Abstract
Anaplastic thyroid carcinoma (ATC) although rare is the most deadly form of thyroid cancer. The fatality rate for ATC is high-pitched, the survival rate at 1 year after diagnosis is <20%. Control of ATC is severely hard and widespread with unpredictability. We Previous proved that histone gene reviser and epigenetic changes role significant parts in papillary and anaplastic thyroid cancer tumorigenesis. Herein, the goal of this study was to investigate the anti-tumor activities of a HDAC inhibitor, HNHA alone and in combination with sorafenib in ATC cells in vitro and in vivo and to explore its effects on apoptotic cell death pathways. Three ATC cell lines were exposed to sorafenib in the presence or absence of HNHA, and cell viability was determined by MTT assay. Effects of combined treatment on cell cycle and intracellular signaling pathways were assessed by flow cytometry and western blot analysis. The ATC cell lines xenograft model was used to examine the anti-tumor activity in vivo. Our data showed that HNHA and sorafenib synergistically decreased cell viability in ATC cells, and also significantly increased apoptotic cell death in these cells, as proved by the cleavage of caspase-3 and DNA fragmentation. HNHA and sorafenib combination was reduced anti-apoptotic factor in ATC. Thus, combination therapy with HNHA and sorafenib significantly decreased vessel density, and most significantly reduced tumor volume and increased survival in ATC xenografts.
These results propose that HNHA in combination with sorafenib has significant anti-cancer activity in preclinical models, potentially suggesting a new clinical approach for patients of advanced thyroid cancer type.
Introduction
A significant event in the tumorigenic transformation of thyroid follicular cells is the constitutive stimulation of a single signaling pathway. This pathway, known the RAS-BRAF-ERK pathway, is triggered by RET/PTC rearrangement [1]. A high prevalence of BRAF point mutations occurs in papillary thyroid cancers (PTCs; about 30–70%) and in anaplastic thyroid carcinomas (ATCs; about 10–40%) [2]. In PTCs, BRAF mutations, RET/PTC rearrangements, and RAS mutations are mostly mutually exclusive [3].
Patients with the most ordinary type of thyroid cancer, PTC, have low hazard of recurrence and high survival. However, some patients with ATC exhibit high levels of invasiveness and metastasis, and do not respond to any chemotherapy, typically dying in a few months [4], [5]. Although rare, ATC is the deadliest form of thyroid cancer; its fatality rate is high, with a 20% survival rate 1 year after diagnosis.
Many studies have shown histone deacetylase (HDAC) inhibitors to be effective anticancer agents; therefore, the US Food and Drug Administration (USFDA) has approved the use of such substances for treating several cancer types [6], [7]. Some HDAC inhibitors are currently in clinical trials as therapeutic agents alone or in combination with other anticancer drugs [8]. N-hydroxy-7-(2-naphthylthio) hepatonomide (HNHA) is a novel HDAC inhibitor that demonstrates significantly higher anticancer activity than other HDAC inhibitors such as trichostatin A and suberoylanilide hydroxamic acid [9], [10], [11].
The USFDA recently expanded the permitted use of sorafenib in treating advanced thyroid cancer [12]. Sorafenib is a multi-kinase inhibitor that obstructs different signaling pathways, including Raf kinases, vascular endothelial growth factor receptor (VEGFR), and platelet-derived growth factor receptors (PDGFRs) [13]. Furthermore, sorafenib has also been approved for the therapy of advanced renal cell carcinoma (RCC) and various other human cancers, including thyroid cancer [13], [14], [15], [16]. The anticancer activity of sorafenib occurs via the Raf/Mek/Erk pathway, inducing cell apoptosis and blocking tumorigenesis [17]. Like HDAC inhibitors, sorafenib suppresses tumorigenesis by translationally restraining the anti-apoptotic Bcl-2 family member, Mcl-1 [18], [19]. Recently, Stat3 was shown to be a major kinase-independent target of sorafenib [20], [21]. Unfortunately, however, a majority of the patients does not respond to sorafenib and HDAC inhibitors, and several patients who initially do respond subsequently become resistant, with the continuation of tumor progression [22], [23]. Consequently, several researchers have sought to target human cancer with a combination of sorafenib and chemotherapy [24], [25]. Since most patients with ATC are diagnosed at an advanced stage, there is a desperate need for new cancer therapies. The present study suggests a new clinical approach for ATC treatments by combination therapy with an HDAC inhibitor and sorafenib. The aim of this study was to determine the antitumor activities of HNHA alone and in combination with sorafenib in ATC cells.
Materials and Methods
Tissue Specimens
Fresh tumors were obtained from one patient with biochemically and histologically proven advanced metastatic ATC, who was treated at the Thyroid Cancer Center, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. The tumor tissue were acquired during surgical resection of primary and metastatic ATC sites. The research protocol was approved by the Institutional Review Board of the Thyroid Cancer Center, Gangnam Severance Hospital, Yonsei University College of Medicine (IRB Protocol: 3–2016-0076).
Tumor Cell Isolation and Primary Culture
After resection, tumors were kept in normal saline with antifungal and antibiotic agents. Normal tissue and fat were removed, and the tissues were rinsed with 1× Hanks' balanced salt solution (HBSS). Tumor tissue was minced with dissociation medium, which contained RPMI-1640 medium (Hyclone, South Logan, UT, USA) supplemented with 20% FBS and 1 mg/mL of type IV collagenase (Sigma-Aldrich, St Louis, MO, USA). Minced and suspended tumor cells were filtered through sterile 70-μm-pore nylon cell strainers (BD Falcon, Franklin Lakes, NJ, USA), rinsed with 50 mL 1× HBSS, and centrifuged at 1400 rpm for 5 min. Cells were resuspended with RPMI-1640 medium containing 10% fetal bovine serum (FBS; Hyclone) and 2% penicillin/streptomycin solution (Gibco, Grand Island, NY, USA). Cell viability was analyzed by the trypan blue dye exclusion method.
