Abstract
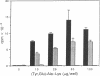
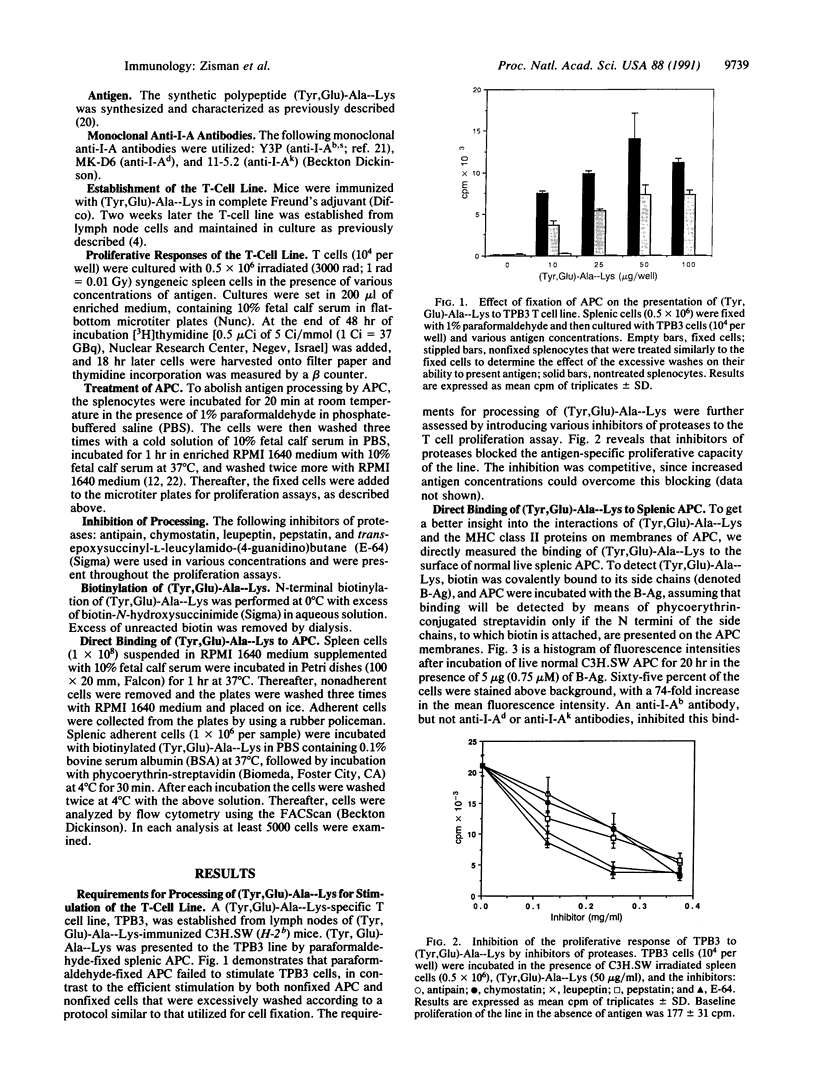
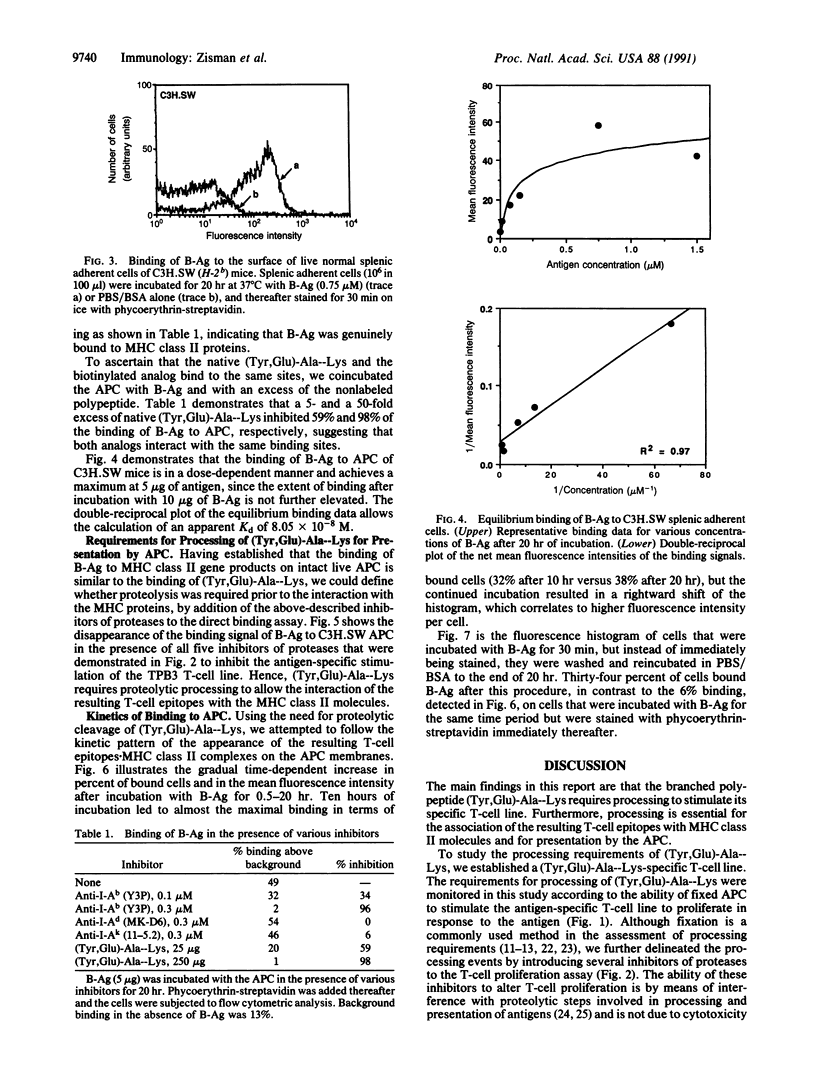
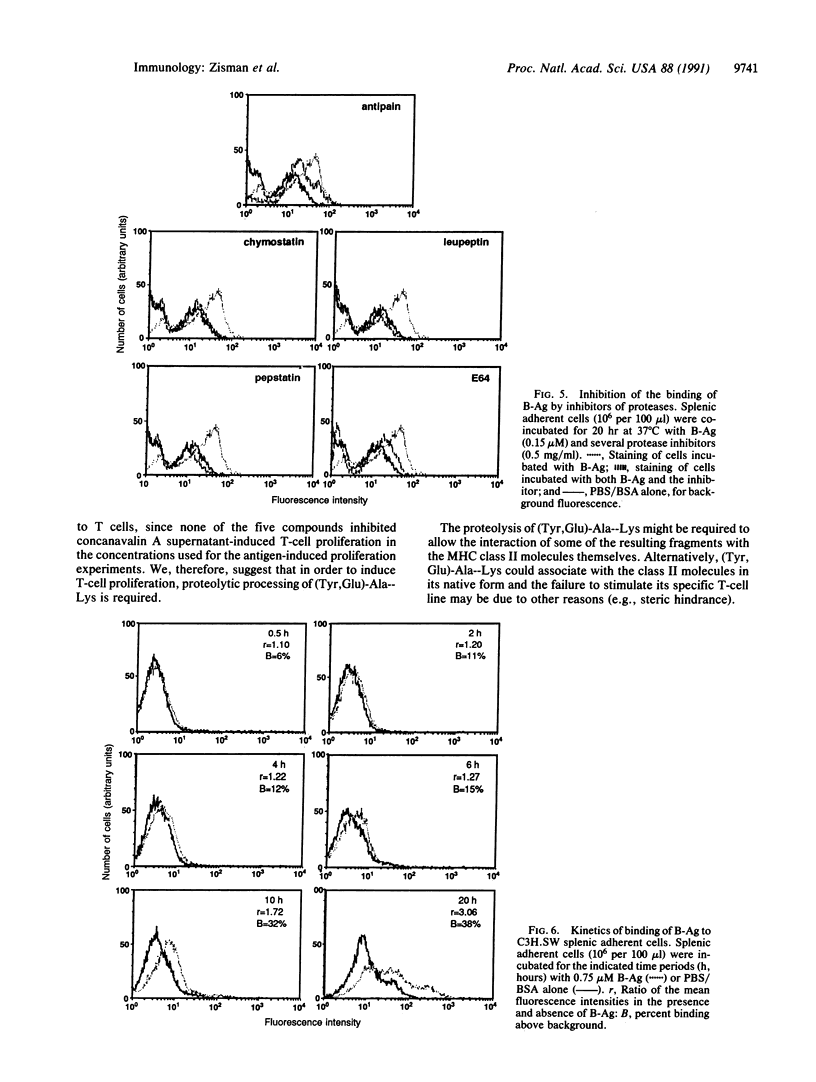
T-cell activation involves the recognition of foreign antigens as a complex with self-major histocompatibility complex (MHC) proteins on the surface of antigen-presenting cells (APC). Protein antigens usually require uptake by the APC and processing that results in the generation of peptide fragments. The branched synthetic polypeptide (Tyr, Glu)-Ala--Lys was chosen as a model antigen to follow the processing requirements, leading to T-cell activation. It has been demonstrated, by using fixed APC and various inhibitors of proteases, that (Tyr, Glu)-Ala--Lys has to be processed to stimulate a (Tyr, Glu)-Ala--Lys-specific T-cell line of C3H.SW (H-2b) origin to proliferate. To determine whether processing of (Tyr,Glu)-Ala--Lys is required to allow its association with the MHC class II molecules, biotin was covalently attached to it. Binding of the biotinylated (Tyr,Glu)-Ala--Lys to MHC class II gene products on the surface of intact normal APC was directly detected by phycoerythrin-streptavidin. The specificity of the binding was confirmed by its inhibition with anti-I-Ab antibodies as well as with excess of nonlabeled (Tyr,Glu)-Ala--Lys. Furthermore, introducing several inhibitors of proteases to the binding assay, we could substantiate that the proteolysis of (Tyr,Glu)-Ala--Lys is required to allow association of the resulting peptidyl T-cell epitopes with the MHC class II molecules themselves. The presence of the biotin moiety in the resulting peptides suggests that the T-cell epitopes of (Tyr,Glu)-Ala--Lys