Abstract
Background
The cJun-N-terminal-kinase (JNK) plays a central role in the cell stress response, with outcomes ranging from cell death to cell proliferation and survival, depending on the specific context. JNK is also one of the most investigated signal transducers in obesity and insulin resistance, and studies have identified new molecular mechanisms linking obesity and insulin resistance. Emerging evidence indicates that whereas JNK1 and JNK2 isoforms promote the development of obesity and insulin resistance, JNK3 activity protects from excessive adiposity. Furthermore, current evidence indicates that JNK activity within specific cell types may, in specific stages of disease progression, promote cell tolerance to the stress associated with obesity and type-2 diabetes.
Scope of review
This review provides an overview of the current literature on the role of JNK in the progression from obesity to insulin resistance, NAFLD, type-2 diabetes, and diabetes complications.
Major conclusion
Whereas current evidence indicates that JNK1/2 inhibition may improve insulin sensitivity in obesity, the role of JNK in the progression from insulin resistance to diabetes, and its complications is largely unresolved. A better understanding of the role of JNK in the stress response to obesity and type-2 diabetes, and the development of isoform-specific inhibitors with specific tissue distribution will be necessary to exploit JNK as possible drug target for the treatment of type-2 diabetes.
Keywords: Oxidative stress, Endoplasmic eeticulum stress, Autophagy, MAPK, Diabetes, Inflammation
1. Introduction
The cJun-N-terminal kinases JNKs are members of the mitogen-activated protein kinases (MAPK) family, which differ from classical MAPK such as ERK in the fact that JNK activity is more potently induced in response to cellular stress than to mitogens [1]. JNKs are named after their capacity to phosphorylate and activate the protein cJun, a member of the AP-1 family of transcription factors [2]. There are three JNK isoforms encoded by three different genes, JNK1 and JNK2, which are ubiquitously expressed, and JNK3 for which expression is restricted to the brain, testis, and pancreatic β-cells [1], [3]. JNKs are well known to play a central role in the cell response to stress, and JNK activity in different cell types was implicated in the pathogenesis of obesity-driven insulin resistance. The mechanisms leading to JNK activation in obesity, its cell-specific action in insulin resistance, and its role in pancreatic β-cell function were previously reviewed [3], [4], [5], [6], [7], [8], [9], [10]. However, to our knowledge, a comprehensive review discussing the potential benefits and possible side effects of JNK inhibition in obese diabetic patients is missing. Here we discuss the role of JNK in the link between obesity, type-2 diabetes, and their complications in light of an increasing body of evidence implicating JNK activity in the complex cellular response to the metabolic stress driven by obesity and type-2 diabetes. As emerging evidence indicates that JNK activity within specific cellular contexts may promote metabolic stress tolerance, a better understanding of this response is necessary to appreciate both the potential and the limitations of JNK as a drug target for the treatment of obesity-driven diabetes.
2. JNK in the pathogenesis of insulin resistance
JNK is one of the most investigated molecules in obesity models of insulin resistance, and studies have led to a new paradigm of how excess adiposity may cause insulin resistance [3], [5], [6], [11]. JNK was proposed to drive insulin resistance in obesity by four distinct mechanisms: direct inhibitory phosphorylation of insulin receptor substrates (IRS) 1 and 2; promotion of metabolic inflammation; promotion of metabolic efficiency and adiposity via inhibition of the TSH-thyroid hormone axis; and negative regulation of the PPARα-FGF21 axis (Figure 1).
Figure 1.
Molecular mechanisms of JNK1 and JNK2 in obesity-driven insulin resistance. The JNK1/2 kinases were proposed to play a central role in obesity-driven insulin resistance by four mechanisms: (A) In insulin-target cells, JNK1/2 directly phosphorylate IRS1 and IRS2 at serine and threonine residues leading to reduced tyrosine-phosphorylation of IRS1/2 molecules and decreased recruitment of the PI3K-AKT signaling pathway in response to insulin. (B) JNK1/2 play a major role in obesity-driven macrophage activation, leading to increased levels of inflammatory “M1” cytokines driving insulin resistance. (C) During obesity JNK1/2 activity in pituitary thyrotropic cells sustains the expression of DIO2 leading to a T3-dependent reduction of TSH production. Reduced TSH level leads to low circulating levels of thyroid hormone, increased metabolic efficiency, and increased adiposity, which drives insulin resistance. (D) During obesity, JNK1/2 activity in the hepatocyte sustains the expression of the transcription co-repressor NCor1, which inhibits PPARα-driven gene-expression leading to reduced FGF21 production, reduced fatty acid oxidation, and ketogenesis, promoting fatty liver and insulin resistance.
2.1. Mechanism 1: direct phosphorylation of IRS1 and IRS2
In 1993, Hotamisligil et al. reported that TNFα mRNA expression is elevated in adipose tissue of obese insulin-resistant rodents compared to control lean animals, and neutralization of TNFα improves insulin sensitivity in obese insulin resistant rats [12]. This study motivated the investigation of the role of signal transducers downstream TNFα and other inflammatory signals in insulin resistance.
One year later, Tanti et al. reported that serine/threonine phosphorylation of IRS1 caused defective IRS1 tyrosine phosphorylation and reduced phosphatidylinositide 3 kinase (PI3K) AKT signaling in response to insulin receptor activation [13]. Altogether, the two studies above indicate that serine/threonine kinases induced by TNFα and inflammation, such as JNK [3], may promote insulin resistance via direct phosphorylation of IRS molecules. It was later found that treatment of cell cultures with the potent JNK inducer anisomycin caused a reduction of IRS1 and IRS2 tyrosine phosphorylation in response to insulin, which was paralleled by increased serine/threonine phosphorylation of IRS1 and IRS2 [14]. The JNK phosphorylation site on IRS1 was mapped at serine-307, and it was shown that phosphorylation of this residue inhibits the interaction of IRS1 with the insulin receptor and tyrosine phosphorylation of IRS1 in response to insulin [14], [15]. Importantly, it was shown that JNK activity in mouse muscle, fat, and liver is elevated during obesity, and obese mice with systemic ablation of JNK1 (JNK1−/−) but not JNK2 (JNK2−/−) display a dramatically improved metabolic phenotype [16]. Indeed, JNK1−/− mice were resistant to diet-induced obesity and showed markedly improved insulin sensitivity, which was correlated with improved insulin-stimulated IRS1 tyrosine phosphorylation and decreased IRS1 serine-307 phosphorylation in liver [16]. Altogether, these studies indicate a preferential role for JNK1 in obesity-driven insulin resistance, possibly via direct phosphorylation of IRS1 at serine-307 (Figure 1A). However, it was shown that mice lacking JNK2 and only one allele of JNK1 (JNK2−/−JNK1+/−) were also protected from diet-induced obesity, steatosis, and insulin resistance, indicating that JNK2 is also involved in obesity driven insulin resistance [17]. Furthermore, JNK1 and JNK2 display the same ability to bind IRS1 and phosphorylate it at serine 307 in-vitro [17]. It is unlikely, however, that IRS1 phosphorylation at serine-307 per se is sufficient to explain the role of JNK in insulin resistance. Indeed, it was shown in mice that replacing IRS1 serine-307 with an alanine, to prevent its phosphorylation, does not protect from obesity-driven insulin resistance [18]. One possibility is that a single serine phosphorylation site (e.g. serine-307) is not sufficient to inhibit insulin signaling and that multiple serine/threonine residues need to be phosphorylated to prevent tyrosine phosphorylation of IRS1/2 by the insulin receptor. One could speculate that phosphorylation of multiple serine/threonine sites on IRS1 could prevent the interaction with the activated insulin receptor, which undergoes autophosphorylation on tyrosine residues because of a charge-repulsion effect. This view is supported by a study showing that mice with muscle-specific expression of an IRS1 mutant with an alanine substitution at serine residues 302, 307, and 612 display improved insulin sensitivity and muscle insulin signaling in a high-fat diet model of insulin resistance [19]. Furthermore, it should be considered that JNK was also shown to phosphorylate IRS2 at serine/threonine residues [14], [20], [21], hence blocking a single phosphorylation site on IRS1, and not on IRS2, is not expected to fully rescue the direct effects of JNK on insulin signaling. The major weakness of this mechanism is that it is largely based on cell-culture experiments and lacks of an in-vivo validation. Indeed, whereas IRS1 serine-307 phosphorylation is reduced in mice with systemic ablation of JNK1 [16], it must be also noted that JNK1−/− mice are resistant to diet-induced obesity and develop less inflammation than control mice when kept on high-fat diet [17]. Hence, reduced IRS1 serine-307 phosphorylation in JNK1−/− mice kept on high-fat diet could be the indirect consequence of their leaner phenotype and reduced inflammation.
In one study, it was reported that blocking JNK1 specifically in muscles of mice kept on high-fat diet improved insulin sensitivity, increased insulin stimulated IRS1 tyrosine phosphorylation, and decreased IRS1 serine-307 phosphorylation [22].Consistent with these results another group showed that elevation of JNK1 activity in tibialis anterior muscle of mice by electroporation of a vector expressing a JNK1 made constitutively active by fusion with the upstream kinase JNKK2 (JNKK2-JNK1) increased IRS1 serine-307 phosphorylation and caused muscle insulin resistance [23].
