Abstract
Non-valvular atrial fibrillation (NVAF) significantly contributes to the burden of stroke, particularly in elderly patients. The challenge of optimizing anticoagulation therapy is balancing efficacy and bleeding risk, especially as the same patients at high risk of stroke also tend to be at high risk of bleeding. Treating the elderly patient with NVAF presents special challenges because of their heightened risk for both stroke and bleeding. Despite clinical trial data and evidence-based guidelines, surveys indicate that physicians underuse anticoagulation in older patients for reasons that include overemphasis of bleeding risk, particularly with the increased risk of falling, at the cost of thromboembolic risk. Clinical trial data are now available, and real-world data are emerging, to illustrate the relative merits of the non-vitamin K antagonist oral anticoagulants compared with conventional anticoagulation in the treatment of elderly patients with this condition, and to suggest some subgroups of older patients who may be more suitable candidates for particular agents. Care of elderly patients with NVAF is often complicated by factors including risk of falling, adherence, health literacy, cognitive function, adverse effects, and involvement of caregivers, as well as other factors including the patient–provider relationship and logistical barriers to obtaining medication. Thus, conversations between clinicians and patients, as well as shared decision making, are important. In addition, elderly patients often suffer from comorbidities including hypertension, coronary heart disease, diabetes mellitus, COPD, and/or heart failure, which necessitate the use of multiple concomitant medications, increasing the risk of drug/drug interactions. This review provides an overview of clinical trial data on the use of non-vitamin K anticoagulant agents in elderly populations, and serves as a practical resource for the management of NVAF in the elderly patient.
Keywords: aged, non-vitamin K antagonist oral anticoagulants, stroke, warfarin, bleeding
Introduction
The prevalence of atrial fibrillation (AF) in the US population (estimated at 5.2 million in 2010) is projected to increase to 12.1 million by 2030.1 While age-adjusted incidence of clinically recognized AF has risen in recent decades, a 1993–2007 Medicare sample found a steady incidence, indicative of the association of AF with an aging population.2,3 AF, the most common cardiac arrhythmia, is a significant risk factor for stroke, increasing the risk fivefold.4,5
In analysis of trial data from ~9,000 patients with AF, increasing age was found to be associated with elevated stroke risk (hazard ratio [HR] per decade increase, 1.45; 95% confidence interval [CI] 1.26–1.66).6 Elderly patients with AF also often suffer from impactful comorbidities, including hypertension, coronary heart disease, diabetes mellitus, COPD, and/or heart failure; the kidney is particularly affected by aging, losing mass and glomerular and tubular function.2,7
Among Medicare beneficiaries with AF, the mean age is 80 years, and 55% are female;2 a meta-analysis has demonstrated women ≥75 years to be at an elevated risk of stroke vs men among patients with AF (relative risk [RR], 1.28; 95% CI 1.15–1.43).8 Female sex and increased age have both been identified as risk factors for stroke and incorporated into the CHA2DS2-VASc (Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke, transient ischemic attack, Vascular disease, Age 65–74 years, Sex category) risk-scoring system, which includes, among other factors, 1 point each for female sex and age 65–74 years, and 2 points for age ≥75 years.9 Table 1 shows factors included in both stroke risk and bleeding risk scores, highlighting the prominence of advanced age in both.9–11 The American College of Cardiology provides tools on its website allowing users to calculate scores including CHA2DS2-VASc, HAS-BLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history, Labile international normalized ratio [INR], Elderly, Drugs/alcohol), and a combination of both.12
Table 1.
Risk scales for predicting stroke and risk of bleeding
| Stroke risk
|
Bleeding risk
|
|||
|---|---|---|---|---|
| CHADS210 | CHA2 DS2-VASc9 | HEMORR2 HAGES59 | HAS-BLED11 | ATRIA60 |
| Age ≥75 years (1 point) | Age ≥75 years (2 points), age 65–74 years (1 point) | Age >75 years (1 point) | Age >65 years (1 point) | Age ≥75 years (2 points) |
| History of stroke or TIA (2 points) | Previous stroke/TIA/thromboembolism (2 points) | Stroke (1 point) | Stroke (previous history, particularly lacunar) (1 point) | |
| Hypertension (1 point) | Hypertension (1 point) | Hypertension (1 point) | Hypertension (1 point) | Hypertension (1 point) |
| CHF (1 point) | CHF/left ventricular dysfunction (1 point) | Hepatic/renal disease (1 point) | Abnormal renal/liver function (1 point each) | Severe renal disease (eGFR <30 mL/min or dialysis-dependent) (3 points) |
| Diabetes mellitus (1 point) | Diabetes mellitus (1 point) | Prior bleed (2 points) | Bleeding history or predisposition (anemia) (1 point) | Any prior hemorrhage diagnosis (1 point) |
| Vascular disease (prior myocardial infarction, peripheral artery disease, or aortic plaque) (1 point) | Anemia (1 point) | Anemia (3 points) | ||
| Female sex (1 point) | Reduced platelet count or function (1 point) | Labile INR (therapeutic time in range <60%) (1 point) | ||
| Ethanol abuse (1 point) | Drugs (antiplatelet agents, nonsteroidal anti-inflammatory drugs) or alcohol excess (≥8 units/week) (1 point each) | |||
| Malignancy (1 point) Genetic factors (CYP2C9 single-nucleotide polymorphisms) (1 point) Excessive fall risk (1 point) |
||||
Notes: Reprinted from Chest, 137(2), Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. 263–272. Copyright 2010 with permission from Elsevier.9 Reprinted from Chest, 138(5), Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. 1093–1100. Copyright 2010, with permission from Elsevier.11
Abbreviations: ATRIA, Anticoagulation and Risk Factors in Atrial Fibrillation; CHA2DS2-VASc, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke, transient ischemic attack, Vascular disease, Age 65–74 years, Sex category; CHADS2, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, prior Stroke, TIA, or non-central nervous system thromboembolism doubled; CHF, congestive heart failure; eGFR, estimated glomerular filtration rate; HAS-BLED, Hypertension, Abnormal renal/liver function, Stroke, Bleeding history, Labile international normalized ratio, Elderly, Drugs/alcohol; HEMORR2HAGES, Hepatic or renal disease, Ethanol abuse, Malignancy, Older age, Reduced platelet count or function, Re-bleeding, Hypertension, Anemia, Genetic factors, Excessive fall risk, and Stroke; INR, international normalized ratio; TIA, transient ischemic attack.
The challenge of optimizing anticoagulation therapy in all patients is balancing efficacy and bleeding risk, especially as the same patients at high risk of stroke also tend to be at high risk of bleeding.13 Vitamin K antagonists (VKAs), most notably warfarin, have been the standard of care for reducing the risk of stroke in patients with AF for over 50 years. Surveys have found physicians to be reluctant to prescribe warfarin for elderly patients, for reasons that include overemphasis of bleeding risk at the cost of thromboembolic risk, as well as the complications inherent to warfarin therapy (ie, drug/food and drug/drug interactions, need for frequent monitoring).14–16 Four non-vitamin K antagonist oral anticoagulants (NOACs) – the direct thrombin inhibitor dabigatran and the direct factor Xa inhibitors rivaroxaban, edoxaban, and apixaban – have been approved by the US Food and Drug Administration (FDA)17–20 for reducing the risk of stroke and systemic embolism (SE) in patients with non-valvular AF (NVAF). The aims of this review are to examine current and emerging data regarding the risks of stroke and bleeding in elderly patients with NVAF, to discuss the risk–benefit balance of various treatment options for NVAF in elderly patients, and to review the unique clinical challenges of managing NVAF in patients of advanced age.