contain the N-terminal portion of the side chains of the branched polypeptide. An apparent Kd of 8.05 x 10(-8) M was determined, and optimal binding was detected after 10 hr of incubation with the antigen. The latter phenomenon is not due to slow uptake, since uptake of (Tyr,Glu)-Ala--Lys occurs mainly during the first 30 min of incubation, but rather reflects the events of processing that precede MHC interaction.
Full text
PDF
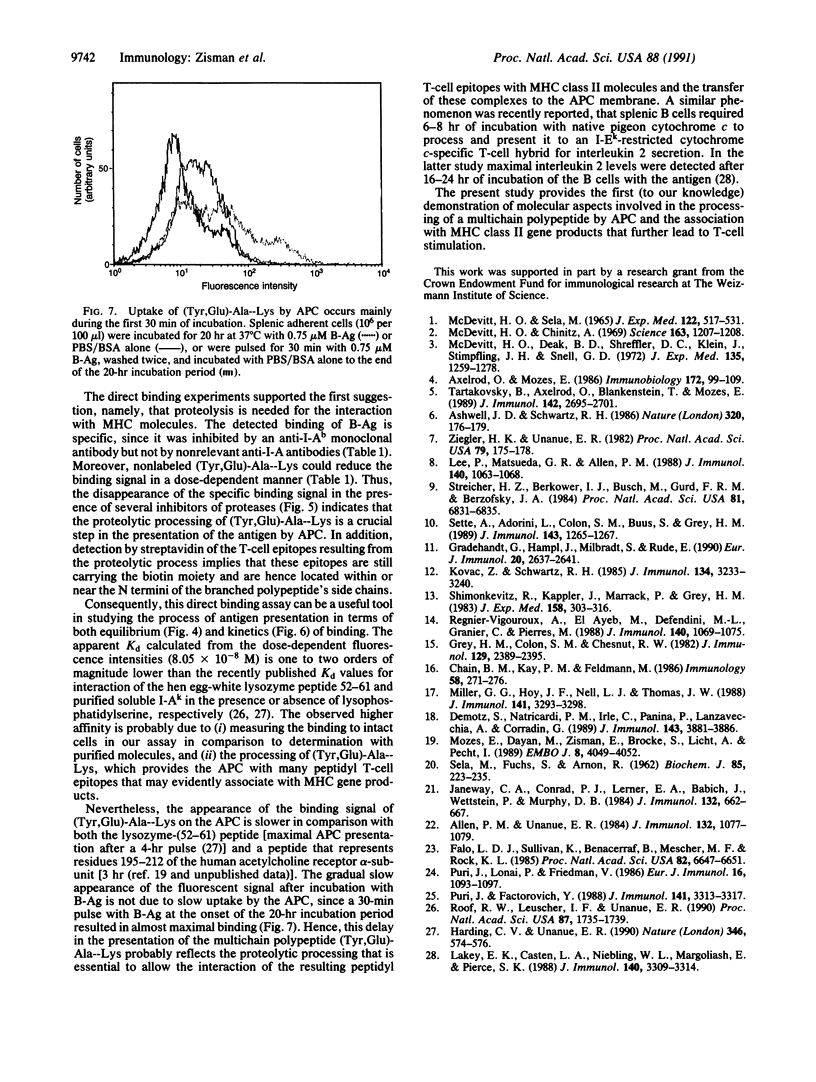
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Ashwell J. D., Schwartz R. H. T-cell recognition of antigen and the Ia molecule as a ternary complex. Nature. 1986 Mar 13;320(6058):176–179. doi: 10.1038/320176a0. [DOI] [PubMed] [Google Scholar]
- Axelrod O., Mozes E. Analysis of the biological functions and fine specificity of (T,G)-A--L specific T cell clones. Immunobiology. 1986 Aug;172(1-2):99–109. doi: 10.1016/S0171-2985(86)80056-0. [DOI] [PubMed] [Google Scholar]
- Chain B. M., Kay P. M., Feldmann M. The cellular pathway of antigen presentation: biochemical and functional analysis of antigen processing in dendritic cells and macrophages. Immunology. 1986 Jun;58(2):271–276. [PMC free article] [PubMed] [Google Scholar]
- Demotz S., Matricardi P. M., Irle C., Panina P., Lanzavecchia A., Corradin G. Processing of tetanus toxin by human antigen-presenting cells. Evidence for donor and epitope-specific processing pathways. J Immunol. 1989 Dec 15;143(12):3881–3886. [PubMed] [Google Scholar]
