Abstract
Objective
Elevated inflammation has been repeatedly observed in posttraumatic stress disorder (PTSD), and it may drive the development of both psychiatric symptoms and physical comorbidities. However, it is not clear if elevated inflammation is a feature of both remitted and current PTSD, and little is known about relationships between specific clusters of PTSD symptoms and inflammation. Exaggerated threat sensitivity, as indexed by threat reactivity and avoidance of perceived threats, may be particularly closely associated with inflammation.
Methods
We assessed PTSD symptoms and threat sensitivity using the Clinician Administered PTSD Scale in 735 Veterans Affairs patients (35% current PTSD; 16% remitted PTSD) who participated in the Mind Your Heart Study (mean age = 59±11; 94% male). High sensitivity C-reactive protein (hsCRP), white blood cell count (WBC), and fibrinogen were used as indices of inflammation. Analysis of covariance models with planned contrasts were used to examine differences in inflammation by PTSD status, adjusting for age, sex, race, kidney function and socioeconomic status.
Results
Individuals with current PTSD had significantly higher hsCRP and WBC than patients with no history of PTSD, but there were no significant differences in inflammatory markers between those with remitted versus no history of PTSD. Within patients with current PTSD, higher threat reactivity was independently associated with higher hsCRP (β = .16, p = .01) and WBC count (β = .24, <.001), and higher effortful avoidance was associated with higher fibrinogen (β = .13, p = .04).
Conclusion
Our data indicate that elevated inflammation may be a feature of current, but not remitted, PTSD. Within patients with PTSD, higher threat reactivity was also associated with elevated inflammation. A better understanding of the relationship between threat sensitivity and inflammation may inform interventions for patients with PTSD.
Keywords: avoidance, C-reactive protein, fibrinogen, inflammation, posttraumatic stress disorder, threat reactivity, threat sensitivity, trauma, veterans, white blood cell count
Posttraumatic stress disorder (PTSD) is a common and disabling disorder that affects approximately 7–10% of the general population and 10–30% of military veterans (Boscarino, 2006; Dohrenwend et al., 2007; Seal et al., 2008). Increased systemic inflammation may contribute to PTSD-related increased risk for chronic physical diseases, including cardiovascular (Cohen et al., 2009a), neurodegenerative (Yaffe et al., 2010), and autoimmune disorders (Boscarino, 2004; Boscarino et al., 2010; O’Donovan et al., 2015b). Accumulating evidence indicates that inflammation may also provoke psychiatric symptoms seen in PTSD (Dantzer et al., 2008; Raison et al., 2013; Slavich and Irwin, 2014). In line with this hypothesis, several studies have reported that PTSD is associated with elevated inflammation. A better understanding of the association between PTSD and inflammation could lead to the development of novel therapies to improve mental and physical health in trauma-exposed individuals.
Multiple studies have found that individuals with current PTSD display elevated levels of systemic inflammatory markers, including C-reactive protein (CRP), white blood cell count (WBC), and fibrinogen (Boscarino and Chang, 1999; Heath et al., 2013; Hoge et al., 2009; Plantinga et al., 2013; Spitzer et al., 2010; Vaccarino et al., 2010; Vidovic et al., 2010; Vidovic et al., 2007; von Kanel et al., 2006; von Kanel et al., 2007). Several lines of evidence suggest that the observed relationship between PTSD and inflammation could be bidirectional. First, traumatic stress exposure itself as well as PTSD symptoms could promote inflammation via the stress response. Studies have demonstrated that inflammation is increased chronically in individuals exposed to traumatic stress and acutely in response to standardized psychosocial stressors (Black, 2002; Cohen et al., 2007; Danese et al., 2007; Dekaris et al., 1993; O’Donovan et al., 2012; Steptoe et al., 2007). Second, inflammation may be causally involved in the development of PTSD symptoms. Animal models and experimental human studies demonstrate that inflammatory activity can evoke symptoms analogous to some of the core symptoms of PTSD (Chen et al., 2013; Dantzer et al., 2008; Engler et al., 2011; Inagaki et al., 2012; Raison et al., 2013). There is also some evidence that elevated inflammation, as indexed by higher levels of the inflammatory marker C-reactive protein (CRP), increases risk for the development of PTSD following trauma exposure (Eraly et al., 2014).
To date, most research examining the relationship between PTSD and inflammation have compared individuals with current PTSD to those without current PTSD. Less attention has been paid to examining inflammation in those with a past history of PTSD that has gone into remission. Among the small studies that have examined inflammation in those with remitted PTSD, one indicated lower levels of some inflammatory markers in individuals with remitted compared to no history of PTSD (Kawamura et al., 2001), and one indicated that individuals with remitted PTSD had levels of inflammatory markers similar to those of controls and significantly lower than those of individuals with current PTSD (Gill et al., 2013). Thus, it remains unclear if elevated inflammation is a correlate of current PTSD symptomatology or a stable factor indexing either vulnerability to develop PTSD or a lingering consequence of PTSD.
Although most studies have observed elevated inflammation in PTSD, there are some notable exceptions finding either similar or even lower levels of inflammatory proteins in individuals with current PTSD compared to healthy controls (O’Donovan et al., 2015a; Sondergaard et al., 2004; von Kanel et al., 2010). These inconsistent findings in the literature may be because PTSD is a heterogeneous disorder and elevated inflammation is likely to be present in only a subset of patients with PTSD. For example, evidence from studies with small sample sizes indicates that some categories of PTSD symptoms may be more strongly associated with elevated inflammation than others, though results have been mixed (Ironson et al., 1997; von Kanel et al., 2006). In general, these studies have used standard re-experiencing, avoidance, and hyperarousal clusters of symptoms from the Diagnostic and Statistical Manual-IV to classify PTSD symptoms (APA, 2000). While these categorizations are highly useful in the clinical setting, they may be less useful in the effort to uncover the underlying biological basis of specific domains of dysfunction because symptoms in a single category may not arise from a common underlying pattern of dysfunction (Insel et al., 2010). Among the various domains identified in the National Institute of Mental Health’s Research Domain Criteria (RDoC), threat sensitivity is particularly important in the context of the relationship between PTSD and inflammation. Patients with PTSD differ in their levels of threat sensitivity, and both threat reactivity and avoidance have been linked with elevated inflammation in clinical and animal research studies (Chen et al., 2013; Engler et al., 2011; Inagaki et al., 2012; Michopoulos et al., 2015). However, little is known about the relationship between threat sensitivity and inflammation in individuals with PTSD.
