Abstract
Previous studies revealed several alterations of the cerebral white matter in patients with major depressive disorder. However, it is unknown if these alterations are associated with vascular changes in the brain and other body parts. We compared diffusion tensor imaging derived fractional anisotropy in a well characterized sample of middle-aged patients with major depressive disorder (n = 290) and never-depressed controls (n = 346) by the method of tract-based spatial statistics. Subsequently, the potential role of pulse wave velocity as a mediator of depression- and age-related changes in extracted estimates of fractional anisotropy were analyzed.
The results of the tract-based analysis revealed significantly reduced fractional anisotropy in the left posterior thalamic radiation associated with depression. Analyses of extracted data indicated additional reductions of fractional anisotropy bilaterally in the posterior thalamic radiation and in the left sagittal stratum. The analyses of indirect effects did not show any significant mediation of depression-related effects on fractional anisotropy via pulse wave velocity. However, age-related effects on fractional anisotropy were partially mediated by pulse wave velocity.
In conclusion, major depressive disorder is associated with detrimental effects on cerebral white matter microstructure properties which are independent of vascular changes, as measured by pulse wave velocity. However, a portion of age-related detrimental effects on white matter is explained by vascular changes. Longitudinal studies are required for investigating changes in white matter and vascular parameters over time and their association with incident depression.
Keywords: Depression, Structural connectivity, White matter, Pulse wave velocity, Vascular stiffness
Highlights
-
•
Depression is associated with reduced fractional anisotropy in the posterior thalamic radiation and in the sagittal stratum.
-
•
Pulse wave velocity is associated with reduced fractional anisotropy across the cerebral white matter.
-
•
Pulse wave velocity does not mediate depression-related effects on white matter microstructure.
-
•
Pulse wave velocity mediates a part of age-related detrimental effects on white matter microstructure.
1. Introduction
Major depressive disorder (MDD) is a severe, recurrent affective disease with a high lifetime prevalence (Bromet et al. 2011). Brain imaging studies revealed that MDD is associated with impaired white matter tissue in the brain. In elderly patients with depression, impairments in white matter tissue have been associated with vascular changes (Bijanki et al., 2015, Herrmann et al., 2007). MRI-based diffusion tensor imaging allows a precise assessment of white matter microstructure, quantified as fractional anisotropy (FA), which reflects a variety of white matter microstructural properties such as myelination and packing density (Jones et al. 2013). Studies investigating FA with a tract-based approach in patients with MDD revealed reduced FA in the frontal lobe of the brain (Guo et al. 2012), in the sagittal stratum, posterior thalamic radiation, external capsule and posterior corona radiata (Korgaonkar et al. 2011) as well as the corpus callosum (Aghajani et al., 2014, Ota et al., 2015). Additionally, depression-related FA reductions in the internal capsule, parahippocampal gyrus and posterior cingulum have been detected (Zhu et al. 2011).
From a clinical point of view, many patients with MDD exhibit unhealthy lifestyle patterns, e.g. physical inactivity, unhealthy diet and smoking (Bonnet et al. 2005). Such patterns are known to result in cumulative subclinical arteriosclerosis of peripheral and central arteries (Copeland et al., 2009) which in turn may mediate adverse effects of these patterns on FA in the brain (Allen et al., 2016, Gons et al., 2011, Gons et al., 2013). Pulse wave velocity (PWV) is a cumulative measure of arterial stiffness and peripheral subclinical arteriosclerosis, reflecting vascular changes over lifetime. Accordingly, PWV is influenced by vascular risk factors, e.g. obesity and high blood pressure (Lurbe et al. 2012), and was inversely associated with FA in a sample of elderly people (Tarumi et al. 2015). This association has also been observed in the community-based participants from the Framingham Heart Study (Maillard et al. 2016). PWV increases with age (Parikh et al. 2016) while a simultaneous decrease of FA has been observed during aging (Pfefferbaum et al. 2000). However, a possible mediation of age effects via PWV has not been investigated. Similarly, in the context of patients with MDD, the role of PWV as an estimate of cumulative vascular changes has not been investigated.