- Falo L. D., Jr, Sullivan K., Benacerraf B., Mescher M. F., Rock K. L. Analysis of antigen presentation by metabolically inactive accessory cells and their isolated membranes. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6647–6651. doi: 10.1073/pnas.82.19.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradehandt G., Hampl J., Milbradt S., Rüde E. Processing without proteolytic cleavage is required for recognition of insulin by T cells. Eur J Immunol. 1990 Dec;20(12):2637–2641. doi: 10.1002/eji.1830201217. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Colon S. M., Chesnut R. W. Requirements for the processing of antigen by antigen-presenting B cells. II. Biochemical comparison of the fate of antigen in B cell tumors and macrophages. J Immunol. 1982 Dec;129(6):2389–2395. [PubMed] [Google Scholar]
- Harding C. V., Unanue E. R. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature. 1990 Aug 9;346(6284):574–576. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Conrad P. J., Lerner E. A., Babich J., Wettstein P., Murphy D. B. Monoclonal antibodies specific for Ia glycoproteins raised by immunization with activated T cells: possible role of T cellbound Ia antigens as targets of immunoregulatory T cells. J Immunol. 1984 Feb;132(2):662–667. [PubMed] [Google Scholar]
- Kovac Z., Schwartz R. H. The molecular basis of the requirement for antigen processing of pigeon cytochrome c prior to T cell activation. J Immunol. 1985 May;134(5):3233–3240. [PubMed] [Google Scholar]
- Lakey E. K., Casten L. A., Niebling W. L., Margoliash E., Pierce S. K. Time dependence of B cell processing and presentation of peptide and native protein antigens. J Immunol. 1988 May 15;140(10):3309–3314. [PubMed] [Google Scholar]
- Lee P., Matsueda G. R., Allen P. M. T cell recognition of fibrinogen. A determinant on the A alpha-chain does not require processing. J Immunol. 1988 Feb 15;140(4):1063–1068. [PubMed] [Google Scholar]