However, others could not observe a significant effect of muscle-specific JNK1 activation or blockage on insulin sensitivity by using cre-Lox mediated deletion of JNK1 or induced expression of JNKK2-JNK1 [24]. We conclude that the in-vivo relevance of direct phosphorylation of IRS molecules by JNK remains an open question. Because JNK1 and JNK2 are equally capable of binding and phosphorylating IRS1 in-vitro [17], it is necessary to investigate obesity-driven IRS1/2 serine/threonine phosphorylation in mice in which both JNK1/2 are inactivated in specific insulin-target tissues.
2.2. Mechanism 2: induction of macrophage M1 gene-expression
JNK1−/− mice and JNK1−/+JNK2−/− mice are protected from diet-induced obesity, inflammation, and insulin resistance, which implicates JNK1 and JNK2 in energy balance, insulin sensitivity, and metabolic inflammation [16], [17]. However, whole-body inactivation of JNK1/2 does not allow the identification of the cause-consequence relationship between the effects of JNK1/2 on adiposity, inflammation, and insulin sensitivity. To address this question, a bone marrow transplantation procedure was adapted to mice models of diet-induced obesity to ablate JNK1 specifically in cells of hematopoietic origin (e.g. macrophages) or in non-hematopoietic tissues [25]. This study showed that loss of JNK1 activity in both compartments, hematopoietic and non-hematopoietic, contributes to improved insulin sensitivity in mice fed a high-fat diet. The obesity-resistant phenotype was due to JNK1 inactivation in the non-hematopoietic compartment, whereas JNK1 activity in hematopoietic cells was necessary for obesity-driven expression of genes associated with classical (M1) macrophage activation independently from differences in adiposity. Interestingly, JNK1 ablation in the hematopoietic compartment reduced serum free fatty acid levels indicating a possible role for JNK1 within adipose tissue leukocytes in the control of lipolysis [25]. Another study using a similar bone marrow transplantation procedure also indicated that loss of JNK1 activity in both compartments, hematopoietic and non-hematopoietic, contributed to improved insulin sensitivity in mice fed a high-fat diet [26]. However, this study came to the conclusion that JNK1 action on insulin sensitivity is predominantly due to its activity in non-hematopoietic cells [26]. Conditional deletion of JNK1 in myeloid cells or adipocytes using transgenic mice expressing the cre recombinase under the control of the lysosome-M promoter (for myeloid cells) or the ap2 promoter (for adipocytes), respectively, indicated that JNK1 action in adipose tissue inflammation is due to its activity in adipocytes [27]. Quantitative and qualitative differences in these studies could be due to several reasons including difficulties in the standardization of bone marrow transplantation protocols, compensation from JNK2, and partial efficiency and specificity of cre recombination. In particular, the use of the ap2 promoter to drive the cre expression was recently shown to cause partial genetic recombination in adipocytes and considerable deletion of target genes in tissues other than fat [28]. Therefore, the role of JNK1 within the adipocyte in driving inflammation and insulin resistance should be investigated using adiponectin promoter-driven cre recombination, which is more efficient and specific for adipose tissue [28].
To clarify the role of JNK in macrophages, mice lacking JNK1 and JNK2 in macrophages were generated and investigated in diet-induced obesity. The results indicate that mice lacking JNK1 and JNK2 in myeloid cells were largely protected from obesity-driven inflammation and insulin resistance independent of differences in adiposity [29]. Furthermore, JNK1/2 inactivation in myeloid cells reduced macrophage accumulation in adipose tissue and the expression of M1 cytokines, thereby indicating that JNK activity in adipose tissue macrophages is required for their activation during obesity [29]. These results were confirmed in a subsequent study, indicating that JNK activity in macrophages promotes adipose tissue IL-6 expression, which increases adipose tissue lipolysis and circulating free-fatty acids, which drive hepatic glucose production in spite of normal liver insulin signaling [30]. Altogether, these studies indicate that JNK activity in myeloid cells is required for obesity-driven M1 activation of macrophages and elevation of pro-inflammatory cytokines levels in the obese adipose tissue (Figure 1B). Adipose tissue pro-inflammatory cytokines may promote insulin resistance by acting directly on insulin signaling within insulin target cells, or indirectly via stimulation of adipose tissue lipolysis and consequent elevation of circulating free fatty acid levels.
2.3. Mechanism 3: inhibition of pituitary thyroid axis
The fact that JNK1−/− mice and JNK1−/+JNK2−/− mice are protected from diet-induced obesity suggests that their improved insulin sensitivity could be, in part, an indirect consequence of their leaner phenotype [16], [17]. This concept is consistent with data from bone marrow transplantation experiments showing that improved insulin sensitivity in obese mice lacking JNK1 in the non-hematopoietic compartment was correlated with their leaner phenotype [25]. To study the role of JNK1 activity in neurons in diet-induced obesity, two different laboratories investigated mice in which JNK1 was specifically ablated in the central nervous system (CNS) and in the hypophysis using the cre recombinase driven by the nestin promoter. One study showed that loss of JNK1 in the CNS causes a defective growth phenotype as body weight and naso-anal length were reduced in mice lacking JNK1 in CNS that were kept on chow diet [31]. This defective growth phenotype observed in mice with specific CNS deletion of JNK1 is surprising as mice with whole body ablation of JNK1 do not display this phenotype but show reduced fat mass only when placed on an obesogenic diet [16], [17], [25]. When placed on a high-fat diet, mice lacking JNK1 in CNS showed increased T3 levels and pituitary expression of TSHβ, indicating activation of the pituitary thyroid axis. These mice also showed reduced body mass, reduced epididymal fat, smaller adipocytes, and dramatically reduced hepatic triglyceride content compared to control mice placed on a high-fat diet [31]. Another laboratory reported that JNK1 ablation in the CNS specifically reduced adiposity in mice placed on a high-fat diet without affecting growth on standard chow diet, a phenotype, which was explained by increased circulating levels of thyroid hormones and pituitary expression of TSHβ. Overall, these two studies consistently indicate that JNK1 promotes adiposity during high-fat diet feeding by inhibiting the pituitary-thyroid axis [32].
To learn more about the role of JNK in the pituitary-thyroid axis, mice lacking JNK1 and JNK2 in the anterior pituitary were generated and investigated in diet-induced obesity [33]. The results indicate that JNK activity in the pituitary was necessary to sustain the expression of TSHβ and that the action of JNK on TSHβ expression is indirect. Indeed, it was shown that JNK activity in pituitary cells promotes the expression of type-2 deiodinase (Dio2) gene, which mediates the conversion of T4 to T3 leading to T3-mediated inhibition of TSHβ gene expression. JNK ablation in pituitary cells thus reduces intracellular T3 levels and decreases inhibition of TSHβ gene expression, resulting in increased pituitary-thyroid activation, elevated thyroid hormone levels, and thereby a leaner phenotype (Figure 1C). Importantly, mice lacking JNK1 in the central nervous system (including the pituitary) and mice lacking JNK1 and JNK2 in the pituitary displayed improved glucose tolerance and insulin tolerance compared to their controls when placed on high-fat diet [31], [32], [33]. The latter is consistent with the idea that improved insulin sensitivity caused by JNK deletion in high-fat diet fed mice is in part an indirect consequence of their leaner phenotype.
The role of the neuron-specific isoform JNK3 in metabolism was recently investigated using mice models of diet-induced obesity and insulin resistance [34]. The data indicate that JNK3 is activated in hypothalamic neurons by high-fat diet feeding and that such activity is required for normal leptin action on the control of food intake. Compared to control animals, mice with ablation of JNK3 in the germline, mice lacking JNK3 in leptin receptor expressing cells, and mice lacking JNK3 in Agrp neurons, all display increased food intake, increased adiposity, and insulin resistance specifically when placed on high-fat diet [34].
Collectively, these studies indicate that whereas central JNK1 and JNK2 inhibition is expected to protect from obesity and insulin resistance by decreasing metabolic efficiency, central inhibition of JNK3 may increase caloric intake and thus exacerbate adiposity and insulin resistance.
2.4. Mechanism 4: negative regulation of PPARα activity and FGF21 expression by hepatic JNK
In recent studies, mice lacking JNK1 and JNK2 in hepatocytes were investigated to learn about the role of hepatic JNK activity in diet-induced obesity and insulin resistance [35], [36]. JNK1/2 ablation in the hepatocyte improved insulin sensitivity and markedly improved fatty liver in a mouse model of diet-induced obesity, a phenotype which was largely dependent on increased FGF21 expression [35], [36]. The results indicate that during high-fat diet feeding, hepatic JNK activity is required to sustain the expression of the transcriptional corepressors Ncor1 and Nrip1 and that JNK acts as a negative regulator of the transcription factor PPARα [35]. Decreased PPARα transcriptional activity resulted in reduced expression of genes involved in fatty acid oxidation, ketogenesis, and of the hormone FGF21. Ncor1, but not Nrip1, binding to the Fgf21 promoter region depended on JNK signaling, and the improved metabolic phenotype caused by ablation of hepatic JNK activity was not observed in mice which do not express a functional FGF21 in their hepatocytes [35], [36].
Altogether, these results indicate that JNK acts as a negative regulator of PPARα activity and FGF21 expression in the hepatocyte, via induction of the Ncor1 corepressor, thereby reducing hepatic fatty acid oxidation, ketogenesis, and promoting hepatic steatosis and insulin resistance during diet-induced obesity (Figure 1D).