Conventional therapy for elderly patients with NVAF
Numerous trials have shown the benefits of warfarin treatment over placebo in patients with NVAF.21 Antiplatelet therapy has also been shown to reduce the risk of stroke in NVAF patients, albeit less effectively than anticoagulation and with less consistency among studies.21,22 Aspirin use continues to be prevalent in patients with AF, including older patients, as aspirin may be associated with lower bleeding risk vs warfarin.23,24 Physician surveys identify fear of bleeding risk as the most commonly reported reason for not using anticoagulation in elderly patients.14
Despite physicians’ concerns, evidence suggests a generally positive balance of stroke risk and bleeding risk for warfarin in older patients. In 13,559 patients with NVAF (median age 73 years), patients aged ≥85 years were found to obtain particular benefit from VKA therapy, according to an analysis that accounted for both the rate of VKA-associated intracranial hemorrhage (ICH) and the rate of prevented ischemic strokes and systemic emboli. In patients aged ≥85 years receiving a VKA, the adjusted annual rate of thromboembolism was 2.86 events per 100 patients lower and the adjusted annual rate of ICH was 0.35 events per 100 patients higher than those not receiving a VKA; corresponding rates for the entire cohort showed a reduction of 1.04% in thromboembolism and a 0.24% increase in ICH.25
Clinical trial data: NOACs in patients with NVAF
In four Phase III trials, patients with NVAF at moderate (CHADS2 [Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Prior Stroke, transient ischemic attack, or non-central nervous system thromboembolism doubled] score ≥1) to high risk (CHADS2 score ≥2) of stroke were randomly assigned to receive NOAC or VKA treatment.26–29 Primary findings from each of the trials are summarized in Table 2. As there are no trials directly comparing the NOACs, and each trial enrolled different baseline populations and used different methodologies, care must be taken when making comparisons between agents.
Table 2.
Results of trials of NOACs for stroke prevention in NVAF
| Characteristic | RE-LY
|
RE-LY
|
ROCKET AF
|
ENGAGE AF-TIMI 48
|
ENGAGE AF-TIMI 48
|
ARISTOTLE
|
AVERROES
|
|---|---|---|---|---|---|---|---|
| Dabigatran 110 mg26,30,61 | Dabigatran 150 mg26,30,61 | Rivaroxaban 20 mg28,a | Edoxaban 30 mg29,b | Edoxaban 60 mg29,b | Apixaban 5 mg27,c | Apixaban 5 mg31,d | |
| Comparator | Warfarin, target INR 2.0–3.0 | Warfarin, target INR 2.0–3.0 | Warfarin, target INR 2.0–3.0 | Warfarin, target INR 2.0–3.0 | Warfarin, target INR 2.0–3.0 | Warfarin, target INR 2.0–3.0 | Aspirin 81–324 mg |
| CHADS2(mean) | 2.1 | 2.2 | 3.5 | 2.8 | 2.8 | 2.1 | 2.0/2.1 (apixaban/aspirin) |
| Age, mean (years) | 71 | 72 | 73 | 72 (median) | 72 (median) | 70 (median) | 70 |
| Female | 36% | 37% | 40% | 39% | 38% | 36% | 41% |
| Prior stroke/TIA | 20% | 20% | 55% (includes SE) | 28% | 28% | 19% | 14% |
| Efficacy | |||||||
| Stroke or SE (noninferiority analysis) | 1.54 vs 1.71; RR: 0.90 (0.74–1.10); P<0.001e | 1.11 vs 1.71; RR: 0.65 (0.52–0.81); P<0.001e | PP: 1.7 vs 2.2; HR: 0.79 (0.66–0.96); P<0.001 | mITT: 1.61 vs 1.50; HR: 1.07 (0.87–1.31); P=0.005 | mITT: 1.18 vs 1.50; HR: 0.79 (0.63–0.99); P<0.001 | 1.27 vs 1.60; HR: 0.79 (0.66–0.95); P<0.001 | |
| Stroke or SE (superiority analysis) | 1.54 vs 1.72; RR: 0.89 (0.73–1.09); P=0.27f | 1.12 vs 1.72; RR: 0.65 (0.52–0.81); P<0.001f | 2.1 vs 2.4; HR: 0.88 (0.75–1.03); P=0.12 OT: 1.7 vs 2.2; HR: 0.79 (0.66–0.96); P=0.02 |
2.04 vs 1.80; HR: 1.13 (0.96–1.34); P=0.10 | 1.57 vs 1.80; HR: 0.87 (0.73–1.04); P=0.08 | 1.27 vs 1.60; HR: 0.79 (0.66–0.95); P=0.01 | 1.6 vs 3.7; HR: 0.45 (0.32–0.62); P<0.001 |
| Hemorrhagic stroke | 0.12 vs 0.38; RR: 0.31 (0.17–0.56); P<0.001 | 0.10 vs 0.38; RR: 0.26 (0.14–0.49); P<0.001 | SOT: 0.26 vs 0.44; HR: 0.59 (0.37–0.93); P=0.024 | 0.16 vs 0.47; HR: 0.33 (0.22–0.50); P<0.001 | 0.26 vs 0.47; HR: 0.54 (0.38–0.77); P<0.001 | 0.24 vs 0.47; HR: 0.51 (0.35–0.75); P<0.001 | 0.2 vs 0.3; HR: 0.67 (0.24–1.88); P=0.45 |
| Ischemic stroke | Ischemic or nonspecified: 1.34 vs 1.22; RR: 1.10 (0.88–1.37); P=0.42 | Ischemic or nonspecified: 0.93 vs 1.22; RR: 0.76 (0.59–0.97); P=0.03 | SOT: 1.34 vs 1.42; HR: 0.94 (0.75–1.17); P=0.581 | 1.77 vs 1.25; HR: 1.41 (1.19–1.67); P<0.001 | 1.25 vs 1.25; HR: 1.00 (0.83–1.19); P=0.97 | Ischemic or nonspecified: 0.97 vs 1.05; HR: 0.92 (0.74–1.13); P=0.42 | 1.1 vs 3.0; HR: 0.37 (0.25–0.55); P<0.001 |
| Myocardial infarction | 0.82 vs 0.64; RR: 1.29 (0.96–1.75); P=0.09 | 0.81 vs 0.64; RR: 1.27 (0.94–1.71); P=0.12 | SOT: 0.91 vs 1.12; HR: 0.81 (0.63–1.06); P=0.121 | 0.89 vs 0.75; HR: 1.19 (0.95–1.49); P=0.13 | 0.70 vs 0.75; HR: 0.94 (0.74–1.19); P=0.60 | 0.53 vs 0.61; HR: 0.88 (0.66–1.17); P=0.37 | 0.8 vs 0.9; HR: 0.86 (0.50–1.48); P=0.59 |
| All-cause mortality | 3.75 vs 4.13; RR: 0.91 (0.80–1.03); P=0.13 | 3.64 vs 4.13; RR: 0.88 (0.77–1.00); P=0.051 | SOT: 1.87 vs 2.21; HR: 0.85 (0.70–1.02); P=0.073 | 3.80 vs 4.35; HR: 0.87 (0.79–0.96); P=0.006 | 3.99 vs 4.35; HR: 0.92 (0.83–1.01); P=0.08 | 3.52 vs 3.94; HR: 0.89 (0.80–0.998); P=0.047 | 3.5 vs 4.4; HR: 0.79 (0.62–1.02); P=0.07 |
