ABSTRACT
Several compounds have been proposed to stimulate TRPM3 Ca2+ channels. We recently showed that stimulation of TRPM3 channels with pregnenolone sulfate activated the transcription factor AP-1, while other proposed TRPM3 ligands (nifedipine, D-erythro-sphingosine) exhibited either no or TRPM3-independent effects on gene transcription. Here, we have analyzed the transcriptional activity of CIM0216, a synthetic TRPM3 ligand proposed to have a higher potency and affinity for TRPM3 than pregnenolone sulfate. The results show that CIM0216 treatment of HEK293 cells expressing TRPM3 channels activated AP-1 and stimulated the transcriptional activation potential of c-Jun and c-Fos, 2 basic region leucine zipper transcription factors that constitute AP-1. CIM0216-induced gene transcription was attenuated by knock-down of TRPM3 or treatment with mefenamic acid, a TRPM3 inhibitor. CIM0216 was similarly or less capable in activating TRPM3-mediated gene transcription, suggesting that pregnenolone sulfate is still the ligand of choice for changing the gene expression pattern via TRPM3.
KEYWORDS: c-Fos, c-Jun, gene expression, lentivirus, TRPM3
Introduction
Transient receptor potential M3 (TRPM3) is a Ca2+ permeable cation channel and stimulation of TRPM3 triggers a rise in the intracellular Ca2+ concentration. We discovered that TRPM3 stimulation induces a intracellular signaling cascade that results in the activation of the stimulus-responsive transcription factors AP-1, c-Fos, c-Jun, CREB, Egr-1, and Elk-1.1-5 The natural ligands for TRPM3 channels are still unknown, but several compounds have been suggested to function as TRPM3-specific agonists. In most of these studies, the influx of Ca2+ ions into the cells and the subsequent rise in the intracellular Ca2+ concentration was used as an indication of activated TRPM3 channels. However, experiments with cultured neurons and neuronal cell lines revealed that the influx of Ca2+ ions into the cells is not always sufficient to induce an intracellular signaling cascade that leads to changes in the gene expression pattern.6-8 Recently, we compared the ability of putative activators of TRPM3 to induce gene transcription.3 The result revealed that pregnenolone sulfate is a powerful activator of TRPM3 mediated gene transcription, while other proposed TRPM3 ligands (nifedipine, D-erythro-sphingosine) exhibited either no or TRPM3-independent effects on gene transcription. These compounds induce a rise in intracellular Ca2+, but the Ca2+ signal is insufficient to induce an intracellular signaling cascade that changes the gene expression pattern of the cells. Recently, a synthetic compound termed CIM0216 has been described whose potency in generating a Ca2+ influx via TRPM3 channels greatly exceeded that of pregnenolone sulfate.9 We therefore asked whether CIM0216 is a potent activator of gene transcription as well, mediated by the stimulation of TRPM3 channels.
Results and discussion
As a sensor to measure AP-1 regulated transcription we used lentiviral gene transfer to implant a collagenase promoter/luciferase reporter gene into the chromatin of HEK293 cells that contain a tetracycline-inducible TRPM3 transcription unit (Fig. 1A). We tested several concentrations of CIM0216 (Fig. 1B) for induction of AP-1-responsive reporter gene transcription. As a control, we stimulated the cells with pregnenolone sulfate (Fig. 1C). The results show that CIM0216 significantly stimulated transcription from the Coll.luc reporter gene. However, pregnenolone sulfate was more than twice as potent as CIM0216 in upregulating AP-1 via TRPM3 channels (Fig. 1D).
Figure 1.
