Abstract
Objective
Recent data show that iNOS has an essential role in ER stress in obesity. However, whether iNOS is sufficient to account for obesity-induced ER stress and Unfolded Protein Response (UPR) has not yet been investigated. In the present study, we used iNOS knockout mice to investigate whether high-fat diet (HFD) can still induce residual ER stress-associated insulin resistance.
Methods
For this purpose, we used the intraperitoneal glucose tolerance test (GTT), euglycemic-hyperinsulinemic clamp, western blotting and qPCR in liver, muscle, and adipose tissue of iNOS KO and control mice on HFD.
Results
The results of the present study demonstrated that, in HFD fed mice, iNOS-induced alteration in insulin signaling is an essential mechanism of insulin resistance in muscle, suggesting that iNOS may represent an important target that could be blocked in order to improve insulin sensitivity in this tissue. However, in liver and adipose tissue, the insulin resistance induced by HFD was only partially dependent on iNOS, and, even in the presence of genetic or pharmacological blockade of iNOS, a clear ER stress associated with altered insulin signaling remained evident in these tissues. When this ER stress was blocked pharmacologically, insulin signaling was improved, and a complete recovery of glucose tolerance was achieved.
Conclusions
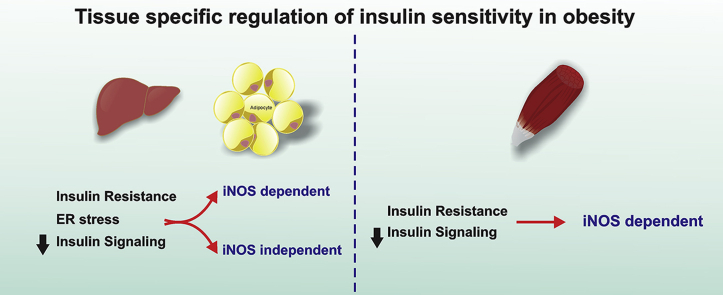
Taken together, these results reinforce the tissue-specific regulation of insulin signaling in obesity, with iNOS being sufficient to account for insulin resistance in muscle, but in liver and adipose tissue ER stress and insulin resistance can be induced by both iNOS-dependent and iNOS-independent mechanisms.
Keywords: Blocking, iNOS, Endoplasmic reticulum stress, Improving, Insulin resistance
Abbreviations: AKT, Protein kinase B; ATF6, activating transcription factor 6; ER, endoplasmic reticulum; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GTT, glucose tolerance test; HFD, high-fat diet; IKK, kappa α/β kinase; iNOS, inducible nitric oxide synthase; IRE1, inositol requiring enzyme 1; ITT, insulin tolerance test; JNK, c-JunN-terminal kinase; NO, nitric oxide; PERK, protein kinase RNA-like ER kinase; qPCR, real time PCR; UPR, unfolded protein response
Graphical abstract
1. Introduction
It is well established that chronic, low-grade inflammation is implicated in the dysfunctional insulin signaling associated with major metabolic diseases such as obesity. Several pathways and intracellular mechanisms are involved in triggering these diseases, including the production of nitric oxide (NO) by the inducible nitric oxide synthase (iNOS) [1], [2]. Activation of iNOS, which can be induced by proinflammatory cytokines [3], [4], has also been described as a potential cause of insulin resistance, a condition which often precedes these metabolic disorders [5], [6], [7]. Although this enzyme performs an important function in the innate immune system [8], [9], higher concentrations of NO generated from overexpression of iNOS can negatively modulate insulin signaling and action [10], [11], [12], [13], [14], [15].
The endoplasmic reticulum (ER) is an organelle responsible for the synthesis, folding, maturation, translocation, and processing of almost all proteins that reside or pass through the endomembrane system of eukaryotic cells [16], [17]. However, in some pathological states, stress disrupts ER homeostasis, leading to the accumulation of misfolded proteins in its lumen. The ER, in turn, responds to this accumulation by activating an intracellular signal transduction pathway termed the unfolded protein response (UPR) [18], [19]. Previous studies have shown a significant association between the activation of inflammation and ER stress in obesity, wherein obesity seems to be a chronic stimulus for the development of ER stress in peripheral tissues, and this is the likely mechanism involved in the onset of insulin resistance and type 2 diabetes [20], [21]. However, therapeutic proposals, such as treatment with chemical chaperones that increase the ability of the ER folding machinery, seem to improve insulin sensitivity and action in peripheral tissues [22], [23].
The interactions between iNOS and ER stress are complex and bidirectional [24]; an increase in iNOS can induce ER stress and later increase iNOS expression [25], [26], [27], [28]. Very recently, Yang et al. [29] showed that, in obesity, the increase in iNOS causes S-nitrosylation of IRE1α, which is a key UPR regulator, leading to a reduction in IRE1α-mediated XBP1 protein splicing activity. This study demonstrated a mechanism by which inflammatory iNOS contributes to ER stress in obesity. However, whether iNOS is sufficient to account for obesity-induced ER stress and UPR has not yet been investigated. In the present study, using iNOS knockout mice, we aimed to investigate whether high-fat diet (HFD) can induce residual ER stress-associated insulin resistance in these mice.
2. Material and methods
2.1. Animal studies
Four week old C57BL6/J and iNOS KO mice, obtained from the Multidisciplinary Center for Biological Research in Laboratory Animal Science Area (CEMIB) of Unicamp, were subjected to standard rodent chow or high-fat diet (HFD) with 55% calories resulting from fat [30], for 12 weeks. The animals were housed under constant conditions of temperature (23 ± 2 °C) and light/dark cycle (12 h/12 h). Water and food were provided ad libitum. All experiments were approved by the Ethics Committee of the State University of Campinas (Unicamp).
2.2. PBA (4-phenyl butyric acid) treatment
C57BL6/J and iNOS KO mice fed a standard or HFD were treated for 7 days with the ER stress inhibitor PBA (1 g/kg of body weight; Sigma–Aldrich) or saline. This treatment protocol was adapted from Won et al. [31]. The inhibitor was diluted in sodium hydroxide, as previously described by Luo et al. [32], and administered via gavage.
2.3. L-NIL (N6-(1-iminoethyl)-l-lysine, dihydrochloride) treatment
C57BL6/J mice fed a standard or HFD were treated with the selective iNOS inhibitor L-NIL (80 mg/kg of body weight; Cayman Chemical) or saline twice a day. This treatment protocol was adapted from Pauli et al. [33]. The inhibitor was dissolved in injection water and administered via intraperitoneal injections for 7 days.
2.4. Glucose tolerance test
After 12 h of fasting and subsequent intraperitoneal anesthesia (ketamine/xylazine), the animals received a 20% glucose solution (2.0 g/kg of body weight) intraperitoneally. The plasma glucose concentration was evaluated in blood samples collected from the tail at 0 (basal), 30, 60, 90, and 120 min after glucose injection.
2.5. Euglycemic hyperinsulinemic clamp
After 12 h of fasting, the animals were anesthetized, and catheters were inserted into the left jugular vein (for infusion of marker) and the carotid artery (for blood collection) according to the protocol described by Prada et al. [34]. The animals received continuous infusion of insulin (3 mU/kg/min) and blood samples were collected at intervals of 5 min for a period of 2 h for the immediate evaluation of blood glucose; 10% (vol./vol.) glucose was also infused at variable rates to maintain blood glucose close to 100 mg/dl.
2.6. Insulin measurement (ELISA)
Serum insulin levels of all groups after 12 h of fasting were measured using the ELISA Rat/Mouse Insulin Kit obtained from Millipore Corporation.
2.7. Extraction of tissues
The animals were intraperitoneally injected with insulin (1 U/kg) or saline, and, after 5 min, liver, gastrocnemius muscle, and epididymal adipose tissue were extracted and homogenized in buffer as described in Saad et al. [35]. Samples of all tissue extracts were subjected to electrophoresis and western blotting [35], [36], [37]. Bands were detected using the chemiluminescence method (West Pico Chemiluminescent Substrate Kit, Thermo Scientific, USA). The antibodies used were anti-phospho-JNK, anti-phospho-IKKα/β, anti-phospho-PERK, anti-phospho-IRE1α, and anti-ATF6α (all obtained from Santa Cruz Technology, Santa Cruz CA, USA) and anti-phospho-Akt and anti-phospho-IRS-1 (Cell Signaling, Boston, MA, USA).
2.8. Immunoprecipitation
For immunoprecipitation, 600 μg of whole lysate from liver, epididymal adipose tissue, and gastrocnemius muscle was incubated with insulin receptor substrate 1 antibody (IRS-1, Cell Signaling, Boston, MA, USA) and protein A-Sepharose 6 MB (Pharmacia, Uppsala, Sweden) for 2 h. Samples were then boiled in Laemmli sample buffer for 5 min and subjected to Western blotting analysis.
