Abstract
Transcutaneous vagus nerve stimulation (tVNS), a non-invasive method of brain stimulation through the auricular branch of the vagus nerve, has shown promising results in treating major depressive disorder (MDD) in several pilot studies. However, the neural mechanism by which the effect on depression might be achieved has not been fully investigated, with only a few neuroimaging studies demonstrating tVNS-induced changes in the brains of healthy volunteers. Identifying specific neural pathways, which are influenced by tVNS compared with sham in depressed individuals, as well as determining neurobiomarkers of tVNS treatment success are needed to advance the application of tVNS for MDD. In order to address these questions, we measured fMRI brain activity of thirty-eight depressed patients assigned to undergo tVNS (n = 17) or sham (n = 21) treatment for 4 weeks, during the first stimulation session. The results showed significant fMRI signal increases in the left anterior insula, revealed by a direct comparison of tVNS and sham stimulation. Importantly, the insula activation level during the first stimulation session in the tVNS group was significantly associated with the clinical improvement at the end of the four-week treatment, as indicated by the Hamilton Depression Rating Scale (HAM-D) score. Our findings suggest that anterior insula fMRI activity could serve as a potential cortical biomarker and an early predictor of tVNS longitudinal treatment success.
Keywords: Transcutaneous vagus nerve stimulation tVNS, Major depressive disorder (MDD), Functional magnetic resonance imaging (fMRI)
Highlights
-
•
Transcutaneous vagus nerve stimulation (tVNS) was used to treat depression (75).
-
•
Significant fMRI activation in the left anterior insula was observed during tVNS (81).
-
•
Insula activation during the first treatment was associated with clinical improvement (85).
1. Introduction
Characterized by persistent sadness, pessimism, social withdrawal, low self-confidence, and compromised cognitive abilities, major depressive disorder (MDD) affects a large proportion of the population by significantly impairing their occupational, social and academic functioning (de Graaf et al., 2010, Johnson et al., 1992, Lehtinen and Joukamaa, 1994). Changes in affective/emotional and cognitive processing accompanying MDD have been related to abnormal functioning of the insular, frontal (dlPFC, vlPFC, and mPFC), limbic (ACC, amygdala), hippocampal and thalamo-striatal (thalamus, globus pallidus, putamen, and caudate) brain regions (Davidson et al., 2002, Hastings et al., 2004, Pizzagalli, 2011, Sacher et al., 2012, Singh and Gotlib, 2014). Furthermore, several neural networks, such as the default mode network (DMN), cognitive control network, as well as emotional regulation and saliency networks have been implicated in MDD neuropathology (Greicius et al., 2007, Sheline et al., 2009, Sheline et al., 2010, Wagner et al., 2015). In addition to documenting illness biomarkers, recent advances in neuroimaging and brain stimulation methods have allowed for the development of new anti-depression treatments to complement traditional behavioral therapies and pharmacological approaches, further calling for the identification of reliable treatment biomarkers, for a review see (Lener and Iosifescu, 2015).
More severe and treatment-resistant cases of MDD have been recently treated with vagus nerve stimulation (VNS), an invasive surgical procedure that involves implanting a stimulation device (Chae et al., 2003, Howland, 2014, Nahas et al., 2006, Nemeroff et al., 2006, Rush and Siefert, 2009). Vagus nerve has been shown to have direct and indirect connections to the cortical-limbic-thalamic-striatal neural circuits relevant for depression, specifically influencing the dorsolateral prefrontal cortex, anterior cingulate, insula, and precuneus (Conway et al., 2006, Kosel et al., 2011, Ruffoli et al., 2011, Rush et al., 2005). However, VNS being invasive and expensive to implement (Sperling et al., 2009), transcutaneous VNS (tVNS) has been suggested as an alternative (Ellrich, 2011).
The method of tVNS targets the cutaneous receptive field of the auricular branch of the vagus nerve (ABVN) by applying an electrical stimulus to the left cymba conchae (Peuker and Filler, 2002). As a non-invasive stimulation method tVNS has shown promising results for treating MDD (Hein et al., 2013, Rong et al., 2016), however, optimizing the protocols could benefit from a clearer understanding of what specific changes in the brain contribute to the treatment success of tVNS. Neuroimaging could be instrumental for elucidating the mechanism of tVNS. Yet still little is known about the neural activations during tVNS in general, let alone specifically in depression. Several recent studies in healthy subjects showed that applying tVNS specifically affects brain stem structures (the ipsilateral nucleus tractus solitarii, bilateral spinal trigeminal nucleus, dorsal raphe, locus coeruleus), as well as cortical areas, including the insula, hippocampus, thalamus, nucleus accumbens, amygdala and paracentral gyrus (Dietrich et al., 2008, Fang et al., 2014, Frangos et al., 2015, Kraus et al., 2007, Kraus et al., 2013b, Liu et al., 2016). The most reliably identified brain region in all studies has been the insula, although activation in the thalamus and deactivation in the hippocampus have also been consistently reported. The regions identified in these studies are potentially relevant for depression. However, these previous studies have not directly investigated the relationship between depression and tVNS. In a previous study, we found that longitudinal tVNS can alter resting state functional connectivity in the insula and the DMN (Fang et al., 2015). The brain response to tVNS during the treatment itself (compared with sham) and its association with the treatment-induced improvement, however, remain unknown (Fang et al., 2014).
In order to provide the crucial link showing the association between the activation during tVNS stimulation and the clinical outcome, in this study we set out to 1) reveal the patterns of fMRI signal changes during tVNS and sham treatment directly comparing the two to detect the specific effect of tVNS and understand the basis for the therapeutic effect of sham tVNS; 2) explore neuroimaging biomarkers that might predict the success of tVNS longitudinal treatment already during the first treatment session. We hypothesized that increased activation in the insula and decreased activation in the DMN, as suggested by resting stage changes in our previous study based on the same sample would show differential activation during tVNS compared with sham and predict longitudinal treatment success.
