Abstract
“Which is the dominant hemisphere?” is a question that arises frequently in patients considered for neurosurgery. The concept of the dominant hemisphere implies uniformity of language lateralisation throughout the brain. It is increasingly recognised that this is not the case in the healthy control brain, and it is especially not so in neurological diseases such as epilepsy.
In the present work we adapt our published objective lateralisation method (based on the construction of laterality curves) for use with sub-lobar cortical, subcortical and cerebellar regions of interest (ROIs). We apply this method to investigate regional lateralisation of language activation in 12 healthy controls and 18 focal epilepsy patients, using three different block design language fMRI paradigms, each tapping different aspects of language processing. We compared lateralisation within each ROI across tasks, and investigated how the quantity of data collected affected the ability to robustly estimate laterality across ROIs.
In controls, lateralisation was stronger, and the variance across individuals smaller, in cortical ROIs, particularly in the Inferior Frontal (Broca) region. Lateralisation within temporal ROIs was dependent on the nature of the language task employed. One of the healthy controls was left lateralised anteriorly and right lateralised posteriorly. Consistent with previous work, departures from normality occurred in ~ 15–50% of focal epilepsy patients across the different ROIs, with atypicality most common in the Lateral Temporal (Wernicke) region. Across tasks and ROIs the absolute magnitude of the laterality estimate increased and its across participant variance decreased as more cycles of task and rest were included, stabilising at ~ 4 cycles (~ 4 min of data collection).
Our data highlight the importance of considering language as a complex task where lateralisation varies at the subhemispheric scale. This is especially important for presurgical planning for focal resections where the concept of ‘hemispheric dominance’ may be misleading. This is a precision medicine approach that enables objective evaluation of language dominance within specific brain regions and can reveal surprising and unexpected anomalies that may be clinically important for individual cases.
Abbreviations: LI, Laterality index; ROI, Region of interest; fMRI, Functional magnetic resonance imaging; OLR, Orthographical lexical retrieval; NV, Noun verb; PR, Pseudoword rhyming
Keywords: Language lateralisation, Regional, Subcortical, Laterality, Statistical power, Presurgical planning
Graphical abstract
Highlights
-
•
Different brain regions support different aspects of language function.
-
•
The degree of language lateralisation varies in different brain regions.
-
•
Atypical lateralisation is common in focal epilepsy patients, particularly in the temporal lobe.
-
•
Even in normal controls, frontal and temporal language systems can be in opposite hemispheres.
-
•
Language dominance is more complex than often thought.
1. Introduction
Language fMRI is frequently performed during the pre-operative evaluation of patients being considered for neurosurgery. The objective is to localise and lateralise language function and estimate the risk of post-surgical dysphasia, enabling patient specific counselling and potentially tailoring surgical procedures (Devinsky et al., 1993, Nagata et al., 2001, Adcock et al., 2003, Woermann et al., 2003, Anderson et al., 2006, Binder et al., 2008, Abbott et al., 2010). At the clinical level, this objective is often expressed by the question, “which is the dominant hemisphere?”
It is increasingly recognised, however, that this question is ill posed. Implicit in the notion of the dominant hemisphere is the assumption that lateralisation is homogenous throughout different neuroanatomical components of the language system. It is now recognised that such homogeneity is generally not observed in the control brain (Hickok and Poeppel, 2007, Berl et al., 2014), with departures from such homogeneity even more pronounced in the setting of neurological disease. For instance, a number of studies – both from our own group (Briellmann et al., 2003, Briellmann et al., 2006a, Sveller et al., 2006, Everts et al., 2010, Tailby et al., 2014) and others (Springer et al., 1999, Adcock et al., 2003, Woermann et al., 2003, Benke et al., 2006, Janszky et al., 2006, Berl et al., 2014, Norrelgen et al., 2015) – have shown considerable heterogeneity of language activation patterns in focal epilepsy, with a variety of departures from the typical left lateralised pattern. This includes an elevated incidence of right lateralisation and bilaterality (considered either globally or regionally), and cases in which anterior and posterior language regions are in opposite hemispheres (Kurthen et al., 1992, Baciu et al., 2003, Ries et al., 2004).
Nonetheless, it is not uncommon in contemporary practice for groups to only consider lateralisation at the hemispheric level. For instance, a PubMed search using the expression “(((((language lateralisation) OR (language lateralisation) OR (language laterality))) AND epilepsy) AND (surgery OR neurosurgery))” yields 28 papers published since the beginning of 2015, in 12 of which language lateralisation was considered in the context of presurgical planning for epilepsy. Of these 12 papers, only one considered separate lateralisation indices for different regions of the language network.
In the present paper we compare the degree of language lateralisation observed in different brain areas as a function of different language tasks. Insofar as the objective of pre-surgical language fMRI is to localise language function in order to estimate post-surgical risk it is important to characterise (i) the variability of lateralisation across different brain areas in controls, (ii) whether this variability is increased in patients, and (iii) determine empirically whether particular regional lateralisation patterns in patients carry prognostic implications. This study addresses the first two of these points.
Studies of language lateralisation typically focus on perisylvian regions, such as Broca and Wernicke areas. In our clinical experience, some degree of lateralisation is also often apparent in subcortical (e.g. basal ganglia (Donnan et al., 1991, Booth et al., 2007) and cerebellar regions (Booth et al., 2007, O'Halloran et al., 2012, Gelinas et al., 2014, Orellana et al., 2015). In the present work we therefore extend the consideration of regional lateralisation to encompass these non-cortical areas as well, to evaluate whether they might be suitable for consideration as prognostic markers. Our data confirm previous reports, showing non-uniformity of language lateralisation across the language system, and an elevated prevalence of anomalous lateralisation in focal epilepsy patients.
Our data highlight the importance of considering regional lateralisation patterns in the context of presurgical planning. In Discussion we consider some of the important challenges that will have to be addressed in translating from an awareness of the importance of regional lateralisation in the setting of focal resection to utilising such information to inform neurosurgical decision making.
2. Materials and methods
2.1. Subjects
Thirty subjects participated, all of whom completed each of the three language paradigms described below. The 30 participants comprise twelve healthy controls (all right handed) and eighteen focal epilepsy patients (three left handed, three ambidextrous) studied as part of their assessment in the Comprehensive Epilepsy Program (CEP) at Austin Health. This includes eight patients with temporal lobe epilepsy (six left), three with lesions in the left parietal/parieto-temporal region, two with left frontal lesions, one with periventricular nodular heterotopia, one with bilateral perisylvian polymicrogyria, one with double cortex, one with a fronto-temporal focus, and one with a focal cortical dysplasia in the right precuneus. The sample of patients is heterogenous epileptologically, though representative of the variety of patients referred for language lateralisation in the CEP. Of the eighteen patients studied seven have had surgery since their language scans (three right and one left anterior temporal lobectomies; one right and two left parietal lesionectomies). Postoperative language assessment was qualitative. There were no language disturbances in the right sided operations and two of the left sided operations had disturbance of language functions. All participants provided written informed consent. The Austin Health Human Research Ethics Committee approved this study.
2.2. Language activation paradigms
In an effort to sample multiple cognitive aspects of language processing, thereby recruiting multiple regions of the language network, we used three different language activation paradigms: orthographic lexical retrieval (OLR) (Wood et al., 2004), noun-verb generation (NV) (Wood et al., 2004), and pseudoword rhyming (PR). The OLR task emphasises language production and strategic search of the lexicon, the NV task emphasises semantic aspects of language processing, and the PR task emphasises grapheme-phoneme conversion and phonological analysis.
Each paradigm was block design in nature, alternating task active and baseline phases of 30s duration (OLR and NV used four active phases total, PR used five active phases total) plus an initial 30s baseline. During the active phase of the OLR paradigm a single letter is visual presented to the participant, who is instructed to silently think of words beginning with that letter. A letter is displayed continuously for 15 s, then replaced by a second letter for another 15 s. During the active phase of the NV paradigm a noun is visually presented and participants instructed to think of an associated verb (“an action or doing word”; e.g. broom → “sweep”). Nine nouns were presented in each 30 s task active phase, one every 3.3 s. During the 30s baseline phases of the OLR and NV tasks a “+” is displayed, with participants instructed to rest. In the active phases of the PR task (Pugh et al., 1996) participants are shown two nonwords (e.g. crute and doot), presented one above the other, and instructed to decide whether they would rhyme if pronounced aloud. During baseline phases, participants are shown two patterns of forward and backslashes (e.g. “/ / \ /” and “/ \ \ /”) and instructed to decide whether the two patterns were identical. A new nonword/pattern pair was shown every 4.5 s, with six consecutive pairs per active/baseline phase (27 s total). The transition between active and baseline phases was indicated by a 3 s cue screen displaying the word “RHYME” or “PATTERN”, respectively.
The OLR, NV, and PR paradigms were presented in separate runs. Participants were instructed to execute the tasks silently, without overt vocalisation. The ability to execute the task was assessed prior to the scan and in scanner performance confirmed by participants after the study.
