Abstract
Fatigue is a frequent and distressing symptom in children undergoing leukemia treatment; however, little is known about factors influencing this symptom. Antioxidants such as glutathione can decrease symptom severity in adult oncology patients, but no study has evaluated antioxidants’ effects on symptoms in pediatric oncology patients. This study describes fatigue patterns and associations of fatigue with antioxidants represented by reduced glutathione (GSH) and the reduced/oxidized glutathione (GSH/GSSG) ratio among children receiving leukemia treatment. A repeated measures design assessed fatigue and antioxidants among 38 children from two large U.S. cancer centers. Fatigue was assessed among school-age children and by parent proxy among young children. Antioxidants (GSH and GSH/GSSG ratio) were assessed from cerebrospinal fluid at four phases during leukemia treatment. Young children had a steady decline of fatigue from the end of induction treatment through the continuation phase of treatment, but no significant changes were noted among the school-age children. Mean antioxidant scores varied slightly over time; however, the GSH/GSSG ratios in these children were significantly lower than the normal ratio. Mean GSH/GSSG ratios significantly correlated to fatigue scores of the school-age children during early phases of treatment. Children with low mean GSH/GSSG ratios demonstrated oxidative stress. The low ratios noted early in therapy were significantly correlated with higher fatigue scores during induction and postinduction treatment phases. This finding suggests that increased oxidative stress during the more intensive phases of therapy may explain the experience of fatigue children report.
Keywords: fatigue, oxidative stress, pediatric, leukemia
Children diagnosed with cancer face many challenges. Among these are the pervasive symptoms that frequently occur during cancer treatment that pediatric patients often find distressing (Baggott et al., 2011; Kestler & LoBiondo-Wood, 2012). One of the symptoms children with cancer report most frequently is fatigue (Baggott et al., 2010; Kestler & LoBiondo-Wood, 2012; Miller, Jacob, & Hockenberry, 2011). Fatigue, defined as a state of decreased energy level fluctuating from tiredness to exhaustion, can interfere with the ability to accomplish daily tasks (Miller et al., 2011). Patients undergoing cancer treatment have significantly higher fatigue levels when compared to patients without a chronic health condition (Daniels, Brumley, & Schwartz, 2013), and this fatigue negatively impacts patients’ quality of life (Baggott et al., 2010; Erickson et al., 2010; Ream, Gibbons, Edwards, & Sepion, 2006).
Assessing fatigue is a priority when caring for pediatric oncology patients. It is important to understand the patterns of fatigue in order to develop effective interventions (Kestler & LoBiondo-Wood, 2012). Fatigue studies illustrate a pattern of fatigue that declines during treatment but fluctuates with administration of additional courses of chemotherapy or radiation treatment. Among 36 pediatric patients undergoing treatment for leukemia, Hockenberry, Taylor, Pasvogel, et al. (2014) found that the occurrence of fatigue was highest in the induction phase of treatment and then declined during the postinduction and continuation treatment phases. In another study, adolescent patients receiving chemotherapy every 1–2 weeks reported fatigue peaks and troughs and described fatigue as a “declining roller coaster” until the next cycle began (Erickson et al., 2010). Although children in previous studies reported an overall decline of fatigue throughout treatment, it never completely resolved, and their ratings of fatigue severity and distress did not significantly change over time (Baggott et al., 2010; Hockenberry, Taylor, Pasvogel, et al., 2014; Ream et al., 2006).
The variability of fatigue patterns illustrates the importance of identifying specific factors influencing fatigue. Fatigue is associated with anemia, malnutrition, infection, stress, and sleep–wake disturbances (Crabtree et al., 2015; Erickson et al., 2010; Hockenberry, Hooke, Gregurich, & McCarthy, 2009; Kestler & LoBiondo-Wood, 2012; Orsey, Wakefield, & Cloutier, 2013; Yeh et al., 2008), and treatment is targeted at eliminating these specific causes. However, the persistence of fatigue and fatigue-related distress demonstrates that treatment of these causes is not sufficient to provide sustained relief of fatigue and illustrates the importance of evaluating other related mechanisms that influence this symptom’s severity. Identifying mediators associated with fatigue, such as oxidative stress, is important for advancing the understanding of fatigue and developing more effective treatments (Gilliam & St. Clair, 2011; Gutstein, 2001; Hockenberry, Taylor, Pasvogel, et al., 2014).