In the present study, we assessed inflammation as indexed by hsCRP, WBC, and fibrinogen in a large sample of Veterans Affairs (VA) patients with current, remitted, or no history of PTSD. To our knowledge, our sample of 735 patients is the first to include large numbers of patients with both current and remitted PTSD in a single study. In addition, we explored associations of these inflammatory markers with threat reactivity and avoidance in individuals with current PTSD. No prior studies have attempted to examine if PTSD symptoms specifically related to threat sensitivity are associated with inflammation. Given that adverse health behaviors may play a key role in the relationship between PTSD and inflammation (O’Connor and Irwin, 2010; Spoormaker and Montgomery, 2008; Talbot et al., 2013; Zen et al., 2012), we also examined BMI, smoking, physical inactivity, sleep quality, and alcohol use as potential contributors to the association of PTSD and threat sensitivity with inflammation. We hypothesized that current, but not remitted PTSD would be associated with elevated inflammation, and that exaggerated threat reactivity and avoidance would be associated with elevated levels of inflammatory markers in individuals with current PTSD.
Methods
Participants
The Mind Your Heart Study is a prospective cohort study designed to examine the effects of PTSD on health outcomes in VA patients. Between February 2008 and June 2010, data were gathered from adult patients recruited from outpatient clinics affiliated with two Bay Area Departments of VA Medical Centers: the San Francisco VA Medical Center and the VA Palo Alto Health Care System, California. Patients who planned on leaving the area within three years or who did not have stable mailing or contact information for follow-up were excluded during the recruitment process. Because the study involved an exercise treadmill test (see Turner et al., 2013 for details), potential participants were also excluded if they had a myocardial infarction in the prior six months or if they were unable to walk one block. If participants reported symptoms of acute illness, their appointment was rescheduled. Targeted mailings were used to oversample for people with current and remitted PTSD. Overall, 1020 patients were recruited and assessed for eligibility. Of these, 104 were ineligible and 170 were eligible but did not complete enrollment, leaving 746 patients who enrolled in the study. Eleven patients were excluded from the present analyses because they did not have complete data on their PTSD assessments or because the supervising psychologist had concerns regarding the accuracy of their PTSD diagnoses. Thus, the sample for the present study includes 735 VA patients. The study was approved by the Committee on Human Research at the University of California, San Francisco, and all participants provided written informed consent.
Psychiatric Diagnoses
The Clinician-Administered PTSD Scale (CAPS), a structured interview measure that corresponds to DSM-IV criteria for PTSD, was used to assess PTSD symptoms. The CAPS has excellent convergent and discriminant validity, diagnostic utility and inter-item and inter-rater reliability and is highly sensitive to clinical change, based on a review of over 200 studies (Weathers et al., 2001). Interviewers assessed symptoms experienced in the previous month for current PTSD, and symptoms experienced during the worst episode associated with the subject’s self-identified worst traumatic event for lifetime PTSD. We used the standard DSM-IV scoring rule to determine symptom positivity, requiring a score of at least 1 for frequency and 2 for intensity (Weathers et al., 2001). In addition to full PTSD, we assessed partial PTSD, which has been associated with significant impairment in health and functioning (Marshall et al., 2001; Sayer et al., 2010). Though there are multiple definitions of partial PTSD, we chose a conservative definition: meeting criteria for the re-experiencing symptom cluster and either the avoidance or hyperarousal symptom cluster (Blanchard et al., 1994) as well as all other CAPS criteria. We also required this group to have a total CAPS score of ≥40, which is defined by the authors of the CAPS as moderate or threshold PTSD (Weathers et al., 2001). Twenty-eight participants in this study met these criteria for partial PTSD; similar to prior analyses, they were combined with participants with full PTSD in our analyses (Cohen et al., 2013; Turner et al., 2013; Wingenfeld et al., 2015). All diagnoses were made by trained clinical interviewers who calibrated their assessments at weekly case consensus meetings, supervised by an experienced PhD-level clinical psychologist.
To assess comorbid psychiatric diagnoses, we used the depression and anxiety modules of the World Health Organization CIDI Computer Assisted Personal Interview (Version 21.1.1), which generated diagnoses based on DSM-IV criteria for major depressive disorder (MDD) and generalized anxiety disorder (GAD).
Inflammatory Markers
Morning fasting venous blood samples were drawn from participants to measure levels of inflammatory biomarkers. High sensitivity C-reactive protein (hsCRP) was measured using a BN II nephelometer (Seimens Health Care Diagnostics, Tarrytown, New York) with interassay coefficients of variation < 5%. White blood cell (WBC) count, measured as the number of WBC/volume of blood, was determined using the Beckman Coulter LH 750 analyzer (Beckman Coulter, Inc., California) with an interassay coefficient of variation < 2%. Fibrinogen levels were determined from plasma and serum samples. Serum fibrinogen concentrations were obtained by the Clauss assay with coefficients of variation < 3%. Dilutions of the plasma standard (of known concentrations) were clotted with a high concentration of thrombin, with the resultant clotting time being proportional to the fibrinogen concentration. The clotting time of the participant’s plasma was used to read the fibrinogen concentration from the standard curve. The standard assay range is from 60 to 10,000 mg/dL and the interassay and intraassay coefficient of variation were both < 3%.
Threat Sensitivity
Based on a published model of threat sensitivity (O’Donovan et al., 2013), we created measures of threat reactivity and threat avoidance using four items from the CAPS to index threat reactivity and two to index threat avoidance. For threat reactivity, we included psychological and physiological reactivity to triggers (B4 and B5), conceptualizing triggers as perceived threats, as well as hypervigilance (D4) and startle (D5). For threat avoidance, we included only effortful avoidance symptoms related to avoidance of external and internal trauma reminders, also conceptualizing reminders as perceived threats (C1 and C2). Based on the validated scoring system for the CAPS, we added together intensity and frequency scores for each individual CAPS item and then created a total scores for threat reactivity and avoidance by adding together the total scores for each of the included items.