| Vascular death | 2.43 vs 2.69; RR: 0.90 (0.77–1.06); P=0.21 | 2.28 vs 2.69; RR: 0.85 (0.72–0.99); P=0.04 | SOT: 1.53 vs 1.71; HR: 0.89 (0.73–1.10); P=0.289 | CV death: 2.71 vs 3.17; HR: 0.85 (0.76–0.96); P=0.008 | CV death: 2.74 vs 3.17; HR: 0.86 (0.77–0.97); P=0.013 | CV death: 1.80 vs 2.02; HR: 0.89 (0.76–1.04); P=NS | 2.7 vs 3.1; HR: 0.87 (0.65–1.17); P=0.37 |
| Safety | |||||||
| Major bleeding | 2.92 vs 3.61; RR: 0.80 (0.70–0.93); P=0.003 | 3.40 vs 3.61; RR: 0.94 (0.82–1.08); P=0.41 | SOT: 3.6 vs 3.4; HR: 1.04 (0.90–1.20); P=0.58 | SOT: 1.61 vs 3.43; HR: 0.47 (0.41–0.55); P<0.001 | SOT: 2.75 vs 3.43; HR: 0.80 (0.71–0.91); P<0.001 | SOT: 2.13 vs 3.09; HR: 0.69 (0.60–0.80); P<0.001 | 1.4 vs 1.2; HR: 1.13 (0.74–1.75); P=0.57 SOT: 1.4 vs 0.9; HR: 1.54 (0.96–2.45); P=0.07 |
| Intracranial hemorrhage | 0.23 vs 0.76; RR: 0.30 (0.19–0.45); P<0.001 | 0.32 vs 0.76; RR: 0.41 (0.28–0.60); P<0.001 | SOT: 0.5 vs 0.7; HR: 0.67 (0.47–0.93); P=0.02 | SOT: 0.26 vs 0.85; HR: 0.30 (0.21–0.43); P<0.001 | SOT: 0.39 vs 0.85; HR: 0.47 (0.34–0.63); P<0.001 | SOT: 0.33 vs 0.80; HR: 0.42 (0.30–0.58); P<0.001 | 0.4 vs 0.4; HR: 0.85 (0.38–1.90); P=0.69 |
| CRNM bleeding | SOT: 11.8 vs 11.4; HR: 1.04 (0.96–1.13); P=0.35 | SOT: 6.60 vs 10.15; HR: 0.66 (0.60–0.71); P<0.001 | SOT: 8.67 vs 10.15; HR: 0.86 (0.79–0.93); P<0.001 | 3.1 vs 2.7; HR: 1.15 (0.86–1.54); P=0.35 | |||
| Major GI bleeding | 1.15 vs 1.07; RR: 1.08 (0.85–1.38); P=0.52 | 1.56 vs 1.07; RR: 1.48 (1.18–1.85); P=0.001 | SOT: 3.2 vs 2.2; P<0.001 | SOT: 0.82 vs 1.23; HR: 0.67 (0.53–0.83); P<0.001 | SOT: 1.51 vs 1.23; HR: 1.23 (1.02–1.50); P=0.03 | SOT: 0.76 vs 0.86; HR: 0.89 (0.70–1.15); P=0.37 | 0.4 vs 0.4; HR: 0.86 (0.40–1.86); P=0.71 |
Notes: All columns show NOAC vs warfarin except AVERROES, which compared apixaban vs aspirin. All data are presented as annual rates per 100 patients and RRs/HRs with 95% confidence intervals. All analyses were performed in intent-to-treat populations unless otherwise specified.
Fifteen milligrams daily for those with moderately impaired renal function (CrCl 30–49 mL/min). The 2,950 (20.7%) patients with CrCl 30–49 mL/min had a mean age of 79 years.62
Dose of edoxaban 30 or 60 mg daily or placebo was halved if any of the following characteristics were present at the time of randomization or during the study: estimated CrCl 30–50 mL/min; body weight ≤60 kg; or the concomitant use of verapamil, quinidine, or dronedarone. 25.4% of each group had dose reduction.
A reduced dose of apixaban 2.5 mg twice daily or placebo was administered to patients with two or more of the following: age ≥80 years, body weight ≤60 kg, or serum creatinine ≥1.5 mg/dL (133 μmol/L) (428 [4.7%] and 403 [4.4%] patients in apixaban and warfarin groups, respectively).
A reduced dose of apixaban (2.5 mg twice daily) was used throughout the study for patients with two or more of the following: age ≥80 years, body weight ≤60 kg, or serum creatinine ≥1.5 mg/dL (133 μmol/L). A total of 6% of the patients in the apixaban group and 7% in the aspirin group received 2.5 mg twice a day according to protocol. A daily dose of 81 mg of aspirin or aspirin placebo was used in 65% of patients in the apixaban group and 64% in the aspirin group.
Based on the noninferiority analysis in the revised 2010 results.
Based on the superiority analysis in the revised 2014 results.
Abbreviations: ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; AVERROES, Apixaban Versus Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment; CHADS2, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, prior Stroke, TIA, or non-central nervous system thromboembolism doubled; CrCl, creatinine clearance; CRNM, clinically relevant nonmajor; CV, cardiovascular; ENGAGE AF-TIMI 48, Evaluation of Efficacy and Safety of Edoxaban versus Warfarin in Subjects with Atrial Fibrillation – Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation; GI, gastrointestinal; HR, hazard ratio; INR, international normalized ratio; mITT, modified intent-to-treat; NOAC, non-vitamin K antagonist oral anticoagulant; NS, nonsignificant; NVAF, non-valvular atrial fibrillation; OT, on treatment; PP, per protocol; RE-LY, Randomized Evaluation of Long-Term Anticoagulation Therapy; ROCKET AF, Rivaroxaban Once-Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation; RR, relative risk; SE, systemic embolism; SOT, safety on-treatment; TIA, transient ischemic attack.
A total of 18,113 patients (mean CHADS2 score 2.1; mean age 71 years) were randomized to dabigatran 110 or 150 mg or adjusted-dose warfarin in the Randomized Evaluation of Long Term Anticoagulant Therapy with Dabigatran (RE-LY) trial. In revised results from the intent-to-treat analysis, annual rates of stroke or SE in the dabigatran 150 mg group were superior vs warfarin (1.12% vs 1.72%; P<0.001), while rates in the dabigatran 110 mg group were comparable to warfarin (1.54% vs 1.72%).26,30 In the Rivaroxaban Once-daily oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF), 14,264 patients (mean CHADS2 score 3.5; mean age 73 years) were randomized to rivaroxaban 20 mg once daily (15 mg if creatinine clearance [CrCl] was 30–49 mL/min) or warfarin. Rivaroxaban was noninferior to warfarin in the intent-to-treat analysis (annual rates of stroke/SE of 2.1% vs 2.4%; P<0.001 for noninferiority) and superior to warfarin in prespecified analyses of events during treatment (annual rates of 1.7% vs 2.2%; P=0.02).28 The Evaluation of Efficacy and Safety of Edoxaban