3. Role of jnk in the cell response to stress, basic concepts
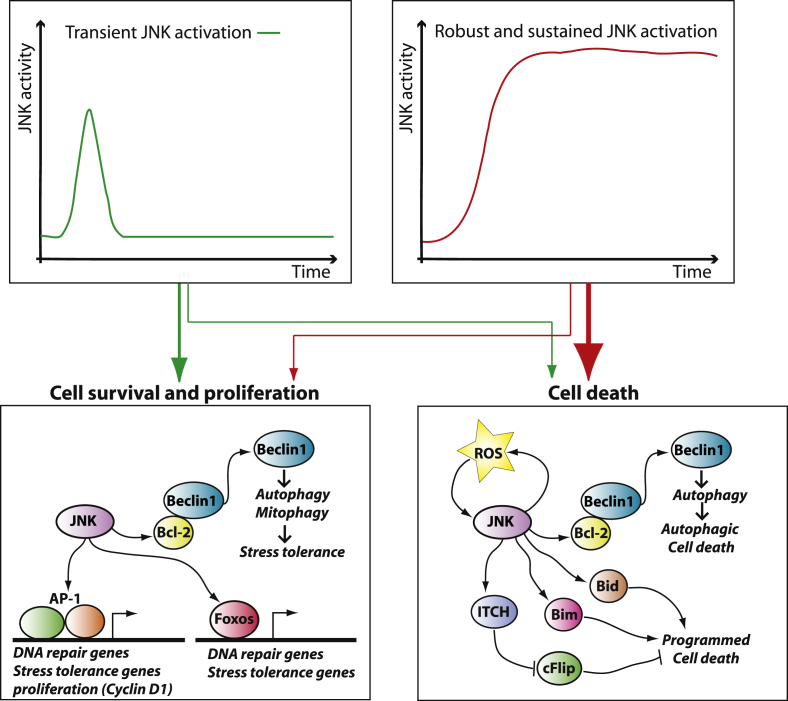
The four mechanisms described above explain the role of JNK in diet-induced obesity and insulin resistance. However, to fully appreciate the potential impact of JNK inhibition in obesity and type-2 diabetes, it is important to consider the role of JNK in the cellular response to stress. JNK is well known to be a major signal transducer driving the physiological response to several cellular stressors, including ultraviolet (UV)-radiation, genotoxic damage, oxidative stress, endoplasmic reticulum stress, long-chain saturated fatty acids, inflammatory cytokines, and microbial byproducts. Indeed, JNK was identified as an UV-responsive protein kinase mediating the phosphorylation and activation of the cJun transcription factor in response to a variety of stressors [2], [37], [38]. The role of JNK in the cellular stress response has been extensively investigated, and emerged that JNK works as a sort of “yin-yang” transducer capable of driving opposite outcomes depending on context, magnitude, and duration of its activation (Figure 2). The AP-1 transcription factors, which are regulated by JNK, have been linked to cell survival and cell cycle progression, but also to programmed cell death, although evidence indicates that cJun is mainly a positive regulator of cell proliferation [39], [40], [41], [42], [43]. Indeed, the JNK-cJun pathway was shown to be an early event driving proliferation during liver regeneration by a mechanism involving increased cyclin D1 expression [44], [45], [46], [47]. Furthermore, JNK was implicated in stress tolerance via its action on AP-1 and FOXO transcription factors [48], [49], [50], [51], [52], [53], [54]. Importantly, JNK1 was shown to induce autophagy by direct phosphorylation of Bcl-2 at threonine-69, serine-70, and serine-87, which leads to disruption of the Bcl-2/beclin-1 complex and consequent activation of autophagy [55]. Autophagy, “self-eating,” is a process of lysosomal mediated degradation of cellular components that can promote tolerance to stress by removing damaged organelles such as mitochondria (e.g. mitophagy). However, excessive autophagy can also lead to autophagic cell death.
Figure 2.
A general model for JNK action in the cell stress response. The JNK kinases are activated in response to several cellular stressors and inflammatory mediators, and JNK activation can either promote cell death or survival depending on the specific context. An important variable in determining the outcome of JNK signaling is the length and intensity of its activation. Early-transient activation of JNK was linked to stress tolerance and survival, whereas intense and sustained JNK activation often correlates with cell death. JNK action on stress tolerance and proliferation depends on its activity on the transcription factors AP-1 and Foxo, whereas JNK promotes cell death by phosphorylating cytoplasmic proteins implicated in programmed cell death including Bid, Bim, and ITCH which controls cFlip stability. JNK was also shown to promote autophagy by phosphorylating Bcl-2, which causes the release of Beclin1. Autophagy is a process of lysosomal-mediated cellular self-digestion, which can promote stress tolerance by removing damaged organelles but, when excessive, can also lead to autophagic cell death.
Sustained activation of JNK by the upstream kinase MEKK induces cell death in fibroblasts [56]; however, JNK activation is not always correlated with cell death and transient JNK activation can drive proliferation [57] (Figure 2). The role of JNK in cell death was extensively investigated in tumor necrosis factor (TNF) signaling, which induces a transient JNK activation. TNF drives distinct signaling cascades with opposing actions on cell survival [58]. Inhibition of NF-kB mediated gene expression changes the dynamic of JNK activation by TNF from transient to sustained and sensitizes cells to TNF-driven cell death in a JNK-dependent manner [58]. It was shown that the early-transient JNK activation by TNF is a signal promoting cell survival, whereas the long-sustained activation of JNK by TNF in cells with inhibited NF-kB driven gene expression causes cell death [59], [60]. Because JNK promotes cell death in cells in which gene-expression is blocked, it is evident that JNK-driven cell death can be largely independent from its action on AP-1 transcriptional activity. Indeed, JNK controls the activity of cytoplasmic proteins implicated in the control of programmed cell death, including Bim, Bid, and cFlip [61], [62], [63], [64], [65]. Sustained JNK activation following TNF treatment in cells in which NF-kB is blocked was also linked to increased production of reactive oxygen species, which contributes to cell death and play an important role in sustaining JNK activity at late time-points [66], [67], [68]. This dual action of JNK, with an early transient activation driving stress tolerance and a late sustained activation driving cell death, was also observed in response to DNA damage [48], [49], [50], [69] and, more recently, in response to endoplasmic reticulum stress [70], [71]. A general model for the role of JNK in stress response is summarized in Figure 2: JNK activation drives signals promoting survival, stress tolerance, and proliferation but also signals leading to programmed cell death. Pro-survival signals involve activation of AP-1 and Foxo dependent gene expression, whereas signals driving cell death are largely independent of changes in gene expression and are mediated by direct phosphorylation of cytoplasmic targets (Figure 2). The initial phase of JNK activation was shown to promote survival and proliferation in different experimental conditions, whereas at later time points JNK activity is often correlated with cell death (Figure 2). Thereby, a good generalization is that transient moderate JNK activation may promote stress tolerance, whereas for sustained JNK activation the expected outcome is cell death.
In obesity, moderate and sustained JNK activation is observed in different tissues [16], [17], a signaling dynamic which is in between the two extremes described above (Figure 2). The role of JNK activation in the cell response to the stress associated with obesity and diabetes in the different cell-types involved in the pathogenesis of obesity, type-2 diabetes, and their complications is still not completely understood.
4. Role of jnk in the cell response to metabolic stress: emerging concepts
Research on the role of JNK in obesity and type-2 diabetes has mainly focused on its action on insulin resistance (Figure 1). However, increasing evidence indicates that, in specific cellular contexts, JNK may mediate a cellular response to the metabolic stress associated with obesity and diabetes similar to the one described above (Figure 2). Below, we discuss the evidence indicating a role for JNK in the cell response to metabolic stress.
4.1. JNK in metabolic stress, lessons from Drosophila and Caenorhabditis elegans
It is established that reduction of insulin and IGF signaling leads to a major life-span extension in Drosophila and C. elegans because of increased transcriptional activity of their Foxo homologues (DFoxo and DAF-16), due to lack of repression from the PI3K-AKT pathway [72], [73]. It later emerged that JNK is a most important signal transducer in the adaptive response to stress in Drosophila and C. elegans [74], [75], [76]. Indeed, it was shown that Drosophila mutants with systemic reduction of JNK activity are more sensitive to the oxidative stress inducer paraquat and display reduced life-span [74]. Conversely, Drosophila mutants in which JNK activity is systemically induced are resistant to paraquat-induced oxidative damage and display extended longevity [74]. It was later found that JNK action on stress tolerance and longevity in Drosophila and C. elegans depends on JNK activity on FOXO homologue proteins, as JNK promotes FOXO nuclear localization and increases the expression of genes implicated in oxidative stress tolerance [75], [76]. Furthermore, JNK mediated nuclear translocation of DFoxo in insulin producing cells resulted in reduced expression of Drosophila insulin like-peptide-2 (dilp2), leading to lower systemic activation of insulin and IGF receptors [76]. Hence, in Drosophila and C. elegans, insulin and JNK signaling are two major determinants of life-span and oxidative stress tolerance because of their opposite actions on FOXO nuclear localization [75], [76]. More recently, it was shown that JNK activity in C. elegans is also required for the longevity effects of intermittent fasting and that JNK action on the expression of genes promoting oxidative stress tolerance depends also on AP-1 transcription activity [77]. Overall, these studies indicate that JNK promotes stress tolerance by driving FOXO-dependent gene-expression in antagonism with the action of insulin/IGF signaling, by reducing insulin production, and by activating AP-1 transcription factors.