Covariates
Self-report questionnaires were administered to all patients to assess medical history and demographic information, including age, sex, ethnicity, income, and education (Whooley et al., 2008). Because impaired kidney function has been associated with systemic inflammation, such as elevated C-reactive protein (Abraham et al., 2009; Ix et al., 2003; Stuveling et al., 2003), kidney dysfunction was assessed by estimated glomerular filtration rate (eGFR). This was calculated from serum creatinine levels obtained through fasting morning venous blood samples using the Modification of Diet in Rental Disease (MDRD) equation.
Standard questionnaires were used to assess health behaviors, including current tobacco and alcohol use, physical activity, and sleep quality (Cohen et al., 2009b). Participants were asked to indicate on a 6-point Likert Scale how often they engaged in vigorous activity for at least 15–20 minutes at a time: not at all, a little (1–2 times a month), fairly (3–4 times per month), quite (1–2 times a week), very (3–4 times a week), or extremely (5 or more times a week). The variable was then dichotomized, so that physical inactivity was defined as being not at all or a little active. Self-report has been shown to be a reliable, valid, and accurate method of assessing physical activity with single-response items, in particular, having demonstrated excellent construct validity (Aadahl et al., 2007; Ainsworth et al., 1993; Bowles et al., 2004; Jackson et al., 2007; Kurtze et al., 2008). Participants self-rated their overall sleep quality in the prior month, using a 5-point Likert Scale from very bad to very good, with ratings of very or fairly bad being coded as poor sleep quality. The Alcohol Use Disorders Identification Test-consumption questions (AUDIT-C) was used to identify possible problematic alcohol use, using the recommended cutoff scores of 3 for women and 4 for men (Bradley et al., 1998; Bush et al., 1998). BMI was assessed by a trained technician. Self-reported use of immunosuppressant or steroid medication was recorded and then verified by chart review.
Statistical Analyses
Differences in sample characteristics among patients with current PTSD, remitted PTSD, and no PTSD were estimated using analysis of variance (ANOVA) for continuous variables and chi-square tests for dichotomous variables (see Table 1). Analysis of covariance (ANCOVA) models with follow-up planned contrasts were used to test for differences in levels of inflammatory markers across the current, remitted, and never PTSD groups with adjustment for covariates. Hierarchical linear regression was then used to evaluate associations of overall PTSD severity, threat reactivity, and avoidance with inflammatory markers among patients with current PTSD. All models were adjusted for age, sex, race, education, income, and kidney function. We also examined the potential role of health behaviors by including them as covariates if they were associated with inflammatory markers at p ≤ .10 in Student’s t-tests or Pearson’s correlations in the full sample. We did not adjust for comorbid psychiatric conditions, such as depression and anxiety, because their symptoms overlap with PTSD symptoms and therefore their presence can act as a marker of PTSD severity. In addition, we conducted sensitivity analyses excluding individuals with hsCRP levels greater than 10 mg/L and use of immunosuppressant and steroid medications to test whether these individuals impacted our pattern of results. For all analyses, hsCRP, WBC count, and fibrinogen were log-transformed to produce a more normal distribution. All statistical analyses were performed with SPSS 23.0.
Table 1.
Demographic and Clinical Characteristics Based on Posttraumatic Stress Disorder (PTSD) Status
| Characteristic | No PTSD (N = 363) |
Remitted PTSD (N = 115) |
Current PTSD (N = 257) |
||||
|---|---|---|---|---|---|---|---|
| Mean | ±SD or (%) |
Mean or N |
±SD or (%) |
Mean or N |
±SD or (%) |
pa | |
| Age (years) | 59.5 | ±12.2 | 56.5 | ±10.9 | 58.0 | ±10.1 | .04 |
| Male sex | 355 | (97.8) | 109 | (94.8) | 229 | (89.1) | <.001 |
| White race | 209 | (58.2) | 66 | (58.4) | 151 | (59.9) | .91 |
| College graduate | 119 | (33.0) | 26 | (22.6) | 73 | (28.5) | .09 |
| Annual income <$20,000 | 123 | (34.1) | 36 | (31.6) | 72 | (28.2) | .31 |
| Current smoker | 79 | (21.9) | 37 | (32.5) | 63 | (25.1) | .08 |
| Problematic Alcohol use | 159 | (45.0) | 48 | (42.5) | 89 | (36.8) | .13 |
| Physical inactivity | 76 | (21.1) | 33 | (28.9) | 101 | (40.2) | <.001 |
| Poor sleep quality | 90 | (25.0) | 38 | (33.3) | 153 | (61.0) | <.001 |
| eGFRb (mL/min/1.73 m2) | 85.0 | ±23.2 | 85.5 | ±20.1 | 79.2 | ±20.6 | .002 |
| Body mass index (kg/m2) | 28.4 | ±5.3 | 29.0 | ±5.6 | 30.1 | ±6.0 | <.001 |
| Major depressive disorder | 7 | (1.9) | 14 | (12.4) | 48 | (20.0) | <.001 |
| Generalized anxiety disorder | 6 | (1.7) | 5 | (4.4) | 18 | (17.5) | .002 |
| C-reactive protein (mg/L)c | 2.53 | ±5.94 | 2.80 | ±4.30 | 2.85 | ±3.67 | .004 |
| White blood cell count (× 109/L)c | 6.35 | ±1.91 | 6.43 | ±2.04 | 6.81 | ±2.21 | .03 |
| Fibrinogen (mg/dL)c | 333.8 | ±67.5 | 325.1 | ±74.5 | 343.3 | ±71.5 | .04 |
P values based on one-way ANOVAs or chi square tests
eGFR, estimated glomerular filtration rate
Mean and SD values for inflammatory markers are presented as non-transformed values
Results
Sample Characteristics
Of the 735 patients analyzed, 257 (35.0%) had current PTSD, 115 (15.6%) had remitted PTSD, and 363 (49.4%) had no PTSD (Table 1). The groups differed on most study variables, including sociodemographic, co-morbidities, and health behaviors.