- McDevitt H. O., Chinitz A. Genetic control of the antibody response: relationship between immune response and histocompatibility (H-2) type. Science. 1969 Mar 14;163(3872):1207–1208. doi: 10.1126/science.163.3872.1207. [DOI] [PubMed] [Google Scholar]
- McDevitt H. O., Deak B. D., Shreffler D. C., Klein J., Stimpfling J. H., Snell G. D. Genetic control of the immune response. Mapping of the Ir-1 locus. J Exp Med. 1972 Jun 1;135(6):1259–1278. doi: 10.1084/jem.135.6.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt H. O., Sela M. Genetic control of the antibody response. I. Demonstration of determinant-specific differences in response to synthetic polypeptide antigens in two strains of inbred mice. J Exp Med. 1965 Sep 1;122(3):517–531. doi: 10.1084/jem.122.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. G., Hoy J. F., Nell L. J., Thomas J. W. Antigen processing and the human T cell receptor repertoire for insulin. J Immunol. 1988 Nov 15;141(10):3293–3298. [PubMed] [Google Scholar]
- Mozes E., Dayan M., Zisman E., Brocke S., Licht A., Pecht I. Direct binding of a myasthenia gravis related epitope to MHC class II molecules on living murine antigen-presenting cells. EMBO J. 1989 Dec 20;8(13):4049–4052. doi: 10.1002/j.1460-2075.1989.tb08588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri J., Factorovich Y. Selective inhibition of antigen presentation to cloned T cells by protease inhibitors. J Immunol. 1988 Nov 15;141(10):3313–3317. [PubMed] [Google Scholar]
- Puri J., Lonai P., Friedman V. Antigen-Ia interaction and the proteolytic processing of antigen: the structure of the antigen determines its restriction to the A or E molecule of the major histocompatibility complex. Eur J Immunol. 1986 Sep;16(9):1093–1097. doi: 10.1002/eji.1830160911. [DOI] [PubMed] [Google Scholar]