In the present study, we examined FA in a large sample of patients with MDD and never-depressed controls of the same source population by the method of tract based spatial statistics (TBSS) (Smith et al. 2006). We hypothesized that FA is primarily reduced in the posterior thalamic radiation, corpus callosum and the sagittal stratum in patients with MDD. Mean FA across the entire white matter skeleton and in hypothesized tracts were subsequently extracted to analyze an association with PWV as an indicator of peripheral vascular changes and subclinical arteriosclerosis. Finally, we examined a possible mediation of depression- and age-related effects on FA via PWV, respectively.
2. Materials and methods
2.1. Participants
A detailed description regarding design and purpose of the BiDirect study has been provided elsewhere (Teismann et al., 2014, Teuber et al., 2016). Aim of the BiDirect study is to investigate the relationship between depression and subclinical arteriosclerosis. The study comprised 2257 participants aged between 35 and 65 years, among them 911 controls and 999 patients with depression, recruited during treatment for an acute episode of depression. For the purpose of providing homogenous groups, the patient population was restricted to those with an ICD-10 diagnosis of MDD (F32 & F33; n = 927). Controls were randomly drawn from the population register of the city of Münster and invited to participate in the study. Control participants with a history of physician diagnosed depression or a positive screening (total score > 15) (Radloff 1977) on the Center for Epidemiologic Studies Depression Scale (CES-D) were excluded from the present analysis (n = 229). In both groups, participants with epilepsy, multiple sclerosis, Parkinson's disease, brain tumors, anomalies on DTI scans, a history of anxiety disorders, psychotic symptoms and substance abuse were excluded (n = 434). Participants with incomplete data (n = 372) or substandard DTI quality (e.g. defective slice acquisitions, stripe artifacts, venetian blind artifacts, large echo-planar imaging distortions and intensity inconsistencies; n = 167) were also excluded. The final sample included 636 participants, 290 patients with MDD and 346 controls. The BiDirect study was carried out in accordance with the provisions of the Declaration of Helsinki and approved by the ethics committee of the University of Münster and the Westphalian Chamber of Physicians in Münster. Written informed consent was received from each participant.
2.2. Pulse wave velocity
Assessment of PWV and blood pressure was done after a resting period of approximately 10 min. Brachial-ankle PWV was assessed with the automated Vascular Explorer device (enverdis, Jena, Germany) in accordance with the guidelines provided by the manufacturer. Prior to the assessment of PWV, participants were instructed to lie down in a comfortable position and to remain motionless during measurement. Inflatable cuffs were attached to the participants' upper arm (brachial artery) and ankle (posterior tibial artery). Pulse transit time (PTT) between the upper arm and ankle was determined using simultaneous measures of diastolic pressure levels. Body distances between suprasternal notch and the respective arm cuff (L1) as well as the distance between suprasternal notch and the respective ankle cuff (L3) were measured. PWV was computed depending on PTT and body distances with the formula:
2.3. MRI acquisition
MRI acquisition in all participants was performed on a 3 Tesla scanner (Intera, Philips, Best, NL). Diffusion weighted images were collected using a single-shot 2D echo-planar imaging sequence with the following parameters: repetition time (TR): 5900 ms, echo time (TE): 95 ms, 90° flip angle, 128 × 128 matrix, 240 × 240 mm2 field of view (FOV), 36 axial slices, 3.6 mm slice thickness, reconstructed to pixel size: 0.94 × 0.94 mm2, b = 1000 s/mm2. Diffusion gradients were applied along 20 non-collinear directions along with a single image without diffusion weighting (b = 0 s/mm2).
2.4. MRI preprocessing
Diffusion weighted scans were preprocessed using tools implemented in the FMRIB software library version 5 (FSL, http://www.fmrib.ox.ac.uk/fsl). In a first step, FMRIB's Diffusion Toolbox (FDT) was used for correction of eddy current distortions and head motions. Next, diffusion weighted images were skull stripped with the Brain Extraction Tool (BET). Diffusion tensor models were then fitted on corrected data in order to construct FA maps using the linear regression method implemented in DTIFIT. Subsequently, individual FA maps were nonlinearly registered to the FMRIB58_FA template in MNI space. Resulting FA maps were used to create a white matter skeleton representing the core of white matter tracts in the brain. This skeleton was set to an FA threshold value of 0.2 in order to avoid inclusion of non-white matter tissue. Individual FA maps were then projected onto the skeleton.