versus Warfarin in Subjects with Atrial Fibrillation – Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation (ENGAGE AF-TIMI 48) trial randomized 21,105 patients with NVAF (mean CHADS2 score 2.8; median age 72 years) to once-daily edoxaban 60 or 30 mg (in either group, the dose was halved if any of the following applied: estimated CrCl 30–50 mL/min; body weight ≤60 kg; or concomitant use of verapamil, quinidine, or dronedarone) or VKA. Both edoxaban doses demonstrated noninferiority to VKA in reducing the risk of stroke or SE in the primary analysis, including patients in the intent-to-treat population who received study drug during the treatment period (annual rates of 1.61%, 1.18%, and 1.50% for low-dose edoxaban, high-dose edoxaban, and warfarin, respectively); high-dose edoxaban showed a trend toward better efficacy vs warfarin in a prespecified superiority analysis of the intent-to-treat population during the entire study period (1.57% vs 1.80%; P=0.08).29 In Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE), 18,201 patients (mean CHADS2 score 2.1; median age 70 years) were randomized to apixaban 5 mg twice daily (2.5 mg doses were used in patients with two or more of the following: age ≥80 years, body weight ≤60 kg, or serum creatinine level ≥1.5 mg/dL) or warfarin. Apixaban was superior to warfarin in the primary intent-to-treat analysis of the primary end point of stroke or SE (1.27% vs 1.60% annually; P=0.01).27
In RE-LY, dabigatran 110 mg reduced the risk of major bleeding vs VKA (2.92% vs 3.61% annually; P=0.003), while dabigatran 150 mg was associated with a similar rate of major bleeding vs VKA (3.40% vs 3.61%; P=0.41).26,30 Whereas major bleeding was the primary end point in other Phase III studies of NOACs in patients with NVAF, the principal safety end point in ROCKET AF was a composite of major and clinically relevant nonmajor bleeding; annual rates were 14.9% for rivaroxaban 20 mg and 14.5% for warfarin (P=0.44).28 Annualized rates of major bleeding specifically were 1.61%, 2.75%, and 3.43% for low-dose edoxaban, high-dose edoxaban, and warfarin, respectively (P<0.001 for superiority for both edoxaban doses vs warfarin) in ENGAGE AF-TIMI 48.29 In ARISTOTLE, apixaban 5 mg was associated with reduced major bleeding vs warfarin (2.13% vs 3.09% annually; P<0.001).27
In an additional study, 5,599 patients with NVAF (mean CHADS2 score 2.1; mean age 70 years) who were unsuitable for VKA therapy were randomized to apixaban or aspirin. Apixaban was superior in reducing the risk of the primary outcome of stroke or SE (annual rates were 1.6% and 3.7% for apixaban and aspirin, respectively; P<0.001). The risk of major bleeding was similar between apixaban and aspirin (1.4% and 1.2% per year in the apixaban and aspirin groups, respectively).31
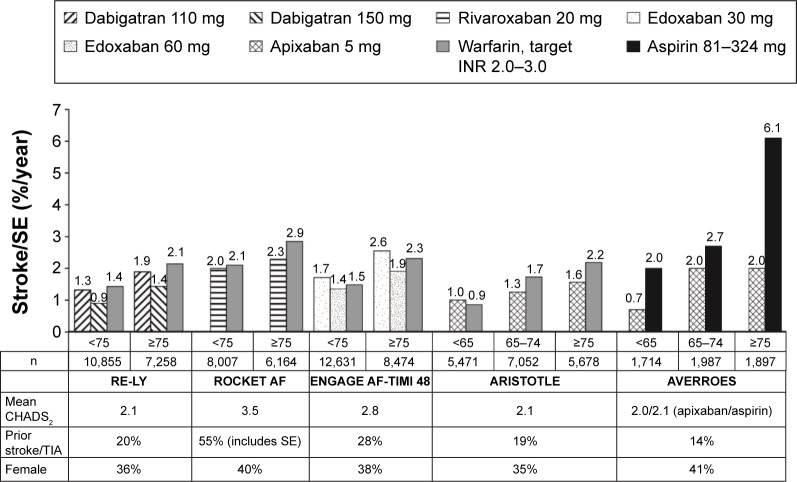
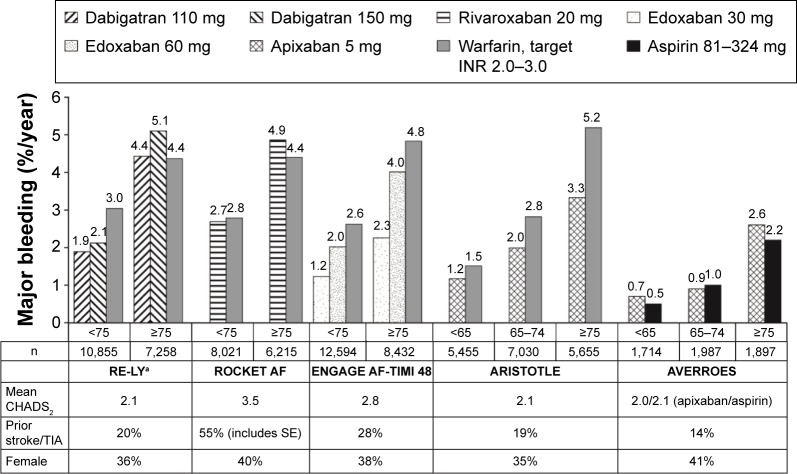
Figures 1 and 2 show rates of stroke/SE and major bleeding, respectively, in subgroups divided by age in the Phase III trials of NOACs for reducing the risk of stroke/SE in patients with NVAF. Table 3 shows more detailed efficacy and safety results from pivotal trials of NOACs examining reduction of stroke in NVAF in subgroups of patients aged ≥75 years.
Figure 1.
Rates of stroke or systemic embolism by age subgroup in Phase III trials of NOACs in patients with NVAF.
Notes: Values represent rates per 100 patient-years. Data from the following studies.27,29,31–33
Abbreviations: ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; AVERROES, Apixaban Versus Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment; CHADS2, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, prior Stroke, TIA, or non-central nervous system thromboembolism doubled; ENGAGE AF-TIMI 48, Evaluation of Efficacy and Safety of Edoxaban versus Warfarin in Subjects with Atrial Fibrillation – Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation; INR, international normalized ratio; NOAC, non-vitamin K antagonist oral anticoagulant; NVAF, non-valvular atrial fibrillation; RE-LY, Randomized Evaluation of Long-Term Anticoagulation Therapy; ROCKET AF, Rivaroxaban Once-Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation; SE, systemic embolism; TIA, transient ischemic attack.
Figure 2.
Rates of major bleeding by age subgroup in Phase III trials of NOACs in patients with NVAF.
Notes: aP<0.05 for interaction between age and treatment. Values represent rates per 100 patient-years. Data from the following studies.27,29,31–33
Abbreviations: ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; AVERROES, Apixaban Versus Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment; CHADS2, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, prior Stroke, TIA, or non-central nervous system thromboembolism doubled; ENGAGE AF-TIMI 48, Evaluation of Efficacy and Safety of Edoxaban versus Warfarin in Subjects with Atrial Fibrillation – Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation; INR, international normalized ratio; NOAC, non-vitamin K antagonist oral anticoagulant; NVAF, non-valvular atrial fibrillation; RE-LY, Randomized Evaluation of Long-Term Anticoagulation Therapy; ROCKET AF, Rivaroxaban Once-Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation; SE, systemic embolism; TIA, transient ischemic attack.
Table 3.
