ABSTRACT
Two-pore channels (TPC1-3) comprise a subfamily of the eukaryotic voltage-gated ion channels (VGICs) superfamily that are mainly expressed in acidic stores in plants and animals. TPCS are widespread across the animal kingdom, with primates, mice and rats lacking TPC3, and mainly act as Ca+ and Na+ channels, although it was also suggested that they could be permeable to other ions. Nowadays, TPCs have been related to the development of different diseases, including Parkinson´s disease, obesity or myocardial ischemia. Due to this, their study has raised the interest of the scientific community to try to understand their mechanism of action in order to be able to develop an efficient drug that could regulate TPCs activity. In this review, we will provide an updated view regarding TPCs structure, function and activation, as well as their role in different pathophysiological processes.
KEYWORDS: endolysosome; NAADP; PI(3,5)P2; TPC1; TPC2
Introduction
Two-pore channels (TPCs) comprise a subfamily (TPC1-3) of the eukaryotic voltage-gated ion channels (VGICs) superfamily that were originally sequenced from a rat kidney-cell cDNA library in 20001 and subsequently identified in Arabidopsis thaliana in 20012 as a result of their structural homology with the voltage-gated Na+ and Ca2+ channels, although their function was unknown until 2005, when TPC1 was confirmed as a plant vacuole Ca2+ release channel.3 Subsequently, in 2009, it was demonstrated that mammalian TPCs are expressed intracellularly in acidic organelles (endo-lysosomes), with TPC2 being specifically targeted to lysosomal membranes, and that they function as Ca2+ release channels when activated by nicotinic acid adenine dinucleotide phosphate (NAADP).4 Still, on the other hand, in 2012 and 2013 2 independent studies refuted that mammalian TPCs were Ca2+ release channels activated by NAADP, probing that they are not Ca2+ but Na+ release channels that are not activated by NAADP but by phosphatidylinositol 3,5-bisphosphate (PtdIns(3,5)P2) and inhibited by the mammalian target of rapamycin (mTOR),5,6 which caused a great commotion regarding TPCs regulation and function among the scientific community.7-10 Nowadays, it is accepted that mammalian TPCs not only function as Ca2+ or Na+ release channels, but also as H+ and K+ channels,11,12 and it has been demonstrated that TPCs can be activated by other signals apart from NAADP and PI(3,5)P2, such as the leucine-rich repeat kinase 2 (LRRK2)13 or action potentials,14 and inhibited by Mg2+ concentrations,15 Ca2+ and Na+ ion channels inhibitors,16,17 or c-Jun N-terminal kinase (JNK) and p38 kinase,15 apart from mTOR. So that, it seems reasonable that, according to the cellular context, TPCs could be differentially regulated and exert different functions.
Today it is known that TPCs have a role not only in ion signaling but also in intracellular vesicle trafficking,18-20 participating in a wide range of pathophysiological processes; that is why in the last years TPCs have received an increased attention from the main journals in the field, and they have been related to the regulation of many biological functions at different levels, including pancreatic β-cell function,21,22 thermogenesis,23 nutrient sensing,6 endolysosomal transport and functions,19,20 exocytosis,24,25 cytokinesis,26 fertilization and embryogenesis,27 cell differentiation,28-31 angiogenesis,32 endothelium activation mediated by histamine,33 smooth muscle contraction,34 autophagy,35-40 skin pigmentation,41 or even to the Ebola virus infection mechanism.42 As well, recent studies have suggested their possible implication in the pathogenesis of Alzheimer disease,36 myocardial ischemia,43 fatty liver disease,44 type 2 diabetes mellitus,45 obesity23 or Parkinson disease.46 Thus, TPCs have become attractive therapeutic targets, although it is necessary to go deeper into their main functions and mechanisms of action not only to clinically relevant compounds could be designed but also to understand the pathophysiology of an increased wide range of diseases related to TPCs function/dysfunction.
Structure
The TPC gene (TPCN) is widespread across the animal kingdom, which is indicative of its phylogenetic and functional relevance, and has undergone multiplication (TPCN1-3), with different species containing different types of TPCs.47 Plants express only a single 2-pore channel protein, TPC1,2 (or in the case of tobacco have 2 very homologous channel proteins48); primates, mice and rats have only 2 members (TPC1-2; TPC3 seems to be a pseudogene);4,47,49 and the 3 TPC proteins are expressed in most other mammals, birds, frogs, fishes or sea urchins, for instace,47 being TPC1 and TPC2 the deepest studied forms throughout the literature.
TPCs belong to the superfamily of the voltage-gated ion channels (VGICs),1 which are large transmembrane proteins that enable the passage of ions through their pore across the cell membrane, being imperative for neuronal signaling, muscle contraction or secretion.50 VGICs are composed by α-subunits or Shaker like-domains containing 6 transmembrane segments (S1–S6), being required to constitute a functional ion channel the assemblage of 4 domains, which is the common structural motif for this family.51 The common 4-domain structure of the VGICs could be formed by the association of proteins containing just one domain that bind to form tetramers (K+ channels), or by the rearrangement of one protein with 4 domains (Na+ and Ca2+ channels).52 Based on their structural similarity, it was proposed that an ancestral Na+ and Ca2+ α-subunit could be formed by 2 rounds of duplication of a single channel domain similar to voltage-activated K+ channels.53 Their activation is enabled by conserved positively charged residues at every third position in S4 (voltage sensor) of the 4 domains, which move outwards upon changes in membrane potential, inducing a conformational change that results in the opening of the channel pore, which is formed by the segments S5 and S6.50 The selectivity to specific ions is enabled by the selectivity filter, which is composed of conserved residues, specific for the ion conducted by the channel, that are situated at the pore-lining loops (p-loops) connecting S5 to S6 in the domains.50 Moreover, many VGICs include in addition to the α-subunit one or more auxiliary subunits that modify their expression levels, folding efficiency, functional properties or subcellular localization.50
TPCs are so-called because they contain 2 homologous Shaker-like domains with 6 transmembrane regions and a re-entrant pore loop between S5 and S6 arranged in tandem and connected by a cytosolic linker, so that it is necessary the dimerization of TPCs to form the 4-domain structure that enables the channel functionality, which can be composed by homo- or heterodimers that interact in a rotational/head-to-tail symmetry (the NH2-terminus of one monomer with the COOH-terminus of the other monomer, both of them facing the cytosol) (Fig. 1).54-57 This structural organization places TPCs in a key position in the evolution of VGICs, being an evolutionary intermediate linker between tetrameric one-domain channels and monomeric 4-domain channels.9 TPCs have been found to have structural homologies to the transient receptor potential (TRP) channel superfamily and the cation channel of sperm (CatSper), both of them tetrameric one-domain channels,55 and recent phylogenetic analyses have related each domain of TPCs to counterparts in Ca2+ and Na+ channels, supporting a common ancestry,17 although it was suggested that TPCs are more closely related to TRP channels, followed by CatSper channels and, more distantly, to Na+ and Ca2+ channels.58 Moreover, many of the TRP channels also have preferential interaction in rotational symmetry,59 which supports again the evolutionary role of TPCs between tetrameric one-domain channels and monomeric 4-domain channels.
Figure 1.