2.3. Functional image acquisition
Imaging was performed on a 3 T Siemens Trio scanner (Siemens, Erlangen, Germany) equipped with a Siemens body coil and a twelve-channel head coil. A gradient-echo, echo-planar T2*-weighted sequence was used to acquire data sensitive to the BOLD signal (repetition time = 3000 ms; field of view = 216 mm × 216 mm; 72 × 72 imaging matrix; 44 contiguous slices). Resolution was 3 mm × 3 mm in-plane, with 3-mm thick slices in the plane 30° to the AC-PC line.
Pre-processing of fMRI data was performed using Statistical Parametric Mapping software (SPM8; Wellcome Department of Imaging Neuroscience, University College London, London, UK) and included: slice-timing correction; motion correction (Friston et al., 1995); nonlinear normalisation to standard space (“MNI space”; Montreal Neurological Institute, McGill University, Quebec, Canada) (Ashburner and Friston, 1999); re-sampling into 2 × 2 × 2 mm voxels; and spatial smoothing (Gaussian FWHM = 8 mm).
Statistical parametric maps were calculated using SPM8. The design matrix for each paradigm contained one regressor of interest, obtained by convolving the canonical HRF with a task boxcar function, and the six motion correction parameters as regressors of no interest (Friston et al., 1996a, Friston et al., 1996b). The model included a high-pass filter with a cut-off of 128 s and pre-whitening with an AR(1) model. The contrast of interest coded task-minus-baseline.
2.4. Definition of ROIs and rationale for their selection
We created a set of language related ROIs using the Harvard-Oxford cortical and subcortical probability atlases (HO-cpa and HO-spa, respectively) distributed with FSL (Smith et al., 2004), the automatic anatomical labelling (aal) tool (Tzourio-Mazoyer et al., 2002), the anatomy toolbox available for SPM8 (Eickhoff et al., 2005), and independent functional activation data acquired in our laboratory. ROIs generated from probabilistic atlases were created by binarising them at a threshold of 20%; this yielded relatively large ROIs, allowing some dispersion in functional localisation across individuals. Each ROI was also initially made symmetric about the midline by taking the union of the ROI and its left-right flipped counterpart, thereby accommodating subtle left-right differences of neuroanatomy. Left and right ROIs were then created by, respectively, zeroing those voxels located to the right or left of the midline in the binarised summed image.
We defined: an Inferior Frontal/Broca area [IF (Broca)] ROI using volumes 5, 6, and 41 of the HO-cpa (4808 voxels, 38.5 cm3, in extent bilaterally); a Middle Frontal Gyrus [MFG] ROI using volume 4 of the HO-cpa (7868 voxels, 62.9 cm3); a superior medial frontal [SMA] ROI using volumes 3, 26, and 28 of the HO-cpa (excluding voxels > 14 mm from the midline; 8762 voxels, 70.1 cm3); a Lateral Temporal/Wernicke area [LT (Wernicke)] ROI using volumes 10, 12, and 13 of the HO-cpa (8296 voxels, 66.4 cm3); a Ventral Temporal/Visual Word Form Area [VT (VWFA)] ROI using volumes 15 and 38 of the HO-cpa (4072 voxels, 32.6 cm3); an Intraparietal Sulcus [IPS] ROI, obtained from a one-sample t-test performed on data obtained from 12 controls using an in-house block design n-back fMRI task (thresholded at p < 0.001, retaining the cluster anchored in the IPS bilaterally); an Inferior Parietal Lobule [IPL] ROI using volumes 19, 20 and 21 of the HO-cpa combined with the angular gyrus as identified in the aal package (excluding voxels present in the LT (Wernicke) and IPS ROIs defined above; 9628 voxels, 77.0 cm3); a Thalamus and Basal Ganglia [Thal & BG] ROI using volumes 4, 5, 6, 7, 15, 16, 17 and 18 of the HO-spa (7936 voxels, 63.5 cm3); and a Cerebellum ROI using Lobules 1-X from the anatomy toolbox, which was then eroded to prevent overlap with the ventral temporal region (11,546 voxels, 92.4 cm3). We also used a Multi-lobe ROI, constructed as the union of the IF (Broca), MFG, SMA, VT (VWFA), LT (Wernicke), IPL and IPS ROIs, thus collating the presumptive cortical language ROIs into a single ROI spanning multiple lobes. ROIs are shown in Fig. 1.
Fig. 1.
ROIs used to evaluate regional language lateralisation. ROIs are bilaterally symmetric, but for clarity are shown here as unilateral.
Inclusion of ROIs covering Broca's area, MFG, SMA, Wernicke's area and the IPL was based on observation from classical aphasiology (Benson and Ardila, 1996); the ventral temporal ROI on the basis of the purported role of this region in skilled reading (Dehaene and Cohen, 2011), and the IPS, subcortical and cerebellar ROIs on the basis of our clinical experience using language fMRI.
2.5. Laterality curves and laterality indices
From the SPM-t image for a given language task we derived a laterality curve – a plot of laterality index (LI, described below) as a function of the number of voxels contributing to that index (Abbott et al., 2010). The curve is obtained by thresholding the SPM-t image to include only the n most active voxels (Nactive) within the left and right ROIs, counting the number of suprathreshold voxels within the left and right hemisphere ROIs (Nleft and Nright, respectively), and calculating the LI for that particular value of Nactive as (Nleft − Nright)/(Nleft + Nright). Hence, the LI quantifies, at a given threshold, the extent to which task-related activations within left-right symmetric ROIs show hemispheric bias. The LI takes values between + 1 and − 1, with a value of + 1/− 1 indicating suprathreshold voxels exclusively within the left/right hemisphere ROI; values near 0 indicate balanced numbers of suprathreshold voxels in the left and right ROIs. We calculated separate laterality curves for each individual on each task in each ROI.
For the purposes of this paper we summarised the resulting laterality curves using methods based upon those reported in Abbott et al. (2010). We first constructed mean laterality curves and their one-sided confidence intervals, obtained as the mean absolute LI and the lower confidence interval (calculated as the mean absolute LI minus 1.65 standard deviations of the absolute LI). The active voxel range of the mean laterality curve (and its associated confidence interval) was specified according to the following constraints: (1) only t-values > 0 are considered (i.e. activations). (2) As the number of t-values > 0 (Ntotal) varies across participants, we limit the upper voxel count range to ensure that an LI can be calculated for at least 90% of the control population. (3) At low values of Nactive the behaviour of the LI is potentially unstable. Further, activation will likely encompass relatively extended clusters of voxels. For these reasons we imposed a minimum Nactive of 125 voxels (1000 mm3) for calculating an LI. While there is a degree of arbitrariness to this criterion, it does serve the important purpose of excluding undue emphasis on only a small handful of voxels. (4) In order to ensure that the distribution of absolute LIs (across participants, at a given value of Nactive) is normal, and can therefore be accurately summarised using the standard deviation, we consider only those values of Nactive for which a Jarque-Bera test on the absolute LI values is significant (Matlab's jbtest function, at alpha < 0.001). As the Jarque-Bera test can indicate normality even when the vast majority of values in the distribution are identical (such as at low values of Nactive, where all but one or two participants may have an absolute LI = 1) we also required that at least 75% of participants had an absolute LI value ≤ 0.9 (see also Seghier (2008) for discussion of additional issues surrounding the highly nonlinear relationship between activation asymmetries and LI for high LI values; and Jansen et al. (2006) for a related discussion on imposing constraints while estimating laterality).
In order to summarise the strength of lateralisation of a given laterality curve for a given participant, language task, and ROI we used the (raw) LI obtained at the lowest value of Nactive satisfying the above constraints (Fig. 2). We refer to this lateralisation summary statistic as LInorm. We consider only confidence intervals and LInorm as measures of lateralisation in this paper, though note that a variety of approaches to quantifying laterality have been advocated, with their relative strengths and weaknesses extensively discussed (see, for example (Jansen et al., 2006, Seghier, 2008)).
