Abstract
The striatum plays an important role in controlling motor function in humans, and its degeneration has the ability to cause severe motor disorders. More specifically, previous studies have demonstrated a disruption in the connectivity of the cortico-striatal loop in patients suffering from motor disorders caused by dopamine dysregulation, such as Parkinson's disease. However, little is known about striatal functional connectivity in patients with motor dysfunction not caused by dopamine dysregulation. In this study, we used early-state Bell's palsy (BP) patients (within 14 days of onset) to investigate how functional connectivity between the striatum and motor cortex is affected by peripheral nerve injury in which the dopamine system remains fully functional. We found a significant increase in the connectivity between the contralateral putamen, and the ipsilateral primary sensory (S1) and motor cortex (M1) in BP patients compared to healthy controls. We also found increased connectivity between the ventral striatum and supplementary motor area (SMA), and the dorsal caudate and medial prefrontal lobe in BP patients compared to healthy controls. Our results demonstrate that the entirety of the striatum is affected following acute peripheral nerve injury, and suggests that this disrupted striatal functional connectivity may reflect a compensatory mechanism for the sensory-motor mismatch caused by BP.
Keywords: Bell's palsy, Striatum, Functional connectivity, Motor disorder
Highlights
-
•
We used 12 striatal sub-regions to investigate rsFC changes in acute BP patients.
-
•
rsFC was disrupted in many sub-regions of the striatum in BP patients.
-
•
Striatal sub-regions work together to solve the sensory-motor mismatch problem.
-
•
The putamen may have its own somatotopic body representation.
-
•
The putamen's body representation may be remapped following motor nerve injury.
1. Introduction
The human striatum is a complex structure that belongs to the extrapyramidal system and is integral to motor, cognitive, and affective functions in humans. The various structures within the striatum such as the putamen, the caudate, and the ventral striatum have been found to play different roles in the brain. More specifically, previous neuroanatomical and neuroimaging studies of the human striatum suggest that the association cortex projects to the caudate, the sensorimotor cortex projects to the putamen, and the limbic area projects to the ventral striatum (Alexander et al., 1990, Vanderah and Gould, 2015, Draganski et al., 2008, Di Martino et al., 2008, Choi et al., 2012, Lehericy et al., 2004).
The striatum, especially the putamen, plays an important role in motor function, by adjusting the amplitude and velocity of muscle contractions through both the corticostriatal loop and the dopamine system (Loonen and Ivanova, 2013, Grillner et al., 2005). In some motor disorders, such as Parkinson's disease and Huntington's disease, degeneration of the central nervous system can cause a disorder of the dopamine system and affect the functional connectivity between the striatum and motor cortex (Hacker et al., 2012, Helmich et al., 2010, Kwak et al., 2010, Luo et al., 2014, Unschuld et al., 2012), thereby suggesting that the corticostriatal motor loop is impaired due to the reduced dopamine levels in the striatum (Helie et al., 2013). These results further prove that the striatum is a crucial region in motor function, and plays an important role in the development of motor diseases. In Parkinson's disease the lesion is directly in the striatum, which allows us to explore the function of the striatum, but limits our exploration of how the striatum modulates movement, especially when motor dysfunction occurs with a completely normal dopamine system.
Bell's palsy (BP) is the paralysis of the unilateral facial expression muscles caused by reactivation of the herpes virus in the facial nerve. This reactivation impairs the signaling from the motor cortex to the affected facial muscle, which results in the paralysis of the facial muscles. In turn, the sensory cortex detects that the facial muscles do not move, despite the motor cortex giving the muscle the command. We call this discrepancy the sensory-motor mismatch. Compared to other conditions such as Parkinson's disease, BP has fewer complications, fewer or no medications, and importantly, no effect on the dopamine system, thereby allowing researchers to explore the physiological function of the striatum following peripheral nerve injury (Vakharia and Vakharia, 2016). BP offers an ideal model to investigate how the striatum modulates motor function following peripheral nerve injury.
Recently, investigators have applied resting state functional connectivity (rsFC) analysis to sub-regions of the striatum (Di Martino et al., 2008). More recently, rsFC has also been used to investigate the pathological changes of different disorders such as Parkinson's disease, depression, autism, and obsessive-compulsive disorder (Di Martino et al., 2011, Felger et al., 2015, Gabbay et al., 2013, Hacker et al., 2012, Harrison et al., 2009, Helmich et al., 2010, Padmanabhan et al., 2013). The results obtained have significantly enhanced our understanding on the pathophysiology of these disorders and the function of the striatum.
In this study, we compared the rsFC changes in 12 sub-regions of the striatum (left and right side, each with 6 sub-regions), which were identified (Di Martino et al., 2008) and applied in previous studies (Kwak et al., 2010, Subira et al., 2016, Lin et al., 2015, Kerestes et al., 2015, Bell et al., 2015, Di Martino et al., 2011, Gabbay et al., 2013, Wang et al., 2017), in BP patients. Our objective was to explore if the rsFC between the striatum and the rest of the brain, especially the motor cortex, was similarly affected in BP patients as with patients with reduced dopamine levels in the striatum. We hypothesized that the functional connectivity between the striatum and other brain regions, especially between the putamen and motor cortex, would be increased at the contralateral side in BP patients. Additionally, we aimed to test if there was any disruption between the ipsilateral and contralateral striatum in BP patients. We hypothesized that the connectivity between the ipsilateral and contralateral striatum should also be moderately disrupted in the early stage of BP.
2. Method
2.1. Subjects
Twenty-five right-handed patients with left or right side Bell's palsy (House-Brackmann Scale ≥ 3, age 36 ± 7 years old ranging from 23 to 50, 15 males, 10 females, 14 right side facial paralysis) were recruited from the First Affiliated Hospital of Zhejiang Chinese Medical University. All patients with Bell's palsy onset of fewer than 14 days underwent an MRI scan (He et al., 2014). We recruited 25 age- and gender-matched healthy controls. No participants had a history of physical or mental disorders. The study was approved by the ethics committee of the First Affiliated Hospital of Zhejiang Chinese Medical University.