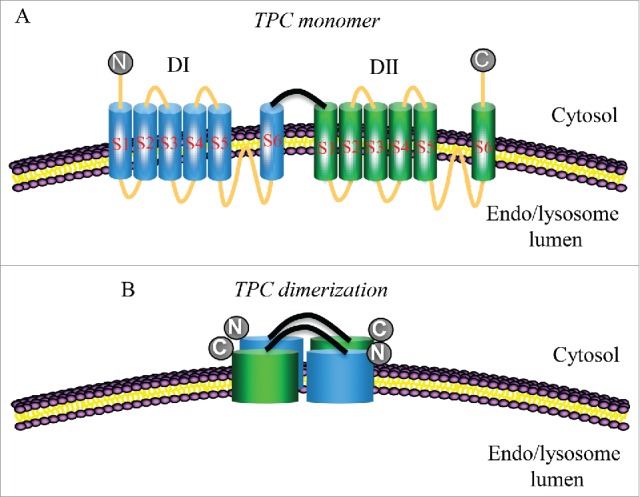
Schematic drawing of endolysosomal membrane showing the localization of the transmembrane topology of TPCs. They have 2 putative pore-forming repeats (DI-II), each containing 6 transmembrane segments (S1-6) and an intervening pore-loop between S5 and S6. N: NH2-terminal region. C: COOH-terminal region.
Studies performed in Human Embrionic Kidney 293 (HEK293) and SkBr3 human breast cancer cells have recently shown that TPC1 and TPC2 are N-glycosylated.54,60,61 N-Linked glycosylation is the process whereby carbohydrate residues are added to asparagine residues of proteins, specifically a subset residing in the Asn-X-Ser/Thr motif (where X is any residue except proline), that can affect many key biological processes including cell adhesion, molecular trafficking and clearance, receptor activation, signal transduction, and endocytosis.62 Both TPC1 and TPC2 are N-glycosylated conforming to the consensus Asn-X-Ser/Thr at residues 599, 611, and TPC1 has an additional glycosylation site at residue 616.54 These N-glycosylations seems to inhibit NAADP-evoked Ca2+ release through TPCs.54
The three types of TPC have positively charged voltage-sensing motifs in the S4 transmembrane domain characteristic of the VGICs superfamily, however, it was suggested that only TPC1 and TPC3 are regulated by voltage,14,63 whereas TPC2 is not.8,16
TPC1 mRNA transcripts have a molecular weight around 5 kb and those for TPC2 around 3 kb in murine (Table 1).1,60 However, northern blot and RT-PCR analysis of TPC1 expression in rats and mice confirmed the presence of 2 TPC1 transcripts with different size, being now referred as TPC1A (the first characterized isoform, ∼ 5 kb) and TPC1B (a smaller isoform, ∼ 4 kb).1,20 TPC1B contains an alternative 5 untranslated region (UTR) by initiating from sequences immediately upstream from exon 3 that are intronic relative to TPC1A.20 The 2 TPC1 isoforms are likely to originate from different promoters and have different 5´-UTRs, which suggests that their expression can be differentially regulated and could result in distinct relative levels of expression in a tissue- and/or cell-type-specific manner and differential regulation by intracellular signals.20 As well, searches of human cDNA libraries also revealed the presence of equivalent human TPC1B transcripts, suggesting that the expression of the TPC1B isoform might be evolutionarily conserved.20
Table 1.
Summary of TPCs gene, mRNA and protein location, mass and length according to the NCBI Gene database90 and the UniProt database.88
| TPC1 |
|||||
|---|---|---|---|---|---|
| Gene ID | Chromosome location | Exon count | Protein mass (Da) | mRNA lenght (pb) | |
| Rattus norvergicus | 246215 | 12q16 | 26 | 94,405 | 4,735 |
| Mus musculus | 252972 | 5; 5 F | 30 | 94,496 | 4,666 |
| Homo sapiens | 53373 | 12q24.13 | 34 | 94,147 | 5,489 |
| TPC2 | |||||
| Rattus norvergicus | 309139 | 1q42 | 25 | 59,848 | 2,879 |
| Mus musculus | 233979 | 7; 7 F5 | 36 | 83,595 | 2,993 |
| Homo sapiens | 219931 | 11q13.3 | 30 | 85,243 | 5,026 |
Plant TPC1 is located in vacuoles (acidic Ca2+ store in plants) and acts as a Ca2+ release channel that mediates long-range Ca2+ waves.3,64 Unlike mammalian TPCs, plant TPC1 has an EF-hand domain separating the 2 Shaker-like pore-forming subunits, which contributes to the Ca2+-sensitive activity of TPC1,56,65 although plant TPC1 has been shown to be a non selective ion channel.56
Both TPC1 (Fig. 2) and TPC2 (Fig. 3) proteins are highly conserved between human, mice and rats, with more than a 70% identity, which suggests an important biological function for these channels.
Figure 2.

Multiple alignment of TPC1 sequence from the UniProt database.88 (a) Clustal-Omega alignment89 between TPC1 sequences of Homo sapiens, Mus musculus and Rattus norvergicus. *(asterisk) indicates positions which have a single, fully conserved residue, :(colon) indicates conservation between groups of strongly similar properties - scoring > 0.5 in the Gonnet PAM 250 matrix, and . (period) indicates conservation between groups of weakly similar properties - scoring =< 0.5 in the Gonnet PAM 250 matrix. (b) Percent identity matrix derived from the alignment.
Figure 3.

Multiple alignment of TPC2 sequence from the UniProt database.88 (a) Clustal-Omega alignment89 between TPC2 sequences of Homo sapiens, Mus musculus and Rattus norvergicus. *(asterisk) indicates positions which have a single, fully conserved residue, :(colon) indicates conservation between groups of strongly similar properties - scoring > 0.5 in the Gonnet PAM 250 matrix, and . (period) indicates conservation between groups of weakly similar properties - scoring = < 0.5 in the Gonnet PAM 250 matrix. (b) Percent identity matrix derived from the alignment.
Intracellular and tissue expression
Consistent with their role in Ca2+ release from acidic organelles, TPCs localize to the endo-lysosomal system, with TPC2 located predominantly to lysosomes through an N-terminal dileucine motif, whereas TPC1 is more broadly distributed throughout the endo-lysosomal system in animals. In plants, an N-terminal dileucine motif also directs TPC1 to the vacuoule membrane.66 This particular localization of TPCs in acidic organelles, which are Ca2+ stores that mediate the main intracellular signaling cascades throughout kingdoms,9 suggests an important role for these channels in animal and plant cell physiology.