Oxidative stress occurs when there is an imbalance between reactive oxygen species (free radicals) and antioxidant defenses, causing a chain reaction that results in cell death (Betteridge, 2000). When free radicals accumulate in the body, antioxidants such as glutathione serve as electron donors to stabilize the free radicals and protect the body against oxidative damage (Moore et al., 2013). This process causes reduced glutathione (GSH) to oxidize into glutathione disulfide (GSSG). Thus, the ratio of GSH/GSSG is a useful indicator of oxidative stress, as it signifies the availability of GSH to protect against oxidative reactions and the generation of GSSG from the oxidative reactions (Jones, 2006).
Normal GSH/GSSG ratios can be as high as 100:1, signifying the high availability of GSH and the normally low presence of GSSG. However, during oxidative stress, the GSH/GSSG ratio can be decreased to 10:1 or even as low as 1:1 (Zitka et al., 2012). Oxidative stress has been reported in children with severe traumatic brain injury (Bayir et al., 2002) and patients with aseptic and bacterial meningitis (de Menezes et al., 2009), with significantly lower levels of GSH found in the cerebrospinal fluid (CSF). Administration of chemotherapy agents creates a state of oxidative stress, yet few studies have evaluated oxidative stress in oncology patients and none have evaluated biomarkers of oxidative stress in the CSF of oncology patients. Jonas et al. (2000) found that patients receiving high-dose chemotherapy had a 20% decrease in plasma GSH, and the GSH/GSSG ratio became more oxidized acutely and during 2 weeks following treatment. Results from two randomized, double-blind trials showed that patients who received chemotherapy plus GSH had improved quality of life, decreased treatment-related symptoms, and decreased neurotoxicity compared to those who received chemotherapy plus placebo (Cascinu et al., 2002; Smyth et al., 1997). Collectively, these findings support our hypothesis that patients undergoing chemotherapy could have a decrease in the GSH/GSSG ratio and that decrease would be associated with an increase in symptoms such as fatigue.
Chemotherapy agents commonly administered to children undergoing treatment for acute lymphoblastic leukemia (ALL) are linked to oxidative stress. Anthracycline medications increase production of free radicals, while methotrexate decreases production of antioxidants (Gilliam & St. Clair, 2011; Hockenberry, Taylor, Pasvogel, et al., 2014). Despite these known associations, no study has correlated oxidative stress with fatigue in children undergoing ALL treatment. Evaluating antioxidants as an indicator of oxidative stress may provide crucial information for understanding how fatigue patterns are influenced by oxidative stress, which, in turn, could lead to identification of targeted interventions to relieve this distressing symptom. In the present study, we describe the prevalence of fatigue among children receiving treatment for ALL and identify associations of fatigue with oxidative stress as measured by the GSH/GSSG ratio in the CSF.
Method
Design
We used a repeated-measures design to assess fatigue and antioxidants at several time points during leukemia treatment to explore the patterns of fatigue and oxidative stress throughout all phases of treatment. Specifically, we obtained fatigue assessments at the end of induction (average 45 days from diagnosis), during postinduction (average 142 days from diagnosis), and during continuation phases of treatment (average of 338 days from diagnosis). We obtained CSF for the measurement of antioxidants during routine therapeutic lumbar punctures at approximately Day 8 postdiagnosis and during induction, postinduction, and continuation phases of therapy.