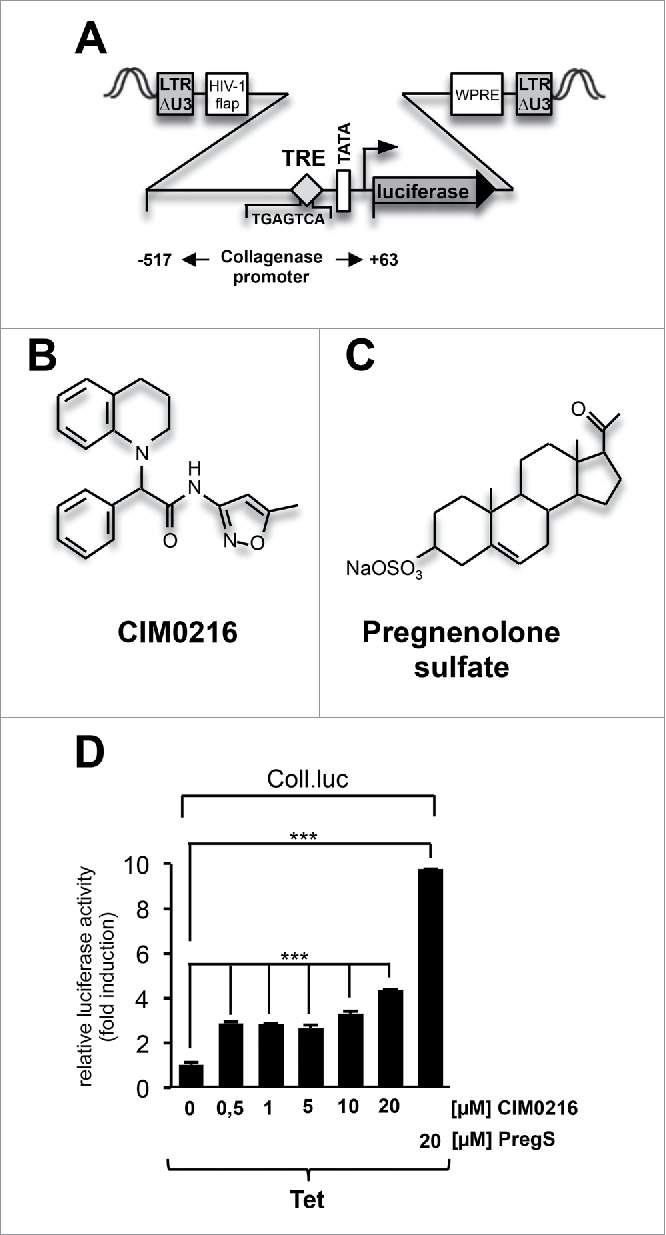
Stimulation of TRPM3 channels with either pregnenolone sulfate or CIM0216 activates AP-1. (A) Schematic representation of the integrated provirus containing the AP-1 responsive collagenase promoter/luciferase reporter gene (Coll.luc). The AP-1 binding site, termed the phorbol 12-O-tetradecanoylphorbol-13-acetate (TPA)-responsive element (TRE), is depicted. The location of the woodchuck hepatitis virus post-transcriptional regulatory element (WPRE) and the HIV flap element are shown. (B) CIM0216 (C) Pregnenolone sulfate. (D) HEK293 cells containing a tetracycline-inducible TRPM3 expression unit were infected with a recombinant lentivirus containing the collagenase promoter/luciferase reporter gene (Coll.luc). The cells were cultured for 24 hours in medium containing 0.05% serum in presence of tetracycline. Stimulation with CIM0216 (0.5-20 μM) or pregnenolone sulfate (20 μM) was performed with medium containing 0.05% serum for 24 hours. Cell extracts were prepared and analyzed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean +/− SD of 3 experiments performed in quadruplicate; (☆☆☆, P < 0.001).
Originally, the transcription factor AP-1 was identified as a dimer of the basic region leucine zipper transcription factors c-Fos and c-Jun. To measure the transcriptional activation potentials of c-Fos and c-Jun, fusion proteins were expressed consisting of the DNA binding domain of the yeast transcription factor GAL4 and the activation domains of c-Fos and c-Jun that are regulated via phosphorylation (Fig. 2A, 2B). To measure the transcriptional response, we integrated a reporter gene into the genome that had GAL4 binding sites (termed UAS, upstream activation sequence) in its regulatory region (Fig. 2C). The results show that the transcriptional activation potential of c-Fos and c-Jun were significantly increased in TRPM3-expressing HEK293 cells that had been stimulated with either CIM0216 or pregnenolone sulfate (Fig. 2D, E).
