Abstract
Objectives
To summarize and meta-analyze studies on changes in grey matter (GM) in patients with migraine. We aimed to determine whether there are concordant structural changes in the foci, whether structural changes are concordant with functional changes, and provide further understanding of the anatomy and biology of migraine.
Methods
We searched PubMed and Embase for relevant articles published between January 1985 and November 2015, and examined the references within relevant primary articles. Following exclusion of unsuitable studies, meta-analysis were performed using activation likelihood estimation (ALE).
Results
Eight clinical studies were analyzed for structural changes, containing a total of 390 subjects (191 patients and 199 controls). Five functional studies were enrolled, containing 93 patients and 96 controls. ALE showed that the migraineurs had concordant decreases in the GM volume (GMV) in the bilateral inferior frontal gyri, the right precentral gyrus, the left middle frontal gyrus and the left cingulate gyrus. GMV decreases in right claustrum, left cingulated gyrus, right anterior cingulate, amygdala and left parahippocampal gyrus are related to estimated frequency of headache attack. Activation was found in the somatosensory, cingulate, limbic lobe, basal ganglia and midbrain in migraine patients.
Conclusion
GM changes in migraineurs may indicate the mechanism of pain processing and associated symptoms. Changes in the frontal gyrus may predispose a person to pain conditions. The limbic regions may be accumulated damage due to the repetitive occurrence of pain-related processes. Increased activation in precentral gyrus and cingulate opposed to GMV decrease might suggest increased effort duo to disorganization of these areas and/or the use of compensatory strategies involving pain processing in migraine. Knowledge of these structural and functional changes may be useful for monitoring disease progression as well as for therapeutic interventions.
Keywords: Migraine, Magnetic resonance imaging, Grey matter changes, Meta-analysis, Disease progression
Highlights
-
•
There are some concordant structural changes in migraine.
-
•
Some structural changes like frontal lobe and cingulate are also over-activated in interictal phase.
-
•
Frontal gyrus may predispose a person to pain condition.
-
•
Limbic regions may be accumulating brain damage due to pain-related processes.
1. Introduction
Migraine is a primary headache that is characterized by mostly unilateral pulsating head pain, is aggravated by routine physical activity, and is associated with nausea and/or photophobia and phonophobia (Headache Classification Committee of the International Headache, 2013). Migraine may be accompanied by a variety of autonomic, cognitive, and emotional disturbances (Grassini and Nordin, 2015). Epidemiological studies have documented its high prevalence and high socio-economic and personal impact. Migraine has a 1-year prevalence of 14% in the general population (Vos et al., 2012) and 9.3% in China (Yu et al., 2012). It was ranked as the sixth greatest cause of disability worldwide in the Global Burden of Disease Survey 2013 (2015). It is generally believed that migraine is abnormal brain function that depends on the activation and sensitization of the trigeminovascular pathway (Borsook and Burstein, 2012, Noseda and Burstein, 2013). Cortical spreading depression (CSD) is the electrophysiological correlate of migraine aura (Ferrari et al., 2015, Pietrobon and Moskowitz, 2013). Questions remain, however, concerning the mechanisms of initiation, continuation, and termination of migraine.
Neuroimaging has led to advances in the understanding of primary headache mechanisms, and to better identification of the causes of headache, as well as the structures that are responsible for initiation of the pain. Co-localization of structural changes (i.e., an increase in voxel-based morphometry [VBM]) as well as changes in localized brain activity characterized using positron emission tomography (PET) have been found in the same area of the brain (hypothalamus) in cluster headache patients (May et al., 1999). Hypothalamus is undoubtedly crucial for the pathogenesis of the trigeminal autonomic syndromes, so anatomical co-localization of functional and structural changes (especially increase in GM) raises the possibility that the observed changes may be causal—as opposed to a consequence—of pain (May, 2009). Combined analysis of structural and functional changes in migraine may be urgent to elaborate the pathophysiological mechanism.
Because of the episodic nature and unpleasantness of migraine attacks, imaging during spontaneous migraine has proven difficult. Therefore, most magnetic resonance imaging (MRI) studies of migraine have focused on the migraineurs between attacks, during the so-called interictal phase. In recent years, grey matter (GM) morphology based on MR, particularly VBM, has been carried out on migraineurs. VBM involving statistical voxel-wise testing of the local concentration of GM is a whole-brain method for analysis of automatically pre-processed structural high-resolution MRI data (Draganski and May, 2008). However, not all the studies reported entirely consistent findings. Some researchers have meta-analyzed VBM studies in migraine, but they did not combine them with functional changes and had not taken frequency of migraine attacks into account (Dai et al., 2015, Wenting Hu et al., 2015).
There have been several studies on the functional changes that occur in interictal migraineurs. However, variations like nociceptive, olfactory and visual stimuli in the methods used complicate a meta-analysis of all studies showing brain regions with atypical activation in migraine sufferers (Chen et al., 2015, Schwedt et al., 2015). Interictal cutaneous pain thresholds are lower in migraine patients compared to controls, so we chose articles used nociceptive stimuli for a meta-analysis (Schwedt et al., 2011, Weissman-Fogel et al., 2003). Here, we systematically summarize and meta-analyze the voxel-wise changes and functional changes in GM in patients with migraine. Combined with previously published studies on pain disorders, we aimed to answer the following questions. i) Are there concordant structural changes in the foci (increase or decrease) in migraine? ii) Are these changes concurrent with changes in function? iii) What can be deduced from GM changes in migraine? We describe how these studies have helped our understanding of the anatomy and biology of migraine, discuss their limitations, and propose avenues for future research using MRI to study migraine.
2. Materials and methods
2.1. Search strategy
We searched PubMed and Embase for manuscripts published between January 1985 and November 2015 using the following search terms: (“MRI” OR “magnetic resonance imaging”) AND “migraine” AND “structur*” for VBM analysis. Search words: (“fMRI” OR “functional magnetic resonance imaging”) AND (“migraine disorders” OR (“migraine” AND “disorders”) OR “migraine”) were used for functional studies. Literatures were screened from the title, abstract, intensive reading full-text. We limited the search to studies published in the English language and where the subjects were human. We also examined the references of relevant primary articles and review articles to identify studies that may have been missed in the search.
2.2. Inclusion and exclusion criteria
The meta-analysis included only articles (i) that evaluated the association of grey matter changes and migraine based on a case-control or cohort design; (ii) that contained information on the sample sizes, disease conditions; (iii) patients with migraine were diagnosed according to the International Classification of Headache Disorders (ICHD); (iv) reported whole-brain results of changes in stereotactic coordinates; v) nociceptive stimuli (either heat or ammonia) were used for functional studies and (vi) used thresholds for significance corrected for multiple comparisons or uncorrected with special extent thresholds. We excluded the following: a) non-original studies; b) studies in which the field of view was confined to a restricted region of the cortex; c) studies in which the comparisons between patients with migraine and healthy subjects did not include changes in GM; d) comorbidity with other diseases; e) migraine-like syndromes that were secondary to other diseases; f) articles that concerned other types of headache or a special subtype of migraine (like vestibular migraine); g) articles that reported no significant results; h) articles in which there was no healthy control (HC) group or no comparison with HC; i) studies with unspecified VBM analyses; j) grey matter functional changes due to other stimuli (like visual or olfactory) or combined with other tasks or intervention; k) only functional connectivity was conducted; l) resting-state functional studies in migraineurs; m) duplicate articles; and n) case reports. The selection process is shown as a flow chart in Fig. 1.
Fig. 1.
Flow chart describing the study selection process. Number of publications (n) and number of individuals (N) are indicated.
2.3. Data extraction
The two authors (ZJ and SY) independently extracted data from each study using a predefined data extraction form, any lack of clarity or disagreement was resolved through discussion. The investigators abstracted data from each study to obtain information on author, publication year, sample size, characteristics of the study population (age, gender), types of migraine, disease information and technical details (MRI scanner, region studied, timing, methods and main findings). The coordinates in each study were independently extracted according to the ALE Method.
2.4. Statistical analysis
We used Ginger ALE 2.3.5 (http://www.brainmap.org/) to evaluate the presence of common patterns of GM alterations. Ginger ALE is a BrainMap application that can be used to perform an ALE meta-analysis on coordinates in a Talairach or MNI space. ALE is probably the most widely used algorithm for coordinate-based meta-analyses. The approach treats activation foci reported in neuroimaging studies as spatial probability distributions centered at given coordinates rather than as single points. For each voxel, Ginger ALE estimates the cumulative probabilities that at least one study reports activation for that locus. ALE maps are then obtained by computing the union of activation probabilities for each voxel. Differentiation between true convergence of foci and random clustering is tested using a permutation procedure (Eickhoff et al., 2009, Laird et al., 2005, Turkeltaub et al., 2012). With version 2.0, Ginger ALE switched the ALE methods from fixed effects to random effects, and incorporated variable uncertainty based on the sample size (Eickhoff et al., 2012). A random effects model assumes a higher than chance likelihood of consensus between different experiments, but not in relation to activation variance within each study. During an ALE analysis, each activation focus is modeled as the center of a Gaussian probability distribution, and is used to generate a modeled activation (MA) map for each study. Where we used data that were not expressed in Talairach coordinates, these were transformed into Talairach space using icbm2tal, as implemented in Ginger ALE 2.3.5 (Lancaster et al., 2007). A very conservative threshold of P < 0.001 was chosen, and the minimum cluster size was 100 mm3. All of these steps combined help to control for publication bias with regard to reported foci.