The relevance of the opposing actions of insulin and JNK on Foxo-driven stress tolerance to human health is not known and requires further investigation. However, FOXO action on the expression of genes implicated in oxidative stress tolerance is conserved in mammalian cells [78], [79], and some studies have associated genetic variation of the FOXO3a gene with longevity in humans [80], [81].
4.2. Long-term effects of JNK1 ablation in obese mice
The studies described above in Drosophila and C. elegans indicate that JNK activity is required to maintain normal tolerance to systemic oxidative stress, thereby promoting longevity, which poses a possible concern for the long–term effects of JNK inhibition.
To learn about the long-term effects of JNK1 ablation in mammals undergoing metabolic stress from obesity, we have investigated mice lacking JNK1 (JNK1−/−) that are chronically fed an obesogenic high-fat diet for the duration of their lifetime [82]. The results showed that, compared to control mice, JNK1−/− mice maintained on high-fat diet display normal life-span and long-term sustained protection from obesity, hepatic lipid accumulation, adipose tissue inflammation, and insulin resistance. However, JNK1−/− mice exposed to high-fat feeding for 40 weeks were not protected from liver inflammation and were predisposed to alopecia and hair depigmentation, the latter typically being associated with oxidative damage and aging. Furthermore, JNK1−/− mice developed increased epidermal thickness and inflammation and displayed increased expression of genes involved in skin inflammation and regeneration, compared to control mice. Measurement of lipid peroxidation in different tissues showed that oxidative damage in JNK1−/− mice fed a high-fat diet for 40 weeks was similar-to-controls in kidney and heart, slightly improved in the liver and largely improved in adipose tissue, but was significantly worsened in the skin. The protective effects of JNK1 ablation on liver and fat oxidative stress were proposed to be, at least in part, a consequence of the reduced steatosis and reduced adipose tissue inflammation, whereas increased skin oxidative damage was correlated with a local reduction of the expression of antioxidant genes [82].
Altogether, this study indicates that JNK1 ablation confers long-term metabolic protection in a mouse model of diet-induced obesity, with reduced oxidative damage in liver and, to a larger extent, white adipose tissue. However, JNK1 ablation exacerbated oxidative damage in the skin indicating that, at least in specific cell types and in specific conditions, JNK1 may play an important role in promoting oxidative stress tolerance during obesity.
4.3. JNK activity in the pancreatic β-cell
The role of JNK in pancreatic β-cell function was not investigated to the same extent as the role of JNK in insulin resistance, and further studies are necessary. However, current evidence indicates that JNK activity may play a role in loss of β-cell function and mass under certain conditions. Similar to what was observed in Drosophila [76], it was shown that JNK activity in primary rat islets is required for oxidative stress mediated inhibition of insulin gene-expression [83]. Furthermore, it was shown that JNK activity in β-cell reduces insulin gene-expression by inducing nuclear translocation of Foxo1 and cytoplasmic exclusion of the transcription factor PDX-1 [84]. JNK activity was also implicated in the negative effects of the glucose secondary metabolite glucosamine on insulin gene-expression in primary rat islets and in RIN pancreatic β-cell [83], [85]. Treatment of primary mouse islets with long-chain saturated fatty acids caused a sustained JNK activation, which was necessary for the negative effects of saturated fatty acids on insulin gene expression [20]. The effects of JNK on insulin gene expression in primary mouse islets exposed to palmitate and to RIN pancreatic β-cell exposed to glucosamine depended on JNK negative action on autocrine insulin signaling [20], [85]. Indeed, in these experimental models, JNK activity was associated with increased IRS1 serine phosphorylation, reduced IRS1 tyrosine phosphorylation, and decreased IRS1-associated PI3K activity [20], [85]. Consistent with the studies above, it was shown that transgenic mice in which a sustained JNK activation is specifically driven in β-cells by a constitutive active mutant of the JNK activating kinase MKK7 develop glucose intolerance, reduced insulin secretion, and defective insulin signaling in their β-cells [86]. Altogether, these studies indicate that sustained JNK activation in β-cells may contribute to loss of β-cell function because of decreased insulin production.
Several studies implicated JNK in β-cell death caused by several stressors inducing a robust and prolonged or sustained JNK activation, including cytokines (IL-1β; TNFα; IFNγ), islets isolation, islets transplantation, and streptozotocin-induced genotoxic and oxidative stress [87], [88], [89], [90], [91], [92], [93], [94]. Collectively, these studies indicate that, at least in specific conditions, a robust-sustained JNK activation may contribute to loss of pancreatic β-cell mass and function. However, it must be noted that selective blockage of JNK3, which is expressed in β-cells, leads to the opposite outcome than blockage of JNK1 and JNK2. Indeed, it was reported that JNK3 silencing in β-cells enhanced sensitivity to cytokine-induced apoptosis and caused defective insulin signaling [95], [96]. Moreover, JNK3 activity in β-cells is required for the protective effects of extendin-4 on pancreatic β-cells exposed to inflammatory cytokines [97]. Interestingly, β-cell death was not always correlated with JNK activity. Indeed, one study reported that JNK inhibition in the NES2Y β-cell line had no effects on stearic acid-induced cell death [98], and one study reported that JNK1 silencing promoted glucolipotoxicity-mediated cell death in the INS1 β-cell line [99]. Furthermore, another study proposed that JNK activity is required for palmitate-mediated induction of autophagy in the MIN6 mouse insulinoma cell line and inhibition of autophagy promoted palmitate-induced cell death in this model [100]. Interestingly, it was reported that short-term exposure of β-cells to a low concentration of IL-1β for only two hours increased glucose-stimulated insulin secretion in a JNK-dependent manner [101]. Hence, it should be considered that specific JNK isoforms (e.g. JNK3) or specific JNK signaling dynamics, as with transient stimulation with low doses of IL-1β, may promote β-cell stress tolerance and function in specific experimental conditions.
4.4. JNK activity in non-alcoholic fatty liver disease
Non-alcoholic fatty liver disease (NAFLD) is a pathological condition initiated by excessive lipid storage in the hepatocyte (fatty liver), a condition that is per se harmless but that can progress to non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma. Because NAFLD progresses to more advanced stages of the disease only in some patients, a two-hits model for NAFLD was proposed [102]. The first hit, which initiates the disease, is the metabolic derangement leading to steatosis; the second hit, which drives the progression of the disease, is less understood, but oxidative stress was proposed to be an essential promoting factor [102]. JNK was implicated in both the initiation (first hit) and progression (second hit) of NAFLD. As described above, mice lacking JNK1, mice lacking JNK2 and heterozygous for JNK1 loss of function, and mice lacking JNK1 and JNK2 in their livers are largely protected from steatosis in models of diet-induced obesity [16], [17], [35]. These data indicate that blocking JNK may prevent the development of steatosis by the direct and indirect mechanisms described in Figure 1C,D. Furthermore, JNK1 activity in hematopoietic cells was implicated in the development of NASH in mice fed a choline-deficient l-amino acid defined diet, suggesting a major role for JNK1 in the progression of steatohepatitis [103]. Finally, sustained JNK1 activity in hepatocytes [104] and JNK activity in myeloid cells [105] were implicated in the promotion of chemically–induced hepatocellular carcinoma. Altogether, these studies indicate that blocking JNK, in particular JNK1, may prevent the development of steatosis, steatohepatitis, and hepatocellular carcinoma. However, it must also be considered that other studies indicate that the role of JNK in NAFLD is more complex and that, at least in specific conditions, JNK inhibition may promote NAFLD and liver damage. Indeed, it was reported that mice lacking JNK1 specifically in hepatocytes spontaneously develop microsteatosis, hepatic insulin resistance, and glucose intolerance on a chow-diet [27]. Furthermore, mice lacking a functional JNK2 placed on a high-fat diet developed similar steatosis than control mice but were predisposed to liver damage [106], and antisense oligonucleotide against JNK2 markedly increased liver damage in obese mice [106]. Importantly, it was recently shown that mice lacking JNK1 and JNK2 in their hepatocytes are predisposed to liver injury induced by acetaminophen or by carbon tetrachloride, two potent oxidative stress inducers [107]. Furthermore, mice lacking JNK1 and JNK2 in their hepatocytes developed larger tumors in a model of chemically induced hepatocellular carcinoma [108]. Finally, several studies indicate that JNK, and most notably JNK1, plays an important role in liver regeneration [43], [46], [108], [109]. Altogether, these studies indicate that JNK blockage may prevent obesity-driven NAFLD indirectly, by preventing obesity itself (Figure 1C), and may prevent the development of NASH (Figure 1B). However, the role of JNK activity in hepatocytes in NAFLD is less obvious, as JNK1 and JNK2 play specific and sometimes opposite actions in NAFLD initiation and progression in a context dependent manner.
