Abstract
Behavioral research has revealed deficits in the development of joint attention (JA) as one of the earliest signs of autism. While the neural basis of JA has been studied predominantly in adults, we recently demonstrated a protracted development of the brain networks supporting JA in typically developing children and adolescents. The present eye-tracking/fMRI study now extends these findings to adolescents with autism. Our results show that in adolescents with autism JA is subserved by abnormal activation patterns in brain areas related to social cognition abnormalities which are at the core of ASD including the STS and TPJ, despite behavioral maturation with no behavioral differences. Furthermore, in the autism group we observed increased neural activity in a network of social and emotional processing areas during interactions with their mother. Moreover, data indicated that less severely affected individuals with autism showed higher frontal activation associated with self-initiated interactions. Taken together, this study provides first-time data of JA in children/adolescents with autism incorporating the interactive character of JA, its reciprocity and motivational aspects. The observed functional differences in adolescents ASD suggest that persistent developmental differences in the neural processes underlying JA contribute to social interaction difficulties in ASD.
Keywords: Social interaction, Eye-tracking, Familiarity, Functional magnetic resonance imaging, Temporoparietal junction, Superior temporal sulcus
Highlights
-
•
Gaze-contingent fMRI task to study joint attention in a developmental sample with autism
-
•
JA in the autism group elicited abnormal activation in social cognition related areas.
-
•
The interaction partner's familiarity modulated brain activity in the autism group.
-
•
In the autism group frontal activation is related to the severity of communication deficits.
1. Introduction
Social interactions form a substantial part of human daily living and typically develop from fundamental processes during infancy to highly sophisticated processes in adolescence and adulthood. Impaired social interactions constitute a core deficit of autism spectrum disorder (ASD; American Psychiatric Association, 2013) and deficits in the development of joint attention (JA) are one of the earliest signs to be at risk for ASD (Charman, 2003). JA, i.e., the shared attentional focus of two people on an object or third person, typically develops within the first two years of life. Other-initiated JA refers to the ability to follow the direction of gaze and gestures of others, whereas self-initiated JA refers to the ability to use gaze and gestures to direct the attention of others (e.g., Mundy et al., 2007, Mundy and Newell, 2007). Several lines of research suggest that other-initiated and self-initiated JA reflect distinct but interacting processes. Other-initiated and self-initiated JA follow dissociated developmental pathways during typical early childhood (Brooks and Meltzoff, 2005) as well as during atypical development (Naber et al., 2008) and may rely on potentially distinct neural networks (Mundy and Newell, 2007). In general, JA is considered as a prerequisite for sophisticated social processes, such as false-belief reasoning. In fact, JA is assumed to be an important developmental precursor to overall social and cognitive abilities facilitating human learning and development (Bruinsma et al., 2004, Charman, 2003, Dawson et al., 2002, Freeman et al., 2015, Mundy and Newell, 2007). Accordingly, early interventions focusing on JA also improve other social communicative skills (Dallaire and Schreibman, 2003, Schertz and Odom, 2007, Whalen et al., 2006).
Despite extensive behavioral research on JA (Bean and Eigsti, 2012, Charman, 2003, Dawson et al., 2004, Naber et al., 2008), data on the underlying neuronal mechanisms remain scarce, to date (Pfeiffer et al., 2014, Schilbach et al., 2010). Previous fMRI studies relied on subjects' mere observation of gaze cues to investigate JA (Materna et al., 2008, Williams et al., 2005). However, interactive paradigms, simulating social interaction by means of gaze-contingent responses of the interaction partner, are better suited to capture the interactive character and reciprocity of JA. They may thus be more appropriate to investigate the neural mechanisms underlying deficits in ASD (Schilbach et al., 2010, Schilbach et al., 2013). To this end, we recently adapted a combined eye-tracking/fMRI paradigm investigating (self- and other-initiated) JA in healthy adults (Redcay et al., 2010, Wilms et al., 2010) for the use in children and adolescents and could, thus, investigate the developmental trajectories of brain networks supporting JA in typically developing (TD) children and adolescents (Oberwelland et al., 2016).
To date, only one study has used an interactive task to investigate the neural correlates of JA in ASD (Redcay et al., 2013). Importantly, this study focused on adults. Authors reported aberrant activation in the posterior superior temporal sulcus (STS) and dorsal medial prefrontal cortex (dmPFC) during JA in adults with ASD compared to healthy controls, despite successful established JA, i.e., no behavioral differences between groups. Consistently, the STS has been identified as part of the JA network in TD children/adolescents (Oberwelland et al., 2016) and healthy adults (Redcay et al., 2010, Schilbach et al., 2010), supporting its crucial role in various aspects of social functioning (Bzdok et al., 2016, Zilbovicius et al., 2006). In contrast, in adults with ASD JA has not been found to elicit STS activity (Redcay et al., 2013). On the other hand, we could not observe dmPFC activation as part of the JA network in TD children/adolescents (Oberwelland et al., 2016), suggesting that dmPFC involvement in JA settings may be less relevant for JA in children and adolescents and subject to further development into adulthood.
The temporoparietal junction (TPJ) is located adjacent to the STS and is known to be involved in basic spatial attention functions (in particular reorienting of attention), but has also been implicated in social and emotional processing (Bilek et al., 2015, Frith and Frith, 2003, Krall et al., 2015, Schulte-Rüther et al., 2011, Schulte-Rüther et al., 2007) including JA (Oberwelland et al., 2016, Redcay et al., 2010, Schilbach et al., 2010). Several studies revealed abnormal activation patterns of the STS and TPJ in participants with ASD during various aspects of social and emotional processing (Chaminade et al., 2015, Frith, 2001, Pitskel et al., 2011, Schulte-Rüther et al., 2011, Wang et al., 2004, Williams et al., 2006), suggesting that both regions are critically involved in JA and also implicated in ASD.
In the context of truly interactive paradigms, it is essential to consider that social interactions depend on the relationship of the people interacting with each other (Oberwelland et al., 2016). In neurotypical individuals and individuals with ASD, behavior and neural responses differ as a function of how familiar the interaction partner is (Deaner et al., 2007, Doyle et al., 1980, Gobbini and Haxby, 2006, Hudry and Slaughter, 2009, Natu and O'Toole, 2011, Nomi and Uddin, 2015, Pierce et al., 2004, Pierce and Redcay, 2008, Shah et al., 2001, Sterling et al., 2008). Consistently, the JA network in TD children/adolescents is modulated by the familiarity of the interaction partner (Oberwelland et al., 2016). It remains to be investigated whether familiarity may similarly increase or “amplify” the motivational engagement in JA in children/adolescents with ASD.
To sum up, the present study was designed to investigate differences between young adolescents with and without ASD with respect to the (1) neural substrates of JA. We hypothesized abnormal activation patterns in areas previously implicated in ASD and JA in children and adolescents (Oberwelland et al., 2016), namely the STS and TPJ. Furthermore, we expected (2) a modulatory effect by the type of initiation (i.e. self- vs. other-initiation) and (3) familiarity of the interaction partner. To this end, we devised a task that used a virtual character set-up (Oberwelland et al., 2016, Schilbach et al., 2010, Wilms et al., 2010), including both a familiar (i.e., the participant's mother) and an unfamiliar interaction partner (i.e., identical female stranger for all participants).
2. Material and methods
2.1. Subjects
In total 16 male participants with ASD between 8 and 18 years of age and 16 TD participants were included in the analyses (see Table 1). TD participants were selected from a larger dataset (Oberwelland et al., 2016) to provide a close match in age and IQ in comparison the ASD group. All participants with ASD had been diagnosed by an independent clinician and reached cut-offs on the ADOS-G (Autism Diagnostic Observation Schedule) and ADI-R (Autism Diagnostic Interview). TD participants did not show any indication of developmental delay and had no history of any psychiatric disorder as assessed by a structured screening interview assessing various psychiatric disorders and symptoms before participation.
Table 1.