2.5. Statistical analysis
Demographic variables were compared across patients and controls with student's t-tests for continuous variables and χ2 test for categorical variables. Next, an analysis of covariance was conducted in order to compare PWV between patients and controls, while adjusting for age, sex and education. Statistical thresholds were set to p < 0.05 (two-tailed).
Statistical analysis of TBSS data was conducted with ‘FSL Randomise’ (Winkler et al. 2014). Skeletonized FA maps of patients with MDD and controls were compared by voxel wise tests implemented in the general linear model while adjusting for age, sex and education. Adjustment for education was done since white matter microstructure varies with different levels of education (Noble et al. 2013). Statistical maps were thresholded at p < 0.05 and corrected for multiple comparisons using threshold free cluster enhancement (TFCE) employing 5000 permutations. Also, individual mean FA values were extracted in the hypothesized tracts of interest (TOIs) according to the JHU ICBM-DTI-81 white-matter labels atlas (Mori et al. 2008) and across the entire skeleton.
The general linear model was used to analyze age, sex and education adjusted effects of MDD on FA in the respective TOIs and across the entire skeleton (model 1). These analyses were subsequently repeated with additional inclusion of smoking status and hypertension (model 2) as well as additional adjustment for PWV (model 3). With the same models, we also compared effects of age on FA with and without inclusion of smoking status, hypertension and PWV as additional covariates. Subsequently, possible indirect effects of MDD via PWV on FA were tested by utilizing a bootstrapping approach (5000 resamples) implemented in the PROCESS macro (Hayes 2013) for SPSS. Correspondingly, we analyzed potential indirect effects of age via PWV on FA with the PROCESS macro. For the analyses of extracted FA data in the three models, statistical thresholds were Bonferroni-corrected for the five hypothesized tracts plus the averaged FA across the entire skeleton. This yields a threshold of p < 0.008 (= 0.05/6). Data analysis was conducted with SPSS version 22 (IBM, Armonk, N.Y., U.S.A.).
3. Results
3.1. Sample characteristics
Patients with depression and controls differed significantly in age, sex and education (see Table 1). Furthermore, prevalences of diagnosed hypertension and smoking were significantly higher in patients with MDD. Patients with depression had significantly lower PWV (p < 0.01) in univariate analysis. However, after adjustment for age, sex and education, this difference was no longer statistically significant (p = 0.87). Of note, this was exclusively driven by age differences across groups since inclusion of age renders group differences on PWV non-significant (p = 0.42).
Table 1.
Comparison of demographic characteristics across cohorts.
| Patients with MDD | Controls | ||
|---|---|---|---|
| Variable | n = 290 | n = 346 | p |
| Age: mean (SD) | 48.93 (7.26) | 51.87 (8.20) | < 0.01 |
| Women: n (%) | 191 (65.9%) | 164 (47.4%) | < 0.01 |
| Education: n (%) | < 0.01 | ||
| Primary of general secondary school | 73 (25.2%) | 56 (16.2%) | |
| Intermediate secondary school | 88 (30.8%) | 78 (22.5%) | |
| High school | 63 (21.7%) | 60 (17.3%) | |
| University graduates (> 13 years) | 66 (22.8%) | 152 (43.9%) | |
| CES-D total score: mean (SD) | 25.02 (11.98) | 6.33 (4.02) | < 0.01 |
| History of hypertension: n (%) | 90 (31.0%) | 70 (20.2%) | < 0.01 |
| Current smokers: n (%) | 113 (39.0%) | 56 (16.2%) | < 0.01 |
| Pulse wave velocity:a m/s (SE) | 9.39 (0.07) | 9.38 (0.06) | 0.87 |
Abbreviations: MDD, major depressive disorder: SD, standard deviation; SE, standard error.
Adjusted for age, sex and education.
3.2. TBSS analysis of fractional anisotropy
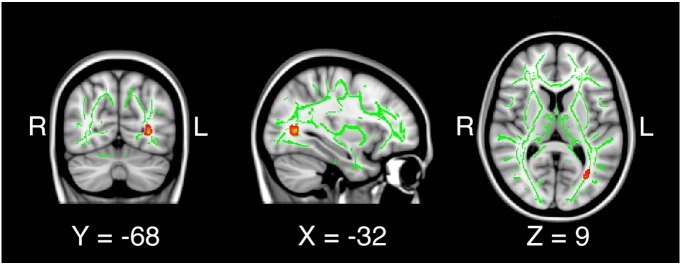
Results of the whole-brain TBSS analysis are presented in Fig. 1. Patients with MDD exhibited decreased FA in the left posterior thalamic radiation. No region displayed increased FA in patients with MDD compared to controls.