2.2. fMRI imaging acquisition
All scans were performed with a 3.0 Tesla MR scanner (Magnetom Verio, Siemens, Germany) in order to obtain T1-weighted structural images and echo-planar T2*-weighted images (EPI). Structural images were obtained by MP-RAGE sequence: TR = 1900 ms, TE = 2.45 ms, FA = 9 degrees, voxel size = 1 × 1 × 1 mm, matrix = 256 × 256. Two hundred time points of functional resting state data were acquired by EPI session: TR = 2000 ms, TE = 30 ms, FA = 90 degrees, slices = 33, voxel size = 4.0 × 4.0 × 4.0 mm, matrix = 256 × 256. All subjects were required to keep their eyes open during the scan.
2.3. fMRI data preprocessing
Before any processing, all patients with left-side Bell's palsy and their matched healthy controls were flipped along the y-axis (R-L flip) in order to directly compare them to the patients with right-side Bell's palsy (Klingner et al., 2014).
Functional MRI data analysis was carried out by applying a seed-based approach using the CONN toolbox v15.g (Whitfield-Gabrieli and Nieto-Castanon, 2012) (http://www.nitrc.org/projects/conn). Similar to our previous study (Tao et al., 2016), the preprocessing of fMRI data was performed using Statistical Parametric Mapping (SPM8) (Welcome Department of Cognitive Neurology, University College, London, UK) in MATLAB (Mathworks, Inc., Natick, MA, USA). The preprocessing steps included realignment, coregistration of subjects' respective functional and structural images, normalization, and smoothing with an 8-mm full width at half maximum (FWHM) kernel. In addition to these steps, we employed segmentation of gray matter, white matter, and cerebrospinal fluid (CSF) areas in order to remove temporal confounding factors (Whitfield-Gabrieli and Nieto-Castanon, 2012). Band-pass filtering was performed with a frequency window of 0.008–0.9 Hz.
To eliminate correlations caused by head motion and artifacts, we identified outlier time points in the motion parameters and global signal intensity using ART (http://www.nitrc.org/projects/artifact_detect). For each subject, we treated images as outliers if composite movement from a preceding image exceeded 0.5 mm, or if the global mean intensity was > 3 SDs from the mean image intensity for the entire resting scan. Outliers were included as regressors in the first-level General Linear Model along with motion parameters.
2.4. Functional resting state connectivity analysis
We used six striatum sub-regions in each hemisphere as 3 mm spherical seeds. The centers of the seeds were: dorsal caudal putamen (DCP, NMI coordinate, ± 28, 1, 3), dorsal rostral putamen (DRP, ± 25, 8, 6), ventral rostral putamen (VRP, ± 20, 12, − 3), dorsal caudate (DC, ± 13, 15, 9), inferior ventral striatum (VSi, ± 9, 9, − 8), and superior ventral striatum (VSs, ± 10, 15, 0) (Di Martino et al., 2008). Seeds were selected using WFU-Pick Atlas software (Maldjian et al., 2003) according to NMI coordinates.
First-level correlation maps were produced by extracting the average BOLD time course from each striatum seed and by computing Pearson's correlation coefficients between that time course and the time courses of all other voxels in the brain. Correlation coefficients were Fisher transformed into “Z” scores, which increased normality and allowed for improved second-level General Linear Model analyses.
A whole brain group analysis was performed using two-sample t-tests to compare changes in the 12 striatal sub-regions' functional connectivity between the healthy control group and the BP patient group. To explore the association between the observed clusters and the House-Brackmann Scale (HBS) scores of the BP patients, we extracted average Fisher z-values for the survived clusters and performed multiple regression analyses across all patients using SPSS 18.0 Software (SPSS Inc., Chicago, IL, USA). Age, gender, and duration of onset (days) were included in the analysis as covariates of non-interest. A threshold of voxel-wise p < 0.005 uncorrected and cluster-level p < 0.05 family wise error (FWE) correction was applied to all fMRI data analyses.
In addition, we also calculated the functional connectivity between the 12 (6 × 2 hemispheres) seeds to explore the network change within the striatum between the healthy controls and patients. We applied a threshold of p < 0.05 FDR (false discovery rate) corrected.
3. Results
3.1. Behavioral results
All patients' had lost their left or right facial muscle function due to BP, with HBS grades ≥ 3. The MR scan was performed 3–14 days after onset.