Cell Culture
ATC cell lines 8505C, SNU-80, and GSA1 were obtained from the European Collection of Cell Cultures (ECACC, Salisbury, United Kingdom) or the Korea Cell Line Bank (Seoul National University, Seoul, Korea) or by tumor cell isolation from the current patient and grown in RPMI-1640 medium with 10% FBS (Table 1). Authentication was performed by short tandem repeat profiling, karyotyping, and isoenzyme analysis.
Table 1.
Cell line characteristics, viability after drug treatment of all thyroid carncer cell lines examined
| 8505C | SNU-80 | GSA1 | |
|---|---|---|---|
| Age at diagnosis | 78 | 59 | 74 |
| Gender | Female | Female | Female |
| Primary disease site | Thyroid | Thyroid | Thyroid |
| Stage | - | - | IVc |
| Primary pathology | Anaplastic thyroid cancer | Anaplastic thyroid cancer | Anaplastic thyroid cancer |
| Classification of specimen used for culture | - | - | Fresh tumor |
| Obtained from | ECACC | Korea Cell Line Bank | Gangnam Severance Hospital, Seoul, Korea |
Cell Viability Assay
Cells were seeded in 96-well plates at 5 × 103 cells per well and incubated overnight to achieve 70% confluency. The indicated drugs were added to achieve final concentrations of 0–100 μM. Cells were then incubated for the indicated time points before the determination of cell viability, using the MTT reagent according to the manufacturer's protocol, and absorbance was measured at 490 nm. Data were expressed as percentages of the signal observed in vehicle-treated cells and shown as the mean ± SD of triplicate experiments.
Flow Cytometry Analysis of the Cell Cycle
Cells were treated with HNHA and sorafenib alone or in combination in RPMI-1640 medium containing 10% FBS for 40 h, harvested by trypsinization, and fixed with 70% ethanol. Cells were stained for total DNA, using PBS containing 40 μg/mL propidium iodide and 100 μg/mL RNase I for 30 min at 37°C. Cell cycle distribution was then analyzed in the FACSCalibur Flow Cytometer (BD Biosciences, San Jose, CA, USA). The proportions of cells in the sub-G0/G1, G0/G1, S, and G2/M phases were analyzed by FlowJo v8 software for MacOSX (Tree Star, Ashland, OR, USA). This experiment was repeated thrice, and the results were averaged.
Evaluation of Apoptotic Cell Death
Cells were fixed with 4% paraformaldehyde solution for 48 h and then analyzed using a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) kit (Promega, Madison, WI, USA). The apoptotic cells (fluorescent green) and total cells were counted by fluorescence microscopy. Images were acquired under a confocal microscope (LSM Meta 700, Carl Zeiss, Oberkochen, Germany) and analyzed using the Zeiss LSM Image Browser software, version 4.2.0121.
Immunoblot Analysis
Equal amounts of protein (20 μg) were separated by 8–10% SDS-PAGE. The antibodies for p53 and p21 were obtained from Abcam (Cambridge, UK). Apaf-1, CDK 4, CDK 6, cyclin D1, Bcl-2, p-NF-κB, caspase-3, and β-actin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies for GRP78, PERK, p-PERK, eIF2α, p-eIF2α, ATF4, and CHOP were purchased from Cell Signaling Technology (Danvers, MA, USA).
Human ATC Cell Xenograft
Human ATC cells (2.0 × 107 cells/mouse) were cultured in vitro and then injected subcutaneously into the upper left flank region of female BALB/c nude mice. After 7 days, tumor-bearing mice were grouped randomly (n = 10/group) and treated with 25 mg/kg HNHA (intraperitoneally) alone, 80 mg/kg sorafenib (orally) alone, or a combination of 6.5 mg/kg HNHA and 25 mg/kg sorafenib, once every 2 days for a total of 10~ 12 injections. Tumor size was measured every other day using calipers. Tumor volume was estimated using the following formula: L × S/2 (where L is the longest diameter and S is the shortest diameter). Animals were maintained under specific pathogen-free conditions. All experiments were approved by the Animal Experiment Committee of Yonsei University.
In Vivo Toxicity Study
In vivo toxicity assays were performed using female BALB/c nude mice. Six-week-old mice were caged for 1 week for acclimatization. Each group of 10 mice was administered HNHA or sorafenib alone or the combination of HNHA plus sorafenib in the previous subsection. The animals were monitored regularly for external signs of toxicity or lethality. All animals were housed in cages with five mice per cage, with a 12-h/12-h light:dark cycle and temperature and humidity of 22 °C and 40–60%, respectively. A standard diet of rodent pellets and tap water (membrane filter-purified and autoclaved) were provided ad libitum.
Immunohistochemistry
All tissues were fixed in 10% neutral-buffered formalin and embedded in paraffin wax by standard protocols. Tissue sections (5 μm) were dewaxed, and antigen retrieval was performed in citrate buffer (pH 6), using an electric pressure cooker set at 120 °C for 5 min. Sections were incubated for 5 min in 3% hydrogen peroxide to quench endogenous tissue peroxidase. All tissue sections were counterstained with hematoxylin, dehydrated, and mounted.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA). Immunohistochemistry results were subjected to ANOVA followed by a Bonferroni post hoc test. Values are expressed as means ± SD. P<.05 indicated statistical significance.