Northern blot analysis have shown a wide expression of TPC1 mRNA in rats, including kidney, liver, lung, spleen, heart, brain, skeletal muscle, and testis,1 and in heart, brain, liver, spleen, kidney, embryo, lung, thymus, testis and ovary in mice.60 As well, mRNA expression of TPC1 was detected by RT-PCR in heart, kidney, spleen, liver, lung and adipose tissue in mice,20 in heart tissue in rats,40 and in human myocardium, where TPC1 has increased expression in patients with dilated cardiomyopathy67 and in women compared to men,40 a gender difference also confirmed in rats. Protein levels of TPC1 were observed by western blot in human and rat heart tissue.40,67
Regarding TPC2, RNA gel blot analysis showed the same tissue distribution as TPC1 in mice, although with lower expression,60 and, in human, TPC2 was detectable in lung, placenta, small intestine, liver, kidney, spleen, thymus, colon, skeletal muscle, heart and brain.4 RT-PCR showed TPC2 mRNA expression in pancreas in mice,21 in liver and heart in rats,40,68 and in the human heart.40,67 Protein expression of TPC2 was detectable by protein gel blot in liver in mice,44 and in human and rat hearts,40,67 and by immunofluorescence in human pancreatic β-cells.21
Activity regulation
TPCs were initially described as Ca2+ release channels activated by NAADP, so that over-expression of TPCs enhanced NAADP-induced responses whereas knockdown (KD) or knockout (KO) reduced or prevented them.4,10,60,69-71 Soon after, TPCs were also described as PtdIns(3,5)P2-regulated Na+ selective channels,5,6 and, again, overexpression of TPCs stimulated the PI(3,5)P2-induced current, while this current was abrogated in double-KO mice lacking TPC1 and TPC2.5,6,71
Nowadays, different molecules have been suggested as regulators of TPCs activity. Ca2+ has been shown to activate TPCs either from the endo-lysosome lumen or from the cytosol.11,12,14 NAADP-induced Ca2+ release from acidic stores seems to be dependent on Ca2+ load, so that depletion of Ca2+ stores below a threshold level could terminate TPCs Ca2+ flux even in the maintained presence of NAADP, allowing for refilling of the stores until the threshold is again reached.12 This mechanism of luminal Ca2+ control could lead to the Ca2+ oscillations that are characteristic of NAADP signaling in physiological systems.12
On the other hand, TPC2 has been suggested to be strongly inhibited by Mg2+ ions, which selectively inhibitis outward Na+ currents regardless of NAADP or PtdIns(3,5)P2.15 This inhibition is more effective from the cytoplasmic free Mg2+ than from the luminal face, and small changes in cytoplasmic free Mg2+ markedly affect the activity of TPC2 and thus the lysosomal membrane potential.15 A decrease in cytoplasmic free Mg2+ is suggested to depolarize the lysosomal membrane potential to affect the transport of all electrogenic coupled and uncoupled transporters, including facilitation of Ca2+ release from the lysosomes.15 In this line, TPC1 and TPC3 have been probed to be regulated by membrane potential,11,14,16,63 whereas TPC2 seems to be independent of membrane potential changes,12,14,72 in spite of the presence of the positively charged voltage-sensing motifs in the S4 transmembrane domain. And some studies have reported that acidic luminal pH activates TPCs,11,14,72 however, in others it was found that acidic pH inhibits TPCs opening.12,16
TPCs have been also suggested to function as nutrient sensors linked to mTOR action: nutrient replete cells have high adenosine triphosphate (ATP), which presumably enables mTOR to phosphorylate TPCs and/or its associated proteins and maintain the channel in a closed state. During cell starvation, ATP levels fall, mTOR delocalizes from TPCs, and the channel opens.6,15,38 As well, due to the fact that TPCs are involved in the regulation of multiple biological functions, different protein kinasas have been suggested to regulate TPCs activity: the leucine-rich repeat kinase 2 (LRRK2) has been proposed to regulate autophagy through TPC2 activation13 while JNK and P38 kinases have shown to inhibit TPC2 NAADP-mediated Ca2+ release.15
Finally, it has been recently demonstrated that TPC1 function is dependent of a COOH-terminal helix that allow channel dimerization and, consequently, its function.73
NAADP-dependent biological functions
Differentiation
NAADP signaling through the action of TPCs is essential for the differentiation of many cell types.29 TPCs have an important role in neuronal development; in fact, it has been shown a liposomal delivery of NAADP mediated release of Ca2+ from acidic Ca2+ stores, and that this stimulus was enough to drive differentiation of the PC12 cells (derived from a pheochromocytoma of the rat adrenal medulla) to a neuronal-like phenotype.74 Moreover, Zhang et al.28 have observed that the expression of TPC2 was markedly decreased during the initial stem cell entry into neural progenitors, and that it was gradually rebounded during the late stages of neurogenesis. Additionally, TPC2 KD accelerated mouse stem cell differentiation into neural progenitors, but inhibited these neural progenitors from committing to neurons, and had no effect on the differentiation of astrocytes and oligodendrocytes of mouse stem cells.28
Otherwise, in skeletal muscle cells, Aley et al.29 studies strongly supported a role of the TPCs in the differentiation process of skeletal muscle cells: treatment of C2C12 cells (a mouse myoblast cell line) with TPC2 siRNA resulted in a decreased differentiation and fusion index,29 while TPC1 siRNA treated C2C12 cells showed a reduced number of multinucleated myotubes; although the number of nuclei per myotube did not differ from control cells and, additionally, treatment with Ned-19 (an NAADP agonist) interfere with the differentiation of C2C12 cells.29
Ultimately, it has been demonstrated that the expression levels of TPC2 were enhanced during osteoclast differentiation and,30 although the mechanism which causes this suppression remains to be determined, current findings indicate that TPC2 could be a differentiation indicator of osteoclastic cells.30
Angiogenesis
It has been shown that in the endothelium there are Ca2+ receptors activated by NAADP able to regulate vascular smooth muscle contractility and blood pressure.75 Futhermore, it has been identified NAADP as a crucial second messenger in histamine-induced Ca2+ release via H1 receptors (H1R) in endothelial cells:33 it have been shown that selective H1R activation increases intracellular NAADP levels, and that H1R induced Ca2+ release and Von Willebrand factor secretion;33 however, TPC1 and TPC2 simultaneous silencing block this secretion, suggesting an important role of TPCs in this process.33
Favia et al.32 demonstrated in a recent report in human umbilical vein endothelial cells (HUVEC) cells and in TPC1/TPC2 KO mice that vascular endothelial growth factor receptor 2 (VEGFR2) activation induced NAADP-dependent Ca2+ release and,32 in fact, both the use of Ned-19 or TPC2 silencing in vitro, and also in vivo studies in TPC2 KO mice, showed an inhibition of angiogenesis induced by VEGFR2 activation.32
Smooth muscle contraction
In the detrusor muscle, activation of muscarinic receptors (the main physiological mechanism for emptying the urinary bladder) by carbachol results in a contractile response through the release of Ca2+ dependent acidic organelles.34 It has been also shown that this response is mediated by NAADP, disappearing into estrusor muscle preparations from TPC2 KO mice.34 Similarly, in smooth muscle preparations of rat gastric fundus, it has also been shown that carbachol induces the release of Ca2+ dependent from TPCs and NAADP.76
Metabolism
The endolysosomal organelles play a key role in trafficking and recycling different macromolecules such as low-density lipoprotein (LDL)-cholesterol, epithelial growth factor (EGF) or transferrin.77,78 In fact, Grimm et al.44 have demonstrated in primary fibroblasts and hepatocytes cultures from TPC2 KO mice that, in the absence of this channel, there is a damage in cholesterol trafficking and in the epidermal growth factor receptor (EGFR), that could be attributed to a dysfunction in endolysosomal degradation due to the absence of fusion between the lysosome and the endosome.44 To note, mice deficient in TPC2 had liver damage, compatible with a non alcoholic esteohepatitis.44
Recently, it has been shown that TPC1/2 KO mice have a respiratory quotient higher than the wild-type (WT) mice and that develop obesity between 6 and 9 months of age.23 Expression levels of hormone-sensitive lipase (HSL) and lipid availability are reduced in brown adipose tissue in TPC1/2 KO mice, in which also exist a defect in β-adrenergic receptor signaling that leads to all these metabolic alterations.23
Autophagy
