ABSTRACT
Extracellular S3-S4 linkers of domain IV (IVS3-S4) of L-type Ca2+ channels (CaV1) are subject to alternative splicing, resulting into distinct gating profiles serving for diverse physiological roles. However, it has remained elusive what would be the determining factor of IVS3-S4 effects on CaV1 channels. In this study, we systematically compared IVS3-S4 variants from CaV1.1-1.4, and discover that the flexibility of the linker plays a prominent role in gating characteristics. Chimeric analysis and mutagenesis demonstrated that changes in half activation voltage (V1/2) or activation time constant (τ) are positively correlated with the numbers of flexible glycine residues within the linker. Moreover, antibodies that reduce IVS3-S4 flexibility negatively shifted V1/2, emerging as a new category of CaV1 enhancers. In summary, our results suggest that the flexibility or rigidity of IVS3-S4 linker underlies its modulations on CaV1 activation (V1/2 and τ), paving the way to dissect the core mechanisms and to develop innovative perturbations pertaining to voltage-sensing S4 and its vicinities.
KEYWORDS: L-type Ca2+ channels, linker flexibility, S3-S4 loop, voltage-dependent activation, voltage sensing domain
Introduction
L-type Ca2+ channels (LTCCs), also named CaV1 family, play a crucial role in numerous physiological functions by mediating Ca2+ influx, including muscle contraction, hormone secretion, gene transcription, synaptic transmission and cardiac pacemaking.1 LTCC is formed as multimeric channel complex, by auxiliary subunits such as β and α2δ, and the prominent pore-forming α1 subunit, composed of 4 homologous but non-identical domains, each of which contains a series of 6 transmembrane α-helical segments, numbered S1–S6, which are linked by both intracellular and extracellular loops.2,3 Segments S1-S4 form voltage sensing domains (VSDs), and segments S5-S6 constitute the permeation pore.2 VSDs could act as transducers of transmembrane voltage potentials by positively charged S4, leading to subsequent conformational changes.4,5
The extracellular loops linking S3 and S4 (S3-S4 loops or linkers) may influence the function of VSDs presumably by controlling the downstream molecular interactions, e.g., between S3 and S4.6-10 It has been reported that certain subtypes of CaV channels, including CaV1.1, CaV1.2, CaV2.1 and CaV2.2 channels, are subject to alternative splicing of short exons within the S3-S4 loops of domain IV (IVS3-S4). These splice variants exhibit distinct gating characteristics, particularly the voltage-dependent activation, implying an important role of IVS3-S4 in VSD behaviors thus the channel gating.7,11-15 However, it is still unclear about the determining factor underlying differential modulations by diverse variants of the linker. Such determinant is potentially shared as common principles to different subtypes and variants across the LTCC family. It would be intriguing and beneficial to systematically compare all the family members, i.e., CaV1.1–1.4, in the context of IVS3-S4 modulations and mechanisms. First, IVS3-S4 of natural variants would be properly and quantitatively evaluated for its effects on gating, which are supposedly in part responsible for different gating profiles of CaV1 subtypes. Sporadic evidence on the IVS3-S4 effects has been reported, such as from CaV1.1 and CaV1.2, but it is imperative to extend to the whole CaV1 family. Second, the mechanisms of IVS3-S4 would help provide further insights into the working principles of VSDs and channel activations of CaV1 channels. Understanding toward voltage-dependent activation specific to CaV1 channels has been limited thus far.2,7,16 Third, new strategies could be devised to modulate CaV1 channels for potential therapeutics, e.g., small molecules or antibodies, by targeting and perturbing the key activation mechanism related to the IVS3-S4 loops.
In this study, we systematically examined and compared IVS3-S4 linkers across LTCCs, which contain the exons with different abundance of glycine residues (GX linkers). Further analysis by chimeric channels, mutagenesis and specific antibodies suggest that the flexibility of GX linker would be the major index to determine the mobility of VSD thus the potency of modulatory effects on CaV1 activation.
Results
IVS3-S4 loops from CaV1 channel family differentially modulate voltage-dependent activation
In LTCCs, 4 pore-forming α1 subunit isoforms (α1S, α1C, α1D and α1F) exhibit distinct biophysical properties and expression profiles, to accommodate different cellular-specific or tissue-specific needs.11,12,17-21 The IVS3-S4 loop of all α1 subunits contains the splicing exon: exon 29 in the skeletal muscle CaV1.1 channel, exon 33 in the cardiac CaV1.2 channel, exon 32 in the neuronal CaV1.3 channel, and exon 32 in the retinal CaV1.4 channel (Fig. 1A). The coding sequences of these exons (yellow colored) appear to differ in the length and also in the number of glycines (red colored). To examine the role of various IVS3-S4 loops in voltage-dependent activation, we constructed CaV1.3 chimeric channels by inserting corresponding exons from the 4 α1 subunits into a natural splice variant of α1DE321DΔ (lacking exon E321D), which has rather short linker and decent current in HEK cells. In response to membrane potentials (Vm) of the step protocol, current traces color-coded for Vm of −30 mV, −10 mV and +10 mV indicated significant differences in channel activation between α1DE321DΔ control and chimeric channels (Fig. 1B). For both rows of current traces, in accordance with longer exons/linkers, less activation could be achieved at particular Vm, indicated by the traces appearing at lower positions for −30 mV step (blue traces). As quantified by half-activation voltage (V1/2) values, the activation curves were significantly shifted to the positive direction by the linkers (Fig. 1C). Apparently the length of the linker plays an important role in the modulation of channel activation, as suggested by the comparison between α1DE321DΔ and α1DE291S, the latter of which has the longest linker and also the most significant V1/2 shift (ΔV1/2) (Fig. 1D). The positive shift of α1DE321F activation as compared with α1DE321DΔ can also be explained by their difference in the linker length. However, we noticed that although E321F is the shortest exon, the E321F linker also produced a significant ΔV1/2, just second to that of E291S. Closer examinations suggested that the richness of glycines within the linker could also play a prominent role, which led us to hypothesize that the flexibility of linkers modulates the activation of CaV1 channels.
Figure 1.
