: 10.1016/S1808-8694(15)30603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batson OV. The function of the vertebral veins and their role in the spread of metastases. Ann Surg. 1940;112(1):138–149. doi: 10.1097/00000658-194007000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya I, Williamson A, Cohen DM, Bever JL. Metastatic neuroblastoma with ganglioneuromatous differentiation and mandibular involvement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88(5):586–592. doi: 10.1016/s1079-2104(99)70090-9. [DOI] [PubMed] [Google Scholar]
- Bodner L, Sion-Vardy N, Geffen DB, Nash M. Metastatic tumors to the jaws: a report of eight new cases. Med Oral Patol Oral Cir Bucal. 2006;11(2):E132–E135. [PubMed] [Google Scholar]
- Boutet A, Esteban MA, Maxwell PH, Nieto MA. Reactivation of Snail genes in renal fibrosis and carcinomas: a process of reversed embryogenesis? Cell Cycle. 2007;6(6):638–642. doi: 10.4161/cc.6.6.4022. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Cumming J, Hacking N, Fairhurst J, Ackery D, Jenkins JD. Distribution of bony metastases in prostatic carcinoma. Br J Urol. 1990;66(4):411–414. doi: 10.1111/j.1464-410x.1990.tb14964.x. [DOI] [PubMed] [Google Scholar]
- D'Silva NJ, Summerlin DJ, Cordell KG, Abdelsayed RA, Tomich CE, Hanks CT, et al. Metastatic tumors in the jaws: a retrospective study of 114 cases. J Am Dent Assoc. 2006;137(12):1667–1672. doi: 10.14219/jada.archive.2006.0112. [DOI] [PubMed] [Google Scholar]
- Damodaran S, Berger MF, Roychowdhury S. Clinical tumor sequencing: opportunities and challenges for precision cancer medicine. Am Soc Clin Oncol Educ Book. 2015:e175–e182. doi: 10.14694/EdBook_AM.2015.35.e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8(8):579–591. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- Herranz N, Pasini D, Diaz VM, Franci C, Gutierrez A, Dave N, et al. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol Cell Biol. 2008;28(15):4772–4781. doi: 10.1128/MCB.00323-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshberg A, Berger R, Allon I, Kaplan I. Metastatic tumors to the jaws and mouth. Head Neck Pathol. 2014;8(4):463–474. doi: 10.1007/s12105-014-0591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshberg A, Leibovich P, Buchner A. Metastases to the oral mucosa: analysis of 157 cases. J Oral Pathol Med. 1993;22(9):385–390. doi: 10.1111/j.1600-0714.1993.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Hirshberg A, Leibovich P, Buchner A. Metastatic tumors to the jawbones: analysis of 390 cases. J Oral Pathol Med. 1994;23(8):337–341. doi: 10.1111/j.1600-0714.1994.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Hirshberg A, Shnaiderman-Shapiro A, Kaplan I, Berger R. Metastatic tumours to the oral cavity—pathogenesis and analysis of 673 cases. Oral Oncol. 2008;44(8):743–752. doi: 10.1016/j.oraloncology.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Hyman DM, Solit DB, Arcila ME, Cheng DT, Sabbatini P, Baselga J, et al. Precision medicine at Memorial Sloan Kettering Cancer Center: clinical next-generation sequencing enabling next-generation targeted therapy trials. Drug Discov Today. 2015;20(12):1422–1428. doi: 10.1016/j.drudis.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SA, Movahed R, Salama A, Ord RA. Maxillofacial metastases: a retrospective review of one institution's 15-year experience. J Oral Maxillofac Surg. 2013;71(1):178–188. doi: 10.1016/j.joms.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Mirra JM. Bone Tumors. Lea & Febiger: 1989. Metastasis; pp. 1495–1517. [Google Scholar]
- Murillo J, Bagan JV, Hens E, Diaz JM, Leopoldo M. Tumors metastasizing to the oral cavity: a study of 16 cases. J Oral Maxillofac Surg. 2013;71(9):1545–1551. doi: 10.1016/j.joms.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ishimaru J, Mizui T, Kobayashi A, Toida M, Makita H, et al. Osteosarcoma metastatic to the mandible: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(4):452–454. doi: 10.1067/moe.2001.113107. [DOI] [PubMed] [Google Scholar]
- Ost P, Bossi A, Decaestecker K, De Meerleer G, Giannarini G, Karnes RJ, et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2015;67(5):852–863. doi: 10.1016/j.eururo.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7(6):415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- Pesis M, Taicher S, Greenberg G, Hirshberg A. Metastasis to the jaws as a first manifestation of hepatocellular carcinoma: report of a case and analysis of 41 cases. J Craniomaxillofac Surg. 2014;42(8):1997–2001. doi: 10.1016/j.jcms.2014.09.005. [DOI] [PubMed] [Google Scholar]
- Sauerborn D, Vidakovic B, Baranovic M, Mahovne I, Danic P, Danic D. Gastric adenocarcinoma metastases to the alveolar mucosa of the mandible: a case report and review of the literature. J Craniomaxillofac Surg. 2011;39(8):645–648. doi: 10.1016/j.jcms.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Summerlin DJ, Tomich C, Abdelsayed R. American Academy of Oral Pathology Annual Meeting. Santa Fe, New Mexico: 1994. Metastatic disease to the jaws. [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- van der Waal RI, Buter J, van der Waal I. Oral metastases: report of 24 cases. Br J Oral Maxillofac Surg. 2003;41(1):3–6. doi: 10.1016/s0266-4356(02)00301-7. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Zachariades N. Neoplasms metastatic to the mouth, jaws and surrounding tissues. J Craniomaxillofac Surg. 1989;17(6):283–290. doi: 10.1016/s1010-5182(89)80098-8. [DOI] [PubMed] [Google Scholar]