2. Methods
2.1. Subjects
Forty-nine subjects MDD patients naïve to tVNS were enrolled in this single blinded clinical trial from three participating hospitals. The inclusion criteria were 1) meeting ICD-10 diagnosis (2 typical and 2–3 other core symptoms); 2) depressive symptoms exhibited for at least 2 months but < 2 years; 3) aged between 16 and 70; 4) anti-depressive and other psychiatric medication free for 2 weeks before the intervention; 5) education above junior high school and ability to understand the scales. Subjects with very severe depression and suicidal thoughts, drug addiction, or other severe health problems, such as heart disease, kidney failure were excluded from the study. All subjects provided informed consent before the start of the study. Only subjects that underwent fMRI scanning during the first treatment session were included in the current analysis, resulting in the total of 38 subjects. Table 1 shows the demographic characteristics of the subject cohort.
Table 1.
Demographic information. HAM-D, Hamilton Depression Scale; SDS, Self-Rating Depression Scale; tVNS, transcutaneous vagus nerve stimulation.
| Variable | tVNS | Sham | p-value |
|---|---|---|---|
| N (male) | 17 (5) | 21 (7) | 0.79 (Chi-square test, 2-tailed) |
| Age mean (SE) | 40.35 (3.2) | 40.38 (2.4) | 0.99 (2-sample t-test, 2-tailed) |
| SDS (pre) score mean (SE) | 70.35 (2.0) | 67.57(1.8) | 0.31 (2-sample t-test, 2-tailed) |
| HAM-D (pre) score mean (SE) | 30.47 (1.0) | 28.52 (0.9) | 0.18 (2-sample t-test, 2-tailed) |
2.2. Intervention
All treatments were applied with an ear vagus nerve stimulator developed through the cooperation of the Institute of Acupuncture and Moxibustion, China Academy to Chinese Medicine Science (Beijing, China) and Suzhou Medical Appliance Factory (Jiangsu Province, China), featuring custom-designed ear clips (electrodes) (Huang et al., 2014, Rong et al., 2012, Rong et al., 2014).
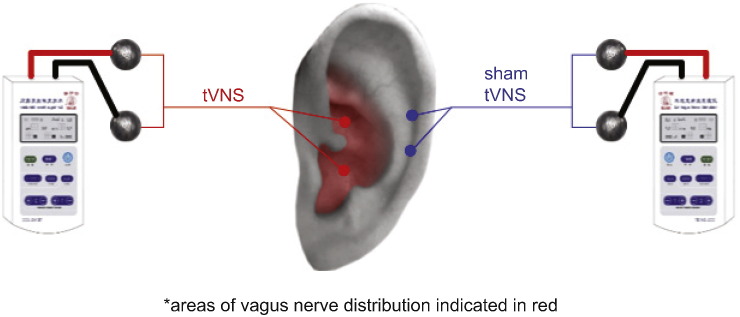
Participants were trained to apply tVNS or sham and subsequently self-administered all treatments at home. Patients were instructed to sit or lie on their side, disinfect the stimulation points and attach ear clips on the stimulation sites. Stimulation parameters were as follows: a 20 Hz continuous sinusoidal wave (wave width of 0.2 ms); stimulation intensity increased gradually (starting from 0) to the highest point that the patients could tolerate without it being painful (typically between 4 and 6 mA); stimulation lasted 30 min and was applied twice a day, at least 5 days a week for 4 weeks. Patients were also instructed to daily complete a patient diary booklet to describe any side effects of treatment. All procedures for the tVNS and sham treatment groups were identical except the location of the stimulation. The points for the tVNS were located in the auricular concha area known to have vagus nerve branch distributions; the stimulation points for the sham treatment were located at the superior scapha (outer ear margin midpoint) devoid of vagus nerve distribution (Peuker and Filler, 2002) (Fig. 1). To avoid the noise evoked by the electrical current of tVNS, the connecting line between the stimulator and the ear was electromagnetically shielded. A round tin electrode, about 5 mm in diameter, was installed at the distant end of the line. The electrodes were fixed by tape during the fMRI scan (Fig. 1).
Fig. 1.
Locations for the tVNS and sham stimulation.
2.3. Behavioral data analysis
All dependent variables were measured at week 0 and week 4. The primary measurement was the 24-item Hamilton Depression Rating Scale (HAM-D), secondary measurements included the Hamilton Anxiety Rating Scale (HAM-A), Self-Rating Anxiety Scale (SAS), and Self-Rating Depression Scale (SDS). All behavioral results of the trial were analyzed and reported elsewhere (Fang et al., 2015), we here only focus on the HAM-D score relationship to the brain activation during the first fMRI session. To assess the effect tVNS and sham on MDD in the sample used here, we performed a repeated measures ANOVA with Group (tVNS vs. sham) and Treatment time point (pre vs. post) on subjects' HAM-D scores, followed by planned comparisons for pre- and post- results within and between each group.
2.4. Imaging data acquisition and analysis
Brain imaging was performed with a 1.5 Tesla GE Signa MRI system (GE Healthcare, Buckinghamshire, United Kingdom) equipped with the standard two-channel birdcage head coil. T1-weighted high-resolution structural images were acquired with the three-dimensional fast spoiled gradient-echo sequence (matrix 192 × 256, field of view [FOV] 200 mm, flip angle 15°, slice thickness 1.4 mm). T2-weighted functional images encompassing the whole brain were acquired with the gradient echo echo-planar imaging sequence (echo time 30 ms, repetition time 2500 ms, matrix 64 × 64, FOV 240 mm, flip angle 90°, slice thickness 3.0 mm, gap 0.5 mm, 41 slices, paralleled by anterior commissure-posterior commissure line). Task functional scans consisted of two 6-min tVNS or sham stimulation sessions performed in the scanner (Fig. 2). Image collection was preceded by four dummy scans to allow for equilibration of the MRI signal. Two 6-min resting state fMRI scans were applied while the subjects were required to keep their eyes closed.
Fig. 2.
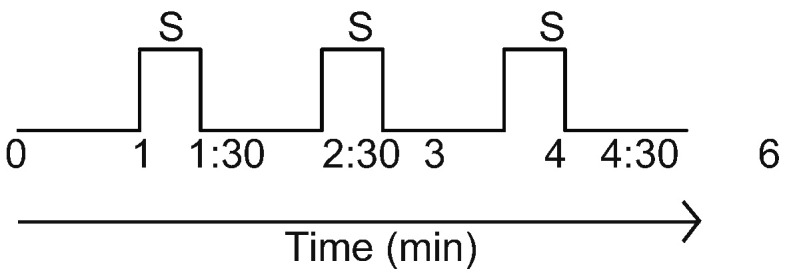
Stimulation protocol. Each session took 6 min to complete. Two stimulation sessions were recorded per subject. S: stimulation.