Figure 2.
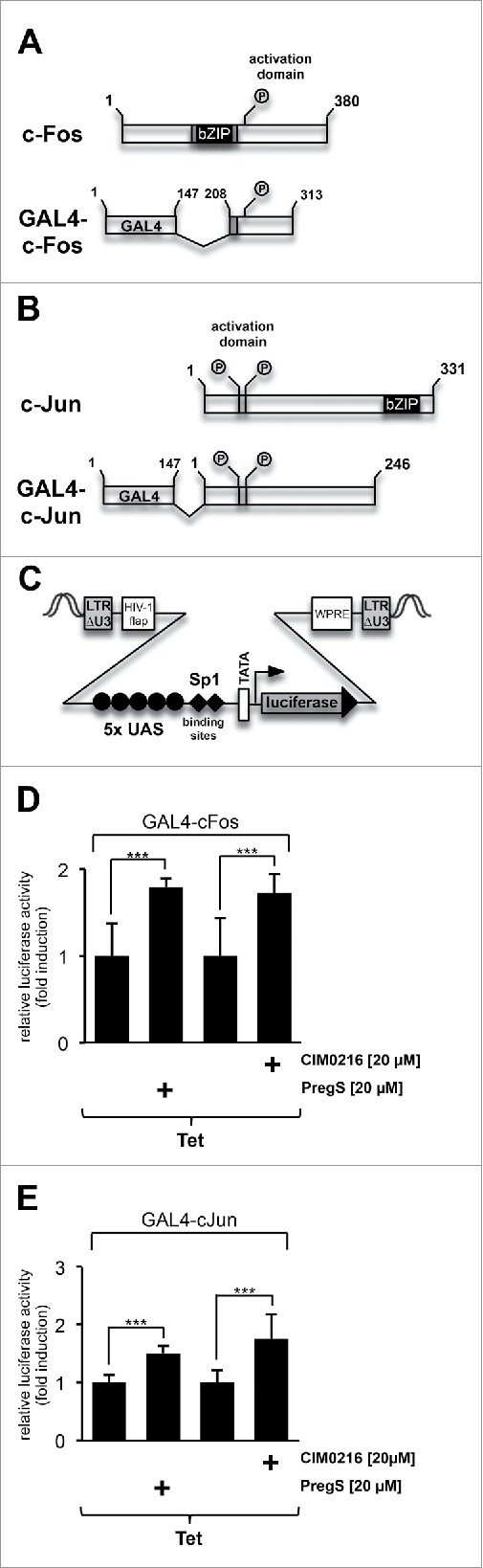
(see previous page) Stimulation of TRPM3 channels with CIM0216 or pregnenolone sulfate upregulates the transcriptional activation potentials of c-Fos and c-Jun. (A, B) Schematic representation of the modular structure of c-Fos and GAL4-c-Fos (A) and c-Jun and GAL4-c-Jun (B). The bZIP DNA binding and the dimerization domains are depicted. The GAL4 fusion proteins lack the bZIP domain, but retain the C-terminal activation domain of c-Fos (GAL4-cFos), or the N-terminal activation domain of c-Jun (GAL4-c-Jun). The truncated c-Fos and c-Jun proteins are expressed as fusion proteins together with the N-terminal DNA binding domain of GAL4. (C) Schematic representation of the integrated provirus encoding a luciferase reporter gene under the control of the minimal promoter, consisting of 5 GAL4 binding sites (UAS, upstream activating sequence), 2 Sp1 binding sites, a TATA box and an initiator element. (D, E) HEK293 cells containing a tetracycline-inducible TRPM3 expression unit were double-infected with a lentivirus containing the GAL4-responsive luciferase reporter gene and a lentivirus encoding either GAL4-c-Fos (D) or GAL4-c-Jun (E). The cells were serum-starved for 24 hours in the presence of tetracycline (1 μg/ml) and then stimulated with either CIM0216 (20 μM) or pregnenolone sulfate (PregS, 20 μM) for 24 hours as indicated. Cell extracts were prepared and analyzed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean +/− SD of 3 experiments performed in quadruplicate (☆☆☆, P < 0.001).