3. Results
Lots of studies have been performed on grey matter (structural or functional) changes in migraine patients, but the results were not consistent and different areas were found in them. After a careful screen, we identified 8 clinical studies that used VBM to assess changes in GM regions in migraineurs and controls, containing a total of 390 subjects (191 patients and 199 controls). The patient group was comprised of 24 men and 167 women, where 155 cases of migraine were without aura and 36 were with aura. The control group comprised 28 men and 171 women. There were 11 subjects diagnosed as chronic migraineurs. The clinical manifestations and technical details of the structural changes on migraine are listed in Table 1, Table 2. The studies we analyzed compared the whole brain grey matter volume (GMV) of 191 migraine patients (mean age: 37.94 years), and 199 healthy controls (mean age: 36.71 years). There were no significant age or gender differences between the patients with migraine and the HC subjects (t-test; P < 0.05). The relationship between changes in GMV and frequency of attack and disease duration were also performed in five out of the eight studies (Kim et al., 2008, Maleki et al., 2012a, Rocca et al., 2006, Schmitz et al., 2008a, Valfre et al., 2008). Four of them assessing the correlation of GM changes with clinical data through multiple comparison or Pearson correlation test, we chose them for subtraction meta-analysis to explore the correlation of GMV changes with frequency of attack and disease duration (Kim et al., 2008, Rocca et al., 2006, Schmitz et al., 2008a, Valfre et al., 2008). As they are correlation analysis, the concept ‘estimated frequency of migraine attacks (frequency of migraine attacks or duration of the disease) was used and a dichotomous grouping based on disease duration -related GMV changes may present the complete picture of development in migraine (DeRamus and Kana, 2015, Kim et al., 2008, Nellessen et al., 2015). Using these data, group analyses were performed between patients with migraine and HC subjects, lower estimated frequency of migraine attacks and higher estimated frequency of migraine attacks patients, respectively. Only one study included chronic migraine, and the number of migraines with aura was small; hence, a group analysis among migraine subtypes was not possible.
Table 1.
Demographic and clinical characteristics of structural changes in migraine.
| First author (year) | Total migraine |
Subgroup |
HC |
Disease |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Number (male) | Age (years) | Number (male) | Age (years) | Number (male) | Age (years) | Duration (years) | Attacks (per month) | Attacks (h) | |
| Rocca MA (2006) (Rocca et al., 2006) | 16 (1) | 42.7 | MO 9 (0) |
NA | 15 (2) | 38.6 | 24.8 | 1.7 | NA |
| Kim JH (2008) (Kim et al., 2008) | 20 (3) | 33.97 ± 11.3 | MO 15 (NA) |
NA | 33 (4) | 33.8 ± 10.5 | 9.8 ± 6.0 | 2.7 ± 0.9 | 21.8 ± 13.1 |
| Schmidt-Wilcke T (2008) (Schmidt-Wilcke et al., 2008) | MO 35 (3) |
32.4 ± 9.2 | mM 19 (0) |
NA | 31 (0) | 32.3 ± 12.6 | NA | NA | NA |
| Schmitz N (2008) (Schmitz et al., 2008a) | 28 (0) | 43.5 ± 8.21 | MO 20 (0) |
44.03 ± 9.43 | 28 (0) | 42.5 ± 9.31 | 30.50 ± 11.43 | 3.5 ± 1.97 | NA |
| Schmitz N (2008) (Schmitz et al., 2008b) | 24 (0) | 45.5 ± 9.31 | MO 16 (0) |
NA | 24 (0) | 41.5 ± 12.9 | 30.62 ± 12.23 | 3.48 ± 2.38 | NA |
| Valfre W (2008) (Valfre et al., 2008) | 27 (6) | 34.9 ± 8.4 | EM 16 (4) |
32.1 ± 8.7 | 27 (7) | 34.9 ± 8.6 | 20.7 ± 3.8 | 11.8 ± 9.7 | 20.6 ± 8.9 |
| Maleki N (2012) (Maleki et al., 2012a) | 20 (6) | 42.1 ± 3.5 | HF (> 8) 10 (3) |
43.9 ± 83.4 | 20 (10) | 41.8 ± 9.9 | 21.4 ± 3.1 | NA | NA |
| Jin C (2013) (Jin et al., 2013) | 21 (5) | 31.2 ± 11.3 | MO 21 (5) |
31.2 ± 11.3 | 21 (5) | 30.7 ± 10.5 | 10.6 ± 6.6 | 4.7 ± 2.0 | 14.1 ± 6.6 |
HC: healthy control; MO: migraine without aura; NA: not available; mM: menstrual migraine; EM: episodic migraine; HF: higher frequency.
Table 2.
Technique details of structural changes in migraine.
| First author (year) | MRI scanner | Region studied | Timing | Methods | Main findings |
|---|---|---|---|---|---|
| Rocca MA (2006) (Rocca et al., 2006) | 3.0T | Whole brain | Interictal | VBM | Reduced GM density located in the frontal and temporal lobes, increased PAG density; reduced GM density was strongly related to age, disease duration |
| Kim JH (2008) (Kim et al., 2008) | 1.5T | Whole brain | Interictal | VBM | GMV reductions in the bilateral insula, motor/premotor, prefrontal, cingulated cortex, right posterior parietal cortex, and orbitofrontal cortex; GMV changes were negatively correlated with headache duration and lifetime headache frequency |
| Schmidt-Wilcke T (2008) (Schmidt-Wilcke et al., 2008) | 1.5T | Whole brain (cingulate cortex, anterior insulae, thalamus, brainstem) | Interictal | VBM | Reduced grey matter density in the anterior and posterior part of the cingulate cortex and the right insular cortex |
| Schmitz N (2008) (Schmitz et al., 2008a) | 3.0T | Whole brain | Interictal | VBM, DTI | Frontal GM density reduction; reduced FA values in the superior frontal lobe, the medial frontal lobe, the brainstem and the cerebellum; high attack frequency show reduced left parahippocampal, left superior frontal gyrus and the inferior parietal lobe GM density; long disease duration(> 15 years) showed decreased GM density in the basal ganglia and the brainstem (medulla), decreased FA in the right frontal lobe |
| Schmitz N (2008) (Schmitz et al., 2008b) | 3.0T | Whole brain | Interictal | VBM | Reduced GM density in the right middle frontal and left inferior parietal lobe |
| Valfre W (2008) (Valfre et al., 2008) | 1.0T | Whole brain | Interictal | VBM | Grey matter reduction in the Right Superior Temporal, Right Inferior Frontal and Left Precentral Gyrus. Chronic migraine compared to episodic, showed a grey matter decrease in the bilateral Anterior Cingulate Cortex. A significant correlation between grey matter reduction in anterior cingulated cortex and frequency of migraine attacks was found. |
| Maleki N (2012) (Maleki et al., 2012a) | 3.0T | Whole brain | Interictal | Cortical thickness, volumetric comparisons | HF patients showed higher thickness in the post-central Gyrus, temporal; smaller cortical volume was observed in the cingulate cortex, insula |
| Jin C (2013) (Jin et al., 2013) | 3.0T | Whole brain | Interictal | VBM, FC | Decreased grey matter volume in: the left medial prefrontal cortex(MPFC), dorsal anterior cingulate cortex (dACC), right occipital lobe, cerebellum and brainstem, the grey matter volume of the dACC was correlated with the duration of disease in migraine patients; increased functional connectivity between the bilateral middle temporal lobe, orbitofrontal cortex, left dorsolateral prefrontal cortex and the left dACC |
VBM: voxel-based morphometry; GM: grey matter; DTI: diffusion tensor imaging; GMV: grey matter volume; FC: functional connectivity.
Five functional MRI studies used nociceptive stimuli were included to assess functional changes in the whole brain GM regions in migraine patients containing a total of 189 subjects (93 patients and 96 controls). 32 men and 61 women were contained in patient group. The clinical manifestations and technical details of the functional changes on migraine can be found in Table 3, Table 4. There was no significant gender difference between the migraineurs and the HC subjects (t-test; P < 0.05). Age information are not available in some researches, however, gender and age were exactly matched in each study.
Table 3.