Results
Synergistic Inhibition by HNHA and Sorafenib in 8505C, SNU-80, and GSA1 Cells
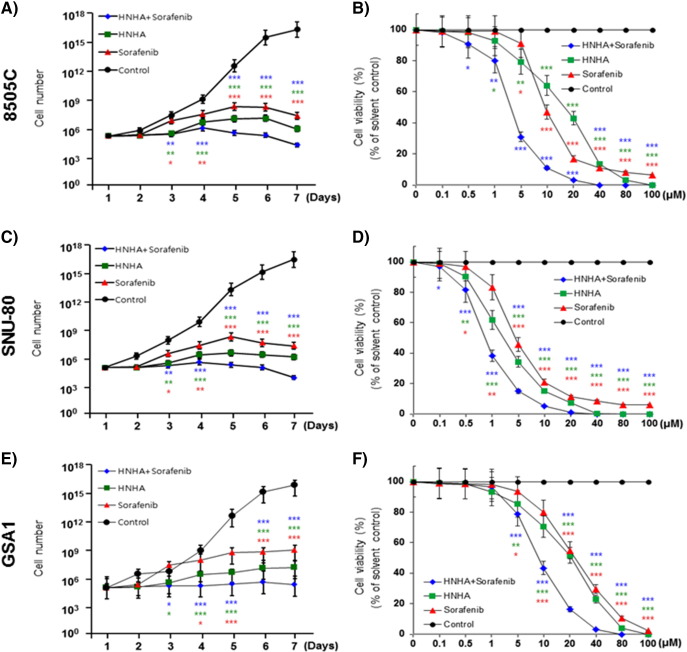
To investigate the anticancer effects of the synergistic interaction between HNHA and sorafenib in combination, as well as their individual effects, on ATC, we assayed 8505C, SNU-80, and GSA1 cell proliferation in the presence and absence of these compounds by MTT assay (Table 2). HNHA or sorafenib treatment alone had a lower IC50 in ATC than the combined treatment. Further characterization of the synergistic effect of HNHA and sorafenib on ATC cell viability showed that the combination reduced the viability of ATC cells to a greater extent than by either agent alone. The combination of HNHA and sorafenib suppressed cell proliferation better than either agent used singly (Figure 1,A, C, and E); moreover, this effect was concentration-dependent (Figure 1,B, D, and F).
Table 2.
IC50 (half maximal inhibitory concentration) determination using a cell proliferation assay
| Cell line | Hisopathology | Animal | Cell proliferation IC50⁎ (μM) |
||
|---|---|---|---|---|---|
| HNHA ± Sorafenib | HNHA | Sorafenib | |||
| 8505C | Thyroid cancer: anaplastic | Human | 3.82 (±0.5)* | 17.42 (±0.6) | 10.21 (±0.9) |
| SNU-80 | Thyroid cancer: anaplastic | Human | 0.87 (±1.1)* | 2.28 (±0.4) | 5.14 (±1.1) |
| GSA1 | Thyroid cancer: anaplastic | Human | 8.72 (±0.5)* | 20.14 (±0.5) | 23.45 (±0.6) |
HNHA and sorafenib combination treatment is a lower IC50 than HNHA or sorafenib alone. Each data point represents the mean of 3 independent MTT assays for IC50 performed in triplicate. SD, standard deviation.
Figure 1.
Combination of HNHA and sorafenib suppressed ATC cell proliferation more efficiently than either agent alone. Cell viability and proliferation assay of HNHA and sorafenib combined and HNHA and sorafenib alone in ATC cells (8505C, A and B; SNU-80, C and D; GSA1, E and F). Points indicate mean % of the value observed in the solvent-treated control. All experiments were repeated at least 3 times. The data represent the mean ± SD. Experiments were repeated at least 3 times with similar results. * P < .05 vs. control, ** P < .01 vs. control, *** P < .005 vs. control.
The HNHA and Sorafenib Combination Significantly Induced Endoplasmic Reticulum Stress-Dependent Cell Cycle Arrest in ATC
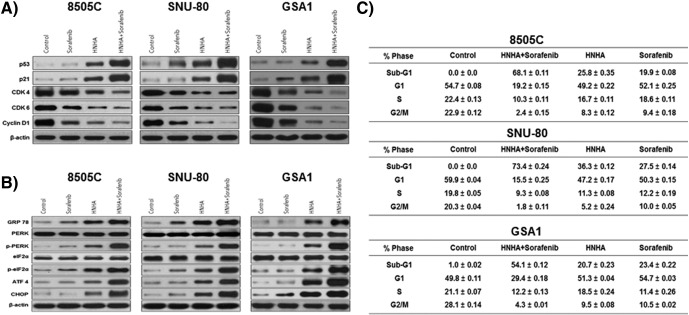
Immunoblot analyses of protein levels in ATC (8505C, SNU-80, and GSA1) cell lines indicated that the HNHA and sorafenib combination induced more marked increases in the levels of p53 and p21—well-known arrestors of the cell cycle—and decreases in the levels of cyclins D1, CDK 4, and CDK 6—positive regulators of the cell cycle—as compared with HNHA or sorafenib alone (Figure 2A). We also tested whether these compounds induced endoplasmic reticulum (ER) stress by treating 8505C, SNU-80, and GSA1 cells with HNHA or sorafenib alone or in combination for 24 h and analyzing the expression of GRP78, ATF4, CHOP, PERK, p-PERK, eIF2α, and p-eIF2α by immunoblotting (Figure 2B). The HNHA and sorafenib combination-treated cells showed the highest increase in these markers of ER stress. Flow cytometry was performed to investigate the effects of these compounds on cell cycle progression. The HNHA and sorafenib combination showed the most significant induction of G0/G1 phase arrest and increase in the sub-G0/G1 population (P < .05), indicating the induction of cell cycle arrest and cell death in the ATC cell lines (Figure 2C). Thus, the synergistic effect of HNHA and sorafenib most potently induced ER stress, leading to ER stress-dependent apoptosis, cell cycle arrest, and strong inhibition of ATC and PTC cell viability.