PTSD and Inflammation
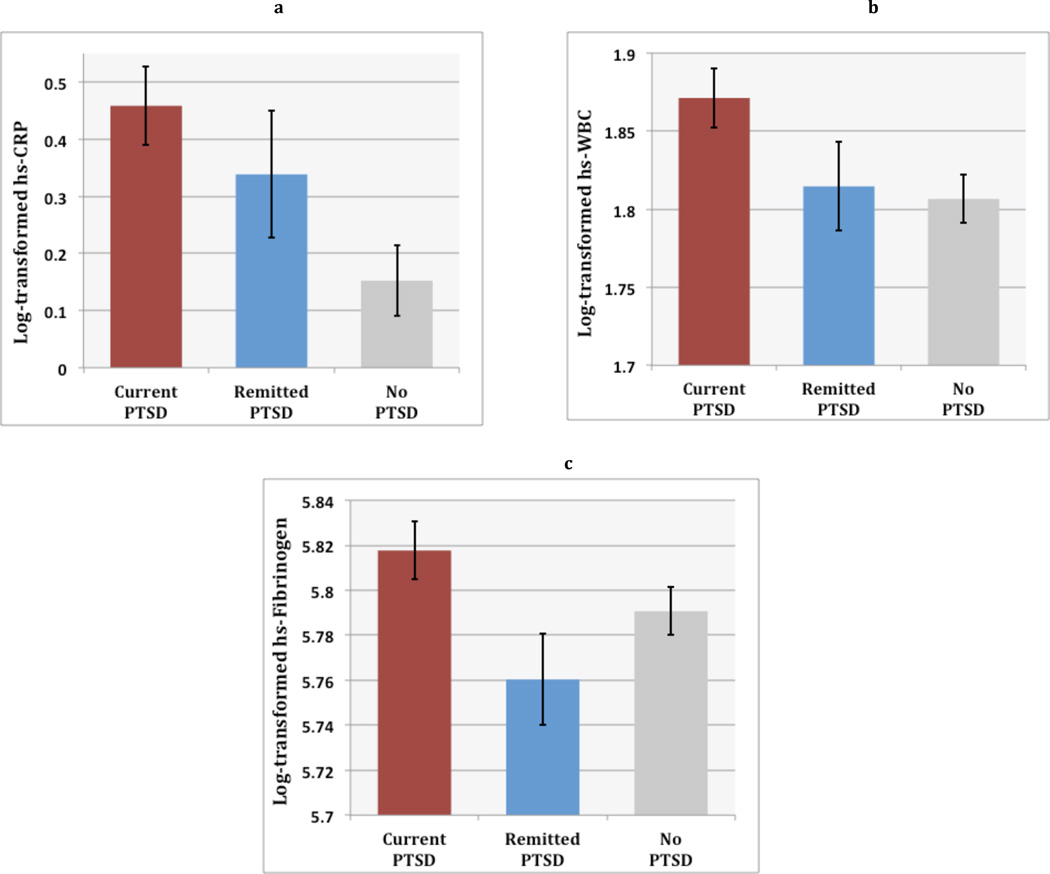
In unadjusted analyses, there were significant differences among the three PTSD groups in levels of hsCRP [F(2,725) = 5.46, p = .004], WBC [F(2,728) = 3.71, p = .03], and fibrinogen [F(2,728) = 3.24, p = .04]. Follow-up planned comparisons indicated that those with current PTSD had higher levels of hsCRP (Estimate = −.306, SE = .094, p = .001) and WBC count (Estimate = −.065, SE = .024, p = .008), but not fibrinogen (Estimate = −.027, SE = .017, p = .11), compared to those with no PTSD. There were no significant group differences in inflammation between individuals with remitted versus no history of PTSD (Figure 1). In models adjusted for age, sex, race, education, income, and kidney function, group differences remained significant for hsCRP [F(2, 700) = 2.95, p = .05] and WBC count [F(2, 705) = 3.00, p = .05], but differences in levels of fibrinogen became nonsignificant ([F(2,705) = 1.99, p = .14]. Adjusted follow-up planned comparisons showed the same pattern of results, with higher hsCRP and WBC count in current PTSD compared to no PTSD (Estimate = −.227, SE = .097, p = .02; Estimate = −.060, SE = .025, p = .02), and no other significant group differences.
Figure 1. PTSD Status and Inflammation.
Figure shows mean levels and standard error of the mean of log-transformed inflammatory markers in groups with current versus remitted versus no history of posttraumatic stress disorder (PTSD). Results indicated that individuals with current, but not remitted, PTSD had significantly elevated levels of high sensitivity C-reactive protein (hsCRP) and white blood cell (WBC) count than those with no history of PTSD.
The following behaviors had at least a trend-level relationship (p ≤ .10) with inflammatory markers in the full sample and were included as potential contributors to the relationship between PTSD status and inflammation: BMI, smoking, physical inactivity, and poor sleep for hsCRP; and BMI, smoking, alcohol use, physical inactivity, and poor sleep for WBC count. After adjustment for potential mediators, PTSD group status was no longer significantly associated with the inflammatory markers, indicating that health behaviors may play a role in the relationship between current PTSD status and inflammatory markers.
PTSD Severity and Inflammation
Finally, we examined if current PTSD severity as indexed by total CAPS score was associated with inflammatory activity within individuals with current PTSD. In unadjusted analyses, higher severity of PTSD was associated with higher levels of WBC (β = .16, p = .01) and fibrinogen β = .11, p = .05), but not higher levels of hsCRP (β = .05, p = .44). Adjusted analyses demonstrated a similar pattern, with higher severity of PTSD associated with higher levels of WBC (β = .18, p = .005) and fibrinogen (β = .15, p = .02), but not higher levels of hsCRP (β = .08, p = .21). In contrast with our group effect, adjusting for health behaviors did not change this general pattern of findings (WBC: β = .18, p = .004; fibrinogen: β = .12, p = .06; hsCRP β = .03, p = .66).