2.9. RNA extraction and real time-PCR
The RNA was extracted from liver and epididymal adipose tissue from mice using TRIzol reagent (Life Technologies, USA). To synthesize cDNA, we used the Maxima First Strand cDNA Synthesis Kit with dsDNase (Thermo Scientific, USA). PCR was performed using the PCR Quant Studio 6 Flex System from Applied Biosystems (Life Technologies) and SYBR Green PCR Master Mix (Sigma–Aldrich). The primers used were:
Eif2ak3(PERK) F5′-GGTATTTCAACGCCTGGCTG-3′ and R5′GGCCAGTCTGTGCTTTCGTC-3′;
IRE1_alpha F5′GAGCAAGCTAACGCCTACTCTGT-3′ and R5′CACCATTGAGGGAGAGGCATA-3′;
Actb F5′GTCATCACTATTGGCAACGAGC-3′ and R5′GCACTGTGTTGGCATAGAGGTCT-3′;
Gapdh F5′GTCGTGGAGTCTACTGGTGTCTTC-3′ and R5′AGTTGTCATATTTCTCGTGGTTCA-3.
We used the Data Assist program to calculate relative amounts of mRNA using the 2(−Delta Delta C(T)) method.
2.10. Statistical analysis
All results were expressed as mean ± SEM, and the statistical significance was set to 5% (p < 0.05). The statistical analysis was performed using Graph Pad Prism software, and one or two-way analysis of variance (ANOVA) followed by Bonferroni post-test, delta percentage of baseline, and area under curve were used as appropriate.
3. Results
3.1. iNOS KO mice show partial protection from HFD-induced insulin resistance and glucose intolerance
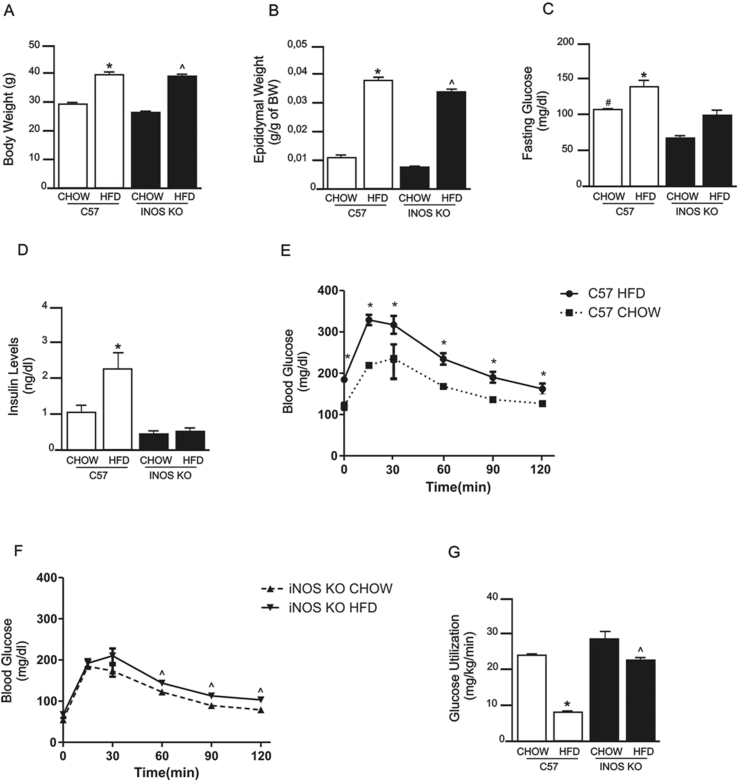
To assess whether the iNOS enzyme can modify the characteristics and metabolic profile of mice fed a HFD, we used animals with selective deletion of the gene encoding this enzyme. When fed a HFD, iNOS KO mice gained weight and increased adipose tissue mass in a similar fashion to controls (Figure 1A, B). Fasting blood glucose levels were decreased in iNOS KO mice fed a CHOW and HFD diet compared with their respective controls (Figure 1C). Serum insulin levels increased in control mice on HFD but not in iNOS KO mice on the same diet (Figure 1D). HFD induced glucose intolerance in control mice, but iNOS KO mice were partially protected from this diet-induced glucose intolerance (Figure 1E, F). Insulin sensitivity, assessed through glucose utilization in hyperinsulinemic-euglycemic clamp, was not significant different between control mice and iNOS KO mice in CHOW diet, was decreased in control mice fed a HFD but was partially preserved in HFD-fed iNOS KO mice (Figure 1G). It is interesting that although insulin sensitivity was similar between controls and iNOS KO mice chow-fed, there was an approximately 30% reduction in fasting blood glucose. However, it is important to mention that fasting blood glucose has a more clear correlation with hepatic glucose output and hepatic insulin resistance, than with peripheral insulin sensitivity [38].
Figure 1.
Metabolic parameters of iNOS KO and control mice fed a standard (chow) or high-fat diet (HFD). (A) Body weight, (B) epididymal fat mass, (C) fasting blood glucose, (D) insulin levels, and (E and F) glucose tolerance, measured by the 120 min glucose tolerance test in (E) C57BL/6J mice and (F) iNOS KO mice. (G) Glucose utilization, as measured by euglycemic-hyperinsulinemic clamp. *p < 0.05 vs. C57BL/6J HFD, ˆp < 0.05 vs. iNOS KO HFD, #p < 0.05 vs. C57BL/6J CHOW. Bars represent mean ± SEM from 4 to 8 mice.
3.2. HFD induces tissue-specific modulation of insulin signaling in iNOS KO mice
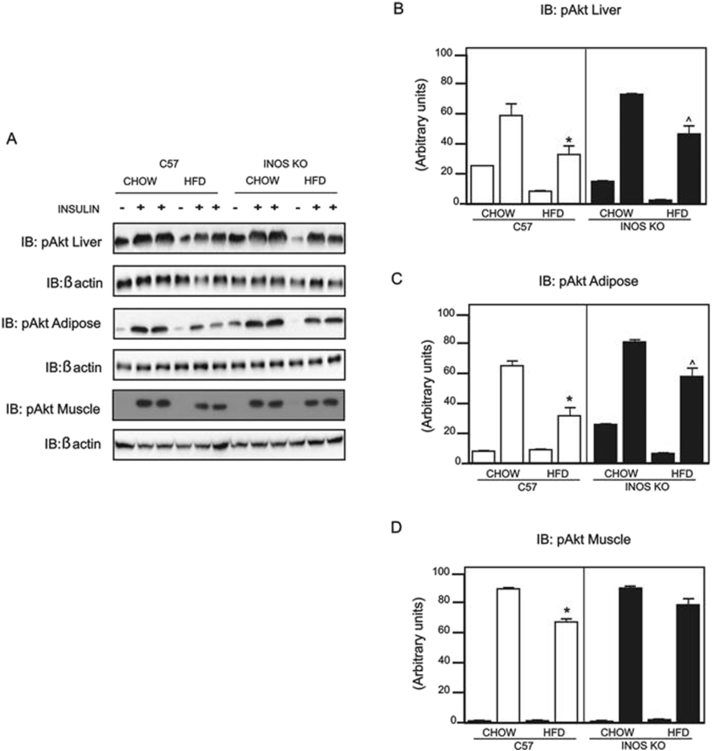
Insulin-induced Akt phosphorylation was markedly reduced in the liver, muscle, and adipose tissue of HFD controls, but in iNOS KO mice fed a HFD, we observed a moderate reduction in insulin-induced Akt phosphorylation in liver and a marked reduction in adipose tissue; this effect of insulin was preserved in gastrocnemius muscle (Figure 2A–D).
Figure 2.
Evaluation of insulin signaling in iNOS KO and control mice in response to intraperitoneal insulin. (A) Insulin-induced Protein kinase B (Akt) phosphorylation in liver, epididymal adipose tissue, and gastrocnemius muscle. (B–D) Akt phosphorylation densitometry in (B) liver, (C) epididymal adipose tissue, and (D) gastrocnemius muscle. *p < 0.05 vs. C57BL/6J HFD, ˆp < 0.05 vs. iNOS KO HFD. Bars represent mean ± SEM from 4 to 8 mice. Similar results were observed when using delta percentage of baseline.