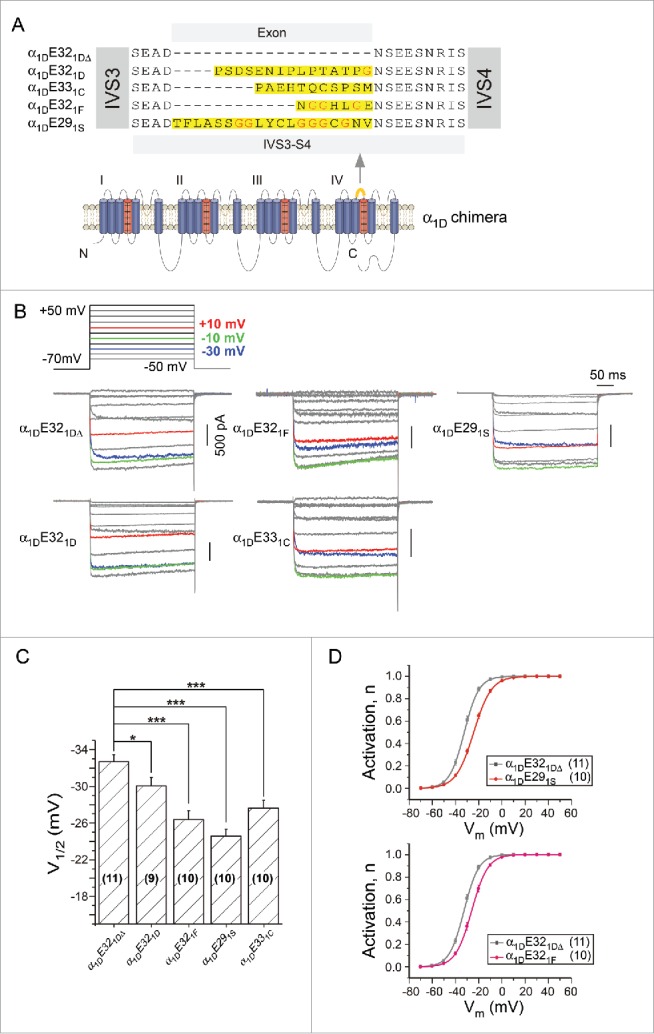
Comparison among different IVS3-S4 variants from CaV1 family. (A) Construction scheme for α1D chimeras targeting the splicing exon. Based on 2 natural splice variants of exon32: α1DE321DΔ and α1DE321D, additional chimeric channels were constructed with α1DE321DΔ as the backbone inserted into E32-equivalent exons from α1S, α1F and α1C, respectively. E32 or E32-equivalent exons are highlighted as yellow in both the sequence alignment and the cartoon of α1D topology. Glycine residues are marked in red. (B) Modulations of voltage-dependent activation of α1D-based chimeric channels. The voltage protocol was composed of 10 mV steps ranging from −50 mV to +50 mV. Representative whole-cell Ba2+ currents were from HEK293T cells expressing α1D chimeras, with traces at −30 mV, −10 mV and +10 mV colored as shown. (C) Comparison of V1/2 values (voltage at 50% activation, n) between α1DE321DΔ and α1D chimeras of α1DE321F, α1DE291S, and α1DE331C respectively. *, p < 0.05 and ***, p < 0.001. (D) Voltage dependence of channel activation of α1DE291S, or α1DE321F compared with that of α1DE321DΔ.
Enrichment of glycines within IVS3-S4 loops further attenuates voltage-dependent activation
To test the flexibility hypothesis, we gradually substituted original amino acids of exons E321F and E291S with glycines, achieving mutant channels containing different numbers of glycines: G3, G4 and G7; and G6, G10 and G19, based on α1DE321F and α1DE291S respectively (Fig. 2A). Glycine enrichment of IVS3-S4 loops further positively shifted the activation of the channels with more positive V1/2, evidenced from their exemplar traces before and after full glycine substitutions (Fig. 2B). Moreover, gradual changes of V1/2 can be observed from both groups of α1DE321F and α1DE291S channels, indicating an apparent trend of V1/2 modulations in accordance to the number of glycines (Fig. 2C). Glycines add more flexibility to the motif in the protein structure.22 We reason that the glycine-mediated flexibility may be the more direct factor underlying the voltage-dependent activation of channel variants containing different linkers.
Figure 2.
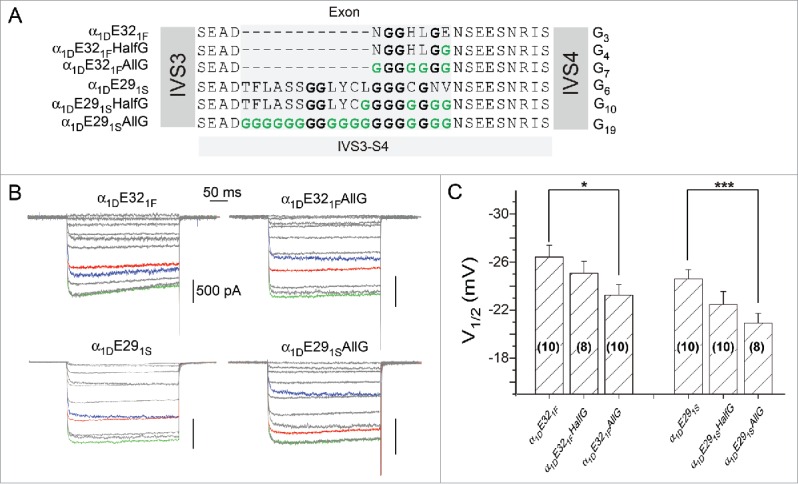
The strategy to change the flexibility of IVS3-S4 linkers by enrichment of glycine residues. (A) The summary of point mutations to glycine residues. Based on chimeric channels of α1DE321F and α1DE291S containing native variants of IVS3-S4, glycine (G) residues gradually replaced the original sites of amino acids for each chimera. According to the percentage of glycine residues, the constructs are named as HalfG or AllG. (B) Exemplar current traces of selected mutant channels in (A), in response to the same voltage protocols and the color scheme as in Figure 1B. Top row and bottom row are based on α1DE321F and α1DE291S, respectively. According to the number of glycine residues (GX), for top row from the left to right, X = 3 or 7; and X = 6 or 19 for the bottom row. The statistics of the group data is shown in (C), with the number of cells indicated for each chimera. *, p < 0.05 and ***, p < 0.001.