Efficacy and safety results (patients aged ≥75 years) in trials of NOACs for reduction in the risk of stroke in NVAF
| Characteristic | RE-LY
|
RE-LY
|
ROCKET AF
|
ENGAGE AF-TIMI 48
|
ENGAGE AF-TIMI 48
|
ARISTOTLE
|
AVERROES
|
|---|---|---|---|---|---|---|---|
| Dabigatran 110 mg32 | Dabigatran 150 mg32 | Rivaroxaban 20 mg33,a | Edoxaban 30 mg29,b | Edoxaban 60 mg29,b | Apixaban 5 mg27,34,c | Apixaban 5 mg31,36,d | |
| N (aged ≥75 years) | 7,258 | 6,229 | 8,474 | 5,678 | 1,898 | ||
| Comparator | Warfarin, target INR 2.0–3.0 | Warfarin, target INR 2.0–3.0 | Warfarin, target INR 2.0–3.0 | Warfarin, target INR 2.0–3.0 | Warfarin, target INR 2.0–3.0 | Warfarin, target INR 2.0–3.0 | Aspirin 81–324 mg |
| Efficacy | |||||||
| Stroke or SE | 1.89 vs 2.14; RR: 0.88 (0.66–1.17) | 1.43 vs 2.14; RR: 0.67 (0.49–0.90) | 2.29 vs 2.85; HR: 0.80 (0.63–1.02) | 2.55 vs 2.31 | 1.91 vs 2.31 | 1.56 vs 2.19; HR: 0.71 (0.53–0.95) | 2.0 vs 6.1; HR: 0.33 (0.20–0.54) |
| Stroke, SE, vascular death | 5.27 vs 5.74; HR: 0.92 (0.78–1.087) | ||||||
| Stroke, SE, MI, vascular death | 6.07 vs 6.68; HR: 0.91 (0.78–1.06) | ||||||
| Hemorrhagic stroke | 0.34 vs 0.49; HR: 0.70 (0.39–1.25) | ||||||
| Ischemic stroke | 1.71 vs 1.95; HR: 0.88 (0.67–1.16) | 1.6 vs 5.0; HR: 0.33 (0.18–0.56) | |||||
| Stroke (undetermined) | 0.09 vs 0.16; HR: 0.55 (0.19–1.65) | ||||||
| All-cause mortality | 5.42 vs 5.97; HR: 0.91 (0.77–1.07) | 5.6 vs 7.8; HR: 0.71 (0.50–0.99) | |||||
| Safety | |||||||
| Major bleeding | 4.43 vs 4.37; RR: 1.01 (0.83–1.23) | 5.10 vs 4.37; RR: 1.18 (0.98–1.42) | 4.86 vs 4.40; HR: 1.11 (0.92–1.34) | 2.26 vs 4.83 | 4.01 vs 4.83 | 3.33 vs 5.19; HR: 0.64 (0.52–0.79) | 2.6 vs 2.2; HR: 1.21 (0.69–2.12) |
| Hb drop | 3.85 vs 2.98; HR: 1.29 (1.03–1.61) | ||||||
| Transfusion | 2.28 vs 1.86; HR: 1.23 (0.93–1.64) | ||||||
| Clinical organ | 1.07 vs 1.42; HR: 0.75 (0.52–1.08) | ||||||
| Fatal bleeding | 0.28 vs 0.61; HR: 0.45 (0.23–0.87) | ||||||
| Intracranial hemorrhage | 0.37 vs 1.00; RR: 0.37 (0.21–0.64) | 0.41 vs 1.00; RR: 0.42 (0.25–0.70) | 0.66 vs 0.83; HR: 0.80 (0.50–1.28) | 0.43 vs 1.29; HR: 0.34 (0.20–0.57) | 0.6 vs 0.7; HR: 0.84 (0.28–2.41) | ||
| Major or CRNM bleeding | 19.83 vs 17.55; HR: 1.13 (1.02–1.25) | 6.7 vs 5.3; HR: 1.26 (0.88–1.80) | |||||
| GI bleeding | 2.19 vs 1.59; RR: 1.39 (1.03–1.98) | 2.80 vs 1.59; RR: 1.79 (1.35–2.37) | |||||
| Extracranial hemorrhage | 4.10 vs 3.44; RR: 1.20 (0.97–1.48) | 4.68 vs 3.44; RR: 1.39 (1.13–1.70) | |||||
| Non-GI extracranial hemorrhage | 2.00 vs 1.95; RR: 1.02 (0.76–1.36) | 2.26 vs 1.95; RR: 1.16 (0.88–1.53) | |||||
| Any bleeding | 23.5 vs 33.7; HR: 0.71 (0.65–0.78) | ||||||
| Net clinical events | 8.91 vs 10.9; HR: 0.82 (0.72–0.93) | ||||||
Notes: All columns show NOAC vs warfarin except AVERROES, which compared apixaban vs aspirin. All data are presented as annual rates per 100 patients and RRs/HRs with 95% confidence intervals. Population numbers represent subjects in the total randomized population who were aged ≥75 years at baseline.
Fifteen milligrams daily for those with moderately impaired renal function (CrCl 30–49 mL/min). The 2,950 (20.7%) patients with CrCl 30–49 mL/min had a mean age of 79 years.62
For patients in either group, dose was halved if any of the following characteristics were present at the time of randomization or during the study: estimated CrCl 30–50 mL/min; body weight ≤60 kg; or the concomitant use of verapamil, quinidine, or dronedarone.
A reduced dose of apixaban 2.5 mg twice daily or placebo was administered in 790 patients ≥75 years of age (13.9% of patients ≥75 years).
A reduced dose of apixaban (2.5 mg twice daily) was used throughout the study for patients who met two of the following criteria: age ≥80 years, body weight ≤60 kg, or serum creatinine ≥1.5 mg/dL (133 μmol/L). A total of 6% of the patients in the apixaban group and 7% in the aspirin group received 2.5 mg twice a day according to protocol. A daily dose of 81 mg of aspirin or aspirin placebo was used in 65% of patients in the apixaban group and 64% in the aspirin group.
Abbreviations: ARISTOTLE, Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation; AVERROES, Apixaban Versus Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment; CrCl, creatinine clearance; CRNM, clinically relevant nonmajor; ENGAGE AF-TIMI 48, Evaluation of Efficacy and Safety of Edoxaban versus Warfarin in Subjects with Atrial Fibrillation – Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation; HR, hazard ratio; INR, international normalized ratio; MI, myocardial infarction; NOAC, non-vitamin K antagonist oral anticoagulant; NVAF, non-valvular atrial fibrillation; RE-LY, Randomized Evaluation of Long-Term Anticoagulation Therapy; ROCKET AF, Rivaroxaban Once-Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation; RR, relative risk; SE, systemic embolism.
In subgroups aged <75 or ≥75 years from RE-LY, no interactions between age and treatment (dabigatran/warfarin) were evident for the outcome of stroke or SE. RRs in the aged <75 years cohort were 0.93 (95% CI 0.70–1.22) for dabigatran 110 mg vs warfarin and 0.63 (95% CI 0.46–0.86) for dabigatran 150 mg vs warfarin; in patients aged ≥75 years, the respective RRs were 0.88 (95% CI 0.66–1.17) and 0.67 (95% CI 0.49–0.90). Additionally, both dabigatran 110 and 150 mg were associated with lower risks of major bleeding compared with warfarin in those aged <75 years (RR, 0.62 [95% CI 0.50–0.77] and 0.70 [95% CI 0.57–0.86] for dabigatran 110 and 150 mg, respectively, vs warfarin). However, in the older subgroup, both doses of dabigatran were associated with more gastrointestinal (GI) bleeding vs warfarin (RR, 1.39 [95% CI 1.03–1.98] and 1.79 [95% CI 1.35–2.37] for dabigatran 110 and 150 mg, respectively, vs warfarin).32 The US prescribing information for dabigatran (150/75 mg tablets) notes the elevated bleeding risk in geriatric patients.19
In the ROCKET AF subanalysis including 6,229 patients aged ≥75 years and 8,035 younger patients, rivaroxaban 20 mg once daily (15 mg daily for those with moderately impaired renal function [CrCl 30–49 mL/min]) resulted in rates of stroke or SE and major bleeding similar to those with warfarin. In patients aged ≥75 years, the HR for rivaroxaban vs warfarin for stroke/SE was 0.80 (95% CI 0.63–1.02); in patients >75 years of age, it was 0.95 (95% CI 0.76–1.19; P=0.313 for interaction). The HR for major bleeding (rivaroxaban vs warfarin) in the ≥75 years subgroup was 1.11 (95% CI 0.92–1.34); in the younger subgroup, the HR was 0.96 (95% CI 0.78–1.19; P=0.336 for interaction). Hemorrhagic stroke rates were similar in both age groups; there was no interaction between age and rivaroxaban response.33
No significant interaction was seen between treatment (edoxaban 30 mg vs warfarin or edoxaban 60 mg vs warfarin) and subgroups defined according to age <75 or ≥75 years in subgroup analysis of ENGAGE AF-TIMI 48 for the primary efficacy end point of stroke or SE, or for the primary safety end point of major bleeding.29
In data from ARISTOTLE, no significant interaction by age category was observed for stroke/SE, all-cause mortality, major bleeding, all bleeding, ICH, or net clinical effects for apixaban vs warfarin. The HR for stroke or SE (apixaban vs warfarin) was 1.16 (95% CI 0.77–1.73) in patients aged <65 years, 0.72 (95% CI 0.54–0.96) in patients aged 65 to <75 years, and 0.71 (95% CI 0.53–0.95) in patients aged ≥75 years; the respective HRs in these groups for major bleeding were 0.78 (95% CI 0.55–1.11), 0.71 (95% CI 0.56–0.89), and 0.64 (95% CI 0.52–0.79).34 Most patients who received the reduced 2.5 mg dose were ≥75 years; this dose was associated with reductions in stroke and major bleeding similar to those with the normal 5 mg dose. Exploratory analysis found that age predicted major bleeding but was not associated with a differential treatment effect on major hemorrhage between warfarin and apixaban.35
In an analysis from Apixaban Versus Acetylsalicylic Acid [ASA] to Prevent Stroke in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES), apixaban was more efficacious than aspirin for reducing stroke risk in patients aged ≥75 years (HR 0.33) compared with patients <75 years (HR, 0.68; P=0.06 for age interaction).36 No significant interaction with age was found for the risk of major bleeding.