3.3. HFD affects IRS-1 serine phosphorylation and JNK phosphorylation in liver, muscle, and adipose tissue of iNOS KO mice
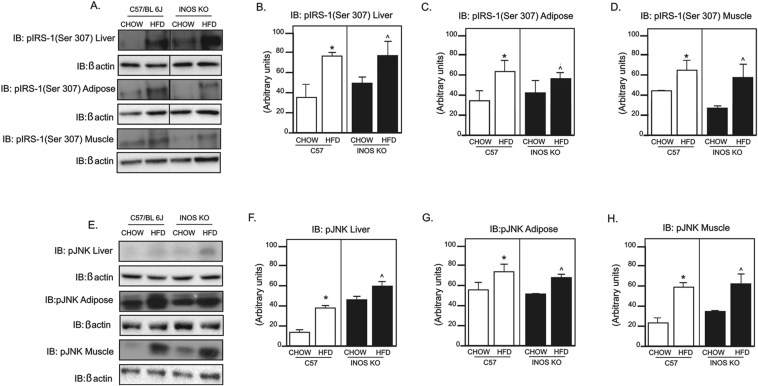
HFD induced a marked increase in IRS-1 serine phosphorylation in liver (Figure 3B), adipose tissue (Figure 3C), and gastrocnemius muscle (Figure 3D) of controls and iNOS KO mice. Since JNK protein is a serine kinase that may phosphorylate IRS-1, impairing the insulin signaling, we investigated the effect of HFD on JNK phosphorylation in the liver, adipose tissue, and muscle of iNOS KO mice and controls. As expected, HFD increased JNK phosphorylation in the liver, muscle and adipose tissue of controls and iNOS KO mice (Figure 3E–H).
Figure 3.
Phosphorylation of IRS-1 and inflammatory markers. (A) Insulin receptor substrate 1 (IRS-1) serine 307 phosphorylation in liver and epididymal adipose tissue. (B–D) IRS-1 serine 307 phosphorylation densitometry in (B) liver, (C) adipose tissue, and (D) gastrocnemius muscle. (E) c-JunN-terminal kinase (JNK) phosphorylation in liver, epididymal adipose tissue, and gastrocnemius muscle. (F–H) JNK phosphorylation densitometry in (F) liver, (G) adipose, and (H) gastrocnemius muscle. *p < 0,05 vs. C57BL/6J HFD, ˆp < 0,05 vs. iNOS KO HFD. Bars represent mean +/− SEM from 4 to 8 mice. Similar results were observed when using delta percentage of baseline.
3.4. iNOS KO mice fed a HFD still have ER stress
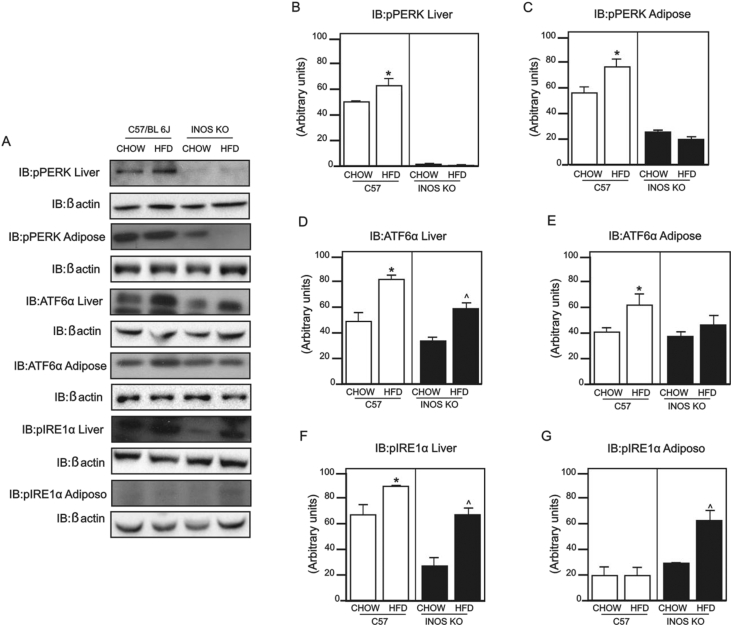
Previous data showed that HFD induces ER stress in liver and adipose tissue and that iNOS might play an important role in this process. Given this, we investigated the main proteins of UPR through the phosphorylation of the protein kinase RNA-like ER kinase (PERK) and inositol requiring enzyme 1 (IRE1α) and the expression of activating transcription factor 6 (ATF6α) in these tissues. There was an increase in PERK phosphorylation in liver and adipose tissue (Figure 4A–C) in the control fed a HFD but not in the iNOS KO mice on the same diet. However, ATF6α expression was higher in liver (Figure 4D) of control and iNOS KO mice, but, in adipose tissue (Figure 4D), this difference was only observed in control mice. HFD induced an increase in IRE1α phosphorylation in both tissues of control and iNOS KO mice (Figure 4F–G).
Figure 4.
Expression and phosphorylation of proteins involved in the unfolded proteins response (UPR). (A) Protein kinase RNA-like ER kinase (PERK) phosphorylation in liver and epididymal adipose tissue; activating transcription factor 6 (ATF6α) expression in liver and epididymal adipose tissue; inositol requiring enzyme 1 (IRE1α) phosphorylation in liver and adipose tissue. (B and C) PERK phosphorylation densitometry in (B) liver and (C) adipose tissue. (D and E) ATF6α expression densitometry in (D) liver and (E) adipose tissue. (F and G) IRE1α phosphorylation densitometry in (F) liver and (G) adipose tissue. *p < 0.05 vs. C57BL/6J HFD, ˆp < 0.05 vs. iNOS KO HFD. Bars represent mean ± SEM from 4 to 8 mice. Similar results were observed when using delta percentage of baseline.
In HFD fed mice, we did not find activation of pPERK, pIRE1α, and expression of ATF6 in muscle (data not show) indicating that this diet is not able to induce ER stress in muscle. The basal phosphorylation of PERK in liver and adipose tissue and of IRE1α in liver was lower in iNOS KO mice. Previous data showed that, in some tissues, basal ER stress may be a physiological phenomenon [39]. In this regard, we can suggest that in iNOS KO mice on CHOW diet there is a reduction in basal ER stress in liver and adipose tissue.
3.5. Pharmacological inhibition of ER stress in iNOS KO mice further improves the metabolic profile and insulin sensitivity of these animals
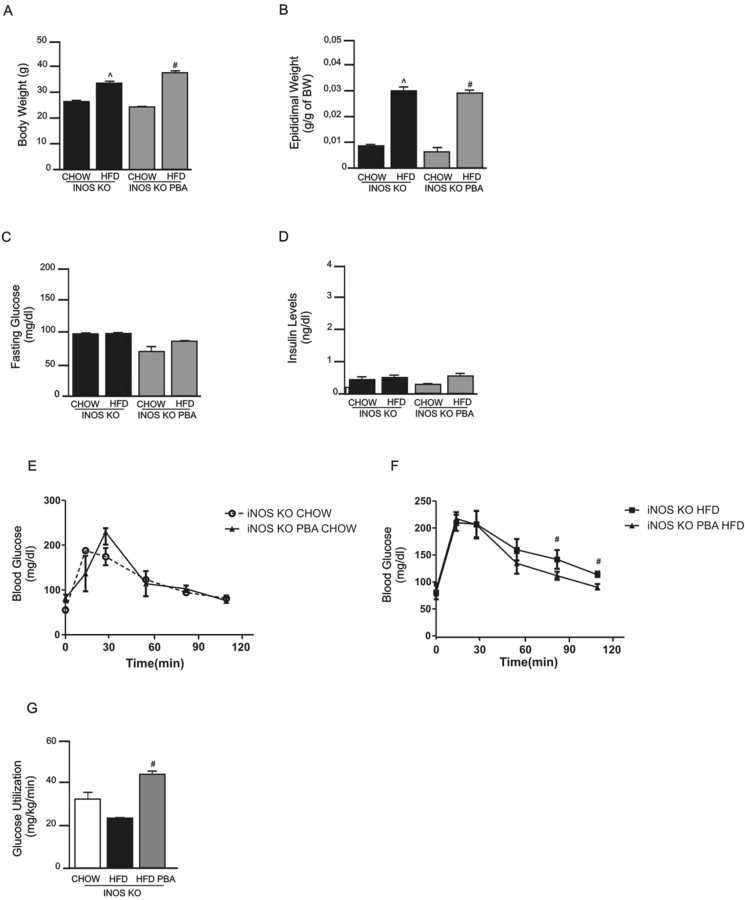
Since iNOS KO mice fed a HFD were not completely protected from ER stress, we investigated whether the pharmacological inhibition of ER stress would have a synergistic effect on the improvement of insulin sensitivity in these mice. Treatment with the ER stress blocker PBA had no effect on body weight and adipose tissue mass between groups on the same diet (Figure 5A, B, Supplemental 1A, B). Fasting blood glucose levels (Figure 5C) and serum insulin levels (Figure 5D) in iNOS KO mice fed a HFD did not change after treatment with PBA compared to iNOS KO mice on HFD without treatment. Glucose tolerance was similar between the iNOS KO groups fed the chow diet (Figure 5E) and although HFD induced mild glucose intolerance in iNOS KO mice, the treatment with PBA reversed this intolerance (Figure 5F). As previously shown, iNOS KO mice fed a HFD showed a moderate improvement in insulin sensitivity, as evaluated by euglycemic-hyperinsulinemic clamp, but after treatment with PBA there was a complete normalization of insulin sensitivity in these mice (Figure 5G).