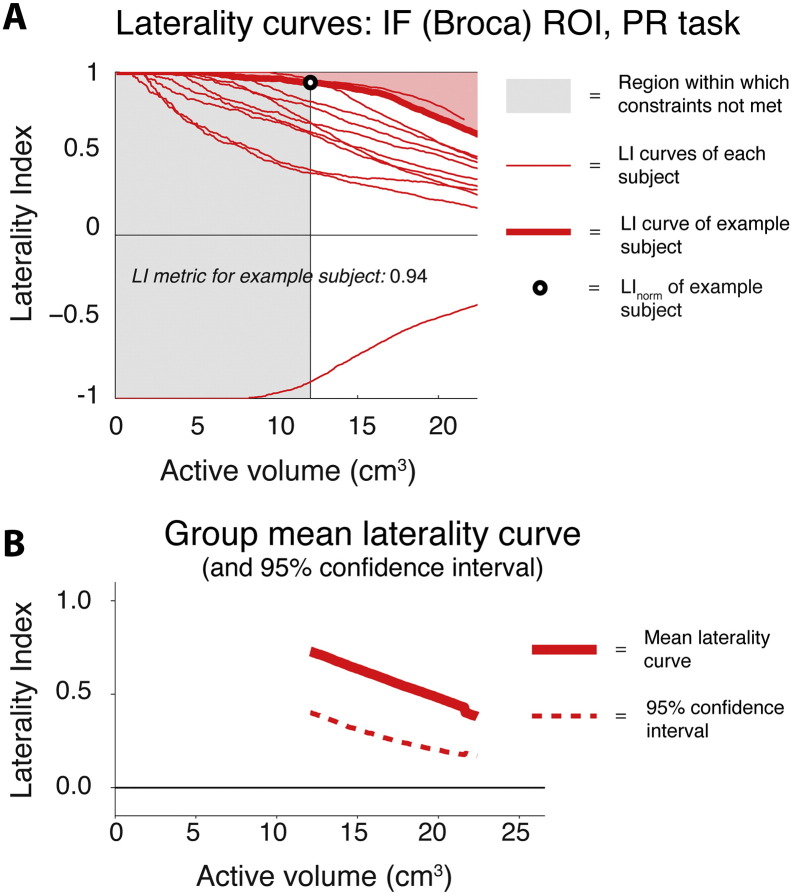
Fig. 2.
Illustration of laterality curves and associated metrics. A: LI curves obtained from the IF (Broca) ROI on the PR task are shown for all 12 control participants (thin red lines), one of whom is right hemisphere dominant (negative LI values). LI curves are plotted here, and throughout, as a function of active brain volume (cm3, N active voxels multiplied by voxel volume). The grey shaded region at left indicates the initial portion of the curves that lies outside of the valid LI range (see Materials and Methods). The LI curve for one participant is highlighted (heavy red line) for the purposes of illustrating the LI metric used throughout this paper. B: group mean laterality curve and associated confidence interval, derived from the data shown in A.
Note that while we construct confidence intervals using absolute LI values, we consider individual laterality curves, and their associated LInorm values, on the basis of their raw (as opposed to absolute) LI values, for which sign denotes hemisphere. In defining the normative range on the basis of absolute LI we are effectively treating left and right dominant laterality curves as equivalent but for a change of sign (i.e. but for reflection about the LI = 0 axis) (Knecht et al., 2003). In this manner, right lateralised control participants contribute to the definition of the confidence interval, even though they themselves would be considered to fall outside of the so defined confidence interval.
2.6. Impact of the number of active blocks on reliability of laterality estimate
It is known that block design tasks are optimal in terms of activation detection (Friston et al., 1999, Liu et al., 2001). In our previous work we have used four blocks of a 30 s language task alternating with rest to derive lateralisation indices from activation images (Briellmann et al., 2002, Briellmann et al., 2003, Anderson et al., 2006, Briellmann et al., 2006a, Sveller et al., 2006, Abbott et al., 2010, Everts et al., 2010, Tailby et al., 2014). In the context of multi-region laterality assessment, using methods exploiting a range of thresholds, it is not clear whether fewer task blocks would suffice. In order to examine how laterality estimates varied with the number of active blocks collected we calculated laterality curves and laterality metrics as described above, but using different subsets of the data. Specifically, we analysed (i) the first, (ii) first and second, (iii) first to third, (iv) first to fourth, and, in the case of the Pseudoword task (v) first to fifth task active epochs (and their temporally adjacent baseline periods). These correspond to 90s, 150 s, 210 s, 270 s, and 300 s of data collection, respectively.
3. Results
3.1. Individual laterality curves
Fig. 2A illustrates the basic lateralisation method used here. It shows laterality curves obtained within the IF (Broca) ROI on the PR task for all 12 control participants. All curves necessarily begin at an LI of 1 (left lateralised) or − 1 (right lateralised), then smoothly approach an LI of 0 (bilaterality) as the active volume (the number of voxels included in the calculation) increases. The LI curve for one participant remains negative across its full range, indicating right lateralisation. Fig. 2B shows the mean laterality curve derived from the data in Fig. 2A, and its associated 95% confidence interval (see Methods for details).
3.2. Control data: laterality varies with ROI and task
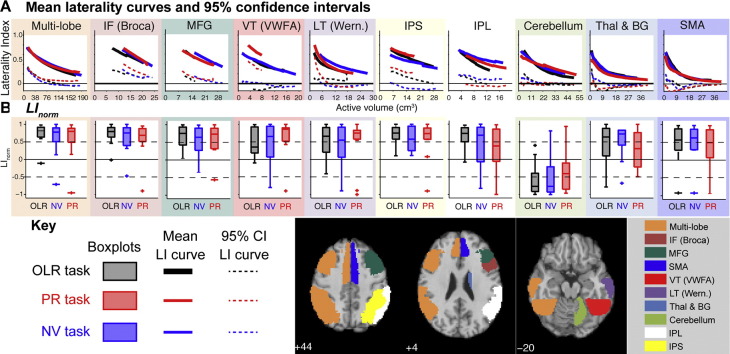
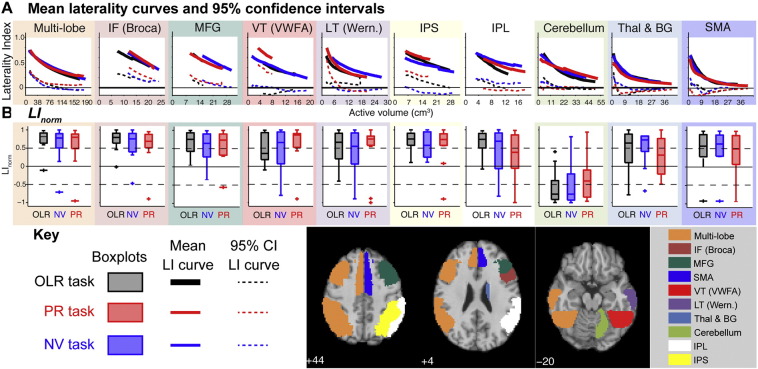
Laterality within each ROI on each of the language tasks is summarised across the 12 control participants in Fig. 3. Each column summarises data from a single ROI. The top row plots mean laterality curves and their one-sided 95% confidence interval. Underneath these are shown boxplots of LInorm, for which increasingly positive/negative values indicate increasing left/right dominance. The boxplots therefore capture whether individual LI curves are lateralised to the left or the right hemisphere.
Fig. 3.
Summary of regional language lateralisation in control participants across the eight ROIs using the three language tasks. A, mean laterality curves (solid lines) and their associated 95% confidence intervals (dashed lines, plotted as mean minus 1.65 standard deviations). The different language tasks can give rise to different valid LI ranges, resulting in curves of different lengths within a given ROI. Note that ROIs differ in volume. B, boxplots of LInorm (increasingly positive/negative indicates increasingly left/right lateralised). Within each “box” the central line indicates the median value, the edges indicate the 25th and 75th percentiles, and the whiskers extend to the most extreme data points not considered outliers (which are plotted as crosses).
There is strong and consistent lateralisation across tasks within our published Multi-lobe ROI and the IF (Broca) and MFG ROIs (second and third columns of Fig. 3). Within the IF (Broca) ROI, the mean LI curves for each language task remain above an LI of 0.4 across their full extent, and their associated confidence intervals remain clear of abs(LI) = 0. Similarly, the boxplots of LInorm indicate strong lateralisation on all tasks.
The fusiform gyrus is implicated in graphemic analysis. Within the VT (VWFA) ROI all three tasks produced, on average, reasonably strong lateralisation. The tasks, however, differed in the consistency of that lateralisation across participants. The PR task, which draws most heavily on grapheme-phone conversion, showed strong lateralisation across control participants. The OLR and NV tasks, however, were associated with considerable spread: the 95% confidence interval for the mean laterality curve, and the observed ranges of LInorm, encompassed values of zero, indicating bilaterality in some participants.
A similar pattern was produced within the LT (Wernicke) ROI. Again, the PR task was the most consistently lateralising, as assessed by the confidence interval of the mean laterality curve, or the boxplot of LInorm. The PR task is the task that makes the heaviest demands on phonemic processing. Interestingly, the boxplot of LInorm for the PR task indicates that two participants are right lateralised within the LT (Wernicke) region (compared with only one in the more anterior ROIs) – a point we return to below.
Within the IPL and IPS ROIs lateralisation was generally strong (averaged across participants) across all tasks, however the associated confidence intervals tended to be relatively broad. Among these ROIs, the confidence interval was tightest for the IPS when using the PR task (and to a lesser extent on the OLR task). The confidence intervals for all tasks in the IPL ROI are close to zero (< 0.2) across the full extent of the curves.