Preprocessing and statistical analyses were performed using SPM12 software (Wellcome Department of Cognitive Neurology, London, UK). Preprocessing included realignment, normalization to MNI stereotactic space, and spatial smoothing with a 6 mm Gaussian kernel. For each subject, a GLM (general linear model) design matrix was calculated, including all stimulation events (2 sessions of 3 stimulations lasting 30-second each). In addition, head motion analysis was performed using the artifact detection toolbox (ART) (https://www.nitrc.org/projects/artifact_detect/). Time points were marked as outliers if global signal exceeded three standard deviations of the mean and if movement exceeded 0.5 mm of scan-to-scan deviation. As head motion can differ from subject to subject (Power et al., 2012), we included ART-identified movement artifacts as covariates in first-level analyses.
After computing the contrast between stimulation ‘on’ and implicit baseline for each subject, we performed one-sample t-tests to identify activations and deactivations during stimulation vs. baseline separately for tVNS and sham groups. Finally, we directly compared stimulation-induced activations in the two groups by using a 2-sample t-test. A threshold of voxel-wise p < 0.001, cluster-corrected at p < 0.05 (FWE) was used for the one-sample t-tests. A threshold of voxel-wise p < 0.05 (FWE) after small volume correction (svc) was set for the group comparison with the initial thresholding of p < 0.005. Cortical brain regions previously implicated in tVNS and depression were used as independent regions of interest (ROIs) for svc. These included the bilateral anterior insula, precuneus, thalamus and hippocampus (Kraus et al., 2013a). All regions of interest were anatomically defined using the Harvard-Oxford Atlas-based parcellation previously reported in (Hashmi et al., 2014). All coordinates were reported in MNI space, as used in SPM.
In order to check whether there was an association between insular activity during the first tVNS session and HAM-D improvement following the real tVNS treatment, we have performed a partial correlation between the post-treatment HAM-D scores (adjusting for age, gender and pre-treatment HAM-D scores) and the signal extracted from the insula (a sphere with a 6 mm radius around the peak coordinate of the small volume-corrected contrast between tVNS and sham).
3. Results
3.1. Sample characteristics
Data from 38 subjects were included in the current analysis (tVNS N = 17; sham N = 21). Note that this was part of the same tVNS research study reported in (Fang et al., 2015). The current sample is, however, slightly different, as not all tVNS subjects from the previously reported cohort underwent fMRI scanning during the first session and more sham subjects had fMRI data during the stimulation available.
3.2. Behavioral data results
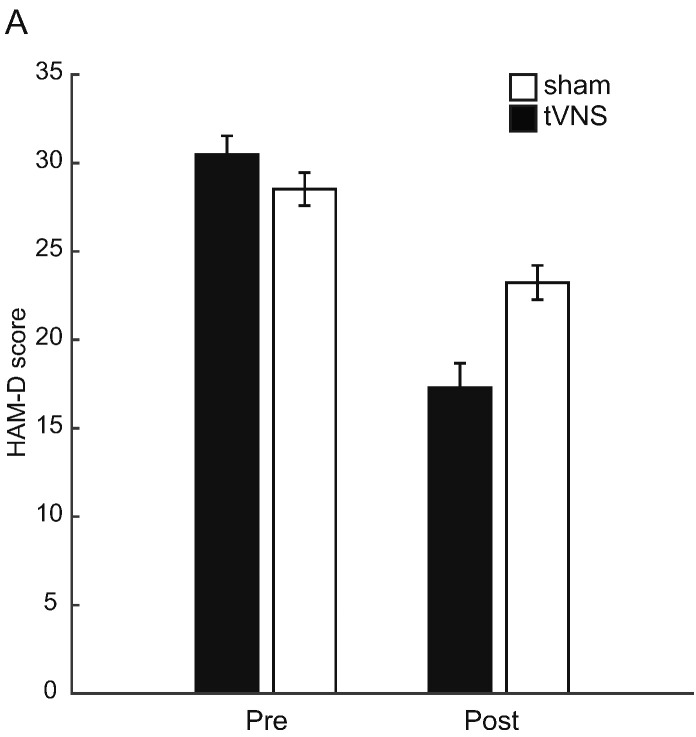
The results of the repeated measures ANOVA on HAM-D scores with the factors Group and Treatment time point showed a significant main effect of Treatment time point (F(1,36) = 78.02, p < 0.01), suggesting that in both treatment groups there was a decrease in HAM-D scores (tVNS: t(16) = 8.06, p < 0.01; sham: t(20) = 4.29, p < 0.01)) (Fig. 3A). Importantly, there was also a significant interaction between Group and Treatment time point (F(1.36) = 15.45, p < 0.01), suggesting that there was greater improvement in the tVNS group, compared with the sham group. Planned comparisons between the groups showed that there was a significant difference between the groups post-treatment (t(36) = 3.44, p < 0.01) but not pre-treatment (t(36) = 1.37, p = 0.18). The decrease in HAM-D score following treatment constituted 42.45% in the tVNS and 17.15% in the sham group.
Fig. 3.
Bar graph demonstrating HAM-D scores pre- and post-treatment by group (tVNS vs. sham).
3.3. Imaging data results
First we investigated patterns of activation and deactivation during tVNS and sham treatment; they are reported in Table 2. In the tVNS group, the results showed increased activation in the left insula and bilateral cerebellum and a deactivation in the DMN (MPFC and precuneus). In the sham group, there was a significant activation in the right inferior frontal gyrus, as well as a deactivation in the DMN (MPFC, posterior cingulate and precuneus) and the left hippocampus.
Table 2.