Fig. 1.
Significantly reduced fractional anisotropy in the left posterior thalamic radiation in patients with depression versus controls.
3.3. TOI-based analysis of fractional anisotropy
The results obtained by the TOI-based analysis extend findings from TBSS analysis: Effects of depression without (model 1) and with additional adjustment for hypertension and smoking status (model 2) as well as PWV (model 3) are presented in Table 2. In model 1, patients with MDD showed significantly decreased FA bilaterally in the posterior thalamic radiation and in the left sagittal stratum. As can be seen across the three consecutive models, inclusion of hypertension, smoking status and PWV led to virtually no changes in the estimated mean FA for patients with MDD. However, despite no meaningful changes in estimated mean FA between patients and controls across the three consecutive models, group effects did not reach significance in the right posterior thalamic radiation and the left sagittal stratum in model 2 and model 3 after Bonferroni-correction. Accordingly, analyses of indirect effects of MDD via PWV on FA were non-significant in any respective TOI as the bias-corrected, bootstrapped 95% confidence intervals always included zero (not shown).
Table 2.
Adjusted mean fractional anisotropy for patients with depression versus controls and corresponding p-values.
| Tracts of interest | Model 1 |
Model 2 |
Model 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Depression | Controls | p | Depression | Controls | p | Depression | Controls | p | |
| Corpus callosum | 0.664 | 0.667 | 0.379 | 0.664 | 0.665 | 0.540 | 0.664 | 0.666 | 0.495 |
| Left posterior thalamic radiation | 0.616 | 0.624 | 0.001 | 0.615 | 0.622 | 0.006 | 0.615 | 0.623 | 0.005 |
| Right posterior thalamic radiation | 0.621 | 0.628 | 0.007 | 0.620 | 0.627 | 0.019 | 0.621 | 0.627 | 0.017 |
| Left sagittal stratum | 0.536 | 0.544 | 0.002 | 0.535 | 0.541 | 0.012 | 0.535 | 0.542 | 0.010 |
| Right sagittal stratum | 0.555 | 0.561 | 0.021 | 0.554 | 0.558 | 0.076 | 0.554 | 0.559 | 0.071 |
| Complete skeleton | 0.449 | 0.451 | 0.161 | 0.449 | 0.450 | 0.363 | 0.449 | 0.450 | 0.331 |
Model 1 includes group, age, sex and education; Model 2 includes group, age, sex, education, hypertension and smoking status. Model 3 includes group, age, sex, education, hypertension, smoking status and pulse wave velocity. Bonferroni-corrected p-threshold is 0.008. Significant group differences are indicated by bold type.
PWV itself was inversely associated with FA and reached significance in the corpus callosum, the left sagittal stratum and globally across the entire skeleton (see Table 3). As presented in Table 4, inclusion of PWV consistently attenuates β-coefficients of age on FA in all respective TOIs. Analyses of indirect effects of age via PWV on FA yielded significant estimates in the left sagittal stratum, the left posterior thalamic radiations, the corpus callosum and across the entire white matter skeleton as shown in Table 5.
Table 3.
Regression coefficients for PWV along with corresponding p-values.
| Tracts of interest | PWV | ||
|---|---|---|---|
| β | Std. coefficient | p | |
| Corpus callosum | − 0.0037 | − 0.160 | 0.001 |
| Left posterior thalamic radiation | − 0.0024 | − 0.098 | 0.036 |
| Right posterior thalamic radiation | − 0.0021 | − 0.085 | 0.076 |
| Left sagittal stratum | − 0.0035 | − 0.149 | 0.002 |
| Right sagittal stratum | − 0.0018 | − 0.074 | 0.122 |
| Complete skeleton | − 0.0017 | − 0.132 | 0.005 |
Abbreviations: PWV, pulse wave velocity; Std, standardized. Estimates are derived from model 3 including group, sex, age, education, hypertension, smoking status and pulse wave velocity. Bold type indicates significant associations.
Bonferroni-corrected p-threshold is 0.008.
Table 4.