Setting/Sample
The sample consisted of children newly diagnosed with ALL who were participating in a larger study funded by the National Institute of Nursing Research (NR010889). Children were eligible for the study if they were diagnosed with pre-B-cell ALL or T-cell ALL, treated according to the Children’s Oncology Group protocols, diagnosed between the ages of 3 and 15 years, English speaking, and had complete data at all time points. The sample for this analysis consisted of children aged 3–12 years. Children with a history of neurological disorders, psychiatric disorders, traumatic brain injury, or developmental disability were excluded from the study. Children were recruited from two large cancer centers in the southwestern area of the United States. Each center’s institutional review board approved the study, and we obtained both parent consent and patient assent when applicable.
Measurements
We obtained demographic information including age, gender, and ethnicity from participants’ medical records upon their enrollment into the study. We assessed fatigue and CSF antioxidants during multiple phases of the child’s leukemia treatment, as described above, and assessed baseline antioxidant levels in the CSF at approximately Day 8 postdiagnosis. Induction therapy lasts 1 month and is initiated upon confirmation of the child’s diagnosis of leukemia. Postinduction begins immediately after induction and lasts 6–8 months. Continuation therapy begins immediately after postinduction therapy and lasts 2–3 years.
We used the Parent Fatigue Scale (PFS) to capture the parent’s perception of fatigue for the young child aged 3–6 years. Other researchers have confirmed that parent proxy reports of symptoms are valid in young children (Chang & Yeh, 2005; Varni et al., 2015). The PFS consists of 17 items that ask the parent to report his or her perceptions of the amount of fatigue-related behaviors his or her child exhibited in the past week. Parents rate these behaviors on a 5-point Likert-type scale ranging from a rating of 0 for not at all to 4 for always. Total scores range from 0 to 68 with higher scores indicating more fatigue. We used the Child Fatigue Scale (CFS) to assess reports of fatigue among school-age children aged 7–12 years. The CFS consists of 10 items asking the child to rate feelings associated with fatigue on a 5-point Likert-type scale. Total scores range from 0 to 40 with higher scores indicating more fatigue. These scales have acceptable reliability and validity (Hinds et al., 2010; Hockenberry et al., 2003).
We measured antioxidants in the CSF at four time points during the first 14 months of treatment at approximately Day 8 postdiagnosis and during induction, postinduction, and continuation phases of treatment. We took special precautions because antioxidant results can be influenced by sample handling, and some autooxidation of GSH will occur spontaneously in the presence of oxygen. We placed all CSF samples on ice immediately to slow/prevent reactions, kept samples cold during the entire process, stored them at −80°C, and thawed them on ice before analysis to further minimize auto-oxidation. We measured antioxidant levels with the Promega GSH Glo™ glutathione assay and reported them as the concentration of GSH, concentration of GSSG, and the GSH/GSSG ratio. The Promega GSH Glo assay is a luminescence-based assay that requires 100 μl of CSF for analysis. The luminescence assay utilizes a luciferin derivative that is converted to luciferin by the glutathione S-transferase enzyme in the presence of GSH. A stable luminescent signal is then generated when firefly luciferase is added. This signal is proportional to the amount of GSH in the sample. Wells on the plate measure existing GSH and duplicate wells contain the reducing agent on the plate to measure total reduced GSH. Each well contains 25 μl. First, the CSF was analyzed for reduced GSH, then a reducing agent was added to convert any oxidized GSSG to reduced GSH, and the CSF was analyzed again. The reducing agent is a thiol-free compound, tris(2-carboxyethyl)phosphine hydrochloride, which reduces disulfide bonds without any deleterious effects on the assay itself. A concentration of 0.5 mM was used. GSSG could then be calculated as (the total reduced [GSH] − the initial reduced [GSH])/2 (2 GSH = 1 GSSG).
Statistical Analysis
We analyzed all data using IBM SPSS™, Version 22. We used descriptive statistics to summarize the characteristics of the sample, fatigue scores, and biomarkers (GSH and GSH/GSSG ratio) and evaluated significant differences of fatigue and biomarker levels across time with repeated measures analysis of variance followed by multiple comparison testing. We used Pearson correlation to determine the association between fatigue scores and GSH/GSSG ratio during induction, postinduction, and continuation phases of treatment.