One-sample t-tests, whole-brain p < 0.001, k = 20, cluster-corrected FWE-corr. p < 0.05.
| Brain region | Hemisphere | MNI coordinates |
k | T | Z | Peak p(unc) | Cluster p(FWE-corr) | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| tVNS treatment (one-samplet-test) | |||||||||
| Activation | |||||||||
| Cerebelum Crus1 | L | − 27 | − 73 | − 34 | 96 | 9.11 | 5.33 | < 0.001 | < 0.001 |
| Cerebelum Crus1 | R | 36 | − 67 | − 28 | 132 | 7.97 | 5 | < 0.001 | < 0.001 |
| Operculum/Insula/Precentral gyrus | L | − 57 | − 1 | 8 | 46 | 6.4 | 4.45 | < 0.001 | 0.003 |
| Deactivation | |||||||||
| Medial Frontal Gyrus | R | 12 | 44 | − 10 | 39 | 6.33 | 4.42 | < 0.001 | 0.008 |
| Precuneus | R | 9 | − 58 | 44 | 38 | 5 | 3.83 | < 0.001 | 0.009 |
| Sham treatment (one-samplet-test) | |||||||||
| Activation | |||||||||
| Middle Frontal/Inferior Frontal Gyrus | R | 51 | 23 | 26 | 68 | 7.98 | 5.29 | < 0.001 | 0.001 |
| Inferior Frontal Gyrus | R | 54 | 11 | 5 | 37 | 5.25 | 4.12 | < 0.001 | 0.023 |
| Deactivation | |||||||||
| Posterior Cingulate/Calcarine | R | 15 | − 55 | 11 | 48 | 7.16 | 4.99 | < 0.001 | 0.006 |
| Medial Frontal Gyrus | R | 3 | 44 | − 10 | 140 | 5.97 | 4.47 | < 0.001 | < 0.001 |
| Precuneus | R | 12 | − 52 | 59 | 55 | 6.12 | 4.54 | < 0.001 | 0.003 |
| Hippocampus | L | − 12 | − 7 | − 22 | 31 | 5.03 | 4 | < 0.001 | 0.048 |
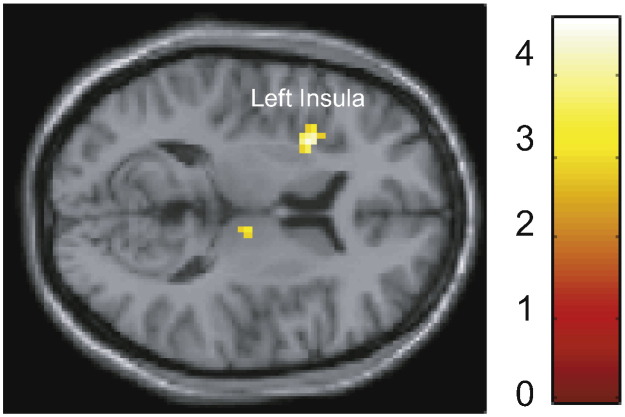
Direct comparison of the two stimulation groups revealed more activation in the tVNS, compared with the sham group, in the left anterior insula (peak MNI coordinate [− 30, 8, 8]), small-volume corrected at FWE p < 0.05 (Fig. 4). No significant difference was observed for the opposite contrast.
Fig. 4.
Results of a 2-sample t-test, tVNS > sham, whole-brain p = 0.005 (SVC for multiple comparisons).
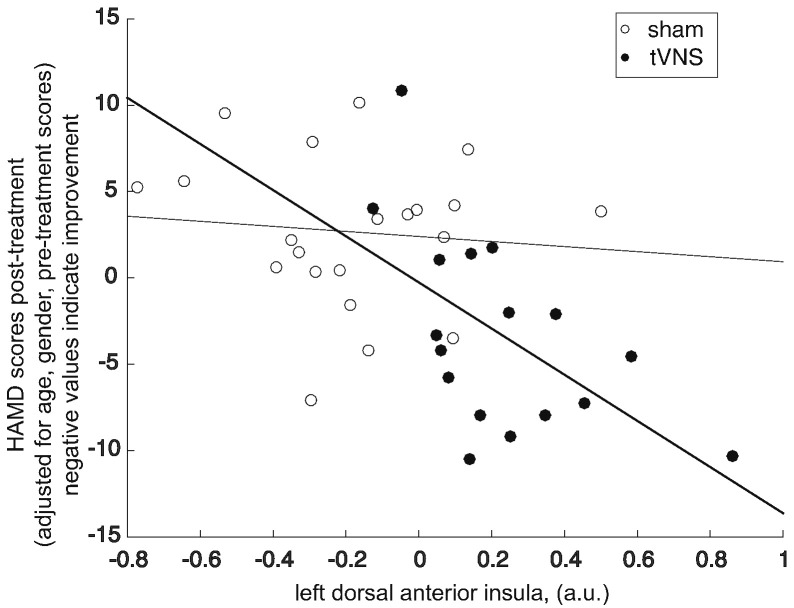
We further investigated the association between the insula activity during first stimulation session and longitudinal treatment outcome (post-treatment HAM-D scores adjusted for age, gender and pre-treatment HAM-D scores) in real and sham tVNS groups separately. The partial correlation between the insula (6-mm radius sphere at MNI [− 30, 8, 8]) and treatment outcome (HAM-D score after 4 weeks) was significant in the real tVNS group (r(17) = − 0.65, p = 0.01), suggesting that the more activated the insula was during the first stimulation treatment the lower HAM-D score at the end of 4 weeks of treatment was observed (Fig. 5). There was no significant association in the sham group (p = 0.6), suggesting that the activation of the insula does not generally reflect the state of depression but is associated with treatment efficacy in the tVNS group.
Fig. 5.
Scatter plot showing a significant negative correlation between HAM-D scores at 4 weeks and activation in the left dorsal anterior insula during the first treatment session (values extracted from a sphere with 6 mm radius around the peak coordinate (− 30, 8, 8)) in the tVNS group, suggesting that greater activation of the insula during treatment is associated with better treatment outcome. The lines represent least squares linear fits (darker line for the tVNS group correlation).
No association was observed in the sham group.
4. Discussion
The goal of the study was twofold: 1) to reveal patterns of fMRI activation during tVNS and sham treatment in depressed patients and directly compare them to detect the specific effect of tVNS on MDD-related neuropathology; 2) to identify neuroimaging biomarkers that could predict the success of tVNS longitudinal treatment already during the first treatment session. The results indicate that the activation level in the left anterior insula might be enhanced by tVNS compared with sham. Specifically for the real tVNS insula activation was associated with the longitudinal treatment outcome and could in principle be used to predict the results of a 4-week treatment already at the first session. Other brain regions, including the cerebellum appear to be modulated more by tVNS than sham, while the overall HAM-D score improvement observed in both tVNS and sham can be related to the modulation of parts of the DMN (MFPC, precuneus) and hippocampus previously shown to influence depression symptoms.