Demographic and clinical characteristics of functional changes in migraine.
| Study | Total migraine |
Subgroup |
HC |
Disease |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Number (male) | Age (years) | Number (male) | Age (years) | Number (male) | Age (years) | Duration (years) | Attacks (per month) | Attacks (h) | |
| Moulton et al. (2011) | 11 (3) | 42.5 ± 11.9 | MO 6 (NA) |
NA | 11 (3) | 42.3 ± 11.9 | NA | 4–8 | NA |
| Stankewitz et al. (2011) | 20 (5) | 20–39 | MO 13 (NA) |
NA | 20 (5) | 18–37 | 12.7 ± 8.1 | 3 | NA |
| Maleki et al. (2012) | 22 (11) | NA | Male (11) | 42.7 ± 9.3 | 22 (11) | NA | NA | NA | NA |
| Russo et al. (2012) | 16 (8) | 27.83 ± 1.26 | MO (8) | 27.83 ± 1.26 | 16 (8) | 27.50 ± 1.70 | 9.58 ± 1.53 | 6.83 ± 1.12 | NA |
| Schwedt et al. (2014) | 24 (5) | 36.2 ± 11.3 | NA | NA | 27 (5) | 33.7 ± 12.5 | 15.0 ± 9.3 | 6.5 ± 3.0 | NA |
HC: healthy control; MO: migraine without aura; NA: not available.
Table 4.
Technique details of functional changes in migraine.
| Study | MRI scanner | Region studied | Timing | Stimulus | Main findings |
|---|---|---|---|---|---|
| Moulton et al. (2011) | 3.0T | Whole brain | Interictal | Heat | Increased activation in migraine patients in the contralateral anterior temporal pole, the ipsilateral parahippocampal gyrus, pulvinar nucleus, periaqueductal grey and decreases in the dorsolateral prefrontal cortex |
| Stankewitz et al. (2011) | 3.0T | Whole brain | Interictal | Ammonia | Controls showed significantly stronger activation in a brainstem area corresponding to the trigeminal nuclei |
| Maleki et al. (2012) | 3.0T | Whole brain | Interictal | Heat | Female migraineurs showed significant increased activation in paracingulate, ipsilateral superior frontal gyrus, contralateral hippocampus, parahippocampal and precentral gyrus |
| Russo et al. (2012) | 3.0T | Whole brain | Interictal | Heat | Stronger activation in anterior cingulate cortex; and weaker activation in secondary somatosensory cortex and pons in episodic migraineurs than in healthy controls |
| Schwedt et al. (2014) | 3.0T | Whole brain | Interictal | Heat | Greater activation of lentiform nucleus, fusiform gyrus, subthalamic nucleus, hippocampus, middle cingulate cortex, premotor cortex, somatosensory cortex and dorsolateral prefrontal cortex, and less activation in precentral gyrus and superior temporal gyrus in migraineurs |
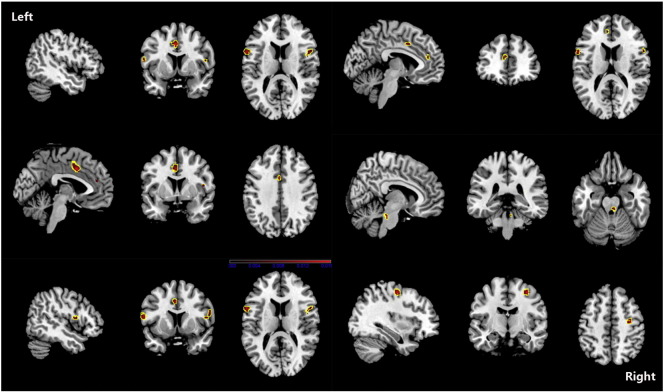
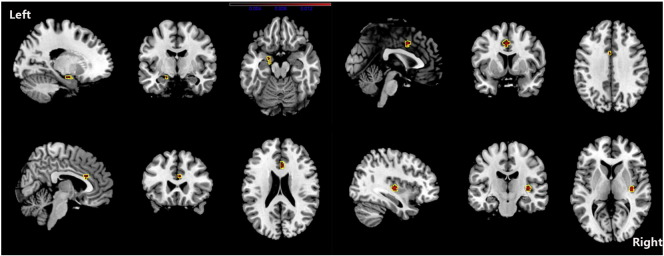
Although PAG increase was found in one study (Rocca et al., 2006), we found no overall increase in GM in patients with migraine. The ALE results showed that, compared with HC, migraine was associated with a common core set of decreases in GM volume in the bilateral inferior frontal gyri, the right precentral gyrus, the right cerebellar culmen, the left middle frontal gyrus and the left cingulate gyrus (see Fig. 2 and Table 5). Changes in the volume of GM in the right claustrum, left cingulated gyrus, right anterior cingulate, amygdala, and left parahippocampal gyrus were negatively related to the estimated frequency of attack (see Fig. 3 and Table 6).
Fig. 2.
ALE map investigating differences in GMV between migraine patients and HC. This image summarizes the results of all the papers involved in this meta-analysis. Red colour show grey matter decreases, they include bilateral inferior frontal gyri, right precentral gyrus, right cerebellar culmen, left middle frontal gyrus and left cingulate gyrus. (ALE maps were computed at a threshold of p < 0.001, with a minimum cluster size of K > 100 mm3 and visualized using MRIcron). Talairach coordinates of clusters showed in this image are reported in Table 5.
Table 5.
Regional difference of GMV between Migraine patients and HC.
| Cluster no. | Volume (mm3) | Weighted center (x, y, z) | x | y | z | ALE value (× 103) | Label | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 672 | − 2.2 | 5.8 | 40.6 | − 2 | 6 | 40 | 16.268 | Left cingulate gyrus BA32 |
| − 2 | 0 | 46 | 9.502 | Left cingulate gyrus BA24 | |||||
| 2 | 488 | 33.4 | − 11.4 | 54.2 | 34 | − 12 | 54 | 16.521 | Right precentral gyrus BA6 |
| 3 | 392 | − 58.2 | 9.2 | 14.1 | − 58 | 10 | 14 | 31.043 | Left inferior frontal gyrus BA44 |
| 4 | 344 | 53.1 | 8.8 | 15.6 | 52 | 10 | 14 | 13.420 | Right inferior frontal gyrus BA44 |
| 56 | 12 | 16 | 9.479 | Right inferior frontal gyrus BA44 | |||||
| 48 | 4 | 12 | 8.818 | Right precentral gyrus BA44 | |||||
| 58 | 10 | 20 | 8.778 | Right inferior frontal gyrus BA45 | |||||
| 58 | 10 | 24 | 8.601 | Right inferior frontal gyrus BA9 | |||||
| 5 | 296 | 5.3 | − 30.4 | − 24.8 | 6 | − 30 | − 26 | 11.372 | Right cerebellum culmen |
| 6 | 120 | − 4.2 | 41.6 | 18.7 | − 4 | 42 | 18 | 9.696 | Left medial frontal gyrus BA9 |
Fig. 3.
ALE map investigating regional difference of GMV related to estimated frequency of headache attack in Migraine patients. Red colour show grey matter decreases, they include right claustrum, left cingulated gyrus, right anterior cingulate, amygdala and left parahippocampal gyrus. (ALE maps were computed at a threshold of p < 0.001, with a minimum cluster size of K > 100 mm3 and visualized using MRIcron). Talairach coordinates of clusters showed in this image are reported in Table 6.
Table 6.
Regional difference of GMV related to estimated frequency of headache attack in migraine patients.
| Clusterno. | Volume (mm3) | Weighted center (x, y, z) | x | y | z | ALE value (× 103) | Label | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 496 | 36.8 | − 15.9 | 3.4 | 36 | − 16 | 4 | 15.792 | Right claustrum |
| 2 | 424 | − 1.4 | 6.7 | 39.4 | − 2 | 6 | 40 | 13.774 | Left cingulated gyrus B32 |
| 3 | 264 | 4.1 | 23.2 | 22.9 | 4 | 22 | 22 | 10.652 | Right anterior cingulate BA24 |
| 4 | 256 | − 18.7 | − 6.7 | − 17.3 | − 20 | − 4 | − 18 | 9.541 | Left parahippocampal gyrus amygdala |
| − 18 | − 10 | − 16 | 9.409 | Left parahippocampal gyrus BA34 | |||||
In individual studies, both activated and deactivated areas were found in migraineurs. Several areas containing the bilateral lentiform nucleus, putamen and claustrum, the right precentral gyrus, the right midbrain, the right postcentral gyrus, the left limbic lobe and the left cingulate gyrus were activated in the migraine patients after ALE analysis. There were also area deactivations in the left precentral gyrus and the right superior temporal gyrus in the migraineurs (shown in Fig. 4 and Table 7).
Fig. 4.
Contrast maps for nociceptive stimuli functional MRI activation. Contrast analysis of the functional differences in migraineurs versus healthy control subjects revealed significant activation (in red) and deactivation (in blue) in migraine patients. A common core set of activations are shown in the bilateral basal ganglia, the right paracentral gyrus, the right midbrain, the left limbic lobe and the left cingulate gyrus. The left precentral gyrus and the right superior temporal gyrus are deactivated in migraine patients. (ALE maps were computed at a threshold of p < 0.001, with a minimum cluster size of K > 100 mm3 and visualized using MRIcron). Talairach coordinates of clusters showed in this image are reported in Table 7.
Table 7.