Figure 5.
Metabolic profile of iNOS KO mice fed a standard (chow) or high-fat diet (HFD) and treated with the ER stress blocker 4-phenyl butyric acid (PBA). (A) Body weight, (B) epididymal fat mass, (C) fasting blood glucose, (D) insulin levels, and (E and F) glucose tolerance, measured by the 120 min glucose tolerance test in the (E) chow groups and (F) high fat diet groups, and (G) glucose utilization, as measured by euglycemic-hyperinsulinemic clamp. ˆp < 0.05 vs. iNOS KO HFD and #p < 0.05 vs. iNOS KO PBA HFD. Bars represent mean ± SEM from 4 to 8 mice.
3.6. Pharmacological inhibition of ER stress normalizes insulin signaling through Akt phosphorylation and decreases JNK phosphorylation and ER stress in iNOS KO mice
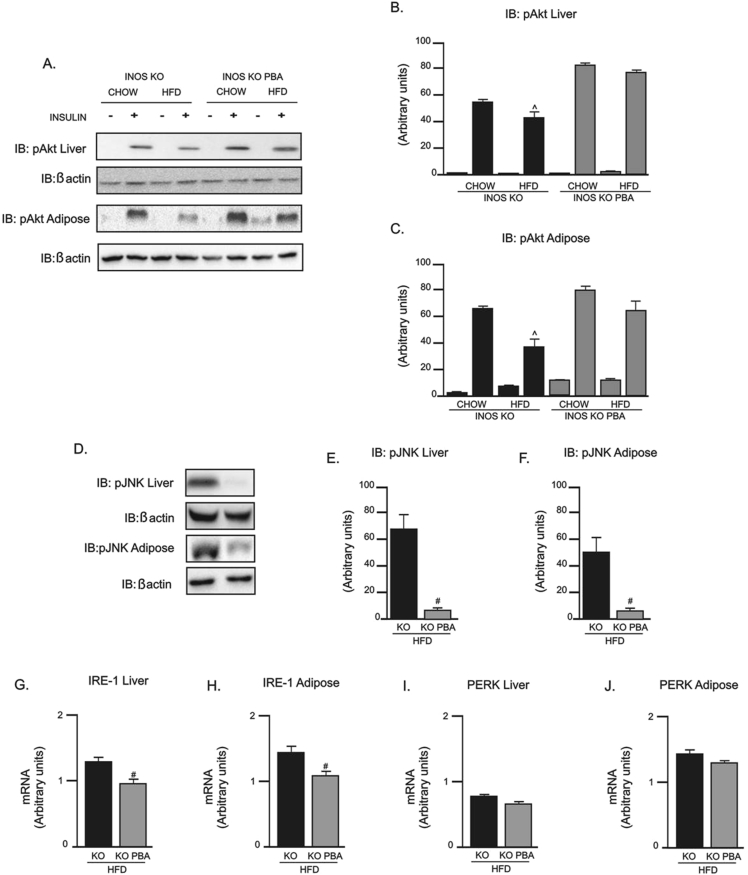
After treatment with PBA in iNOS KO mice on HFD, there was a complete normalization of insulin-induced Akt phosphorylation in liver and adipose tissue (Figure 6A–C). In addition, PBA treatment of iNOS KO mice fed a HFD markedly reduced JNK phosphorylation in liver and adipose tissue (Figure 6D–F) compared to a HFD iNOS KO mice without treatment. Similarly, iNOS KO mice fed a HFD showed a decreased expression of IRE-1α mRNA in liver (Figure 6G) and adipose tissue (Figure 6H) after PBA treatment; however, PERK expression was not affected by PBA treatment in these mice (Figure 6I and J).
Figure 6.
Evaluation of insulin signaling and mediators of the inflammatory and unfolded protein response (UPR) pathways in iNOS KO mice treated with PBA. (A) Insulin-induced Protein kinase B (Akt) phosphorylation in liver and epididymal adipose tissue. (B and C) Akt phosphorylation densitometry in (B) liver and (C) adipose tissue. (D) c-JunN-terminal kinase (JNK) phosphorylation in liver and epididymal adipose tissue. (E and F) JNK phosphorylation densitometry in (E) liver and (F) adipose tissue. (G and H) IRE-1α mRNA expression in (G) liver and (H) adipose tissue. (I and J) PERK mRNA expression in (I) liver and (J) adipose tissue. ˆp < 0.05 vs. iNOS KO HFD, #p < 0.05 vs. iNOS KO PBA HFD. Bars represent mean ± SEM from 4 to 8 mice. Similar results were observed when using delta percentage of baseline.
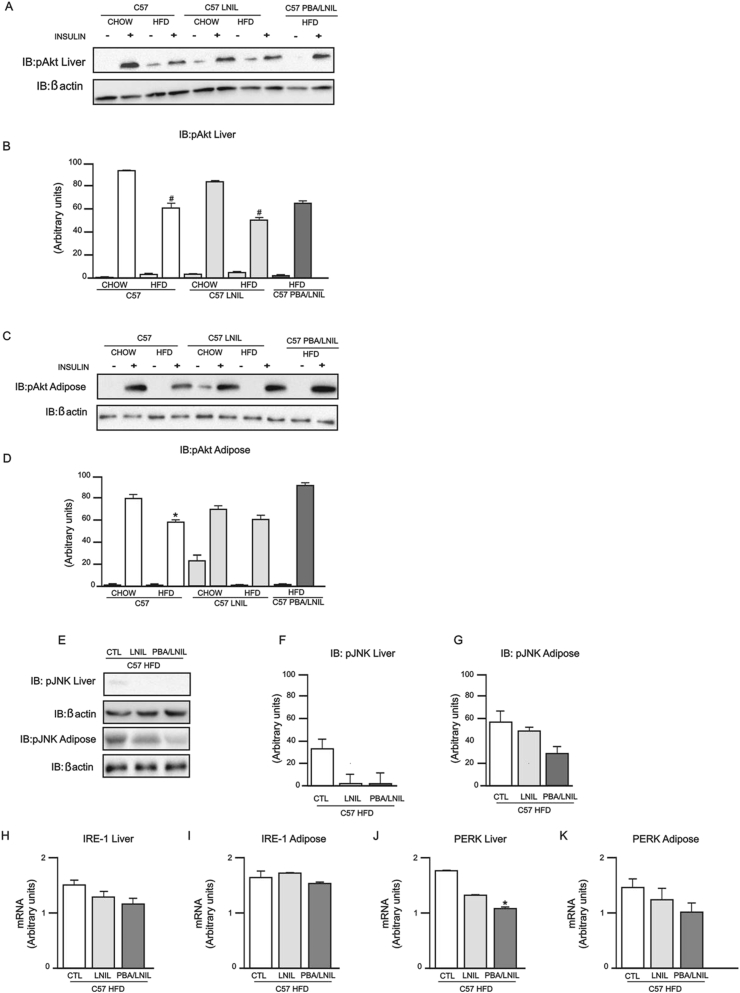
3.7. Pharmacological blockade of ER stress combined with inhibition of iNOS synergistically improves insulin resistance in C57BL/6J mice
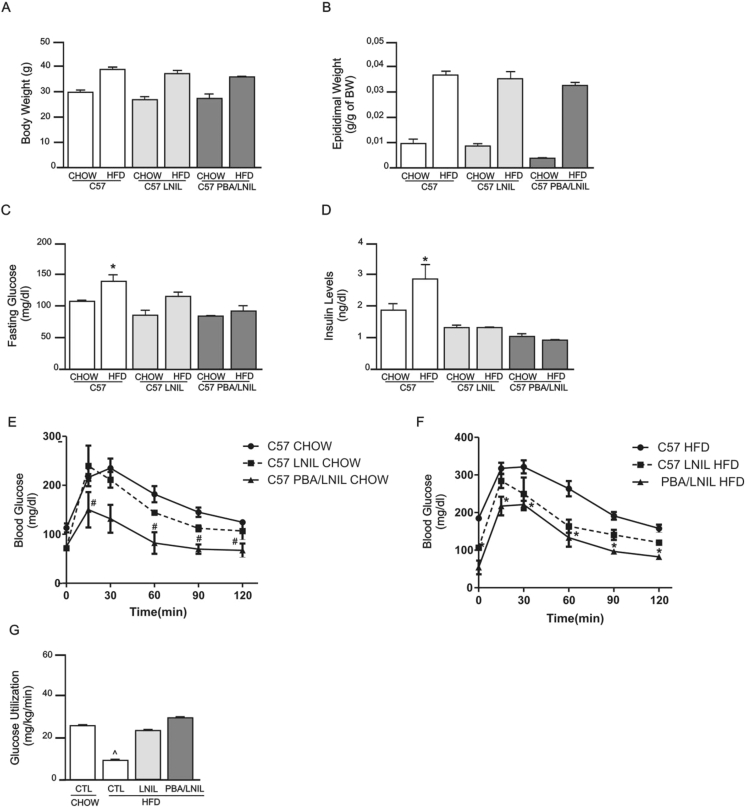
In order to assess whether the results obtained with iNOS KO mice could also be reproduced with pharmacological blockage of the iNOS enzyme, we treated control and HFD mice with the iNOS inhibitor L-NIL and investigated glucose tolerance, insulin sensitivity and signaling, and ER stress. In addition, we also investigated the effect of double blockage with L-NIL and PBA in these animals. L-NIL and L-NIL/PBA treatments had no effect on body weight (Figure 7A) and epididymal fat mass (Figure 7B) of mice whether on chow or HFD. On the other hand, these pharmacological treatments reduced fasting plasma glucose (Figure 7C) and serum insulin levels (Figure 7D) in HFD fed animals. Glucose intolerance in HFD mice was partially improved with L-NIL treatment alone but was only completely normalized when mice were treated with L-NIL plus PBA (Figure 7F). Similarly, insulin sensitivity, as assessed through glucose utilization in hyperinsulinemic-euglycemic clamp, was partially improved in HFD mice treated with L-NIL and was completely normalized with double pharmacological blockade (Figure 7G).