Threat Sensitivity
Mean scores were 16.65 (SD = 6.21, range 0 – 31) on the threat reactivity measure and 9.01 (SD = 3.69, range 0 −16) on the avoidance measure in our sample of participants with current PTSD. Scores on our two threat sensitivity measures, threat reactivity and avoidance, were positively and significantly correlated amongst those with current PTSD (r = .34, p < .001). Avoidance was positively and significantly correlated with Criterion B (r = .34, p < .001), Criterion C (r = .60, p < .001), and Criterion D (r = .28, p < .001), as well as with the total CAPS severity score (r = .53). Threat reactivity was more highly correlated with CAPS scores, showing the following associations with Criterion B (r = .69, p < .001), Criterion C (r = .44, p < .001), Criterion D (r = .72, p < .001), and the total CAPS severity score (r = .77, p < .001). Although these represent relatively strong correlations between threat reactivity, avoidance and the subscale scores, which would be expected given the overlap in items, the amount of variance shared varies from a low of 8% (avoidance and Criterion D) to a high of 52% (threat reactivity and Criterion D) between our scales and the traditional subscales of the CAPS. Thus, despite strong associations among these variables, they appear distinct from one another.
Threat Sensitivity and Inflammation
To examine whether threat reactivity and avoidance were associated with levels of inflammatory markers among patients with current PTSD, we conducted hierarchical linear regression models in patients with current PTSD only. Results of unadjusted and adjusted models are displayed in Table 2. Results indicated that higher threat reactivity was associated with significantly elevated levels of hsCRP (β = .16, p = .01) and WBC count (β = .24, <.001), and with trend-level elevated levels of fibrinogen (β = .11, p = .07), independent of age, sex, race, education, income, and kidney function. Furthermore, greater severity of avoidance was associated with elevated levels of fibrinogen (β = .18, p = .04), but not hsCRP or WBC, independent of age, sex, race, education, income, and kidney function.
Table 2.
Associations between threat reactivity and avoidance and inflammatory markers in patients with current PTSD
| Unadjusted |
Adjusted for confoundsb |
|||||
|---|---|---|---|---|---|---|
| FChange | β | 95% CI | FChange | β | 95% CI | |
| Threat reactivitya | ||||||
| hsCRP | 5.23* | .14 | .003 – .05 | 6.54* | .16 | .006 – .05 |
| WBC count | 11.85** | .21 | .004 – .02 | 14.60** | .24 | .005 – .02 |
| Fibrinogen | 1.97 | .09 | −.001 – .01 | 3.30 | .11 | .000 – .01 |
| Avoidancea | ||||||
| hsCRP | 1.16 | .07 | −.02 – .06 | 0.92 | .06 | −.02 – .05 |
| WBC count | 1.74 | .08 | −.003 – .02 | 0.73 | .06 | −.01 – .01 |
| Fibrinogen | 6.58* | .16 | .002 – .02 | 4.22* | .18 | .000 – .01 |
Inflammation markers were log-transformed to produce a more normal distribution
Confounds include age, sex, race, education, income, and kidney function.
p < .05;
p < .01
We repeated our analyses controlling for health behaviors associated with each inflammatory marker at p ≤ .10. Health behaviors used in these adjustments included: BMI, smoking, physical activity, and sleep for hsCRP; BMI, smoking, alcohol, physical activity, and sleep for WBC count; and smoking, BMI, and activity for fibrinogen. Higher threat reactivity maintained its association with both elevated hsCRP (β = .13, p = .03) and WBC count (β =.21, p = .001). However, greater severity of avoidance was no longer associated with elevated fibrinogen (β = .09, p = .17).
Secondary Analyses
In secondary analyses, we examined if extremely high levels of hsCRP or use of immunosuppressant or steroid medications impacted our pattern of results. There were 8 people (3.4%) with levels of hsCRP greater than 10 mg/L in the current PTSD group, 4 (3.5%) in the past PTSD group, and 12 (3.3%) in the no history of PTSD group; these group differences were not statistically significant (Chi Square = .04, p = .98). In follow-up sensitivity analyses, excluding individuals with hsCRP levels > 10mg/L, our pattern of significant and non-significant results remained the same for the group differences and for the threat sensitivity and avoidance analyses. There were 3 people (1.2%) on immunosuppressant or steroid medications in the current PTSD group, 1 (0.9%) in the past PTSD group, and 9 (2.5%) in the no history of PTSD group; these group differences were also not statistically significant (Chi Square = 2.13, p = .35). We also reran our analyses excluding patients on immunosuppressant and steroid medications at the time of the study. In these models, our pattern of significant and non-significant results remained the same, with the exception of the association between avoidance and fibrinogen, which became marginally non-significant (β = .12, p = .06).
Discussion
In this large cohort of VA patients with and without PTSD, we found that patients with current PTSD had elevated levels of hsCRP and WBC. In contrast, levels of inflammation in those with remitted PTSD were not significantly different from those with no history of PTSD. Greater overall severity of PTSD was also associated with elevated levels of WBC and fibrinogen. These data support the idea that elevated inflammation may be more closely related to current PTSD symptomatology than to the vulnerability to develop PTSD. Although the relationship between PTSD status and inflammation was independent of our confounders, health behaviors did appear to contribute to elevated inflammation in PTSD. Among patients with current PTSD, exaggerated threat reactivity was associated with elevated hs-CRP and WBC and effortful avoidance was associated with elevated fibrinogen, independent of confounders. Health behaviors appeared to contribute to the relationship between avoidance and fibrinogen, but not the relationship between threat reactivity and hsCRP or WBC. Overall, our data show elevated inflammation as a feature of current, but not remitted, PTSD and also that threat reactivity may be independently associated with inflammatory markers within patients with PTSD.