Positive correlations between the flexibility of GX linkers and the activation characteristics of V1/2 and τ
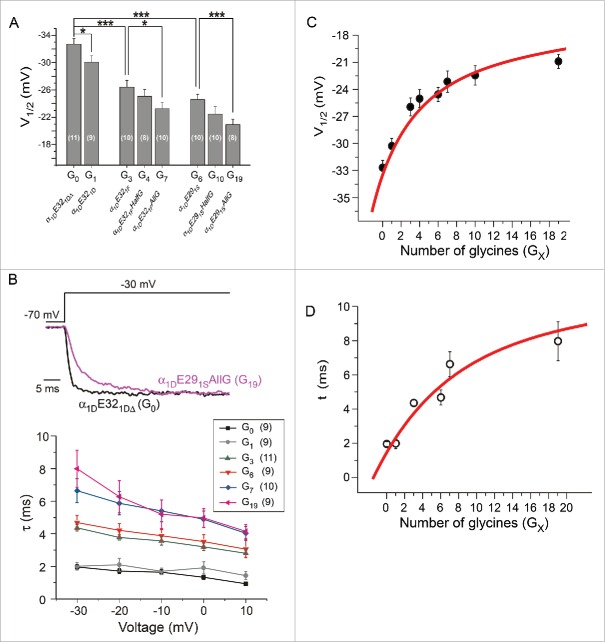
Collecting up all channel variants with different IVS3-S4 loops, our results show V1/2 values of the mutant channels became more positive with the number of glycine residues being increased (Fig. 3A). The shift in V1/2 was accompanied by the change in slope factor (S): 3.0 ± 0.2 (n = 11) for variant G0 and 3.6 ± 0.1 (n = 11) for variant G19, p < 0.01. This is within expectations as both V1/2 and S could be the manifestation of decreased apparent sensitivity to Vm along with increased number of glycines. Meanwhile, either insertion of native alternative splicing exons or enrichment of glycine residues slowed down the activation kinetics of the channel (Fig. 3B). In addition, the statistic results demonstrate that the number of glycines within IVS3-S4 loop is positively correlated with V1/2 and the time constant (τ) of the channel (Fig. 3C and D), confirming the flexibility of IVS3-S4 loop provided by glycine residues can significantly impair the activation of Cav1.3 channels.
Figure 3.
Effects of IVS3-S4 on CaV1.3 activation are correlated with the number of glycine residues of the linker. (A) Summary of V1/2 for various α1D channels including the native forms and chimeric mutants. These channel constructs were also named according to the number of glycines (GX) within the linker. *, p < 0.05 and ***, p < 0.001. (B) Flexibility of different GX linkers affected the kinetics of channel activation. At −30 mV, time constant (τ) values of current traces were fitted, as shown by the exemplars of G0 and G19 (upper), and summarized for voltages from −30 mV to +10 mV (lower). (C and D) Correlations between the linker flexibility (GX) and voltage-dependence (V1/2) or time-dependence (τ, at −30 mV) of activation. The red lines are visually appended to illustrate the trends in relation to GX and loop flexibility.
Antibodies that bind and constrain IVS3-S4 enhance channel activation by negative ΔV1/2
Inspired by prior report that antibodies binding onto extracellular loops could act as innovative channel modulators,23 we devised a similar strategy to target the IVS3-S4 linker based on our findings here, in hope to specifically modulate channel activation. As the proof of concept, we first inserted the His-tag (6 histidine residues) into IVS3-S4 loop of α1DE321DΔ to construct α1DE321DΔ6His, which would be recognized and bound by antibodies of Anti-His-tag right at the loop of IVS3-S4. TIRF imaging confirmed that only cells expressed with α1DE321DΔ6His channels, but not control cells, can be recognized by the antibody (Fig. 4A). As expected, the activation of α1DE321DΔ6His channels was significantly shifted to the left after incubating with Anti-His-tag, as evidenced by the exemplar traces (Fig. 4B) and the statistical analysis of the activation curves (Fig. 4C). Supposedly, the antibody constrains the flexibility of the loop, thus limits the randomized mobility of VSD, which would counteract with the rightward activation shift due to the flexible linker. As further assurance of our design, the effect of Anti-His-tag did not cause any significant shift of V1/2, for control channels (α1DE321DΔ without His-tag, p > 0.6) or under control conditions (intracellular application of Anti-His-tag, p > 0.5). In addition, we also performed another set of experiments based on the channel variant with G19 linker, which confirmed the antibody effects of activation enhancement (Fig. S1).
Figure 4.
