Abstract
Background
Adult studies suggest antibodies to infliximab (ATI) correlate with loss of response in inflammatory bowel disease but pediatric data are limited.
Methods
We conducted a cross-sectional study of trough infliximab levels and ATI in 134 pediatric and young adult patients receiving infliximab. At the time serum was obtained demographics, disease phenotype, duration of infliximab therapy, use of combination therapy (methotrexate or 6-mercaptopurine with infliximab), and surgery were recorded.
Results
Assays were performed on 134 subjects currently receiving infliximab (85 male; mean age, 17.3 ± 4.3 years; 114 Crohn’s disease and 20 ulcerative colitis). Infliximab use ranged from 12 days to 12 years: median 2.0 (interquartile range [1.1–4.3]) years. Twenty-seven of 134 (20%) patients had ATI ≥5 U/mL. Of patients with ATI ≥5 U/mL, 59% had infliximab levels <5 μg/mL, compared with 14% of patients with ATI <5 U/mL (P < 0.001). Ten (7%) patients (9 Crohn’s disease, 1 ulcerative colitis) underwent bowel resections after beginning infliximab infusions. Sixty percent who underwent surgery had ATI ≥12 U/mL; in contrast, only 8% of patients who did not undergo surgery had ATI ≥12 U/mL (P = 0.01). At the time of serum sampling, 50 (37%) patients were receiving combination therapy, compared with 84 (63%) on infliximab alone. Combination therapy at the time of serum sampling did not correlate with either increase infliximab levels or lower ATI compared with infliximab monotherapy. However, prior immunomodulator use was associated with lower antibody levels (P = 0.007).
Conclusions
ATI correlates with reduction in infliximab level and a higher risk of surgery in patients with inflammatory bowel disease.
Keywords: inflammatory bowel disease, infliximab, Crohn’s disease, ulcerative colitis, infliximab level, antibodies to infliximab, loss of response
Infliximab (Remicade, Janssen Pharmaceuticals, Inc., Titusville, NJ) is a chimeric monoclonal antibody to tumor necrosis factor alpha. Infliximab treatment has revolutionized therapy of inflammatory bowel disease (IBD) by inducing and maintaining clinical remission, reducing corticosteroid use, and lowering rates of both hospitalization and surgery in patients with Crohn’s disease (CD) and ulcerative colitis (UC).1–3 Unfortunately, 25% to 40% of patients who initially benefit from infliximab treatment develop adverse reactions or loss of response over time.2,4,5 Loss of response has been correlated with both the formation of anti-infliximab antibodies5,6 and low serum infliximab levels (IFXL).7,8 These data are from adult studies and do not include pediatric subjects who may have different pharmacodynamics and are an understudied group. Furthermore, much of these data were generated using the prior solid phase assay where the presence of circulating drug could mask the presence of anti-infliximab antibodies decreasing the assay’s sensitivity9 and leads to uninterpretable results.6,10 The newer homogenous mobility shift assay (HMSA) (Prometheus Laboratories Inc., San Diego, CA), a liquid-phase assay capable of measuring anti-infliximab antibodies (ATI) independently of the circulating drug, has been developed and clinically validated in adults.11–14 This assay allows for more accurate assessment of the importance of ATI and IFXL.
The potential benefit from combining infliximab with a second immunosuppressive agent remains an area of controversy. In 2008, Van Assche et al performed a randomized controlled trial in which adult patients with CD were assigned to either withdrawal of immunomodulators (thiopurines or methotrexate) after 6 months of combination therapy or continuation of combination therapy with immunomodulators and infliximab. Although the clinical remission rates after 2 years were comparable, combination therapy was associated with a higher median infliximab trough level and a lower C-reactive protein (CRP).15 In 2006, a pediatric study, a randomized, multicenter, open-label study to evaluate the safety and efficacy of infliximab in moderate-to-severe CD (REACH) more than 95% of patients were on concomitant immunomodulators at enrollment, the response rate was 88% at week 10 and ATI prevalence was 2.5%.10 In contrast, in an adult study (ACCENT), only 27% of patients were treated with immunomodulators and 10% of patients developed ATI during the study.6 Notably, the majority of subjects in REACH (77.1%) and 46% of subjects in ACCENT had inconclusive results for ATI due to the detection of infliximab in the serum, which would interfere with interpretation of the previous assay.