Demographic and questionnaire data. Mean values and standard deviations are given for IQ, SRS and FSK.
| TD participants | ASD participants | T | p | Cohen's d | |
|---|---|---|---|---|---|
| N | 16 | 16 | |||
| Male | 16 | 16 | |||
| Age | 13.2 (3.20) | 14.2 (3.52) | − 0.89 | 0.382 | |
| IQ | 118 (6.33) | 111a (17.40) | 1.55 | 0.133 | |
| SRS⁎⁎ | 19.94 (13.74) | 100.31 (23.46) | − 10.77 | < 0.001 | 3.81 |
| FSK⁎⁎ | 4.00 (3.61) | 20.69 (7.22) | − 11.70 | < 0.001 | 4.14 |
Significant difference at p < 0.01.
IQ score from 15 ASD participants.
All participants and a caregiver filled in various questionnaires in order to assess current psychiatric disorders (Child Behavior Checklist (CBCL); (Achenbach, 1991)), screening for autistic symptoms (SCQ; Berument et al., 1999; German version “Fragebogen zur sozialen Kommunikation”; FSK; Bölte and Poustka, 2006) and dimensional assessment of social functioning (Social Responsiveness Scale (SRS); Constantino et al., 2003). The FSK provides a sensitive screening for ASD (sensitivity of 92% against healthy controls). The German version of the SRS has high internal consistency between 0.91 and 0.97 (Bölte et al., 2008). The study was carried out at the Research Center Jülich, Germany, and was approved by the ethics committee of the University Hospital Aachen, Germany. All participants and/or their caregivers gave written informed consent/assent to participate in the study.
2.2. fMRI task
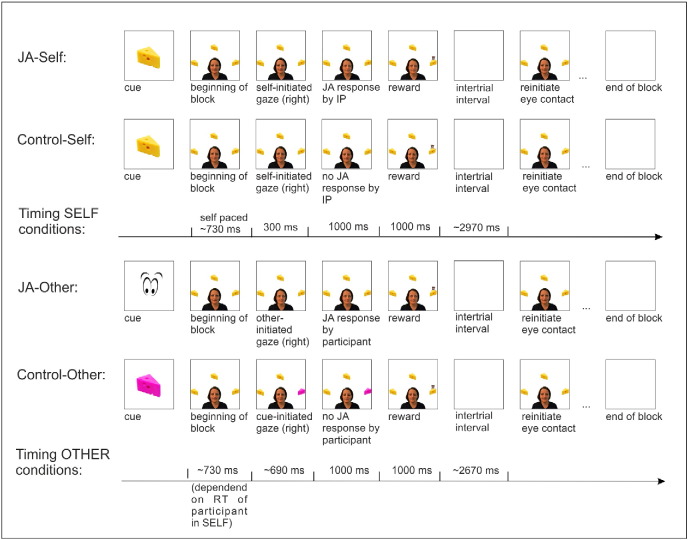
We used an interactive, gaze-contingent JA task which has been described in detail elsewhere (Oberwelland et al., 2016). In short, participants were asked to either initiate (i.e., self-initiation) or respond (i.e., other-initiation) to gaze interactions in four different conditions that either resulted in JA or non-JA (i.e., Control) situations: (1) JA-Self, (2) Control-Self, (3) JA-Other and (4) Control-Other.
All four conditions were completed with a virtually interacting avatar that depicted an unfamiliar (i.e., identical female stranger for all participants) or a familiar interaction partner (i.e., the participant's mother) resulting in a total of eight conditions. The avatar's behavior consisted of gaze shifts towards the targets, gaze shifted downwards, and gaze contact with the participant. The virtually avatar's reaction (i.e. gaze behavior) was contingent upon the participant's gaze behavior (depending on the experimental condition, see Fig. 1 for details). Additionally, a low-level baseline condition was included, using a black fixation cross on a white screen displayed for 18 s. In total, the participants completed two runs, each consisting of 27 blocks, presenting each condition three times (in total 144 interaction and 18 low level baseline blocks). Blocks were presented in a pseudo-randomized order ensuring that two identical blocks were not presented in a row.
Fig. 1.
Illustration and timing of all condition-specific gaze-based interaction sequences (IP = Interaction partner). Conditions were presented in blocks of 18 s, with 3 trials per block. After each picture cue the participants completed three corresponding interaction trials (~ 6 s). The intertrial interval varied depending on the participant's reaction time toll fill up ~ 6 s before the beginning of the next trial. Note that each time indicated with tilde indicates an average time across participants (Oberwelland et al., 2016).
We used an fMRI compatible eye-tracking system (Eyelink 1000 system (SR Research, Canada)), allowing for gaze-contingent experimental set-ups. Fixation within a predefined screen area (384 × 384 pixels) centered on the targets (i.e., a piece of cheese) for at least 150 ms was required for detection of target fixation. If no target fixation was detected within 2400 ms after trial onset the next trial was shown.
2.3. Behavioral data
Gaze data of participants were analyzed using MATLAB 8.1 and IBM SPSS Statistics 21. Onset, duration, and location of target fixation during all conditions were extracted to calculate (1) accuracy, (2) latency to JA, (3) direction of gaze shift towards the target (left, right or top), and (4) number of saccades after successful fixation of a target until the appearance of reward. General Linear Models (GLM; univariate and repeated-measures) were computed in order to test for interactions and main effects. Mixed ANOVAs with the within-group factors (JA/Control, Self/Other, Familiar/Unfamiliar) and between-group factor diagnosis (TD/ASD) were computed. Post-hoc t-tests were performed to determine differences between certain conditions.
2.4. Functional magnetic resonance imaging (fMRI)
The fMRI protocol and analysis is identical to the one described in detail in Oberwelland et al. (2016). In short, scans were acquired on a 3-Tesla Siemens Trio scanner (Erlangen, Germany) and analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Functional images were realigned to the mean image and co-registered with the participant's individual T1 weighted 3-D MP-RAGE image. Normalization parameters were determined and functional volumes were smoothed. Boxcar functions (aligned with the eye-tracking data) were convolved with a model of the hemodynamic response function (HRF) and its first-order temporal derivative. The unsquared and square roots of the movement parameters were included as additional regressors. All conditions were modeled separately in a block design (i.e., one block lasting 18 s) with error trials modeled as an additional nuisance regressor of no interest (i.e., trials without appearance of reward). The simple effect of each experimental condition was taken to the second-level and a random effects analysis was performed (mixed ANOVA model with within-participant factors ‘condition’ and one group factor). Initial analyses including age as a covariate revealed no influence of age (i.e., main effect and interaction) within this sample. In a second step, we examined the effect of type of initiation and familiarity separately within the ASD and TD group for specific contrasts in which activation did not exceed the threshold for statistical significance at the chosen threshold (i.e., at p < 0.05 cluster-level FWE corrected (p < 0.001 voxel-level). Moreover, we also investigated brain-behavior correlations in order to increase validity of our findings. The SPM8 anatomy toolbox (Eickhoff et al., 2007) was used to assign anatomical labels to the functional results of the second-level analysis, which were additionally compared to Brodmann and AAL templates in MRICroN.
3. Results
3.1. Demographic data and questionnaires
Table 1 summarizes the sample that was included in the analyses and their average scores on the questionnaire data. As expected, ASD participants scored significantly higher on the FSK and SRS.
3.2. Behavioral data
3.2.1. Accuracy
Successful trials were noted if the participants fixated target location and hence the reward (i.e., a mouse behind the cheese) appeared at the target location. Unsuccessful trials were primarily due to technical problems or inaccuracy of the eye-tracking set-up (Oberwelland et al., 2016). TD participants had on average 69% successful trials and participants with ASD 73%, which were included in fMRI condition regressors. A mixed ANOVA with the within-group factors (JA/Control, Self/Other, Familiar/Unfamiliar) and the between-group factor (TD/ASD) were computed to examine significant differences between the number of errors made for specific conditions. First, there was a main effect for JA (F(1,30) = 7.04, p < 0.01, ηp2 = 0.32), with more correct trials in Control (M = 73.74%; SD = 21.00) compared to JA (M = 69.44%; SD = 20.52) conditions across groups. Second, there was a significant Self × Familiar × Group (F(1,30) = 10.21, p < 0.01, ηp2 = 0.25) interaction. Post-hoc paired sample t-test revealed that when interacting with the familiar interaction partner, TD participants had significantly more correct trials (t(30) = 2.31, p < 0.05) in the other-initiated (M = 76.90%; SD = 19.60) compared to the self-initiated interactions (M = 71.53%; SD = 23.02), but no difference in accuracy between self- and other-initiation with the unfamiliar interaction partner (t(30) = 0.07, p > 0.05). ASD participants had significantly more correct trials (t(30) = 2.69, p < 0.05) during interactions with the familiar interaction partner when self-initiated (M = 75.00%; SD = 20.79) than when other-initiated (M = 65.10%; SD = 24.30), and also no difference between self- and other-initiation with the unfamiliar interaction partner (t(30) = 0.15, p > 0.05). Importantly, even though this three-way interaction was significant, differences in the average percentage of correct trials were small. Of note, all of these behavioral differences are unlikely to affect the results on the neural level, since the analyses of neural correlates only included correct trials. For a complete overview of the percentages of correct trials per condition per group please refer to Supplement Table 1.