Results
The sample consisted of 38 children, including 23 children aged 3–6 years who supplied CSF samples and for whom their parents completed fatigue assessments, and 15 children aged 7–12 years, who provided CSF samples and fatigue assessments. Table 1 reports the children’s characteristics. Because children were often sedated for their lumbar puncture procedure, we did not usually collect fatigue assessments on the same day as the CSF.
Table 1.
Sample Characteristics.
| Characteristic | n | % |
|---|---|---|
| Age at diagnosis | ||
| 3–6 Years | 23 | 61 |
| 7–12 Years | 15 | 39 |
| Gender | ||
| Female | 21 | 55 |
| Ethnicity/race | ||
| Caucasian | 16 | 42 |
| Hispanic | 10 | 26 |
| African American | 2 | 5 |
| Other | 6 | 16 |
| Missing | 4 | 11 |
Note. N = 38.
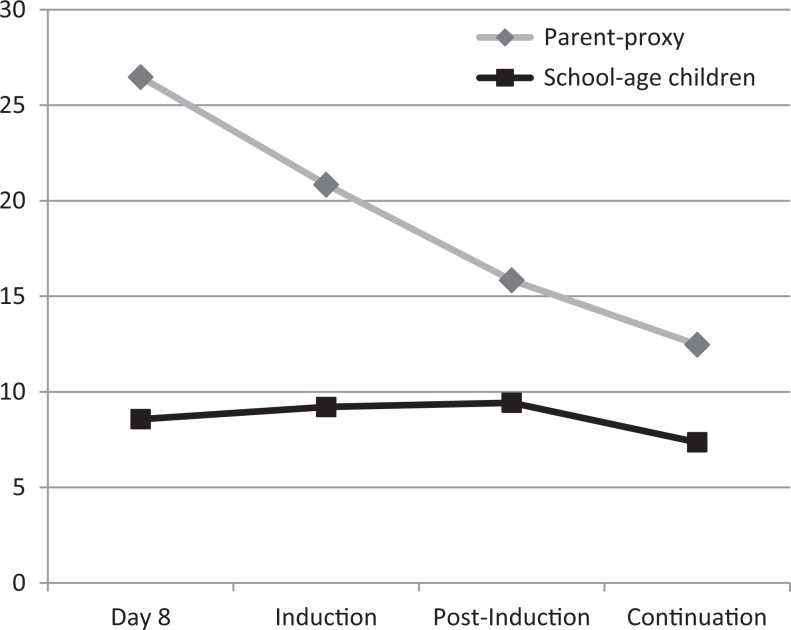
Figure 1 illustrates fatigue levels as reported by the parents for the young children and as the school-age children reported for themselves. Parents reported low levels of fatigue among the young children, as demonstrated by low mean fatigue scores. With a possible maximum score of 68, the mean score was highest for these children at Day 8 postdiagnosis ( = 26.5) and lowest at the continuation phase ( = 12.5). Scores steadily and significantly declined over time, F(3, 54) = 11.64, p = .00, illustrating that fatigue levels decreased over time. The school-age children also reported low levels of fatigue across time, as evidenced by the low fatigue scores at the four time points. There was no statistical change in fatigue levels over time for the school-age children, F(3, 39) = 0.36, p = .78.
Figure 1.
Mean fatigue scores for young (parent-proxy report, n = 23) and school-age (n = 15) children over time.
Table 2 lists mean GSH levels and GSH/GSSG ratios among the young and school-age children. Mean GSH levels in this study were less than 200 nM, considerably lower than the 800–1,200 nM mean GSH levels authors have reported in healthy children’s CSF (Bayir et al., 2002; Kawakami et al., 2006). Levels of both biomarkers varied slightly over time with no statistically significant changes. These findings suggest persistent oxidative stress as evidenced by the low GSH and extremely low GSH/GSSG ratios.