The Cerebellum, the Thalamus and Basal Ganglia, and the SMA ROIs are notable for rapid convergence towards bilaterality and confidence intervals that quickly encompasses an LI of 0. These data indicate that while an LI curve for a given individual can be lateralising within these ROIs, there are a number of individuals for whom the LI curve rapidly converges to 0. Note that lateralisation within the Cerebellar ROI is generally crossed with respect to the other ROIs (compare boxplots in Fig. 3B), especially on the OLR task (which produced the most consistent cerebellar lateralisation among the tasks used here). This likely reflects the decussation of cerebrocerebellar connections.
Overall, the data of Fig. 3 indicate that lateralisation varies across ROIs and tasks. Across ROIs, the PR task provides the most consistent indication of lateralisation of the tasks in this sample. All tasks lateralise in the IF (Broca) and MFG ROIs. At the group level, the PR task also consistently reveals hemispheric dominance in the VT (VWFA), LT (Wernicke), and IPS ROIs. Lateralisation is less consistent in the IPL, Cerebellum, Thalamus & Basal Ganglia, and SMA ROIs.
3.3. Anterior (Broca) and posterior (Wernicke) laterality can dissociate in controls
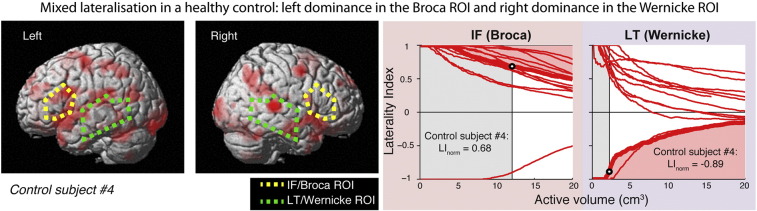
Our random sample of controls were all right handed and free from language or intellectual difficulties. Eleven of these 12 controls could be characterised as having a ‘dominant hemisphere’ (in the sense of having similarly signed LInorm values across cortical ROIs) but one showed dissociation of laterality with left dominance in the IF (Broca) ROI, and right dominance in the LT (Wernicke) ROI (Fig. 4). This indicates that anterior and posterior language areas can be strongly lateralised to opposite hemispheres even in the healthy control brain (see also Berl et al., 2014). Such information is lost however if one considers only the Multi-lobe LI estimate in this individual (0.14, suggesting bilaterality throughout the language system).
Fig. 4.
Control participant with mixed lateralisation. This individual shows left dominance within the IF (Broca) ROI and right dominance in the LT (Wernicke) ROI. Left panels show surface overlays of activation on the PR task, displayed on the default SPM surface render. Yellow shows outline of the IF (Broca) ROI; green shows outline of the LT (Wernicke) ROI. Right panels show the laterality curves in these ROIs for this individual (heavy red curve), along with those of the remaining participants (thin red curves). Conventions otherwise the same as in Fig. 3.
3.4. Atypical language activation is common in the lateral temporal region in focal epilepsy patients
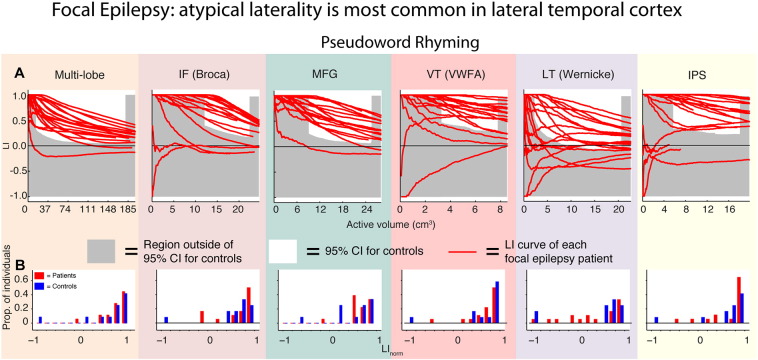
So far we have presented data only in Controls. One of the main aims for this paper is to highlight the importance of considering regional variation of lateralisation in patients being considered for neurosurgery. To this end, Fig. 5A shows laterality curves for the PR task obtained in a consecutive series of 18 focal epilepsy patients scanned as part of the clinical evaluation of their suitability for neurosurgery (see Methods). The sample is epileptologically heterogeneous; we collect them here to illustrate the range of patterns observed in a typical sample of patients referred for language fMRI in the context of presurgical planning. The data show that while the majority of focal epilepsy patients have LI curves that fall within the normal range (the 95% CI of controls), clear departures from normality are observed in each ROI. Departures from normality are most common in the LT (Wernicke) ROI (Fig. 5A, column 5), where ~ 50% of patients show bilateral or right lateralisation (see histograms of LInorm in patients and controls, Fig. 5B). Were a global laterality index to be calculated (Fig. 5A, column 1) many of these abnormal lateral temporal lateralisation patterns would be overlooked; only one of 18 cases appears unequivocally abnormal in the Multi-lobe ROI (Fig. 5B, column 1), compared with nine of 18 cases for the LT (Wernicke) ROI (Fig. 5B, column 5).
Fig. 5.
Laterality curves across ROIs for the PR task in focal epilepsy cases. A: Laterality curves obtained from all 18 focal epilepsy patients (red curves) in the Multi-lobe, IF (Broca), MFG, VT (VWFA), LT (Wernicke), and IPS ROIs; these are the ROIs that showed the most consistent lateralisation in controls. Grey shaded areas show regions falling outside of the 95% confidence interval defined in controls (as in Fig. 3). Atypical lateralisation is therefore shown by curves falling outside the white area in each of the graphs. Atypical laterality is most common in the LT (Wernicke) ROI, as shown in the histograms (B) of LInorm obtained in focal epilepsy patients and controls using the PR task.
3.5. How much data is required to characterise language lateralisation?
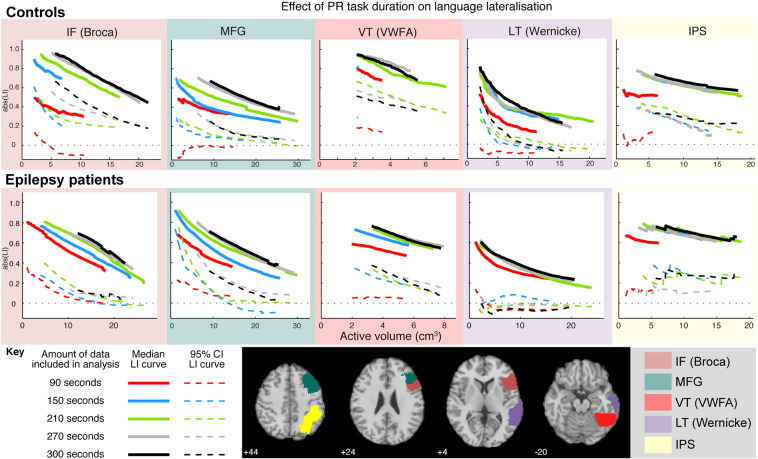
While not a principal aim of this study, our data afforded us the opportunity to examine the effect of task duration on laterality estimates in different regions of the language network. Fig. 6 shows mean laterality curves and their associated 95% confidence intervals as increasing amounts of data contribute to the analysis. Curves are shown only for the PR task (the most consistently lateralising task used here), and only in those ROIs in which consistent lateralisation was observed across control participants (the IF (Broca), MFG, VT (VWFA), LT (Wernicke), and IPS ROIs).
Fig. 6.
Effect of task duration on language lateralisation. Laterality curves (solid lines) and their associated confidence intervals (dotted lines) within a subset of ROIs (represented by background frame colour - see legend and images), plotted as a function of the amount of data (coloured lines) included in the analysis. Only data from the PR task is shown. Data for the IF (Broca), MFG, VT (VWFA), LT (Wernicke), and IPS ROIs are shown. Top row: controls; middle row: patients; bottom row: key.
Patterns are comparable across ROIs, and across patients and controls. In all ROIs both the strength (displacement from LI = 0) and consistency (width of the confidence interval) of the LI curves across individuals increases as more task-active blocks are analysed. The most dramatic increase occurs in going from one task-active block (red curves, 90 s of data) to two task-active blocks (orange curves, 150 s of data), with further improvement up to four blocks of task-active data (grey curves, 270 s of data); there is little gain from inclusion of the fifth task-active block (black curves, 300 s of data). Similar results were observed for the NV and OLR tasks (not shown). Thus, for the tasks and ROIs explored here, four task-active blocks (270 s of data) appears to produce relatively strong and reliable lateralisation in controls, with the laterality curves obtained in patients stabilising (though with wider variance than in controls) over the same period of data collection.
4. Discussion
We examined regional language lateralisation patterns on three different language fMRI tasks in a sample of healthy control participants, and a consecutive series of focal epilepsy patients. The principal observations stemming from this approach are:
-
1.
Lateralisation is not homogenous throughout the language network. As has been shown previously, this is the case in the healthy control brain (Hickok and Poeppel, 2007, Berl et al., 2014), and is especially so in focal epilepsy (Spreer et al., 2002, Benke et al., 2006, Sveller et al., 2006, Weber et al., 2006, Gaillard et al., 2007, Everts et al., 2010, Berl et al., 2014).