3.2.2. Latency to JA and NoJA
Latency was defined as the reaction time of the participant either in response to a social (i.e., JA-Other) or external non-social cue (Control-Other) or the time to freely choose a target location in response to trial onset (JA-Self/Control-Self). A mixed ANOVA with the within-group factors (JA/Control, Self/Other, Familiar/Unfamiliar) and the between-group factor (TD/ASD) revealed a significant JA x Self interaction (F(1,30) = 25.84, p < 0.001, ηp2 = 0.44) and a main effect for JA (F(1,30) = 11.61, p < 0.01, ηp2 = 0.28). Post-hoc paired sample t-test showed that participants were significantly faster (t(30) = 5.69, p < 0.001) in the Control-Other condition (M = 0.65; SD = 0.14) compared to the JA-Other condition (M = 0.80; SD = 0.14), but showed comparable reaction times for JA-Self and Control-Self (t(30) = 0.75, p > 0.05). Importantly, there was no difference between TD and ASD (no main effect of group nor any interaction with group). The difference in latency for the other-initiated conditions is most likely due to the higher saliency of the external non-social cue in the Control-Other conditions compared to the more subtle gaze cue in the JA-Other condition (Oberwelland et al., 2016).
3.2.3. Direction of JA
To ensure that participants actually made choices during self-initiated conditions and selected targets at different locations, we compared the mean number of target choices for all locations. TD and ASD participants distributed their choices among all target locations (TD: top target: M = 45.77%; left target: M = 24.00%; and right target: M = 30.50%; ASD: top target: M = 48.65%; left target: M = 25.44%; and right target: M = 26.30%). In accordance with previous results (Oberwelland et al., 2016), participants made more gaze shifts towards the top target. A mixed ANOVA with within-group factors (JA/Control and Familiar/Unfamiliar) and between-group factor (TD/ASD) revealed no significant interaction or main effect supporting the conclusion that the preference for the top-target is due to a tendency for our technical set-up to recognize fixations at the top target most reliably (Oberwelland et al., 2016).
3.2.4. Saccades: number of saccades between target fixation and reward appearance
Since participants were uncertain whether the interaction partner followed their gaze or not during self-initiated conditions, they could potentially shift their gaze back and forth between target and interaction partner. Mixed ANOVA of the number of saccades for the within-group factors (JA/Control and Familiar/Unfamiliar) and between-group factor (TD/ASD) revealed no significant interaction or main effect. On average participants shifted 2.01 times back and forth between target and interaction partner indicating that the reaction of the interaction partner was important to the participant.
3.3. Neural correlates
3.3.1. Between-group differences for joint attention (JA > Control)
We hypothesized that young adolescents with ASD elicit differential activation patterns during JA compared to Control conditions in areas previously implicated in ASD and JA in children and adolescents (Oberwelland et al., 2016), namely the STS and TPJ.
3.3.1.1. TD versus ASD participants
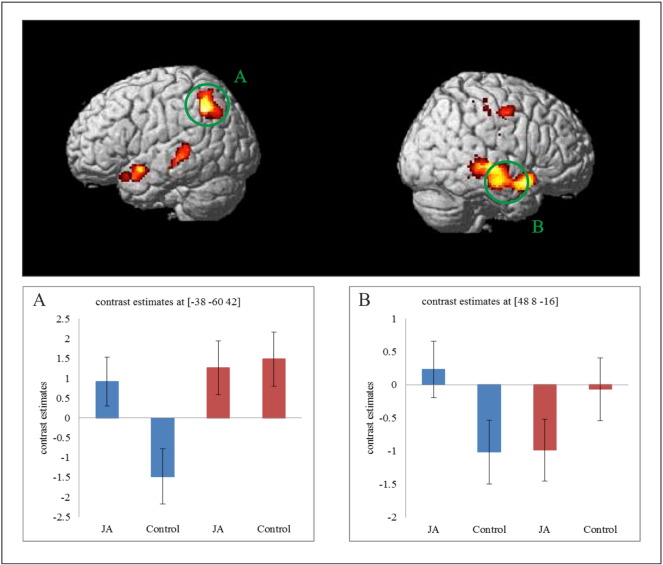
Direct comparison of engagement in JA (irrespective of type of initiation and familiarity of the interaction partner [(JA-Self + JA-Other) > (Control-Self + Control-Other)]) revealed a significant difference between TD and ASD participants in two clusters of brain regions (Fig. 2 and Table 2). One cluster mainly comprised the right temporal lobe including the superior temporal pole, middle temporal gyrus (MTG) and STS. A second cluster was located at the left parietal lobe including the TPJ, precuneus and inferior parietal lobe. Examination of the contrast estimates at the peak voxels of the two clusters revealed a significant group x condition interaction (F(1,30) = 8.43, p < 0.01) that in the right temporal pole TD participants showed greater activation during JA than Control, while ASD participants had no significant difference in activation during JA and Control (see Fig. 2B). In the left precuneus we also observed a significant group x condition interaction (F(1,30) = 14.71, p < 0.01), TD participants showed a differential activation for JA and Control whereas ASD participants showed comparable activation (see Fig. 2A). The inverse contrast, ASD > TD, did not reveal any significant difference in activation between groups. Furthermore, direct group comparisons related to specific effects for familiarity and type of initiation did not exceed the threshold for statistical significance. Therefore, in the next step, modulation of neural activation patterns by type of initiation and familiarity were examined separately within the ASD and TD group and brain-behavior correlations were investigated within the ASD group.
Fig. 2.
Neural correlates of the main effect for JA comparing TD > ASD participants and contrast estimates at the location of the corresponding peak voxel; blue: TD participants, red: ASD participants. All activations depict statistically significant neural activity at cluster-level p < 0.05 FWE corrected, voxel-level p < 0.01. Panels A and B are bar graphs depicting contrast estimates at the two corresponding peak voxel (JA: JAself + JAother; Control: Controlself + Controlother, for each group separately).
Table 2.
Neural correlates comparing TD > ASD participants for the main effect of JA (at p < 0.05 cluster-level FWE corrected (p < 0.001 voxel-level); MNI coordinate of principally activated voxels for each cluster are given).
| Brain region | x | y | z | k | T |
|---|---|---|---|---|---|
| Group differences (TD > ASD) for joint attention (JA > Control) | |||||
| Right temporal pole | 48 | 8 | − 16 | 2062 | 4.60 |
| Right middle temporal gyrus (MTG) | 56 | − 18 | − 8 | 4.25 | |
| Right superior temporal sulcus (STS) | 48 | − 18 | 8 | 4.22 | |
| Left angular gyrus | − 38 | -60 | 42 | 1537 | 3.87 |
| Left precuneus | − 14 | − 50 | 40 | 3.70 | |
| Left TPJ | − 58 | − 56 | 40 | 3.69 | |
3.3.2. Neural correlates of within – group differences for TD and ASD participants
3.3.2.1. Common activations for self-sustained interactions (SELF > OTHER)
With respect to the within-group differences for TD participants we expected a broad bilateral network including frontal and parietal brain areas (Oberwelland et al., 2016). With respect to ASD participants we hypothesized abnormalities in self-other distinction and difficulties in initiating social interaction, probably associated with less distinct activation in brain areas underlying self- and other initiated interactions (Kennedy and Courchesne, 2008, Lombardo et al., 2010, Schulte-Rüther et al., 2013, Vogeley et al., 2004, Vogeley and Fink, 2003).