Table 2.
Oxidative Stress Biomarker Levels Over Time in Young (3–6 Years; n = 23) and School-Age (7–12 Years; n = 15) Children.
| Biomarker | Day 8 Mean (SD) | Induction Mean (SD) | Postinduction Mean (SD) | Continuation Mean (SD) | F(df) p Value |
|---|---|---|---|---|---|
| GSH (nM) | |||||
| 3–6 Years | 160.62 (70.90) | 188.34 (69.60) | 194.72 (80.00) | 189.39 (75.86) | 2.24(3, 63) .09 |
| 7–12 Years | 142.67 (86.84) | 143.32 (49.73) | 167.83 (74.08) | 150.47 (67.91) | 0.71(3, 36) .56 |
| GSH/GSSG | |||||
| 3–6 Years | 0.54 (0.31) | 0.55 (0.23) | 0.62 (0.27) | 0.62 (0.23) | 1.43(3, 63) .24 |
| 7–12 Years | 0.54 (0.17) | 0.60 (0.25) | 0.66 (0.22) | 0.61 (0.21) | 1.20(3, 36) .32 |
Note. GSH = reduced glutathione; GSSG = glutathione disulfide.
Mean GSH/GSSG ratios significantly correlated to fatigue scores of the school-age children during early phases of treatment. The negative correlation illustrates the association of oxidative stress (low GSH/GSSG ratios) with higher fatigue levels. Specifically, low mean GSH/GSSG ratios measured at both Day 8 postdiagnosis and during induction therapy negatively correlated with mean fatigue levels at postinduction (r = −.51, p = .04 and r = −.45, p = .05, respectively), and low mean GSH/GSSG ratios measured at induction and postinduction significantly correlated to high mean fatigue levels during induction (r = −.44, p = .05 and r = −.52, p = .02, respectively). We found no correlation between GSH/GSSH ratio and fatigue levels among the 3- to 6-year-old children.
Discussion
This study described fatigue and measured oxidative stress among young and school-age children during the induction, postinduction, and continuation phases of leukemia treatment. Although fatigue showed no variation over time in school-age children, among young children, it decreased in the postinduction and continuation phases of treatment. Fatigue never completely resolved during the study in either group of children as evidenced by fatigue scores greater than 0 at all time points. This pattern is similar to other reports of fatigue in children undergoing cancer therapy, where fatigue remained present throughout continuation therapy (Erickson et al., 2010; Ream et al., 2006; Zupanec, Jones, & Stremler, 2010). Persistence of symptoms is distressing to patients (Hockenberry, Taylor, Pasvogel, et al., 2014) and results in a lower quality of life (Baggott et al., 2011). The National Cancer Institute identified cancer-related fatigue as a high-priority research area due to the limited scientific progress with both measuring fatigue and understanding the biology behind it (Barsevick et al., 2013).
Evaluating fatigue in relation to oxidative stress increases our knowledge of the biology of fatigue. Antioxidant levels were low among children in this study, with mean GSH levels less than 200 nM and GSH/GSSG ratios less than 1:1. These mean GSH levels are lower than the mean levels of 800–1,200 nM reported in CSF among children who underwent lumbar punctures to rule out meningitis (Bayir et al., 2002; Kawakami et al., 2006). We could find no study that has reported GSH/GSSG ratios among healthy children. Exposure to chemotherapy agents has decreased antioxidant levels in individuals undergoing cancer treatment in prior studies (Gilliam & St. Clair, 2011). A previous report of increased concentrations of F2 isoprostanes, an established measure of oxidative stress, in CSF samples obtained from children with ALL during central nervous system (CNS)-directed therapy supports the proposal that this type of therapy increases oxidative stress (Hockenberry, Taylor, Gundy, et al., 2014). The low mean GSH levels and GSH/GSSG ratios that we noted among children in the present study could have been due to the repeated administration of chemotherapy medications during their treatment. However, these values could also have been influenced by the storing of samples at −80°C.