Figure 2.
Combination of HNHA and sorafenib induced cell cycle arrest and endoplasmic reticulum stress in ATC cells more potently than either agent alone. Immunoblot analysis of the indicated cell lines following exposure to the HNHA and sorafenib combination or each agent singly. The HNHA and sorafenib combination potently induced the expression of cell cycle arrest proteins and reduced the expression of positive regulators of the cell cycle (A). 8505C, SNU 80, and GSA1 were exposed to the indicated inhibitors for 24 h prior to the analysis of the expression of GRP78, ATF4, CHOP, PERK, p-PERK, eIF2α, and p-eIF2α (markers of endoplasmic reticulum stress) by immunoblot analysis (B). Cells were exposed to the indicated inhibitors, harvested, and stained with propidium iodide before analysis by flow cytometry and FlowJo v8 software (C).
The HNHA and Sorafenib Combination Induced Caspase-Mediated Apoptosis of ATC Cell Lines
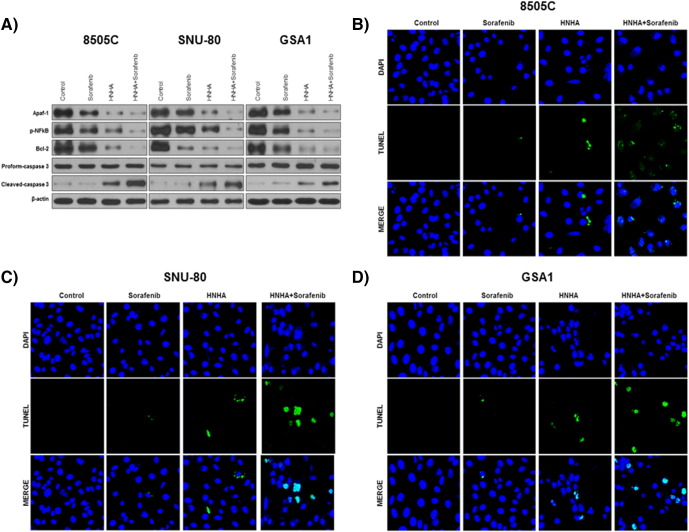
To study the pro-apoptotic signaling pathways activated by exposure of ATC cells to HNHA and sorafenib, the expression of pro-apoptotic (Apaf-1) and anti-apoptotic (phosphorylated NF-κB p65 and Bcl-2) members of the Bcl-2 family, as well as the cleavage of caspase-3, were analyzed by immunoblot analysis (Figure 3A). The results showed that the HNHA and sorafenib combination enriched the “pro” form of caspase-3 and induced the cleavage of pro-caspase-3 more potently than did HNHA or sorafenib alone (Figure 3A). The TUNEL assay confirmed that the combination induced apoptosis in ATC cell lines more potently than did either agent alone (Figure 3, B–D). These data demonstrated that the synergistic effect of HNHA and sorafenib efficiently induces apoptosis in ATC cells and that it exerts this effect via caspase cleavage and inhibition of the Bcl-2 pathway.
Figure 3.
Combination of HNHA and sorafenib significantly induced apoptotic death in ATC cells. Immunoblot analyses suggested that the indicated inhibitors increased the levels of apoptotic proteins and reduced those of anti-apoptotic proteins in ATC cells (A). TUNEL assay of ATC cells; TUNEL-positive (apoptotic) cells are indicated (×400) (B, 5805C; C, SNU-80; and D, GSA1).
The HNHA and Sorafenib Combination Reduced Xenograft Growth and Improved Survival In Vivo
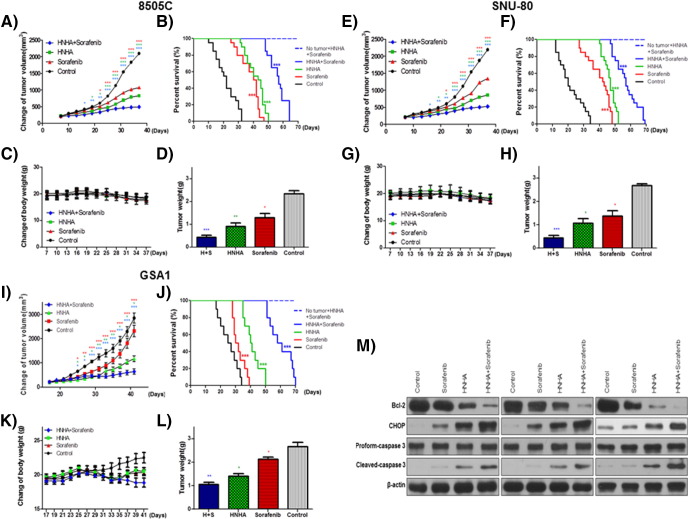
HNHA and sorafenib markedly suppressed 8505C and SNU-80 cell xenograft tumors; however, their combination exhibited a greater suppression of these tumors (Figure 4, A, E, and I). Mouse survival was prolonged significantly by both agents, but their combination yielded better survival rates (Figure 4, B, F, and J). No evidence of systemic toxicity or treatment-related death was found in any group. There was no significant effect on the body weight of mice treated with HNHA and sorafenib (Figure 4, C, G, and K). The combination-treated group showed significantly smaller tumor volumes compared to the groups treated with HNHA or sorafenib alone (Figure 4, D, H, and L). HNHA and sorafenib synergistically reduced Bcl-2 (anti-apoptotic protein) levels, increased CHOP (ER stress protein) levels, and cleaved caspase to a greater extent than HNHA or sorafenib alone. This implies a more efficient induction of cell cycle arrest and apoptosis due to ER stress in ATC mouse xenografts by the combination treatment (Figure 4M).