Autophagy is an essential biological process that provides for cellular “quality control” and determines the turnover of long-lived proteins and the selective degradation of damaged cellular components to maintain tissue homeostasis.38 This process involves a degradation by the autolysosome, which is formed by fusion of an autophagosome and a lysosome, which suggests that NAADP signaling and TPCs could be involved in this process.79 The first study that established a possible relationship between TPCs and autophagy showed that in rat astrocytes NAADP increased acidic vesicular organelle formation and contributed to increased autophagy markers as LC3II and Beclin1;35 this data provided an evidence that NAADP-evoked Ca2+ signals mediated by TPCs regulated autophagy in astrocytes.35 Later, Kayala et al.36 associated the absence of presenilins (which are important for the proteolysis process during autophagy) with abnormalities in lysosomal Ca2+ and changes in expression levels and dimerization of TPCs, which they hypothesized that could lead to an autophagy disruption in these cells. In 2013, Lu et al.80 found that overexpression of TPC2 in HeLa or mouse embryonic stem cells leaded to an acumulattion of LC3II, p62 and autophagosomes, suggesting an inhibition of fusion between the lysosome and the autophagosome. This group postulated that this overexpression of TPC2, through NAADP and Ca2+ pathway, could alkalinize lysosomal pH and prevent the recruitment of Rab7 (an important protein involved in late endocytic membrane traffic regulation); therefore, the fusion between the lysosome and the autophagosome would be inhibited.37 However, TPC2 KD or treatment of cells with Ned-19, an NAADP antagonist, reversed this process and markedly reduced the accumulation of autophagosomes.37 Later, in 2014, Lin et al.38 showed that TPC2 contributed to autophagy through the regulation of the protein homeostasis in skeletal muscle: TPC2 KO mice muscles showed atrophy compared to WT mice, which resulted from a harmful increase of autophagosomes accumulation during autophagy induced by energy deprivation and chloroquine, suggesting that these effects could be mediated by a change in pH or a reduced activity of the lysosomal proteases.38 It has also been observed an association between mTOR and TPC2 in skeletal muscle: the absence of TPC2 prevents reactivation of mTOR during prolonged deprivation, suggesting that there is a relationship between both, and that no proper termination of autophagy occurs in those conditions.38
Moreover, it has been determined an interaction between human TPC1/2 and the anti-apoptotic protein Hax-1 (that is a negative regulator of autophagy and apoptosis); and this link may represent a conserved mechanism by which these endolysosomal ion channels are regulated.39
Recently, out group has demonstrated that, in cultured cardiomyocytes, starvation induced a significant increase in TPC1 and TPC2 transcript and protein levels that paralleled the increase in autophagy identified by increased LC3-II and decreased p62 levels.40 Moreover, we proved that small interfering RNA depletion of TPC2 alone or together with TPC1 increased both LC3-II and p62 levels under basal conditions and in response to serum starvation, suggesting that, under conditions of severe energy depletion, changes in the autophagic flux occurred either when TPC1 or TPC2 were downregulated.40 Finally, we reported that the TPC1/2 double KO mice cardiomyocytes contained larger numbers of immature lysosomes with diameters significantly smaller tan those of WT mice; and that in cardiac tissues from humans and rats, TPCs transcripts and protein levels were higher in females than in males.40 Therefore, this study concludes that the endolysosomal channels TPC1 and TPC2 are essential for appropriate basal and induced autophagic flux in cardiomyocytes, and also that they are differentially expressed in male and female hearts.40
Control of Ebola virus host cell entry
Ebola viruses (EBOVs) are the causative agents of a severe form of viral hemorrhagic fever in man, designated Ebola hemorrhagic fever, constituting this virus Marburg virus the family Filoviridae.81 Infections caused for these virus are characterized by immune suppression and systemic inflammatory responses that causes impairment of the vascular, coagulation, and immune systems, leading to multiorgan failure and shock, and thus, in some ways, resembling septic shock.81 Like the majority of the viruses, EBOV depends on its host to complete its cycle, therefore, understanding how this mechanism occur is highly important to be able to develop an effective cure.82
Previously, it has been proposed that EBOV joined glycoproteins from the cell surface which led to its internalization through the endocytic pathway.83 In 2015, Sakurai et al, demonstrated that TPC2 is necessary for the release of the viral genome EBOV within the host cell.42 To reach these results, they first observed that mouse embryonic fibroblasts from TPC1 and TPC2 KO mice resisted EBOV infection,42 however, TPCs overexpression in human mutant cells retrieve this infectivity.42 The silencing of TPCs through interfering RNAs in HeLa cells, prevented complete EBOV infections.42 Moreover, overexpression of a dominant negative form of TPC2 (NAADP blocking and therefore blocking the cycle by which the Ca2+ induces the release of more Ca2+) also inhibited EBOV infection.42 Finally, this group also showed that tetrandrine (a type of alkaloid) was able to block the activity of TPC2 and therefore could block EBOV infection.42
PI(3,5)P2-dependent biological functions
Ion homeostasis
The PtdIns(3,5)P2 is a phosphoinositide formed by the action of the PI(3)P 5-kinase (PIKfyve) complex that phosphorylates PtdIns(3)P to yield PI(3,5)P2).84 Phosphoinositides (PIPs), specifically PtdIns(3,5)P2, can determine the vesicular identity and the direction of membrane trafficking, both of them essential functions for endolysosomal trafficking.85 Shen et al.85 demonstrated that PI(3,5)P2-deficient cells are characterized for enlarged endolysosomes/vacuoles and trafficking defects in the late endocytic pathways; however, it was not until 2012 when, based on patch-clamp studies, in the fibroblast-like cell line derived from monkey kidney tissue (COS-1) endolysosomes, and in TPC1 and TPC2 KO mice, was proposed the hypothesis that the TPCs are Na+ channels activated by PtdIns(3,5)P2.5
Metabolism/nutrient sensing
Cang et al.6 showed in HEK293 cells and in TPC1/2 KO mice that mTOR is capable of associating and controlling the activation of an endolysosomal Na+ channel adenosine triphosphate (ATP) (lysoNaATP) sensitive, formed by TPCs and other proteins. Thus, when cells have enough energy or, what is the same, when cells have a high concentration of ATP, mTOR phosphorylates the TPCs and/or their associated proteins to maintain the channel in a closed state and, conversely, when ATP levels are low (for energy deprivation, for example), mTOR is delocalized of TPCs, and this causes the channel opening, which allows Na+ and other ions leave the lysosome.6 Therefore, TPCs have been proposal to be the link between mTOR signaling and lysosomes, which monitors the response to nutrient deprivation, hypoxia and cell growth.86
Parkinson's disease
Parkinson disease is a common incurable neurodegenerative disorder whose origin is still unknown, and mainly characterized by the loss of dopaminergic neurons in the midbrain (specifically, substantia nigra pars compacta).9 Also, this disease is characterized for the presence of intraneuronal proteinaceous inclusions called Lewy bodies.87 The presence of this proteinaceous aggregates in the brain suggests a defect in protein handling system as part of the etiology of the disease.87 Different reports confirmed this hypothesis, as evidenced by an accumulation of autophagic vesicles associated with a lysosomal depletion.87 In fact, a recent report evidenced altered lysosome morphology in fibroblasts from patients with the common G2019S mutation in LRRK2 (also known as PKR8), which causes an autosomal dominant form of the disease.46 Patients with this mutation present an endolysosomal system morphology markedly disrupted and characterized by clumped and swollen lysosomes, which could reflect a trafficking defect within the endolysosomal system.46 Defects in LRRK2G2019S fibroblasts were reversed by: 1) molecular silencing of TPC2 but not TPC1; 2) pharmacological inhibition of TPC regulators (NAADP or Rab7); and 3) buffering local Ca2+ increases.46 Such interactions suggest that TPC2 acts downstream of pathogenic LRRK2 to regulate trafficking within the endolysosomal system in a pathway of potential relevance to the pathology of LRRK2-mediated Parkinson disease; and this interactions are of potential therapeutic relevance to Parkinson disease, although it is not yet known whether TPCs deregulate trafficking in neurons.46
Conclusion
Regardless of the lack of knowledge about their exact mechanism of action, TPCs are beginning to be linked to the development of different pathologies, suggesting that the pharmacological targeting of these channels may be clinically beneficial (Fig. 4). However, it is too soon to try to develop an effective drug to regulate TPCs activity in order to improve/prevent a concrete pathology because 1) TPCs have been proved to participate in a wide range of pathophysiological processes, so their modulation could affect other processes, and 2) there exist different and, even more, contradictory results regarding TPCs function and regulation, so it is probable that under different experimental conditions/physiological situations TPCs could act in a different manner.