Regression coefficients for age and corresponding p-values.
| Tracts of interest | Model 1 |
Model 2 |
Model 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | Std. coefficient | p | β | Std. coefficient | p | β | Std. coefficient | p | |
| Corpus callosum | − 0.0008 | − 0.221 | < 0.001 | − 0.0008 | − 0.216 | < 0.001 | − 0.0005 | − 0.139 | 0.004 |
| Left posterior thalamic radiation | − 0.0013 | − 0.322 | < 0.001 | − 0.0012 | − 0.301 | < 0.001 | − 0.001 | − 0.253 | < 0.001 |
| Right posterior thalamic radiation | − 0.001 | − 0.239 | < 0.001 | − 0.0009 | − 0.225 | < 0.001 | − 0.0007 | − 0.184 | < 0.001 |
| Left sagittal stratum | − 0.0007 | − 0.188 | < 0.001 | − 0.0006 | − 0.162 | < 0.001 | − 0.0003 | − 0.09 | 0.058 |
| Right sagittal stratum | − 0.0009 | − 0.219 | < 0.001 | − 0.0007 | − 0.190 | < 0.001 | − 0.0006 | − 0.155 | 0.001 |
| Complete skeleton | − 0.0007 | − 0.319 | < 0.001 | − 0.0007 | − 0.305 | < 0.001 | − 0.0005 | − 0.241 | < 0.001 |
Abbreviations: Std. coefficient, standardized coefficient. Model 1 includes group, age, sex and education; Model 2 includes group, age, sex, education, hypertension and smoking status; Model 3 includes group, age, sex, education, hypertension, smoking status and pulse wave velocity. Bold type indicates significant associations. Bonferroni-corrected p-threshold is 0.008.
Table 5.
Indirect effect for age on fractional anisotropy via PWV.
| Tracts of interest | PWV |
|
|---|---|---|
| β | 95% CI | |
| Corpus callosum | − 0.0003 | − 0.00047 to − 0.00013 |
| Left posterior thalamic radiation | − 0.0002 | − 0.00037 to − 0.00002 |
| Right posterior thalamic radiation | − 0.0002 | − 0.00036–0.00002 |
| Left sagittal stratum | − 0.0003 | − 0.00047 to − 0.00011 |
| Right sagittal stratum | − 0.0001 | − 0.00031–0.00002 |
| Complete skeleton | − 0.0001 | − 0.00023 to − 0.00005 |
Abbreviations: PWV, pulse wave velocity; CI, bootstrapping-based confidence intervals (5000 resamples). The analyses are adjusted for group, sex, education, hypertension and smoking status. Bold type indicates significant indirect effects for age on fractional anisotropy via pulse wave velocity.
4. Discussion
We aimed to analyze potential FA differences in patients with MDD compared to never-depressed controls and to evaluate a potential mediating role of PWV, a marker of arterial stiffness and subclinical arteriosclerosis. In summary, our results indicate that MDD is accompanied by detrimental effects on white matter microstructure properties in the left sagittal stratum and bilaterally in the posterior thalamic radiations. While whole-brain TBSS analysis revealed decreased FA only in the left posterior thalamic radiation, the TOI-based analysis suggests that decreased FA is present in the left sagittal stratum and bilaterally in the posterior thalamic radiation. These current results support previous studies showing depression-related reductions of FA in the posterior thalamic radiation and sagittal stratum (Kieseppä et al., 2010, Korgaonkar et al., 2011, Ota et al., 2015). Importantly, the inclusion of hypertension, smoking and PWV as an additional covariates did not attenuate estimated mean FA in patients with MDD compared to controls. In line with these indications, the analyses of indirect effects did not yield any significant mediation of depression-related effects on FA via PWV, a marker of arterial stiffness and subclinical arteriosclerosis. This finding supports the concept of vascular depression to be a distinct diagnostic subtype of depression, limited to the elderly (Sneed et al. 2006), who presumably suffer from advanced vascular disease.
Previous findings of reduced FA in the corpus callosum (Ota et al. 2015) and frontal lobe tracts revealed in adolescents (Aghajani et al. 2014) and young adults with MDD (Guo et al. 2012) could not be confirmed in our sample of middle-aged patients. As the thalamus orchestrates cortical activity (Malekmohammadi et al. 2015), disruption of thalamocortial connections such as the sagittal stratum and posterior thalamic radiation may disturb the complex interplay between functional network nodes with a potential impact on functional connectivity patterns. Accordingly, it has been shown that specific functional networks in the brain are reflected by underlying white matter connections (Greicius et al. 2009).
