Abstract
To describe the appearance of a scleral-derived feeder vessel in a highly myopic eye with secondary choroidal neovascularization (CNV) as visualized on both en face high-speed swept-source (SS) optical coherence tomography angiography (OCTA) prototype, and a commercially available spectral-domain (SD) OCTA, with the corresponding en face and cross-sectional structural OCT images. In this case report, a 60-year-old white male presented with high myopia and secondary CNV in the right eye, previously treated with anti-vascular endothelial growth factor, and was imaged on both SD-OCT and SS-OCT. The neovascular complex could be visualized on both devices. Structural en face SS-OCT images demonstrated a large choroidalscleral feeder vessel that was not visualized with SD-OCT. The authors concluded that structural en face SS-OCT better visualizes scleral feeder vessel compared to SD-OCT due to the longer wavelength (~1,050 nm) with increased choroidal penetration and decreased sensitivity roll-off in the SS-OCT system.
INTRODUCTION
Type 2 choroidal neovascularization (CNV) is a relatively common complication in myopic patients that can result in significant visual loss. Detection of CNV by fluorescein angiography or indocyanine-green angiography requires intravenous administration of a dye, which may limit frequent application in the clinical setting.1
Optical coherence tomography angiography (OCTA) is a noninvasive imaging technique that enables high-speed, high-resolution, and depth-resolved imaging of the retinal and choroidal vasculatures. Images are generated by comparing the decor-relation signal (differences in the backscattered OCT signal) between rapidly repeated cross-sectional OCT scans to identify blood flow. OCTA imaging can be performed with either spectral-domain (SD) or swept-source (SS) technologies.2–12 The first recently U.S. Food and Drug Administration-approved devices for OCTA are SD devices. However, SS prototype devices are available and have been shown to be especially valuable to image the choroid. OCTA has been utilized to diagnose the presence of CNV in various pathological conditions of the eye.13,14 Using OCTA to image a myopic CNV can be difficult to interpret due to image artifacts caused by the increased axial ocular length, as well as artifacts such as blocked sign by the hemorrhage in the CNV or by the presence of pigment epithelial detachment.
This is a case report of a scleral-based feeder vessel in a highly myopic patient with secondary CNV imaged on en face high-speed SS-OCTA prototype, and a commercially available SD-OCTA, with the corresponding en face and cross-sectional structural OCT images.
CASE REPORT
A 60-year-old white male with high myopia presented in February 2015 with acutely decreased vision in the right eye; best-corrected visual acuity (BCVA) was 20/70. Funduscopy exam revealed myopic degeneration in both eyes with a central Förster-Fuchs retinal spot in the right eye, and a retinal break without retinal detachment in the left eye. Fluorescein angiography showed leakage due to the CNV in the right eye. The SD-OCTA (Optovue, Freemont, CA), operating at an approximately 840-nm wavelength with a rate of 70,000 A-scans per second, identified the CNV complex, and the cross-sectional OCT scan showed subretinal fluid (Figure 1C).15 The patient was treated with intravitreal anti-vascular endothelial growth factor (VEGF) bevacizumab 1.25 mg (Avastin; Genentech, South San Francisco, CA) at this visit. He subsequently received two additional anti-VEGF treatments during the following 2 months.16
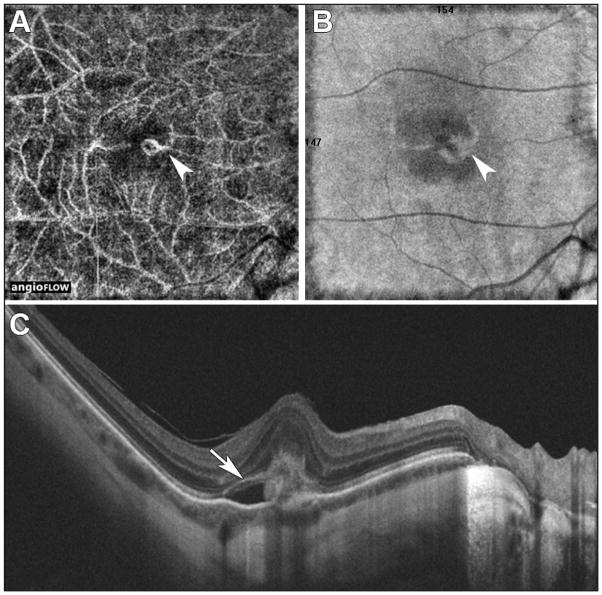
Figure 1.