3.2. rsFC results in the healthy control group and BP patient group
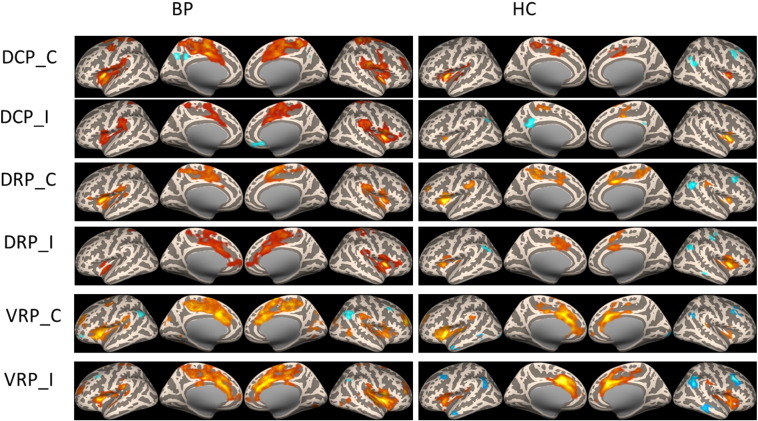
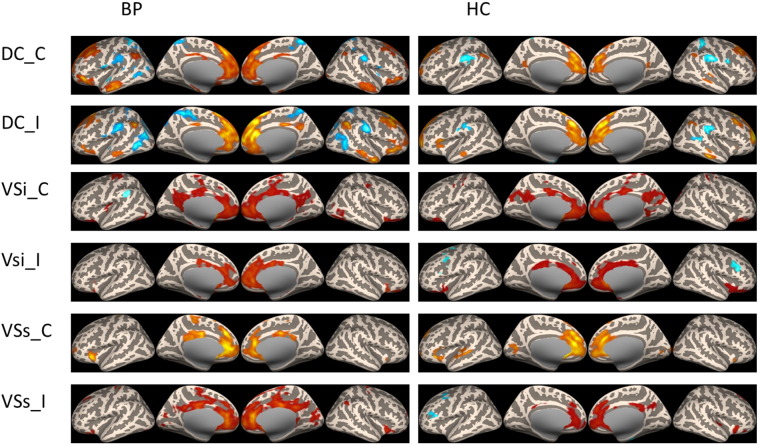
The rsFC of the two groups were similar (Fig. 1 & Fig. 2). The putamen seeds were associated with the middle cingulate, paracentral lobe and insula. The dorsal caudate was associated with anterior cingulate cortex (ACC) and prefrontal lobe. The ventral striatum seeds were associated with ACC and posterior cingulate cortex (PCC). Moreover, in the BP patient group, the putamen showed increased connectivity with the sensorimotor area, while the ventral striatum showed significant increased connectivity with insula.
Fig. 1.
Resting state functional connectivity of putamen seeds in BP patients and healthy controls.
Fig. 2.
Resting state functional connectivity of caudate and ventral striatum seeds in BP patients and healthy controls.
3.3. Comparison between the healthy control group and BP patients
3.3.1. Dorsal caudal putamen (DCP)
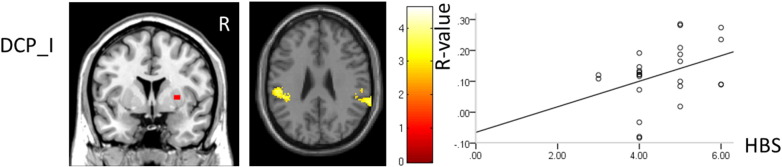
The connectivity between the contralateral DCP, and the ipsilateral primary sensory (S1) and motor cortex (M1), premotor cortex, SMA, insular and central opercular cortex in BP patients was significantly increased compared to healthy controls (Table 1; Fig. 3). The connectivity between the ipsilateral DCP and the bilateral secondary somatosensory area (S2) was significantly increased in BP patients compared to healthy controls. Additionally, we found the connectivity between the ipsilateral DCP and ipsilateral S2 was positively associated with HBS scores in patients (p = 0.05) (Fig. 5).
Table 1.
Comparison of the connectivity in the putamen seeds between two groups.
| Seeds | Contrast | Brain region | Cluster size | Peak z score | MNI coordinates (mm) |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| DCP_C | BP > HC | I S1/M1 | 1216 | 3.79 | 34 | − 26 | 62 |
| I premotor cortex | 34 | − 6 | 62 | ||||
| I insula | 261 | 3.66 | 38 | − 8 | − 6 | ||
| I temporal lobe | 3.3 | 60 | 0 | 6 | |||
| DRP_C | BP > HC | I premotor cortex | 700 | 3.95 | 34 | − 2 | 64 |
| I S1/M1 | 28 | − 22 | 66 | ||||
| VRP_C | BP > HC | I temporal lobe | 404 | 4.37 | 50 | − 54 | − 4 |
| I S1/M1 | 952 | 3.93 | 46 | 0 | 22 | ||
| C temporal lobe | 247 | 3.56 | − 64 | − 60 | − 4 | ||
| DRP_I | BP > HC | I S1/M1 | 1225 | 4.28 | 36 | − 16 | 68 |
| DCP_I | BP > HC | C S2 | 633 | 4.18 | − 40 | − 36 | 20 |
| I S2 | 371 | 3.69 | 48 | − 36 | 20 | ||
| VRP_I | BP > HC | I fusiform | 327 | 3.79 | 22 | − 72 | − 12 |
| VRP_C | HC > BP | I thalamus | 362 | 3.64 | 10 | − 4 | 6 |
| C caudate | 3.27 | − 4 | 10 | 0 | |||
| DCP_C | HC > BP | No region above the threshold | |||||
| DRP_C | HC > BP | No region above the threshold | |||||
| VRP_I | HC > BP | No region above the threshold | |||||
| DRP_I | HC > BP | No region above the threshold | |||||
| DCP_I | HC > BP | No region above the threshold | |||||
Abbreviation: BP, Bell's palsy; C, contralateral; DCP, dorsal caudal putamen; DRP, dorsal rostral putamen; HC, healthy control; I, ipsilateral; M1, primary motor cortex; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; VRP, ventral rostral putamen.
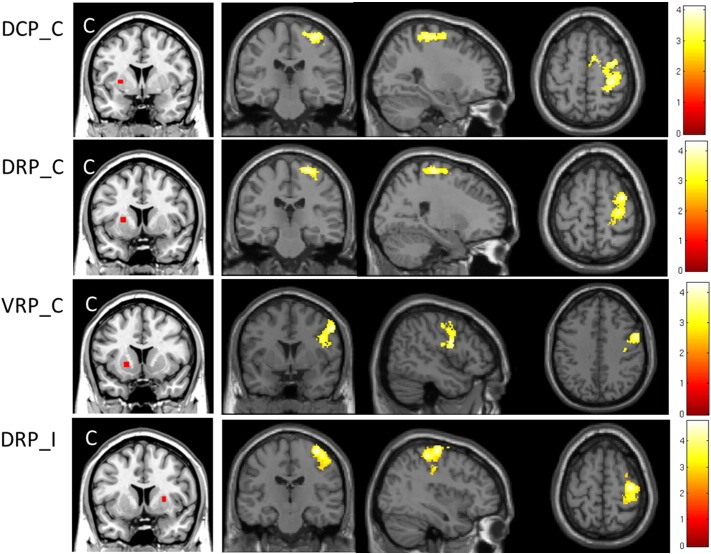
Fig. 3.