Figure 4.
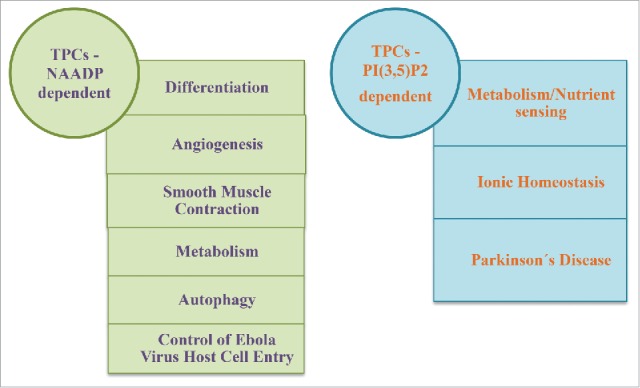
Main functions mediated by TPCs activated by NAADP or by PI(3,5)P2.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Ishibashi K, Suzuki M, Imai M. Molecular cloning of a novel form (two-repeat) protein related to voltage-gated sodium and calcium channels. Biochem Biophys Res Commun 2000; 270:370-6; PMID:10753632; http://dx.doi.org/ 10.1006/bbrc.2000.2435 [DOI] [PubMed] [Google Scholar]
- [2].Furuichi T, Cunningham KW, Muto S. A putative two pore channel AtTPC1 mediates Ca(2+) flux in Arabidopsis leaf cells. Plant Cell Physiol 2001; 42:900-5; PMID:11577183; http://dx.doi.org/ 10.1093/pcp/pce145 [DOI] [PubMed] [Google Scholar]
- [3].Peiter E, Maathuis FJM, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 2005; 434:404-8; PMID:15772667; http://dx.doi.org/ 10.1038/nature03381 [DOI] [PubMed] [Google Scholar]
- [4].Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang K-T, et al.. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 2009; 459:596-600; PMID:19387438; http://dx.doi.org/ 10.1038/nature08030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang X, Zhang X, Dong X-P, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, et al.. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell 2012; 151:372-83; PMID:23063126; http://dx.doi.org/ 10.1016/j.cell.2012.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cang C, Zhou Y, Navarro B, Seo Y, Aranda K, Shi L, Battaglia-Hsu S, Nissim I, Clapham DE, Ren D. mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell 2013; 152:778-90; PMID:23394946; http://dx.doi.org/ 10.1016/j.cell.2013.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marchant JS, Patel S. Questioning regulation of two-pore channels by NAADP. Messenger (Los Angeles, Calif Print) 2013; 2:113-9; PMID:24829847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morgan AJ, Davis LC, Ruas M, Galione A. TPC: the NAADP discovery channel? Biochem Soc Trans 2015; 43:384-9; PMID:26009180; http://dx.doi.org/ 10.1042/BST20140300 [DOI] [PubMed] [Google Scholar]
- [9].Patel S. Function and dysfunction of two-pore channels. Sci Signal 2015; 8:re7-re7; PMID:26152696; http://dx.doi.org/ 10.1126/scisignal.aab3314 [DOI] [PubMed] [Google Scholar]
- [10].Morgan AJ, Galione A. Two-pore channels (TPCs): current controversies. Bioessays 2014; 36:173-83; PMID:24277557; http://dx.doi.org/ 10.1002/bies.201300118 [DOI] [PubMed] [Google Scholar]
- [11].Pitt SJ, Lam AKM, Rietdorf K, Galione A, Sitsapesan R. Reconstituted human TPC1 is a proton-permeable ion channel and is activated by NAADP or Ca2+. Sci Signal 2014; 7:ra46; PMID:24847115; http://dx.doi.org/ 10.1126/scisignal.2004854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pitt SJ, Funnell TM, Sitsapesan M, Venturi E, Rietdorf K, Ruas M, Ganesan A, Gosain R, Churchill GC, Zhu MX, et al.. TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+. J Biol Chem 2010; 285:35039-46; PMID:20720007; http://dx.doi.org/ 10.1074/jbc.M110.156927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gómez-Suaga P, Luzón-Toro B, Churamani D, Zhang L, Bloor-Young D, Patel S, Woodman PG, Churchill GC, Hilfiker S. Leucine-rich repeat kinase 2 regulates autophagy through a calcium-dependent pathway involving NAADP. Hum Mol Genet 2012; 21:511-25; PMID:22012985; http://dx.doi.org/ 10.1093/hmg/ddr481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rybalchenko V, Ahuja M, Coblentz J, Churamani D, Patel S, Kiselyov K, Muallem S. Membrane potential regulates Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) dependence of the pH- and Ca2+-sensitive organellar two-pore channel TPC1. J Biol Chem 2012; 287:20407-16; PMID:22500018; http://dx.doi.org/ 10.1074/jbc.M112.359612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jha A, Ahuja M, Patel S, Brailoiu E, Muallem S. Convergent regulation of the lysosomal two-pore channel-2 by Mg2+, NAADP, PI(3,5)P2 and multiple protein kinases. EMBO J 2014; 33:501-11; PMID:24502975; http://dx.doi.org/ 10.1002/embj.201387035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cang C, Bekele B, Ren D. The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat Chem Biol 2014; 10:463-9; PMID:24776928; http://dx.doi.org/ 10.1038/nchembio.1522 [DOI] [PubMed] [Google Scholar]
- [17].Rahman T, Cai X, Brailoiu GC, Abood ME, Brailoiu E, Patel S. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci Signal 2014; 7:ra109-ra109; PMID:25406377; http://dx.doi.org/ 10.1126/scisignal.2005450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Churamani D, Hooper R, Rahman T, Brailoiu E, Patel S. The N-terminal region of two-pore channel 1 regulates trafficking and activation by NAADP. Biochem J 2013; 453:147-51; PMID:23634879; http://dx.doi.org/ 10.1042/BJ20130474 [DOI] [PubMed] [Google Scholar]
- [19].Ruas M, Rietdorf K, Arredouani A, Davis LC, Lloyd-Evans E, Koegel H, Funnell TM, Morgan AJ, Ward JA, Watanabe K, et al.. Purified TPC isoforms form NAADP receptors with distinct roles for Ca2+ signaling and endolysosomal trafficking. Curr Biol 2010; 20:703-9; PMID:20346675; http://dx.doi.org/ 10.1016/j.cub.2010.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ruas M, Chuang K-T, Davis LC, Al-Douri A, Tynan PW, Tunn R, Teboul L, Galione A, Parrington J. TPC1 has two variant isoforms, and their removal has different effects on endo-lysosomal functions compared to loss of TPC2. Mol Cell Biol 2014; 34:3981-92; PMID:25135478; http://dx.doi.org/ 10.1128/MCB.00113-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Arredouani A, Ruas M, Collins SC, Parkesh R, Clough F, Pillinger T, Coltart G, Rietdorf K, Royle A, Johnson P, et al.. Nicotinic acid adenine dinucleotide phosphate (NAADP) and endolysosomal two-pore channels modulate membrane excitability and stimulus-secretion coupling in mouse pancreatic beta cells. J Biol Chem 2015; 290:21376-92; PMID:26152717; http://dx.doi.org/ 10.1074/jbc.M115.671248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cane MC, Parrington J, Rorsman P, Galione A, Rutter GA. The two pore channel TPC2 is dispensable in pancreatic β-cells for normal Ca(2+) dynamics and insulin secretion. Cell Calcium 2016; 59:32-40; PMID:26769314; http://dx.doi.org/ 10.1016/j.ceca.