The low GSH/GSSG ratios at Day 8, early in therapy, were significantly correlated with higher fatigue scores during the induction and postinduction treatment phases in school-age children in the present study. This finding suggests that increased oxidative stress during the more intensive phases of ALL therapy may help to explain the experience of fatigue children report in later stages of treatment. However, further studies should be conducted to confirm our findings, as the low levels of fatigue and small changes among the biomarkers in this study make it challenging to identify statistically significant correlations.
A strength of the present study is that we obtained biomarkers and fatigue measures from the same children over time, providing a robust data set. However, we did not obtain fatigue scores and CSF samples on the same day due to the need to sedate the children for their lumbar puncture. Also, CSF samples were collected at various times during the day depending on the timing of the lumbar puncture, which limited our ability to consider the influences of diurnal rhythm on the biomarkers. On the other hand, since most children were sedated prior to their lumbar puncture, the potential influence of diet on GSH was minimized. Finally, we collected fatigue scores for the young children in this sample via parent report rather than self-report. There is no evidence to support the association of biomarkers to parent proxy data, so this method of measuring fatigue may have affected our ability to detect correlations between fatigue and oxidative stress in these subjects. Despite these limitations, we were still able to detect, for the first time, correlations between a biomarker of oxidative stress and fatigue among children receiving treatment for ALL. These significant findings highlight the need for further evaluation. In future studies, researchers should attempt to collect fatigue and oxidative stress data simultaneously and note the timing of CSF collection to comprehensively evaluate correlations.
Conclusion
Although fatigue is a highly prevalent and distressing symptom in children undergoing cancer treatment, no previous study has evaluated the relationship between antioxidant levels or other biomarkers of oxidative stress to fatigue among these patients. Researchers need to further evaluate potential biological mechanisms of fatigue, such as oxidative stress, in order to identify preventive strategies and develop effective interventions to alleviate this distressing symptom.
Footnotes
Author Contribution: Cheryl Rodgers contributed to conception, design, and interpretation; drafted and critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Chelse Sanborn contributed to conception and design, drafted and critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Olga Taylor contributed to acquisition and interpretation, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Patricia Gundy contributed to acquisition and interpretation, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Alice Pasvogel contributed to analysis and interpretation, critically revised the manuscript, gave final approval, and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Ida M. (Ki) Moore contributed to conception, design, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. Marilyn J. Hockenberry contributed to conception, design, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support was provided by the National Institute of Nursing Research at the National Institutes of Health (NR010889, Ida M. (Ki) Moore and 1R01-CA169398-01, Marilyn Hockenberry).
References
- Baggott C., Dodd M., Kennedy C., Marina N., Matthay K. K., Cooper B. A., Miaskowski C. (2010). Changes in children’s reports of symptom occurrence and severity during a course of myelosupporessive chemotherapy. Journal of Pediatric Oncology Nursing, 27, 307–315. doi:10.1177/1043454210377619 [DOI] [PubMed] [Google Scholar]