Contrast analysis results for nociceptive stimuli functional MRI activation.
| Cluster no. | Volume (mm3) | Weighted center (x, y, z) | x | y | z | ALE value (× 103) | Label | ||
|---|---|---|---|---|---|---|---|---|---|
| Activations migraine patients > healthy controls | |||||||||
| 1 | 600 | − 21.8 | − 5.2 | − 29 | − 20 | − 6 | − 30 | 13.068 | Left limbic lobe BA 36 |
| 2 | 440 | 28.8 | 8 | 24.9 | 30 | 8 | 26 | 11.233 | Right precentral gyrus BA6 |
| 3 | 200 | 21.8 | 7.8 | 9.8 | 20 | 8 | 10 | 9.033 | Right lentiform nucleus, putamen |
| 28 | 6 | 10 | 7.346 | Right claustrum | |||||
| 5 | 112 | 10.4 | − 15.6 | − 6 | 10 | − 16 | − 6 | 8.983 | Right midbrain |
| 6 | 112 | − 21.3 | 0.4 | 2.1 | − 22 | 0 | 2 | 8.821 | Left lentiform nucleus, putamen |
| 7 | 104 | − 36 | − 19.2 | − 7.1 | − 36 | − 20 | − 8 | 8.665 | Left claustrum |
| 9 | 104 | 41.1 | − 24.8 | 26 | 42 | − 24 | 26 | 8.632 | Right postcentral gyrus BA2 |
| 10 | 104 | − 3.4 | − 12 | 31.7 | − 4 | − 12 | 32 | 8.834 | Left cingulate gyrus BA23 |
| Activations healthy controls > migraine patients | |||||||||
| 4 | 152 | − 16 | − 24.6 | 64.5 | − 16 | − 24 | 64 | 8.972 | Left precentral gyrus BA4 |
| 8 | 104 | 58 | − 49.4 | 7.7 | 58 | − 50 | 8 | 8.863 | Right superior temporal gyrus BA22 |
4. Discussion
Initiation of a migraine attack is associated with genetic and environmental factors. Genome-wide association studies of migraine have revealed that genetics are involved in migraine (Anttila et al., 2010, Chasman et al., 2011, Freilinger et al., 2012); however, only limited variance can be explained by genetics. Environmental factors, such as stress, weather, hormonal fluctuations, sleep disturbances, meal skipping, and sensory overload, are clearly involved in migraine (Kelman, 2007, Levy et al., 2009). Whatever the cause, imaging during migraine pain has led to a shift from vascular hypotheses of migraine pathophysiology to neurovascular or central nervous system (CNS) theories.
MRI is a non-invasive procedure with the potential to determine a morphological basis of neurological diseases, as well as to investigate functional–structural relationships. In idiopathic or primary headaches, including migraine, tension-type headaches, and cluster headaches, the accepted view is that these conditions are due to abnormal brain function that occurs with normal brain structure. Patients with chronic tension-type headaches exhibit a decrease in GM volume in the structures that are responsible for transmitting pain (Schmidt-Wilcke et al., 2005). Data on cluster headaches describe an increase in the volume of GM in the hypothalamus which is associated with functional areas involved during acute headache attacks (May et al., 1999).
In recent years, there have been a number of studies on GM morphology using MR (especially VBM) to investigate the mechanisms of initiation, continuation and termination of migraine (Hougaard et al., 2014). Lots of researches also be conducted on GM function in migraine patients used various methods. Nevertheless, single imaging study carries several limitations, including small sample sizes, low reliability and its inherent subtraction logic which is only sensitive to differences between conditions (Feredoes and Postle, 2007, Price et al., 2005). ALE treats activation foci, assesses the overlap between foci based on modelling them as probability distributions centered at the respective coordinates and finds agreement across subject groups. Either way, single study results are driven by dataset's foci as well as how they are grouped. A P value is used to set a significance threshold on the ALE scores. ALE was widely applied to meta-analyze the structural and functional brain changes of various neuropsychiatric disorders, such as autism spectrum disorders, Alzheimer's Disease, anhedonia, idiopathic dystonia and physiological conditions, such as aging and touch (Chapleau et al., 2016, DeRamus and Kana, 2015, Di et al., 2014, Lokkegaard et al., 2016, Morrison, 2016, Nickl-Jockschat et al., 2012).
After ALE meta-analysis of structural and functional changes in the GM in migraine patients, combined with previous studies in pain disorder, our study demonstrates: i) migraine was associated with a common core set of decreases in the volume of GM in the bilateral inferior frontal gyri, the right precentral gyrus, the left middle frontal gyrus and the left anterior cingulate gyrus; changes in the volume of GM in the cingulated gyrus were negatively related to the estimated frequency of headache attack; ii) the precentral gyrus and the cingulate gyrus were also over-activated in interictal migraineurs during nociceptive stimuli; and iii)structural changes in the frontal gyrus may predispose a person to pain, and the cingulate gyrus may accumulate damage due to the repeated occurrence of migraine; functional activation in the precentral and postcentral gyrus, the limbic region, the basal ganglia and the right midbrain may be associated with pain processing and associated symptoms in migraine patients.
4.1. Changes in brain function
Brain function activation in primary headache syndromes contains two groups: areas generally involved in pain processing, such as the somatosensory, cingulate, thalamus et al.; and areas specifically activated in primary headaches, such as the hypothalamus, which is activated in cluster headache attacks (May et al., 1999). One review of stimulus-induced brain activation in migraine found atypical brain responses to sensory stimuli, an absence of the normal habituating response between attacks, and atypical functional connectivity of sensory processing regions (Schwedt et al., 2015). Migraine sensory hypersensitivities may be induced by a combination of enhanced sensory facilitation and reduced inhibition in response to sensory stimuli (Russo et al., 2012, Schwedt et al., 2014), as well as reduced or absent habituation to stimuli interictally (Burstein et al., 2010). They may also contribute to a transformation from episodic to chronic migraine (Moulton et al., 2008). A concordant activation was found in the somatosensory, cingulate, limbic lobe, basal ganglia and midbrain in migraine patients in our study. All these areas are related to pain processing, modulation and associated symptoms (like emotional disturbances) in migraineurs. The activation areas of somatosensory, basal ganglia and midbrain coincided with previous studies and further confirms that activation and sensitization of the trigeminovascular pathway are involved in migraine pathophysiology (DaSilva et al., 2007, Noseda and Burstein, 2013, Schulte and May, 2016, Stankewitz et al., 2011). Studies of changes in brain function during the ictal phase may improve our understanding of migraine mechanisms. Some investigators captured the onset of a migraine attack (Afridi et al., 2005a, Bahra et al., 2001, Welch et al., 1998), and others exposed migraine subjects to attack triggers in order to initiate migraine (Afridi et al., 2005b, Cao et al., 2002, Maniyar et al., 2014). During the premonitory phase of nitroglycerin-triggered migraine attacks, the following areas of the brain were found to be activated: the posterolateral hypothalamus, the brainstem, and various cortical areas, including the occipital, temporal, and prefrontal cortices (Maniyar et al., 2014). Studies that captured the onset of migraine found an increase in the signal from the brainstem (Bahra et al., 2001). Comparisons of ictal images with interictal scans revealed significant activation in the anterior and posterior cingulate, as well as the cerebellum, thalamus, insula, prefrontal cortex, temporal lobes, and dorsal pons during migraine (Afridi et al., 2005a). Several studies found that, following treatment of the migraine with sumatriptan and suboccipital stimulator therapy, the dorsal pons remained active (Bahra et al., 2001, Matharu et al., 2004). Taken together, these studies show that the brainstem is continuously activated during a migraine, and that this may be specific to migraine and it may act as a ‘generator’ of migraine. A longitudinal fMRI study on a migraine patient found that hypothalamic activity increases towards the next migraine attack and shows altered functional coupling with the spinal trigeminal nuclei and the dorsal rostral pons during the preictal day and the pain phase (Schulte and May, 2016). Combined with previous study, this may indicate that the real driver of attacks might be the functional changes in hypothalamo–brainstem connectivity (Maniyar et al., 2014). If there are concordant structural changes, especially increases in the GMV, these areas may be the cause of migraine.
4.2. Changes in brain structure
MRI studies of patients with migraine have consistently shown a distributed pattern of morphological brain abnormalities characterized by regions of decreased and increased GMV (Jin et al., 2013, Kim et al., 2008, Maleki et al., 2012a, Rocca et al., 2006, Schmidt-Wilcke et al., 2008, Schmitz et al., 2008a, Schmitz et al., 2008b, Valfre et al., 2008). The role of such abnormalities in the pathogenesis of this condition remains a matter of debate; histological measures, such as neuronal density, do not correlate with VBM GM probability maps (Eriksson et al., 2009), and changes in cerebral blood flow produce ‘apparent’ GMV changes in VBM analyses (Franklin et al., 2013). This may reflect alterations in the dendritic complexity or changes in the number of synapses, or simply changes in water content. An increase in GMV may reflect structural brain plasticity as a result of exercise and learning (May, 2009). A decrease in GMV suggest that the central reorganization processes in chronic pain syndromes may involve degeneration of anti-nociceptive brain areas.