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lear P V, González-Touceda D, Porteiro Couto B, Viaño P, Guymer V, Remzova E, Tunn R, Chalasani A, García-Caballero T, Hargreaves IP, et al.. Absence of intracellular ion channels TPC1 and 2 leads to mature-onset obesity in male mice, due to impaired lipid availability for thermogenesis in brown adipose tissue. Endocrinology 2014; 156:en20141766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Davis LC, Morgan AJ, Chen J-L, Snead CM, Bloor-Young D, Shenderov E, Stanton-Humphreys MN, Conway SJ, Churchill GC, Parrington J, et al.. NAADP activates two-pore channels on T cell cytolytic granules to stimulate exocytosis and killing. Curr Biol 2012; 22:2331-7; PMID:23177477; http://dx.doi.org/ 10.1016/j.cub.2012.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Arndt L, Castonguay J, Arlt E, Meyer D, Hassan S, Borth H, Zierler S, Wennemuth G, Breit A, Biel M, et al.. NAADP and the two-pore channel protein 1 participate in the acrosome reaction in mammalian spermatozoa. Mol Biol Cell 2014; 25:948-64; PMID:24451262; http://dx.doi.org/ 10.1091/mbc.E13-09-0523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Horton JS, Wakano CT, Speck M, Stokes AJ. Two-pore channel 1 interacts with citron kinase, regulating completion of cytokinesis. Channels (Austin) 2015; 9:21-9; PMID:25665131; http://dx.doi.org/ 10.4161/19336950.2014.978676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ramos I, Reich A, Wessel GM. Two-pore channels function in calcium regulation in sea star oocytes and embryos. Development 2014; 141:4598-609; PMID:25377554; http://dx.doi.org/ 10.1242/dev.113563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhang Z-H, Lu Y-Y, Yue J. Two pore channel 2 differentially modulates neural differentiation of mouse embryonic stem cells. PLoS One 2013; 8:e66077; PMID:23776607; http://dx.doi.org/ 10.1371/journal.pone.0066077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Aley PK, Mikolajczyk AM, Munz B, Churchill GC, Galione A, Berger F. Nicotinic acid adenine dinucleotide phosphate regulates skeletal muscle differentiation via action at two-pore channels. Proc Natl Acad Sci U S A 2010; 107:19927-32; PMID:21041635; http://dx.doi.org/ 10.1073/pnas.1007381107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Notomi T, Ezura Y, Noda M. Identification of Two-pore Channel 2 as a Novel Regulator of Osteoclastogenesis. J Biol Chem 2012; 287:35057-64; PMID:22833668; http://dx.doi.org/ 10.1074/jbc.M111.328930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Parrington J, Tunn R. Ca(2+) signals, NAADP and two-pore channels: role in cellular differentiation. Acta Physiol (Oxf) 2014; 211:285-96; PMID:24702694; http://dx.doi.org/ 10.1111/apha.12298 [DOI] [PubMed] [Google Scholar]
- [32].Favia A, Desideri M, Gambara G, D'Alessio A, Ruas M, Esposito B, Del Bufalo D, Parrington J, Ziparo E, Palombi F, et al.. VEGF-induced neoangiogenesis is mediated by NAADP and two-pore channel-2-dependent Ca2+ signaling. Proc Natl Acad Sci U S A 2014; 111:E4706-15; PMID:25331892; http://dx.doi.org/ 10.1073/pnas.1406029111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Esposito B, Gambara G, Lewis AM, Palombi F, Alessio AD, Taylor LX, Genazzani AA, Ziparo E, Galione A, Churchill GC, et al.. NAADP links histamine H1 receptors to secretion of von Willebrand factor in human endothelial cells. Blood 2011; 117:4968-77; PMID:21364192; http://dx.doi.org/ 10.1182/blood-2010-02-266338 [DOI] [PubMed] [Google Scholar]
- [34].Tugba Durlu-Kandilci N, Ruas M, Chuang K-T, Brading A, Parrington J, Galione A. TPC2 proteins mediate nicotinic acid adenine dinucleotide phosphate (NAADP)- and agonist-evoked contractions of smooth muscle. J Biol Chem 2010; 285:24925-32; PMID:20547763; http://dx.doi.org/ 10.1074/jbc.M110.129833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Pereira GJS, Hirata H, Fimia GM, do Carmo LG, Bincoletto C, Han SW, Stilhano RS, Ureshino RP, Bloor-Young D, Churchill G, et al.. Nicotinic acid adenine dinucleotide phosphate (NAADP) regulates autophagy in cultured astrocytes. J Biol Chem 2011; 286:27875-81; PMID:21610076; http://dx.doi.org/ 10.1074/jbc.C110.216580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Neely Kayala KM, Dickinson GD, Minassian A, Walls KC, Green KN, Laferla FM. Presenilin-null cells have altered two-pore calcium channel expression and lysosomal calcium: implications for lysosomal function. Brain Res 2012; 1489:8-16; PMID:23103503; http://dx.doi.org/ 10.1016/j.brainres.2012.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lu Y, Hao B-X, Graeff R, Wong CWM, Wu W-T, Yue J. Two pore channel 2 (TPC2) inhibits autophagosomal-lysosomal fusion by alkalinizing lysosomal pH. J Biol Chem 2013; 288:24247-63; PMID:23836916; http://dx.doi.org/ 10.1074/jbc.M113.484253 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [38].Lin P-H, Duann P, Komazaki S, Park KH, Li H, Sun M, Sermersheim M, Gumpper K, Parrington J, Galione A, et al.. Lysosomal two-pore channel subtype 2 (TPC2) regulates skeletal muscle autophagic signaling. J Biol Chem 2015; 290:3377–89; PMID: 25480788; http://dx.doi.org/24188827 10.1074/jbc.M114.608471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Lam AKM, Galione A, Lai FA, Zissimopoulos S. Hax-1 identified as a two-pore channel (TPC)-binding protein. FEBS Lett 2013; 587:3782-6; PMID:24188827; http://dx.doi.org/ 10.1016/j.febslet.2013.10.031 [DOI] [PubMed] [Google Scholar]
- [40].García-Rúa V, Feijóo-Bandín S, Rodríguez-Penas D, Mosquera-Leal A, Abu-Assi E, Beiras A, Seoane L, Lear P, Parrington J, Portolés M, et al.. Endolysosomal two-pore channels regulate autophagy in cardiomyocytes. J Physiol 2016; 594:3061-77; http://dx.doi.org/ 10.1113/JP271332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ambrosio AL, Boyle JA, Aradi AE, Christian KA, Di Pietro SM. TPC2 controls pigmentation by regulating melanosome pH and size. Proc Natl Acad Sci U S A 2016; PMID:27140606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sakurai Y, Kolokoltsov AA, Chen C-CC-C, Tidwell MW, Bauta WE, Klugbauer N, Grimm C, Wahl-Schott C, Biel M, Davey RA. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science (80-) 2015; 347:995-8; PMID:25722412; http://dx.doi.org/ 10.1126/science.1258758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Davidson SM, Foote K, Kunuthur S, Gosain R, Tan N, Tyser R, Zhao YJ, Graeff R, Ganesan A, Duchen MR, et al.. Inhibition of NAADP signalling on reperfusion protects the heart by preventing lethal calcium oscillations via two-pore channel 1 and opening of the mitochondrial permeability transition pore. Cardiovasc Res 2015; 108:357-66; PMID:26395965; http://dx.doi.org/ 10.1093/cvr/cvv226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Grimm C, Holdt LM, Chen C-C, Hassan S, Müller C, Jörs S, Cuny H, Kissing S, Schröder B, Butz E, et al.. High susceptibility to fatty liver disease in two-pore channel 2-deficient mice. Nat Commun 2014; 5:4699; PMID:25144390; http://dx.doi.org/ 10.1038/ncomms5699 [DOI] [PubMed] [Google Scholar]