In 2009, Oussalah et al16 performed an observational study of azathioprine–infliximab combination therapy among patients with CD (16 [33%], younger than 16 years), which showed an association between the discontinuation of azathioprine and a higher loss of response (inflammation and disease flares) and lower infliximab trough levels. Finally, the SONIC trial (Study of Biologic and Immunomodulator-Naive patients in Crohn’s disease), a double-blind randomized controlled trial of azathioprine or infliximab monotherapy compared with combination of azathioprine and infliximab therapy showed higher rates of corticosteroid-free remission and mucosal healing among adult subjects on combination therapy compared with infliximab monotherapy.17 Additionally, patients receiving combination of azathioprine and infliximab had a lower ATI prevalence of 1%, compared with 14% in patients on infliximab monotherapy.
Pediatric data on the prevalence of ATI and how ATI correlates with IFXL and clinical outcome are lacking. The aim of our study was to determine whether monitoring IFXL and ATI has clinical utility in the treatment of patients with IBD who have received or are receiving treatment with infliximab. Because IFXL and ATI are often only checked in the setting of loss of response, the prevalence of ATI in children receiving infliximab is unknown. We performed a cross-sectional study to assess IFXL and the prevalence of ATI in children and young adults currently receiving infliximab for the treatment of IBD and compared these values with clinical parameters.
METHODS
We conducted a cross-sectional study of ATI and IFXL in children and young adults with IBD followed at a single tertiary care institution. The study was approved by the Institutional Review Board at Boston Children’s Hospital, and parental and/or subject consent was obtained before study enrollment. From June 14, 2012 to November 2, 2013, we recruited 134 patients into this study. These patients were currently receiving infliximab in our infusion center. As a comparison group, we also obtained serum from 18 additional patients who had previously been treated with infliximab but had the drug discontinued either because of infusion reactions or loss of efficacy.
For this cross-sectional study, we obtained a single serum sample from each patient. In the 134 patients currently receiving infliximab, serum was obtained immediately before the patient’s infusion. Consecutive patients were recruited for study. Patients were most often enrolled at one infusion, and samples were obtained just before their subsequent infusion. In the 18 comparison patients no longer receiving infliximab, serum was obtained during a follow-up clinic visit. Serum was stored at −80°C and shipped to Prometheus Laboratories, where assays were performed. Clinical information gathered at the time serum was obtained included age, sex, race, weight, height, IBD type (CD or UC), and concomitant medications (azathioprine, 6-mercaptopurine, methotrexate, or corticosteroids). Disease phenotype was recorded using the Paris classification.18 We characterized disease activity using the pediatric Crohn’s disease activity index (PCDAI19) or pediatric ulcerative colitis activity index (PUCAI20). We also documented the duration of infliximab therapy, dose, infusion frequency, and history of infusion reactions. The patient’s medical record was reviewed to identify prior interventions, including infliximab dose escalation, decreased interval between doses, or change to another agent (e.g., adalimumab). Surgical history was also recorded and subdivided into whether surgery occurred before the start of infliximab or after infliximab was initiated. Additional documented laboratory parameters at the time of infusion included: hematocrit (in percentage), albumin (in grams per deciliter), erythrocyte sedimentation rate (in millimeter per hour), and CRP (in milligrams per deciliter).
Loss of response to infliximab was defined as an increase in infliximab dose over the standard 5 mg/kg, a decrease in dosing interval (i.e., infusion more often than every 8 wk) to treat IBD disease activity, or progression to surgery while on infliximab.16 All treatment decisions were made by the primary treating gastroenterologist. Patients who developed infusion reactions but were able to be successfully rechallenged were not counted as loss of response.