4.5. JNK activity in the complications of type-2 diabetes
Several studies indicate that JNK may be involved in the promotion of cardiovascular diseases associated with obesity and type-2 diabetes. Primary human endothelial cells (HUVEC) exposed to high glucose levels accumulate reactive oxygen species, driving a robust and sustained JNK activation, and JNK1 ablation with antisense oligonucleotides protected these cells from high-glucose induced apoptosis [110]. One study reported that mice lacking JNK2 in macrophages decreased atherogenesis in the ApoE−/− mouse model of atherosclerosis because of defective foam-cell formation [111]. Furthermore, other studies indicated that JNK1 activity promotes atherosclerosis by driving endothelial cell damage in models of atherogenesis [112], [113]. However, it was recently reported that JNK1 ablation in hematopoietic cells increases atherosclerosis in the low-density lipoprotein receptor null mouse model by preventing macrophage apoptosis [114]. Hence, in models of atherogenesis, JNK1 promotes apoptosis of endothelial cells and macrophages, the former being linked to increased endothelium damage and promotion of atherogenesis, whereas the latter is linked to the delay of the development of atherosclerotic lesions.
Mice with compound deletions of all JNK isoforms in endothelial cells were generated, and these mice were predisposed to ischemic injury following arterial occlusion [115]. However, this was due to a role of JNK during development in native collateral arteries formation [115]. Thrombotic events such as hearth attack and stroke are major causes of mortality in type-2 diabetics [116]. A recent study indicates that JNK activity in platelets of diabetic patients may be part of an important mechanism protecting platelets from oxidative stress mediated cell damage, which leads to platelet activation and apoptosis causing thrombosis [117]. This study showed that platelets from diabetic subjects display increased oxidative stress, increased JNK activation, and increased autophagy/mitophagy compared to platelets from healthy control individuals [117]. Furthermore, exposure of platelets from healthy donors to hydrogen peroxide was sufficient to induce mitophagy, an effect that was blocked by co-treatment with a chemical inhibitor of JNK [117]. Importantly, blockage of JNK-driven autophagy made platelets more sensitive to oxidative stress-driven activation and apoptosis [117]. Hence, this study indicates that JNK activity in human platelets promotes oxidative stress tolerance by induction of adaptive mitophagy and may protect diabetic patients from thrombosis. A similar mechanism was described in human retinal capillary pericytes (HRCP), a cell type responsible of maintaining retinal capillary functional integrity and the loss of which is considered an initiator of diabetic retinopathy [118]. The authors observed that autophagy is induced in the retina of diabetic patients, and, to further investigate the role and the mechanism of autophagy in diabetic retinopathy, the authors exposed cultured HRCP to different doses of oxidized glycated LDL (HOG-LDL) [118]. HOG-LDL induced JNK activity and autophagy, and inhibition of JNK blocked the induction of autophagy by HOG-LDL. Importantly, JNK-driven autophagy protected HRCP from apoptosis induced by a low-dose of HOG-LDL but contributed to cell death induced by high-doses of HOG-LDL [118]. This dual role of JNK-driven autophagy in the response of HRCP cells to the stress caused by HOG-LDL is largely consistent with the general role of JNK in cell stress response (Figure 2).
The role of JNK in diabetic nephropathy was investigated in mice models of diabetes. One study used a pharmacological approach in which a cell permeable peptide inhibitor was given to db/db mice and a genetic approach in which hyperglycemia was induced with streptozotocin in mice lacking either JNK1 (JNK1−/−) or JNK2 (JNK2−/−) or in control mice [119]. The results indicate that JNK inhibition worsens albuminuria in db/db diabetic mice and that JNK1 genetic ablation does not affect the development of albuminuria in streptozotocin-induced diabetes, whereas loss of JNK2 dramatically worsens albuminuria in streptozotocin-induced diabetes [119]. Altogether, these results indicate that JNK activity, and in particular JNK2 activity, protects the kidney from the stress caused by hyperglycemia. More recently, another laboratory investigated the role of JNK in the kidney damage caused by hyperglycemia by treating mice made diabetic with streptozotocin with a small molecule JNK inhibitor (SP00125) [120]. The results are in sharp contrast with those from the study above [119] and showed that pharmacological inhibition of JNK decreases albuminuria, proteinuria, and blood urea nitrogen [120].
The role of JNK in the diabetic kidney remains therefore an open question. Opposing outcomes of JNK inhibition on cellular damage should not be surprising, although, for the future, it will be important to determine whether differences from the studies above are due to the use of different inhibitors or to different experimental conditions.
5. Conclusions
JNK is one of the most investigated signal transducers in models of obesity and insulin resistance, and the results from these studies have led to a new paradigm of the molecular mechanisms linking obesity and insulin resistance (Figure 1 and Figure 3). Based on these mechanisms, pharmacological inhibition of JNK1 and JNK2 is predicted to have beneficial metabolic effects in obesity by preventing excessive adiposity, inflammation, and insulin resistance. However, JNK3 inhibition is expected to cause deleterious effects in obesity by further promoting positive energy balance, adiposity, insulin resistance, and possible loss of β-cell function (Figure 3) [34], [95], [96], [97]. The latter poses a major obstacle to the exploitation of JNK as drug target for the treatment of obesity-driven insulin resistance. Indeed, in spite of intensive efforts to develop small molecules JNK inhibitors, to date, no compound inhibiting JNK1 and JNK2 with selectivity against JNK3 was reported [121], [122].
Figure 3.
JNK at the crossroad of obesity, insulin resistance, and the cell stress response. The role of JNK in obesity-driven insulin resistance was extensively investigated and the evidence indicates that JNK1 and JNK2 isoforms promote positive energy balance, adiposity, metabolic inflammation, and insulin resistance by the mechanisms described in Figure 1. However, JNK3 was reported to play an important homeostatic function in leptin control of food intake, and its inhibition is expected to increase food intake and aggravate adiposity and insulin resistance in obese subjects. The role of JNK in the β-cell failure occurring in type-2 diabetes and in the complications of obesity and type-2 diabetes was not investigated to the same extent as the role of JNK in insulin resistance. However, emerging evidence indicates that the action of JNK in loss of β-cell function, NAFLD progression, and diabetes complications may be more complex, with JNK activity driving disease promotion or being protective depending on the context. This dual action of JNK in the response to the metabolic stress associated to obesity and type-2 diabetes is reminiscent of the general role of JNK in cellular stress response described in Figure 2.
The role of JNK activity in β-cell-failure and complications of type-2 diabetes was not investigated to the same extent as for obesity and insulin resistance. However, emerging evidence indicates that JNK may act either as a promoting factor in disease progression or could induce stress tolerance and protect from disease progression depending on the model, cell type, and specific JNK isoform (Figure 3). The emerging picture is similar to the general mechanism for the JNK action in cellular stress response described in Figure 2, with a dual role for JNK in promoting either cell survival or cell death in response to stress. JNK action on cell survival in pathological conditions associated with obesity and type-2 diabetes such as NAFLD, β-cell death, and vascular complications seems to depend on the specific isoform, cell type, and magnitude of its activation. Indeed, some studies indicate that, at least under specific experimental conditions, JNK activity may promote tolerance to specific metabolic stressors and improve cell survival and functionality. Most notably, it was recently reported that JNK activity promotes insulin secretion in β-cells exposed to low concentrations of IL-1β [101], supports survival of human pericytes exposed to low-concentrations of HOG-LDL [118], and protects human platelets from oxidative stress induced activation and cell death [117]. Importantly, the same biological outcome in different cell-types may have opposite effects on disease progression. This may be the case for atherosclerosis, where JNK1 activity drives cell death in endothelial cells and in macrophages, with the former promoting atherosclerosis [112], [113] and the latter reducing the formation of atherosclerotic lesions [114]. We conclude that to exploit JNK inhibition as an insulin sensitizing therapy for the treatment of type-2 diabetes, it is necessary to better understand the role of specific JNK isoforms in the cell response to the metabolic stress driving loss of β-cell function and in pathological conditions associated with type-2 diabetes. In particular, a possible role for JNK activity in specific cell types in supporting tolerance to metabolic stress in type-2 diabetes models needs to be investigated.
It is intriguing that JNK activity was shown to be induced in cultured myotubes in response to contraction and in mice muscles in response to exercise [123], as this raises the question of a possible role for JNK in the metabolic stress tolerance induced by exercise. It is also remarkable that mice expressing a mutated Bcl-2 at its JNK1 phosphorylation sites display defective autophagy induction in response to exercise and are refractory to the beneficial effects of exercise on high-fat diet-induced glucose intolerance [124]. Although the dissociation of the Bcl-2 beclin1 complex was not correlated with JNK phosphorylation in this study, the role of JNK in exercise-induced metabolic stress tolerance deserves further investigations.
Acknowledgments
This work is supported by grants from the Swiss National Science Foundation, the Swedish Research Council, the Swedish Diabetes Foundation, and the Novo Nordisk Foundation.
Conflict of interest
The authors declare to have no conflict of interest in the topic covered by this manuscript.