Recently, we showed that the upregulation of AP-1 in pregnenolone sulfate-stimulated HEK293 cells expressing TRPM3 was almost completely blocked by the preincubation of the cells with mefenamic acid.3 Figure 3A shows that incubation of the cells with mefenamic acid attenuated CIM0216-induced AP-1 activation in HEK293 cells expressing TRPM3 channels as well. Furthermore, expression of a TRPM3-specific shRNA significantly reduced AP-1 activity in TRPM3-expressing HEK293 cells following stimulation of the cells with either CIM0216 or pregnenolone sulfate (Fig. 3B). Thus, both CIM0216 and pregnenolone sulfate-induced gene transcription relies on the activation of TRPM3 channels.
Figure 3.
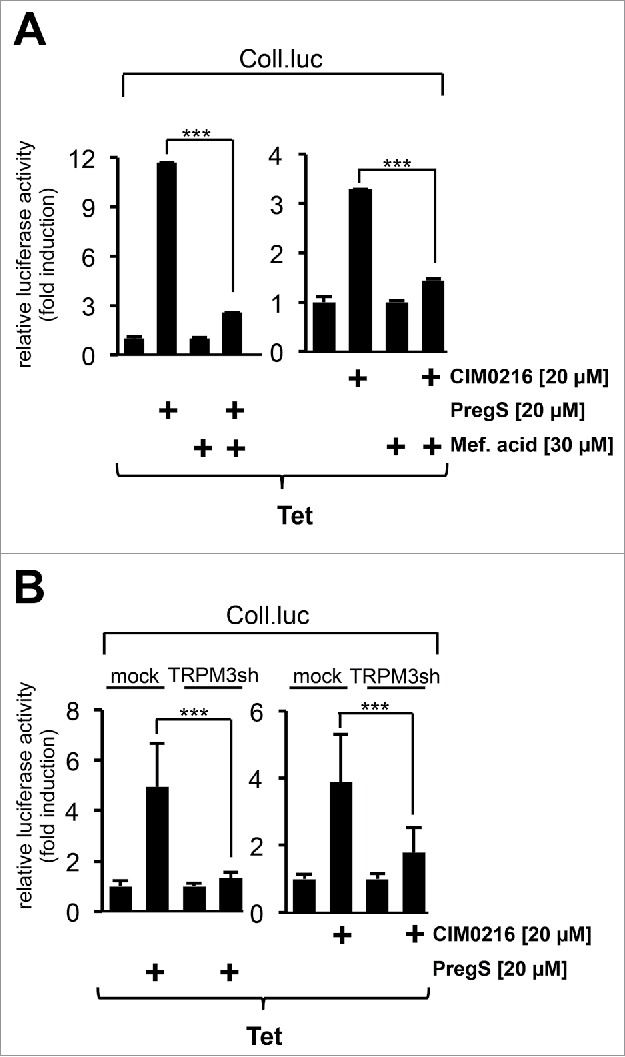
CIM0216 and pregnenolone sulfate are TRPM3 ligands. (A) HEK293 cells containing a tetracycline-inducible TRPM3 transcription unit were infected with recombinant lentiviruses encoding the collagenase promoter/luciferase reporter gene. The cells were serum-starved for 24 hours in the presence of tetracycline (1 μg/ml) and then stimulated with either CIM0216 (20 μM) or pregnenolone sulfate (PregS, 20 μM) in the presence or absence of mefenamic acid (Mef, 30 μM) for 24 hours. Cell extracts were prepared and analyzed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean +/− SD of 3 experiments performed in quadruplicate; (☆☆☆, P < 0.001). (B) HEK293 cells containing a tetracycline-inducible TRPM3 transcription unit were infected with a lentivirus encoding the collagenase promoter/luciferase reporter gene (Coll.luc). In addition, cells were infected with a lentivirus encoding a TRPM3-specific shRNA. As a control, cells were infected with a lentivirus generated with the lentiviral transfer vector pLL3.7 (mock). The cells were serum-starved for 24 hours in the presence of tetracycline (1 μg/ml) and then stimulated with either CIM0216 (20 μM) or pregnenolone sulfate (PregS, 20 μM) for 24 hours as indicated. Cell extracts were prepared and analyzed for luciferase activities. Luciferase activity was normalized to the protein concentration. Data shown are mean +/− SD of 3 experiments performed in quadruplicate (☆☆☆, P < 0.001).