Figure 4.
Combination of HNHA and sorafenib produced antitumor effects in ATC cell xenografts in vivo. Athymic nude mice with established tumors were treated with the indicated inhibitors. Data represent the mean tumor volumes. HNHA and sorafenib combination therapy induced more potent inhibition of tumor progression than did HNHA or sorafenib alone, resulting in the maximum prolongation of survival in mice with ATC (8505C, A–D; SNU-80, E–H; and GSA1, I–L) xenografts (n = 10 mice/group) (A, E, and I). “No tumor + HNHA + sorafenib” indicates HNHA and sorafenib combination-treated mice with no xenograft; no evidence of systemic toxicity or treatment-related death was found in HNHA and sorafenib combination-treated groups (B, F, and J). The compounds had no significant effect on mouse body weight (C, G, and K). Weights of the dissected tumors (D, H, and L). Immunoblot analysis of total proteins isolated from the tumors (M). * P < .05 vs. control, ** P < .01 vs. control, *** P < .005 vs. control.
The HNHA and Sorafenib Combination Therapy Suppressed Tumor Growth by Down-Regulating the Expression of an Anti-Apoptotic Factor in ATC Xenografts
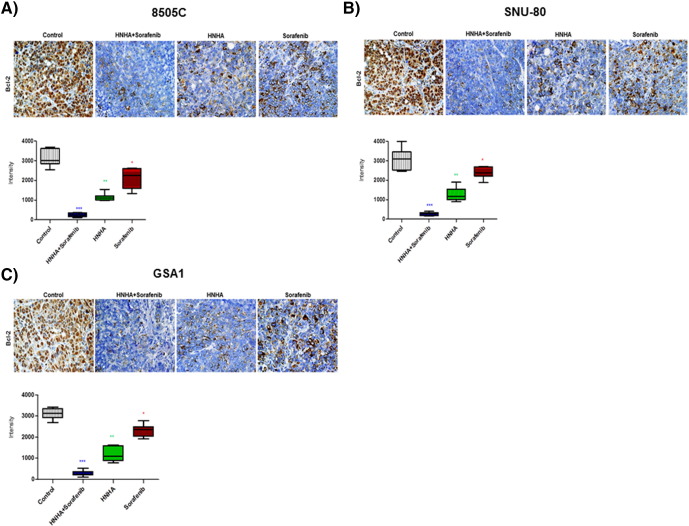
Anti-apoptotic activity is an important factor in the assessment of the biological behavior of tumorigenesis. At present, Bcl-2 is the most useful marker of anti-apoptosis. We detected this marker by immunohistochemical examination of 8505C, SNU-80, and GSA1 cell xenograft tumors and found that the combination-treated group showed the strongest decrease in Bcl-2 expression (Figure 5, A–C), further confirming that HNHA and sorafenib combination therapy has potent anticancer activity in the ATC xenograft model.
Figure 5.
HNHA and sorafenib combination therapy significantly reduced tumor Bcl-2 expression. Immunohistochemical analysis of the Bcl-2 protein levels in paraffin-embedded tumor tissues from mice with ATC xenografts. Synergistic activity of the HNHA and sorafenib combination induced more potent inhibition of tumor Bcl-2 expression than HNHA or sorafenib did alone. MetaMorph 4.6 image-analysis software was used to quantify Bcl-2 immunostaining. * P < .05; ** P < .01; *** P < .005 for the comparison with the control.
Discussion
Thyroid cancer is a common endocrine malignancy [26]. Recent times have witnessed dramatic improvements in the perception of the molecular pathogenesis of this cancer, best explained by the essential roles of certain signaling pathways. The key mechanisms are epigenetic and genetic modifications in these pathways, such as uncontrolled methylation and mutation of genes [27], [28]. A great number of these molecular modifications provide clues about novel prognostic and diagnostic therapeutic targets in thyroid cancer, thereby providing immense chances for further study and progress in the development of novel treatment strategies for this cancer.
BRAF, a serine/threonine kinase well-known to stimulate MAPK pathway mutations, has been implicated in melanoma, colon cancer, and thyroid cancer [3], [29], [30]. A high prevalence of BRAF point mutations in PTCs and ATCs has been reported [2]. However, in thyroid carcinomas, the pathways and molecules connected to the effect of BRAF suppression in cellular survival are poorly understood. Notably, patients with PTC have a low risk of recurrence and high survival, whereas some patients with ATC show considerable metastasis and respond poorly to chemotherapy [31], [32], [33]. Therefore, combinations of targeted therapies that shrink ATCs are warranted.