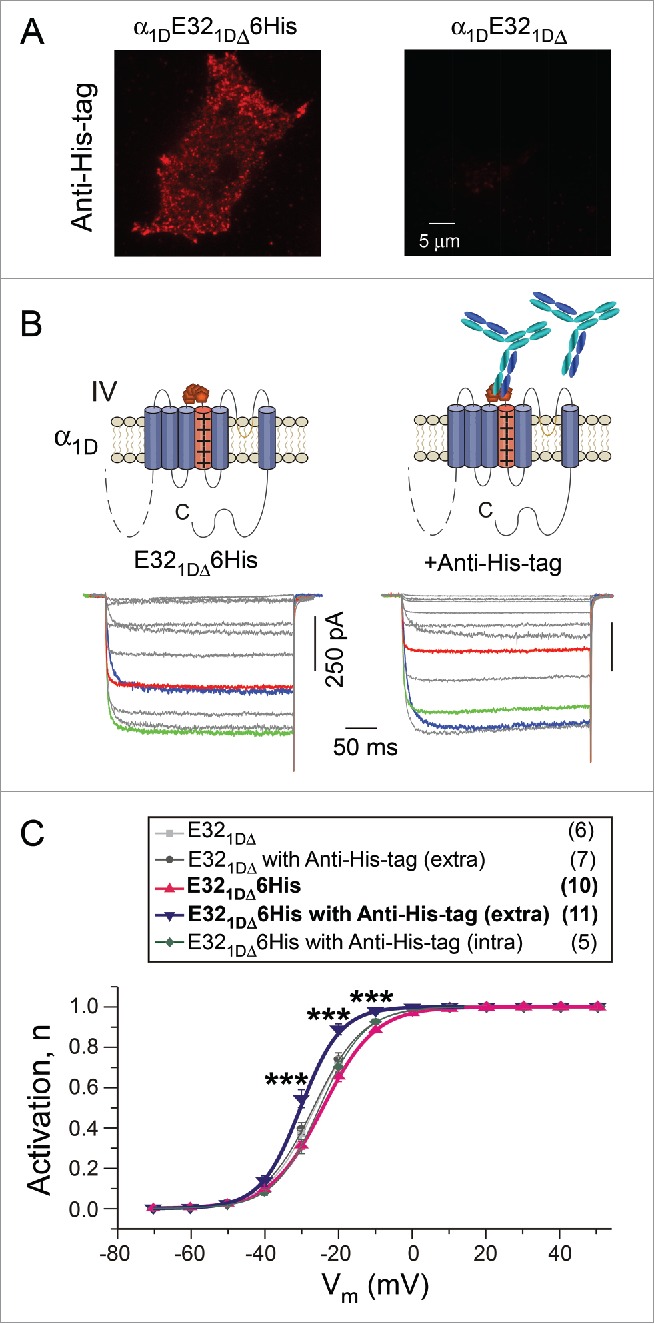
Antibody targeting IVS3-S4 loop modulated the activation of channels. (A) His-tag antibodies (Anti-His-tag) recognized α1DE321DΔ6His on the cell membrane, compared with α1DE321DΔ as the control group. (B) Exemplar current traces of the α1DE321DΔ6His channels without/with extracellular incubation (ex) of Anti-His-tag antibodies. The voltage protocol and color scheme are the same as in Figure 1B. (C) Voltage dependence of the activation curves. A significant leftward shift in α1DE321DΔ6His activation was evidenced without/with His-tag antibodies in the extracellular solution (extra), as indicated by the differences in the level of activation at particular voltages (***, p < 0.001). No difference existed among channels of E321DΔ, E321DΔ with antibodies extracellular applied (extra), E321DΔ6His, or E321DΔ6His with antibodies intracellular applied through pipettes (intra).
Effects on channel activation arising from IVS3-S4 and C-terminus are additive
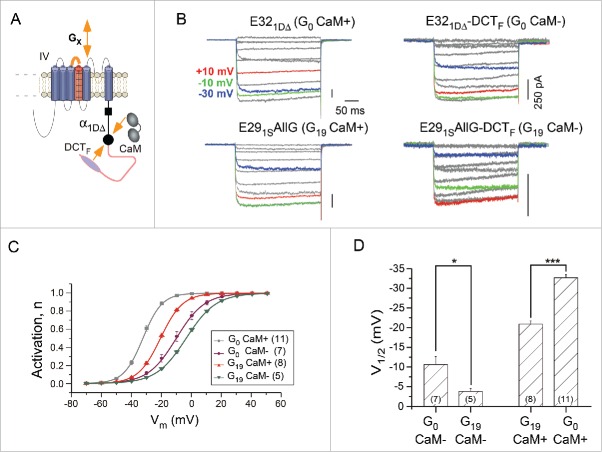
We next explored another established modulation of channel gating mediated by the competition between distal carboxyl terminus (DCT) and apo-calmodulin (Ca2+-free CaM)16,24 (Fig. 5A). One major phenotype according to the modulation profiles is the shift of activation: DCT such as DCTF (strong DCT from α1F) would positively shift V1/2 whereas CaM would leftward shift V1/2. Although the detailed mechanism about how CaM pre-associated channel affects the gating is unclear, a reasonable assumption is that channels fully bound with CaM reach the maximum activation (most negative V1/2); in parallel, every DCT-bound channel such as α1DΔ-DCTF is essentially CaM-less thus approaching the most positive V1/2 (rightward shifted). We were asking about the potential relationship between these 2 types of modulation: GX and CaM. DCTF caused additional shift of V1/2 for channels with either short (G0) or long (G19) linkers (Fig. 5B and C). From the perspective of GX effects, increasing number of glycines (enriched GX, from G0 to G19) produced further rightward shift for channels with (CaM+) or without (CaM-) pre-associated CaM. Alternative interpretations would be: for CaM- channels, GX changing from G0 to G19 made the channel even more difficult to open as compared with the V1/2 limit of “CaM-” channels; on the other hand, for CaM+ channels, GX changing from G19 to G0 negatively shifts the channel beyond the V1/2 limit of “G19 CaM+” channels (Fig. 5D). The latter view emphasizes the additive nature of IVS3-S4 and CaM effects, strongly suggesting that the 2 mechanisms should follow different (non-overlapped) downstreams in modulation of CaV1 activation.
Figure 5.
The additive effects on channel activation due to the flexible IVS3-S4 linker and DCT/CaM competition. (A) Two different mechanisms that modulate the voltage-dependent activation of CaV1.3 channels. In addition to the IVS3-S4 linker where the flexibility presumably modulates the function of VSD, CaV1.3 channels are also subject to the competitive tuning between DCT and CaM for which the channel activation is dependent on whether CaM is preassociated with the channel. Both GX linker (increasing flexibility) and competitive DCT (kicking-off apoCaM) would cause the positive shift of channel activation. (B and C) Exemplar traces for different combinations of GX and DCT effects. The native form of α1DE321DΔ (G0 CaM+) contains a DCT motif with much weaker strength in competition with CaM than DCT from α1F (DCTF). E291SAllG consists of the linker from exon 29 of α1S but with glycine substitutions (G19 CaM+). α1DΔE321DΔ-DCTF (G0 CaM-) is the chimeric channel fused with DCTF to α1DΔ, essentially replacing the DCT of the native form in α1DE321DΔ (G0 CaM+). α1DΔE291SAllG-DCTF (G19 CaM-) is to incorporate DCTF while containing the G19 linker within the loop. Experimental conditions and color schemes are similar to Figure 1B. (C and D) Comparison among the voltage dependence of the activation curves, for the 4 kinds of channels under tests. Activation curves (C) and statistics of V1/2 values (D) are shown for each channel subtypes. *, p < 0.05; ***, p < 0.001.