The contralateral putamen had significantly increased connectivity with S1/M1 in BP patients compared to healthy controls. However, different regions of S1/M1 had increased connectivity with different sub-regions of the putamen, most notably the ventral and dorsal putamen. In the ipsilateral putamen, only the DRP seed showed significantly increased connectivity with the ipsilateral S1/M1 in BP patients compared to healthy controls.
Fig. 5.
The ipsilateral DCP showed significantly increased connectivity with bilateral S2. The connectivity between ipsilateral S2 and ipsilateral DCP was positively associated with BP severity (p = 0.05).
3.3.2. Dorsal rostral putamen (DRP)
The bilateral DRP both showed significantly increased connectivity with the ipsilateral S1/M1 in BP patients compared to healthy controls (Table 1; Fig. 3).
3.3.3. Ventral rostral putamen (VRP)
The contralateral VRP had significantly increased connectivity with the ipsilateral S1/M1 and bilateral temporal lobe, and significantly decreased connectivity with the bilateral thalamus and ventral striatum in BP patients compared to healthy controls (Table 1; Fig. 3). We found the ipsilateral VRP had significantly increased connectivity with the ipsilateral fusiform in BP patients compared to healthy controls.
3.3.4. Dorsal caudate (DC)
The ipsilateral DC had significantly decreased connectivity with the bilateral cerebellum, and a significantly increased connectivity with the ipsilateral temporal pole, medial pre-frontal cortex, and orbital cortex in BP patients compared to healthy controls (Table 2; Fig. 4). Using the contralateral DC as a seed, we found no significant connectivity differences between healthy controls and BP patients.
Table 2.
Comparison of the connectivity in the caudate and ventral striatum seeds between two groups.
| Seeds | Contrast | Brain regions | Cluster size | Peak z value | MNI coordinates (mm) |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| DC_C | HC > BP | C temporal lobe | 267 | 3.92 | − 68 | − 22 | 10 |
| DC_I | HC > BP | C cerebelum | 353 | 4.1 | − 28 | − 30 | − 38 |
| I cerebelum | 330 | 3.95 | 38 | − 56 | − 30 | ||
| DC_I | BP > HC | I mPFC | 345 | 4.33 | 8 | 60 | 44 |
| I frontal obital lobe | 265 | 4.29 | 16 | 32 | − 14 | ||
| I temporal pole | 548 | 3.7 | 44 | 4 | − 38 | ||
| Vsi_C | BP > HC | C thalamus | 325 | 3.87 | − 18 | − 16 | 12 |
| C insula | 3.77 | − 38 | − 12 | 8 | |||
| C putamen | 3.56 | − 32 | − 6 | 10 | |||
| VSs_C | BP > HC | B paracentral lobe | 452 | 3.98 | − 6 | − 34 | 58 |
| B SMA | 3.83 | 0 | − 30 | 54 | |||
| VSs_I | BP > HC | B mid-cingulate | 518 | 3.46 | 14 | − 12 | 34 |
| DC_C | BP > HC | No region above the threshold | |||||
| Vsi_I | BP > HC | No region above the threshold | |||||
| Vsi_I | HC > BP | No region above the threshold | |||||
| Vsi_C | HC > BP | No region above the threshold | |||||
| VSs_C | HC > BP | No region above the threshold | |||||
| VSs_I | HC > BP | No region above the threshold | |||||
Abbreviation: B, bilateral; BP, Bell's palsy; C, contralateral; DC, dorsal caudate; HC, healthy control; I, ipsilateral; mPFC, medial prefrontal cortex; VSi, inferior ventral striatum; VSs, superior ventral striatum.
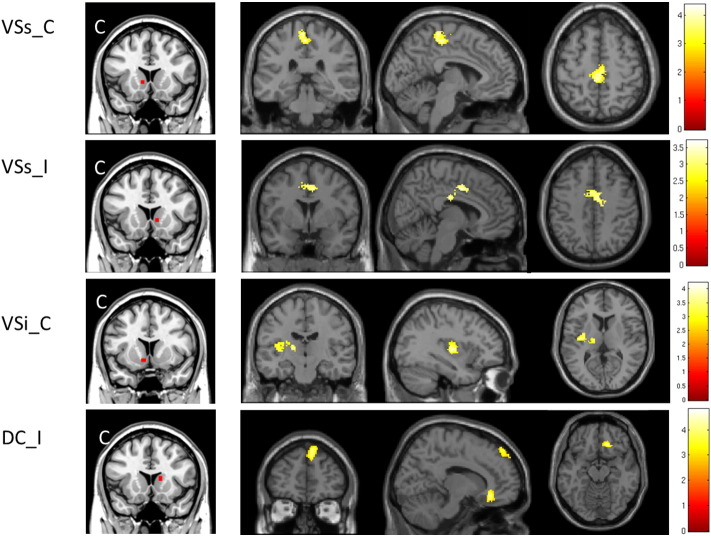
Fig. 4.
The contralateral VSs showed significantly increased connectivity with the bilateral paracentral lobe and SMA in BP patients compared to healthy controls. The ipsilateral VSs showed significantly increased connectivity with the bilateral mid-cingulate in BP patients compared to healthy controls. The contralateral VSi showed significantly increased connectivity with the contralateral insula and thalamus in BP patients compared to healthy controls. The ipsilateral DC showed significantly increased connectivity with the medial prefrontal lobe and orbital cortex in BP patients compared to healthy controls.