High myopia and secondary choroidal neovascularization (CNV) active in the right eye. Optical coherence tomography angiography (OCTA) 6 mm × 6 mm on spectral-domain (SD)-OCTA device showing the CNV (A) (white head arrow) surrounded with projection artifacts or decorrelation tails. On structural en face OCT (B), white head arrow indicates the CNV and the feeder vessel projection. The OCT B-scan high-definition one-line scan using SD-OCT shows the CNV (C) (white arrow) with pigment epithelium detachment and subretinal fluid.
Examination at the most recent visit in November 2015 showed inactive CNV on clinical examination. At this time, the patient had no visual complaints; BCVA was 20/30 in the right eye and 20/25 in the left eye. A commercially available SD-OCTA (Carl Zeiss Meditec, Dublin, CA) that operates at an approximately 840-nm wavelength and 68,000 A-scans per second was performed at this visit, showing no fluid on structural cross-sectional OCT scan. One large feeder vessel could be seen extending downward through the choroid on the corresponding en face image (Figure 2).
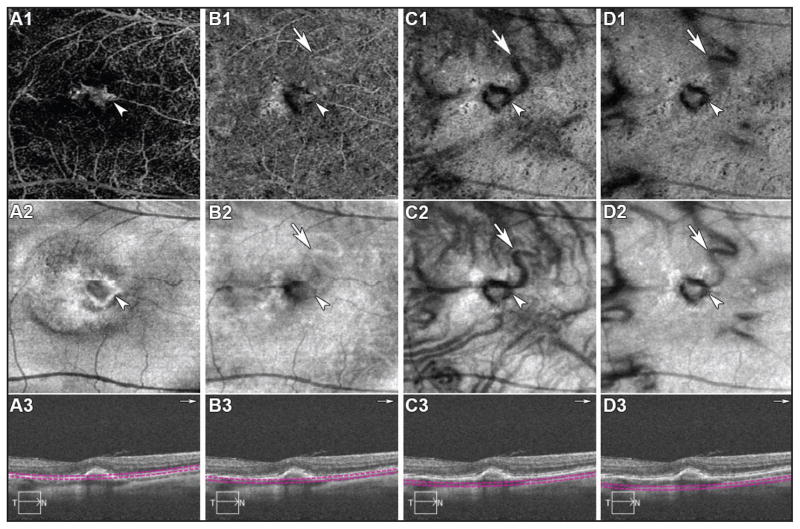
Figure 2.
Spectral-domain optical coherence tomography angiography (SD-OCTA) from the right eye demonstrates multiples depths of scans (A, B, C, D). OCTA (upper row), en face structural OCT (middle row), and cross-sectional OCT (lower row) are shown. (A, B, C, D) White head arrows indicate the choroidal neovascularization and white arrows indicate the feeder vessel in all scans.
Signed informed consent was obtained and the patient was examined with the SS-OCT device, which is an ultra–high-speed, long-wavelength prototype that uses a high speed vertical cavity surface emitting laser as the light source and operates at a ~1,050 nm wavelength achieving a speed of 400,000 A-scans per second (prototype developed at the Massachusetts Institute of Technology, Cambridge, MA) and deployed to New England Eye Center, Boston (Figure 3). All OCTA scans were centered at the fovea. The SS-OCT en face image showed a large, tortuous feeder vessel extending below the retinal pigment epithelium and penetrating into the sclera (video available at www.Healio.com/OSLIRetina). This vessel was not readily appreciated on SD-OCT scans. The patient has been followed with continued preservation of visual acuity and CNV activity has not recurred so far.