4.1. Left anterior insula is activated more by tVNS than sham and predicts treatment outcome
Previous neuroimaging studies investigating the effect of tVNS in healthy subjects have consistently reported an increase in the activation of the anterior insula during the stimulation (Frangos et al., 2015, Kraus et al., 2007, Kraus et al., 2013a). In fact in the study by Kraus et al. (Kraus et al., 2013a) insula was the only region that was positively activated regardless of whether anterior or posterior part of the left auditory canal was stimulated, while in most other cortical areas changes were in the opposite direction between the stimulation sites.
Being a hub for emotional and saliency processing, insula is a key region in MDD neuropathology previously shown to be involved in both clinical (Sprengelmeyer et al., 2011) and subclinical depression (Hwang et al., 2015). Previous studies found abnormal insula task activation (Groenewold et al., 2013) and decreased regional homogeneity in resting state functional MRI in the right insula in participants with MDD (Liu et al., 2010). A number of studies showed MDD-related reduction in insular volume (Sprengelmeyer et al., 2011), specifically in the left anterior insula (Cohen et al., 2013, Hatton et al., 2012, Soriano-Mas et al., 2011, Takahashi et al., 2010). Crucially these studies demonstrated correlations between the left anterior insula volume and depressive and psychotic symptoms. Furthermore, anterior insula, albeit right, has been previously proposed as a biomarker for the treatment selection for MDD. Specifically, fluorodeoxyglucose (FDG) positron emission tomography (PET) signature in the insula was found to discriminate between cognitive behavioral therapy and antidepressants responders (McGrath et al., 2013).
Our behavioral results show that tVNS significantly decreased HAM-D scores compared to the pre-treatment level and also compared with the sham treatment results. In addition, fMRI results further confirm that the left anterior insula was significantly activated during the tVNS treatment but not sham. Together these findings suggest that the effect of tVNS treatment might be primarily achieved through the modulation of the left anterior insula activity. This result is further corroborated by our previous resting state connectivity study showing changes in insula-DMN connectivity for pre-post tVNS compared to sham (Fang et al., 2015). Crucially, this new finding of the association between the insula activation during tVNS and depression improvement was obtained during the very first stimulation session, suggesting that it might be useful for an early prediction of longitudinal tVNS treatment outcome (Lener and Iosifescu, 2015, McGrath et al., 2013), e.g., if the insula activation is observed patients are likely to benefit from tVNS treatment, if the insula is not active during the stimulation, the treatment is not likely to be effective. While the results of this task fMRI and our previous resting state fMRI studies agree well, they are part of the same trial involving the same cohort (although note that the number of subjects in the two studies is not exactly the same, since our previous study included only subjects with resting state fMRI data both at baseline and post-treatment). Further replication in independent samples is necessary to confirm the role of insula in tVNS effect on depression and the association between the brain activation during stimulation and long-term changes in the resting state.
Although not revealed by the direct comparison between sham and tVNS, bilateral cerebellum was significantly activated during tVNS but not during sham, as suggested by the one-sample t-tests (Table 2). The cerebellum (Konarski et al., 2005) has been shown to be dysregulated in MDD. Often the cerebellum was implicated in MDD together with the insula, e.g. regional homogeneity decreases in the insula and cerebellum (Liu et al., 2010). In addition, cerebellar modulation has been reported for VNS, e.g., (Henry et al., 2004). The role of the cerebellum in tVNS effect for depression should be further explored.
We also were not able to observe any significant differences in the activation of the nucleus of the solitary tract (NTS) that is expected in verum tVNS (Frangos et al., 2015, Kraus et al., 2013a). Most previous studies reporting this activation optimized their fMRI acquisition sequences (e.g., focusing on the brain stem), pre-processing (e.g., omitting the smoothing step), or statistical analysis (e.g., using ROI analysis vs. whole-brain analysis) to have the power and sensitivity to observe the activity in this region. We here focused on the cortical biomarkers of tVNS using standard fMRI acquisition, pre-processing and statistical analysis, that could facilitate assessing treatment success in a clinical setting. Insula activation similar to our report here has also been found in a previous study that showed differential NTS activation (Kraus et al., 2013a), suggesting that there might be co-activation of the two during tVNS. Insula and NTS are known to have direct and indirect anatomical connections (Hayama and Ogawa, 2001, King, 2007, Shipley, 1982), which could be particularly important for the application of tVNS in depression. While the absence of observed NTS activation may present a major limitation in our study, it could be related to a general difficulty of observing NTS activity with conventional fMRI, calling for a more robust biomarker for manipulation success.
4.2. Sham tVNS brain activation and clinical outcome
While a significant difference in both behavioral and imaging results has been observed between tVNS and sham treatment, there was a significant therapeutic effect of sham tVNS, as well as significant activation and deactivation of MDD-related brain regions during the stimulation. Specifically, 17% improvement from the baseline (‘post’ minus ‘pre’ divided by ‘pre’) was observed in the sham condition (compared to 42% in the tVNS condition). The right inferior frontal gyrus has not been related to either depression or tVNS, so its increased activation during sham could be epiphenomenal sensory activation. However, during the sham stimulation, deactivation in the key regions of the DMN were observed – MPFC, precuneus, posterior cingulate, similar to the deactivations seen during tVNS (Table 2). The involvement of the DMN in MDD and its stimulation by the tVNS are discussed in more detail in (Fang et al., 2015). In addition, a deactivation in the hippocampus, previously reported in tVNS imaging studies (Dietrich et al., 2008, Frangos et al., 2015, Kraus et al., 2007), appeared in the sham condition. This suggests that there might be some effect of sham stimulation in the regions relevant for the clinical outcome. It should be noted that previous studies used a different sham stimulation site, namely sham stimulation electrodes were placed in the center of the left earlobe (Frangos et al., 2015, Kraus et al., 2007, Kraus et al., 2013a), known to be free of vagal innervation (Peuker and Filler, 2002). We here used the outer ear margin midpoint for stimulation that has also been shown to be 100% innervated by the great auricular nerve, like the lobule of auricle (Peuker and Filler, 2002). The main reason we chose this location was that it is predominantly controlled by the minor occipitalis nerve of the cervical plexus, and the tissue layer at this location is similar to the location where the tVNS was applied.