3.3.5. Superior ventral striatum (VSs)
The contralateral VSs had significantly increased connectivity with the bilateral paracentral lobe and bilateral supplementary motor area (SMA) in patients compared to healthy controls. The ipsilateral VSs had significantly increased connectivity with the bilateral mid-cingulate in BP patients compared to healthy controls (Table 2; Fig. 4).
3.3.6. Inferior ventral striatum (VSi)
The contralateral VSi had significantly increased connectivity with the contralateral insula, thalamus, and caudal putamen in BP patients compared to healthy controls (Table 2; Fig. 4).
3.4. Within striatum network
We also compared the rsFC between each of the 12 seeds within the striatum. There were no significant changes between the BP patients and healthy controls using a threshold of p < 0.05 FDR corrected. We also applied a less conservative threshold (p < 0.05) as an exploratory analysis. We found the connectivity between the VRP_L and VSs_R was significantly decreased in BP patients compared to the healthy controls (p = 0.019). Similarly, the connectivity between the VRP_R and VSs_R was significantly decreased in BP patients compared to the healthy controls (p = 0.043).
4. Discussion
In this study, we found altered resting state functional connectivity in a majority of the 12 sub-regions of the striatum in Bell's palsy patients. All of the contralateral putamen seeds (DCP, DRP, and VRP) and one ipsilateral seed (DRP) showed significantly increased connectivity with the ipsilateral S1/M1 in BP patients compared to healthy controls. The connectivity between the ipsilateral DCP and ipsilateral S2 was positively associated with HBS scores in patients. The inferior ventral striatum had significantly increased connectivity with the insula and thalamus, and the superior ventral striatum had significantly increased connectivity with the bilateral paracentral lobe and mid-cingulated cortex in patients compared to healthy controls.
The functional connectivity of the healthy control group was consistent with previous studies (Barnes et al., 2010, Di Martino et al., 2008, Kelly et al., 2009). Investigators found that the putamen seeds had stronger functional connectivity with sensorimotor area compare to the ventral striatum. The caudate has stronger functional connectivity with the prefrontal cortex compare to putamen, which may involve in affective processing. The ventral striatum seeds had functional connectivity with cingulate gyrus compare to putamen, which is part of the limbic system. The ventral putamen had stronger connectivity with the dorsolateral prefrontal cortex and rostral ACC compared to the dorsal putamen. In the dorsal putamen, the rostral region had greater correlation with the dorsal ACC compared to the caudal region. The inferior ventral striatum had a greater connectivity with the parahippocampal gyrus compared to the superior ventral striatum, and the superior ventral striatum had a greater connectivity with the dorsolateral prefrontal cortex, inferior frontal gyrus and rostral anterior cingulate compared to the inferior ventral striatum.
Previous studies found disrupted brain networks using resting-state functional connectivity in BP patients (He et al., 2014, Hu et al., 2015, Klingner et al., 2012, Klingner et al., 2014, Klingner et al., 2011, Kong et al., 2015, Li et al., 2013, Smit et al., 2010) In a recent study, investigators found resting state functional connectivity increased between the ipsilateral ACC and other brain regions such as the contralateral M1, SMA, ipsilateral S2, and mid-cingulate cortex with increased duration of BP (ranging from 2 to 55 days) (Hu et al., 2015). In another study, investigators used the hand region of bilateral S1 as a seed to investigate the connectivity with the contralateral side of the hand in BP patients before and after acupuncture treatment. They found significantly decreased resting state functional connectivity between S1, and bilateral S1, S2, and precuneus in BP patients shortly following nerve injury (He et al., 2014). However, all these studies only focused on the cortico-cortical connectivity. In this novel study, we used the sub-regions of striatum explore the functional connectivity between the subcortical region (striatum) and other brain regions.
In this study, we did not find connectivity differences between the ipsilateral and contralateral striatum as we originally hypothesized. Nevertheless, using a less conservative threshold of (p < 0.05), we found significantly decreased connectivity between the VRP_L and VSs_R, as well as between the VRP_R and VSs_R in BP patients compared to healthy controls. We speculate this mild impairment may be due to the short duration of BP. Future studies are needed to further examine this hypothesis.
4.1. Sensory-motor mismatch and a compensatory mechanism
Due to the unilateral facial muscle paralysis, which directly controls the movement of the facial muscles, BP patients have a discrepancy in the signals to and from the motor and sensory cortex (i.e. sensory-motor mismatch). The sensory cortex detects that the facial muscles do not move, despite the motor cortex giving the muscles the command. In a previous study, investigators compared resting state functional connectivity within the motor network as identified by facial movements. They found that in BP patients, the thalamus, insula, and S2 area had decreased connectivity with the other sensory motor areas, and that S2 and the contralateral insula had decreased connectivity that was negatively associated with the severity of BP. They suggested that the sensory-motor mismatch may be the cause of the decreased functional connectivity between areas within the motor-network (Klingner et al., 2014).
In our study, we found the contralateral inferior ventral striatum seed had significantly increased connectivity with the contralateral thalamus and insula. We also found the connectivity between the ipsilateral DCP and bilateral S2 was significantly increased in BP patients, and the connectivity between ipsilateral S2 and ipsilateral DCP was positively associated with BP severity. Previous studies found that these regions play an important role in sensory-motor balance. The thalamus relays and modulates the flow of information to the insula and S2 (Dieterich and Brandt, 2008, Lopez and Blanke, 2011, Suzuki et al., 2001).
In addition, we found two superior ventral striatum seeds had significantly increased connectivity with the bilateral SMA and mid-cingulate. We also found the ipsilateral dorsal caudate had significantly increased functional connectivity with the ipsilateral medial prefrontal lobe and orbital cortex. Previous studies suggest that the SMA and medial frontal cortex such as the orbital frontal cortex play an important role in action monitoring and error processing (Bonini et al., 2014). We speculate that the ventral striatum and caudate may be involved and play a role in action monitoring in BP patients.