In our study, increased PWV was associated with significantly decreased FA and, thus, detrimental effects on white matter microstructure. Particularly, the inverse relationship between PWV and FA was observed in the corpus callosum, the left sagittal stratum and across the entire skeleton. In the remaining regions, effects did not reach significance after Bonferroni-correction. Our results show a consistent attenuation of the β-coefficients for age, particularly after the inclusion of PWV in the third model, which indicates a mediation of age-related effects via PWV. The subsequent analyses of indirect effects of age via PWV on FA yielded significant effects in the corpus callosum, the left posterior thalamic radiation, the left sagittal stratum and across the entire white matter skeleton. There is no evidence for a complete mediation, suggesting that only one part of age-related detrimental effects on white matter microstructure can be attributed to PWV as a measure of subclinical arteriosclerosis. Previous studies (Barton et al., 1997, Jani, 2006, Thijssen et al., 2016, Tsamis et al., 2013) have shown that aging leads to stiffening of the arteries attributable to various mechanisms such as decreased elastin concentration and increased collagen content, as well as increased endothelin and decreased nitric oxide production. Stiffened arteries cause an increase in blood pressure, which represents an additional burden for the vascular system and, in turn, may induce further stiffening of the arteries (Mitchell 2015). It has been suggested that stiffened arteries foster excessive pressure and pulsatile flow in the vascular system, thereby inducing microvascular remodelling that limits blood flow and manifests as microvascular ischaemia (Mitchell et al. 2011). Thus, it appears plausible that increased arterial stiffness has adverse consequences on cerebral white matter at large, and is also a partial mediator for age-related detrimental effects on white matter microstructural properties in the brain.
Our study has limitations and strengths. First, participation in the BiDirect study was restricted to participants aged 35 to 65. This inherently limits the generalizability of the present results to younger and middle-aged individuals. Secondly, the study at hand is cross-sectional and does not allow conclusions about time sequences of onset. Hence, it is uncertain whether the decreased FA in the respective regions is a consequence of MDD or a precursor. We chose PWV as a cumulative measure of changes in the arterial walls over lifetime. While risk factors related to arteriosclerosis such as smoking or hypertension are easier to assess they represent transient but not cumulative exposures. Brachial-ankle PWV represents a valid measure of vascular changes (Yamashina et al. 2003) and has previously been associated with detrimental effects on white matter (Sugawara et al. 2005). Nevertheless, measuring PWV directly at the femoral and carotid arteries may provide preciser information regarding vascular changes relevant for the brain. However, vascular stiffness as measured by PWV is only one parameter describing vascular changes. Other measures for the assessment of vascular disease include the ankle-brachial-index, intima-media thickness and a magnetic resonance angiogram. Further strength of the present study are its detailed classification of a large number of participants from the same source population, which have been investigated both by a whole-brain and a TOI-based approach. Depression subtypes were not considered in the present analysis, which may provide additional information regarding alterations in white matter microstructure. According to previous findings (Jones 2004), diffusion gradients should be applied along at least 20 non-collinear sampling orientations in order to properly estimate FA.
In summary, we found decreased levels of FA in the left sagittal stratum and bilaterally in the posterior thalamic radiation in a well powered sample of patients with MDD compared to population controls without depression. Depression-specific reductions in FA are not mediated by PWV as an indicator of vascular changes. However, PWV itself was associated with reduced FA across the brain and represents a partial mediator for age-related effects on white matter microstructure, irrespective of depression. These findings highlight the concurrent but independent effects of vascular changes and MDD on white matter microstructure. Additional studies are required to explore potential long-term consequences of altered structural connectivity on functional networks and incident depression.
Funding
The present work was funded by the German Federal Ministry of Education and Research (BMBF, grant numbers FZK-01ER0816 and FZK-01ER1205) and the German Research Foundation (DFG, grant number FOR2107; DA1151/5-1).
Acknowledgements
We are grateful to all participants of the BiDirect study for their engagement. We thank Benedikt Sundermann for his advice regarding the TBSS analysis and Jens Minnerup for his role in the management of incidental findings on MRI scans.