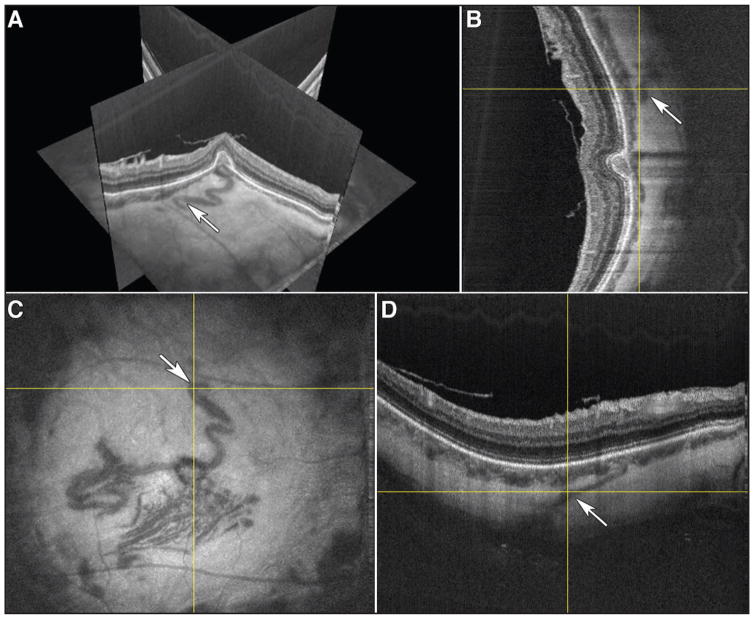
Figure 3.
(A) 3-D Orthoplane view using 3-D Slicer (a free open-source software application for medical image computing available at http://www.slicer.org) from the right eye with X-fast and Y-fast swept-source optical coherence tomography (SS-OCT) cross-sectional scans, on top of structural en face OCT centered on the choroidal neovascularization (CNV). (B, C, D) Orthogonal view with the (C) en face structural scan and the corresponding (D) X-fast OCT cross-sectional scan and the (B) Y-fast cross-sectional scan. White arrow (A, B, C, D) indicates the feeder vessel.
DISCUSSION
In this case, the patient was imaged on SD-OCT and SS-OCT systems. The latter uses longer wavelengths and has less variation in sensitivity with depth compared to SD-OCT, which enables deeper penetration into the choroid.13,17
This case study demonstrates that OCTA and the corresponding structural en face OCT in addition to cross-sectional scans can be helpful in visualizing the morphology of the feeder vessels in myopic CNV. This report also demonstrates that the feeder vessel connected to the sclera in high myopic CNV can be better visualized on structural en face SS-OCT. The signal level of structural en face SD-OCT image was limited at the level of the choroidalscleral interface, whereas OCTA in both SS and SD technologies was not able to document the feeder vessel throughout its pathway deep through the choroid and the sclera.18 The dark appearance of the large choroidal vessel is due to the thresholding artifact. Thresholding is a background image processing step applied to OCTA images to distinguish flow from noise. In areas where there is low OCT signal penetration, such as the choroid and sclera, OCTA decoration signals cannot be reliably interpreted because white pixels that are noise may appear as flow. In a case report, Querques et al. demonstrated a relationship between a similar large vessel and lacquer cracks. This may support our finding since lacquer cracks are often related to CNV in high myopic patients.19,20 The greater dynamic range, decreased sensitivity roll-off, and longer wavelength of the SS-OCT technology allows better in vivo visualization compared to SD-OCT, as it is less prone to signal attenuation through deeper tissues.13
In conclusion, using structural en face SS-OCT imaging, we described the visualization of a feeder vessel in CNV secondary to high myopia connecting to a scleral vessel. Structural en face SD-OCT was not able to document such finding, and neither OCTA.
Acknowledgments
Supported by the Massachusetts Lions Club; a grant from the Macular Vision Research Foundation, New York; National Institute of Health grants R01-EY011289-29, NIH R01-EY011289-28, R44-EY022864-03, and R01-CA075289-17; and Air Force Office of Scientific Research AFOSR grants FA9550-10-1-0551 and FA9550-12-1-0499.
Footnotes
Drs. Louzada and Novais are researchers supported by the CAPES Foundation, Ministry of Education of Brazil, Brasília, DF, Brazil. Dr. Ferrara is an employee of Genentech and has stock/stock options with Roche. Dr. Fujimoto received grants from the National Institutes of Health and the Air Force Office of Scientific Research during the conduct of this study; he is also a scientific advisor for and has stock options with Optovue and receives royalties from a patent owned by MIT and licensed to Optovue. Dr. Duker is a consultant for and receives research funding from Carl Zeiss Meditec and Optovue. The remaining authors report no relevant financial disclosures.