One explanation for the behavioral improvement following the sham treatment could be a placebo response. The large placebo effect observed in our study is in line with findings from a previous clinical trial testing the efficacy of antidepressants (Kirsch et al., 2008). A strong placebo expectation would be associated with both tVNS and sham to the same extent and potentially reflected in the activation of the same brain regions for tVNS and sham. Consistent with that both tVNS and sham conditions produced a decrease in DMN regions. The insula activation was observed only in tVNS, potentially corresponding to the specific treatment effect without the placebo confound. At the same time the brain regions that showed deactivation in the sham condition, such as the posterior cingulate, MPFC, precuneus and hippocampus are not key regions associated with a typical placebo response (Freeman et al., 2015, Tracey, 2010). It is therefore possible that the significant decrease in the DMN and the hippocampus induced by the sham stimulation (as well as tVNS) was more specific to depression.
At the same time, qualitative difference between the depressive symptoms improvement between tVNS and sham could be observed, suggesting that despite some positive effect of the sham stimulation, it is significantly smaller than the therapeutic effect of proper tVNS. In addition, post-treatment HAM-D scores differed between the groups in terms of severity levels (Weeks et al., 2013). At the end of the treatment depression severity in the tVNS group was reduced to the ‘mild’ level (with the score 17 ± 1) and in the sham group it remained at the ‘moderate’ level (with the score 23 ± 1). This qualitative comparison suggests that the distinction between tVNS and sham treatment in our study is clinically meaningful and goes beyond numerical significance, further emphasizing that key MDD neural network was only affected by tVNS but not sham.
5. Summary
This study is the first to investigate the neural effects of tVNS during the stimulation in a sample of depressed patients. We report that tVNS is successful for depression treatment and specifically targets the left anterior insula compared to sham. The activation of the anterior insula by the tVNS stimulation during the first treatment session predicts the outcome of the longitudinal tVNS treatment. Based on the results of our study, we recommend the use of fMRI during tVNS to monitor insula activity during treatment to ensure its efficacy and appropriateness as MDD treatment. Future studies need to further investigate the use of anterior insula as a cortical biomarker for tVNS and adapt the paradigm to allow for the prediction of success at a single subject level.
Author contribution
Design: B Zhu, PJ Rong, Jl Fang, and J Kong; patient enrollment and treatment: Jun Liu, YY Fan, XL Wang, YT Yu, XY Li; fMRI data collection: Y Hong, YY Fan, YY Ma, CH Xu, XL Wang; experiment Quality Monitoring & Controlling: JL Fang, CH Liu; data processing: N Egorova, J Kong, J Liu; manuscript preparation: N Egorova, J Kong, JL Fang, PJ Rong.
Conflict of interest
All authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgements
This scientific work was supported by the Chinese National Natural Science Foundation to Jiliang Fang (No. 30870668, 81273674), to Xiaoling Wang (No. 81303056), to Peijing Rong (No. 30973798, 81473780), to Chunhong Liu (No. 81471389); National Basic Research Program of China to Peijing Rong (973 Program, No. 2012CB518503), grant of technology development research from the Ministry of Science and Technology (2011EG152313), the National Twelfth Five-Year Plan of the National Science and Technology Support Program of China (2012BAF14B10) and the Natural Science Foundation of Beijing China to Peijing Rong (No. 7111007). Jian Kong is supported by R01AT006364 (NIH/NCCIH), R01 AT008563 (NIH/NCCIH); R21AT008707 (NIH/NCCIH), R61 AT009310 and P01 AT006663 (NIH/NCCIH). We would like to thank the reviewers for thoughtful comments and suggestions.
Contributor Information
Peijing Rong, Email: rongpj@cintcm.ac.cn, drrongpj@163.com.
Jian Kong, Email: kongj@nmr.mgh.harvard.edu.
References
- Chae J.-H., Nahas Z., Lomarev M., Denslow S., Lorberbaum J.P., Bohning D.E., George M.S. A review of functional neuroimaging studies of vagus nerve stimulation (VNS) J. Psychiatr. Res. 2003;37:443–455. doi: 10.1016/s0022-3956(03)00074-8. [DOI] [PubMed] [Google Scholar]
- Cohen J.D., Nichols T., Keller J., Gomez R.G., Schatzberg A.F., Reiss A.L. Insular cortex abnormalities in psychotic major depression: relationship to gender and psychotic symptoms. Neurosci. Res. 2013;75:331–339. doi: 10.1016/j.neures.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway C.R., Sheline Y.I., Chibnall J.T., George M.S., Fletcher J.W., Mintun M.A. Cerebral blood flow changes during vagus nerve stimulation for depression. Psychiatry Res. 2006;146:179–184. doi: 10.1016/j.pscychresns.2005.12.007. (S0925-4927(05)00207-6 [pii]) [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Pizzagalli D., Nitschke J.B., Putnam K. Depression: perspectives from affective neuroscience. Annu. Rev. Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. (pii) [DOI] [PubMed] [Google Scholar]
- de Graaf L.E., Huibers M.J., Cuijpers P., Arntz A. Minor and major depression in the general population: does dysfunctional thinking play a role? Compr. Psychiatry. 2010;51:266–274. doi: 10.1016/j.comppsych.2009.08.006. (S0010-440X(09)00092-3 [pii]) [DOI] [PubMed] [Google Scholar]
- Dietrich S., Smith J., Scherzinger C., Hofmann-Preiß K., Freitag T., Eisenkolb A., Ringler R., Hofmann-Preiss K., Freitag T., Eisenkolb A., Ringler R. A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional MRI. Biomed. Tech. 2008;53:104–111. doi: 10.1515/BMT.2008.022. [DOI] [PubMed] [Google Scholar]
- Ellrich J. Transcutaneous vagus nerve stimulation. Eur. Neurol. Rev. 2011;6:254–256. [Google Scholar]
- Fang J., Hong Y., Fan Y., Liu J., Ma Y., Xiu C., Wang H., Ma Y., Wang X., Liu Z., Zhu B., Kong J., Rong P. Brain response to Transcutaneous vagus nerve stimulation, an fMRI study. J. Magn. Reson. Imaging. 2014;5:416–422. [Google Scholar]