Conclusion
The experiments show that CIM0216 and pregnenolone sulfate are powerful activators of AP-1-regulated gene transcription via TRPM3. Moreover, both compounds increased the transcriptional activation potential of c-Jun and c-Fos. A comparison of the efficiency of CIM0216 and pregnenolone sulfate to induce gene transcription revealed that pregnenolone sulfate is stronger in activating AP-1 and exhibits similar activity to CIM0216 in stimulating the transcriptional activation potential of c-Jun and c-Fos.
Materials and methods
Cell culture
HEK293 cells containing the human TRPM3 coding region under the control of a tetracycline-regulated promoter were kindly provided by David Beech and Yasser Majeed, University of Leeds, UK and cultured and stimulated as described.3,10 Stimulation with pregnenolone sulfate (20 μM, Sigma-Aldrich # P162, dissolved in DMSO) or CIM0216 (1-20 μM, Tocris # 5521, dissolved in DMSO) was performed for 24 hours.
Lentiviral gene transfer
The lentiviral transfer vector pFUW-GAL4-c-Fos has been described previously.11 The GAL4-c-Jun expression plasmid pGAL4-c-Jun, expressing the amino acids 1-246 of c-Jun, was a kind gift of Michael Karin, University of California San Diego. To generate the lentiviral transfer vector pFUW-GAL4-c-Jun, plasmid pGAL4-c-Jun was cut with BamHI, filled in with the Klenow fragment of DNA polymerase I, and recut with HpaI. The fragment was cloned into plasmid pFUW-GAL4-NK10,12 replacing the repression domain of NK10 with the phosphorylation-dependent activation domain of c-Jun. The lentiviral transfer vectors pLL.TRPM3, encoding a TRPM3-specific shRNA, has been described.1,4 The viral particles were produced as previously described13,14 by triple transfection of 293T/17 cells with the gag-pol-rev packaging plasmid, the env plasmid encoding VSV glycoprotein, and the transfer vector.
Reporter assays
The lentiviral transfer vectors pFWColl.luc and pFW-UAS5Sp12luc have been described elsewhere.12,15 Cell extracts were prepared using reporter lysis buffer (Promega, Mannheim, Germany) and analyzed for luciferase activities. Luciferase activity was normalized to the protein concentration. Each experiment was performed at least 3 times in quadruplicate giving consistent results.
Statistics
Statistical analysis were done by using the 2-tailed student´s t-test. Data shown are mean +/− SD from 3 to 4 independent experiments performed in quadruplicate. Statistical probability is expressed as P < 0.001. Values were considered significant when P < 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank David Beech and Yasser Majeed for their generous gift of HEK293 cells containing a tetracycline-regulated TRPM3 expression unit. We thank Libby Guethlein for critical reading of the manuscript.
Funding
Funding was provided by Saarland University.