Figure 7.
Metabolic profile of C57BL/6J mice fed standard chow or a high-fat diet and treated with the ER stress blocker 4-phenyl butyric acid (PBA) and the iNOS inhibitor L-NIL. (A) Body weight, (B) epididymal fat mass, (C) fasting blood glucose, (D) insulin levels, and (E–F) glucose tolerance, measured by the 120 min glucose tolerance test in (E) the chow groups and (F) high fat diet groups, (G) Glucose utilization, as measured by euglycemic-hyperinsulinemic clamp. *p < 0.05 vs. C57BL/6J HFD, #p < 0.05 vs. C57BL/6J CHOW and ˆp < 0.05 C57BL/6J PBA/L-NIL HFD. Bars represent mean ± SEM from 4 to 8 mice.
3.8. Pharmacological inhibition of ER stress and iNOS improves insulin signaling through Akt phosphorylation, decreased JNK phosphorylation and ER stress in C57BL/6J mice
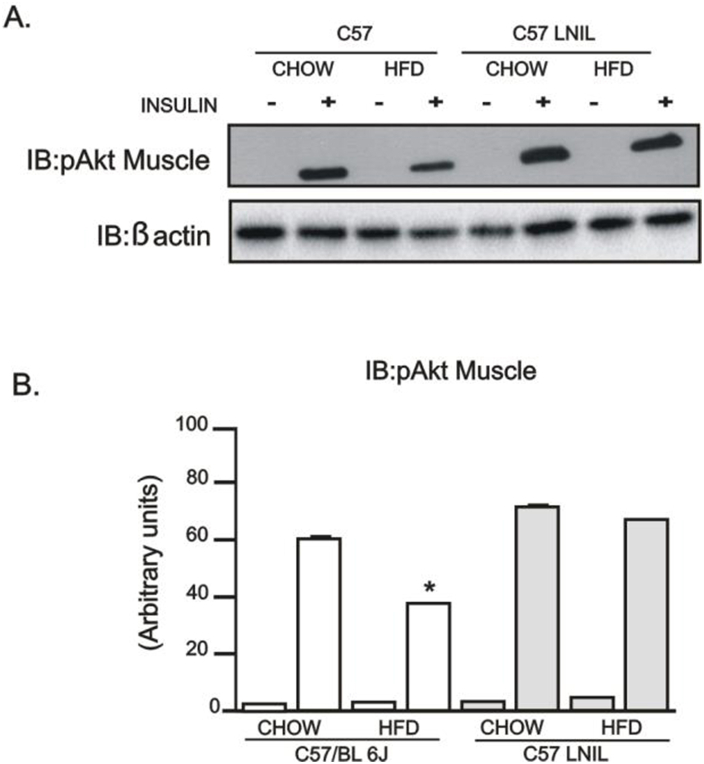
As expected, insulin-induced Akt phosphorylation was blunted in liver and adipose tissue of HFD-fed mice (Figure 8A–D), and L-NIL treatment did not normalize Akt phosphorylation in these tissues. However, Akt phosphorylation was preserved in the muscle of these animals (Supplemental 2). On the other hand, PBA plus L-NIL treatment reversed insulin-induced Akt phosphorylation in liver and adipose tissue of HFD-fed C57BL/6J mice (Figure 8A–D).
Figure 8.
Evaluation of insulin signaling and the unfolded protein response (UPR) pathway in C57BL/6J mice treated with PBA and PBA/L-NIL. (A) Insulin-induced Protein kinase B (Akt) phosphorylation in liver. (B) Akt phosphorylation densitometry in liver. (C) Insulin-induced Akt phosphorylation in epididymal adipose tissue. (D) Akt phosphorylation densitometry in epididymal adipose tissue. (E) c-JunN-terminal kinase (JNK) phosphorylation in liver and epididymal adipose tissue. (F and G) JNK phosphorylation densitometry in (F) liver and (G) adipose. (H and I) IRE-1α mRNA expression in (H) liver and (I) adipose tissue. (J and K) PERK mRNA expression in (J) liver and (K) adipose tissue. *p < 0.05 vs. C57BL/6J L-NIL CHOW, #p < 0.05 vs. C57BL/6J CHOW, +p < 0.05 vs. C57BL/6J PBA/L-NIL CHOW and ˆp < 0.05 C57BL/6J PBA/L-NIL HFD. Bars represent mean ± SEM from 4 to 8 mice. Similar results were observed when we used delta percentage of baseline.
This combined treatment in HFD-fed mice also decreased JNK phosphorylation in liver and adipose tissue compared to HFD-fed mice without treatment (Figure 8E–G). Lastly, we demonstrated a decreased expression of IRE-1α mRNA in liver (Figure 8H) and adipose tissue (Figure 8I), and of PERK mRNA in liver (Figure 8J) and adipose tissue (Figure 8K) of PBA/LNIL HFD-fed mice. These results suggest that pharmacological inhibition of iNOS and ER stress acts synergistically.
4. Discussion
The insulin resistance of obesity and DM2 is linked to a subclinical inflammation, and, at molecular level, this inflammatory state has multiple facets [2], [4], [40], but ER stress seems to be an important mechanism. Although the connection between ER stress and insulin resistance in obesity is well established, a clear molecular mechanism was only recently demonstrated for this interaction. In seminal work, Yang et al. [29] demonstrated that, in obesity, there is evident integration of inflammatory pathways and ER stress showing that UPR function is compromised due to an iNOS-mediated S-nitrosylation of IRE1α, which induces chronic ER stress. Similarly, Nakato et al. [41] recently showed that NO can S-nitrosylate the ER stress sensors IRE1α and PERK by inducing different modulations in these proteins. S-nitrosylation of IRE1α inhibits its ribonuclease activity, while S-nitrosylation of PERK activates its kinase activity, indicating that nitrosative stress leads to dysfunctional ER stress signaling. Taken together, these results suggest that iNOS-induced ER stress is an important molecular modulator of ER stress-induced insulin resistance in obesity. Moreover, iNOS can also induce insulin resistance through nitrosylation of insulin signaling pathway proteins and/or through oxidative stress [42], [43], [44].
In accordance with previous data [15], our data showed that iNOS KO mice are partially protected from diet-induced insulin resistance but still have altered insulin signaling at least in liver and adipose tissue. However, in these mice, there were molecular signals of mild ER stress in liver and adipose tissue, characterized by UPR activation, suggesting that, in obesity, there is also an iNOS-independent ER stress. This residual ER stress in iNOS KO mice on HFD, with increased protein expression of ATF6 in liver and of IRE1α in liver and adipose tissue, was accompanied by an increase in JNK activation, which may account for the mild reduction in insulin sensitivity and glucose intolerance still present in iNOS KO mice on HFD. In agreement, we found in these mice an increase in IRS-1 serine 307 phosphorylation in these tissues. Serine phosphorylation is generally linked to IR, leading to decreased activation of PI-3 kinase [45], [46], [47], which is upstream of Akt. In accordance, we also observed a decrease in insulin-induced Akt activation in these tissues. We also showed that when this residual ER stress was blocked in the liver and adipose tissue of iNOS KO mice fed a HFD, complete recovery of insulin signaling was achieved in these mice. Taken together, these results suggest that in obesity, ER stress can be induced by both iNOS-dependent and iNOS-independent mechanisms.