- Fang J., Rong P., Hong Y., Fan Y., Liu J., Wang H., Zhang G., Chen X., Shi S., Wang L., Liu R., Hwang J., Li Z., Tao J., Wang Y., Zhu B., Kong J. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol. Psychiatry. 2015:1–8. doi: 10.1016/j.biopsych.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangos E., Ellrich J., Komisaruk B.R. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015;8:624–636. doi: 10.1016/j.brs.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman S., Yu R., Egorova N., Chen X., Kirsch I., Claggett B., Kaptchuk T.J., Gollub R.L., Kong J. Distinct neural representations of placebo and nocebo effects. NeuroImage. 2015;112:197–207. doi: 10.1016/j.neuroimage.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Flores B.H., Menon V., Glover G.H., Solvason H.B., Kenna H., Reiss A.L., Schatzberg A.F. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. (S0006-3223(06)01193-0 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewold N.a., Opmeer E.M., de Jonge P., Aleman A., Costafreda S.G. Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 2013;37:152–163. doi: 10.1016/j.neubiorev.2012.11.015. [DOI] [PubMed] [Google Scholar]
- Hashmi J.A., Kong J., Spaeth R., Khan S., Kaptchuk T.J., Gollub R.L. Functional network architecture predicts psychologically mediated analgesia related to treatment in chronic knee pain patients. J. Neurosci. 2014;34:3924–3936. doi: 10.1523/JNEUROSCI.3155-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings R.S., Parsey R.V., Oquendo M.A., Arango V., Mann J.J. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29:952–959. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- Hatton S.N., Lagopoulos J., Hermens D.F., Naismith S.L., Bennett M.R., Hickie I.B. Correlating anterior insula gray matter volume changes in young people with clinical and neurocognitive outcomes: an MRI study. BMC Psychiatry. 2012;12:45. doi: 10.1186/1471-244X-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama T., Ogawa H. Two loci of the insular cortex project to the taste zone of the nucleus of the solitary tract in rats. Neurosci. Lett. 2001;303:49–52. doi: 10.1016/s0304-3940(01)01707-4. [DOI] [PubMed] [Google Scholar]
- Hein E., Nowak M., Kiess O., Biermann T., Bayerlein K., Kornhuber J., Kraus T. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J. Neural Transm. 2013;120:821–827. doi: 10.1007/s00702-012-0908-6. [DOI] [PubMed] [Google Scholar]
- Henry T.R., Bakay R.A., Pennell P.B., Epstein C.M., Votaw J.R. Brain blood-flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: II. Prolonged effects at high and low levels of stimulation. Epilepsia. 2004;45:1064–1070. doi: 10.1111/j.0013-9580.2004.03104.x. [DOI] [PubMed] [Google Scholar]
- Howland R.H. Vagus nerve stimulation. Curr. Behav. Neurosci. Rep. 2014;1:64–73. doi: 10.1007/s40473-014-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Dong J., Kong J., Wang H., Meng H., Spaeth R.B., Camhi S., Liao X., Li X., Zhai X., Li S., Zhu B., Rong P. Effect of transcutaneous auricular vagus nerve stimulation on impaired glucose tolerance: a pilot randomized study. BMC Complement. Altern. Med. 2014;14:203. doi: 10.1186/1472-6882-14-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.W., Egorova N., Yang X.Q., Zhang W.Y., Chen J., Yang X.Y., Hu L.J., Sun S., Tu Y., Kong J. Subthreshold depression is associated with impaired resting-state functional connectivity of the cognitive control network. Transl. Psychiatry. 2015;5 doi: 10.1038/tp.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J., Weissman M.M., Klerman G.L. Service utilization and social morbidity associated with depressive symptoms in the community. JAMA. 1992;267:1478–1483. [PubMed] [Google Scholar]
- King M.S. Anatomy of the Rostral nucleus of the solitary tract. In: Bradley R.M., editor. The Role of the Nucleus of the Solitary Tract in Gustatory Processing. CRC Press/Taylor & Francis; Boca Raton, Florida: 2007. [Google Scholar]
- Kirsch I., Deacon B.J., Huedo-Medina T.B., Scoboria A., Moore T.J., Johnson B.T. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the food and drug administration. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarski J.Z., McIntyre R.S., Grupp L.a., Kennedy S.H. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J. Psychiatry Neurosci. 2005;30:178–186. [PMC free article] [PubMed] [Google Scholar]
- Kosel M., Brockmann H., Frick C., Zobel A., Schlaepfer T.E. Chronic vagus nerve stimulation for treatment-resistant depression increases regional cerebral blood flow in the dorsolateral prefrontal cortex. Psychiatry Res. 2011;191:153–159. doi: 10.1016/j.pscychresns.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Kraus T., Hosl K., Kiess O., Schanze A., Kornhuber J., Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J. Neural Transm. 2007;114:1485–1493. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- Kraus T., Kiess O., Hosl K., Terekhin P., Kornhuber J., Forster C. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal — a pilot study. Brain Stimul. 2013;6:798–804. doi: 10.1016/j.brs.2013.01.011. 10.1016/j.brs.2013.01.011 S1935-861X(13)00034-X [pii] [DOI] [PubMed] [Google Scholar]
- Kraus T., Kiess O., Hösl K., Terekhin P., Kornhuber J., Forster C. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal — a pilot study. Brain Stimul. 2013;6:798–804. doi: 10.1016/j.brs.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Lehtinen V., Joukamaa M. Epidemiology of depression: prevalence, risk factors and treatment situation. Acta Psychiatr. Scand. Suppl. 1994;377:7–10. doi: 10.1111/j.1600-0447.1994.tb05794.x. [DOI] [PubMed] [Google Scholar]
- Lener M.S., Iosifescu D.V. In pursuit of neuroimaging biomarkers to guide treatment selection in major depressive disorder: a review of the literature. Ann. N. Y. Acad. Sci. 2015;1344:50–65. doi: 10.1111/nyas.12759. [DOI] [PubMed] [Google Scholar]
- Liu Z., Xu C., Xu Y., Wang Y., Zhao B., Lv Y., Cao X., Zhang K., Du C. Decreased regional homogeneity in insula and cerebellum: a resting-state fMRI study in patients with major depression and subjects at high risk for major depression. Psychiatry Res. 2010;182:211–215. doi: 10.1016/j.pscychresns.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Liu J., Fang J., Wang Z., Rong P., Hong Y., Fan Y., Wang X., Park J., Jin Y., Liu C., Zhu B., Kong J. Transcutaneous vagus nerve stimulation modulates amygdala functional connectivity in patients with depression. J. Affect. Disord. 2016;205:319–326. doi: 10.1016/j.jad.2016.08.003. [DOI] [PubMed] [Google Scholar]