Previous studies found that in Parkinson's disease, the putamen and sensorimotor area had a disrupted connectivity. Some investigators found that in patients with non-tremor Parkinson's disease, both the anterior and posterior putamen showed increased connectivity with bilateral S1 and M1 (Hacker et al., 2012). Other studies demonstrated significantly decreased connectivity between the putamen and the sensorimotor area in PD patients with akinesia symptoms compared to healthy controls. They also found this striatal-cortical connectivity was negatively correlated with UPDRS motor scores. This diminished connectivity may be a consequence of the dysfunction of the striatum. Furthermore, as the disorder progresses they found that these abnormal interactions among these two brain regions became more exaggerated (Yang et al., 2016, Wu et al., 2011a, Wu et al., 2011b). The main symptom of our BP patients was facial muscle paralysis. In contrast to PD studies, BP patients had significantly increased connectivity between the putamen, and the ipsilateral S1 and M1 compared to healthy controls. This difference may due to the fact that the striatum is not directly affected in BP patients, while it is damaged in PD patients. We speculate the sensorimotor and putamen showed increased connectivity in BP patients in order to provide a compensatory mechanism and solve the mismatch problem, thereby allowing for the recovering of the disease.
We speculate that this increased connectivity between the ventral striatum and SMA, the dorsal caudate and the medial prefrontal lobe, and the putamen and ipsilateral S1 and M1 may represent a compensatory mechanism, in which the ventral striatum and dorsal caudate receives the message regarding the sensory-motor mismatch through the sensorimotor integration area, and attempts to regulate and resolve this mismatch by increasing the connectivity between the putamen and M1/S1. Taken together, these results suggest that not only the putamen, but also other parts of the striatum such as the caudate and ventral striatum may be involved in the recovery of BP, and other peripheral nerve injuries. Different corticostriatal loops may be involved in action monitoring, error detective and error correction.
4.2. Somatotopic body representation
In the putamen, the contralateral dorsal putamen had significantly increased connectivity with the upper portion of M1, while the ventral rostral putamen had significantly increased connectivity with the lower portion of M1 compared to the healthy controls. In addition, we found the increased connectivity between S1/M1 and the ipsilateral dorsal rostral putamen overlapped with both the lower and upper S1 and M1 regions. A previous study using a clustering method defined 17-network parcellations of the cerebral cortex (Yeo et al., 2011), in which M1 consists of an upper and lower part, which corresponds to the dorsal and ventral part of the putamen, respectively (Choi et al., 2012). It is known that the lower part of M1 is the cortical representation of the face (Meier et al., 2008, Penfield and Boldrey, 1937), and considering the nature of Bell's palsy, we suggest that the ventral rostral putamen is more directly associated with facial muscle movement compared to the dorsal rostral putamen, although the dorsal rostral putamen has the ability to become involved in facial muscle movement following facial nerve damage. We speculate that the putamen has its own somatotopic body representation similar to that of S1 and M1 (Gerardin et al., 2003, Selemon and Goldman-Rakic, 1985), and this representation may be remapped following motor nerve injury.
This study is not without limitations. We did not have the opportunity to scan the patients at a later stage or after recovery, so we were not able to explore the dynamic striatum connectivity changes of BP patients. Further studies are needed to investigate the acute, late and recovery period of BP patients in order to define the relationship between human striatum connectivity of all these BP stages and its specific role in the peripheral nerve injury. In addition, since we focused on the functional connectivity changes of subregions of striatum, our study cannot detect the cortico-cortical connectivity changes in BP patients to compare with previous studies (Hu et al., 2015, Klingner et al., 2014).
5. Conclusion
We found disrupted striatal rsFC in BP patients. Our results suggest that 1) the putamen, as well as the rest of the striatum such as the caudate and ventral striatum were affected following unilateral facial paralysis caused by peripheral nerve injury; 2) striatal sub-regions play different roles, but together solve the sensory-motor mismatch problem through the corticostriatal loop; 3) the putamen may have its own somatotopic body representation and the representation may be remapped following motor nerve injury.
Acknowledgement
The study is supported by the Construction Funds of Key Subjects of Colleges and Universities in Zhejiang Province [grant number ZJGK2012-80-160] and Special Fund for Traditional Chinese Medicine Research in the Public Interest [grant number 201507006-01]. Jian Kong is supported by R01AT006364, R01 AT008563, R21AT008707, R61AT009310, and P01 AT006663 from NIH/NCCIH.
Contributor Information
Maosheng Xu, Email: xums166@126.com.
Jian Kong, Email: kongj@nmr.mgh.harvard.edu.