References
- Aghajani M., Veer I.M., van Lang N.D.J., Meens P.H.F., van den Bulk B.G., Rombouts S.A.R.B. Altered white-matter architecture in treatment-naive adolescents with clinical depression. Psychol. Med. 2014;44:2287–2298. doi: 10.1017/S0033291713003000. [DOI] [PubMed] [Google Scholar]
- Allen B., Muldoon M.F., Gianaros P.J., Jennings J.R. Higher blood pressure partially links greater adiposity to reduced brain white matter integrity. Am. J. Hypertens. 2016;29:1029–1037. doi: 10.1093/ajh/hpw026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton M., Cosentino F., Brandes R.P., Moreau P., Shaw S., Luscher T.F. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension. 1997;30:817–824. doi: 10.1161/01.hyp.30.4.817. [DOI] [PubMed] [Google Scholar]
- Bijanki K.R., Matsui J.T., Mayberg H.S., Magnotta V.A., Arndt S., Johnson H.J. Depressive symptoms related to low fractional anisotropy of white matter underlying the right ventral anterior cingulate in older adults with atherosclerotic vascular disease. Front. Hum. Neurosci. 2015;9:408. doi: 10.3389/fnhum.2015.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet F., Irving K., Terra J.-L., Nony P., Berthezène F., Moulin P. Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis. 2005;178:339–344. doi: 10.1016/j.atherosclerosis.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Bromet E., Andrade L.H., Hwang I., Sampson N.A., Alonso J., de Girolamo G. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011;9:90. doi: 10.1186/1741-7015-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland K., Short, Blackett, Gardner Vascular health in children and adolescents: effects of obesity and diabetes. Vasc. Health Risk Manag. 2009;5:973. doi: 10.2147/vhrm.s7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gons R.A.R., van Norden A.G.W., de Laat K.F., van Oudheusden L.J.B., van Uden I.W.M., Zwiers M.P. Cigarette smoking is associated with reduced microstructural integrity of cerebral white matter. Brain. 2011;134:2116–2124. doi: 10.1093/brain/awr145. [DOI] [PubMed] [Google Scholar]
- Gons R.A.R., Tuladhar A.M., de Laat K.F., van Norden A.G.W., van Dijk E.J., Norris D.G. Physical activity is related to the structural integrity of cerebral white matter. Neurology. 2013;81:971–976. doi: 10.1212/WNL.0b013e3182a43e33. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Supekar K., Menon V., Dougherty R.F. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W.-B., Liu F., Xue Z.-M., Gao K., Wu R.-R., Ma C.-Q. Altered white matter integrity in young adults with first-episode, treatment-naive, and treatment-responsive depression. Neurosci. Lett. 2012;522:139–144. doi: 10.1016/j.neulet.2012.06.027. [DOI] [PubMed] [Google Scholar]
- Hayes A.F. Guilford Press; New York, NY: 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach. [Google Scholar]
- Herrmann L.L., Le Masurier M., Ebmeier K.P. White matter hyperintensities in late life depression: a systematic review. J. Neurol. Neurosurg. Psychiatry. 2007;79:619–624. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- Jani B. Ageing and vascular ageing. Postgrad. Med. J. 2006;82:357–362. doi: 10.1136/pgmj.2005.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn. Reson. Med. 2004;51:807–815. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Knösche T.R., Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kieseppä T., Eerola M., Mäntylä R., Neuvonen T., Poutanen V.-P., Luoma K. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J. Affect. Disord. 2010;120:240–244. doi: 10.1016/j.jad.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Korgaonkar M.S., Grieve S.M., Koslow S.H., Gabrieli J.D.E., Gordon E., Williams L.M. Loss of white matter integrity in major depressive disorder: evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Hum. Brain Mapp. 2011;32:2161–2171. doi: 10.1002/hbm.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurbe E., Torro I., Garcia-Vicent C., Alvarez J., Fernández-Fornoso J.A., Redon J. Blood pressure and obesity exert independent influences on pulse wave velocity in youth. Hypertension. 2012;60:550–555. doi: 10.1161/HYPERTENSIONAHA.112.194746. [DOI] [PubMed] [Google Scholar]