References
- 1.Kotsolis AI, Killian FA, Ladas ID, Yannuzzi LA. Fluorescein angiography and optical coherence tomography concordance for choroidal neovascularisation in multifocal choroidtis. Br J Ophthalmol. 2010;94(11):1506–1508. doi: 10.1136/bjo.2009.159913. [DOI] [PubMed] [Google Scholar]
- 2.Makita S, Hong Y, Yamanari M, Yatagai T, Yasuno Y. Optical coherence angiography. Opt Express. 2006;14(17):7821–7840. doi: 10.1364/oe.14.007821. [DOI] [PubMed] [Google Scholar]
- 3.Fingler J, Schwartz D, Yang C, Fraser SE. Mobility and transverse flow visualization using phase variance contrast with spectral domain optical coherence tomography. Opt Express. 2007;15(20):12636–12653. doi: 10.1364/oe.15.012636. [DOI] [PubMed] [Google Scholar]
- 4.Tao YK, Davis AM, Izatt JA. Single-pass volumetric bidirectional blood flow imaging spectral domain optical coherence tomography using a modified Hilbert transform. Opt Express. 2008;16(16):12350–12361. doi: 10.1364/oe.16.012350. [DOI] [PubMed] [Google Scholar]
- 5.An L, Wang RK. In vivo volumetric imaging of vascular perfusion within human retina and choroids with optical microangiography. Opt Express. 2008;16(15):11438–11452. doi: 10.1364/oe.16.011438. [DOI] [PubMed] [Google Scholar]
- 6.Mariampillai A, Standish BA, Moriyama EH, et al. Speckle variance detection of microvasculature using swept-source optical coherence tomography. Opt Lett. 2008;33(13):1530–1532. doi: 10.1364/ol.33.001530. [DOI] [PubMed] [Google Scholar]
- 7.Vakoc BJ, Lanning RM, Tyrrell JA, et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat Med. 2009;15(10):1219–1223. doi: 10.1038/nm.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu L, Chen Z. Doppler variance imaging for three-dimensional retina and choroid angiography. J Biomed Opt. 2010;15(1):016029. doi: 10.1117/1.3302806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonathan E, Enfield J, Leahy MJ. Correlation mapping method for generating microcirculation morphology from optical coherence tomography (OCT) intensity images. J Biophotonics. 2011;4(9):583–587. doi: 10.1002/jbio.201000103. [DOI] [PubMed] [Google Scholar]
- 10.Blatter C, Klein T, Grajciar B, et al. Ultrahigh-speed noninvasive widefield angiography. J Biomed Opt. 2012;17(7):070505. doi: 10.1117/1.JBO.17.7.070505. [DOI] [PubMed] [Google Scholar]
- 11.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitudedecorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710–4725. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi W, Mohler KJ, Potsaid B, et al. Choriocapillaris and choroidal microvasculature imaging with ultrahigh speed OCT angiography. PLoS One. 2013;8(12):e81499. doi: 10.1371/journal.pone.0081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novais EA, Adhi M, Moult EM, et al. Choroidal neovascularization analyzed on ultra-high speed swept source optical coherence tomography angiography compared to spectral domain optical coherence tomography angiography. Am J Ophthalmol. 2016;164:80–88. doi: 10.1016/j.ajo.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moult E, Choi W, Waheed NK, et al. Ultrahigh-speed swept-source OCT angiography in exudative AMD. Ophthalmic Surg Lasers Imaging Retina. 2014;45(6):496–505. doi: 10.3928/23258160-20141118-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Carlo TE, Bonini Filho MA, Chin AT, et al. Spectral-domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmology. 2015;122(6):1228–1238. doi: 10.1016/j.ophtha.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36(4):331–335. [PubMed] [Google Scholar]
- 17.Adhi M, Liu JJ, Qavi AH, et al. Choroidal analysis in healthy eyes using swept-source optical coherence tomography compared to spectral domain optical coherence tomography. Am J Ophthalmol. 2014;157(6):1272–1281. e1. doi: 10.1016/j.ajo.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara D, Mohler KJ, Waheed N, et al. En face enhanced-depth swept-source optical coherence tomography features of chronic central serous chorioretinopathy. Ophthalmology. 2014;121(3):719–726. doi: 10.1016/j.ophtha.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Querques G, Corvi F, Balaratnasingam C, et al. Lacquer cracks and perforating scleral vessels in pathologic myopia: a possible causal relationship. Am J Ophthalmol. 2015;160(4):759–766. e2. doi: 10.1016/j.ajo.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Klein RM, Green S. The development of lacquer cracks in pathologic myopia. Am J Ophthalmol. 1988;106(3):282–285. doi: 10.1016/0002-9394(88)90362-5. [DOI] [PubMed] [Google Scholar]