Discussion
This study focuses on the mechanism of action underlying the modulation of IVS3-S4 loop on voltage-dependent gating and provides the evidence to support the flexibility of IVS3-S4 loop as the major factor.
Implications on physiological roles of IVS3-S4 variants of CaV channels
Several earlier studies reported that S3-S4 loops of voltage-gated ion channels have significant impact on voltage dependence and temporal kinetics of activation.11-15,17,25 In both P/Q-type CaV2.1 and N-type CaV2.2 channels, alternatively spliced exons 31a contain only 6 nucleic acid bases, encoding NP or ET at IVS3-S4 loop, respectively. The presence of NP or ET causes slower activation kinetics for both channels,13-15 but with preferential distributions: NP of CaV2.1 in the central nervous system,15 and ET of CaV2.2 in the peripheral,13 suggesting specific neurons may utilize IVS3-S4 loop as one of the sophisticated mechanisms to gauge channel gating and Ca2+ signaling.
For CaV1.1 and CaV1.2 of LTCCs, different IVS3-S4 spliced variants also exist distinct distributions, suggesting LTCCs may also take advantage of IVS3-S4 to alter channel activities to control Ca2+ signals. In CaV1.1 channel, the variant lacking exon 29 is distributed at low levels in differentiated muscle, but abundantly expressed in myotubules, and this variant shows a significant left-shifted V1/2 and a substantially increased current density.17 For CaV1.2 channels, IVS3-S4 splice variants display different levels of expression in fetal and adult heart and brain, and all the IVS3-S4 variants demonstrate unmistakable V1/2 shifts.12 CaV1.3 splice variants exhibit distinct percentages of abundance in brain and neuroendocrine cells.20 For CaV1.4, splice variants are distributed not only in retina but also in the immune system.21
Systematic comparison across the whole CaV1 family
CaV1.3 and CaV1.4 channels are also subject to alternative splicing within IVS3-S4 loop, however, the gating modulation of this loop is lacking.19,20 In this work, we devised the chimeric analysis to symmetrically evaluate the functional roles of splicing exons within IVS3-S4 loops from the entire LTCC family. Results confirmed the previous observation of inhibitory effects on the channel activation, including positive V1/2 shift (Fig. 1), slower activation τ (Fig. 3), etc., but extended onto the whole CaV1 family. Also, the order of modulation strength as demonstrated by chimeric analysis is E291S > E321F > E331C > E321D, consistent with the activation profiles of CaV1 channels following the same order: CaV1.1 > CaV1.4 > CaV1.2 > CaV1.3,10,16,20,25 where CaV1.1 is the most hard-to-activate CaV1 and CaV1.3 has the most negative V1/2 (Fig. S2).
Mechanistic insights into the effects by IVS3-S4 loops
Regarding the mechanisms underlying IVS3-S4 modulation, it has been speculated that the length of the linker might be the key factor,12 which also appears to be consistent with our data. However, more thorough comparison and mutagenesis suggest that the flexibility of the loop would be the more direct index. Other properties, such as hydrophobicity, of the linker peptide might also underlie the GX modulation. So we examined the hydrophobicity of E321F and E291S, and both linkers turn out to be neural peptides; when the residues are completely replaced with glycines (low hydrophobic index, so producing neutral peptides), the richness of glycines strongly modulates the channel function (G7 and G19) while the overall hydrophobicity remains unaltered. Thus, it is unlikely that hydrophobicity plays any significant role in the GX effects in this work. Whether under certain circumstances the hydrophobicity of the linker could also cause appreciable effects on channel function, as an intriguing perspective, inviting future investigations.
The consensus has been reached that upon depolarization S4 should move to appropriate position (e.g., to interact with S3) and activate the channel (Fig. 6A and B). Meanwhile, S4 as part of the VSDs is also dynamic and subject to random movement which could be highly regulated by the flexible linker of IVS3-S4. It is unlikely that the GX linker directly interferes with the voltage-dependent VSD movement, but instead, adding glycines further randomizes VSD dynamics, reducing the chance for S3-S4 cooperation, under the conditions of both with and without depolarization. In this context, regardless of the actual perturbations, which could be the length or the number of glycines being increased, the same consequence would be caused: the flexibility of VSD is increased, so is the randomness of the movement, eventually the channel would end up with lower open probability at different Vm, i.e., more positive V1/2 or less activation (Fig. 6B). In this view, the degree of freedom in VSD movement (i.e., the number of glycines) would determine the effective concentration for the interaction,22 or in an alternative view the probability of S4 to appear in appropriate positions favoring the S3-S4 cooperation or interaction7. In this context, our study and related work from other groups are complementary to each other: we focus on changes in effective concentrations of the interacting peptides and they emphasize on changes in binding affinity between S3 and S4 motifs7,10, altogether supporting such putative reaction-based scheme we proposed (Fig. 6B).
Figure 6.