- Maillard P., Mitchell G.F., Himali J.J., Beiser A., Tsao C.W., Pase M.P. Effects of arterial stiffness on brain integrity in young adults from the Framingham heart study. Stroke. 2016;47:1030–1036. doi: 10.1161/STROKEAHA.116.012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malekmohammadi M., Elias W.J., Pouratian N. Human thalamus regulates cortical activity via spatially specific and structurally constrained phase-amplitude coupling. Cereb. Cortex. 2015;25:1618–1628. doi: 10.1093/cercor/bht358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G.F. Arterial stiffness. Curr. Opin. Nephrol. Hypertens. 2015;24:1–7. doi: 10.1097/MNH.0000000000000092. [DOI] [PubMed] [Google Scholar]
- Mitchell G.F., van Buchem M.A., Sigurdsson S., Gotal J.D., Jonsdottir M.K., Kjartansson O. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the age, gene/environment susceptibility - Reykjavik study. Brain. 2011;134:3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Oishi K., Jiang H., Jiang L., Li X., Akhter K. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. NeuroImage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble K.G., Korgaonkar M.S., Grieve S.M., Brickman A.M. Higher education is an age-independent predictor of white matter integrity and cognitive control in late adolescence. Dev. Sci. 2013;16:653–664. doi: 10.1111/desc.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota M., Noda T., Sato N., Hattori K., Hori H., Sasayama D. White matter abnormalities in major depressive disorder with melancholic and atypical features: a diffusion tensor imaging study. Psychiatry Clin. Neurosci. 2015;69:360–368. doi: 10.1111/pcn.12255. [DOI] [PubMed] [Google Scholar]
- Parikh J.D., Hollingsworth K.G., Kunadian V., Blamire A., MacGowan G.A. Measurement of pulse wave velocity in normal ageing: comparison of Vicorder and magnetic resonance phase contrast imaging. BMC Cardiovasc. Disord. 2016;16:50. doi: 10.1186/s12872-016-0224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A., Sullivan E.V., Hedehus M., Lim K.O., Adalsteinsson E., Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn. Reson. Med. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Sneed J.R., Roose S.P., Sackeim H.A. Vascular depression: a distinct diagnostic subtype? Biol. Psychiatry. 2006;60:1295–1298. doi: 10.1016/j.biopsych.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Sugawara J., Hayashi K., Yokoi T., Cortez-Cooper M.Y., DeVan A.E., Anton M.A. Brachial–ankle pulse wave velocity: an index of central arterial stiffness? J. Hum. Hypertens. 2005;19:401–406. doi: 10.1038/sj.jhh.1001838. [DOI] [PubMed] [Google Scholar]
- Tarumi T., de Jong D.L.K., Zhu D.C., Tseng B.Y., Liu J., Hill C. Central artery stiffness, baroreflex sensitivity, and brain white matter neuronal fiber integrity in older adults. NeuroImage. 2015;110:162–170. doi: 10.1016/j.neuroimage.2015.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teismann H., Wersching H., Nagel M., Arolt V., Heindel W., Baune B.T. Establishing the bidirectional relationship between depression and subclinical arteriosclerosis – rationale, design, and characteristics of the BiDirect study. BMC Psychiatry. 2014;14:174. doi: 10.1186/1471-244X-14-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuber A., Sundermann B., Kugel H., Schwindt W., Heindel W., Minnerup J. MR imaging of the brain in large cohort studies: feasibility report of the population- and patient-based BiDirect study. Eur. Radiol. 2016;1–8 doi: 10.1007/s00330-016-4303-9. [DOI] [PubMed] [Google Scholar]
- Thijssen D.H.J., Carter S.E., Green D.J. Arterial structure and function in vascular ageing: are you as old as your arteries? J. Physiol. 2016;594:2275–2284. doi: 10.1113/JP270597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsamis A., Krawiec J.T., Vorp D.A. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J. R. Soc. Interface. 2013;10:20121004. doi: 10.1098/rsif.2012.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. NeuroImage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashina A., Tomiyama H., Arai T., Hirose K., Koji Y., Hirayama Y. Brachial-ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens. Res. 2003;26:615–622. doi: 10.1291/hypres.26.615. [DOI] [PubMed] [Google Scholar]
- Zhu X., Wang X., Xiao J., Zhong M., Liao J., Yao S. Altered white matter integrity in first-episode, treatment-naive young adults with major depressive disorder: a tract-based spatial statistics study. Brain Res. 2011;1369:223–229. doi: 10.1016/j.brainres.2010.10.104. [DOI] [PubMed] [Google Scholar]