A meta-analysis of data from trials of the NOACs dabigatran, rivaroxaban, and apixaban in patients with NVAF limited to those aged ≥75 years found a 35% reduction (odds ratio, 0.65; 95% CI 0.48–0.87) vs control (VKA in three studies, aspirin in one) in the risk of stroke or SE.37 Pooled data from ten randomized controlled trials (including trials for reducing stroke and SE in NVAF and trials in other thromboembolic disorders) showed no excess bleeding with NOACs vs conventional therapy (VKA or aspirin) in the ≥75-year population.
Recently, data have begun to emerge on the real-world use of NOACs in patients with NVAF. Two analyses have compared outcomes in Medicare beneficiaries receiving dabigatran vs those receiving warfarin. In a cohort of 134,414 patients, dabigatran was associated with reduced risk of ischemic stroke (HR, 0.80; 95% CI 0.67–0.96; P=0.02), ICH (HR, 0.34; 95% CI 0.26–0.46; P<0.001), and overall mortality (HR, 0.86; 95% CI 0.77–0.96; P=0.006) but an increased risk of GI bleeding (HR, 1.28; 95% CI 1.14–1.44; P<0.001).38 The increase in GI bleeding risk appeared to be driven by the effects in women aged ≥75 years and men aged ≥85 years. In a group including 1,302 patients receiving dabigatran and 8,102 receiving warfarin, dabigatran was associated with a significantly higher risk of major and any bleeding than warfarin, after controlling for patient characteristics; the adjusted major bleeding incidence was 9.0% (95% CI 7.8%–10.2%) for the dabigatran group and 5.9% (95% CI 5.1%–6.6%) for the warfarin group. Additionally, patients receiving dabigatran had increased risk of GI bleeding (HR, 1.85; 95% CI 1.64–2.07) but decreased risk of ICH (HR, 0.32; 95% CI 0.20–0.50); the decrease in ICH associated with dabigatran was significant in patients older than 75 years (HR, 0.10; 95% CI 0.04–0.24) but not in younger patients.39 A retrospective analysis of administrative claims from a large database including privately insured and Medicare Advantage enrollees identified 92,816 new users of anticoagulants. In propensity score match models, the risk of GI bleeding in patients aged ≤75 years favored the NOACs over warfarin. In patients with NVAF aged >75 years, the risk of GI bleeding with dabigatran (n=2,063; HR, 2.49; 95% CI 1.61–3.83 vs the reference warfarin 18–64 age group) exceeded the risk with warfarin (n=2,068; HR, 1.62; 95% CI 1.02–2.58). Similarly, in patients with NVAF aged <75 years, the risk with rivaroxaban (n=1,582; HR, 2.91; 95% CI 1.65–4.81) exceeded that with warfarin (n=1,609; HR, 2.05; 95% CI 1.17–3.59).40
Special clinical considerations in anticoagulation for the elderly patients with NVAF
Comorbidities
Additional factors complicating anticoagulation of elderly patients with NVAF include the frequent presence of multiple comorbidities. In a sample of almost 4 million patients hospitalized for AF (70% >65 years of age; 53% female), the most frequent comorbidities were hypertension (60%), diabetes mellitus (22%), and COPD (20%). Over the time observed (2000–2010), the comorbidity that most increased in prevalence was renal failure, which reached 12% by 2010.41 Among patients with AF, the prevalence of chronic kidney disease (CKD) increases with age, and the addition of CKD as a comorbidity is associated with increased risk of stroke or SE and of bleeding. Patients with NVAF and renal disease have been found to be more likely to experience bleeding when treated with either warfarin or aspirin compared with those with NVAF only.42
NOAC metabolism is altered to varying degrees in patients with renal impairment, while renal clearance is considered to be a minor determinant of anticoagulant response to warfarin, and no warfarin dosage adjustment is necessary for patients with renal impairment.16 Patient characteristics related to renal and hepatic function and age may influence the choice of NOAC or warfarin use; the potential impact of these characteristics is outlined in Table 4.16–19,43 Elderly patients in general are subject to changes in kidney function, which leave them vulnerable to acute renal failure provoked by causes including dehydration, surgery, sepsis, and radiocontrast procedures.44 It should be noted that NVAF is associated not only with the impairment in renal function normally seen in aging patients but also with greater progression of kidney disease. In a cohort of 206,229 adults with CKD (mean age 70.7 [standard deviation 11.0] years), incident AF was associated with a 67% higher relative rate of subsequent end-stage renal disease after adjustment for potential confounders.45
Table 4.
Effect of non-modifiable patient characteristics on oral anticoagulant use
| Characteristic | Dabigatran19 | Rivaroxaban17 | Edoxaban20 | Apixaban18 | Warfarin16 |
|---|---|---|---|---|---|
| Renal impairment | Dosing recommendations cannot be provided for those with CrCl <15 mL/min or on dialysis Use reduced dose (75 mg bid) in patients with CrCl 15–30 mL/min |
Use reduced dose (15 mg qd) in patients with CrCl 15–50 mL/min | Reduce dose to 30 mg qd if CrCl 15–50 mL/min Not recommended if CrCl <15 mL/min |
Reduce dose to 2.5 mg bid if two or more of the following were met: age ≥80 years, body weight ≤60 kg, serum creatinine ≥1.5 mg/dL | No dose adjustment required |
| Hepatic impairment | Administration in patients with moderate hepatic impairment showed large inter-subject variability but no evidence of consistent change in exposure | Avoid use in patients with Child–Pugh B and C hepatic impairment or any degree of hepatic disease associated with coagulopathy | Not recommended in patients with moderate or severe hepatic impairment | Not recommended in patients with severe hepatic impairment Dosing recommendation cannot be provided in patients with moderate hepatic impairment |
Caution needed in patients with moderate-to-severe hepatic impairment |
| Age | Risk of stroke and bleeding increases with age, but risk–benefit profile is favorable in all age groups | Risk of stroke and bleeding increases with age, but risk–benefit profile is favorable in all age groups | Efficacy and safety are similar in elderly and younger patients | Reduce dose to 2.5 mg bid if two or more of the following were met: age ≥80 years, body weight ≤60 kg, serum creatinine ≥1.5 mg/dL | Consider lower initiation and maintenance doses of warfarin in patients ≥60 years |
Abbreviations: bid, twice daily; CrCl, creatinine clearance; qd, once daily.
Interactions
The frequent presence of multiple comorbidities in elderly patients often necessitates multiple concomitant medications. In general, drug/drug interactions with NOACs are few compared with potential interactions with warfarin;16 however, clinicians must be aware of a number of conflicts to avoid. Potential drug interactions of concern for patients taking dabigatran and edoxaban include other agents that affect the P-gp transport system.19,20 For apixaban and rivaroxaban, strong dual CYP3A4/P-gp inhibitors or inducers may have relevant potential for interaction.17,18
Risk of falling
Significant predictors of not receiving warfarin in hospitalized patients aged ≥65 years with AF include increased age, cognitive impairment, history of hemorrhage, advanced malignancy, and history of falling. For patients ≥80 years of age, physicians cited risk of falling as the primary factor discouraging them from warfarin use.46 Retrospective analysis of records from elderly patients with AF or atrial flutter who fell (42,913 on oral anticoagulation vs 334,960 controls) indicated a significantly higher mortality risk in those receiving anticoagulation (6% vs 3.1%; P<0.001). The increase in risk corresponded to a higher CHA2DS2-VASc score; patients with a score of 0–1 showed no additional mortality risk with anticoagulation, while patients with higher scores did show elevated risk.47 As age ≥75 years by itself receives 2 points in calculating CHA2DS2-VASc score, 9these results suggest that older patients with NVAF receiving anticoagulation may be at elevated mortality risk from falls. Indeed, preinjury warfarin use was seen to increase the odds of ICH by 40% and double 30-day mortality among Medicare beneficiaries with head trauma.48 Conversations between clinicians and patients and shared decision making are important in light of these data, which provide another factor to include in the difficult balance of risk and benefit in patients at the lower end of the stroke risk continuum.