References
- Alexander G.E., Crutcher M.D., Delong M.R. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog. Brain Res. 1990;85119–46 [PubMed] [Google Scholar]
- Barnes K.A., Cohen A.L., Power J.D., Nelson S.M., Dosenbach Y.B., Miezin F.M., Petersen S.E., Schlaggar B.L. Identifying Basal Ganglia divisions in individuals using resting-state functional connectivity MRI. Front. Syst. Neurosci. 2010;418 doi: 10.3389/fnsys.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell P.T., Gilat M., O'Callaghan C., Copland D.A., Frank M.J., Lewis S.J., Shine J.M. Dopaminergic basis for impairments in functional connectivity across subdivisions of the striatum in Parkinson's disease. Hum. Brain Mapp. 2015;36(4):1278–1291. doi: 10.1002/hbm.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini F., Burle B., Liegeois-Chauvel C., Regis J., Chauvel P., Vidal F. Action monitoring and medial frontal cortex: leading role of supplementary motor area. Science. 2014;343(6173):888–891. doi: 10.1126/science.1247412. [DOI] [PubMed] [Google Scholar]
- Choi E.Y., Yeo B.T., Buckner R.L. The organization of the human striatum estimated by intrinsic functional connectivity. J. Neurophysiol. 2012;108(8):2242–2263. doi: 10.1152/jn.00270.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Scheres A., Margulies D.S., Kelly A.M., Uddin L.Q., Shehzad Z., Biswal B., Walters J.R., Castellanos F.X., Milham M.P. Functional connectivity of human striatum: a resting state FMRI study. Cereb. Cortex. 2008;18(12):2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Di Martino A., Kelly C., Grzadzinski R., Zuo X.N., Mennes M., Mairena M.A., Lord C., Castellanos F.X., Milham M.P. Aberrant striatal functional connectivity in children with autism. Biol. Psychiatry. 2011;69(9):847–856. doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich M., Brandt T. Functional brain imaging of peripheral and central vestibular disorders. Brain. 2008;131(Pt 10):2538–2552. doi: 10.1093/brain/awn042. [DOI] [PubMed] [Google Scholar]
- Draganski B., Kherif F., Kloppel S., Cook P.A., Alexander D.C., Parker G.J., Deichmann R., Ashburner J., Frackowiak R.S. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J. Neurosci. 2008;28(28):7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger J.C., Li Z., Haroon E., Woolwine B.J., Jung M.Y., Hu X., Miller A.H. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry. 2015 doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V., Ely B.A., Li Q., Bangaru S.D., Panzer A.M., Alonso C.M., Castellanos F.X., Milham M.P. Striatum-based circuitry of adolescent depression and anhedonia. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52(6):628–641. doi: 10.1016/j.jaac.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerardin E., Lehericy S., Pochon J.B., Tezenas D.M.S., Mangin J.F., Poupon F., Agid Y., Le Bihan D., Marsault C. Foot, hand, face and eye representation in the human striatum. Cereb. Cortex. 2003;13(2):162–169. doi: 10.1093/cercor/13.2.162. [DOI] [PubMed] [Google Scholar]
- Grillner S., Hellgren J., Menard A., Saitoh K., Wikstrom M.A. Mechanisms for selection of basic motor programs—roles for the striatum and pallidum. Trends Neurosci. 2005;28(7):364–370. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Hacker C.D., Perlmutter J.S., Criswell S.R., Ances B.M., Snyder A.Z. Resting state functional connectivity of the striatum in Parkinson's disease. Brain. 2012;135(Pt 12):3699–3711. doi: 10.1093/brain/aws281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B.J., Soriano-Mas C., Pujol J., Ortiz H., Lopez-Sola M., Hernandez-Ribas R., Deus J., Alonso P., Yucel M., Pantelis C., Menchon J.M., Cardoner N. Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2009;66(11):1189–1200. doi: 10.1001/archgenpsychiatry.2009.152. [DOI] [PubMed] [Google Scholar]
- He X., Zhu Y., Li C., Park K., Mohamed A.Z., Wu H., Xu C., Zhang W., Wang L., Yang J., Qiu B. Acupuncture-induced changes in functional connectivity of the primary somatosensory cortex varied with pathological stages of Bell's palsy. Neuroreport. 2014;25(14):1162–1168. doi: 10.1097/WNR.0000000000000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helie S., Chakravarthy S., Moustafa A.A. Exploring the cognitive and motor functions of the basal ganglia: an integrative review of computational cognitive neuroscience models. Front. Comput. Neurosci. 2013;7174 doi: 10.3389/fncom.2013.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmich R.C., Derikx L.C., Bakker M., Scheeringa R., Bloem B.R., Toni I. Spatial remapping of cortico-striatal connectivity in Parkinson's disease. Cereb. Cortex. 2010;20(5):1175–1186. doi: 10.1093/cercor/bhp178. [DOI] [PubMed] [Google Scholar]
- Hu S., Wu Y., Li C., Park K., Lu G., Mohamed A.Z., Wu H., Xu C., Zhang W., Wang L., Yang J., Qiu B. Increasing functional connectivity of the anterior cingulate cortex during the course of recovery from Bell's palsy. Neuroreport. 2015;26(1):6–12. doi: 10.1097/WNR.0000000000000295. [DOI] [PubMed] [Google Scholar]
- Kelly C., de Zubicaray G., Di Martino A., Copland D.A., Reiss P.T., Klein D.F., Castellanos F.X., Milham M.P., Mcmahon K. l-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J. Neurosci. 2009;29(22):7364–7378. doi: 10.1523/JNEUROSCI.0810-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerestes R., Harrison B.J., Dandash O., Stephanou K., Whittle S., Pujol J., Davey C.G. Specific functional connectivity alterations of the dorsal striatum in young people with depression. Neuroimage. Clin. 2015;7266–72 doi: 10.1016/j.nicl.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingner C.M., Volk G.F., Maertin A., Brodoehl S., Burmeister H.P., Guntinas-Lichius O., Witte O.W. Cortical reorganization in Bell's palsy. Restor. Neurol. Neurosci. 2011;29(3):203–214. doi: 10.3233/RNN-2011-0592. [DOI] [PubMed] [Google Scholar]
- Klingner C.M., Volk G.F., Brodoehl S., Burmeister H.P., Witte O.W., Guntinas-Lichius O. Time course of cortical plasticity after facial nerve palsy: a single-case study. Neurorehabil. Neural Repair. 2012;26(2):197–203. doi: 10.1177/1545968311418674. [DOI] [PubMed] [Google Scholar]