Quantification of serum levels of infliximab and ATI were performed by Prometheus Laboratories Inc., using the homogenous mobility shift assay (HMSA), allowing for the simultaneous measurement of serum infliximab level (IFXL) and ATI.14 For ATI, the assay has a lower limit of 3.1 U/mL and an upper limit of 100 U/mL. All values equal to 3.1 U/mL and above are considered antibody-positive. The range of assay detection for IFXL is 1.0 to 34.0 μg/mL.
Statistical Methods
Study data were collected and managed using Research Electronic Data Capture tools hosted at Boston Children’s Hospital. REDCap (Research Electronic Data Capture)21 is a secure web-based application designed to support data capture for research studies. Data were characterized by percentages for categorical data, and mean (standard deviation [SD]) or median (interquartile range [IQR]) for continuous data, depending on the degree of skew in the data. Statistical testing of categorical data included the Pearson’s chi-square statistic (when the expected frequency count was at least 5) and the Fisher’s exact test otherwise. Student’s t test and analysis of variance was used to investigate group differences for continuous outcomes that were normally distributed, and the Wilcoxon rank-sum test and Kruskal–Wallis test otherwise. All tests of significance were 2-sided and unadjusted for multiple comparisons, and P < 0.05 was considered statistically significant. All data analysis and figures were performed with SAS version 9.3 (Cary, NC).
RESULTS
Demographics
Assays were performed on 134 subjects currently receiving infliximab infusions (85 male; mean age, 17.3 ± 4.3 yr; 114 CD and 20 UC). Fifteen subjects had been receiving infliximab for less than 6 months, 47 from 6 months to 2 years, 48 from 2 to 5 years, and 24 for more than 5 years. The majority (105; 78%) of patients recruited in this group were in clinical remission (PCDAI <11 or PUCAI <10). In addition, almost all patients recruited (116; 88%) had a normal CRP (≤0.5 mg/dL). Additional information on Paris classification, disease activity, and CRP is noted in Table 1. At the time serum was drawn, 50 patients were receiving concomitant immunomodulators (7 on mercaptopurine, 43 on methotrexate) compared with 84 receiving infliximab alone without an immunomodulator. One hundred nineteen subjects had received an immunomodulator since diagnosis of IBD.
TABLE 1.
Baseline Characteristics for 134 Subjects Currently on Infliximab (n = 134) and 18 Subjects Previously on Infliximab (n = 18) Who Had Discontinued the Drug Secondary to Loss of Response, Infusion Reaction, or Lack of Initial Response
| Characteristic | Current Infliximab
|
P | |
|---|---|---|---|
| Yes (n = 134)
|
No (n = 18)
|
||
| Statistic | Statistic | ||
| Age, yr | 17.3 ± 4.3 | 17.5 ± 2.9 | 0.85 |
| Male sex | 85 (63%) | 11 (61%) | 0.85 |
| Diagnosis | 0.45 | ||
| UC | 20 (15%) | 3 (17%) | |
| CD | 114 (85%) | 15 (83%) | |
| Disease severity | |||
| CD (PCDAI) | 5 (0–10) | 10 (0–25) | 0.03 |
| UC (PUCAI) | 7.5 (0–15) | 10 (10–30) | 0.25 |
| Disease remission | |||
| CD (PCDAI 0–10) | 95 (83%) | 10 (67%) | 0.15 |
| UC (PUCAI 0–9) | 10 (50%) | 0 (0%) | 0.23 |
| Paris classification at diagnosis | |||
| CD | |||
| Age | 0.05 | ||
| 0–10, yr | 31 (27%) | 9 (60%) | |
| 10–17, yr | 79 (69%) | 6 (40%) | |