The results of our analyses indicated elevated levels of hsCRP and WBC in individuals with current PTSD, as well as independent associations between exaggerated threat reactivity and the same two markers. In contrast, fibrinogen was not elevated in current PTSD, but was higher with greater overall PTSD severity and increased avoidance. This lack of convergence across all three markers may indicate that different aspects of inflammatory activity are increased in association with specific aspects of PTSD. WBCs are major mediators of the inflammatory response and an important source of circulating pro-inflammatory cytokines. The acute phase protein CRP is secreted from the liver in response to various inflammatory cytokines, particularly interleukin-6 (IL-6) (Du Clos, 2000). Thus, both WBC and hsCRP may reflect circulating levels of pro-inflammatory cytokines. Fibrinogen, on the other hand, is also an acute phase protein, but it is primarily involved in clotting and may index other inflammatory pathways. It is imperative that future research studies identify the specific aspects of inflammation that are upregulated in response to traumatic stress, perhaps using gene expression assays and bioinformatics to uncover specific transcriptional profiles underlying elevated levels of inflammatory proteins (O’Donovan et al., 2011).
Taken together with previous research linking exaggerated fear-potentiated startle— psychophysiological responses involved in fear, anxiety, and the perception of threat— with elevated hsCRP in PTSD (Michopoulos et al., 2015), our data indicate that threat sensitivity might be one aspect of PTSD that is associated with inflammatory activity. Specifically, we observed that exaggerated threat reactivity and effortful avoidance were associated with specific aspects of inflammation in our sample with current PTSD. Exaggerated threat sensitivity may lead to repeated and prolonged activation of biological stress responses, which may in turn increase inflammation by activating pro-inflammatory transcription factor signaling pathways (O’Donovan et al., 2013; O’Donovan et al., 2011). Considering pre-clinical research data showing that inflammation increases the sensitivity of threat-responsive brain areas, including the amygdala, our data is also consistent with the idea that inflammation may contribute to exaggerated threat sensitivity in PTSD (Chen et al., 2013; Engler et al., 2011; Inagaki et al., 2012). Our data also tentatively suggest that different aspects of threat sensitivity, specifically threat reactivity and avoidance, may be associated with different inflammatory markers.
Several different causal models could underlie our results. For example, it is possible that individuals who develop elevated levels of inflammation following trauma consequently become more sensitive to threat, which in turn promotes the development of more severe PTSD symptoms in general. It is also possible that individuals who develop higher levels of threat sensitivity following trauma are prone to repeated and prolonged activation of the inflammatory response, and that elevated inflammation then promotes other symptoms of PTSD. Unfortunately, our cross-sectional study does not lend itself to addressing such questions. Future longitudinal and experimental studies will be needed to shed light on these causal relationships.
Limitations
The present study benefits from a large sample of VA patients, oversampled for individuals with current and remitted PTSD. Data included multiple widely available inflammatory markers and data on PTSD symptoms collected by trained, calibrated and supervised clinical interviewers. However, several limitations should be considered. First, the cross-sectional observational nature of the study precludes conclusions about causal direction in the relationships between PTSD symptoms and inflammatory markers. It also precludes mediation models, which would test some of our proposed models of associations among the variables included in this study. Experimental pre-clinical and clinical studies will be necessary to uncover causal relationships among trauma exposure, PTSD, threat sensitivity and inflammatory activity. Second, although assays for the markers we employed in this study have the benefit of being available in most clinical settings, we lack data on the specific pro- and anti-inflammatory signaling pathways underlying our results. Third, we assessed health behaviors at a single timepoint using mostly self-report measures. Prospective studies employing objective measures of health behaviors, such as activity and sleep monitors as well as diet logs, will be necessary to more carefully assess the contribution of health behaviors to elevated inflammation in PTSD. Fourth, although in secondary analyses, we found that excluding any patients on immunosuppresants or steroids did not alter our results, we did not exclude patients with major medical conditions, which could bias our findings. Fifth, the symptoms of PTSD and major depression overlap such that it is impossible to fully tease apart the independent associations of depression and PTSD with inflammation in our study. Our focus on dimensional constructs, such as threat sensitivity, should move us away from these issues that arise when we focus on categorical diagnoses. Finally, results are based on VA patients and may not generalize beyond this population.
Conclusions
Our data provide some of the strongest evidence to date that elevated inflammation may be a feature of current PTSD, and that exaggerated threat sensitivity may be a key aspect of PTSD associated with inflammatory activity. Causal directions in these relationships remain unknown. As increased systemic inflammation has been associated with numerous chronic physical illnesses, reducing inflammation through behavioral or pharmacologic treatments may improve disease risk and quality of life in patients with PTSD. Moreover, given known associations of inflammation with threat sensitivity, anti-inflammatory treatments may have effects on both physical and mental health in PTSD.
Highlights.
Current PTSD associated with elevated inflammation in Veterans Affairs patients.
Similar levels of inflammation in patients with remitted PTSD and no PTSD history.
Exaggerated threat sensitivity independently associated with elevated inflammation.
Data support dimensional approach to inflammation-psychiatric symptom links.