References
- 1.Chang L., Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 2.Hibi M., Lin A., Smeal T., Minden A., Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes & Development. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 3.Solinas G., Karin M. JNK1 and IKKbeta: molecular links between obesity and metabolic dysfunction. FASEB Journal. 2010;24:2596–2611. doi: 10.1096/fj.09-151340. [DOI] [PubMed] [Google Scholar]
- 4.Holzer R.G., Park E.J., Li N., Tran H., Chen M., Choi C. Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell. 2011;147:173–184. doi: 10.1016/j.cell.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pal M., Febbraio M.A., Lancaster G.I. The roles of c-Jun NH2-terminal kinases (JNKs) in obesity and insulin resistance. The Journal of Physiology. 2016;594:267–279. doi: 10.1113/JP271457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotamisligil G.S., Davis R.J. Cell signaling and stress responses. Cold Spring Harbor Perspectives in Biology. 2016;8 doi: 10.1101/cshperspect.a006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manieri E., Sabio G. Stress kinases in the modulation of metabolism and energy balance. Journal of Molecular Endocrinology. 2015;55:R11–R22. doi: 10.1530/JME-15-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quan W., Jo E.K., Lee M.S. Role of pancreatic beta-cell death and inflammation in diabetes. Diabetes Obesity and Metabolism. 2013;15(Suppl. 3):141–151. doi: 10.1111/dom.12153. [DOI] [PubMed] [Google Scholar]
- 9.Kaneto H., Matsuoka T.A. Involvement of oxidative stress in suppression of insulin biosynthesis under diabetic conditions. International Journal of Mololecular Science. 2012;13:13680–13690. doi: 10.3390/ijms131013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaneto H. The JNK pathway as a therapeutic target for diabetes. Expert Opinion on Therapeutic Targets. 2005;9:581–592. doi: 10.1517/14728222.9.3.581. [DOI] [PubMed] [Google Scholar]
- 11.Solinas G. Molecular pathways linking metabolic inflammation and thermogenesis. Obesity Reviews. 2012;13(Suppl. 2):69–82. doi: 10.1111/j.1467-789X.2012.01047.x. [DOI] [PubMed] [Google Scholar]
- 12.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 13.Tanti J.F., Gremeaux T., van Obberghen E., Le Marchand-Brustel Y. Serine/threonine phosphorylation of insulin receptor substrate 1 modulates insulin receptor signaling. The Journal of Biological Chemistry. 1994;269:6051–6057. [PubMed] [Google Scholar]
- 14.Aguirre V., Uchida T., Yenush L., Davis R., White M.F. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) The Journal of Biological Chemistry. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 15.Aguirre V., Werner E.D., Giraud J., Lee Y.H., Shoelson S.E., White M.F. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. The Journal of Biological Chemistry. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 16.Hirosumi J., Tuncman G., Chang L., Gorgun C.Z., Uysal K.T., Maeda K. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 17.Tuncman G., Hirosumi J., Solinas G., Chang L., Karin M., Hotamisligil G.S. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10741–10746. doi: 10.1073/pnas.0603509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Copps K.D., Hancer N.J., Opare-Ado L., Qiu W., Walsh C., White M.F. Irs1 serine 307 promotes insulin sensitivity in mice. Cell Metabolism. 2010;11:84–92. doi: 10.1016/j.cmet.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morino K., Neschen S., Bilz S., Sono S., Tsirigotis D., Reznick R.M. Muscle-specific IRS-1 Ser->Ala transgenic mice are protected from fat-induced insulin resistance in skeletal muscle. Diabetes. 2008;57:2644–2651. doi: 10.2337/db06-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solinas G., Naugler W., Galimi F., Lee M.S., Karin M. Saturated fatty acids inhibit induction of insulin gene transcription by JNK-mediated phosphorylation of insulin-receptor substrates. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16454–16459. doi: 10.1073/pnas.0607626103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharfi H., Eldar-Finkelman H. Sequential phosphorylation of insulin receptor substrate-2 by glycogen synthase kinase-3 and c-Jun NH2-terminal kinase plays a role in hepatic insulin signaling. American Journal of Physiology, Endocrinology and Metabolism. 2008;294:E307–E315. doi: 10.1152/ajpendo.00534.2007. [DOI] [PubMed] [Google Scholar]
- 22.Sabio G., Kennedy N.J., Cavanagh-Kyros J., Jung D.Y., Ko H.J., Ong H. Role of muscle JNK1 in obesity-induced insulin resistance. Molecular and Cellular Biology. 2010;30:106–115. doi: 10.1128/MCB.01162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henstridge D.C., Bruce C.R., Pang C.P., Lancaster G.I., Allen T.L., Estevez E. Skeletal muscle-specific overproduction of constitutively activated c-Jun N-terminal kinase (JNK) induces insulin resistance in mice. Diabetologia. 2012;55:2769–2778. doi: 10.1007/s00125-012-2652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pal M., Wunderlich C.M., Spohn G., Bronneke H.S., Schmidt-Supprian M., Wunderlich F.T. Alteration of JNK-1 signaling in skeletal muscle fails to affect glucose homeostasis and obesity-associated insulin resistance in mice. PLoS One. 2013;8:e54247. doi: 10.1371/journal.pone.0054247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solinas G., Vilcu C., Neels J.G., Bandyopadhyay G.K., Luo J.L., Naugler W. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metabolism. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Vallerie S.N., Furuhashi M., Fucho R., Hotamisligil G.S. A predominant role for parenchymal c-Jun amino terminal kinase (JNK) in the regulation of systemic insulin sensitivity. PLoS One. 2008;3:e3151. doi: 10.1371/journal.pone.0003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabio G., Das M., Mora A., Zhang Z., Jun J.Y., Ko H.J. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee K.Y., Russell S.J., Ussar S., Boucher J., Vernochet C., Mori M.A. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes. 2013;62:864–874. doi: 10.2337/db12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han M.S., Jung D.Y., Morel C., Lakhani S.A., Kim J.K., Flavell R.A. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry R.J., Camporez J.P., Kursawe R., Titchenell P.M., Zhang D., Perry C.J. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160:745–758. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belgardt B.F., Mauer J., Wunderlich F.T., Ernst M.B., Pal M., Spohn G. Hypothalamic and pituitary c-Jun N-terminal kinase 1 signaling coordinately regulates glucose metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6028–6033. doi: 10.1073/pnas.1001796107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sabio G., Cavanagh-Kyros J., Barrett T., Jung D.Y., Ko H.J., Ong H. Role of the hypothalamic-pituitary–thyroid axis in metabolic regulation by JNK1. Genes & Development. 2010;24:256–264. doi: 10.1101/gad.1878510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vernia S., Cavanagh-Kyros J., Barrett T., Jung D.Y., Kim J.K., Davis R.J. Diet-induced obesity mediated by the JNK/DIO2 signal transduction pathway. Genes & Development. 2013;27:2345–2355. doi: 10.1101/gad.223800.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vernia S., Morel C., Madara J.C., Cavanagh-Kyros J., Barrett T., Chase K. Excitatory transmission onto AgRP neurons is regulated by cJun NH2-terminal kinase 3 in response to metabolic stress. Elife. 2016;5:e10031. doi: 10.7554/eLife.10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vernia S., Cavanagh-Kyros J., Garcia-Haro L., Sabio G., Barrett T., Jung D.Y. The PPARalpha-FGF21 hormone axis contributes to metabolic regulation by the hepatic JNK signaling pathway. Cell Metabolism. 2014;20:512–525. doi: 10.1016/j.cmet.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vernia S., Cavanagh-Kyros J., Barrett T., Tournier C., Davis R.J. Fibroblast growth factor 21 mediates glycemic regulation by hepatic JNK. Cell Reports. 2016;14:2273–2280. doi: 10.1016/j.celrep.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kyriakis J.M., Banerjee P., Nikolakaki E., Dai T., Rubie E.A., Ahmad M.F. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 38.Derijard B., Hibi M., Wu I.H., Barrett T., Su B., Deng T. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 39.Shaulian E., Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 40.Shaulian E., Schreiber M., Piu F., Beeche M., Wagner E.F., Karin M. The mammalian UV response: c-Jun induction is required for exit from p53-imposed growth arrest. Cell. 2000;103:897–907. doi: 10.1016/s0092-8674(00)00193-8. [DOI] [PubMed] [Google Scholar]
- 41.Karin M., Shaulian E. AP-1: linking hydrogen peroxide and oxidative stress to the control of cell proliferation and death. IUBMB Life. 2001;52:17–24. doi: 10.1080/15216540252774711. [DOI] [PubMed] [Google Scholar]
- 42.Shaulian E., Karin M. AP-1 as a regulator of cell life and death. Nature Cell Biology. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 43.Thevananther S., Sun H., Li D., Arjunan V., Awad S.S., Wyllie S. Extracellular ATP activates c-jun N-terminal kinase signaling and cell cycle progression in hepatocytes. Hepatology. 2004;39:393–402. doi: 10.1002/hep.20075. [DOI] [PubMed] [Google Scholar]
- 44.Alcorn J.A., Feitelberg S.P., Brenner D.A. Transient induction of c-jun during hepatic regeneration. Hepatology. 1990;11:909–915. doi: 10.1002/hep.1840110602. [DOI] [PubMed] [Google Scholar]