3.3.2.1.1. TD participants
Initiating the interaction regardless of JA and familiarity, that is the main effect of SELF [(JA-Self + Control-Self) > (JA-Other + Control-Other)], resulted in the recruitment of the superior medial frontal gyrus (FG) [(0, 30, 40), t = 5.58], bilateral insula [L: (− 36, 20, − 2), t = 4.95; R: (36, 22, − 6), t = 4.56], right middle frontal gyrus (MFG) [(46, 22, 40), t = 4.80] and a cluster in visual areas centered upon the left cuneus [(− 6, − 90, 18), t = 4.71].
3.3.2.1.2. ASD participants
Within the ASD group, no significant activation at the chosen threshold was observed for the main effect of SELF. Therefore, we examined whether neural activity within the SELF-network as revealed in TD participants, was related to the severity of deficits in social communication in ASD as assessed by the respective subscale of the SRS questionnaire. This subscale captures best those skills which are necessary for self-sustained interaction, as evident during SELF conditions. The scale entails items related to initiating interactions or actively reacting to social stimuli (e.g. communicating feelings, maintaining gaze contact, entertaining relationships with peers and following the flow of a normal conversation). We used the individual SRS-Social Communication scores as a regressor and the individual contrast estimates related to SELF conditions in a whole-brain regression analysis within ASD participants, applying the SELF network of TD participants as a functional ROI. This analysis revealed the left superior medial FG [(− 4, 42, 38), t = 4.30] and MFG [(− 50, 28, 32), t = 4.72] to be correlated with the SRS-Social Communication scores, implying that less severely affected participants showed more frontal activation during self-initiated interactions.
3.3.2.2. Common activations for other-sustained interactions (OTHER > SELF)
For the main effect of OTHER [(JA-Other + Control-Other) > (JA-Self + Control-Self)], we hypothesized to obtain similar findings within both groups, mainly activation in visual processing areas.
3.3.2.2.1. TD participants
Responding to the gaze shift by the interaction partner or to the external cue regardless of familiarity, that is the main effect of OTHER, elicited significant activation in the bilateral occipital gyrus [L: (− 28, − 96, − 8), t = 5.60; R: (30, − 92, 2), t = 7.82].
3.3.2.2.2. ASD participants
Similarly, ASD participants elicited significant activation in the bilateral occipital gyrus [L: (− 24, − 94, − 2), t = 5.60; R: (26, − 94, − 2), t = 6.17].
3.3.2.3. Common activations for interactions with a familiar interaction partner (Familiar > Unfamiliar)
With respect to the within-group differences for TD participants, we hypothesized that interactions with a familiar interaction partner recruit additional areas related to social and emotional processing. With respect to the within-group differences for ASD participants, we hypothesized to observe similar findings as within the TD group, yet a more pronounced differentiation in brain activation between familiar and unfamiliar interactions, which has already been reported for familiarity and face processing (Pierce et al., 2004, Pierce and Redcay, 2008, Sterling et al., 2008).
3.3.2.3.1. TD participants
Interacting with a familiar partner, regardless of JA and Self/Other, that is the main effect for FAMILIAR [(JA_Familiar + Control_Familiar > (JA_Unfamiliar + Control_Unfamiliar)], did not result in any significant activation at the chosen threshold. The inverse contrast, that is the main effect for UNFAMILIAR [(JA_Unfamiliar + Control_Unfamiliar > (JA_Familiar + Control_Familiar)], revealed no significant activation at the chosen threshold.
3.3.2.3.2. ASD participants
In contrast, ASD participants showed widespread activations in response to familiarity. Brain regions activated included the striatum [(− 12, − 6, − 8), t = 4.62], left insula [(− 28, 12, − 18), t = 3.92], temporal pole [(− 36, 24, − 22), t = 3.87], right IFG [(50, 32, 12), t = 3.98], bilateral superior medial FG [L: (− 8, 60, 24), t = 4.11; R: (8, 60, 22), t = 4.56], left MTG [(− 56, − 6, − 18), t = 4.47], bilateral fusiform gyrus [L: (− 42, − 56, − 12), t = 4.58; R: (42, − 60, − 16), t = 4.46], left precuneus [(0, − 60, 34), t = 4.56], right inferior occipital gyrus [(38, − 82, − 14), t = 4.46] and right precentral gyrus [(50, 8, 32), t = 4.27]. The inverse contrast, that is the main effect for UNFAMILIAR, did not reveal significant activation at the chosen threshold.
3.3.2.4. Common activations for statistical interaction JA × Self × Familiar
Within the TD group, we hypothesized a modulatory effect of familiarity specifically for self-initiated JA, as already described for our bigger TD developmental sample (Oberwelland et al., 2016). Within the ASD group, we explored whether a modulatory effect of familiarity could be observed at all, or would entail additional brain regions.
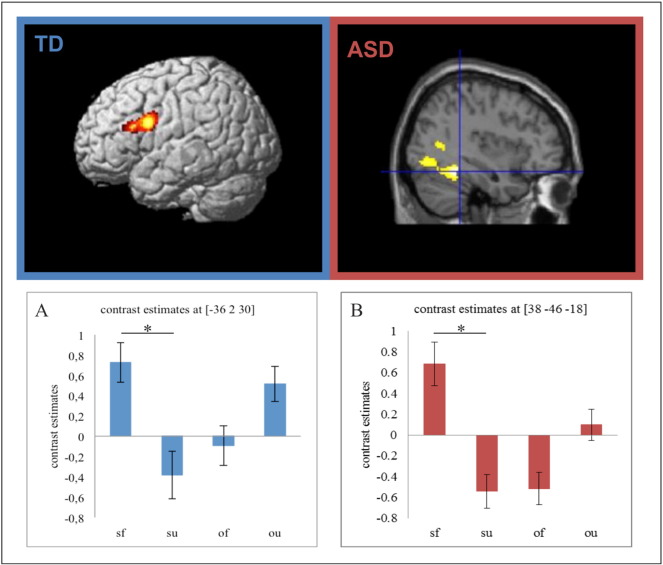
3.3.2.4.1. TD participants
A significant three-way interaction was marginally evident for JA × Self × Familiar [(JA-Self > Control Self) > (JA-Other > Control-Other)]familiar > [(JA-Self > Control-Self) > (JA-Other > Control-Other)]unfamiliar in a cluster centered upon the left inferior FG [(− 36, 2, 30), t = 4.05, p = 0.085] (see Fig. 3A). Post-hoc t-tests on beta estimates extracted from the corresponding regions revealed that the interaction was mostly driven by a familiarity effect for JA-Self (familiar > unfamiliar; respectively, M = 0.65, SD = 0.91 and M = − 0.38, SD = 0.61; p < 0.01) (see Fig. 3B).
Fig. 3.
Neural correlates for the interaction JA × Familiarity × Self within the TD and ASD group separately. Contrast estimates at the location of the corresponding peak voxel are depicted; sf = JA-Self-Familiar, su = JA-Self-Unfamiliar, of = JA-Other-Familiar, ou = JA-Other-Unfamiliar. All activations depict statistically significant neural activity at cluster-level p < 0.05 FWE corrected, voxel-level p < 0.01. *significant at p < 0.01.
3.3.2.4.2. ASD participants
A significant three-way interaction was evident for JA × Self × Familiar [(JA-Self > Control-Self) > (JA-Other > Control-Other)]familiar > [(JA-Self > Control-Self) > (JA-Other > Control-Other)]unfamiliar in the right fusiform gyrus [(38, − 46, − 18), t = 4.52] (see Fig. 3A). Post-hoc t-tests on beta estimates extracted from the corresponding region revealed that the interaction was mostly driven by a familiarity effect for JA-Self (familiar > unfamiliar; respectively, M = 0.68, SD = 0.61 and M = − 0.52, SD = 0.66; p < 0.001) (see Fig. 3B). All further interaction contrasts did not yield significant results.