Monitoring and adherence
Potential barriers to anticoagulation therapy adherence in elderly patients include the following: patient-related factors such as disease-related knowledge, health literacy, and cognitive function; drug-related factors such as adverse effects and polypharmacy; and other factors including the patient–provider relationship and various logistical barriers to obtaining medications.49 Warfarin is associated with the need for regular monitoring and dose adjustment to maintain treatment within the therapeutic range (INR 2.0–3.0),16 and the INR testing at regular visits is used partially as a proxy for adherence to treatment. Although NOACs do not require monitoring,17–20 regular administration is particularly important because of the quick onset/offset of action, making assessment of adherence an important component of follow-up visits. For patients with NVAF, the NOACs apixaban and dabigatran are to be taken twice daily, while rivaroxaban is administered once daily with the evening meal and edoxaban is taken once daily.17–20
Caregivers and coordination of care
Caregivers frequently play an essential participatory role in the care of elderly patients; >65 million people in the US provide this service, which for an elderly patient with NVAF may include confirming dosages, transporting to the primary care physician or anticoagulation clinic, and monitoring for signs of bleeding.50 Caregivers may play essential roles in the coordination of care, as elderly patients with NVAF (who frequently have multiple comorbidities) are treated by an interdisciplinary team. A caregiver may also be important in transitioning between providers, as when an elderly patient with NVAF must move from hospitalization to long-term care, requiring an accurate and complete exchange of information.51
Shared decision making
In addition to balancing stroke/SE and bleeding risks and taking into account special considerations for the elderly (including risk of falls), the recent introduction of NOACs allows individual preferences regarding convenience to be considered in selecting an anticoagulant regimen for each patient. The 2014 American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines recommend that antithrombotic therapy should be individualized for patients with NVAF based on shared decision making after discussion about the absolute risks and RRs of stroke and bleeding and the patient’s values and preferences.52
Emergent reversal
Abundant data testify to the association of advanced age with bleeding risk, which indicates the potential importance of reversal of anticoagulant effect in elderly patients. Although the short half-life of NOACs may decrease the need for immediate reversal, in cases of urgent bleeding or overdose of factor Xa inhibitor, no antidote is readily available, whereas idarucizumab has recently been approved for the reversal of dabigatran,53–55 and the activity of warfarin can be reversed by administration of vitamin K.16–20 Idarucizumab is a humanized monoclonal antibody fragment indicated in dabigatran-treated patients when reversal of the anticoagulant effects of dabigatran is needed for emergency surgery or urgent procedures and for life-threatening or uncontrolled bleeding.55 Idarucizumab received accelerated approval based on a reduction in unbound dabigatran and normalization of coagulation parameters in healthy volunteers. However, continued approval for this indication may be contingent upon the results of an ongoing cohort case series study.55 A recombinant protein for the reversal of factor Xa inhibitors56 and a small synthetic molecule for the reversal of all the NOACs are currently in development; idarucizumab is the only antidote that has yet received FDA approval.57,58 Procoagulant reversal agents such as prothrombin complex concentrate (PCC), activated PCC, and recombinant factor VIIa, although not evaluated in clinical trials, may be considered for reversal of apixaban; activated PCC, recombinant factor VIIa, and/or concentrates of coagulation factors II, IX, or X may be considered for reversal of dabigatran but have not been evaluated in clinical trials; and PCC has partially reversed rivaroxaban-induced prothrombin time prolongation in healthy volunteers.17–19 Additionally, activated charcoal reduces absorption of apixaban, and dabigatran may be removed by hemodialysis, although there is no clinical evidence supporting these strategies in response to emergent bleeding.18,19
Conclusion
Treating the elderly patients with NVAF presents special challenges for many reasons, including, at the most fundamental level, their heightened risk for both stroke and bleeding. Despite clinical trial data and evidence-based guidelines, surveys indicate that many clinicians continue to underuse anticoagulation in those elderly patients who could receive benefit from it. Although clinical experience with the NOACs is relatively limited vs the familiar characteristics of warfarin, subgroup analyses are now available to illustrate the relative merits of the new agents compared with standard anticoagulation in the treatment of elderly patients with NVAF.
Acknowledgments
Professional medical writing and editorial assistance were provided by Robert Coover, MPH, CMPP, and Nicole Draghi, PhD, CMPP, at Caudex, and were funded by Bristol-Myers Squibb Company and Pfizer Inc.
Footnotes
Disclosure
Joanne M Foody, MD, is an employee of Merck & Co. This work was completed when she was a faculty member at Harvard Medical School. At the time of the writing of this article, she was a consultant to Pfizer, Sanofi, Merck, and Janssen. The author reports no other conflicts of interest in this work.
References
- 1.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112(8):1142–1147. doi: 10.1016/j.amjcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 2.Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5(1):85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics – 2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–e60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics – 2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987;147(9):1561–1564. [PubMed] [Google Scholar]
- 6.van Walraven C, Hart RG, Connolly S, et al. Effect of age on stroke prevention therapy in patients with atrial fibrillation: the atrial fibrillation investigators. Stroke. 2009;40(4):1410–1416. doi: 10.1161/STROKEAHA.108.526988. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Bonventre JV, Parrish AR. The aging kidney: increased susceptibility to nephrotoxicity. Int J Mol Sci. 2014;15(9):15358–15376. doi: 10.3390/ijms150915358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagstaff AJ, Overvad TF, Lip GY, Lane DA. Is female sex a risk factor for stroke and thromboembolism in patients with atrial fibrillation? A systematic review and meta-analysis. QJM. 2014;107(12):955–967. doi: 10.1093/qjmed/hcu054. [DOI] [PubMed] [Google Scholar]
- 9.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 10.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 11.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 12.American College of Cardiology Atrial fibrillation toolkit. [Accessed June 8, 2016]. Available from: http://www.acc.org/tools-and-practice-support/clinical-toolkits/atrial-fibrillation-afib.