Acknowledgments
The Mind Your Heart Study is funded by the National Heart, Lung, and Blood Institute (K23 HL 094765-0), the Irene Perstein Foundation, the American Heart Association Clinical Research Program, and Departmental funds from the University of California, San Francisco. Society in Science – The Branco Weiss Fellowship and NIH-KL2TR000143 and NIH-K01MH109871 provided support to AOD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aadahl M, Kjaer M, Kristensen JH, Mollerup B, Jorgensen T. Self-reported physical activity compared with maximal oxygen uptake in adults. Eur J Cardiovasc Prev Rehabil. 2007;14:422–428. doi: 10.1097/HJR.0b013e3280128d00. [DOI] [PubMed] [Google Scholar]
- Abraham G, Sundaram V, Sundaram V, Mathew M, Leslie N, Sathiah V. C-Reactive protein, a valuable predictive marker in chronic kidney disease. Saudi J Kidney Dis Transpl. 2009;20:811–815. [PubMed] [Google Scholar]
- Ainsworth BE, Jacobs DR, Jr, Leon AS. Validity and reliability of self-reported physical activity status: the Lipid Research Clinics questionnaire. Med Sci Sports Exerc. 1993;25:92–98. doi: 10.1249/00005768-199301000-00013. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain Behav Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Hickling EJ, Taylor AE, Loos WR, Gerardi RJ. Psychological morbidity associated with motor vehicle accidents. Behav Res Ther. 1994;32:283–290. doi: 10.1016/0005-7967(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Boscarino JA. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Ann N Y Acad Sci. 2004;1032:141–153. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- Boscarino JA. Posttraumatic stress disorder and mortality among U.S. Army veterans 30 years after military service. Ann Epidemiol. 2006;16:248–256. doi: 10.1016/j.annepidem.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, Chang J. Electrocardiogram abnormalities among men with stress-related psychiatric disorders: implications for coronary heart disease and clinical research. Ann Behav Med. 1999;21:227–234. doi: 10.1007/BF02884839. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, Forsberg CW, Goldberg J. A twin study of the association between PTSD symptoms and rheumatoid arthritis. Psychosom Med. 2010;72:481–486. doi: 10.1097/PSY.0b013e3181d9a80c. [DOI] [PubMed] [Google Scholar]
- Bowles HR, FitzGerald SJ, Morrow JR, Jr, Jackson AW, Blair SN. Construct validity of self-reported historical physical activity. Am J Epidemiol. 2004;160:279–286. doi: 10.1093/aje/kwh209. [DOI] [PubMed] [Google Scholar]
- Bradley KA, Bush KR, McDonell MB, Malone T, Fihn SD. Screening for problem drinking : Comparison of CAGE and AUDIT. J Gen Intern Med. 1998;13:379–388. doi: 10.1046/j.1525-1497.1998.00118.x. [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Chen J, Song Y, Yang J, Zhang Y, Zhao P, Zhu XJ, Su HC. The contribution of TNF-alpha in the amygdala to anxiety in mice with persistent inflammatory pain. Neurosci Lett. 2013;541:275–280. doi: 10.1016/j.neulet.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Cohen BE, Marmar C, Ren L, Bertenthal D, Seal KH. Association of cardiovascular risk factors with mental health diagnoses in Iraq and Afghanistan war veterans using VA health care. JAMA. 2009a;302:489–492. doi: 10.1001/jama.2009.1084. [DOI] [PubMed] [Google Scholar]
- Cohen BE, Marmar CR, Neylan TC, Schiller NB, Ali S, Whooley MA. Posttraumatic stress disorder and health-related quality of life in patients with coronary heart disease: findings from the Heart and Soul Study. Arch Gen Psychiatry. 2009b;66:1214–1220. doi: 10.1001/archgenpsychiatry.2009.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BE, Neylan TC, Yaffe K, Samuelson KW, Li Y, Barnes DE. Posttraumatic stress disorder and cognitive function: findings from the mind your heart study. J Clin Psychiatry. 2013;74:1063–1070. doi: 10.4088/JCP.12m08291. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekaris D, Sabioncello A, Mazuran R, Rabatic S, Svoboda-Beusan I, Racunica NL, Tomasic J. Multiple changes of immunologic parameters in prisoners of war. Assessments after release from a camp in Manjaca, Bosnia. JAMA. 1993;270:595–599. [PubMed] [Google Scholar]
- Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. Continuing controversy over the psychological risks of Vietnam for U.S. veterans. J Trauma Stress. 2007;20:449–465. doi: 10.1002/jts.20296. [DOI] [PubMed] [Google Scholar]
- Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32:274–278. doi: 10.3109/07853890009011772. [DOI] [PubMed] [Google Scholar]
- Engler H, Doenlen R, Engler A, Riether C, Prager G, Niemi MB, Pacheco-Lopez G, Krugel U, Schedlowski M. Acute amygdaloid response to systemic inflammation. Brain Behav Immun. 2011;25:1384–1392. doi: 10.1016/j.bbi.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Eraly A, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, O’Connor DT, Baker DG. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA psychiat. 2014;71(4):423–431. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JM, Saligan L, Lee H, Rotolo S, Szanton S. Women in recovery from PTSD have similar inflammation and quality of life as non-traumatized controls. J Psychosom Res. 2013;74:301–306. doi: 10.1016/j.jpsychores.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Heath NM, Chesney SA, Gerhart JI, Goldsmith RE, Luborsky JL, Stevens NR, Hobfoll SE. Interpersonal violence, PTSD, and inflammation: potential psychogenic pathways to higher C-reactive protein levels. Cytokine. 2013;63:172–178. doi: 10.1016/j.cyto.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety. 2009;26:447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 2012;59:3222–3226. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Ironson G, Wynings C, Schneiderman N, Baum A, Rodriguez M, Greenwood D, Benight C, Antoni M, LaPerriere A, Huang HS, Klimas N, Fletcher MA. Posttraumatic stress symptoms, intrusive thoughts, loss, and immune function after Hurricane Andrew. Psychosom Med. 1997;59:128–141. doi: 10.1097/00006842-199703000-00003. [DOI] [PubMed] [Google Scholar]
- Ix JH, Shlipak MG, Liu HH, Schiller NB, Whooley MA. Association between renal insufficiency and inducible ischemia in patients with coronary artery disease: the heart and soul study. J Am Soc Nephrol. 2003;14:3233–3238. doi: 10.1097/01.asn.0000095642.25603.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AW, Morrow JR, Jr, Bowles HR, FitzGerald SJ, Blair SN. Construct validity evidence for single-response items to estimate physical activity levels in large sample studies. Res Q Exerc Sport. 2007;78:24–31. doi: 10.1080/02701367.2007.10599400. [DOI] [PubMed] [Google Scholar]
- Kawamura N, Kim Y, Asukai N. Suppression of cellular immunity in men with a past history of posttraumatic stress disorder. Am J Psychiatry. 2001;158:484–486. doi: 10.1176/appi.ajp.158.3.484. [DOI] [PubMed] [Google Scholar]