4. Discussion
This is the first study to investigate the JA network in young adolescents with ASD by using an interactive, gaze-contingent eye-tracking paradigm during fMRI. We additionally examined the effects of self- versus other-initiation and the effect of a familiar versus non-familiar interaction partner. We observed abnormal activation during JA in brain regions associated with social cognition (Krall et al., 2015, Olson et al., 2007) and disturbed social and emotional functioning in ASD (Allison et al., 2000, Chaminade et al., 2015, Davies et al., 2011, Frith, 2001, Georgescu et al., 2013, Kuzmanovic et al., 2013, Lombardo et al., 2010, Pitskel et al., 2011, Redcay et al., 2013, Schulte-Rüther et al., 2011, Wang et al., 2004, Watanabe et al., 2012, Zilbovicius et al., 2006).
4.1. The general JA network
Consistent with previous research on social cognition and JA in adults (Redcay et al., 2013), we observed that JA in young adolescents with ASD resulted in deviant activation patterns in a network of areas (see Fig. 2) associated with the so-called “social brain”, including the key nodes temporal pole, STS, TPJ, and precuneus (Schilbach, 2016), despite successfully established JA on the behavioral level. Previously, we could demonstrate that in TD children and adolescents these brain regions play an important role for the experience of JA (Oberwelland et al., 2016). This result suggests subtle, but persistent JA difficulties in children and adolescents with ASD, despite basic functionality, which might be related to difficulties in more sophisticated social interactions, typically accompanying individuals with ASD lifelong.
The temporal pole and STS both play a key role in social and emotional processing and functional abnormalities have consistently been reported for various aspects of social processing in ASD (Allison et al., 2000, Carrington and Bailey, 2009, Gallagher and Frith, 2003, Greimel et al., 2010, Kennedy and Courchesne, 2008, Olson et al., 2007, Schulte-Rüther et al., 2011, Völlm et al., 2006, Zilbovicius et al., 2006). With respect to the STS, these aspects of social processing range from basic perceptual (such as recognizing a specific individual and perceiving a voice) to more complex aspects (such as analyzing perceptual cues with respect to their meaningful contribution to social communication). These processes are crucial for JA because eye movements need to be analyzed with respect to a given social context in order to extract meaningful cues for social communication (Zilbovicius et al., 2006). Note, that we report here a rather anterior region of the STS. This is in line with various studies suggesting that in addition to posterior STS, anterior parts of the STS are also implicated in mentalizing (Schnell, 2014, Schnell et al., 2011, Schulte-Rüther et al., 2012). Olson et al. (2007) suggested that the temporal pole ties complex, highly processed perceptual inputs to instinctive emotional responses. Reduced STS and temporal pole activation in ASD, as revealed in the current study thus, could be taken to suggest less elaborate processing during basic JA within the social cognition network and might underlie the lifelong observed difficulties of ASD individuals in more complex social interactions.
In addition, adolescents with ASD showed abnormal activation in the TPJ and precuneus. These regions have previously been implicated in social information processing (Bilek et al., 2015, Cavanna and Trimble, 2006, Decety and Lamm, 2007, Samson et al., 2004, Saxe, 2006, Schulte-Rüther et al., 2011, Schulte-Rüther et al., 2007), and self-other differentiation (Pfeifer et al., 2007, Platek et al., 2008, Saxe et al., 2006, Vogeley et al., 2004, Vogeley and Fink, 2003). The ability to differentiate mental states as originating from oneself or other people is at the heart of complex social reasoning such as in Theory of Mind (ToM) and false belief processing. Deficits in ToM and false belief processing in children with ASD have been demonstrated and persist during adolescence (Schulte-Rüther et al., 2011, Schulte-Rüther et al., 2013, Senju, 2011). Even though explicit reasoning related to mental states (such as in a classical ToM task) was not involved, our JA task encompassed implicit inferences of the mental state of the interaction partner. In young adolescents with ASD, a ToM deficit appears to be reflected by engagement of the TPJ/precuneus for all conditions involving potential gaze interaction (i.e., JA-Self, Control-Self, JA-Other), thus pointing towards reduced neural specialization in patients, whereas in TD participants activation in the left inferior frontal gyrus is specifically apparent for successfully established JA, but not for control situations (see Fig. 2A). Alternatively, this lack of condition-specific differences may reflect a higher demand on TPJ/precuneus circuitry for basic JA processes in young adolescence with ASD, as opposed to higher demands for evaluating self-initiated interactions in young TD adolescence. A similar lack of neural developmental specialization within the STS has been suggested previously in adults with ASD (Redcay et al., 2013). Both findings might thus suggest a more general lack of neural developmental specialization within “social brain” areas in individuals with ASD. Interestingly, we previously observed a similar lack of specialization in TPJ recruitment during early development in TD children compared to TD adolescents (Oberwelland et al., 2016). The observed lack of neural developmental specialization in TPJ may thus hint at a protracted development of neural specialization in ASD. However, to disentangle this possibility from a fundamental deficiency in TPJ circuitry in JA, further studies are warranted, which need to examine the developmental trajectories during early and late adolescence in ASD in more detail by directly comparing both age groups in a bigger sample with children and adolescents with ASD.
4.2. JA and its modulation by type of initiation
We also observed that parts of the network underlying self-initiated interactions correlated negatively with symptom severity in social communication in ASD. This finding might suggest that young adolescents with ASD who are more severely affected and hence have greater deficits in social communication seem to show reduced activation in frontal brain regions (i.e., MPFC) that are typically recruited during self-initiated interactions and mentalizing. This can be discussed in terms of abnormal self-other distinction (Kennedy and Courchesne, 2008, Lombardo et al., 2010, Schulte-Rüther et al., 2013, Vogeley et al., 2004, Vogeley and Fink, 2003) or impairments in the disengagement of visual attention (see for review: Keehn et al., 2013) in individuals with ASD. First, note that the ability to differentiate oneself from others enables us to take others' perspective, which in turn is the foundation for complex social cognition such as ToM and empathy. Accordingly, individuals with ASD who are less affected had stronger activations related to self-initiated interactions as opposed to other-initiated interactions, suggesting a better self-other distinction represented in their brain. This also concords early behavioral findings, which indicate that specifically self-initiated JA is related to social-communication skills (Pickard and Ingersoll, 2014).
Alternatively, impairment in the disengagement of visual attention has been reported largely in individuals with ASD (Keehn et al., 2013) and has been identified as one the earliest marker in infants at high for ASD (Elsabbagh et al., 2013, Ronconi et al., 2014). In fact, a disengagement deficit may particularly affect self-initiated conditions when participants are required to voluntarily disengage their attention from the face and reorient towards one of the targets.
4.3. JA and its modulation by familiarity
Within the TD and ASD group separately, we found distinct modulatory effects of familiarity. In TD participants, activation was particularly enhanced during self-initiated JA with a familiar interaction partner in the left inferior frontal gyrus (see Fig. 3A), demonstrating modulation of brain regions related to attention control systems and replicating our previous findings in TD participants (Oberwelland et al., 2016).
In ASD participants, conditions with a familiar interaction partner elicited enhanced activation in a broad network including in the left insula, temporal pole, fusiform gyrus, precuneus, MTG, and IFG as well as the right fusiform gyrus, MFG, precentral gyrus and IFG. This is consistent with previous studies suggesting differential brain activity for familiar and unfamiliar faces in individuals with ASD (Pierce et al., 2004, Pierce and Redcay, 2008, Sterling et al., 2008). We here indicate for the first time that a modulatory effect of familiarity in ASD is particularly valid for interactive situations. In contrast, in TD participants, we did not observe such a far-reaching effect of familiarity on brain areas. However, while we can firmly conclude that familiarity influences neural signatures of social interaction in ASD, we can only speculate whether this effect is even stronger than for TD individuals, since the direct interaction of group and familiarity did not exceed the threshold for statistical significance. However, this interesting finding resulting from the analyses within the ASD group may impact upon the development of interventions/trainings for individuals with ASD. It might be particularly beneficial to incorporate familiar others in the training so that skills to be learned can be communicated most efficiently and transferred to daily situations outside the intervention environment.