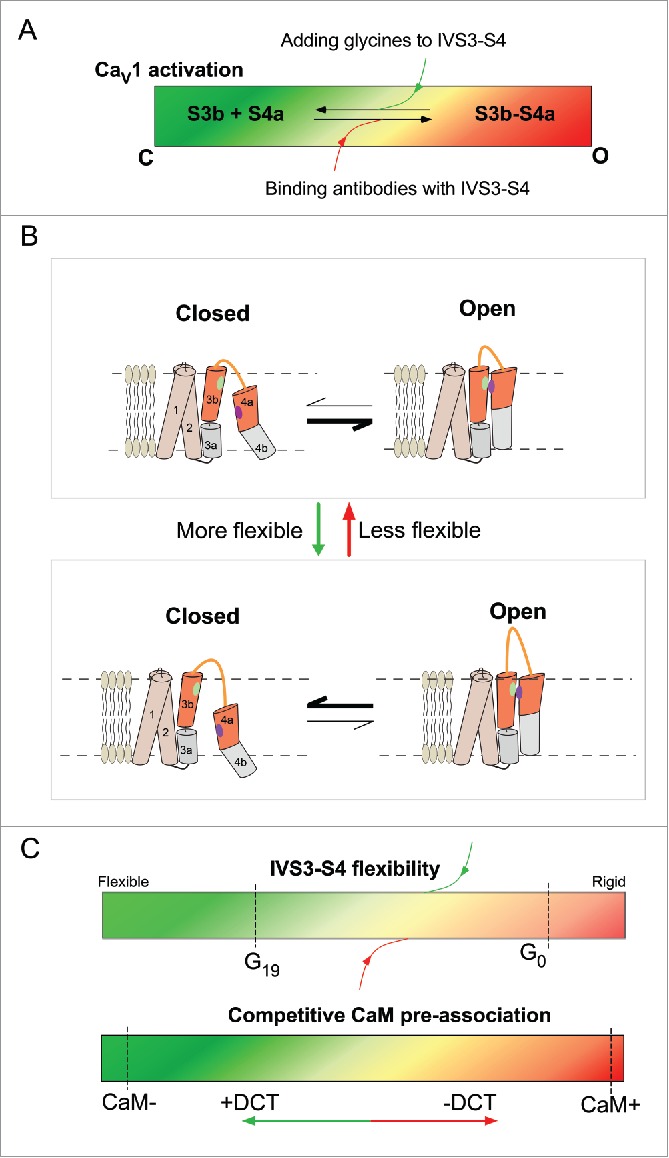
Schematic summary of IVS3-S4 modulation on CaV1 activation. (A) Scheme of summary. Channel activation can be considered being controlled by the cooperative behaviors (binding and downstream events) between S3 and S4. GX linkers attenuate whereas loop-bound antibodies facilitate the activation. The color codes (green, closed; red, open) are to indicate the open probability or the fraction of open channels. (B) Cartoons to illustrate the potential mechanism of flexibility-mediated channel gating. Increasing flexibility of VSD movement would reduce the chance of appropriate VSD positioning for the key sites to interact (indicating by green and violet dots); and decreasing the random movement of VSD would do the opposite. (C) The summary and comparison for the 2 major kinds of modulation in this study. The dynamic range and perturbations related to IVS3-S4 (upper) and CaM (lower) are compared. Color codes are in similar fashion to (A).
Also, applying depolarization or attaching antibody can provide confinement to VSD dynamics, reducing the space of random walk, effectively promoting the probability of S3-S4 binding, as one way to understand the mechanism of voltage-dependent activation. In this work, we explored the dynamic range of the modulation by GX-linker, by chimeric channels, mutagenesis and loop-specific antibodies, which are supposed to be extendable (Fig. 6C, upper panel). In contrast, prior works assure that CaM+ and CaM- channels, if not reaching, are very closely approaching the limits of the dynamic range set by DCT/CaM modulation (Fig. 6C, lower panel). This led us to realize that the loop flexibility is unlikely overlapped with the activation mechanism mediated by CaM modulation, since further V1/2 shifts arising from GX-modulation on CaM+ (from G19 to G0) or CaM- (from G0 to G19) channels were substantially extended beyond the limits of DCT/CaM modulation.
We in this study rely on the analysis of voltage-dependence (V1/2 and S) and temporal-kinetics (τ), which are the 2 most prominent aspects of channel activation. Regarding other channel characteristics, current density should be modulated by GX-linkers as well, presumably similar to V1/2 and τ (e.g., higher current density corresponding to facilitated channel activation), in consistence with our observations and other published reports;7 whereas CDI (Ca2+-dependent inactivation) did not have any appreciable change with GX-linker modulation (Fig. S3).
Proof of concept to inspire strategies of developing innovative CaV1 modulators
Our work demonstrates the potentials to devise more potent perturbations, including longer GX-linkers and corresponding antibodies, both of which would exhibit broader range of activation tuning in both directions (ΔV1/2, Δτ, etc.). Extracellular loop might be an intriguing target to develop specific antibody-based modulators for both research and therapeutic purposes.23 Our success in applying antibodies targeting IVS3-S4 to modulate CaV1 activation, as a proof of principle, opens up the avenue to explore the potentials of such CaV1 openers or enhancers with new mechanism of action. It is worth to further develop more potent (by optimizing antibody properties) and more applicable (by producing antibodies specific to the native loops) antibody-based modulators. Also, we provide the evidence that the facilitation of activation due to CaM16 can be utilized as an additive modulation in parallel with IVS3-S4 effects, altogether would provide much broader space of potency than any strategy currently known. By such “cocktails” of CaV1 inhibition or facilitation combining the effects from both loop flexibility and apoCaM, ΔV1/2 could reach up to −30 mV or more, as estimated from our data (Fig. 5D).
Materials and methods
Molecular biology
Constructs of α1D (AF370009.1), α1C (NM_199460.3), α1F (NP005174) and α1S (XM_983862.1) were generously provided by the groups of D. Yue, K. Beam, J. McRory & T. Snutch and J. Streissnig. For CaV1.3 chimeric channels, α1DE291S, α1DE331C, α1DE321D, α1DE321F or α1DE321DΔ6His were generated by using overlap extension PCR to fuse different exons: exon 29 from CaV1.1 channel, exon 33 from CaV1.2 channel, exon 32 from CaV1.3 channel, exon 32 from CaV1.4 channel or the His-tag (6 histidines) into IVS3-S4 loop of α1DE321DΔ, respectively. For α1DE291S and α1DE321F, glycine residues substitute for original amino acids within IVS3-S4 loop (α1DE291SHalfG, α1DE291SAllG, α1DE321FHalfG, α1DE321FAllG) by QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies). After PCR reaction, the segments were digested with 2 unique sites BamHI and KpnI and inserted to replace the previous region. For α1DΔE321DΔ-DCTF, DCT derived from α1F was amplified by PCR with flanking SpeI and XbaI and fused to mutant α1D (α1DΔ) with the carboxyl terminal being truncated out. To make construct of α1DΔE291SAllG-DCTF, segment from α1DE291SAllG was amplified by PCR with flanking BglII and BstEII and cloned directionally via these 2 unique sites into corresponding region of α1DΔE321DΔ-DCTF. For α1DΔ[6His-E291SAllG-6His]-DCTF, overlap extension PCR was performed, similar to α1DE321DΔ6His.