References
- [1].Mayer SI, Müller I, Mannebach S, Endo T, Thiel G. Signal transduction of pregnenolone sulfate in insulinoma cells. Activation of Egr-1 expression involving TRPM3, voltage-gated calcium channels, ERK, and ternary complex factors. J Biol Chem 2011; 286:10084-96; PMID:21257751; http://dx.doi.org/ 10.1074/jbc.M110.202697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Müller I, Rössler OG, Thiel G. Pregnenolone sulfate activates basic region leucine zipper transcription factors in insulinoma cells: role of voltage-gated Ca2+ channels and transient receptor potential melastatin 3 channels. Mol Pharmacol 2011; 80:1179-89; PMID:21948387; http://dx.doi.org/ 10.1124/mol.111.074781 [DOI] [PubMed] [Google Scholar]
- [3].Lesch A, Rubil S, Thiel G. Activation and inhibition of transient receptor potential TRPM3-induced gene transcription. Br J Pharmacol 2014; 171:2645-58; PMID:24895737; http://dx.doi.org/ 10.1111/bph.12524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lesch A, Hui X, Lipp P, Thiel G. Transient receptor potential melastatin-3 (TRPM3)-induced activation of AP-1 requires Ca2+ ions and the transcription factors c-Jun, ATF2, and ternary complex factor. Mol Pharmacol 2015; 87:617-28; PMID:25576487; http://dx.doi.org/ 10.1124/mol.114.095695 [DOI] [PubMed] [Google Scholar]
- [5].Rubil S, Rössler OG, Thiel G. CREB, AP-1, ternary complex factors and MAP kinases connect transient receptor potential melastatin-3 (TRPM3) channel stimulation with increased c-Fos expression. Brit J Pharmacol 2016; 173:305-18; http://dx.doi.org/ 10.1111/bph.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gallin WJ, Greenberg ME. Calcium regulation of gene expression in neurons: the mode of entry matters. Curr Opin Neurobiol 1995; 5:367-74; PMID:7580160; http://dx.doi.org/ 10.1016/0959-4388(95)80050-6 [DOI] [PubMed] [Google Scholar]
- [7].Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature 1998; 392:198-202; PMID:9515967; http://dx.doi.org/ 10.1038/32448 [DOI] [PubMed] [Google Scholar]
- [8].West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA 2001; 98:11024-31; PMID:11572963; http://dx.doi.org/ 10.1073/pnas.191352298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Held K, Kichko T, De Clercq K, Klaassen H, Van Bree R, Vanherck JC, Marchand A, Reeh PW, Chaltin P, Voets T, et al.. Activation of TRPM3 by a potent synthetic ligand reveals a role in peptide release. Proc Natl Acad Sci USA 2015; 112:E1363-72; PMID:25733887; http://dx.doi.org/ 10.1073/pnas.1419845112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Naylor J, Milligan CJ, Zeng F, Jones C, Beech DJ. Production of a specific extracellular inhibitor of TRPM3 channels. Brit J Pharmacol 2008; 155:567-73; http://dx.doi.org/ 10.1038/bjp.2008.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Thiel G, Rössler OG. Immediate-early transcriptional response to angiotensin II in human adrenocortical cells. Endocrinology 2011; 152:4211-23; http://dx.doi.org/ 10.1210/en.2011-1243 [DOI] [PubMed] [Google Scholar]
- [12].Ekici M, Keim A, Rössler OG, Hohl M, Thiel G. Chromatin structure and expression of the AMPA receptor subunit GluR2 in human glioma cells: major role of REST and Sp1. J Cell Biochem 2012; 113:528-43; PMID:21948504; http://dx.doi.org/ 10.1002/jcb.23376 [DOI] [PubMed] [Google Scholar]
- [13].Keim A, Müller I, Thiel G. Efficient genetic manipulation of 1321N1 astrocytoma cells using lentiviral gene transfer. J Neurosci Meth 2012; 206:138-42; http://dx.doi.org/ 10.1016/j.jneumeth.2012.02.016 [DOI] [PubMed] [Google Scholar]
- [14].Rössler OG, Thiel G. Regulation of gene transcription following stimulation of Gαq-coupled designer receptors. in: Thiel G. (Ed.), Designer receptors exclusivley activated by designer drugs, Humana Press New York, Neuromethods; 2015; 108:49-60 [Google Scholar]
- [15].Rössler OG, Henß I, Thiel G. Transcriptional response to muscarinic acetylcholine receptor stimulation: Regulation of Egr-1 biosynthesis by Elk-1, ERK, MKP-1 and calcineurin in carbachol stimulated human neuroblastoma cells. Arch Biochem Biophys 2008; 470:93-102; PMID:18061571; http://dx.doi.org/ 10.1016/j.abb.2007.11.008 [DOI] [PubMed] [Google Scholar]