Indeed, relationships between decreases in GMV and disease duration or attack frequency have been reported (Kim et al., 2008, Liu et al., 2013, Maleki et al., 2012a, Rocca et al., 2006, Schmidt-Wilcke et al., 2008, Valfre et al., 2008),which suggests that morphological brain abnormalities may be the consequence of repeated attacks. However, some changes in GMV are not correlated with clinical manifestation, suggesting that they may predispose a person to migraine (Liu et al., 2013). In our study, one study without significant result was excluded, it was conducted in 2003 and the possibility may be the differences existed in migraine patients included and image pre-processing procedures (Matharu et al., 2003). We found a set of decreases in the GMV in the bilateral inferior frontal gyri, the right precentral gyrus, the left middle frontal gyrus and the left anterior cingulate gyrus in migraine. Our finding of reliable structural alterations in homologous regions associated with pain processing highlights the importance of these areas in the pathogenesis of migraine. We also found changes in GMV in the right claustrum, the left cingulate gyrus, the right anterior cingulate, the amygdala, and the left parahippocampal gyrus, which were negatively related to the frequency of headache attacks. Our result further indicate that the GMV changes related to the frequency of headache attacks may occur as a consequence of repeated brain insults during migraine attacks. Decreases in GMV in the frontal gyri were not related to the frequency of headache attacks.
The frontal cortex is one of the most important areas associated with brain abnormalities in migraine patients. The role of the frontal lobe in pain processing has been established in several recent morphological studies, including subjects with chronic back pain (Apkarian et al., 2004), fibromyalgia, irritable bowel syndrome, and phantom pain atrophy in the prefrontal cortex (May, 2008). It has recently been reported that significant GM atrophy of the frontal lobe is associated with migraine in pediatric patients, but is not correlated with the disease duration or frequency of attacks (Rocca et al., 2014). A recent longitudinal MRI study using VBM investigated changes in GM related to medication withdrawal in a group of patients with medication overuse headache (Riederer et al., 2013). Patients with clinical improvement showed a significant reversal of the changes in GM in some regions, and patients without treatment response had less GM in the orbitofrontal cortex. Another striking result is a correlation between treatment response and the orbitofrontal GMV. It appears that a decrease in GMV in the frontal gyrus may predispose a person to pain conditions, and may be predictive of poor response to treatment. However, this is not specific to migraine.
PET studies have shown that the limbic regions (the cingulate cortex, amygdala, and parahippocampal gyrus) are activated during migraine attacks (Afridi et al., 2005a, Afridi et al., 2005b, Matharu et al., 2004)and cluster headache attacks (May et al., 1999, May et al., 1998, May et al., 2000). These areas of the brain are also believed to be involved in emotional, cognitive, and autonomic functions (May, 2006, Morgane et al., 2005, Peyron et al., 1999). Alterations in cerebral structures that modulate the affective components of pain that are found in migraine suggest a possible neurobiological mechanism, which may explain the link between chronic migraine and psychiatric disturbances (Buse et al., 2013, Minen et al., 2016). Decreases in GMV in the cingulate cortex were also found in patients with chronic back pain (Schmidt-Wilcke et al., 2006), chronic phantom pain (Henry et al., 2011), and chronic tension-type headaches (Schmidt-Wilcke et al., 2005). These changes are correlated with disease duration and attack frequency, and may result in brain damage accumulated due to repeated occurrences of pain-related processes, and are not specific to migraine.
For better understanding the neuroanatomical and functional brain changes in migraine patients, there are several studies performed on the concurrent functional and structural cortical alterations in migraine (Hubbard et al., 2014, Jin et al., 2013, Maleki et al., 2012a, Maleki et al., 2013). Higher thickness in the post-central gyrus in the higher frequency of attack (HF) correlated with the observed stronger functional activation, suggested adaptation to repeated sensory drive (Maleki et al., 2012a). Decreased dorsal anterior cingulate cortex was correlated with increased functional connectivity between several regions indicate that frequent nociceptive input has modified the structural and functional patterns of the frontal cortex in migraine (Jin et al., 2013). Concordant structural and functional changes in hippocampal in lower frequency of attack (LF) group relative to the HF and HC groups suggestive of an initial adaptive plasticity that may then become dysfunctional with increased frequency (Maleki et al., 2013). In our study, no GMV increase was found, so the “generator” of migraine cannot been deduced. Increased activation in precentral gyrus and cingulate during nociceptive stimuli opposed to GMV decrease might represent increased effort, possibly due to disorganization of these areas and/or the use of compensatory strategies involving pain processing in migraine.
We did not find structural changes in GM in areas that were specifically activated in migraine (i.e., the brainstem, hypothalamus). However, this negative finding does not imply that the brainstem and hypothalamus are not involved in the pathophysiology of migraine. Because most of the studies included in our meta-analysis used whole-brain detection (only in one study was the brainstem subjected to a small-volume correction, but this employed a 1.5-T MRI scanner), and the GM volume of the brainstem is relatively small, we could not draw conclusions on the significance of brainstem morphology in migraine. Further studies are therefore warranted that focus on the brainstem volume using 3.0-T or 7.0-T MRI scanners in order to determine whether there are any significant changes in the volume of the brainstem. At the same time, ALE provides a way of applying statistical thresholds to large sets of coordinate data, in order to identify the most consistently-existed and reproducible loci across many studies, the result can provide a priori hypotheses for further experimental testing. However, it filters out less robust or infrequently-reported foci that may have a greater dependence on the details of individual studies. Longitudinal and combination of structural and functional imaging studies may be needed to better understand the anatomy of migraine. As animal models are available in migraine and their life spans are short, a longitudinal imaging study can also be completed in rodents and brains can be collected ex-vivo for further analysis.
4.3. Limitations of our study
First, we consider limitations that are common to imaging studies. There are no established protocols for imaging studies, and image pre-processing procedures, such as smoothing, registration, the choice of small volume corrections, imaging modalities, and data analysis, differed among studies. The headache characteristics (i.e., attack frequency and disease duration) as well as the HC choices were also inconsistent.
Our meta-analysis was subject to publication bias because unpublished studies and studies without any significant clusters reported were not included, the studies were limited to those published in English and where the subjects were human. The ALE meta-analysis was based on pooling of stereotactic coordinates with significant differences rather than on the raw data from the included studies; this may have limited the accuracy of the results. Nevertheless, previous studies suggested that no individual study could significantly bias the results of ALE meta-analyses after including the sample size and number of reported foci into ALE algorithm, changing from fixed-effects to random-effects inference, and revising for multiple comparison correction (Eickhoff et al., 2012, Eickhoff et al., 2009, Turkeltaub et al., 2012). The inclusion criteria for fMRI were nociceptive stimulation in order to allow for analysis, regardless of stimulation site or stimulus type (e.g., hand, forehead, heat, and ammonia). The result makes clear that the simultaneous activation and deactivation of the relevant regions are implicated in responses to a range of nociceptive stimuli. The sex ratio was not concordant with the prevalence of migraine, and sex differences in brain structure may be present (Maleki et al., 2012b). There were few chronic migraineurs, and the sample may not be broadly representative of migraineurs overall. Migraineurs frequently have several comorbid conditions (e.g., anxiety and mood disorders, altered lifestyles, and drug use that might directly contribute to morphological changes); this was not considered in our study.
5. Conclusion
Whole-brain VBM studies identified consistent widespread reduction in the GMV in migraine, specifically in the frontal cortex and the cingulate gyrus. The cingulate gyrus and the parahippocampal gyrus were related to the estimated frequency of headache attacks. Frontal cortex and cingulate gyrus were over-activated in interictal phase during nociceptive stimuli. These changes in GM may be related to the mechanisms of pain processing and the associated symptoms of migraine, such as cognitive dysfunction, emotional problems, and autonomic dysfunction. Changes in the frontal gyrus may predispose a person to pain conditions. The limbic regions may accumulate damage due to repeated occurrences of pain-related processes. Further studies are required to determine how these changes can be used to monitor disease progression or exploited in therapeutic interventions.
Acknowledgments
The scientific guarantor of this publication is Yu Shengyuan. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. This work was supported by the National Science Foundation of China (grants 81471147) and National Key Technology Support Program (grants 2013BA104B04). No complex statistical methods were necessary for this paper. Written informed consent was not required for this study because this study is a meta-analysis. Methodology: retrospective, meta-analysis, performed at one institution.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.01.019.
Appendix A. Supplementary data
Structural neuroimaging data in NIFTI format.
Structural neuroimaging data related to headache durations in NIFTI format.
Functional neuroimaging data in NIFTI format.
Structural interactive plots.
Structural interactive plots related to headache durations.
Functional interactive plots.