The use of the maternal face as the familiar interaction partner certainly not only includes familiarity of an observed face, but also the complex mother-child relationship, which might have far-reaching effects on social cognition in ASD. For example, specific cues related to ones own mother (e.g. maternal odors) may influence social abilities such as automatic imitation in ASD (Parma et al., 2013). (Casartelli et al., 2016). Further research is needed to disentangle familiarity and mother-child relationship and their specific influence on JA.
Furthermore, we observed an interaction of type of initiation and familiarity on JA both for TD and ASD participants, but within different brain regions (see Fig. 3). Within the ASD group, an effect of familiarity was particularly evident for self-initiated JA in the right fusiform gyrus (see Fig. 3B), showing enhanced activation in these situations when interacting with the mother. The right fusiform gyrus plays an important role in face processing (Kanwisher et al., 1997), with reduced activation in response to faces in individuals with ASD compared to healthy individuals. The fusiform gyrus has thus been suggested to underlie social interaction deficits in ASD (Grelotti et al., 2002, Pierce et al., 2004, Pierce and Redcay, 2008, Schultz, 2005). More recently the fusiform gyrus is also conceived as a more general “expert visual perception” area (Gauthier et al., 2000, Gauthier et al., 1999), showing enhanced activation for any object individuals are experts in. In a single case study, enhanced activation of the fusiform gyrus has been associated with expertise in non-social stimuli such as Cartoon characters (Grelotti et al., 2005). Our results now demonstrate for the first time stronger activation of the fusiform gyrus in response to a familiar face (here the own mother) in ASD, particularly in the context of self-initiated interaction. This pattern of results supports the idea that reduced activation in the fusiform gyrus in ASD may typically reflect reduced expertise in face processing as a consequence of reduced interest in social interaction (Chevallier et al., 2012, Grelotti et al., 2002). At the same time it suggests a greater level of facial processing for the interaction with familiar interaction partners, in particular during self-initiated interaction, again stressing the link between motivational aspects and perceptual processing of facial stimuli.
Interestingly, interactions between familiarity and type of initiation on the neural level were paralleled by similar modulations of the behavioral data. While ASD participants had significantly more correct trials during self-initiated interactions with their mother as opposed to other-initiated interactions, TD participants showed a reversed pattern. In contrast, TD and ASD groups revealed no significant difference in their interactions with the unfamiliar partner. Eye movements typically become more predictable with increased exposure and familiarity (Althoff and Cohen, 1999, Barton et al., 2006) and our data demonstrate that familiarity of a face also influences eye movements and/or patterns of spatial attention during JA. In particular, our data suggest that ASD have a particular advantage for initiating interactions with a familiar interaction partner, whereas TD participants have an advantage for following the gaze of a familiar interaction partner.
5. Conclusion
In summary, the present study compared for the first time brain networks associated with JA in young TD and ASD adolescents using an interactive eye-tracking and fMRI paradigm to study JA during real-time social interaction. We observed profound differences in the JA network in TD and ASD adolescents in a relative late stage of development (young adolescents). Furthermore, our data suggest a modulation of the JA network by type of initiation and ‘familiarity’ of the interaction partner within the ASD. Our results significantly extend previous findings of the behavioral and neural correlates of social interaction and communication in ASD and further advance our understanding of social cognition impairments during development. Our findings underline that even very basic forms of gaze-based social interaction (without noticeable differences in behavioral performance) are associated with profound differences in the underlying neural bases. This might have far-reaching implications for more sophisticated social interactions suggesting continued effects of JA disturbance in infants and young children with ASD into adolescence and adulthood.
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG, IRTG 1328). MSR and KV are supported by the German Federal Ministry for Research and Education (BMBF, 16SV7242). MSR was supported by a Start-Up grant from the RWTH Aachen. LS was supported by the Max-Planck-Society. We would further like to thank all the families who took part in this study and made an indispensable contribution. The authors have no conflict of interest to declare.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.01.006.
Appendix A. Supplementary data
Supplementary material
References
- Achenbach T.M. Department of Psychiatry, University of Vermont; Burlington, VT: 1991. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. [Google Scholar]
- Allison T., Puce A., McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn. Sci. 2000;4(7):267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Althoff R.R., Cohen N.J. Eye-movement-based memory effect: A reprocessing effect in face perception. J. Exp. Psychol. Learn. Mem. Cogn. 1999;25(4):997–1010. doi: 10.1037//0278-7393.25.4.997. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 5th ed. Author; Washington, DC: 2013. Diagnostic and Statisti- cal Manual of Mental Disorders. [Google Scholar]
- Barton J.J.S., Radcliffe N., Cherkasova M.V., Edelman J., Intriligator J.M. Information processing during face recognition: the effects of familiarity, inversion, and morphing on scanning fixations. Perception. 2006;35(8):1089–1105. doi: 10.1068/p5547. [DOI] [PubMed] [Google Scholar]
- Bean J.L., Eigsti I. Assessment of joint attention in school-age children and adolescents. Res. Autism Spectr. Disord. 2012;6:1304–1310. [Google Scholar]
- Berument S., Rutter M., Lord C., Pickles A., Bailey A. Autistic screening questionnaire: diagnostic validity. Br. J. Psychiatry. 1999;175:441–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Bilek E., Ruf M., Schäfer A., Akdeniz C., Calhoun V.D., Schmahl C. Information flow between interacting human brains: identification, validation, and relationship to social expertise. Proc. Natl. Acad. Sci. 2015;112(16):5207–5212. doi: 10.1073/pnas.1421831112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölte S., Poustka F. Huber; Bern: 2006. Fragebogen zur sozialen kommunikation (FSK) [Google Scholar]
- Bölte S., Poustka F., Constantino J.N. Assessing autistic traits: cross-cultural validation of the social responsiveness scale (SRS) Autism Res. 2008;1:354–363. doi: 10.1002/aur.49. [DOI] [PubMed] [Google Scholar]
- Brooks R., Meltzoff A.N. The development of gaze following and its relation to language. Dev. Sci. 2005;8(6):535–543. doi: 10.1111/j.1467-7687.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruinsma Y., Koegel R.L., Koegel L.K. Joint attention and children with autism: a review of the literature. Ment. Retard. Dev. Disabil. Res. Rev. 2004;10(3):169–175. doi: 10.1002/mrdd.20036. [DOI] [PubMed] [Google Scholar]
- Bzdok D., Hartwigsen G., Reid A., Laird A.R., Fox P.T., Eickhoff S.B. Left inferior parietal lobe engagement in social cognition and language. Neurosci. Biobehav. Rev. 2016;68:319–334. doi: 10.1016/j.neubiorev.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington S.J., Bailey A.J. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum. Brain Mapp. 2009;30(8):2313–2335. doi: 10.1002/hbm.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casartelli L., Molteni M., Ronconi L. So close yet so far: motor anomalies impacting on social functioning in autism spectrum disorder. Neurosci. Biobehav. Rev. 2016;63:98–105. doi: 10.1016/j.neubiorev.2016.02.001. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chaminade T., Da Fonesca D., Rosset D., Cheng G., Deruelle C. Atypical modulation of hypothalamic activity by social context in ASD. Res. Autism Spectr. Disord. 2015;10:41–50. [Google Scholar]