Transfection of cDNA constructs
HEK293 cells were cultured in 60 mm dishes, and recombinant channels were transiently transfected according to an established calcium phosphate protocol.24 We applied 5 μg of cDNA encoding the desired α1 subunit, along with 4 μg of rat brain β2a (M80545) and 4 μg of rat brain α2δ (NM012919.2) subunits. All of the above cDNA constructs were driven by a cytomegalovirus promoter. To enhance expression, cDNA for simian virus 40 T antigen (1–2 μg) was also co-transfected. Cells were washed with PBS 6–8 h after transfection and maintained in supplemented DMEM, then incubated for at least 48 h in a water-saturated 5% CO2 incubator at 37°C before whole-cell recordings.
Whole-cell electrophysiology
Whole-cell recordings of transfected HEK293 cells were obtained at room temperature (25°C) using an Axopatch 200B amplifier (Axon Instruments). Electrodes were pulled with borosilicate glass capillaries by a programmable puller (P-1000, Sutter Instruments, USA) and heat-polished by a microforge (MF-900, Narishige, Japan), resulting in 1–3 MΩ resistances, before series resistance compensation of 70% or more. The internal solutions contained, (in mM): CsMeSO3, 135; CsCl2, 5; MgCl2, 1; MgATP, 4; HEPES, 5; and EGTA, 5; at 290 mOsm adjusted with glucose and at pH 7.3 adjusted with CsOH. The bath solution contained (in mM): TEA-MeSO3, 140; HEPES, 10; BaCl2, 10; 300 mOsm, adjusted with glucose and at pH 7.3 adjusted with TEAOH, all according to the previous report.24
Antibody incubation of live cells
HEK293 cells transfected with desired recombinant channels were washed with Tyrode solution contained, (in mM): NaCl, 129; KCl, 5; CaCl2, 2; MgCl2, 1; glucose, 30; Hepes, 25; 300 mOsm, adjusted with glucose and at pH7.3 adjusted with NaOH at room temperature. Cells were then incubated for 1–2 h in Tyrode solution containing THE™ His Tag Antibody, mAb, Mouse (GenScript A00186-100), 1:1000 dilution.
Immunocytochemistry
HEK293 cells on confocal dishes were rinsed briefly in phosphate-buffered saline (PBS), fixed with ice cold 4 % paraformaldehyde in PBS (pH 7.4) for 20 min at the room temperature, then washed 3 times with ice-cold PBS. Fixed cells were then permeabilized with 0.3% Triton X-100, blocked with 10% normal goat serum in PBS for 1 h at room temperature, and incubated overnight at 4°C in primary antibodies: THE™ His Tag Antibody, mAb, Mouse (1:1000 dilution; GenScript). The next day, cells were washed with PBS 3 times, incubated at room temperature for 2 h in a 1:800 dilution of anti-mouse-Alexa 568 (Invitrogen), and washed with PBS 3 times.
TIRF microscopy
Following immunocytochemistry, fluorescence measurements of HEK293 cells were acquired with Nikon Ti-E automatic inverted microscope (A1RSi) through a 100×oil TIRF objective. For Alexa 568 imaging, excitation was delivered by a solid-state laser featuring a 561-nm line. TIRF images were analyzed with Image J and Matlab software.
Analysis and fitting for channel activation
Voltage-dependent activation of the current was fitted according to Boltzmann equation,
where n is the normalized activation, V1/2 is the Vm for half-maximal conductance/activation and S is the slope factor.
The time constant of activation was achieved from the fast activation process of Ba2+ current IBa, by fitting with a single-exponential function,
where I0 is the amplitude of the current, τ is the specific time constant and C is a constant to compensate leakage current when necessary.
Data analysis and statistics
Data were analyzed in Matlab and Origin software. Standard error of the mean (SEM) and student t-test (2-tailed with criteria of significance: *, p < 0.05; **, p < 0.01 and ***, p < 0.001) were calculated when applicable.
Supplementary Material
Abbreviations
- CDI
Ca2+-dependent inactivation
- DCT
distal carboxyl terminus
- LTCCs
L-type Ca2+ channels
- VSDs
voltage sensing domains
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank all Liu-Lab (X-Lab) members for discussions. We acknowledge the researchers who shared constructs as indicated in the Methods section. We also thank Drs. D.T. Yue and M.R. Tadross for providing Matlab-based ZStudio program for whole-cell patch-clamp recording, and Mr. P. Jiang for reprogramming and customizing ZStudio.
Funding
This work is supported by Natural Science Foundation of China (NSFC) grants 81171382, 31370822 and 81371604; Beijing Natural Science Foundation (BNSF) grant 7142089; and Tsinghua National Lab for Information Science and Technology (TNList) Cross-discipline Foundation. XDL also receives support from Tsinghua-Peking Center for Life Sciences (CLS).
Author contributions
NL and YXL conducted the experiments; NL and YXY performed the analyses; XDL and NL wrote the paper; XDL designed the experiments and conceived the project.