- McGrath C.L., Kelley M.E., Holtzheimer P.E., Dunlop B.W., Craighead W.E., Franco A.R., Craddock R.C., Mayberg H.S. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiat. 2013;70:821–829. doi: 10.1001/jamapsychiatry.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahas Z., Burns C., Foust M.J., Short B., Herbsman T., George M.S. Vagus nerve stimulation (VNS) for depression: what do we know now and what should be done next? Curr. Psychiatry Rep. 2006;8:445–451. doi: 10.1007/s11920-006-0049-4. [DOI] [PubMed] [Google Scholar]
- Nemeroff C.B., Mayberg H.S., Krahl S.E., McNamara J., Frazer A., Henry T.R., George M.S., Charney D.S., Brannan S.K. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31:1345–1355. doi: 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- Peuker E.T., Filler T.J. The nerve supply of the human auricle. Clin. Anat. 2002;15:35–37. doi: 10.1002/ca.1089. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. (npp2010166 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong P.J., Fang J.L., Wang L.P., Meng H., Liu J., Ma Y.G., Ben H., Li L., Liu R.P., Huang Z.X., Zhao Y.F., Li X., Zhu B., Kong J. Transcutaneous vagus nerve stimulation for the treatment of depression: a study protocol for a double blinded randomized clinical trial. BMC Complement. Altern. Med. 2012;12:255. doi: 10.1186/1472-6882-12-255. (1472-6882-12-255 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong P., Liu A., Zhang J., Wang Y., Yang A., Li L., Ben H., Li L., Liu R., He W., Liu H., Huang F., Li X., Wu P., Zhu B. An alternative therapy for drug-resistant epilepsy: transcutaneous auricular vagus nerve stimulation. Chin. Med. J. 2014;127:300–304. [PubMed] [Google Scholar]
- Rong P., Liu J., Wang L., Liu R., Fang J., Zhao J., Zhao Y., Wang H., Vangel M., Sun S., Ben H., Park J., Li S., Meng H., Zhu B., Kong J. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J. Affect. Disord. 2016;195:172–179. doi: 10.1016/j.jad.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffoli R., Giorgi F.S., Pizzanelli C., Murri L., Paparelli A., Fornai F. The chemical neuroanatomy of vagus nerve stimulation. J. Chem. Neuroanat. 2011;42:288–296. doi: 10.1016/j.jchemneu.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Rush J., Siefert S.E. Clinical issues in considering vagus nerve stimulation for treatment-resistant depression. Exp. Neurol. 2009;219:36–43. doi: 10.1016/j.expneurol.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Rush A.J., Marangell L.B., Sackeim H.A., George M.S., Brannan S.K., Davis S.M., Howland R., Kling M.A., Rittberg B.R., Burke W.J., Rapaport M.H., Zajecka J., Nierenberg A.A., Husain M.M., Ginsberg D., Cooke R.G. Vagus nerve stimulation for treatment-resistant depression: a randomized, controlled acute phase trial. Biol. Psychiatry. 2005;58:347–354. doi: 10.1016/j.biopsych.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Sacher J., Neumann J., Funfstuck T., Soliman A., Villringer A., Schroeter M.L. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J. Affect. Disord. 2012;140:142–148. doi: 10.1016/j.jad.2011.08.001. 10.1016/j.jad.2011.08.001 S0165-0327(11)00458-7 [pii] [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., Rundle M.M., Vaishnavi S.N., Snyder A.Z., Mintun M.A., Wang S., Coalson R.S., Raichle M.E. The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. U. S. A. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. 10.1073/pnas.0812686106 0812686106 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Price J.L., Yan Z., Mintun M.A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley M.T. Insular cortex projection to the nucleus of the solitary tract and brainstem visceromotor regions in the mouse. Brain Res. Bull. 1982;8:139–148. doi: 10.1016/0361-9230(82)90040-5. [DOI] [PubMed] [Google Scholar]
- Singh M.K., Gotlib I.H. The neuroscience of depression: implications for assessment and intervention. Behav. Res. Ther. 2014;62:60–73. doi: 10.1016/j.brat.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano-Mas C., Hernndez-Ribas R., Pujol J., Urretavizcaya M., Deus J., Harrison B.J., Ortiz H., Lpez-Sol M., Menchn J.M., Cardoner N. Cross-sectional and longitudinal assessment of structural brain alterations in melancholic depression. Biol. Psychiatry. 2011;69:318–325. doi: 10.1016/j.biopsych.2010.07.029. [DOI] [PubMed] [Google Scholar]
- Sperling W., Reulbach U., Kornhuber J. Clinical benefits and cost effectiveness of vagus nerve stimulation in a long-term treatment of patients with major depression. Pharmacopsychiatry. 2009;42:85–88. doi: 10.1055/s-0028-1103294. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R., Steele J.D., Mwangi B., Kumar P., Christmas D., Milders M., Matthews K. The insular cortex and the neuroanatomy of major depression. J. Affect. Disord. 2011;133:120–127. doi: 10.1016/j.jad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Yücel M., Lorenzetti V., Tanino R., Whittle S., Suzuki M., Walterfang M., Pantelis C., Allen N.B. Volumetric MRI study of the insular cortex in individuals with current and past major depression. J. Affect. Disord. 2010;121:231–238. doi: 10.1016/j.jad.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat. Med. 2010;16:1277–1283. doi: 10.1038/nm.2229. [DOI] [PubMed] [Google Scholar]
- Wagner G., Schachtzabel C., Peikert G., Bar K.J. The neural basis of the abnormal self-referential processing and its impact on cognitive control in depressed patients. Hum. Brain Mapp. 2015 doi: 10.1002/hbm.22807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks H.R., Tadler S.C., Smith K.W., Iacob E., Saccoman M., White A.T., Landvatter J.D., Chelune G.J., Suchy Y., Clark E., Cahalan M.K., Bushnell L., Sakata D., Light A.R., Light K.C. Antidepressant and neurocognitive effects of isoflurane anesthesia versus electroconvulsive therapy in refractory depression. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069809. [DOI] [PMC free article] [PubMed] [Google Scholar]