-
2.
On the basis of the width of confidence intervals, for the tasks used here regional hemispheric dominance is most consistently observed anteriorly, in the Inferior Frontal/Broca region and the middle frontal gyrus (Harrington et al., 2006). Using the PR task – which emphasises grapheme-phoneme conversion and phonological analysis – regional hemispheric dominance is also apparent in the ventral occipitotemporal (encompassing the so-called “visual word form area”), Lateral Temporal/Wernicke, and intrapartietal sulcus regions.
-
3.
Within the cerebellum, subcortical (Thalamus & Basal Ganglia), and superior medial frontal (SMA) ROIs, strong language lateralisation can be observed, but is not consistently demonstrated across participants (Wilke et al., 2006, Gelinas et al., 2014).
-
4.
Even in the healthy control brain, major hubs (e.g. Wernicke and Broca areas) of the language network can be lateralised to opposite hemispheres. To our knowledge only one other such case of interhemispheric dissociation has been reported in the healthy control literature (Berl et al., 2014), and it is rare even in epilepsy (4 of 490 cases reported by Lee et al., 2008).
-
5.
Atypical regional language lateralisation, generally expressed as an increased tendency towards bilaterality, is present in between ~ 20–50% of the focal epilepsy patients in our sample, with such atypicality occurring most frequently in the lateral temporal region (Benke et al., 2006, Everts et al., 2010, Tailby et al., 2014).
-
6.
At the group level, lateralisation increases, and becomes more consistent across participants, as more “active vs. rest” cycles are accumulated. For both controls and patients this improvement appears to have largely plateaued after inclusion of ~ 4 active phases (~ 4.5 min of data).
While it has been reported previously that language laterality can vary across elements of the language network, especially in the context of neurological disease, this concept still remains poorly represented in the current literature as it relates to neurosurgical planning. In the following sections we relate our own observations to previous work. We discuss the potential bases and implications of lateralisation within the different ROIs we have considered, and highlight some of the major challenges involved in using language fMRI for prognostic purposes in individual cases being considered for neurosurgery.
4.1. Lateralisation is stronger anteriorly
With the language fMRI paradigms used here lateralisation among controls is strongest in the frontal convexity (the IF (Broca) and MFG ROIs), being slightly less lateralised (though still left dominant in most individuals) in temporal and parietal areas. A degree of posterior bilaterality in controls has been noted previously (Jung-Beeman, 2005, Hickok and Poeppel, 2007, Price, 2010). For instance, functional imaging studies have revealed a tendency for speech processing to recruit posterior middle temporal gyrus and superior temporal sulcus bilaterally, even after subtracting out responses to non-speech sounds (reviewed in (Hickok and Poeppel, 2007)).
Considering our posterior ROIs, consistent hemispheric dominance was observed within the ventral temporal area on the PR task. In the context of epilepsy, this result is of interest given the proximity of this ROI to the mesial temporal lobe. The “visual word form area”, a region showing some specialisation for grapheme processing, is located in the fusiform gyrus/occipitotemporal sulcus (Dehaene and Cohen, 2011), just posterior and lateral to the tail of the hippocampus. It may therefore provide a useful localising role in the setting of presurgical planning for anterior temporal lobectomy in TLE. Indeed, an interesting possibility is that abnormal neural activity (epilepsy) in the affected mesial temporal region could, by virtue of proximity, give rise to altered functionality within this specialist reading system, particularly when the affected side is in the dominant hemisphere. There appears to be an elevated prevalence of reading disorders in epilepsy (Schachter et al., 1993, Breier et al., 2000, Tailby et al., 2014), and we have previously described a failure of language-related specialisation in the temporo-occipital region in focal epilepsy patients with reading disorders (Tailby et al., 2014).
Within the lateral temporal ROI, there was good lateralisation in controls at relatively low active voxel counts (the most active ~ 2–4 cm3 worth of voxels in the ROI), particularly for the PR task. As the active volumes increases the confidence interval quickly encompasses 0 (at > ~ 8 cm3), indicating bilaterality. What underlies the increasing bilaterality of the laterality curves as activation threshold decreases (i.e. as more voxels within the ROI are included in the calculation)? When we examined the SPMs for the PR task individually in our 12 controls (feature threshold, p < 0.001; FDRc < 0.05), 11 of 12 participants had significant clusters in the posterior temporal region. In six of these participants significant posterior temporal clusters were observed only within one hemisphere; in five participants with clusters in both hemispheres there was nonetheless hemispheric dominance when considered in terms of cluster extent and strength of activation (with geometric ratios of dominant/minor hemisphere cluster extent and strength of 2.52 and 2.08, respectively). This suggests that within the posterior temporal region, on the PR task, the rapid approach of the LI curve towards 0 reflects a relatively small area of strong lateralised activity in one hemisphere and slightly weaker activity both ipsilaterally and contralaterally within the larger ROI.
4.2. Language activation in the intraparietal sulcus, but not the inferior parietal lobule, consistently lateralises
Our control data indicates that within the parietal lobe, language related activation is more consistently lateralised within the IPS than within the IPL. The relatively wide confidence intervals for the laterality curves in the IPL is perhaps surprising given the significance of the angular gyrus and supramarginal gyrus in classical aphasiology (Benson and Ardila (1996); also discussed in Binder et al. (1997)). Random effects analyses of the different language tasks used here yielded group-wise significant activation within the (left) IPS but not the IPL (not shown, though see Fig. 1 in (Tailby et al., 2014) for a comparable dataset). This may relate to the task design: the OLR and NV tasks used a passive fixation baseline which might allow semantic activity (commonly present in the IPL (Binder et al., 2009, Price, 2010)) in task and baseline to cancel one another out. Language tasks using meaningful linguistic stimuli, when contrasted with an active baseline state, have been shown to yield reliable activation in the IPL (Binder et al., 1997). IPL activity is not expected in the PR task as active and baseline stimuli are matched and devoid of semantic content.
The IPS has been implicated in a variety of cognitive operations (reviewed in Culham and Kanwisher (2001)), including (with respect to the tasks employed here) attention, eye movements, and visual processing. The lateralising value of the task related activation we observed may reflect visual attention directed towards graphemic elements, which are represented with a left hemisphere bias (Dehaene and Cohen, 2011), during the task active periods.
4.3. Language lateralisation within the cerebellum
In our clinical practice, we often note the presence of cerebellar activation contralateral to anterior brain activation (presumably reflecting the decussation of key cerebrocerebellar connections). This pattern has also recently been noted by Gelinas et al. (2014). We examined whether cerebellar activation is consistently lateralised across controls, as a preliminary step towards determining whether it might be a useful surrogate marker of cortical lateralisation. For instance, if cerebellar lateralisation were a consistent finding across controls and patients, such lateralisation might prove a useful prognostic marker for focal cortical resections in the contralateral hemisphere, potentially providing useful information in clinical cases where cortical lateralisation is unclear. Across tasks the median laterality curve obtained from the cerebellar ROI showed moderate lateralisation in controls, especially on the OLR task, however its associated confidence interval was very broad. Thus, while language related activation within the cerebellum shows strong lateralisation in many control participants, this is not always the case (see also Wilke et al., 2006). The cerebellum has long been recognised as having a role in articulation (Ackermann, 2008), which is involved (covertly) in all of the tasks used here. Non-motor cerebellar functions are also increasingly recognised, including language and executive processes (Schmahmann, 2004, O'Halloran et al., 2012). Fluency, naming, and grammatical deficits have been observed in cases of cerebellar pathology (tumour, stroke, degeneration; reviewed in O'Halloran et al. (2012)), and previous imaging has revealed right cerebellar activation on fluency (Schlösser et al., 1998), speech comprehension (Papathanassiou et al., 2000), semantic (Gelinas et al., 2014) and verb generation tasks (Frings et al., 2006).
4.4. How much data should be collected?
Our data allowed us to address the question of how regional lateralisation, estimated using methods exploiting a range of thresholds, is affected by the duration of data collection. Previous theoretical work has shown that the optimum number of cycles (and subjects) in a block design experiment varies as a function of numerous factors, such as whether errors are correlated, the nature of the statistical test employed (t or F), and the ratio of within- to between-subject variance (Desmond and Glover, 2002, Mumford and Nichols, 2008, Maus et al., 2011). Here, we have assessed this empirically. Further, laterality curves, and the LI derived from them, are aggregate (ROI-based) measures derived from the underlying activation maps, so may have a different dependence on task duration than the activation images themselves (e.g. through the averaging that is inherent to the LI calculation). Our data suggest that in both controls and patients, after 4 phases (4.5 mins of data, 90 volumes at a TR of 3 s) there is convergence of laterality curves with an approximately stable confidence interval on the group mean laterality curve. Further reductions in time may be possible by using a shorter block length (e.g. between 10 and 20 s active and rest phases) (Aguirre and D'Esposito, 1999, Wager and Nichols, 2003, Birn et al., 2004, Henson, 2007), depending on the amplitude of activation (Murphy et al., 2007). The data requirements inferred here are based on averages across participants for a covertly performed task. Additional factors will likely, however, also influence the duration of scanning required (or indeed whether scanning is even appropriate) in individual patients, including overall level of intellectual functioning, ability to sustain attention, reading proficiency, degree of head movement, and so forth. Such factors are, however, beyond the scope of the present study.