HDAC inhibitors have been used against ATC cells [34], [35], and HNHA has been found to be effective at low doses [11]. HDACs are histone-modifying enzymes that play major roles in the control of several proteins that are involved in tumorigenesis. Deviant expression of some HDACs has been demonstrated in diverse human cancers [36]. High HDAC1 expression has been linked with a more advanced tumor–node–metastasis stage, greater cancer cell invasion, and a lower survival rate in human cancers [37]. Accordingly, HDACs are promising drug targets for treating several human cancers. Some HDAC inhibitors are being investigated for their effects against solid and hematologic malignancies [38]. HDAC inhibitors are also being assessed in combination with radiotherapy, chemotherapy, and molecular targeted agents [8], [39]. In an earlier study, we reported the anticancer activity of HNHA, an N-hydroxyacrylamide-derived HDAC inhibitor, in ATC and PTC cell lines [11]. Furthermore, in ATC and PTC tumor xenograft models, HNHA was demonstrated to be more effective than established HDAC inhibitors in suppressing tumor growth without triggering body weight loss [11].
Sorafenib is an established multi-kinase inhibitor with activity against the Ser/Thr kinase Raf, which plays a crucial role in tumor cell proliferation and signaling, as well as angiogenesis-related receptor tyrosine kinases such as VEGFR2 and PDGFR [13], [40], [41]. Sorafenib also targets the Raf/Mek/Erk pathway. One study proved that patients with high levels of p-Erk have a greater survival rate [14], [17]. The use of this multi-kinase inhibitor was recently permitted for the therapy of advanced thyroid cancer [42]. In the present study, we found that the combination therapy of HNHA and sorafenib had a lower IC50 in ATC than that of either agent alone. The mechanisms underlying these synergistic anticancer effects of both agents on ATC cell lines included the induction of cell cycle arrest and apoptosis. Apoptosis was shown by the increased proportion of cells in sub-G1 and by the stimulation of caspase 3. The combination therapy also demonstrated a characteristic effect on cell cycle progression, whereby G1 arrest was evident in the presence of lower concentrations of HNHA and sorafenib, as compared to the levels of HNHA or sorafenib that individually produced this effect. This finding was consistent with those of previous studies showing that HNHA or sorafenib when used alone are cytotoxic and induce G1 arrest at lower concentrations [11], [14], [43].
This study showed synergistic cytotoxic effects of combined HNHA plus sorafenib therapy on ATC cell lines, both in vitro and in vivo. The combination therapy induced a more marked rise in the apoptosis of ATC cells than did HNHA or sorafenib singly. Consistent with this, the capacity of HDAC inhibitors to induce p21 expression has been described in a number of cell types and has been shown to occur through promoter hyperacetylation [44]. The inhibition of p21 expression affects the lethality of HDAC inhibitors and DNA-damaging agents in various cancer cell types, such as leukemia, thyroid cancer, and RCC [10], [11], [45], [46], [47]. It has been suggested that p21 is cleaved by caspase-3 for DNA injury-mediated apoptosis [48]. Sorafenib-mediated transcriptional suppression may cause the down-regulation of p21. Besides its role as a cyclin-mediated kinase inhibitor, some investigations propose that p21 might engage in DNA repair by controlling the interaction between PARP-1 and base excision repair factors, thereby participating in resistance to chemotherapeutic agents [49], [50]. Therefore, it will be interesting to investigate whether the sorafenib-mediated down-regulation of p21 causes the synergistic interaction between HNHA and sorafenib.
Acknowledgements
The authors thank Dr. Seung Won Kim for critical brainstorming and Dr. Kyung Hwa Choi for assistance with tumor cell isolation from patient specimen. This work was supported by a faculty research grant from Yonsei University College of Medicine for 6-2016-0123 and the Brain Korea 21 Project for Medical Science, Yonsei University.
References
- 1.Piscazzi A, Costantino E, Maddalena F, Natalicchio MI, Gerardi AM, Antonetti R, Cignarelli M, Landriscina M. Activation of the RAS/RAF/ERK signaling pathway contributes to resistance to sunitinib in thyroid carcinoma cell lines. J Clin Endocrinol Metab. 2012;97:E898–E906. doi: 10.1210/jc.2011-3269. [DOI] [PubMed] [Google Scholar]
- 2.Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008;21(Suppl. 2):S37–S43. doi: 10.1038/modpathol.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soares P, Trovisco V, Rocha AS, Lima J, Castro P, Preto A, Maximo V, Botelho T, Seruca R, Sobrinho-Simoes M. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22:4578–4580. doi: 10.1038/sj.onc.1206706. [DOI] [PubMed] [Google Scholar]
- 4.Begum S, Rosenbaum E, Henrique R, Cohen Y, Sidransky D, Westra WH. BRAF mutations in anaplastic thyroid carcinoma: implications for tumor origin, diagnosis and treatment. Mod Pathol. 2004;17:1359–1363. doi: 10.1038/modpathol.3800198. [DOI] [PubMed] [Google Scholar]