References
- Afridi S.K., Giffin N.J., Kaube H., Friston K.J., Ward N.S., Frackowiak R.S., Goadsby P.J. A positron emission tomographic study in spontaneous migraine. Arch. Neurol. 2005;62:1270–1275. doi: 10.1001/archneur.62.8.1270. [DOI] [PubMed] [Google Scholar]
- Afridi S.K., Matharu M.S., Lee L., Kaube H., Friston K.J., Frackowiak R.S., Goadsby P.J. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain. 2005;128:932–939. doi: 10.1093/brain/awh416. [DOI] [PubMed] [Google Scholar]
- Anttila V., Stefansson H., Kallela M., Todt U., Terwindt G.M., Calafato M.S., Nyholt D.R., Dimas A.S., Freilinger T., Muller-Myhsok B., Artto V., Inouye M., Alakurtti K., Kaunisto M.A., Hamalainen E., de Vries B., Stam A.H., Weller C.M., Heinze A., Heinze-Kuhn K., Goebel I., Borck G., Gobel H., Steinberg S., Wolf C., Bjornsson A., Gudmundsson G., Kirchmann M., Hauge A., Werge T., Schoenen J., Eriksson J.G., Hagen K., Stovner L., Wichmann H.E., Meitinger T., Alexander M., Moebus S., Schreiber S., Aulchenko Y.S., Breteler M.M., Uitterlinden A.G., Hofman A., van Duijn C.M., Tikka-Kleemola P., Vepsalainen S., Lucae S., Tozzi F., Muglia P., Barrett J., Kaprio J., Farkkila M., Peltonen L., Stefansson K., Zwart J.A., Ferrari M.D., Olesen J., Daly M., Wessman M., van den Maagdenberg A.M., Dichgans M., Kubisch C., Dermitzakis E.T., Frants R.R., Palotie A. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nat. Genet. 2010;42:869–873. doi: 10.1038/ng.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian A.V., Sosa Y., Sonty S., Levy R.M., Harden R.N., Parrish T.B., Gitelman D.R. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahra A., Matharu M.S., Buchel C., Frackowiak R.S.J., Goadsby P.J. Brainstem activation specific to migraine headache. Lancet. 2001;357:1016–1017. doi: 10.1016/s0140-6736(00)04250-1. [DOI] [PubMed] [Google Scholar]
- Borsook D., Burstein R. The enigma of the dorsolateral pons as a migraine generator. Cephalalgia. 2012;32:803–812. doi: 10.1177/0333102412453952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R., Jakubowski M., Garcia-Nicas E., Kainz V., Bajwa Z., Hargreaves R., Becerra L., Borsook D. Thalamic sensitization transforms localized pain into widespread allodynia. Ann. Neurol. 2010;68:81–91. doi: 10.1002/ana.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse D.C., Silberstein S.D., Manack A.N., Papapetropoulos S., Lipton R.B. Psychiatric comorbidities of episodic and chronic migraine. J. Neurol. 2013;260:1960–1969. doi: 10.1007/s00415-012-6725-x. [DOI] [PubMed] [Google Scholar]
- Cao Y., Aurora S.K., Nagesh V., Patel S.C., Welch K.M. Functional MRI-BOLD of brainstem structures during visually triggered migraine. Neurology. 2002;59:72–78. doi: 10.1212/wnl.59.1.72. [DOI] [PubMed] [Google Scholar]
- Chapleau M., Aldebert J., Montembeault M., Brambati S.M. Atrophy in Alzheimer's disease and semantic dementia: an ALE meta-analysis of voxel-based morphometry studies. J. Alzheimers Dis. 2016;54:941–955. doi: 10.3233/JAD-160382. [DOI] [PubMed] [Google Scholar]
- Chasman D.I., Schurks M., Anttila V., de Vries B., Schminke U., Launer L.J., Terwindt G.M., van den Maagdenberg A.M., Fendrich K., Volzke H., Ernst F., Griffiths L.R., Buring J.E., Kallela M., Freilinger T., Kubisch C., Ridker P.M., Palotie A., Ferrari M.D., Hoffmann W., Zee R.Y., Kurth T. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat. Genet. 2011;43:695–698. doi: 10.1038/ng.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhang J., Wang P., Guo J., Zhou M., He L. Functional alterations of pain processing pathway in migraine patients with cutaneous allodynia. Pain Med. 2015;16:1211–1220. doi: 10.1111/pme.12690. [DOI] [PubMed] [Google Scholar]
- Dai Z., Zhong J., Xiao P., Zhu Y., Chen F., Pan P., Shi H. Gray matter correlates of migraine and gender effect: a meta-analysis of voxel-based morphometry studies. Neuroscience. 2015;299:88–96. doi: 10.1016/j.neuroscience.2015.04.066. [DOI] [PubMed] [Google Scholar]
- DaSilva A.F., Granziera C., Tuch D.S., Snyder J., Vincent M., Hadjikhani N. Interictal alterations of the trigeminal somatosensory pathway and periaqueductal gray matter in migraine. Neuroreport. 2007;18:301–305. doi: 10.1097/WNR.0b013e32801776bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRamus T.P., Kana R.K. Anatomical likelihood estimation meta-analysis of grey and white matter anomalies in autism spectrum disorders. Neuroimage Clin. 2015;7:525–536. doi: 10.1016/j.nicl.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X., Rypma B., Biswal B.B. Correspondence of executive function related functional and anatomical alterations in aging brain. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;48:41–50. doi: 10.1016/j.pnpbp.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B., May A. Training-induced structural changes in the adult human brain. Behav. Brain Res. 2008;192:137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B., Laird A.R., Grefkes C., Wang L.E., Zilles K., Fox P.T. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff S.B., Bzdok D., Laird A.R., Kurth F., Fox P.T. Activation likelihood estimation meta-analysis revisited. NeuroImage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S.H., Free S.L., Thom M., Symms M.R., Martinian L., Duncan J.S., Sisodiya S.M. Quantitative grey matter histological measures do not correlate with grey matter probability values from in vivo MRI in the temporal lobe. J. Neurosci. Methods. 2009;181:111–118. doi: 10.1016/j.jneumeth.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feredoes E., Postle B.R. Localization of load sensitivity of working memory storage: quantitatively and qualitatively discrepant results yielded by single-subject and group-averaged approaches to fMRI group analysis. NeuroImage. 2007;35:881–903. doi: 10.1016/j.neuroimage.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari M.D., Klever R.R., Terwindt G.M., Ayata C., van den Maagdenberg A.M.J.M. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol. 2015;14:65–80. doi: 10.1016/S1474-4422(14)70220-0. [DOI] [PubMed] [Google Scholar]
- Franklin T.R., Wang Z., Shin J., Jagannathan K., Suh J.J., Detre J.A., O'Brien C.P., Childress A.R. A VBM study demonstrating ‘apparent’ effects of a single dose of medication on T1-weighted MRIs. Brain Struct. Funct. 2013;218:97–104. doi: 10.1007/s00429-012-0385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freilinger T., Anttila V., de Vries B., Malik R., Kallela M., Terwindt G.M., Pozo-Rosich P., Winsvold B., Nyholt D.R., van Oosterhout W.P., Artto V., Todt U., Hamalainen E., Fernandez-Morales J., Louter M.A., Kaunisto M.A., Schoenen J., Raitakari O., Lehtimaki T., Vila-Pueyo M., Gobel H., Wichmann E., Sintas C., Uitterlinden A.G., Hofman A., Rivadeneira F., Heinze A., Tronvik E., van Duijn C.M., Kaprio J., Cormand B., Wessman M., Frants R.R., Meitinger T., Muller-Myhsok B., Zwart J.A., Farkkila M., Macaya A., Ferrari M.D., Kubisch C., Palotie A., Dichgans M., van den Maagdenberg A.M. Genome-wide association analysis identifies susceptibility loci for migraine without aura. Nat. Genet. 2012;44:777–782. doi: 10.1038/ng.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassini S., Nordin S. Comorbidity in migraine with functional somatic syndromes, psychiatric disorders and inflammatory diseases: a matter of central sensitization? Behav. Med. 2015:1–9. doi: 10.1080/08964289.2015.1086721. [DOI] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache, S The international classification of headache disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- Henry D.E., Chiodo A.E., Yang W. Central nervous system reorganization in a variety of chronic pain states: a review. PM R. 2011;3:1116–1125. doi: 10.1016/j.pmrj.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Hougaard A., Amin F.M., Ashina M. Migraine and structural abnormalities in the brain. Curr. Opin. Neurol. 2014;27:309–314. doi: 10.1097/WCO.0000000000000086. [DOI] [PubMed] [Google Scholar]
- Hubbard C.S., Khan S.A., Keaser M.L., Mathur V.A., Goyal M., Seminowicz D.A. Altered brain structure and function correlate with disease severity and pain catastrophizing in migraine patients. eNeuro. 2014;1(e20):14. doi: 10.1523/ENEURO.0006-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Yuan K., Zhao L., Zhao L., Yu D., von Deneen K.M., Zhang M., Qin W., Sun W., Tian J. Structural and functional abnormalities in migraine patients without aura. NMR Biomed. 2013;26:58–64. doi: 10.1002/nbm.2819. [DOI] [PubMed] [Google Scholar]
- Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia. 2007;27:394–402. doi: 10.1111/j.1468-2982.2007.01303.x. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Suh S.I., Seol H.Y., Oh K., Seo W.K., Yu S.W., Park K.W., Koh S.B. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008;28:598–604. doi: 10.1111/j.1468-2982.2008.01550.x. [DOI] [PubMed] [Google Scholar]
- Laird A.R., Fox P.M., Price C.J., Glahn D.C., Uecker A.M., Lancaster J.L., Turkeltaub P.E., Kochunov P., Fox P.T. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum. Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas-Gutierrez D., Martinez M., Salinas F., Evans A., Zilles K., Mazziotta J.C., Fox P.T. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum. Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D., Strassman A.M., Burstein R. A critical view on the role of migraine triggers in the genesis of migraine pain. Headache. 2009;49:953–957. doi: 10.1111/j.1526-4610.2009.01444.x. [DOI] [PubMed] [Google Scholar]
- Liu J., Lan L., Li G., Yan X., Nan J., Xiong S., Yin Q., von Deneen K.M., Gong Q., Liang F., Qin W., Tian J. Migraine-related gray matter and white matter changes at a 1-year follow-up evaluation. J. Pain. 2013;14:1703–1708. doi: 10.1016/j.jpain.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Lokkegaard A., Herz D.M., Haagensen B.N., Lorentzen A.K., Eickhoff S.B., Siebner H.R. Altered sensorimotor activation patterns in idiopathic dystonia-an activation likelihood estimation meta-analysis of functional brain imaging studies. Hum. Brain Mapp. 2016;37:547–557. doi: 10.1002/hbm.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N., Becerra L., Brawn J., Bigal M., Burstein R., Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia. 2012;32:607–620. doi: 10.1177/0333102412445622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N., Linnman C., Brawn J., Burstein R., Becerra L., Borsook D. Her versus his migraine: multiple sex differences in brain function and structure. Brain. 2012;135:2546–2559. doi: 10.1093/brain/aws175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N., Becerra L., Brawn J., McEwen B., Burstein R., Borsook D. Common hippocampal structural and functional changes in migraine. Brain Struct. Funct. 2013;218:903–912. doi: 10.1007/s00429-012-0437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniyar F.H., Sprenger T., Monteith T., Schankin C., Goadsby P.J. Brain activations in the premonitory phase of nitroglycerin-triggered migraine attacks. Brain. 2014;137:232–241. doi: 10.1093/brain/awt320. [DOI] [PubMed] [Google Scholar]
- Matharu M.S., Good C.D., May A., Bahra A., Goadsby P.J. No change in the structure of the brain in migraine: a voxel-based morphometric study. Eur. J. Neurol. 2003;10:53–57. doi: 10.1046/j.1468-1331.2003.00510.x. [DOI] [PubMed] [Google Scholar]
- Matharu M.S., Bartsch T., Ward N., Frackowiak R.S., Weiner R., Goadsby P.J. Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain. 2004;127:220–230. doi: 10.1093/brain/awh022. [DOI] [PubMed] [Google Scholar]
- May A. A review of diagnostic and functional imaging in headache. J. Headache Pain. 2006;7:174–184. doi: 10.1007/s10194-006-0307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- May A. Morphing voxels: the hype around structural imaging of headache patients. Brain. 2009;132:1419–1425. doi: 10.1093/brain/awp116. [DOI] [PubMed] [Google Scholar]
- May A., Bahra A., Buchel C., Frackowiak R.S., Goadsby P.J. Hypothalamic activation in cluster headache attacks. Lancet. 1998;352:275–278. doi: 10.1016/S0140-6736(98)02470-2. [DOI] [PubMed] [Google Scholar]
- May A., Ashburner J., Buchel C., McGonigle D.J., Friston K.J., Frackowiak R.S., Goadsby P.J. Correlation between structural and functional changes in brain in an idiopathic headache syndrome. Nat. Med. 1999;5:836–838. doi: 10.1038/10561. [DOI] [PubMed] [Google Scholar]
- May A., Bahra A., Buchel C., Frackowiak R.S., Goadsby P.J. PET and MRA findings in cluster headache and MRA in experimental pain. Neurology. 2000;55:1328–1335. doi: 10.1212/wnl.55.9.1328. [DOI] [PubMed] [Google Scholar]
- Minen M.T., Begasse De Dhaem O., Kroon Van Diest A., Powers S., Schwedt T.J., Lipton R., Silbersweig D. Migraine and its psychiatric comorbidities. J. Neurol. Neurosurg. Psychiatry. 2016;87:741–749. doi: 10.1136/jnnp-2015-312233. [DOI] [PubMed] [Google Scholar]
- Morgane P.J., Galler J.R., Mokler D.J. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog. Neurobiol. 2005;75:143–160. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Morrison I. ALE meta-analysis reveals dissociable networks for affective and discriminative aspects of touch. Hum. Brain Mapp. 2016;37:1308–1320. doi: 10.1002/hbm.23103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton E.A., Burstein R., Tully S., Hargreaves R., Becerra L., Borsook D. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton E.A., Becerra L., Maleki N., Pendse G., Tully S., Hargreaves R., Burstein R., Borsook D. Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine states. Cereb. Cortex. 2011;21:435–448. doi: 10.1093/cercor/bhq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellessen N., Rottschy C., Eickhoff S.B., Ketteler S.T., Kuhn H., Shah N.J., Schulz J.B., Reske M., Reetz K. Specific and disease stage-dependent episodic memory-related brain activation patterns in Alzheimer's disease: a coordinate-based meta-analysis. Brain Struct. Funct. 2015;220:1555–1571. doi: 10.1007/s00429-014-0744-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl-Jockschat T., Habel U., Michel T.M., Manning J., Laird A.R., Fox P.T., Schneider F., Eickhoff S.B. Brain structure anomalies in autism spectrum disorder–a meta-analysis of VBM studies using anatomic likelihood estimation. Hum. Brain Mapp. 2012;33:1470–1489. doi: 10.1002/hbm.21299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda R., Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain. 2013;154(Suppl. 1):S44–S53. doi: 10.1016/j.pain.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Peyron R., Garcia-Larrea L., Gregoire M.C., Costes N., Convers P., Lavenne F., Mauguiere F., Michel D., Laurent B. Haemodynamic brain responses to acute pain in humans: sensory and attentional networks. Brain. 1999;122(Pt 9):1765–1780. doi: 10.1093/brain/122.9.1765. [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Moskowitz M.A. Pathophysiology of migraine. Annu. Rev. Physiol. 2013;75:365–391. doi: 10.1146/annurev-physiol-030212-183717. [DOI] [PubMed] [Google Scholar]
- Price C.J., Devlin J.T., Moore C.J., Morton C., Laird A.R. Meta-analyses of object naming: effect of baseline. Hum. Brain Mapp. 2005;25:70–82. doi: 10.1002/hbm.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer F., Gantenbein A.R., Marti M., Luechinger R., Kollias S., Sandor P.S. Decrease of gray matter volume in the midbrain is associated with treatment response in medication-overuse headache: possible influence of orbitofrontal cortex. J. Neurosci. 2013;33:15343–15349. doi: 10.1523/JNEUROSCI.3804-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca M.A., Ceccarelli A., Falini A., Colombo B., Tortorella P., Bernasconi L., Comi G., Scotti G., Filippi M. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke. 2006;37:1765–1770. doi: 10.1161/01.STR.0000226589.00599.4d. [DOI] [PubMed] [Google Scholar]
- Rocca M.A., Messina R., Colombo B., Falini A., Comi G., Filippi M. Structural brain MRI abnormalities in pediatric patients with migraine. J. Neurol. 2014;261:350–357. doi: 10.1007/s00415-013-7201-y. [DOI] [PubMed] [Google Scholar]