- Charman T. Why is joint attention a pivotal skill in autism? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2003;358:315–324. doi: 10.1098/rstb.2002.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C., Kohls G., Troiani V., Brodkin E.S., Schultz R.T. The social motivation theory of autism. Trends Cogn. Sci. 2012;16(4):231–238. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino J.N., Davis S.A., Todd R.D., Schindler M.K., Gross M.M., Brophy S.L., Reich W. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J. Autism Dev. Disord. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Dallaire C.W., Schreibman L. Joint attention training for children with autism using behavior modification procedures. J. Child Psychol. Psychiatry. 2003;44(3):456–468. doi: 10.1111/1469-7610.00135. [DOI] [PubMed] [Google Scholar]
- Davies M.S., Dapretto M., Sigman M., Sepeta L., Bookheimer S.Y. Neural bases of gaze and emotion processing in children with autism spectrum disorders. Brain Behav. 2011;1(1):1–11. doi: 10.1002/brb3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G., Munson J., Estes A., Osterling J., McPartland J., Toth K., Abbott R. Neurocognitive function and joint attention ability in young children with autism spectrum disorder versus developmental delay. Child Dev. 2002;73(2):345–358. doi: 10.1111/1467-8624.00411. http://www.ncbi.nlm.nih.gov/pubmed/11949896 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Dawson G., Toth K., Abbott R., Osterling J., Munson J., Estes A., Liaw J. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev. Psychol. 2004;40(2):271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Deaner R.O., Shepherd S.V., Platt M.L. Familiarity accentuates gaze cuing in women but not men. Biol. Lett. 2007;3(1):65–68. doi: 10.1098/rsbl.2006.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J., Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13(6):580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Doyle A.-B., Connolly J., Riovest L.-P. The effect of playmate familiarity on the social interactions of young children. Child Dev. 1980;51(1):217–223. [Google Scholar]
- Eickhoff S.B., Paus T., Caspers S., Grosbras M.H., Evans A., Zilles K., Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage. 2007;36(3):511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M., Fernandes J., Jane Webb S., Dawson G., Charman T., Johnson M.H. Disengagement of visual attention in infancy is associated with emerging autism in toddlerhood. Biol. Psychiatry. 2013;74(3):189–194. doi: 10.1016/j.biopsych.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S.F.N., Gulsrud A., Kasari C. Brief report: linking early joint attention and play abilities to later reports of friendships for children with ASD. J. Autism Dev. Disord. 2015:2259–2266. doi: 10.1007/s10803-015-2369-x. [DOI] [PubMed] [Google Scholar]
- Frith U. Mind blindness and the brain in autism. Neuron. 2001;32(6):969–979. doi: 10.1016/s0896-6273(01)00552-9. [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C.D. Development and neurophysiology of mentalizing. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2003;358(1431):459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher H.L., Frith C.D. Functional imaging of “theory of mind.”. Trends Cogn. Sci. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gauthier I., Tarr M.J., Anderson A.W., Skudlarski P., Gore J.C. Activation of the middle fusiform “face area” increases with expertise in recognizing novel objects. Nat. Neurosci. 1999;2(6):568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Gauthier I., Skudlarski P., Gore J.C., Anderson A.W. Expertise for cars and birds recruits brain areas involved in face recognition. Nat. Neurosci. 2000;3(2):191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- Georgescu A.L., Kuzmanovic B., Schilbach L., Tepest R., Kulbida R., Bente G., Vogeley K. Neural correlates of “social gaze” processing in high-functioning autism under systematic variation of gaze duration. NeuroImage Clin. 2013;3:340–351. doi: 10.1016/j.nicl.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbini M.I., Haxby J.V. Neural response to the visual familiarity of faces. Brain Res. Bull. 2006;71(1–3):76–82. doi: 10.1016/j.brainresbull.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Greimel E., Schulte-Rüther M., Kircher T., Kamp-Becker I., Remschmidt H., Fink G.R., Konrad K. Neural mechanisms of empathy in adolescents with autism spectrum disorder and their fathers. NeuroImage. 2010;49(1):1055–1065. doi: 10.1016/j.neuroimage.2009.07.057. [DOI] [PubMed] [Google Scholar]
- Grelotti D.J., Gauthier I., Schultz R.T. Social interest and the development of cortical face specialization: what autism teaches us about face processing. Dev. Psychobiol. 2002;40(3):213–225. doi: 10.1002/dev.10028. [DOI] [PubMed] [Google Scholar]
- Grelotti D.J., Klin A.J., Gauthier I., Skudlarski P., Cohen D.J., Gore J.C., Schultz R.T. fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia. 2005;43(3):373–385. doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Hudry K., Slaughter V. Agent familiarity and emotional context influence the everyday empathic responding of young children with autism. Res. Autism Spectr. Disord. 2009;3(1):74–85. [Google Scholar]
- Kanwisher N., McDermott J., Chun M.M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn B., Müller R.A., Townsend J. Atypical attentional networks and the emergence of autism. Neurosci. Biobehav. Rev. 2013;37(2):164–183. doi: 10.1016/j.neubiorev.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.P., Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Soc. Cogn. Affect. Neurosci. 2008;3(2):177–190. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall S.C.C., Rottschy C., Oberwelland E., Bzdok D., Fox P.T.T., Eickhoff S.B.B., Konrad K. The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta-analysis. Brain Struct. Funct. 2015;220(2):587–604. doi: 10.1007/s00429-014-0803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmanovic B., Bente G., von Cramon D.Y., Schilbach L., Tittgemeyer M., Vogeley K. Imaging first impressions: distinct neural processing of verbal and nonverbal social information. NeuroImage. 2013;60(1):179–188. doi: 10.1016/j.neuroimage.2011.12.046. [DOI] [PubMed] [Google Scholar]
- Lombardo M.V., Chakrabarti B., Bullmore E.T., Sadek S.A., Pasco G., Wheelwright S.J., Baron-Cohen S. Atypical neural self-representation in autism. Brain. 2010;133(Pt 2):611–624. doi: 10.1093/brain/awp306. [DOI] [PubMed] [Google Scholar]
- Materna S., Dicke P.W., Thier P. Dissociable roles of the superior temporal sulcus and the intraparietal sulcus in joint attention: a functional magnetic resonance imaging study. J. Cogn. Neurosci. 2008;20(1):108–119. doi: 10.1162/jocn.2008.20.1.108. [DOI] [PubMed] [Google Scholar]
- Mundy P., Newell L. Attention, joint attention, and social cognition. Curr. Dir. Psychol. Sci. 2007;16:269–274. doi: 10.1111/j.1467-8721.2007.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P., Block J., Delgado C., Pomares Y., Van Hecke A.V., Parlade M.V. Individual differences and the development of joint attention in infancy. Child Dev. 2007;78(3):938–954. doi: 10.1111/j.1467-8624.2007.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber F., Bakermans-Kranenburg M.J., van Ijzendoorn M.H., Dietz C., van Daalen E., Swinkels S.H.N., van Engeland H. Joint attention development in toddlers with autism. Eur. Child Adolesc. Psychiatry. 2008;17(3):143–152. doi: 10.1007/s00787-007-0648-6. [DOI] [PubMed] [Google Scholar]
- Natu V., O'Toole A.J. The neural processing of familiar and unfamiliar faces: a review and synopsis. Br. J. Psychol. 2011;102(4):726–747. doi: 10.1111/j.2044-8295.2011.02053.x. [DOI] [PubMed] [Google Scholar]
- Nomi J.S., Uddin L.Q. Face processing in autism spectrum disorders: from brain regions to brain networks. Neuropsychologia. 2015;71:201–216. doi: 10.1016/j.neuropsychologia.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberwelland E., Schilbach L., Barisic I., Krall S.S.C., Vogeley K., Fink G.G.R., Schulte-Rüther M. Look into my eyes: investigating joint attention using interactive eye-tracking and fMRI in a developmental sample. NeuroImage. 2016;130:248–260. doi: 10.1016/j.neuroimage.2016.02.026. [DOI] [PubMed] [Google Scholar]
- Olson I.R., Plotzker A., Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130(7):1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Parma V., Bulgheroni M., Tirindelli R., Castiello U. Body odors promote automatic imitation in autism. Biol. Psychiatry. 2013;74(3):220–226. doi: 10.1016/j.biopsych.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H., Lieberman M.D., Dapretto M. “I know you are but what am I?!”: neural bases of self- and social knowledge retrieval in children and adults. J. Cogn. Neurosci. 2007;19(8):1323–1337. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer U.J., Schilbach L., Timmermans B., Kuzmanovic B., Georgescu A.L., Bente G., Vogeley K. Why we interact: on the functional role of the striatum in the subjective experience of social interaction. NeuroImage. 2014;101:124–137. doi: 10.1016/j.neuroimage.2014.06.061. [DOI] [PubMed] [Google Scholar]