- Kurtze N, Rangul V, Hustvedt BE, Flanders WD. Reliability and validity of self-reported physical activity in the Nord-Trondelag Health Study: HUNT 1. Scand J Public Health. 2008;36:52–61. doi: 10.1177/1403494807085373. [DOI] [PubMed] [Google Scholar]
- Marshall RD, Olfson M, Hellman F, Blanco C, Guardino M, Struening EL. Comorbidity, impairment, and suicidality in subthreshold PTSD. Am J Psychiatry. 2001;158:1467–1473. doi: 10.1176/appi.ajp.158.9.1467. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, Gillespie CF, Ressler KJ. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am J Psychiatry. 2015;172:353–362. doi: 10.1176/appi.ajp.2014.14020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MF, Irwin MR. Links between behavioral factors and inflammation. Clinical Pharmacology and Therapeutics. 2010;87:479–482. doi: 10.1038/clpt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Chao LL, Paulson J, Samuelson KW, Shigenaga JK, Grunfeld C, Weiner MW, Neylan TC. Altered inflammatory activity associated with reduced hippocampal volume and more severe posttraumatic stress symptoms in Gulf War veterans. Psychoneuroendocrinology. 2015a;51:557–566. doi: 10.1016/j.psyneuen.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Cohen BE, Seal KH, Bertenthal D, Margaretten M, Nishimi K, Neylan TC. Elevated risk for autoimmune disorders in iraq and afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015b;77:365–374. doi: 10.1016/j.biopsych.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Neylan TC, Metzler T, Cohen BE. Lifetime exposure to traumatic psychological stress is associated with elevated inflammation in the Heart and Soul Study. Brain Behav Immun. 2012;26:642–649. doi: 10.1016/j.bbi.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Slavich GM, Epel ES, Neylan TC. Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neurosci Biobehav Rev. 2013;37:96–108. doi: 10.1016/j.neubiorev.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Sun B, Cole S, Rempel H, Lenoci M, Pulliam L, Neylan T. Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Dis Markers. 2011;30:123–132. doi: 10.3233/DMA-2011-0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga L, Bremner JD, Miller AH, Jones DP, Veledar E, Goldberg J, Vaccarino V. Association between posttraumatic stress disorder and inflammation: a twin study. Brain Behav Immun. 2013;30:125–132. doi: 10.1016/j.bbi.2013.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer NA, Noorbaloochi S, Frazier P, Carlson K, Gravely A, Murdoch M. Reintegration problems and treatment interests among Iraq and Afghanistan combat veterans receiving VA medical care. Psychiatr Serv. 2010;61:589–597. doi: 10.1176/ps.2010.61.6.589. [DOI] [PubMed] [Google Scholar]
- Seal KH, Metzler T, Gima K, Bertenthal D, Maguen S, Marmar CR. Growing burden of mental disorders among Iraq and Afghanistan Veterans: Trends and risk factors for mental health diagnoses in new users of VA healthcare, 2002–2008. 2008 Under Review. [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140:774–815. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondergaard HP, Hansson LO, Theorell T. The inflammatory markers C-reactive protein and serum amyloid A in refugees with and without posttraumatic stress disorder. Clin Chim Acta. 2004;342:93–98. doi: 10.1016/j.cccn.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Spitzer C, Barnow S, Volzke H, Wallaschofski H, John U, Freyberger HJ, Lowe B, Grabe HJ. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. J Psychiatr Res. 2010;44:15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, Montgomery P. Disturbed sleep in post-traumatic stress disorder: secondary symptom or core feature? Sleep Med Rev. 2008;12:169–184. doi: 10.1016/j.smrv.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Stuveling EM, Hillege HL, Bakker SJ, Gans RO, De Jong PE, De Zeeuw D. C-reactive protein is associated with renal function abnormalities in a non-diabetic population. Kidney Int. 2003;63:654–661. doi: 10.1046/j.1523-1755.2003.00762.x. [DOI] [PubMed] [Google Scholar]
- Talbot LS, Maguen S, Epel ES, Metzler TJ, Neylan TC. Posttraumatic stress disorder is associated with emotional eating. J Trauma Stress. 2013;26:521–525. doi: 10.1002/jts.21824. [DOI] [PubMed] [Google Scholar]
- Turner JH, Neylan TC, Schiller NB, Li Y, Cohen BE. Objective evidence of myocardial ischemia in patients with posttraumatic stress disorder. Biol Psychiatry. 2013;74:861–866. doi: 10.1016/j.biopsych.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Bremner JD, Afzal N, Veledar E, Goldberg J. Posttraumatic stress disorder is associated with higher C-reactive protein levels. J Am Coll Cardiol. 2010;55 A176.E1656-A1176.E1656. [Google Scholar]
- Vidovic A, Grubisic-Ilic M, Kozaric-Kovacic D, Gotovac K, Rakos I, Markotic A, Rabatic S, Dekaris D, Sabioncello A. Exaggerated platelet reactivity to physiological agonists in war veterans with posttraumatic stress disorder. Psychoneuroendocrinology. 2010;36:161–172. doi: 10.1016/j.psyneuen.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Vidovic M, Hisheh S, Schmitt LH. Cortisol and testosterone levels on a weekend and a work day in three mountain villages in the Selska Valley of northwest Slovenia. Ann Hum Biol. 2007;34:26–33. doi: 10.1080/03014460601054624. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Begre S, Abbas CC, Saner H, Gander ML, Schmid JP. Inflammatory biomarkers in patients with posttraumatic stress disorder caused by myocardial infarction and the role of depressive symptoms. Neuroimmunomodulation. 2010;17:39–46. doi: 10.1159/000243084. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Hepp U, Buddeberg C, Keel M, Mica L, Aschbacher K, Schnyder U. Altered blood coagulation in patients with posttraumatic stress disorder. Psychosom Med. 2006;68:598–604. doi: 10.1097/01.psy.0000221229.43272.9d. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res. 2007;41:744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, Ali S, Dowray S, Na B, Feldman MD, Schiller NB, Browner WS. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingenfeld K, Whooley MA, Neylan TC, Otte C, Cohen BE. Effect of current and lifetime posttraumatic stress disorder on 24-h urinary catecholamines and cortisol: results from the Mind Your Heart Study. Psychoneuroendocrinology. 2015;52:83–91. doi: 10.1016/j.psyneuen.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, Kluse M, Marmar C. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67:608–613. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen AL, Whooley MA, Zhao S, Cohen BE. Post-traumatic stress disorder is associated with poor health behaviors: Findings from the Heart and Soul Study. Health Psychol. 2012;31:194–201. doi: 10.1037/a0025989. [DOI] [PMC free article] [PubMed] [Google Scholar]