- 45.Westwick J.K., Weitzel C., Leffert H.L., Brenner D.A. Activation of Jun kinase is an early event in hepatic regeneration. Journal of Clinical Investigation. 1995;95:803–810. doi: 10.1172/JCI117730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwabe R.F., Bradham C.A., Uehara T., Hatano E., Bennett B.L., Schoonhoven R. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003;37:824–832. doi: 10.1053/jhep.2003.50135. [DOI] [PubMed] [Google Scholar]
- 47.Stepniak E., Ricci R., Eferl R., Sumara G., Sumara I., Rath M. c-Jun/AP-1 controls liver regeneration by repressing p53/p21 and p38 MAPK activity. Genes & Development. 2006;20:2306–2314. doi: 10.1101/gad.390506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potapova O., Haghighi A., Bost F., Liu C., Birrer M.J., Gjerset R. The Jun kinase/stress-activated protein kinase pathway functions to regulate DNA repair and inhibition of the pathway sensitizes tumor cells to cisplatin. The Journal of Biological Chemistry. 1997;272:14041–14044. doi: 10.1074/jbc.272.22.14041. [DOI] [PubMed] [Google Scholar]
- 49.Potapova O., Basu S., Mercola D., Holbrook N.J. Protective role for c-Jun in the cellular response to DNA damage. The Journal of Biological Chemistry. 2001;276:28546–28553. doi: 10.1074/jbc.M102075200. [DOI] [PubMed] [Google Scholar]
- 50.Hayakawa J., Mittal S., Wang Y., Korkmaz K.S., Adamson E., English C. Identification of promoters bound by c-Jun/ATF2 during rapid large-scale gene activation following genotoxic stress. Molecular Cell. 2004;16:521–535. doi: 10.1016/j.molcel.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 51.Fuest M., Willim K., MacNelly S., Fellner N., Resch G.P., Blum H.E. The transcription factor c-Jun protects against sustained hepatic endoplasmic reticulum stress thereby promoting hepatocyte survival. Hepatology. 2012;55:408–418. doi: 10.1002/hep.24699. [DOI] [PubMed] [Google Scholar]
- 52.Kietzmann T., Samoylenko A., Immenschuh S. Transcriptional regulation of heme oxygenase-1 gene expression by MAP kinases of the JNK and p38 pathways in primary cultures of rat hepatocytes. The Journal of Biological Chemistry. 2003;278:17927–17936. doi: 10.1074/jbc.M203929200. [DOI] [PubMed] [Google Scholar]
- 53.Shaukat Z., Liu D., Hussain R., Khan M., Gregory S.L. The role of JNK signalling in responses to oxidative DNA damage. Current Drug Targets. 2015;17:154–163. doi: 10.2174/1389450116666150126111055. [DOI] [PubMed] [Google Scholar]
- 54.Eijkelenboom A., Burgering B.M. FOXOs: signalling integrators for homeostasis maintenance. Nature Reviews Molecular Cell Biology. 2013;14:83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- 55.Wei Y., Pattingre S., Sinha S., Bassik M., Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Molecular Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson N.L., Gardner A.M., Diener K.M., Lange-Carter C.A., Gleavy J., Jarpe M.B. Signal transduction pathways regulated by mitogen-activated/extracellular response kinase kinase kinase induce cell death. The Journal of Biological Chemistry. 1996;271:3229–3237. doi: 10.1074/jbc.271.6.3229. [DOI] [PubMed] [Google Scholar]
- 57.Chen Y.R., Wang X., Templeton D., Davis R.J., Tan T.H. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. The Journal of Biological Chemistry. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- 58.Luo J.L., Kamata H., Karin M. IKK/NF-kappaB signaling: balancing life and death – a new approach to cancer therapy. Journal of Clinical Investigation. 2005;115:2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roulston A., Reinhard C., Amiri P., Williams L.T. Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor alpha. The Journal of Biological Chemistry. 1998;273:10232–10239. doi: 10.1074/jbc.273.17.10232. [DOI] [PubMed] [Google Scholar]
- 60.Ventura J.J., Hubner A., Zhang C., Flavell R.A., Shokat K.M., Davis R.J. Chemical genetic analysis of the time course of signal transduction by JNK. Molecular Cell. 2006;21:701–710. doi: 10.1016/j.molcel.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 61.Putcha G.V., Le S., Frank S., Besirli C.G., Clark K., Chu B. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 62.Lei K., Davis R.J. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lei K., Nimnual A., Zong W.X., Kennedy N.J., Flavell R.A., Thompson C.B. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Molecular and Cellular Biology. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deng Y., Ren X., Yang L., Lin Y., Wu X. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell. 2003;115:61–70. doi: 10.1016/s0092-8674(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 65.Chang L., Kamata H., Solinas G., Luo J.L., Maeda S., Venuprasad K. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 2006;124:601–613. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 66.Ventura J.J., Cogswell P., Flavell R.A., Baldwin A.S., Jr., Davis R.J. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes & Development. 2004;18:2905–2915. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamata H., Honda S., Maeda S., Chang L., Hirata H., Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 68.Matsuzawa A., Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochimica et Biophysica Acta. 2008;1780:1325–1336. doi: 10.1016/j.bbagen.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 69.Sanchez-Perez I., Murguia J.R., Perona R. Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene. 1998;16:533–540. doi: 10.1038/sj.onc.1201578. [DOI] [PubMed] [Google Scholar]
- 70.Sano R., Reed J.C. ER stress-induced cell death mechanisms. Biochimica et Biophysica Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brown M., Strudwick N., Suwara M., Sutcliffe L.K., Mihai A.D., Ali A.A. An initial phase of JNK activation inhibits cell death early in the endoplasmic reticulum stress response. Journal of Cell Science. 2016;129:2317–2328. doi: 10.1242/jcs.179127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kenyon C., Chang J., Gensch E., Rudner A., Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 73.Hwangbo D.S., Gershman B., Tu M.P., Palmer M., Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- 74.Wang M.C., Bohmann D., Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Developmental Cell. 2003;5:811–816. doi: 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
- 75.Oh S.W., Mukhopadhyay A., Svrzikapa N., Jiang F., Davis R.J., Tissenbaum H.A. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4494–4499. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang M.C., Bohmann D., Jasper H. JNK extends life span and limits growth by antagonizing cellular and organism-wide responses to insulin signaling. Cell. 2005;121:115–125. doi: 10.1016/j.cell.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 77.Uno M., Honjoh S., Matsuda M., Hoshikawa H., Kishimoto S., Yamamoto T. A fasting-responsive signaling pathway that extends life span in C. elegans. Cell Reports. 2013;3:79–91. doi: 10.1016/j.celrep.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 78.Kops G.J., Dansen T.B., Polderman P.E., Saarloos I., Wirtz K.W., Coffer P.J. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 79.Nemoto S., Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 80.Flachsbart F., Caliebe A., Kleindorp R., Blanche H., von Eller-Eberstein H., Nikolaus S. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Willcox B.J., Donlon T.A., He Q., Chen R., Grove J.S., Yano K. FOXO3A genotype is strongly associated with human longevity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Becattini B., Zani F., Breasson L., Sardi C., D'Agostino V.G., Choo M.K. JNK1 ablation in mice confers long-term metabolic protection from diet-induced obesity at the cost of moderate skin oxidative damage. FASEB Journal. 2016;30:3124–3132. doi: 10.1096/fj.201600393R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kaneto H., Xu G., Fujii N., Kim S., Bonner-Weir S., Weir G.C. Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. The Journal of Biological Chemistry. 2002;277:30010–30018. doi: 10.1074/jbc.M202066200. [DOI] [PubMed] [Google Scholar]
- 84.Kawamori D., Kaneto H., Nakatani Y., Matsuoka T.A., Matsuhisa M., Hori M. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. The Journal of Biological Chemistry. 2006;281:1091–1098. doi: 10.1074/jbc.M508510200. [DOI] [PubMed] [Google Scholar]
- 85.Andreozzi F., D'Alessandris C., Federici M., Laratta E., Del Guerra S., Del Prato S. Activation of the hexosamine pathway leads to phosphorylation of insulin receptor substrate-1 on Ser307 and Ser612 and impairs the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin insulin biosynthetic pathway in RIN pancreatic beta-cells. Endocrinology. 2004;145:2845–2857. doi: 10.1210/en.2003-0939. [DOI] [PubMed] [Google Scholar]
- 86.Lanuza-Masdeu J., Arevalo M.I., Vila C., Barbera A., Gomis R., Caelles C. In vivo JNK activation in pancreatic beta-cells leads to glucose intolerance caused by insulin resistance in pancreas. Diabetes. 2013;62:2308–2317. doi: 10.2337/db12-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonny C., Oberson A., Negri S., Sauser C., Schorderet D.F. Cell-permeable peptide inhibitors of JNK: novel blockers of beta-cell death. Diabetes. 2001;50:77–82. doi: 10.2337/diabetes.50.1.77. [DOI] [PubMed] [Google Scholar]
- 88.Ammendrup A., Maillard A., Nielsen K., Aabenhus Andersen N., Serup P., Dragsbaek Madsen O. The c-Jun amino-terminal kinase pathway is preferentially activated by interleukin-1 and controls apoptosis in differentiating pancreatic beta-cells. Diabetes. 2000;49:1468–1476. doi: 10.2337/diabetes.49.9.1468. [DOI] [PubMed] [Google Scholar]