Our results showed that insulin signaling was completely protected in the muscle of iNOS KO mice fed a HFD, suggesting that obesity-induced alterations in insulin signaling in muscle represent a mechanism of insulin resistance mediated by iNOS. This tissue-specific regulation of insulin signaling observed in the genetic absence of iNOS was also demonstrated by pharmacological blockade of iNOS. These results led us to propose that iNOS-mediated alterations in insulin signaling may represent an important target for treating diet-induced insulin resistance in muscle. It is important to mention that this preserved insulin signaling in muscle of iNOS KO mice on HFD occurred in spite of an increase in JNK activation and IRS-1 serine phosphorylation, which is in accordance with recent data that showed that activation of JNK in muscle fails to induce insulin resistance [48]. Moreover, it was also demonstrated that the increased IRS-1 ser307 phosphorylation in muscle of animal models of insulin resistance seems to have a more adaptive rather than pathogenic function [49]. On the other hand, the iNOS-dependent mechanisms only partially protect insulin signaling in liver and adipose tissue, and the residual ER stress observed in these tissues may also play an important role in the mild insulin resistance and glucose intolerance in these animals. It is tempting to speculate that iNOS may have an important role in the tissue-specific modulation of insulin signaling/resistance in obesity. Previous data showed that in humans and animal models, high-fat diet/obesity is able to induce ER stress in liver and adipose tissue but not in muscle [21], [50], [51]. In accordance with these previous studies, our data showed that control mice on HFD presented ER stress in liver and adipose tissue, and in iNOS KO mice on HFD, the ER stress in these tissues was only partial. It is important to mention that HFD was unable to induce ER stress in muscle of control and iNOS KO mice. In this regard, the absence of ER stress in muscle after HFD may partially explain the tissue specific regulation of insulin signaling in iNOS KO mice on HFD. In addition, one of the most interesting results from our study is that the insulin resistance observed in muscle of HFD animals can be completely prevented by genetic or pharmacological blockage of iNOS.
At the molecular level, the mechanisms involved in the induction of insulin resistance are complex and certainly involve multiple pathways. Although inflammation and ER stress seem to be the unifying mechanisms that can contribute to explaining the increased activity of different serine kinases and of some PTPases [16], [52], other mechanisms and connections have also been involved in the insulin resistance of obesity, including increased lipid storage in tissues and mitochondrial dysfunction [53]. Recently, a clear connection between mitochondrial dysfunction associated oxidative stress with ER stress became more evident and suggests that these alterations may have many mechanisms in common [54], [55]. In this regard, iNOS was recently suggested to play an important role not only in the induction of ER stress but also in the cellular consequences of ER stress, including oxidative stress [56]. Hsieh et al. [24] showed that oxidative stress induced by ER stress signaling is mediated through both iNOS-dependent and independent pathways. Taken together with our results, we suggest that iNOS might have a partial role in both the induction and the consequences of ER stress. In this sense, our results are of crucial importance for the better understanding and treatment of insulin resistance, because in obesity and metabolic disorders that lead to insulin resistance, the expression and activity of iNOS is increased not only in macrophages [57] but also in many other tissues [15], [58], [59].
In conclusion, the results of the present study demonstrated that, in HFD fed mice, iNOS-induced alteration in insulin signaling is an essential mechanism of insulin resistance in muscle, suggesting that iNOS may represent an important target that could be blocked in order to improve insulin sensitivity in this tissue. However, in liver and adipose tissue, the insulin resistance induced by HFD was only partially dependent on iNOS, and even in the presence of genetic or pharmacological blockade of iNOS, a clear ER stress, associated with increased JNK activity and altered insulin signaling, remained evident in these tissues. When this ER stress was blocked pharmacologically, insulin signaling was improved and a complete recovery of glucose tolerance was achieved. Taken together, these results reinforce the tissue-specific regulation of insulin signaling in obesity, with iNOS been sufficient to account for insulin resistance in muscle, but, in liver and adipose tissue, ER stress and insulin resistance can be induced by both iNOS-dependent and iNOS-independent mechanisms.
Disclosure statement
The authors have nothing to disclose.
Acknowledgements
We would like to thank Luis Janeri, Jósimo Pinheiro, Dioze Guadagnini, and Andrey Santos from the Laboratory of Clinical Investigation in Insulin Resistance (LICRI), Department of Internal Medicine, State University of Campinas (UNICAMP), for their technical assistance.
We also acknowledge the financial support from State University of Campinas (FAEPEX), CEPID 2013/07607-8, OCRC (Centro de Pesquisa em Obesidade e Comorbidades), INCT (Instituto Nacional de Ciência e Tecnologia de Obesidade e Diabetes) 573856/2008-7 and from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) 2012/15009-0, 2016/07122-2 and CAPES/CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.12.005.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplemental Figure 1Weight during treatment of all groups. Weight during treatment with PBA in iNOS KO mice fed (A) standard chow and (B) high-fat diet. Weight during treatment with L-NIL in C57BL/6J mice fed (C) standard chow and (D) high-fat diet. Weight during treatment with PBA plus L-NIL in C57BL/6J mice fed (E) standard chow and (F) high-fat diet. Bars represent mean ± SEM from 4 to 8 mice.
Supplemental Figure 2Evaluation of insulin signaling in C57BL/6J mice treated with L-NIL. (A) Insulin-induced Protein kinase B (Akt) phosphorylation in gastrocnemius muscle. (B) AKT phosphorylation densitometry in gastrocnemius muscle. *p < 0.05 vs. C57BL/6J L-NIL HFD. Bars represent mean ± SEM from 4 to 8 mice. Similar results were observed when using delta percentage of baseline.
References
- 1.Gotoh T., Mori M. Nitric oxide and endoplasmic reticulum stress. Arteriosclerosis Thrombosis and Vascular Biology. 2006;26(7):1439–1446. doi: 10.1161/01.ATV.0000223900.67024.15. Epub 2006/04/29, PubMed PMID: 16645155. [DOI] [PubMed] [Google Scholar]
- 2.Litvinova L.S., Kirienkova E.V., Mazunin I.O., Vasilenko M.A., Fattakhov N.S. Pathogenesis of insulin resistance in metabolic obesity. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry. 2014;8(3):192–202. [Google Scholar]
- 3.Kapur S., Bedard S., Marcotte B., Cote C.H., Marette A. Expression of nitric oxide synthase in skeletal muscle: a novel role for nitric oxide as a modulator of insulin action. Diabetes. 1997;46(11):1691–1700. doi: 10.2337/diab.46.11.1691. Epub 1997/11/14. PubMed PMID: 9356014. [DOI] [PubMed] [Google Scholar]
- 4.Martyn J.A., Kaneki M., Yasuhara S. Obesity-induced insulin resistance and hyperglycemia: etiologic factors and molecular mechanisms. Anesthesiology. 2008;109(1):137–148. doi: 10.1097/ALN.0b013e3181799d45. Epub 2008/06/27, PubMed PMID: 18580184; PubMed Central PMCID: PMCPmc3896971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotamisligil G.S., Arner P., Caro J.F., Atkinson R.L., Spiegelman B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. Journal of Clinical Investigation. 1995;95(5):2409–2415. doi: 10.1172/JCI117936. Epub 1995/05/01, PubMed PMID: 7738205; PubMed Central PMCID: PMCPmc295872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaneki M., Shimizu N., Yamada D., Chang K. Nitrosative stress and pathogenesis of insulin resistance. Antioxidants & Redox Signaling. 2007;9(3):319–329. doi: 10.1089/ars.2006.1464. Epub 2006/12/23, PubMed PMID: 17184170. [DOI] [PubMed] [Google Scholar]