- Klingner C.M., Volk G.F., Brodoehl S., Witte O.W., Guntinas-Lichius O. The effects of deefferentation without deafferentation on functional connectivity in patients with facial palsy. Neuroimage. Clin. 2014;626–31 doi: 10.1016/j.nicl.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong S.P., Tan Q.W., Liu Y., Jing X.H., Zhu B., Huo Y.J., Nie B.B., Yang D.H. Specific correlation between the Hegu Point (LI4) and the orofacial part: evidence from an fMRI study. Evid. Based Complement. Alternat. Med. 2015;2015585493 doi: 10.1155/2015/585493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y., Peltier S., Bohnen N.I., Muller M.L., Dayalu P., Seidler R.D. Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson's disease. Front. Syst. Neurosci. 2010;4143 doi: 10.3389/fnsys.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S., Ducros M., Van de Moortele P.F., Francois C., Thivard L., Poupon C., Swindale N., Ugurbil K., Kim D.S. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann. Neurol. 2004;55(4):522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Li C., Yang J., Sun J., Xu C., Zhu Y., Lu Q., Yuan A., Zhu Y., Li L., Zhang W., Liu J., Huang J., Chen D., Wang L., Qin W., Tian J. Brain responses to acupuncture are probably dependent on the brain functional status. Evid. Based Complement. Alternat. Med. 2013;2013175278 doi: 10.1155/2013/175278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Zhou Y., Du Y., Zhao Z., Qin L., Xu J., Lei H. Aberrant corticostriatal functional circuits in adolescents with Internet addiction disorder. Front. Hum. Neurosci. 2015;9356 doi: 10.3389/fnhum.2015.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loonen A.J., Ivanova S.A. New insights into the mechanism of drug-induced dyskinesia. CNS Spectr. 2013;18(1):15–20. doi: 10.1017/s1092852912000752. [DOI] [PubMed] [Google Scholar]
- Lopez C., Blanke O. The thalamocortical vestibular system in animals and humans. Brain Res. Rev. 2011;67(1–2):119–146. doi: 10.1016/j.brainresrev.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Luo C., Song W., Chen Q., Zheng Z., Chen K., Cao B., Yang J., Li J., Huang X., Gong Q., Shang H.F. Reduced functional connectivity in early-stage drug-naive Parkinson's disease: a resting-state fMRI study. Neurobiol. Aging. 2014;35(2):431–441. doi: 10.1016/j.neurobiolaging.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Meier J.D., Aflalo T.N., Kastner S., Graziano M.S. Complex organization of human primary motor cortex: a high-resolution fMRI study. J. Neurophysiol. 2008;100(4):1800–1812. doi: 10.1152/jn.90531.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A., Lynn A., Foran W., Luna B., O'Hearn K. Age related changes in striatal resting state functional connectivity in autism. Front. Hum. Neurosci. 2013;7814 doi: 10.3389/fnhum.2013.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W., Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60(4):389–443. [Google Scholar]
- Selemon L.D., Goldman-Rakic P.S. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J. Neurosci. 1985;5(3):776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit A., van der Geest J., Metselaar M., van der Lugt A., Vanderwerf F., De Zeeuw C. Long-term changes in cerebellar activation during functional recovery from transient peripheral motor paralysis. Exp. Neurol. 2010;226(1):33–39. doi: 10.1016/j.expneurol.2010.07.026. [DOI] [PubMed] [Google Scholar]
- Subira M., Cano M., de Wit S.J., Alonso P., Cardoner N., Hoexter M.Q., Kwon J.S., Nakamae T., Lochner C., Sato J.R., Jung W.H., Narumoto J., Stein D.J., Pujol J., Mataix-Cols D., Veltman D.J., Menchon J.M., van den Heuvel O.A., Soriano-Mas C. Structural covariance of neostriatal and limbic regions in patients with obsessive-compulsive disorder. J. Psychiatry Neurosci. 2016;41(2):115–123. doi: 10.1503/jpn.150012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Kitano H., Ito R., Kitanishi T., Yazawa Y., Ogawa T., Shiino A., Kitajima K. Cortical and subcortical vestibular response to caloric stimulation detected by functional magnetic resonance imaging. Brain Res. Cogn. Brain Res. 2001;12(3):441–449. doi: 10.1016/s0926-6410(01)00080-5. [DOI] [PubMed] [Google Scholar]
- Tao J., Liu J., Egorova N., Chen X., Sun S., Xue X., Huang J., Zheng G., Wang Q., Chen L., Kong J. Increased hippocampus-medial prefrontal cortex resting-state functional connectivity and memory function after Tai Chi Chuan practice in elder adults. Front. Aging Neurosci. 2016;825 doi: 10.3389/fnagi.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unschuld P.G., Joel S.E., Liu X., Shanahan M., Margolis R.L., Biglan K.M., Bassett S.S., Schretlen D.J., Redgrave G.W., van Zijl P.C., Pekar J.J., Ross C.A. Impaired cortico-striatal functional connectivity in prodromal Huntington's disease. Neurosci. Lett. 2012;514(2):204–209. doi: 10.1016/j.neulet.2012.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakharia K., Vakharia K. Bell's palsy. Facial Plast. Surg. Clin. North Am. 2016;24(1):1–10. doi: 10.1016/j.fsc.2015.08.001. [DOI] [PubMed] [Google Scholar]
- Vanderah T., Gould D. Elsevier Health Sciences; 2015. Nolte’s the human brain: an introduction to its functional anatomy[M] [Google Scholar]
- Wang Z., Wang X., Liu J. Acupuncture treatment modulates the corticostriatal reward circuitry in major depressive disorder[J] J. Psychiatr. Res. 2017;84:18–26. doi: 10.1016/j.jpsychires.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Wu T., Long X., Wang L., Hallett M., Zang Y., Li K., Chan P. Functional connectivity of cortical motor areas in the resting state in Parkinson's disease. Hum. Brain Mapp. 2011;32(9):1443–1457. doi: 10.1002/hbm.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Wang L., Hallett M., Chen Y., Li K., Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson's disease. NeuroImage. 2011;55(1):204–215. doi: 10.1016/j.neuroimage.2010.11.074. [DOI] [PubMed] [Google Scholar]
- Yang W., Liu B., Huang B., Huang R., Wang L., Zhang Y., Zhang X., Wu K. Altered resting-state functional connectivity of the Striatum in Parkinson's disease after Levodopa Administration. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0161935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Roffman J.L., Smoller J.W., Zollei L., Polimeni J.R., Fischl B., Liu H., Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]