- 89.Abdelli S., Ansite J., Roduit R., Borsello T., Matsumoto I., Sawada T. Intracellular stress signaling pathways activated during human islet preparation and following acute cytokine exposure. Diabetes. 2004;53:2815–2823. doi: 10.2337/diabetes.53.11.2815. [DOI] [PubMed] [Google Scholar]
- 90.Abdelli S., Abderrahmani A., Hering B.J., Beckmann J.S., Bonny C. The c-Jun N-terminal kinase JNK participates in cytokine- and isolation stress-induced rat pancreatic islet apoptosis. Diabetologia. 2007;50:1660–1669. doi: 10.1007/s00125-007-0704-2. [DOI] [PubMed] [Google Scholar]
- 91.Fornoni A., Pileggi A., Molano R.D., Sanabria N.Y., Tejada T., Gonzalez-Quintana J. Inhibition of c-jun N terminal kinase (JNK) improves functional beta cell mass in human islets and leads to AKT and glycogen synthase kinase-3 (GSK-3) phosphorylation. Diabetologia. 2008;51:298–308. doi: 10.1007/s00125-007-0889-4. [DOI] [PubMed] [Google Scholar]
- 92.Varona-Santos J.L., Pileggi A., Molano R.D., Sanabria N.Y., Ijaz A., Atsushi M. c-Jun N-terminal kinase 1 is deleterious to the function and survival of murine pancreatic islets. Diabetologia. 2008;51:2271–2280. doi: 10.1007/s00125-008-1169-7. [DOI] [PubMed] [Google Scholar]
- 93.Cheon H., Cho J.M., Kim S., Baek S.H., Lee M.K., Kim K.W. Role of JNK activation in pancreatic beta-cell death by streptozotocin. Molecular and Cellular Endocrinology. 2010;321:131–137. doi: 10.1016/j.mce.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 94.Brozzi F., Nardelli T.R., Lopes M., Millard I., Barthson J., Igoillo-Esteve M. Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia. 2015;58:2307–2316. doi: 10.1007/s00125-015-3669-6. [DOI] [PubMed] [Google Scholar]
- 95.Abdelli S., Puyal J., Bielmann C., Buchillier V., Abderrahmani A., Clarke P.G. JNK3 is abundant in insulin-secreting cells and protects against cytokine-induced apoptosis. Diabetologia. 2009;52:1871–1880. doi: 10.1007/s00125-009-1431-7. [DOI] [PubMed] [Google Scholar]
- 96.Abdelli S., Bonny C. JNK3 maintains expression of the insulin receptor substrate 2 (IRS2) in insulin-secreting cells: functional consequences for insulin signaling. PLoS One. 2012;7:e35997. doi: 10.1371/journal.pone.0035997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ezanno H., Pawlowski V., Abdelli S., Boutry R., Gmyr V., Kerr-Conte J. JNK3 is required for the cytoprotective effect of exendin 4. Journal of Diabetes Research. 2014;2014:814854. doi: 10.1155/2014/814854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nemcova-Furstova V., Balusikova K., Sramek J., James R.F., Kovar J. Caspase-2 and JNK activated by saturated fatty acids are not involved in apoptosis induction but modulate ER stress in human pancreatic beta-cells. Cellular Physiology and Biochemistry. 2013;31:277–289. doi: 10.1159/000343367. [DOI] [PubMed] [Google Scholar]
- 99.Prause M., Christensen D.P., Billestrup N., Mandrup-Poulsen T. JNK1 protects against glucolipotoxicity-mediated beta-cell apoptosis. PLoS One. 2014;9:e87067. doi: 10.1371/journal.pone.0087067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Y.Y., Sun L.Q., Wang B.A., Zou X.M., Mu Y.M., Lu J.M. Palmitate induces autophagy in pancreatic beta-cells via endoplasmic reticulum stress and its downstream JNK pathway. International Journal of Molecular Medicine. 2013;32:1401–1406. doi: 10.3892/ijmm.2013.1530. [DOI] [PubMed] [Google Scholar]
- 101.Arous C., Ferreira P.G., Dermitzakis E.T., Halban P.A. Short term exposure of beta cells to low concentrations of interleukin-1beta improves insulin secretion through focal adhesion and actin remodeling and regulation of gene expression. The Journal of Biological Chemistry. 2015;290:6653–6669. doi: 10.1074/jbc.M114.611111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Day C.P., James O.F. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 103.Kodama Y., Kisseleva T., Iwaisako K., Miura K., Taura K., De Minicis S. c-Jun N-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice. Gastroenterology. 2009;137 doi: 10.1053/j.gastro.2009.06.045. 1467–1477 e1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sakurai T., Maeda S., Chang L., Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10544–10551. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Han M.S., Barrett T., Brehm M.A., Davis R.J. Inflammation mediated by JNK in myeloid cells promotes the development of hepatitis and hepatocellular carcinoma. Cell Reports. 2016;15:19–26. doi: 10.1016/j.celrep.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singh R., Wang Y., Xiang Y., Tanaka K.E., Gaarde W.A., Czaja M.J. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology. 2009;49:87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cubero F.J., Zoubek M.E., Hu W., Peng J., Zhao G., Nevzorova Y.A. Combined activities of JNK1 and JNK2 in hepatocytes protect against toxic liver injury. Gastroenterology. 2016;150:968–981. doi: 10.1053/j.gastro.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Das M., Garlick D.S., Greiner D.L., Davis R.J. The role of JNK in the development of hepatocellular carcinoma. Genes & Development. 2011;25:634–645. doi: 10.1101/gad.1989311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hui L., Zatloukal K., Scheuch H., Stepniak E., Wagner E.F. Proliferation of human HCC cells and chemically induced mouse liver cancers requires JNK1-dependent p21 downregulation. Journal of Clinical Investigation. 2008;118:3943–3953. doi: 10.1172/JCI37156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ho F.M., Liu S.H., Liau C.S., Huang P.J., Lin-Shiau S.Y. High glucose-induced apoptosis in human endothelial cells is mediated by sequential activations of c-Jun NH(2)-terminal kinase and caspase-3. Circulation. 2000;101:2618–2624. doi: 10.1161/01.cir.101.22.2618. [DOI] [PubMed] [Google Scholar]
- 111.Ricci R., Sumara G., Sumara I., Rozenberg I., Kurrer M., Akhmedov A. Requirement of JNK2 for scavenger receptor A-mediated foam cell formation in atherogenesis. Science. 2004;306:1558–1561. doi: 10.1126/science.1101909. [DOI] [PubMed] [Google Scholar]
- 112.Chaudhury H., Zakkar M., Boyle J., Cuhlmann S., van der Heiden K., Luong le A. c-Jun N-terminal kinase primes endothelial cells at atheroprone sites for apoptosis. Arteriosclerosis Thrombosis and Vascular Biology. 2010;30:546–553. doi: 10.1161/ATVBAHA.109.201368. [DOI] [PubMed] [Google Scholar]
- 113.Amini N., Boyle J.J., Moers B., Warboys C.M., Malik T.H., Zakkar M. Requirement of JNK1 for endothelial cell injury in atherogenesis. Atherosclerosis. 2014;235:613–618. doi: 10.1016/j.atherosclerosis.2014.05.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Babaev V.R., Yeung M., Erbay E., Ding L., Zhang Y., May J.M. Jnk1 deficiency in hematopoietic cells suppresses macrophage apoptosis and increases atherosclerosis in low-density lipoprotein receptor null mice. Arteriosclerosis Thrombosis and Vascular Biology. 2016;36:1122–1131. doi: 10.1161/ATVBAHA.116.307580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ramo K., Sugamura K., Craige S., Keaney J.F., Davis R.J. Suppression of ischemia in arterial occlusive disease by JNK-promoted native collateral artery development. Elife. 2016;5:1–28. doi: 10.7554/eLife.18414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ferreiro J.L., Angiolillo D.J. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation. 2011;123:798–813. doi: 10.1161/CIRCULATIONAHA.109.913376. [DOI] [PubMed] [Google Scholar]
- 117.Lee S.H., Du J., Stitham J., Atteya G., Lee S., Xiang Y. Inducing mitophagy in diabetic platelets protects against severe oxidative stress. EMBO Molecular Medicine. 2016;8:779–795. doi: 10.15252/emmm.201506046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fu D., Yu J.Y., Yang S., Wu M., Hammad S.M., Connell A.R. Survival or death: a dual role for autophagy in stress-induced pericyte loss in diabetic retinopathy. Diabetologia. 2016;59:2251–2261. doi: 10.1007/s00125-016-4058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ijaz A., Tejada T., Catanuto P., Xia X., Elliot S.J., Lenz O. Inhibition of C-jun N-terminal kinase improves insulin sensitivity but worsens albuminuria in experimental diabetes. Kidney International. 2009;75:381–388. doi: 10.1038/ki.2008.559. [DOI] [PubMed] [Google Scholar]
- 120.Hong Z., Wu D., Nie H. Specific MAPK inhibitors prevent hyperglycemia-induced renal diseases in type 1 diabetic mouse model. Molecular and Cellular Biochemistry. 2016;419:1–9. doi: 10.1007/s11010-016-2722-1. [DOI] [PubMed] [Google Scholar]
- 121.Siddiqui M.A., Reddy P.A. Small molecule JNK (c-Jun N-terminal kinase) inhibitors. Journal of Medicinal Chemistry. 2010;53:3005–3012. doi: 10.1021/jm9003279. [DOI] [PubMed] [Google Scholar]
- 122.Gehringer M., Muth F., Koch P., Laufer S.A. c-Jun N-terminal kinase inhibitors: a patent review (2010–2014) Expert Opinion Therapeutic Patents. 2015;25:849–872. doi: 10.1517/13543776.2015.1039984. [DOI] [PubMed] [Google Scholar]
- 123.Whitham M., Chan M.H., Pal M., Matthews V.B., Prelovsek O., Lunke S. Contraction-induced interleukin-6 gene transcription in skeletal muscle is regulated by c-Jun terminal kinase/activator protein-1. The Journal of Biological Chemistry. 2012;287:10771–10779. doi: 10.1074/jbc.M111.310581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.He C., Bassik M.C., Moresi V., Sun K., Wei Y., Zou Z. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]