- 5.Patel KN, Shaha AR. Poorly differentiated and anaplastic thyroid cancer. Cancer Control. 2006;13:119–128. doi: 10.1177/107327480601300206. [DOI] [PubMed] [Google Scholar]
- 6.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124:30–39. doi: 10.1172/JCI69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolden JE, Shi W, Jankowski K, Kan CY, Cluse L, Martin BP, MacKenzie KL, Smyth GK, Johnstone RW. HDAC inhibitors induce tumor-cell-selective pro-apoptotic transcriptional responses. Cell Death Dis. 2013;4:e519. doi: 10.1038/cddis.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mund C, Lyko F. Epigenetic cancer therapy: Proof of concept and remaining challenges. Bioessays. 2010;32:949–957. doi: 10.1002/bies.201000061. [DOI] [PubMed] [Google Scholar]
- 9.Park KC, Kim SW, Park JH, Song EH, Yang JW, Chung HJ, Jung HJ, Suh JS, Kwon HJ, Choi SH. Potential anti-cancer activity of N-hydroxy-7-(2-naphthylthio) heptanomide (HNHA), a histone deacetylase inhibitor, against breast cancer both in vitro and in vivo. Cancer Sci. 2011;102:343–350. doi: 10.1111/j.1349-7006.2010.01798.x. [DOI] [PubMed] [Google Scholar]
- 10.Park KC, Heo JH, Jeon JY, Choi HJ, Jo AR, Kim SW, Kwon HJ, Hong SJ, Han KS. The novel histone deacetylase inhibitor, N-hydroxy-7-(2-naphthylthio) hepatonomide, exhibits potent antitumor activity due to cytochrome-c-release-mediated apoptosis in renal cell carcinoma cells. BMC Cancer. 2015;15:19. doi: 10.1186/s12885-014-1003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SM, Park KC, Jeon JY, Kim BW, Kim HK, Chang HJ, Choi SH, Park CS, Chang HS. Potential anti-cancer effect of N-hydroxy-7-(2-naphthylthio) heptanomide (HNHA), a novel histone deacetylase inhibitor, for the treatment of thyroid cancer. BMC Cancer. 2015;15:1003. doi: 10.1186/s12885-015-1982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Y, Shi Y, Xing P, Wang L, Feng Y, Han X, He X. Sorafenib in metastatic radioactive iodine-refractory differentiated thyroid cancer: A pilot study. Mol Clin Oncol. 2014;2:87–92. doi: 10.3892/mco.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broecker-Preuss M, Muller S, Britten M, Worm K, Schmid KW, Mann K, Fuhrer D. Sorafenib inhibits intracellular signaling pathways and induces cell cycle arrest and cell death in thyroid carcinoma cells irrespective of histological origin or BRAF mutational status. BMC Cancer. 2015;15:184. doi: 10.1186/s12885-015-1186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moreno-Aspitia A. Clinical overview of sorafenib in breast cancer. Future Oncol. 2010;6:655–663. doi: 10.2217/fon.10.41. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 18.Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J Biol Chem. 2005;280:35217–35227. doi: 10.1074/jbc.M506551200. [DOI] [PubMed] [Google Scholar]
- 19.Yu C, Bruzek LM, Meng XW, Gores GJ, Carter CA, Kaufmann SH, Adjei AA. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene. 2005;24:6861–6869. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- 20.Rosmorduc O, Desbois-Mouthon C. Targeting STAT3 in hepatocellular carcinoma: sorafenib again. J Hepatol. 2011;55:957–959. doi: 10.1016/j.jhep.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Tai WT, Cheng AL, Shiau CW, Huang HP, Huang JW, Chen PJ, Chen KF. Signal transducer and activator of transcription 3 is a major kinase-independent target of sorafenib in hepatocellular carcinoma. J Hepatol. 2011;55:1041–1048. doi: 10.1016/j.jhep.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Choy ML, Marks PA. Mechanisms of resistance to histone deacetylase inhibitors. Adv Cancer Res. 2012;116:39–86. doi: 10.1016/B978-0-12-394387-3.00002-1. [DOI] [PubMed] [Google Scholar]
- 23.Zhai B, Sun XY. Mechanisms of resistance to sorafenib and the corresponding strategies in hepatocellular carcinoma. World J Hepatol. 2013;5:345–352. doi: 10.4254/wjh.v5.i7.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CH, Chen MC, Wang JC, Tsai AC, Chen CS, Liou JP, Pan SL, Teng CM. Synergistic interaction between the HDAC inhibitor, MPT0E028, and sorafenib in liver cancer cells in vitro and in vivo. Clin Cancer Res. 2014;20:1274–1287. doi: 10.1158/1078-0432.CCR-12-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lachenmayer A, Toffanin S, Cabellos L, Alsinet C, Hoshida Y, Villanueva A, Minguez B, Tsai HW, Ward SC, Thung S. Combination therapy for hepatocellular carcinoma: additive preclinical efficacy of the HDAC inhibitor panobinostat with sorafenib. J Hepatol. 2012;56:1343–1350. doi: 10.1016/j.jhep.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen QT, Lee EJ, Huang MG, Park YI, Khullar A, Plodkowski RA. Diagnosis and treatment of patients with thyroid cancer. Am Health Drug Benefits. 2015;8:30–40. [PMC free article] [PubMed] [Google Scholar]
- 27.Faam B, Ghaffari MA, Ghadiri A, Azizi F. Epigenetic modifications in human thyroid cancer. Biomed Rep. 2015;3:3–8. doi: 10.3892/br.2014.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brehar AC, Brehar FM, Bulgar AC, Dumitrache C. Genetic and epigenetic alterations in differentiated thyroid carcinoma. J Med Life. 2013;6:403–408. [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveira C, Pinto M, Duval A, Brennetot C, Domingo E, Espin E, Armengol M, Yamamoto H, Hamelin R, Seruca R. BRAF mutations characterize colon but not gastric cancer with mismatch repair deficiency. Oncogene. 2003;22:9192–9196. doi: 10.1038/sj.onc.1207061. [DOI] [PubMed] [Google Scholar]