4.5. The contribution of task design to observed lateralisation
The degree of lateralisation observed in a given brain region will vary with the task used to derive that lateralisation (Fig. 3). This is most clearly illustrated in the VT (VWFA) ROI (Fig. 3), for which the PR task produced strong consistent lateralisation, whereas the OLR and NV produced weaker and more variable lateralisation. Consequently, a number of groups have advocated for the use of a battery of language tasks tapping a range of language functions (Rutten et al., 2002, Gaillard et al., 2004, Thivard et al., 2005, Arora et al., 2009, Binder, 2010), an approach that we ourselves use in standard practice. The tasks used in such a battery should be selected so as to incorporate demands across a range of language processes, ensuring that important language functions are not overlooked.
The tasks we have used all place demands on covert articulation, likely accounting for the good inferior frontal lateralisation across tasks (Costafreda et al., 2006). Graphemic and phonological analysis are most heavily recruited by the PR task, explaining its better lateralisation in ventral occipitotemporal cortex (Cohen and Dehaene, 2004) and lateral temporal cortex (Hickok and Poeppel, 2007), respectively. The PR task also uses an active baseline that controls for non-graphemic visual analysis (as opposed to the passive “rest” baseline used in the NV and OLR tasks), enabling (in principle, though see Friston et al., 1996a, Friston et al., 1996b) exclusion of regions supporting basic visual and attentional processes common to the active and baseline conditions.
The tasks that we have used place minimal demands on language comprehension/perception, perhaps contributing to the weaker and less consistent lateralisation observed across tasks within our temporal ROIs. Inclusion of language task(s) that robustly recruit comprehension systems would be important for refining localisation and lateralisation within the temporal lobe, the most common target in epilepsy surgery. For instance, tasks that require processing of phrase and sentence length language input for meaning (such as sentence completion or story comprehension tasks) produce strong and extensive activation in temporal regions, extending from parietotemporal cortex to temporal pole (Ashtari et al., 2005, Thivard et al., 2005, Wilke et al., 2006, Arora et al., 2009, Binder et al., 2011). If presented in written form such tasks would have the added advantage of strongly recruiting the ventral temporal region.
4.6. Interhemispheric dissociation of language functions: the importance of considering regional, rather than whole brain, laterality
The importance of considering regional lateralisation is perhaps best illustrated by considering the mixed lateralisation case shown in Fig. 4. Deriving a single metric to characterise language lateralisation is clearly misleading in instances such as this. A single lateralisation value calculated across frontal and temporal regions would imply bilaterality in this case even though strong laterality is apparent in either region individually. Cases such as this highlight the potential inappropriateness of the question, “which is the dominant hemisphere?”
Such crossing of anterior (Broca) and posterior (Wernicke) language hubs is relatively uncommon in the normal population. Berl et al. (2014) reported only 1 of the 118 healthy controls in their sample with crossed frontal and temporal laterality. Relative to healthy controls, however, mixed lateralisation (in the form of either crossed anterior and posterior language systems, or lateralisation in one region and bilaterality in another) is more common among epilepsy patients (Berl et al., 2014). In particular, a number of authors have noted that in focal epilepsy patients deviations from typical left-hemisphere dominance are most common in the temporoparietal region (fMRI: (Benke et al., 2006, Everts et al., 2010, Tailby et al., 2014)), and in speech perception/comprehension systems (WADA: (McGlone, 1984, Zaidel, 1985, Boatman et al., 1998, Wada and Rasmussen, 2007, Hickok et al., 2008)), as we have observed here (Fig. 5).
While language lateralisation patterns in many neurosurgical candidates approximate that of the normal brain (Berl et al., 2014), and hence permit prognostic inference on the basis of typical neuroanatomical-functional patterns, there are nonetheless a non-negligible number of atypical cases for whom such inference is not straightforward. The preponderance of “typical” lateralisation patterns in patients cohorts means that in many group studies the prognostic implications for atypical cases may be “overlooked”, in the sense that their statistical contribution to a group-wise result (such as a regression of lateralisation against outcome) is swamped by that of more typical cases. To state the obvious, surgery is carried out on a given individual, not the average individual, and so detection of anomalous cases – even if only encountered rarely – is important (Kurthen et al., 1992, Baciu et al., 2003, Ries et al., 2004). Ideally one would like to be able to study sufficient numbers of “similarly atypical” subjects to aggregate them into their own groups for prognostic purposes. For instance, one might imagine that in patients being considered for left temporal resection the significance of the strength of lateral temporal lateralisation might vary depending on whether the inferior frontal region is left or right dominant. This ties in to broader questions, such as: Does activation in a region imply that it is necessary to support function (Price et al., 1999)? What is the clinical significance of activation in a region not typically activated in controls (Anderson et al., 2002), or in abnormal/heterotopic tissue (Briellmann et al., 2006b)? Does bilateral language activation in a region imply redundancy such that unilateral resection is safe? Does unilateral temporal lobe language activation imply that verbal memory is ipsilateral to that activation?
Studies comparing pre-surgical regional language activation patterns, tissue resection location, and post-surgical outcomes are required in order to evaluate these issues (Binder, 2010, Rosazza et al., 2013). While various methods have been advocated for determining language lateralisation from activation images (see, for instance, Wilke and Schmithorst, 2006, Seghier, 2008, Abbott et al., 2010), the primary methodological issue we stress here is the need to consider lateralisation regionally. In order to clarify the prognostic implications of different lateralisation patterns for different resection targets very large patient populations will have to be studied. This can only realistically be accomplished using large, multi-site data sets (Binder et al., 2011), ideally with a standard acquisition and regional analysis protocol.
Funding
This study was supported by the National Health and Medical Research Council (NHMRC) of Australia (grants no. 1081151 and 1091593). DFA is supported by fellowship funding from the Australian National Imaging Facility. GDJ is supported by an NHMRC Practitioner Fellowship (grant no. 1060312). The Florey Institute of Neuroscience and Mental Health acknowledges the strong support from the Victorian Government and in particular the funding from the Operational Infrastructure Support Grant.
Acknowledgements
The authors wish to thank all of the participants for giving of their time to be involved in this study.