- [45].Fan Y, Li X, Zhang Y, Fan X, Zhang N, Zheng H, Song Y, Shen C, Shen J, Ren F, et al.. Genetic Variants of TPCN2 Associated with Type 2 Diabetes Risk in the Chinese Population. PLoS One 2016; 11:e0149614; PMID:26918892; http://dx.doi.org/ 10.1371/journal.pone.0149614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hockey LN, Kilpatrick BS, Eden ER, Lin-Moshier Y, Brailoiu GC, Brailoiu E, Futter CE, Schapira AH, Marchant JS, Patel S. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J Cell Sci 2015; 128:232-8; PMID:25416817; http://dx.doi.org/ 10.1242/jcs.164152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhu MX, Ma J, Parrington J, Calcraft PJ, Galione A, Evans AM. Calcium signaling via two-pore channels: local or global, that is the question. Am J Physiol Cell Physiol 2010; 298:C430-41; PMID:20018950; http://dx.doi.org/ 10.1152/ajpcell.00475.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kadota Y, Furuichi T, Ogasawara Y, Goh T, Higashi K, Muto S, Kuchitsu K. Identification of putative voltage-dependent Ca2+-permeable channels involved in cryptogein-induced Ca2+ transients and defense responses in tobacco BY-2 cells. Biochem Biophys Res Commun 2004; 317:823-30; PMID:15081414; http://dx.doi.org/ 10.1016/j.bbrc.2004.03.114 [DOI] [PubMed] [Google Scholar]
- [49].Brailoiu E, Hooper R, Cai X, Brailoiu GC, Keebler M V, Dun NJ, Marchant JS, Patel S. An ancestral deuterostome family of two-pore channels mediates nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J Biol Chem 2010; 285:2897-901; PMID:19940116; http://dx.doi.org/ 10.1074/jbc.C109.081943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Moran Y, Barzilai MG, Liebeskind BJ, Zakon HH. Evolution of voltage-gated ion channels at the emergence of Metazoa. J Exp Biol 2015; 218:515-25; PMID:25696815; http://dx.doi.org/ 10.1242/jeb.110270 [DOI] [PubMed] [Google Scholar]
- [51].Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev 2005; 57:387-95; PMID:16382097; http://dx.doi.org/ 10.1124/pr.57.4.13 [DOI] [PubMed] [Google Scholar]
- [52].Bezanilla F. Voltage-gated ion channels. IEEE Trans Nanobioscience 2005; 4:34-48; PMID:15816170; http://dx.doi.org/ 10.1109/TNB.2004.842463 [DOI] [PubMed] [Google Scholar]
- [53].Strong M, Chandy KG, Gutman GA. Molecular evolution of voltage-sensitive ion channel genes: on the origins of electrical excitability. Mol Biol Evol 1993; 10:221-42; PMID:7680747 [DOI] [PubMed] [Google Scholar]
- [54].Hooper R, Churamani D, Brailoiu E, Taylor CW, Patel S. Membrane topology of NAADP-sensitive two-pore channels and their regulation by N-linked glycosylation. J Biol Chem 2011; 286:9141-9; PMID:21173144; http://dx.doi.org/ 10.1074/jbc.M110.189985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rietdorf K, Funnell TM, Ruas M, Heinemann J, Parrington J, Galione A. Two-pore channels form homo- and heterodimers. J Biol Chem 2011; 286:37058-62; PMID:21903581; http://dx.doi.org/ 10.1074/jbc.C111.289835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kintzer AF, Stroud RM. Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature 2016; 531:258-64; PMID:26961658; http://dx.doi.org/ 10.1038/nature17194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Churamani D, Hooper R, Brailoiu E, Patel S. Domain assembly of NAADP-gated two-pore channels. Biochem J 2012; 441:317-23; PMID:21992073; http://dx.doi.org/ 10.1042/BJ20111617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Clapham DE, Garbers DL. International Union of Pharmacology. L. Nomenclature and structure-function relationships of CatSper and two-pore channels. Pharmacol Rev 2005; 57:451-4; PMID:16382101; http://dx.doi.org/ 10.1124/pr.57.4.7 [DOI] [PubMed] [Google Scholar]
- [59].Fujiwara Y, Minor DL. X-ray crystal structure of a TRPM assembly domain reveals an antiparallel four-stranded coiled-coil. J Mol Biol 2008; 383:854-70; PMID:18782578; http://dx.doi.org/ 10.1016/j.jmb.2008.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rötzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch Eur J Physiol 2009; 458:891-9; PMID:19557428; http://dx.doi.org/ 10.1007/s00424-009-0690-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Brailoiu E, Rahman T, Churamani D, Prole DL, Brailoiu GC, Hooper R, Taylor CW, Patel S. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J Biol Chem 2010; 285:38511-6; PMID:20880839; http://dx.doi.org/ 10.1074/jbc.M110.162073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell 2006; 126:855-67; PMID:16959566; http://dx.doi.org/ 10.1016/j.cell.2006.08.019 [DOI] [PubMed] [Google Scholar]
- [63].Cang C, Aranda K, Ren D. A non-inactivating high-voltage-activated two-pore Na+ channel that supports ultra-long action potentials and membrane bistability. Nat Commun 2014; 5:5015; PMID:25256615; http://dx.doi.org/ 10.1038/ncomms6015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Choi W-G, Toyota M, Kim S-H, Hilleary R, Gilroy S. Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci U S A 2014; 111:6497-502; PMID:24706854; http://dx.doi.org/ 10.1073/pnas.1319955111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Schulze C, Sticht H, Meyerhoff P, Dietrich P. Differential contribution of EF-hands to the Ca 2+-dependent activation in the plant two-pore channel TPC1. Plant J 2011; 68:424-32; PMID:21736651; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04697.x [DOI] [PubMed] [Google Scholar]
- [66].Larisch N, Schulze C, Galione A, Dietrich P. An N-terminal dileucine motif directs two-pore channels to the tonoplast of plant cells. Traffic 2012; 13:1012-22; PMID:22490017; http://dx.doi.org/ 10.1111/j.1600-0854.2012.01366.x [DOI] [PubMed] [Google Scholar]