- Roof R. W., Luescher I. F., Unanue E. R. Phospholipids enhance the binding of peptides to class II major histocompatibility molecules. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1735–1739. doi: 10.1073/pnas.87.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Régnier-Vigouroux A., el Ayeb M., Defendini M. L., Granier C., Pierres M. Processing by accessory cells for presentation to murine T cells of apamin, a disulfide-bonded 18 amino acid peptide. J Immunol. 1988 Feb 15;140(4):1069–1075. [PubMed] [Google Scholar]
- SELA M., FUCHS S., ARNON R. Studies on the chemical basis of the antigenicity of proteins. 5. Synthesis, characterization and immunogenicity of some multichain and linear polypeptides containing tyrosine. Biochem J. 1962 Oct;85:223–235. doi: 10.1042/bj0850223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Adorini L., Colon S. M., Buus S., Grey H. M. Capacity of intact proteins to bind to MHC class II molecules. J Immunol. 1989 Aug 15;143(4):1265–1267. [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher H. Z., Berkower I. J., Busch M., Gurd F. R., Berzofsky J. A. Antigen conformation determines processing requirements for T-cell activation. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6831–6835. doi: 10.1073/pnas.81.21.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakovsky B., Axelrod O., Blankenstein T., Mozes E. Correlation between the biologic functions and the generation of lymphokines in T cell clones. J Immunol. 1989 Apr 15;142(8):2695–2701. [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]