References
- Abbott D.F., Waites A.B., Lillywhite L.M., Jackson G.D. fMRI assessment of language lateralization: an objective approach. NeuroImage. 2010;50:1446–1455. doi: 10.1016/j.neuroimage.2010.01.059. [DOI] [PubMed] [Google Scholar]
- Ackermann H. Cerebellar contributions to speech production and speech perception: psycholinguistic and neurobiological perspectives. Trends Neurosci. 2008;31:265–272. doi: 10.1016/j.tins.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Adcock J.E., Wise R.G., Oxbury J.M., Oxbury S.M., Matthews P.M. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. NeuroImage. 2003;18:423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- Aguirre G.K., D'Esposito M. Experimental design for brain fMRI. Funct. MRI. 1999;369–380 [Google Scholar]
- Anderson D.P., Harvey A.S., Saling M.M., Anderson V., Kean M., Abbott D.F. fMRI lateralization of expressive language in children with cerebral lesions. Epilepsia. 2006;47:998–1008. doi: 10.1111/j.1528-1167.2006.00572.x. [DOI] [PubMed] [Google Scholar]
- Anderson D.P., Harvey A.S., Saling M.M., Anderson V., Kean M., Jacobs R. Differential functional magnetic resonance imaging language activation in twins discordant for a left frontal tumor. J. Child Neurol. 2002;17:766–769. doi: 10.1177/08830738020170101801. [DOI] [PubMed] [Google Scholar]
- Arora J., Pugh K., Westerveld M., Spencer S., Spencer D.D., Todd C.R. Language lateralization in epilepsy patients: fMRI validated with the Wada procedure. Epilepsia. 2009;50:2225–2241. doi: 10.1111/j.1528-1167.2009.02136.x. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Nonlinear spatial normalization using basis functions. Hum. Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashtari M., Perrine K., Elbaz R., Syed U., Thaden E., McIlree C. Mapping the functional anatomy of sentence comprehension and application to presurgical evaluation of patients with brain tumor. Am. J. Neuroradiol. 2005;26:1461–1468. [PMC free article] [PubMed] [Google Scholar]
- Baciu M.V., Watson J.M., McDermott K.B., Wetzel R.D., Attarian H., Moran C.J. Functional MRI reveals an interhemispheric dissociation of frontal and temporal language regions in a patient with focal epilepsy. Epilepsy Behav. 2003;4:776–780. doi: 10.1016/j.yebeh.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Benke T., Köylü B., Visani P., Karner E., Brenneis C., Bartha L. Language lateralization in temporal lobe epilepsy: a comparison between fMRI and the Wada Test. Epilepsia. 2006;47:1308–1319. doi: 10.1111/j.1528-1167.2006.00549.x. [DOI] [PubMed] [Google Scholar]
- Benson D.F., Ardila A. Oxford University Press; 1996. Aphasia: A Clinical Perspective. [Google Scholar]
- Berl M.M., Zimmaro L.A., Khan O.I., Dustin I., Ritzl E., Duke E.S. Characterization of atypical language activation patterns in focal epilepsy. Ann. Neurol. 2014;75:33–42. doi: 10.1002/ana.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R. Use of fMRI language lateralization for quantitative prediction of naming and verbal memory outcome in left temporal lobe epilepsy surgery. In: Ulmer S., Jansen O., editors. fMRI: Basics and Clinical Applications. Berlin: Springer-Verlag; 2010. pp. 77–93. [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Frost J.A., Hammeke T.A., Cox R.W., Rao S.M., Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J. Neurosci. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Gross W.L., Allendorfer J.B., Bonilha L., Chapin J., Edwards J.C. Mapping anterior temporal lobe language areas with fMRI: a multicenter normative study. NeuroImage. 2011;54:1465–1475. doi: 10.1016/j.neuroimage.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Sabsevitz D.S., Swanson S.J., Hammeke T.A., Raghavan M., Mueller W.M. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia. 2008;49:1377–1394. doi: 10.1111/j.1528-1167.2008.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R.M., Cox R.W., Bandettini P.A. Experimental designs and processing strategies for fMRI studies involving overt verbal responses. NeuroImage. 2004;23:1046–1058. doi: 10.1016/j.neuroimage.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Boatman D., Hart J., Lesser R.P., Honeycutt N., Anderson N.B., Miglioretti D. Right hemisphere speech perception revealed by amobarbital injection and electrical interference. Neurology. 1998;51:458–464. doi: 10.1212/wnl.51.2.458. [DOI] [PubMed] [Google Scholar]
- Booth J.R., Wood L., Lu D., Houk J.C., Bitan T. The role of the basal ganglia and cerebellum in language processing. Brain Res. 2007;1133:136–144. doi: 10.1016/j.brainres.2006.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier J.I., Fletcher J.M., Wheless J.W., Clark A., Cass J., Constantinou J.E.C. Profiles of cognitive performance associated with reading disability in temporal lobe epilepsy. J. Clin. Exp. Neuropsychol. 2000;22:804–816. doi: 10.1076/jcen.22.6.804.948. [DOI] [PubMed] [Google Scholar]
- Briellmann R.S., Abbott D.F., Caflisch U., Archer J.S., Jackson G.D. Brain reorganisation in cerebral palsy: a high-field functional MRI study. Neuropediatrics. 2002;33:162–165. doi: 10.1055/s-2002-33680. [DOI] [PubMed] [Google Scholar]
- Briellmann R.S., Labate A., Harvey A.S., Saling M.M., Sveller C., Lillywhite L. Is language lateralization in temporal lobe epilepsy patients related to the nature of the epileptogenic lesion? Epilepsia. 2006;47:916–920. doi: 10.1111/j.1528-1167.2006.00513.x. [DOI] [PubMed] [Google Scholar]
- Briellmann R.S., Little T., Harvey A.S., Abbott D.F., Jacobs R., Waites A.B. Pathologic and physiologic function in the subcortical band of double cortex. Neurology. 2006;67:1090–1093. doi: 10.1212/01.wnl.0000237554.39283.6b. [DOI] [PubMed] [Google Scholar]
- Briellmann R.S., Mitchell L.A., Waites A.B., Abbott D.F., Pell G.S., Saling M.M. Correlation between language organization and diffusion tensor abnormalities in refractory partial epilepsy. Epilepsia. 2003;44:1541–1545. doi: 10.1111/j.0013-9580.2003.19403.x. [DOI] [PubMed] [Google Scholar]
- Cohen L., Dehaene S. Specialization within the ventral stream: the case for the visual word form area. NeuroImage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Costafreda S.G., Fu C.H., Lee L., Everitt B., Brammer M.J., David A.S. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum. Brain Mapp. 2006;27:799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham J.C., Kanwisher N.G. Neuroimaging of cognitive functions in human parietal cortex. Curr. Opin. Neurobiol. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Dehaene S., Cohen L. The unique role of the visual word form area in reading. Trends Cogn. Sci. 2011;15:254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Desmond J.E., Glover G.H. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J. Neurosci. Methods. 2002;118:115–128. doi: 10.1016/s0165-0270(02)00121-8. [DOI] [PubMed] [Google Scholar]
- Devinsky O., Perrine K., Llinas R., Luciano D.J., Dogali M. Anterior temporal language areas in patients with early onset of temporal lobe epilepsy. Ann. Neurol. 1993;34:727–732. doi: 10.1002/ana.410340517. [DOI] [PubMed] [Google Scholar]
- Donnan G.A., Bladin P.F., Berkovic S.F., Longley W.A., Saling M.M. The stroke syndrome of striatocapsular infarction. Brain. 1991;114:51–70. [PubMed] [Google Scholar]
- Eickhoff S.B., Stephan K.E., Mohlberg H., Grefkes C., Fink G.R., Amunts K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Everts R., Harvey A.S., Lillywhite L., Wrennall J., Abbott D.F., Gonzalez L. Language lateralization correlates with verbal memory performance in children with focal epilepsy. Epilepsia. 2010;51:627–638. doi: 10.1111/j.1528-1167.2009.02406.x. [DOI] [PubMed] [Google Scholar]
- Frings M., Dimitrova A., Schorn C.F., Elles H.-G., Hein-Kropp C., Gizewski E.R. Cerebellar involvement in verb generation: an fMRI study. Neurosci. Lett. 2006;409:19–23. doi: 10.1016/j.neulet.2006.08.058. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Ashburner J., Frith C.D., Poline J.B., Heather J.D., Frackowiak R.S.J. Spatial registration and normalization of images. Hum. Brain Mapp. 1995;3:165–189. [Google Scholar]
- Friston K.J., Price C.J., Fletcher P., Moore C., Frackowiak R.S.J., Dolan R.J. The trouble with cognitive subtraction. NeuroImage. 1996;4:97–104. doi: 10.1006/nimg.1996.0033. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S.J., Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Zarahn E., Josephs O., Henson R.N.A., Dale A.M. Stochastic designs in event-related fMRI. NeuroImage. 1999;10:607–619. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Gaillard W.D., Balsamo L., Xu B., McKinney C., Papero P.H., Weinstein S. fMRI language task panel improves determination of language dominance. Neurology. 2004;63:1403–1408. doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- Gaillard W.D., Berl M.M., Moore E.N., Ritzl E.K., Rosenberger L.R., Weinstein S.L. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology. 2007;69:1761–1771. doi: 10.1212/01.wnl.0000289650.48830.1a. [DOI] [PubMed] [Google Scholar]
- Gelinas J.N., Fitzpatrick K.P.V., Kim H.C., Bjornson B.H. Cerebellar language mapping and cerebral language dominance in pediatric epilepsy surgery patients. NeuroImage Clin. 2014;6:296–306. doi: 10.1016/j.nicl.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington G.S., Buonocore M.H., Farias S.T. Intrasubject reproducibility of functional MR imaging activation in language tasks. Am. J. Neuroradiol. 2006;27:938–944. [PMC free article] [PubMed] [Google Scholar]
- Henson R. Efficient experimental design for fMRI. Stat. Parametr. Mapp. Anal. Funct. Brain Images. 2007:193–210. [Google Scholar]