- [67].Garcia-Rua V, Otero MF, Lear PV, Rodriguez-Penas D, Feijoo-Bandin S, Noguera-Moreno T, Calaza M, Alvarez-Barredo M, Mosquera-Leal A, Parrington J, et al.. Increased expression of fatty-acid and calcium metabolism genes in failing human heart. PLoS One 2012; 7:5-7; http://dx.doi.org/ 10.1371/journal.pone.0037505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tsaih S-W, Holl K, Jia S, Kaldunski M, Tschannen M, He H, Andrae JW, Li S-H, Stoddard A, Wiederhold A, et al.. Identification of a novel gene for diabetic traits in rats, mice, and humans. Genetics 2014; 198:17-29; PMID:25236446; http://dx.doi.org/ 10.1534/genetics.114.162982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, et al.. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol 2009; 186:201-9; PMID:19620632; http://dx.doi.org/ 10.1083/jcb.200904073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Patel S, Ramakrishnan L, Rahman T, Hamdoun A, Marchant JS, Taylor CW, Brailoiu E. The endo-lysosomal system as an NAADP-sensitive acidic Ca2+ store: Role for the two-pore channels. Cell Calcium 2011; 50:157-67; PMID:21529939; http://dx.doi.org/ 10.1016/j.ceca.2011.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ruas M, Galione A, Parrington J. Two-Pore Channels: Lessons from Mutant Mouse Models. Messenger (Los Angeles, Calif Print) 2015; 4:4-22; PMID:27330869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Schieder M, Rötzer K, Brüggemann A, Biel M, Wahl-Schott CA. Characterization of two-pore channel 2 (TPCN2)-mediated Ca2+ currents in isolated lysosomes. J Biol Chem 2010; 285:21219-22; PMID:20495006; http://dx.doi.org/ 10.1074/jbc.C110.143123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Larisch N, Kirsch SA, Schambony A, Studtrucker T, Böckmann RA, Dietrich P. The function of the two-pore channel TPC1 depends on dimerization of its carboxy-terminal helix. Cell Mol Life Sci 2016; 73(13):2565-81; PMID:26781468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Brailoiu E, Churamani D, Pandey V, Brailoiu GC, Tuluc F, Patel S, Dun NJ. Messenger-specific role for nicotinic acid adenine dinucleotide phosphate in neuronal differentiation. J Biol Chem 2006; 281:15923-8; PMID:16595650; http://dx.doi.org/ 10.1074/jbc.M602249200 [DOI] [PubMed] [Google Scholar]
- [75].Brailoiu GC, Gurzu B, Gao X, Parkesh R, Aley PK, Trifa DI, Galione A, Dun NJ, Madesh M, Patel S, et al.. Acidic NAADP-sensitive calcium stores in the endothelium: agonist-specific recruitment and role in regulating blood pressure. J Biol Chem 2010; 285:37133-7; PMID:20876534; http://dx.doi.org/ 10.1074/jbc.C110.169763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Pereira GJS, Hirata H, do Carmo LG, Stilhano RS, Ureshino RP, Medaglia NC, Han SW, Churchill G, Bincoletto C, Patel S, et al.. NAADP-sensitive two-pore channels are present and functional in gastric smooth muscle cells. Cell Calcium 2014; 56:51-8; PMID:24882212; http://dx.doi.org/ 10.1016/j.ceca.2014.04.005 [DOI] [PubMed] [Google Scholar]
- [77].Mayle KM, Le AM, Kamei DT. The intracellular trafficking pathway of transferrin. Biochim Biophys Acta 2012; 1820:264-81; PMID:21968002; http://dx.doi.org/ 10.1016/j.bbagen.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bissig C, Gruenberg J. Lipid sorting and multivesicular endosome biogenesis. Cold Spring Harb Perspect Biol 2013; 5:a016816; PMID:24086044; http://dx.doi.org/ 10.1101/cshperspect.a016816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Parrington J, Lear P, Hachem A. Calcium signals regulated by NAADP and two-pore channels - their role in development, differentiation and cancer. Int J Dev Biol 2015; 59:341-55; PMID:26679949; http://dx.doi.org/ 10.1387/ijdb.150211jp [DOI] [PubMed] [Google Scholar]
- [80].Lu Y, Hao B, Graeff R, Yue J. NAADP/TPC2/Ca(2+) Signaling Inhibits Autophagy. Commun Integr Biol 2013; 6:e27595; PMID:24753792; http://dx.doi.org/ 10.4161/cib.27595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet 2011; 377:849-62; PMID:21084112; http://dx.doi.org/ 10.1016/S0140-6736(10)60667-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Dolnik O, Kolesnikova L, Becker S. Filoviruses: Interactions with the host cell. Cell Mol Life Sci 2008; 65:756-76; PMID:18158582; http://dx.doi.org/ 10.1007/s00018-007-7406-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Saeed MF, Kolokoltsov AA, Albrecht T, Davey RA. Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog 2010; 6:e1001110; PMID:20862315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zolov SN, Bridges D, Zhang Y, Lee W-W, Riehle E, Verma R, Lenk GM, Converso-Baran K, Weide T, Albin RL, et al.. In vivo, Pikfyve generates PI(3,5)P2, which serves as both a signaling lipid and the major precursor for PI5P. Proc Natl Acad Sci U S A 2012; 109:17472-7; PMID:23047693; http://dx.doi.org/ 10.1073/pnas.1203106109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Shen D, Wang X, Xu H. Pairing phosphoinositides with calcium ions in endolysosomal dynamics: phosphoinositides control the direction and specificity of membrane trafficking by regulating the activity of calcium channels in the endolysosomes. Bioessays 2011; 33:448-57; PMID:21538413; http://dx.doi.org/ 10.1002/bies.201000152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012; 149:274-93; PMID:22500797; http://dx.doi.org/ 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Bourdenx M, Dehay B. What lysosomes actually tell us about Parkinson's disease? Ageing Res Rev 2016; pii: S1568-1637(16)30023-X; PMID:26947123; http://dx.doi.org/25348405 10.1016/j.arr.2016.02.008 [DOI] [PubMed] [Google Scholar]
- [88].UniProt Consortium TU . UniProt: a hub for protein information. Nucleic Acids Res 2015; 43:D204-12; PMID:25348405; http://dx.doi.org/ 10.1093/nar/gku989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, Cowley AP, Lopez R. Analysis Tool Web Services from the EMBL-EBI. Nucleic Acids Res 2013; 41:W597-600; PMID:23671338; http://dx.doi.org/ 10.1093/nar/gkt376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Murphy M, Brown G, Wallin C, Tatusova T, Pruitt K, Murphy T, Maglott D. Gene Help [Internet]. 2006 Sep 13 [Updated 2016 Apr 19]. Gene Help [Internet]. Bethesda Natl. Cent. Biotechnol. Inf. (US); 2005. Gene Database [Internet]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK3841/ [Google Scholar]