- 7.Sansbury B.E., Hill B.G. Regulation of obesity and insulin resistance by nitric oxide. Free Radical Biology & Medicine. 2014;73:383–399. doi: 10.1016/j.freeradbiomed.2014.05.016. Epub 2014/06/01, PubMed PMID: 24878261; PubMed Central PMCID: PMCPmc4112002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Green J., Rolfe M.D., Smith L.J. Transcriptional regulation of bacterial virulence gene expression by molecular oxygen and nitric oxide. Virulence. 2014;5(8):794–809. doi: 10.4161/viru.27794. Epub 2015/01/21, PubMed PMID: 25603427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowenstein C.J., Padalko E. iNOS (NOS2) at a glance. Journal of Cell Science. 2004;117(Pt 14):2865–2867. doi: 10.1242/jcs.01166. Epub 2004/06/16, PubMed PMID: 15197240. [DOI] [PubMed] [Google Scholar]
- 10.Bashan N., Kovsan J., Kachko I., Ovadia H., Rudich A. Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiological Reviews. 2009;89(1):27–71. doi: 10.1152/physrev.00014.2008. Epub 2009/01/08, PubMed PMID: 19126754. [DOI] [PubMed] [Google Scholar]
- 11.Newsholme P., Homem De Bittencourt P.I., O'Hagan C., De Vito G., Murphy C., Krause M.S. Exercise and possible molecular mechanisms of protection from vascular disease and diabetes: the central role of ROS and nitric oxide. Clinical Science (London) 2010;118(5):341–349. doi: 10.1042/CS20090433. Epub 2009/11/20, PubMed PMID: 19922417. [DOI] [PubMed] [Google Scholar]
- 12.Henriksen E.J., Diamond-Stanic M.K., Marchionne E.M. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radical Biology & Medicine. 2011;51(5):993–999. doi: 10.1016/j.freeradbiomed.2010.12.005. Epub 2010/12/18, PubMed PMID: 21163347; PubMed Central PMCID: PMCPmc3071882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujimoto M., Shimizu N., Kunii K., Martyn J.A., Ueki K., Kaneki M. A role for iNOS in fasting hyperglycemia and impaired insulin signaling in the liver of obese diabetic mice. Diabetes. 2005;54(5):1340–1348. doi: 10.2337/diabetes.54.5.1340. Epub 2005/04/28. PubMed PMID: 15855318. [DOI] [PubMed] [Google Scholar]
- 14.Noronha B.T., Li J.M., Wheatcroft S.B., Shah A.M., Kearney M.T. Inducible nitric oxide synthase has divergent effects on vascular and metabolic function in obesity. Diabetes. 2005;54(4):1082–1089. doi: 10.2337/diabetes.54.4.1082. Epub 2005/03/29. PubMed PMID: 15793247. [DOI] [PubMed] [Google Scholar]
- 15.Perreault M., Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nature Medicine. 2001;7(10):1138–1143. doi: 10.1038/nm1001-1138. Epub 2001/10/09, PubMed PMID: 11590438. [DOI] [PubMed] [Google Scholar]
- 16.Hotamisligil G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–917. doi: 10.1016/j.cell.2010.02.034. Epub 2010/03/23, PubMed PMID: 20303879; PubMed Central PMCID: PMCPmc2887297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroder M., Kaufman R.J. ER stress and the unfolded protein response. Mutation Research. 2005;569(1–2):29–63. doi: 10.1016/j.mrfmmm.2004.06.056. Epub 2004/12/18, PubMed PMID: 15603751. [DOI] [PubMed] [Google Scholar]
- 18.Hampton R.Y. ER stress response: getting the UPR hand on misfolded proteins. Current Biology. 2000;10(14):R518–R521. doi: 10.1016/s0960-9822(00)00583-2. Epub 2000/07/19. PubMed PMID: 10898996. [DOI] [PubMed] [Google Scholar]
- 19.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews Molecular Cell Biology. 2007;8(7):519–529. doi: 10.1038/nrm2199. Epub 2007/06/15, PubMed PMID: 17565364. [DOI] [PubMed] [Google Scholar]
- 20.Lee J., Ozcan U. Unfolded protein response signaling and metabolic diseases. Journal of Biological Chemistry. 2014;289(3):1203–1211. doi: 10.1074/jbc.R113.534743. Epub 2013/12/11, PubMed PMID: 24324257; PubMed Central PMCID: PMCPmc3894306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozcan U., Cao Q., Yilmaz E., Lee A.H., Iwakoshi N.N., Ozdelen E. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. Epub 2004/10/16, PubMed PMID: 15486293. [DOI] [PubMed] [Google Scholar]
- 22.Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R.O. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. Epub 2006/08/26, PubMed PMID: 16931765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S.W., Ozcan U. Potential for therapeutic manipulation of the UPR in disease. Seminars in Immunopathology. 2013;35(3):351–373. doi: 10.1007/s00281-013-0370-z. Epub 2013/04/11, PubMed PMID: 23572207; PubMed Central PMCID: PMCPmc3641308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh Y.H., Su I.J., Lei H.Y., Lai M.D., Chang W.W., Huang W. Differential endoplasmic reticulum stress signaling pathways mediated by iNOS. Biochemical and Biophysical Research. 2007;359(3):643–648. doi: 10.1016/j.bbrc.2007.05.154. Epub 2007/06/15, PubMed PMID: 17560946. [DOI] [PubMed] [Google Scholar]
- 25.Baeuerle P.A. IkappaB-NF-kappaB structures: at the interface of inflammation control. Cell. 1998;95(6):729–731. doi: 10.1016/s0092-8674(00)81694-3. Epub 1998/12/29. PubMed PMID: 9865689. [DOI] [PubMed] [Google Scholar]
- 26.Xie Q.W., Kashiwabara Y., Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. Journal of Biological Chemistry. 1994;269(7):4705–4708. Epub 1994/02/18. PubMed PMID: 7508926. [PubMed] [Google Scholar]
- 27.Guo F., Lin E.A., Liu P., Lin J., Liu C. XBP1U inhibits the XBP1S-mediated upregulation of the iNOS gene expression in mammalian ER stress response. Cell Signaling. 2010;22(12):1818–1828. doi: 10.1016/j.cellsig.2010.07.006. Epub 2010/07/20, PubMed PMID: 20637858. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X., Fu Y., Xu X., Li M., Du L., Han Y. PERK pathway are involved in NO-induced apoptosis in endothelial cells cocultured with RPE under high glucose conditions. Nitric Oxide. 2014;40:10–16. doi: 10.1016/j.niox.2014.05.001. Epub 2014/05/13, PubMed PMID: 24813399. [DOI] [PubMed] [Google Scholar]
- 29.Yang L., Calay E.S., Fan J., Arduini A., Kunz R.C., Gygi S.P. METABOLISM. S-Nitrosylation links obesity-associated inflammation to endoplasmic reticulum dysfunction. Science. 2015;349(6247):500–506. doi: 10.1126/science.aaa0079. Epub 2015/08/01, PubMed PMID: 26228140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prada P.O., Ropelle E.R., Mourao R.H., de Souza C.T., Pauli J.R., Cintra D.E. EGFR tyrosine kinase inhibitor (PD153035) improves glucose tolerance and insulin action in high-fat diet-fed mice. Diabetes. 2009;58(12):2910–2919. doi: 10.2337/db08-0506. Epub 2009/08/22, PubMed PMID: 19696185; PubMed Central PMCID: PMCPmc2780887. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Won J.C., Jang P.G., Namkoong C., Koh E.H., Kim S.K., Park J.Y. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity (Silver Spring) 2009;17(10):1861–1865. doi: 10.1038/oby.2009.194. Epub 2009/06/23, PubMed PMID: 19543218. [DOI] [PubMed] [Google Scholar]
- 32.Luo Z.F., Feng B., Mu J., Qi W., Zeng W., Guo Y.H. Effects of 4-phenylbutyric acid on the process and development of diabetic nephropathy induced in rats by streptozotocin: regulation of endoplasmic reticulum stress-oxidative activation. Toxicology and Applied Pharmacology. 2010;246(1–2):49–57. doi: 10.1016/j.taap.2010.04.005. Epub 2010/04/20, PubMed PMID: 20399799. [DOI] [PubMed] [Google Scholar]
- 33.Pauli J.R., Ropelle E.R., Cintra D.E., Carvalho-Filho M.A., Moraes J.C., De Souza C.T. Acute physical exercise reverses S-nitrosation of the insulin receptor, insulin receptor substrate 1 and protein kinase B/Akt in diet-induced obese Wistar rats. Journal of Physiology. 2008;586(2):659–671. doi: 10.1113/jphysiol.2007.142414. Epub 2007/11/03, PubMed PMID: 17974582; PubMed Central PMCID: PMCPmc2375587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prada P.O., Zecchin H.G., Gasparetti A.L., Torsoni M.A., Ueno M., Hirata A.E. Western diet modulates insulin signaling, c-Jun N-terminal kinase activity, and insulin receptor substrate-1ser307 phosphorylation in a tissue-specific fashion. Endocrinology. 2005;146(3):1576–1587. doi: 10.1210/en.2004-0767. Epub 2004/12/14, PubMed PMID: 15591151. [DOI] [PubMed] [Google Scholar]