- Hickok G., Okada K., Barr W., Pa J., Rogalsky C., Donnelly K. Bilateral capacity for speech sound processing in auditory comprehension: evidence from Wada procedures. Brain Lang. 2008;107:179–184. doi: 10.1016/j.bandl.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. Opinion - the cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Jansen A., Menke R., Sommer J., Förster A.F., Bruchmann S., Hempleman J. The assessment of hemispheric lateralization in functional MRI—robustness and reproducibility. NeuroImage. 2006;33:204–217. doi: 10.1016/j.neuroimage.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Janszky J., Mertens M., Janszky I., Ebner A., Woermann F.G. Left-sided interictal epileptic activity induces shift of language lateralization in temporal lobe epilepsy: an fMRI study. Epilepsia. 2006;47:921–927. doi: 10.1111/j.1528-1167.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends Cogn. Sci. 2005;9:512–518. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Knecht S., Jansen A., Frank A., Van Randenborgh J., Sommer J., Kanowski M. How atypical is atypical language dominance? NeuroImage. 2003;18:917–927. doi: 10.1016/s1053-8119(03)00039-9. [DOI] [PubMed] [Google Scholar]
- Kurthen M., Helmstaedter C., Linke D.B., Solymosi L., Elger C.E., Schramm J. Interhemispheric dissociation of expressive and receptive language functions in patients with complex-partial seizures: an amobarbital study. Brain Lang. 1992;43:694–712. doi: 10.1016/0093-934x(92)90091-r. [DOI] [PubMed] [Google Scholar]
- Lee D., Swanson S.J., Sabsevitz D.S., Hammeke T.A., Scott Winstanley F., Possing E.T. Functional MRI and Wada studies in patients with interhemispheric dissociation of language functions. Epilepsy Behav. 2008;13:350–356. doi: 10.1016/j.yebeh.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.T., Frank L.R., Wong E.C., Buxton R.B. Detection power, estimation efficiency, and predictability in event-related fMRI. NeuroImage. 2001;13:759–773. doi: 10.1006/nimg.2000.0728. [DOI] [PubMed] [Google Scholar]
- Maus B., Van Breukelen G.J., Goebel R., Berger M.P. Optimal design of multi-subject blocked fMRI experiments. NeuroImage. 2011;56:1338–1352. doi: 10.1016/j.neuroimage.2011.03.019. [DOI] [PubMed] [Google Scholar]
- McGlone J. Speech comprehension after unilateral injection of sodium amytal. Brain Lang. 1984;22:150–157. doi: 10.1016/0093-934x(84)90084-1. [DOI] [PubMed] [Google Scholar]
- Mumford J.A., Nichols T.E. Power calculation for group fMRI studies accounting for arbitrary design and temporal autocorrelation. NeuroImage. 2008;39:261–268. doi: 10.1016/j.neuroimage.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Bodurka J., Bandettini P.A. How long to scan? The relationship between fMRI temporal signal to noise ratio and necessary scan duration. NeuroImage. 2007;34:565–574. doi: 10.1016/j.neuroimage.2006.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S.I., Uchimura K., Hirakawa W., Kuratsu J.I. Method for quantitatively evaluating the lateralization of linguistic function using functional MR imaging. AJNR Am. J. Neuroradiol. 2001;22:985–991. [PMC free article] [PubMed] [Google Scholar]
- Norrelgen F., Lilja A., Ingvar M., Åmark P., Fransson P. Presurgical language lateralization assessment by fMRI and dichotic listening of pediatric patients with intractable epilepsy. NeuroImage Clin. 2015;7:230–239. doi: 10.1016/j.nicl.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Halloran C.J., Kinsella G.J., Storey E. The cerebellum and neuropsychological functioning: a critical review. J. Clin. Exp. Neuropsychol. 2012;34:35–56. doi: 10.1080/13803395.2011.614599. [DOI] [PubMed] [Google Scholar]
- Orellana C.M., Visch-Brink E., Vernooij M., Kalloe S., Satoer D., Vincent A. Crossed cerebrocerebellar language lateralization: an additional diagnostic feature for assessing atypical language representation in presurgical functional MR imaging. Am. J. Neuroradiol. 2015;36:518–524. doi: 10.3174/ajnr.A4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papathanassiou D., Etard O., Mellet E., Zago L., Mazoyer B., Tzourio-Mazoyer N. A common language network for comprehension and production: a contribution to the definition of language epicenters with PET. NeuroImage. 2000;11:347–357. doi: 10.1006/nimg.2000.0546. [DOI] [PubMed] [Google Scholar]
- Price C.J. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann. N. Y. Acad. Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Price C.J., Mummery C.J., Moore C.J., Frackowiak R.S.J., Friston K.J. Delineating necessary and sufficient neural systems with functional imaging studies of neuropsychological patients. J. Cogn. Neurosci. 1999;11:371–382. doi: 10.1162/089892999563481. [DOI] [PubMed] [Google Scholar]
- Pugh K.R., Shaywitz B.A., Shaywitz S.E., Constable R.T., Skudlarski P., Fulbright R.K. Cerebral organization of component processes in reading. Brain J. Neurol. 1996;119(Pt 4):1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Ries M.L., Boop F.A., Griebel M.L., Zou P., Phillips N.S., Johnson S.C. Functional MRI and Wada determination of language lateralization: a case of crossed dominance. Epilepsia. 2004;45:85–89. doi: 10.1111/j.0013-9580.2004.04403.x. [DOI] [PubMed] [Google Scholar]
- Rosazza C., Ghielmetti F., Minati L., Vitali P., Giovagnoli A.R., Deleo F. Preoperative language lateralization in temporal lobe epilepsy (TLE) predicts peri-ictal, pre- and post-operative language performance: an fMRI study. NeuroImage Clin. 2013;3:73–83. doi: 10.1016/j.nicl.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten G.J.M., Ramsey N.F., van Rijen P.C., Alpherts W.C., van Veelen C.W.M. FMRI-determined language lateralization in patients with unilateral or mixed language dominance according to the Wada test. NeuroImage. 2002;17:447–460. doi: 10.1006/nimg.2002.1196. [DOI] [PubMed] [Google Scholar]
- Schachter S.C., Galaburda A.M., Ransil B.J. Associations of dyslexia with epilepsy, handedness, and gender. Ann. N. Y. Acad. Sci. 1993;682:402–403. doi: 10.1111/j.1749-6632.1993.tb23006.x. [DOI] [PubMed] [Google Scholar]
- Schlösser R., Hutchinson M., Joseffer S., Rusinek H., Saarimaki A., Stevenson J. Functional magnetic resonance imaging of human brain activity in a verbal fluency task. J. Neurol. Neurosurg. Psychiatry. 1998;64:492–498. doi: 10.1136/jnnp.64.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J.D. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J. Neuropsychiatr. Clin. Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Seghier M.L. Laterality index in functional MRI: methodological issues. Magn. Reson. Imaging. 2008;26:594–601. doi: 10.1016/j.mri.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spreer J., Arnold S., Quiske A., Wohlfarth R., Ziyeh S., Altenmüller D. Determination of hemisphere dominance for language: comparison of frontal and temporal fMRI activation with intracarotid amytal testing. Neuroradiology. 2002;44:467–474. doi: 10.1007/s00234-002-0782-2. [DOI] [PubMed] [Google Scholar]
- Springer J.A., Binder J.R., Hammeke T.A., Swanson S.J., Frost J.A., Bellgowan P.S.F. Language dominance in neurologically normal and epilepsy subjects - a functional MRI study. Brain. 1999;122:2033–2045. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Sveller C., Briellmann R.S., Saling M.M., Lillywhite L., Abbott D.F., Masterton R.A.J. Relationship between language lateralization and handedness in left-hemispheric partial epilepsy. Neurology. 2006;67:1813–1817. doi: 10.1212/01.wnl.0000244465.74707.42. [DOI] [PubMed] [Google Scholar]
- Tailby C., Weintrob D.L., Saling M.M., Fitzgerald C., Jackson G.D. Reading difficulty is associated with failure to lateralize temporooccipital function. Epilepsia. 2014;55:746–753. doi: 10.1111/epi.12607. [DOI] [PubMed] [Google Scholar]
- Thivard L., Hombrouck J., du Montcel S.T., Delmaire C., Cohen L., Samson S. Productive and perceptive language reorganization in temporal lobe epilepsy. NeuroImage. 2005;24:841–851. doi: 10.1016/j.neuroimage.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wada J., Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance. 1960. J. Neurosurg. 2007;106:1117–1133. doi: 10.3171/jns.2007.106.6.1117. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Nichols T.E. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. NeuroImage. 2003;18:293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Weber B., Wellmer J., Reuber M., Mormann F., Weis S., Urbach H. Left hippocampal pathology is associated with atypical language lateralization in patients with focal epilepsy. Brain. 2006;129:346–351. doi: 10.1093/brain/awh694. [DOI] [PubMed] [Google Scholar]
- Wilke M., Lidzba K., Staudt M., Buchenau K., Grodd W., Krägeloh-Mann I. An fMRI task battery for assessing hemispheric language dominance in children. NeuroImage. 2006;32:400–410. doi: 10.1016/j.neuroimage.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Wilke M., Schmithorst V.J. A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. NeuroImage. 2006;33:522–530. doi: 10.1016/j.neuroimage.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Woermann F.G., Jokeit H., Luerding R., Freitag H., Schulz R., Guertler S. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61:699–701. doi: 10.1212/01.wnl.0000078815.03224.57. [DOI] [PubMed] [Google Scholar]
- Wood A.G., Harvey A.S., Wellard R.M., Abbott D.F., Anderson V., Kean M. Language cortex activation in normal children. Neurology. 2004;63:1035–1044. doi: 10.1212/01.wnl.0000140707.61952.ca. [DOI] [PubMed] [Google Scholar]
- Zaidel E. Language in the right hemisphere. In: Benson D.F., Zaidel E., editors. The Dual Brain: Hemispheric Specialization in Humans. New York: Guilford Press; 1985. pp. 205–231. [Google Scholar]