- Baggott C., Dodd M., Kennedy C., Marina N., Matthay K. K., Cooper B. A., Miaskowski C. (2011). An evaluation of the factors that affect the health-related quality of life of children following myelosuppressive chemotherapy. Supportive Care in Cancer, 19, 353–361. doi:10.1007/s00520-010-0824-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsevick A. M., Irwin M. R., Hinds P., Miller A., Berger A., Jacobsen P., … National Cancer Institute Clinical Trials Planning Meeting. (2013). Recommendations for high-priority research on cancer-related fatigue in children and adults. Journal of the National Cancer Institute, 105, 1432–1440. doi:10.1177/1043454209358890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayir H., Kagan V. E., Tyurina Y. Y., Tyurin V., Ruppel R. A., Adelson P. D.…Kochanek P. M. (2002). Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatric Research, 51, 571–578. [DOI] [PubMed] [Google Scholar]
- Betteridge D. J. (2000). What is oxidative stress? Metabolism, 49, 3–8. [DOI] [PubMed] [Google Scholar]
- Cascinu S., Catalano V., Cordella L., Labianca R., Giordani R., Baldelli A. M.…Catalano G. (2002). Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: A randomized, double-blind, placebo-controlled trial. Journal of Clinical Oncology, 20, 3478–3484. [DOI] [PubMed] [Google Scholar]
- Chang P., Yeh C. (2005). Agreement between child self-report and parent proxy-report to evaluate quality of life in children with cancer. Psycho-Oncology, 14, 125–134. doi:10.1002/pon.828 [DOI] [PubMed] [Google Scholar]
- Crabtree V. M., Rach A. M., Schellinger K. B., Russell K. M., Hammarback T., Mandrell B. N. (2015). Changes in sleep and fatigue in newly treated pediatric oncology patients. Supportive Care in Cancer, 23, 393–401. doi:10.1007/s00520-014-2356-3 [DOI] [PubMed] [Google Scholar]
- Daniels L. C., Brumley L. D., Schwartz L. A. (2013). Fatigue in adolescents with cancer compared to healthy adolescents. Pediatric Blood & Cancer, 60, 1902–1907. doi:10.1002/pbc.24706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Menezes C. C., Dorneles A. G., Sperotto R. L., Duarte M. M. F., Schetinger M. R. C., Loro V. L. (2009). Oxidative stress in cerebrospinal fluid of patients with aseptic and bacterial meningitis. Neurochemical Research, 34, 1255–1260. [DOI] [PubMed] [Google Scholar]
- Erickson J. M., Beck S. L., Christian B., Dudley W. N., Hollen P. J., Albritton K.…Godder K. (2010). Patterns of fatigue in adolescents receiving chemotherapy. Oncology Nursing Forum, 37, 444–455. doi:10.1188/10.ONF.444-455 [DOI] [PubMed] [Google Scholar]
- Gilliam L. A., St.Clair D. K. (2011). Chemotherapy-induced weakness and fatigue in skeletal muscle: The role of oxidative stress. Antioxidants & Redox Signaling, 15, 2543–2563. doi:10.1089/ars.2011.3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein H. B. (2001). The biologic basis of fatigue. Cancer, 92, 1678–1683. [DOI] [PubMed] [Google Scholar]
- Hinds P. S., Yang J., Gattuso J. S., Hockenberry M., Jones H., Zupanec S.…Srivastava D. K. (2010). Psychometric and clinical assessment of the 10-item reduced version of the Fatigue Scale-Child instrument. Journal of Pain and Symptom Management, 39, 572–578. doi:10.1016/j.jpainsymman.2009.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenberry M. J., Hinds P. S., Barrera P., Bryant R., Adams-McNeill J., Hooke C.…Manteuffel B. (2003). Three instruments to assess fatigue in children with cancer: The child, parent and staff perspectives. Journal of Pain and Symptom Management, 25, 319–328. [DOI] [PubMed] [Google Scholar]
- Hockenberry M. J., Hooke M. C., Gregurich M., McCarthy K. (2009). Carnitine plasma levels and fatigue in children/adolescents receiving cisplatin, ifosfamide, or doxorubicin. Journal of Pediatric Hematology/Oncology, 31, 664–669. doi:10.1097/MPH.0b013e3181b259a7 [DOI] [PubMed] [Google Scholar]