- 35.Saad M.J., Araki E., Miralpeix M., Rothenberg P.L., White M.F., Kahn C.R. Regulation of insulin receptor substrate-1 in liver and muscle of animal models of insulin resistance. Journal of Clinical Investigation. 1992;90(5):1839–1849. doi: 10.1172/JCI116060. Epub 1992/11/01, PubMed PMID: 1331176; PubMed Central PMCID: PMCPmc443244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thirone A.C., Carvalheira J.B., Hirata A.E., Velloso L.A., Saad M.J. Regulation of Cbl-associated protein/Cbl pathway in muscle and adipose tissues of two animal models of insulin resistance. Endocrinology. 2004;145(1):281–293. doi: 10.1210/en.2003-0575. Epub 2003/10/04, PubMed PMID: 14525909. [DOI] [PubMed] [Google Scholar]
- 37.Ueno M., Bezerra R.M., Silva M.S., Tavares D.Q., Carvalho C.R., Saad M.J. A high-fructose diet induces changes in pp185 phosphorylation in muscle and liver of rats. Brazilian Journal of Medical and Biological Research. 2000;33(12):1421–1427. doi: 10.1590/s0100-879x2000001200004. Epub 2000/12/06. PubMed PMID: 11105093. [DOI] [PubMed] [Google Scholar]
- 38.Nathan D.M., Davidson M.B., DeFronzo R.A., Heine R.J., Henry R.R., Pratley R. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30(3):753–759. doi: 10.2337/dc07-9920. Epub 2007/03/01, PubMed PMID: 17327355. [DOI] [PubMed] [Google Scholar]
- 39.Yang L., Xue Z., He Y., Sun S., Chen H., Qi L.A. Phos-tag-based approach reveals the extent of physiological endoplasmic reticulum stress. PLoS One. 2010;5(7):e11621. doi: 10.1371/journal.pone.0011621. Epub 2010/07/28, PubMed PMID: 20661282; PubMed Central PMCID: PMCPMC2905412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tripathy D., Daniele G., Fiorentino T.V., Perez-Cadena Z., Chavez-Velasquez A., Kamath S. Pioglitazone improves glucose metabolism and modulates skeletal muscle TIMP-3-TACE dyad in type 2 diabetes mellitus: a randomised, double-blind, placebo-controlled, mechanistic study. Diabetologia. 2013;56(10):2153–2163. doi: 10.1007/s00125-013-2976-z. Epub 2013/07/03, PubMed PMID: 23811853. [DOI] [PubMed] [Google Scholar]
- 41.Nakato R., Ohkubo Y., Konishi A., Shibata M., Kaneko Y., Iwawaki T. Regulation of the unfolded protein response via S-nitrosylation of sensors of endoplasmic reticulum stress. Scientific Reports. 2015;5:14812. doi: 10.1038/srep14812. Epub 2015/10/09, PubMed PMID: 26446798; PubMed Central PMCID: PMCPMC4597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carvalho-Filho M.A., Ueno M., Hirabara S.M., Seabra A.B., Carvalheira J.B., de Oliveira M.G. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes. 2005;54(4):959–967. doi: 10.2337/diabetes.54.4.959. Epub 2005/03/29. PubMed PMID: 15793233. [DOI] [PubMed] [Google Scholar]
- 43.Carvalho-Filho M.A., Ueno M., Carvalheira J.B., Velloso L.A., Saad M.J. Targeted disruption of iNOS prevents LPS-induced S-nitrosation of IRbeta/IRS-1 and Akt and insulin resistance in muscle of mice. American Journal of Physiology. Endocrinology and Metabolism. 2006;291(3):E476–E482. doi: 10.1152/ajpendo.00422.2005. Epub 2006/04/28, PubMed PMID: 16638822. [DOI] [PubMed] [Google Scholar]
- 44.Yasukawa T., Tokunaga E., Ota H., Sugita H., Martyn J.A., Kaneki M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. Journal of Biological Chemistry. 2005;280(9):7511–7518. doi: 10.1074/jbc.M411871200. Epub 2005/01/06, PubMed PMID: 15632167. [DOI] [PubMed] [Google Scholar]
- 45.Folli F., Saad M.J., Backer J.M., Kahn C.R. Insulin stimulation of phosphatidylinositol 3-kinase activity and association with insulin receptor substrate 1 in liver and muscle of the intact rat. Journal of Biological Chemistry. 1992;267(31):22171–22177. Epub 1992/11/05. PubMed PMID: 1385396. [PubMed] [Google Scholar]
- 46.Saad M.J., Folli F., Kahn J.A., Kahn C.R. Modulation of insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of dexamethasone-treated rats. Journal of Clinical Investigation. 1993;92(4):2065–2072. doi: 10.1172/JCI116803. Epub 1993/10/01, PubMed PMID: 7691892; PubMed Central PMCID: PMCPMC288376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Velloso L.A., Folli F., Sun X.J., White M.F., Saad M.J., Kahn C.R. Cross-talk between the insulin and angiotensin signaling systems. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(22):12490–12495. doi: 10.1073/pnas.93.22.12490. Epub 1996/10/29. PubMed PMID: 8901609; PubMed Central PMCID: PMCPMC38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pal M., Wunderlich C.M., Spohn G., Bronneke H.S., Schmidt-Supprian M., Wunderlich F.T. Alteration of JNK-1 signaling in skeletal muscle fails to affect glucose homeostasis and obesity-associated insulin resistance in mice. PLoS One. 2013;8(1):e54247. doi: 10.1371/journal.pone.0054247. Epub 2013/01/26, PubMed PMID: 23349837; PubMed Central PMCID: PMCPMC3547909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Copps K.D., Hancer N.J., Opare-Ado L., Qiu W., Walsh C., White M.F. Irs1 serine 307 promotes insulin sensitivity in mice. Cell Metabolism. 2010;11(1):84–92. doi: 10.1016/j.cmet.2009.11.003. Epub 2010/01/16, PubMed PMID: 20074531; PubMed Central PMCID: PMCPMC3314336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deldicque L., Van Proeyen K., Francaux M., Hespel P. The unfolded protein response in human skeletal muscle is not involved in the onset of glucose tolerance impairment induced by a fat-rich diet. European Journal of Applied Physiology. 2011;111(7):1553–1558. doi: 10.1007/s00421-010-1783-1. Epub 2010/12/29, PubMed PMID: 21188411. [DOI] [PubMed] [Google Scholar]
- 51.Carvalho B.M., Oliveira A.G., Ueno M., Araujo T.G., Guadagnini D., Carvalho-Filho M.A. Modulation of double-stranded RNA-activated protein kinase in insulin sensitive tissues of obese humans. Obesity (Silver Spring) 2013;21(12):2452–2457. doi: 10.1002/oby.20410. Epub 2013/03/23, PubMed PMID: 23519983. [DOI] [PubMed] [Google Scholar]
- 52.Zabolotny J.M., Kim Y.B., Welsh L.A., Kershaw E.E., Neel B.G., Kahn B.B. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. Journal of Biological Chemistry. 2008;283(21):14230–14241. doi: 10.1074/jbc.M800061200. Epub 2008/02/19, PubMed PMID: 18281274; PubMed Central PMCID: PMCPMC2386946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Samuel V.T., Shulman G.I. The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. Journal of Clinical Investigation. 2016;126(1):12–22. doi: 10.1172/JCI77812. Epub 2016/01/05, PubMed PMID: 26727229; PubMed Central PMCID: PMCPMC4701542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arruda A.P., Pers B.M., Parlakgul G., Guney E., Inouye K., Hotamisligil G.S. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nature Medicine. 2014;20(12):1427–1435. doi: 10.1038/nm.3735. Epub 2014/11/25, PubMed PMID: 25419710; PubMed Central PMCID: PMCPMC4412031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lewis S.C., Uchiyama L.F., Nunnari J. ER-mitochondria contacts couple mtDNA synthesis with mitochondrial division in human cells. Science. 2016;353(6296) doi: 10.1126/science.aaf5549. aaf5549. Epub 2016/07/16, PubMed PMID: 27418514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keane K.N., Cruzat V.F., Carlessi R., de Bittencourt P.I., Jr., Newsholme P. Molecular events linking oxidative stress and inflammation to insulin resistance and beta-cell dysfunction. Oxidative Medicine and Cellular Longevity. 2015;2015:181643. doi: 10.1155/2015/181643. Epub 2015/08/11, PubMed PMID: 26257839; PubMed Central PMCID: PMCPMC4516838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fite A., Abou-Samra A.B., Seyoum B. Macrophages inhibit insulin signalling in adipocytes: role of inducible nitric oxide synthase and nitric oxide. Canadian Journal of Diabetes. 2015;39(1):36–43. doi: 10.1016/j.jcjd.2014.02.023. Epub 2014/09/03, PubMed PMID: 25179174. [DOI] [PubMed] [Google Scholar]
- 58.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. Epub 2003/12/18, PubMed PMID: 14679176; PubMed Central PMCID: PMCPMC296995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kapur S., Picard F., Perreault M., Deshaies Y., Marette A. Nitric oxide: a new player in the modulation of energy metabolism. International Journal of Obesity and Related Metabolic Disorders. 2000;24(Suppl. 4):S36–S40. doi: 10.1038/sj.ijo.0801502. Epub 2000/12/29. PubMed PMID: 11126239. [DOI] [PubMed] [Google Scholar]