- Pickard K.E., Ingersoll B.R. Brief report: high and low level initiations of joint attention, and response to joint attention: differential relationships with language and imitation. J. Autism Dev. Disord. 2014:262–268. doi: 10.1007/s10803-014-2193-8. [DOI] [PubMed] [Google Scholar]
- Pierce K., Redcay E. Fusiform function in children with an autism spectrum disorder is a matter of “who”. Biol. Psychiatry. 2008;64(7):552–560. doi: 10.1016/j.biopsych.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K., Haist F., Sedaghat F., Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127:2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- Pitskel N.B., Bolling D.Z., Hudac C.M., Lantz S.D., Minshew N.J., Vander Wyk B.C., Pelphrey K.A. Brain mechanisms for processing direct and averted gaze in individuals with autism. J. Autism Dev. Disord. 2011;41(12):1686–1693. doi: 10.1007/s10803-011-1197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek S.M., Wathne K., Tierney N.G., Thomson J.W. Neural correlates of self-face recognition: an effect-location meta-analysis. Brain Res. 2008;1232:173–184. doi: 10.1016/j.brainres.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Redcay E., Dodell-Feder D., Pearrow M.J., Mavros P.L., Kleiner M., Gabrieli J.D.E., Saxe R. Live face-to-face interaction during fMRI: a new tool for social cognitive neuroscience. NeuroImage. 2010;50:1639–1647. doi: 10.1016/j.neuroimage.2010.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E., Dodell-Feder D., Mavros P.L., Pearrow M.J., Triantafyllou C., Gabrieli J.D., Saxe R. Atypical brain activation patterns during a face-to-face joint attention game in adults with autism spectrum disorder. Hum. Brain Mapp. 2013;34(10):2511–2523. doi: 10.1002/hbm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronconi L., Facoetti A., Bulf H., Franchin L., Bettoni R., Valenza E. Paternal autistic traits are predictive of infants visual attention. J. Autism Dev. Disord. 2014;44(7):1556–1564. doi: 10.1007/s10803-013-2018-1. [DOI] [PubMed] [Google Scholar]
- Samson D., Apperly I.A., Chiavarino C., Humphreys G.W. Left temporoparietal junction is necessary for representing someone else's belief. Nat. Neurosci. 2004;7(5):499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Curr. Opin. Neurobiol. 2006;16(2):235–239. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Saxe R., Moran J.M., Scholz J., Gabrieli J. Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Soc. Cogn. Affect. Neurosci. 2006;1(3):229–234. doi: 10.1093/scan/nsl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schertz H.H., Odom S.L. Promoting joint attention in toddlers with autism: a parent-mediated developmental model. J. Autism Dev. Disord. 2007;37(8):1562–1575. doi: 10.1007/s10803-006-0290-z. [DOI] [PubMed] [Google Scholar]
- Schilbach L. Towards a second-person neuropsychiatry. Philos. Trans. R. Soc. B Biol. Sci. 2016;371(December) doi: 10.1098/rstb.2015.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L., Wilms M., Eickhoff S.B., Romanzetti S., Tepest R., Bente G., Vogeley K. Minds made for sharing: initiating joint attention recruits reward-related neurocircuitry. J. Cogn. Neurosci. 2010;22(12):2702–2715. doi: 10.1162/jocn.2009.21401. [DOI] [PubMed] [Google Scholar]
- Schilbach L., Timmermans B., Reddy V., Costall A., Bente G., Schlicht T., Vogeley K. Toward a second-person neuroscience. Behav. Brain Sci. 2013;36(4):393–414. doi: 10.1017/S0140525X12000660. [DOI] [PubMed] [Google Scholar]
- Schnell K. Mentalizing functions provide a conceptual link of brain function and social cognition in major mental disorders. Psychopathology. 2014;47(6):408–416. doi: 10.1159/000366134. [DOI] [PubMed] [Google Scholar]
- Schnell K., Bluschke S., Konradt B., Walter H. Functional relations of empathy and mentalizing: an fMRI study on the neural basis of cognitive empathy. NeuroImage. 2011;54(2):1743–1754. doi: 10.1016/j.neuroimage.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Schulte-Rüther M., Markowitsch H.J., Fink G.R., Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. J. Cogn. Neurosci. 2007;19(8):1354–1372. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- Schulte-Rüther M., Greimel E., Markowitsch H.J., Kamp-Becker I., Remschmidt H., Fink G.R., Piefke M. Dysfunctions in brain networks supporting empathy: an fMRI study in adults with autism spectrum disorders. Soc. Neurosci. 2011;6(1):1–21. doi: 10.1080/17470911003708032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Rüther M., Mainz V., Fink G.R., Herpertz-Dahlmann B., Konrad K. Theory of mind and the brain in anorexia nervosa: relation to treatment outcome. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(8):832–841. doi: 10.1016/j.jaac.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Schulte-Rüther M., Greimel E., Piefke M., Kamp-Becker I., Remschmidt H., Fink G.R., Konrad K. Age-dependent changes in the neural substrates of empathy in autism spectrum disorder. Soc. Cogn. Affect. Neurosci. 2013:1118–1126. doi: 10.1093/scan/nst088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R.T. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int. J. Dev. Neurosci. 2005;23(2–3):125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Senju A. Spontaneous theory of mind and its absence in autism spectrum disorders. Neuroscientist. 2011;18:108–133. doi: 10.1177/1073858410397208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N.J., Marshall J.C., Zafiris O., Schwab A., Zilles K., Markowitsch H.J., Fink G.R. The neural correlates of person familiarity. A functional magnetic resonance imaging study with clinical implications. Brain. 2001;124(Pt 4):804–815. doi: 10.1093/brain/124.4.804. [DOI] [PubMed] [Google Scholar]
- Sterling L., Dawson G., Webb S., Murias M., Munson J., Panagiotides H., Aylward E. The role of face familiarity in eye tracking of faces by individuals with autism spectrum disorders. J. Autism Dev. Disord. 2008;38(9):1666–1675. doi: 10.1007/s10803-008-0550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley K., Fink G.R. Neural correlates of the first-person-perspective. Trends Cogn. Sci. 2003;7(1):38–42. doi: 10.1016/s1364-6613(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Vogeley K., May M., Ritzl A., Falkai P., Zilles K., Fink G.R. Neural correlates of first-person perspective as one constituent of human self-consciousness. J. Cogn. Neurosci. 2004;16(5):817–827. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- Völlm B.A., Taylor A.N.W., Richardson P., Corcoran R., Stirling J., McKie S.…Elliott R. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. NeuroImage. 2006;29(1):90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Wang A.T., Dapretto M., Hariri A.R., Sigman M., Bookheimer S.Y. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43(4):481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Yahata N., Abe O., Kuwabara H., Inoue H., Takano Y., Yamasue H. Diminished medial prefrontal activity behind autistic social judgments of incongruent information. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0039561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen C., Schreibman L., Ingersoll B. The collateral effects of joint attention training on social initiations, positive affect, imitation, and spontaneous speech for young children with autism. J. Autism Dev. Disord. 2006;36(5):655–664. doi: 10.1007/s10803-006-0108-z. [DOI] [PubMed] [Google Scholar]
- Williams J.H.G., Waiter G.D., Perra O., Perrett D.I., Whiten A. An fMRI study of joint attention experience. NeuroImage. 2005;25(1):133–140. doi: 10.1016/j.neuroimage.2004.10.047. [DOI] [PubMed] [Google Scholar]
- Williams J.H.G., Waiter G.D., Gilchrist A., Perrett D.I., Murray A.D., Whiten A. Neural mechanisms of imitation and “mirror neuron” functioning in autistic spectrum disorder. Neuropsychologia. 2006;44(4):610–621. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Wilms M., Schilbach L., Pfeiffer U., Bente G., Fink G.R., Vogeley K. It's in your eyes—using gaze-contingent stimuli to create truly interactive paradigms for social cognitive and affective neuroscience. Soc. Cogn. Affect. Neurosci. 2010;5(1):98–107. doi: 10.1093/scan/nsq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilbovicius M., Meresse I., Chabane N., Brunelle F., Samson Y., Boddaert N. Autism, the superior temporal sulcus and social perception. Trends Neurosci. 2006;29(7):359–366. doi: 10.1016/j.tins.2006.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material