References
- [1].Catterall WA. International union of pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 2005; 57:411-25; PMID:16382099; http://dx.doi.org/ 10.1124/pr.57.4.5 [DOI] [PubMed] [Google Scholar]
- [2].Wu J, Yan Z, Li Z, Yan C, Lu S, Dong M, Yan N. Structure of the voltage-gated calcium channel Cav1.1 complex. Science 2015; 350:aad2395-aad; PMID:26680202; http://dx.doi.org/ 10.1126/science.aad2395 [DOI] [PubMed] [Google Scholar]
- [3].Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol 2000; 16:521-55; PMID:11031246; http://dx.doi.org/ 10.1146/annurev.cellbio.16.1.521 [DOI] [PubMed] [Google Scholar]
- [4].Tombola F, Pathak MM, Isacoff EY. How does voltage open an ion channel? Ann Rev Cell Dev Biol 2006; 22:23-52; PMID:16704338; http://dx.doi.org/ 10.1146/annurev.cellbio.21.020404.145837 [DOI] [PubMed] [Google Scholar]
- [5].Swartz KJ. Sensing voltage across lipid membranes. Nature 2008; 456:891-7; PMID:19092925; http://dx.doi.org/ 10.1038/nature07620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Xu Y, Ramu Y, Lu Z. A shaker K+ channel with a miniature engineered voltage sensor. Cell 2010; 142:580-9; PMID:20691466; http://dx.doi.org/ 10.1016/j.cell.2010.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tuluc P, Yarov-Yarovoy V, Benedetti B, Flucher BE. Molecular interactions in the voltage sensor controlling gating properties of CaV calcium channels. Structure 2016; 24:261-71; PMID:26749449; http://dx.doi.org/ 10.1016/j.str.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mathur R, Zheng J, Yan Y, Sigworth FJ. Role of the S3-S4 linker in Shaker potassium channel activation. J Gen Physiol 1997; 109:191-9; PMID:9041448; http://dx.doi.org/ 10.1085/jgp.109.2.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bezanilla F. Voltage sensor movements. J Gen Physiol 2002; 120:465-73; PMID:12356849; http://dx.doi.org/ 10.1085/jgp.20028660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tuluc P, Benedetti B, Coste de Bagneaux P, Grabner M, Flucher BE. Two distinct voltage-sensing domains control voltage sensitivity and kinetics of current activation in CaV1.1 calcium channels. J Gen Physiol 2016; 147:437-49; PMID:27185857; http://dx.doi.org/ 10.1085/jgp.201611568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Xu W, Lipscombe D. Neuronal CaV1.3α1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci 2001; 16:5944-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tang ZZ, Liang MC, Lu S, Yu D, Yu CY, Yue DT, Soong TW. Transcript scanning reveals novel and extensive splice variations in human L-type voltage-gated calcium channel, Cav1.2 α1Subunit. J Biol Chem 2004; 279:44335-43; PMID:15299022; http://dx.doi.org/ 10.1074/jbc.M407023200 [DOI] [PubMed] [Google Scholar]
- [13].Lin Z, Lin Y, Schorge S, Pan JQ, Beierlein M, Lipscombe D. Alternative splicing of a short cassette exon in α1B generates functionally distinct N-type calcium channels in central and peripheral neurons. J Neurosci 1999; 13:5322-31; PMID:10377343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hans M, Urrutia A, Deal C, Brust PF, Stauderman K, Ellis SB, Harpold MM, Johnson EC, Williams ME. Structural elements in domain IV that influence biophysical and pharmacological properties of human α1A-containing high-voltage-activated calcium channels. Biophys J 1999; 76:1384-400; PMID:10049321; http://dx.doi.org/ 10.1016/S0006-3495(99)77300-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bourinet E, Soong TW, Sutton K, Slaymaker S, Mathews E, Monteil A, Zamponi GW, Nargeot J, Snutch TP. Splicing of alpha 1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat Neurosci 1999; 2:407-15; PMID:10321243; http://dx.doi.org/ 10.1038/8070 [DOI] [PubMed] [Google Scholar]
- [16].Adams PJ, Ben-Johny M, Dick IE, Inoue T, Yue DT. Apocalmodulin itself promotes ion channel opening and Ca(2+) regulation. Cell 2014; 159:608-22; PMID:25417111; http://dx.doi.org/ 10.1016/j.cell.2014.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tuluc P, Molenda N, Schlick B, Obermair GJ, Flucher BE, Jurkat-Rott K. A CaV1.1 Ca2+ channel splice variant with high conductance and voltage-sensitivity alters EC coupling in developing skeletal muscle. Biophys J 2009; 96:35-44; PMID:19134469; http://dx.doi.org/ 10.1016/j.bpj.2008.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tang ZZ, Zheng S, Nikolic J, Black DL. Developmental control of CaV1.2 L-type calcium channel splicing by fox proteins. Mol Cell Biol 2009; 29:4757-65; PMID:19564422; http://dx.doi.org/ 10.1128/MCB.00608-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tan GMY, Yu D, Wang J, Soong TW. Alternative splicing at C terminus of CaV1.4 calcium channel modulates calcium-dependent inactivation, activation potential, and current density. J Biol Chem 2012; 287:832-47; PMID:22069316; http://dx.doi.org/ 10.1074/jbc.M111.268722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Safa P, Boulter J, Hales TG. Functional properties of CaV1.3 (α1D) L-type Ca2+ Channel Splice Variants Expressed by Rat Brain and Neuroendocrine GH3 Cells. J Biol Chem 2001; 276:38727-37; PMID:11514547; http://dx.doi.org/ 10.1074/jbc.M103724200 [DOI] [PubMed] [Google Scholar]
- [21].McRory JE. The CACNA1F gene encodes an L-type calcium channel with unique biophysical properties and tissue distribution. J Neurosci 2004; 24:1707-18; PMID:14973233; http://dx.doi.org/ 10.1523/JNEUROSCI.4846-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mori MX, Erickson MG, Yue DT. Functional stoichiometry and local enrichment of calmodulin interacting with Ca2+ channels. Science 2004; 304:432-5; PMID:15087548; http://dx.doi.org/ 10.1126/science.1093490 [DOI] [PubMed] [Google Scholar]
- [23].Xu S-Z, Zeng F, Lei M, Li J, Gao B, Xiong C, et al.. Generation of functional ion-channel tools by E3 targeting. Nat Biotechnol 2005; 23:1289-93; PMID:16170312; http://dx.doi.org/ 10.1038/nbt1148 [DOI] [PubMed] [Google Scholar]
- [24].Liu X, Yang PS, Yang W, Yue DT. Enzyme-inhibitor-like tuning of Ca2+ channel connectivity with calmodulin. Nature 2010; 463:968-72; PMID:20139964; http://dx.doi.org/ 10.1038/nature08766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nakai J, Adams BA, Imoto K, Beam KG. Critical roles of the S3 segment and S3-S4 linker of repeat I in activation of L-type calcium channels. Proc Natl Acad Sci U S A 1994; 91:1014-8; PMID:8302825; http://dx.doi.org/ 10.1073/pnas.91.3.1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.