- Russo A., Tessitore A., Esposito F., Marcuccio L., Giordano A., Conforti R., Truini A., Paccone A., d'Onofrio F., Tedeschi G. Pain processing in patients with migraine: an event-related fMRI study during trigeminal nociceptive stimulation. J. Neurol. 2012;259:1903–1912. doi: 10.1007/s00415-012-6438-1. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T., Leinisch E., Straube A., Kampfe N., Draganski B., Diener H.C., Bogdahn U., May A. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65:1483–1486. doi: 10.1212/01.wnl.0000183067.94400.80. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T., Leinisch E., Ganssbauer S., Draganski B., Bogdahn U., Altmeppen J., May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T., Ganssbauer S., Neuner T., Bogdahn U., May A. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia. 2008;28:1–4. doi: 10.1111/j.1468-2982.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- Schmitz N., Admiraal-Behloul F., Arkink E.B., Kruit M.C., Schoonman G.G., Ferrari M.D., van Buchem M.A. Attack frequency and disease duration as indicators for brain damage in migraine. Headache. 2008;48:1044–1055. doi: 10.1111/j.1526-4610.2008.01133.x. [DOI] [PubMed] [Google Scholar]
- Schmitz N., Arkink E.B., Mulder M., Rubia K., Admiraal-Behloul F., Schoonman G.G., Kruit M.C., Ferrari M.D., van Buchem M.A. Frontal lobe structure and executive function in migraine patients. Neurosci. Lett. 2008;440:92–96. doi: 10.1016/j.neulet.2008.05.033. [DOI] [PubMed] [Google Scholar]
- Schulte L.H., May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain. 2016;139:1987–1993. doi: 10.1093/brain/aww097. [DOI] [PubMed] [Google Scholar]
- Schwedt T.J., Krauss M.J., Frey K., Gereau R.W.T. Episodic and chronic migraineurs are hypersensitive to thermal stimuli between migraine attacks. Cephalalgia. 2011;31:6–12. doi: 10.1177/0333102410365108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedt T.J., Chong C.D., Chiang C.C., Baxter L., Schlaggar B.L., Dodick D.W. Enhanced pain-induced activity of pain-processing regions in a case-control study of episodic migraine. Cephalalgia. 2014;34:947–958. doi: 10.1177/0333102414526069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedt T.J., Chiang C.-C., Chong C.D., Dodick D.W. Functional MRI of migraine. Lancet Neurol. 2015;14:81–91. doi: 10.1016/S1474-4422(14)70193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankewitz A., Aderjan D., Eippert F., May A. Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J. Neurosci. 2011;31:1937–1943. doi: 10.1523/JNEUROSCI.4496-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub P.E., Eickhoff S.B., Laird A.R., Fox M., Wiener M., Fox P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valfre W., Rainero I., Bergui M., Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48:109–117. doi: 10.1111/j.1526-4610.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M., Shibuya K., Salomon J.A., Abdalla S., Aboyans V., Abraham J., Ackerman I., Aggarwal R., Ahn S.Y., Ali M.K., Alvarado M., Anderson H.R., Anderson L.M., Andrews K.G., Atkinson C., Baddour L.M., Bahalim A.N., Barker-Collo S., Barrero L.H., Bartels D.H., Basanez M.G., Baxter A., Bell M.L., Benjamin E.J., Bennett D., Bernabe E., Bhalla K., Bhandari B., Bikbov B., Bin Abdulhak A., Birbeck G., Black J.A., Blencowe H., Blore J.D., Blyth F., Bolliger I., Bonaventure A., Boufous S., Bourne R., Boussinesq M., Braithwaite T., Brayne C., Bridgett L., Brooker S., Brooks P., Brugha T.S., Bryan-Hancock C., Bucello C., Buchbinder R., Buckle G., Budke C.M., Burch M., Burney P., Burstein R., Calabria B., Campbell B., Canter C.E., Carabin H., Carapetis J., Carmona L., Cella C., Charlson F., Chen H., Cheng A.T., Chou D., Chugh S.S., Coffeng L.E., Colan S.D., Colquhoun S., Colson K.E., Condon J., Connor M.D., Cooper L.T., Corriere M., Cortinovis M., de Vaccaro K.C., Couser W., Cowie B.C., Criqui M.H., Cross M., Dabhadkar K.C., Dahiya M., Dahodwala N., Damsere-Derry J., Danaei G., Davis A., De Leo D., Degenhardt L., Dellavalle R., Delossantos A., Denenberg J., Derrett S., Des Jarlais D.C., Dharmaratne S.D., Dherani M., Diaz-Torne C., Dolk H., Dorsey E.R., Driscoll T., Duber H., Ebel B., Edmond K., Elbaz A., Ali S.E., Erskine H., Erwin P.J., Espindola P., Ewoigbokhan S.E., Farzadfar F., Feigin V., Felson D.T., Ferrari A., Ferri C.P., Fevre E.M., Finucane M.M., Flaxman S., Flood L., Foreman K., Forouzanfar M.H., Fowkes F.G., Franklin R., Fransen M., Freeman M.K., Gabbe B.J., Gabriel S.E., Gakidou E., Ganatra H.A., Garcia B., Gaspari F., Gillum R.F., Gmel G., Gosselin R., Grainger R., Groeger J., Guillemin F., Gunnell D., Gupta R., Haagsma J., Hagan H., Halasa Y.A., Hall W., Haring D., Haro J.M., Harrison J.E., Havmoeller R., Hay R.J., Higashi H., Hill C., Hoen B., Hoffman H., Hotez P.J., Hoy D., Huang J.J., Ibeanusi S.E., Jacobsen K.H., James S.L., Jarvis D., Jasrasaria R., Jayaraman S., Johns N., Jonas J.B., Karthikeyan G., Kassebaum N., Kawakami N., Keren A., Khoo J.P., King C.H., Knowlton L.M., Kobusingye O., Koranteng A., Krishnamurthi R., Lalloo R., Laslett L.L., Lathlean T., Leasher J.L., Lee Y.Y., Leigh J., Lim S.S., Limb E., Lin J.K., Lipnick M., Lipshultz S.E., Liu W., Loane M., Ohno S.L., Lyons R., Ma J., Mabweijano J., MacIntyre M.F., Malekzadeh R., Mallinger L., Manivannan S., Marcenes W., March L., Margolis D.J., Marks G.B., Marks R., Matsumori A., Matzopoulos R., Mayosi B.M., McAnulty J.H., McDermott M.M., McGill N., McGrath J., Medina-Mora M.E., Meltzer M., Mensah G.A., Merriman T.R., Meyer A.C., Miglioli V., Miller M., Miller T.R., Mitchell P.B., Mocumbi A.O., Moffitt T.E., Mokdad A.A., Monasta L., Montico M., Moradi-Lakeh M., Moran A., Morawska L., Mori R., Murdoch M.E., Mwaniki M.K., Naidoo K., Nair M.N., Naldi L., Narayan K.M., Nelson P.K., Nelson R.G., Nevitt M.C., Newton C.R., Nolte S., Norman P., Norman R., O'Donnell M., O'Hanlon S., Olives C., Omer S.B., Ortblad K., Osborne R., Ozgediz D., Page A., Pahari B., Pandian J.D., Rivero A.P., Patten S.B., Pearce N., Padilla R.P., Perez-Ruiz F., Perico N., Pesudovs K., Phillips D., Phillips M.R., Pierce K., Pion S., Polanczyk G.V., Polinder S., Pope C.A., 3rd, Popova S., Porrini E., Pourmalek F., Prince M., Pullan R.L., Ramaiah K.D., Ranganathan D., Razavi H., Regan M., Rehm J.T., Rein D.B., Remuzzi G., Richardson K., Rivara F.P., Roberts T., Robinson C., De Leon F.R., Ronfani L., Room R., Rosenfeld L.C., Rushton L., Sacco R.L., Saha S., Sampson U., Sanchez-Riera L., Sanman E., Schwebel D.C., Scott J.G., Segui-Gomez M., Shahraz S., Shepard D.S., Shin H., Shivakoti R., Singh D., Singh G.M., Singh J.A., Singleton J., Sleet D.A., Sliwa K., Smith E., Smith J.L., Stapelberg N.J., Steer A., Steiner T., Stolk W.A., Stovner L.J., Sudfeld C., Syed S., Tamburlini G., Tavakkoli M., Taylor H.R., Taylor J.A., Taylor W.J., Thomas B., Thomson W.M., Thurston G.D., Tleyjeh I.M., Tonelli M., Towbin J.A., Truelsen T., Tsilimbaris M.K., Ubeda C., Undurraga E.A., van der Werf M.J., van Os J., Vavilala M.S., Venketasubramanian N., Wang M., Wang W., Watt K., Weatherall D.J., Weinstock M.A., Weintraub R., Weisskopf M.G., Weissman M.M., White R.A., Whiteford H., Wiersma S.T., Wilkinson J.D., Williams H.C., Williams S.R., Witt E., Wolfe F., Woolf A.D., Wulf S., Yeh P.H., Zaidi A.K., Zheng Z.J., Zonies D., Lopez A.D., Murray C.J., AlMazroa M.A., Memish Z.A. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman-Fogel I., Sprecher E., Granovsky Y., Yarnitsky D. Repeated noxious stimulation of the skin enhances cutaneous pain perception of migraine patients in-between attacks: clinical evidence for continuous sub-threshold increase in membrane excitability of central trigeminovascular neurons. Pain. 2003;104:693–700. doi: 10.1016/S0304-3959(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Welch K.M., Cao Y., Aurora S., Wiggins G., Vikingstad E.M. MRI of the occipital cortex, red nucleus, and substantia nigra during visual aura of migraine. Neurology. 1998;51:1465–1469. doi: 10.1212/wnl.51.5.1465. [DOI] [PubMed] [Google Scholar]
- Wenting Hu J.G., Chen N., Guo J., He L. A meta-analysis of voxel-based morphometric studies on migraine. Int. J. Clin. Exp. Med. 2015;8:4311–4319. [PMC free article] [PubMed] [Google Scholar]
- Yu S., Liu R., Zhao G., Yang X., Qiao X., Feng J., Fang Y., Cao X., He M., Steiner T. The prevalence and burden of primary headaches in China: a population-based door-to-door survey. Headache. 2012;52:582–591. doi: 10.1111/j.1526-4610.2011.02061.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Structural neuroimaging data in NIFTI format.
Structural neuroimaging data related to headache durations in NIFTI format.
Functional neuroimaging data in NIFTI format.
Structural interactive plots.
Structural interactive plots related to headache durations.
Functional interactive plots.