- 30.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 31.Ranganath R, Shah MA, Shah AR. Anaplastic thyroid cancer. Curr Opin Endocrinol Diabetes Obes. 2015;22:387–391. doi: 10.1097/MED.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 32.Viola D, Valerio L, Molinaro E, Agate L, Bottici V, Biagini A, Lorusso L, Cappagli V, Pieruzzi L, Giani C. Treatment of advanced thyroid cancer with targeted therapies: ten years of experience. Endocr Relat Cancer. 2016;23:R185–R205. doi: 10.1530/ERC-15-0555. [DOI] [PubMed] [Google Scholar]
- 33.Keutgen XM, Sadowski SM, Kebebew E. Management of anaplastic thyroid cancer. Gland Surg. 2015;4:44–51. doi: 10.3978/j.issn.2227-684X.2014.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baldan F, Mio C, Allegri L, Puppin C, Russo D, Filetti S, Damante G. Synergy between HDAC and PARP Inhibitors on Proliferation of a Human Anaplastic Thyroid Cancer-Derived Cell Line. Int J Endocrinol. 2015;2015:978371. doi: 10.1155/2015/978371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitsiades CS, Poulaki V, McMullan C, Negri J, Fanourakis G, Goudopoulou A, Richon VM, Marks PA, Mitsiades N. Novel histone deacetylase inhibitors in the treatment of thyroid cancer. Clin Cancer Res. 2005;11:3958–3965. doi: 10.1158/1078-0432.CCR-03-0776. [DOI] [PubMed] [Google Scholar]
- 36.Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett. 2009;280:168–176. doi: 10.1016/j.canlet.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 37.Rikimaru T, Taketomi A, Yamashita Y, Shirabe K, Hamatsu T, Shimada M, Maehara Y. Clinical significance of histone deacetylase 1 expression in patients with hepatocellular carcinoma. Oncology. 2007;72:69–74. doi: 10.1159/000111106. [DOI] [PubMed] [Google Scholar]
- 38.Witt O, Deubzer HE, Milde T, Oehme I. HDAC family: What are the cancer relevant targets? Cancer Lett. 2009;277:8–21. doi: 10.1016/j.canlet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 40.Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 41.Schult C, Dahlhaus M, Ruck S, Sawitzky M, Amoroso F, Lange S, Etro D, Glass A, Fuellen G, Boldt S. The multikinase inhibitor Sorafenib displays significant antiproliferative effects and induces apoptosis via caspase 3, 7 and PARP in B- and T-lymphoblastic cells. BMC Cancer. 2010;10:560. doi: 10.1186/1471-2407-10-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White PT, Cohen MS. The discovery and development of sorafenib for the treatment of thyroid cancer. Expert Opin Drug Discov. 2015;10:427–439. doi: 10.1517/17460441.2015.1006194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdulghani J, Allen JE, Dicker DT, Liu YY, Goldenberg D, Smith CD, Humphreys R, El-Deiry WS. Sorafenib sensitizes solid tumors to Apo2L/TRAIL and Apo2L/TRAIL receptor agonist antibodies by the Jak2-Stat3-Mcl1 axis. PLoS One. 2013;8:e75414. doi: 10.1371/journal.pone.0075414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gui CY, Ngo L, Xu WS, Richon VM, Marks PA. Histone deacetylase (HDAC) inhibitor activation of p21WAF1 involves changes in promoter-associated proteins, including HDAC1. Proc Natl Acad Sci U S A. 2004;101:1241–1246. doi: 10.1073/pnas.0307708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Almenara J, Rosato R, Grant S. Synergistic induction of mitochondrial damage and apoptosis in human leukemia cells by flavopiridol and the histone deacetylase inhibitor suberoylanilide hydroxamic acid (SAHA) Leukemia. 2002;16:1331–1343. doi: 10.1038/sj.leu.2402535. [DOI] [PubMed] [Google Scholar]
- 46.Dasmahapatra G, Yerram N, Dai Y, Dent P, Grant S. Synergistic interactions between vorinostat and sorafenib in chronic myelogenous leukemia cells involve Mcl-1 and p21CIP1 down-regulation. Clin Cancer Res. 2007;13:4280–4290. doi: 10.1158/1078-0432.CCR-07-0835. [DOI] [PubMed] [Google Scholar]
- 47.Inoue H, Hwang SH, Wecksler AT, Hammock BD, Weiss RH. Sorafenib attenuates p21 in kidney cancer cells and augments cell death in combination with DNA-damaging chemotherapy. Cancer Biol Ther. 2011;12:827–836. doi: 10.4161/cbt.12.9.17680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Fujita N, Tsuruo T. Caspase-mediated cleavage of p21Waf1/Cip1 converts cancer cells from growth arrest to undergoing apoptosis. Oncogene. 1999;18:1131–1138. doi: 10.1038/sj.onc.1202426. [DOI] [PubMed] [Google Scholar]
- 49.Cazzalini O, Scovassi AI, Savio M, Stivala LA, Prosperi E. Multiple roles of the cell cycle inhibitor p21(CDKN1A) in the DNA damage response. Mutat Res. 2010;704:12–20. doi: 10.1016/j.mrrev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 50.Cazzalini O, Dona F, Savio M, Tillhon M, Maccario C, Perucca P, Stivala LA, Scovassi AI, Prosperi E. p21CDKN1A participates in base excision repair by regulating the activity of poly(ADP-ribose) polymerase-1. DNA Repair (Amst) 2010;9:627–635. doi: 10.1016/j.dnarep.2010.02.011. [DOI] [PubMed] [Google Scholar]