- Hockenberry M. J., Taylor O. A., Gundy P. M., Ross A. K., Pasvogel A., Montgomery D.…Moore I. M. (2014). F2-isoprostanes: A measure of oxidative stress in children receiving treatment for leukemia. Biological Research for Nursing, 16, 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenberry M. J., Taylor O. A., Pasvogel A., Rodgers C., McCarthy K., Gundy P.…Moore I. M. (2014). The influence of oxidative stress on symptom occurrence, severity, and distress during childhood leukemia treatment. Oncology Nursing Forum, 41, e238–e247. doi:10.1188/14.ONF.E238-E247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas C. R., Puckett A. B., Jones D. P., Griffith D. P., Szeszycki E. E., Bergman G. F.…Ziegler T. R. (2000). Plasma antioxidant status after high-dose chemotherapy: A randomized trial of parenteral nutrition in bone marrow transplantation patients. American Journal of Clinical Nutrition, 72, 181–189. [DOI] [PubMed] [Google Scholar]
- Jones D. P. (2006). Redefining oxidative stress. Antioxidants & Redox Signaling, 8, 1865–1879. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Monobe M., Kuwabara K., Fujita T., Maeda M., Fujino O.…Fukunaga Y. (2006). A comparative study of nitric oxide, glutathione, and glutathione peroxidase activities in cerebrospinal fluid from children with convulsive diseases/children with aseptic meningitis. Brain & Development, 28, 243–246. [DOI] [PubMed] [Google Scholar]
- Kestler S. A., LoBiondo-Wood G. (2012). Review of symptom experiences in children and adolescents with cancer. Cancer Nursing, 35, e31–e49. doi:10.1097/NCC.0b013e3182207a2a [DOI] [PubMed] [Google Scholar]
- Miller E., Jacob E., Hockenberry M. (2011). Nausea, pain, fatigue, and multiple symptoms in hospitalized children with cancer. Oncology Nursing Forum, 38, e382–e393. doi:10.1188/11.ONF.E382-E393 [DOI] [PubMed] [Google Scholar]
- Moore T., Le A., Niemi A. K., Kwan T., Cusmano-Ozog K., Enns G. M., Cowan T. M. (2013). A new LC-MS/MS method for the clinical determination of reduced and oxidized glutathione from whole blood. Journal of Chromatography B, 929, 51–55. doi:10.1016/j.jchromb.2013.04.004 [DOI] [PubMed] [Google Scholar]
- Orsey A. D., Wakefield D. B., Cloutier M. M. (2013). Physical activity and sleep among children and adolescents with cancer. Pediatric Blood & Cancer, 60, 1908–1913. doi:10.1002/pbc.24641 [DOI] [PubMed] [Google Scholar]
- Ream E., Gibbons F., Edwards J., Sepion B. (2006). Experience of fatigue in adolescents living with cancer. Cancer Nursing, 29, 317–326. [DOI] [PubMed] [Google Scholar]
- Smyth J. F., Bowman A., Perren T., Wilkinson P., Prescott R. J., Quinn K. J.…Tedeschi M. (1997). Glutathione reduces the toxicity and improves quality of life of women diagnosed with ovarian cancer treated with cisplatin: Results of a double-blind, randomized trial. Annals of Oncology, 8, 569–573. [DOI] [PubMed] [Google Scholar]
- Varni J. W., Thissen D., Stucky B. D., Liu Y., Magnus B., He J.…DeWalt D. A. (2015). Item-level informant discrepancies between children and their parents on the PROMIS® pediatric scales. Quality of Life Research, 24, 1921–1937. doi:10.1007/s11136-014-0914-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C. H., Chiang Y. C., Lin L., Yang C. P., Chien L. C., Weaver M. A., Chuang H. L. (2008). Clinical factors associated with fatigue over time in paediatric oncology patients receiving chemotherapy. British Journal of Cancer, 99, 23–29. doi:10.1038/sj.bjc.6604434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitka O., Skalickova S., Gumulec J., Masarik M., Adam V., Hubalek J.…Kizek R. (2012). Redox status expressed as GSH: GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncology Letters, 4, 1247–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupanec S., Jones H., Stremler R. (2010). Sleep habits and fatigue of children receiving maintenance chemotherapy for ALL and their parents. Journal of Pediatric Oncology Nursing, 27, 217–228. doi:10.1177/1043454209358890 [DOI] [PubMed] [Google Scholar]