- 13.Oldgren J, Alings M, Darius H, et al. Risks for stroke, bleeding, and death in patients with atrial fibrillation receiving dabigatran or warfarin in relation to the CHADS2 score: a subgroup analysis of the RE-LY trial. Ann Intern Med. 2011;155(10):660–667. doi: 10.7326/0003-4819-155-10-201111150-00004. [DOI] [PubMed] [Google Scholar]
- 14.Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing. 2011;40(6):675–683. doi: 10.1093/ageing/afr097. [DOI] [PubMed] [Google Scholar]
- 15.Lane DA, Lip GY. Barriers to anticoagulation in patients with atrial fibrillation: changing physician-related factors. Stroke. 2008;39(1):7–9. doi: 10.1161/STROKEAHA.107.496554. [DOI] [PubMed] [Google Scholar]
- 16.Coumadin® (warfarin sodium) [prescribing information] Bristol-Myers Squibb; [Accessed June 8, 2016]. Available from: http://packageinserts.bms.com/pi/pi_coumadin.pdf. [Google Scholar]
- 17.Xarelto® (rivaroxaban) tablets [prescribing information] Janssen Pharmaceuticals, Inc; [Accessed June 8, 2016]. Available from: http://www.xareltohcp.com/shared/product/xarelto/prescribing-information.pdf. [Google Scholar]
- 18.Eliquis® (apixaban tablets) [prescribing information] Bristol-Myers Squibb Company; [Accessed June 8, 2016]. Available from http://packageinserts.bms.com/pi/pi_eliquis.pdf. [Google Scholar]
- 19.Pradaxa® (dabigatran etexilate mesylate) capsules [prescribing information] Boehringer Ingelheim; [Accessed June 8, 2016]. Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. [Google Scholar]
- 20.Savaysa® (edoxaban) tablets [prescribing information] Daiichi Sankyo; [Accessed June 8, 2016]. Available from: http://dsi.com/prescribing-information-portlet/getPIContent?productName=Savaysa&inline=true. [Google Scholar]
- 21.Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;3:CD006186. doi: 10.1002/14651858.CD006186.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 23.Hansen ML, Sorensen R, Clausen MT, et al. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170(16):1433–1441. doi: 10.1001/archinternmed.2010.271. [DOI] [PubMed] [Google Scholar]
- 24.Goren A, Liu X, Gupta S, Simon TA, Phatak H. Warfarin and aspirin use for stroke prevention among patients with atrial fibrillation: the US National Health and Wellness Survey. Am J Ther. 2015;22(4):248–256. doi: 10.1097/MJT.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 25.Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151(5):297–305. doi: 10.7326/0003-4819-151-5-200909010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 27.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 28.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 29.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 30.Connolly SJ, Wallentin L, Yusuf S. Additional events in the RE-LY trial. N Engl J Med. 2014;371(15):1464–1465. doi: 10.1056/NEJMc1407908. [DOI] [PubMed] [Google Scholar]
- 31.Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364(9):806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 32.Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123(21):2363–2372. doi: 10.1161/CIRCULATIONAHA.110.004747. [DOI] [PubMed] [Google Scholar]
- 33.Halperin JL, Hankey GJ, Wojdyla DM, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF) Circulation. 2014;130(2):138–146. doi: 10.1161/CIRCULATIONAHA.113.005008. [DOI] [PubMed] [Google Scholar]
- 34.Halvorsen S, Atar D, Yang H, et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J. 2014;35(28):1864–1872. doi: 10.1093/eurheartj/ehu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hylek EM, Held C, Alexander JH, et al. Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin in the ARISTOTLE trial: predictors, characteristics, and clinical outcomes. J Am Coll Cardiol. 2014;63(20):2141–2147. doi: 10.1016/j.jacc.2014.02.549. [DOI] [PubMed] [Google Scholar]
- 36.Ng KH, Shestakovska O, Connolly SJ, et al. Efficacy and safety of apixaban compared with aspirin in the elderly: a subgroup analysis from the AVERROES trial. Age Ageing. 2016;45(1):77–83. doi: 10.1093/ageing/afv156. [DOI] [PubMed] [Google Scholar]
- 37.Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc. 2014;62(5):857–864. doi: 10.1111/jgs.12799. [DOI] [PubMed] [Google Scholar]
- 38.Graham DJ, Reichman ME, Wernecke M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for non-valvular atrial fibrillation. Circulation. 2014;131(2):157–164. doi: 10.1161/CIRCULATIONAHA.114.012061. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez I, Baik SH, Pinera A, Zhang Y. Risk of bleeding with dabigatran in atrial fibrillation. JAMA Intern Med. 2014;175(1):18–24. doi: 10.1001/jamainternmed.2014.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857. doi: 10.1136/bmj.h1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel NJ, Deshmukh A, Pant S, et al. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129(23):2371–2379. doi: 10.1161/CIRCULATIONAHA.114.008201. [DOI] [PubMed] [Google Scholar]
- 42.Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367(7):625–635. doi: 10.1056/NEJMoa1105594. [DOI] [PubMed] [Google Scholar]
- 43.Mendell J, Johnson L, Ridout G, He L, Chen S. An open-label, phase 1 study to evaluate the effects of hepatic impairment on edoxaban pharmacokinetics. Eur Heart J. 2012;33:343. doi: 10.1002/jcph.550. [DOI] [PubMed] [Google Scholar]
- 44.Pascual J, Liano F, Ortuno J. The elderly patient with acute renal failure. J Am Soc Nephrol. 1995;6(2):144–153. doi: 10.1681/ASN.V62144. [DOI] [PubMed] [Google Scholar]
- 45.Bansal N, Fan D, Hsu CY, Ordonez JD, Marcus GM, Go AS. Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation. 2013;127(5):569–574. doi: 10.1161/CIRCULATIONAHA.112.123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hylek EM, D’Antonio J, Evans-Molina C, Shea C, Henault LE, Regan S. Translating the results of randomized trials into clinical practice: the challenge of warfarin candidacy among hospitalized elderly patients with atrial fibrillation. Stroke. 2006;37(4):1075–1080. doi: 10.1161/01.STR.0000209239.71702.ce. [DOI] [PubMed] [Google Scholar]
- 47.Inui TS, Parina R, Chang DC, Inui TS, Coimbra R. Mortality after ground-level fall in the elderly patient taking oral anticoagulation for atrial fibrillation/flutter: a long-term analysis of risk versus benefit. J Trauma Acute Care Surg. 2014;76(3):642–649. doi: 10.1097/TA.0000000000000138. [DOI] [PubMed] [Google Scholar]
- 48.Collins CE, Witkowski ER, Flahive JM, Anderson FA, Jr, Santry HP. Effect of preinjury warfarin use on outcomes after head trauma in Medicare beneficiaries. Am J Surg. 2014;208(4):544–549. doi: 10.1016/j.amjsurg.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gellad WF, Grenard JL, Marcum ZA. A systematic review of barriers to medication adherence in the elderly: looking beyond cost and regimen complexity. Am J Geriatr Pharmacother. 2011;9(1):11–23. doi: 10.1016/j.amjopharm.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferguson C, Inglis SC, Newton PJ, Middleton S, Macdonald PS, Davidson PM. The caregiver role in thromboprophylaxis management in atrial fibrillation: a literature review. Eur J Cardiovasc Nurs. 2015;14(2):98–107. doi: 10.1177/1474515114547647. [DOI] [PubMed] [Google Scholar]
- 51.Deitelzweig S. Care transitions in anticoagulation management for patients with atrial fibrillation: an emphasis on safety. Ochsner J. 2013;13(3):419–427. [PMC free article] [PubMed] [Google Scholar]
- 52.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 53. Clinicaltrials.gov [database on the Internet] Study to evaluate the safety, pharmacokinetics and pharmacodynamics of BI 655075 administered alone or with dabigatran etexilate. [Accessed June 8, 2016]. Available from: http://clinicaltrials.gov/show/NCT01688830.
- 54.van Ryn J, Litzenburger T, Waterman A, et al. Dabigatran anticoagulant activity is neutralized by an antibody selective to dabigatran in in vitro and in vivo models. J Am Coll Cardiol. 2011;57(14s1):E1130. [Google Scholar]
- 55.Praxbind® (idarucizumab) injection, for intravenous use [prescribing information] Boehringer Ingelheim; [Accessed June 8, 2016]. Available from: http://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Praxbind/Praxbind.pdf. [Google Scholar]
- 56.Crowther M, Kitt M, Lorenz T, et al. A phase 2 randomized, double-blind, placebo-controlled trial of PRT064445, a novel, universal antidote for direct and indirect factor Xa inhibitors. J Thromb Haemost. 2013;11(s2):30. [Google Scholar]
- 57.Laulicht B, Bakhru S, Lee C, et al. Small molecule antidote for anticoagulants. Circulation. 2012;126:A11395. [Google Scholar]
- 58.Perosphere Inc Antidote for new oral anticoagulants – PER977. [Accessed June 8, 2016]. Available from: http://perosphere.com/content/research/#p7HGMpc_1_2.
- 59.Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006;151(3):713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 60.Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58(4):395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363(19):1875–1876. doi: 10.1056/NEJMc1007378. [DOI] [PubMed] [Google Scholar]
- 62.Fox KA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. 2011;32(19):2387–2394. doi: 10.1093/eurheartj/ehr342. [DOI] [PubMed] [Google Scholar]