| 17–40, yr | 4 (4%) | 0 (0%) | |
| Location | |||
| L1 | 15 (13%) | 0 (0%) | 0.21 |
| L2 | 11 (10%) | 2 (13%) | 0.65 |
| L3 | 85 (75%) | 13 (87%) | 0.52 |
| L4 | 3 (3%) | 0 (0%) | 1.00 |
| L4a | 70 (61%) | 12 (80%) | 0.16 |
| L4b | 8 (7%) | 0 (0%) | 0.60 |
| Behavior | |||
| B1 | 104 (91%) | 14 (93%) | 1.00 |
| B2 | 9 (8%) | 1 (7%) | 1.00 |
| B3 | 3 (3%) | 0 (0%) | 1.00 |
| B4 | 42 (37%) | 4 (27%) | 0.44 |
| Growth | 0.50 | ||
| G0 | 92 (81%) | 11 (73%) | |
| G1 | 22 (19%) | 4 (27%) | |
| UC | |||
| Location | 0.44 | ||
| E1 | 1 (7%) | 0 (0%) | |
| E2 | 1 (7%) | 1 (33%) | |
| E3 | 0 (0%) | 0 (0%) | |
| E4 | 13 (86%) | 2 (67%) | |
| Severity | 0.17 | ||
| S0 | 12 (80%) | 1 (33%) | |
| S1 | 3 (20%) | 2 (67%) | |
| Months on infliximab | 24 (13–51) | 15 (6–46)a | 0.12 |
| Combination therapya | 50 (37%) | 0 (0%) | |
| Immunomodulators since diagnosis | 119 (92%) | 18 (100%) | 0.36 |
| CRP obtained | 132 (99%) | 17 (94%) | 0.32 |
| CRP | 0.10 (0.10–0.27) | 0.12 (0.10–1.26) | 0.09 |
| CRP >0.5 | 16 (12%) | 6 (35%) | 0.02 |
| ATI ≥5 | 27 (20%) | 4 (22%) | 0.76 |
| ATI ≥10 | 18 (13%) | 2 (11%) | 1.00 |
| ATI ≥12 | 14 (10%) | 2 (11%) | 1.00 |
| ATI ≥15 | 13 (10%) | 1 (6%) | 1.00 |
Median and percentage of subjects or median and IQR are shown.
Infliximab with methotrexate or 6-mercaptopurine at the time of serum sampling.
We obtained serum levels of IFX and ATI using HMSA in a comparison group of 18 patients in whom infliximab had already been discontinued (Tables 1 and 2). In this subset, 13 patients had infliximab discontinued because of lack of efficacy or loss of response, and 5 had discontinued treatment for infusion reactions. In this group of 18 patients, 11 patients had previously had ATI levels determined for clinical purposes by their physician using a previously available assay at Prometheus Laboratories Inc.5 For this study, we obtained a follow-up serum sample on this group of 18 patients to ascertain whether antibodies persisted after infliximab discontinuation. Of these 18 patients, 15 were receiving immunosuppressive therapies or biologics (mostly adalimumab or certolizumab) at the time the study serum was drawn. The mean duration between the time infliximab was discontinued, and the time of study serum sampling was 28 months (SD, 19 mo; range, 0–64 mo).
TABLE 2.
Eighteen Subjects Previously on Infliximab
| Subject | Reason for Stopping Infliximab |
Days of Infliximab | IFXL, μg/mL | ATI, U/mL | Medication(s)a | Infliximab Level (Prior Assay) | HACAb |
|---|---|---|---|---|---|---|---|
| 65 | Infusion reaction | 1987 | 11.9 | NDc | Adalimumab, budesonide, methotrexate | 1.6 | ND |
| 67 | Infusion reaction | 745 | ND | 63.7 | Methotrexate | ND | 5.74 |
| 118 | Infusion reaction | 371 | ND | ND | Thalidomide | ND | 8.26 |
| 151 | Infusion reaction | 676 | 7.1 | ND | Adalimumab, methotrexate | NAd | NA |
| 153 | Infusion reaction | 1567 | 4.4 | 8.9 | Adalimumab, methotrexate | ND | 6.45 |
| Mean (+/−SD) | 7.8 (±3.8) | 36.3 (±38.7) | 1.6 | 6.8 (±1.3) | |||
| 20 | Disease activity | 1273 | ND | ND | Tysabri | NA | NA |
| 21 | Disease activity | 188 | 34 | ND | Methotrexate, μertolizumab | ND | 8.58 |
| 26 | Disease activity | 967 | 14.4 | ND | Adalimumab | ND | 10.5 |
| 30 | Disease activity | 92 | 8 | 14.1 | Adalimumab, salicylate | ND | 8.01 |
| 42 | Disease activity | 52 | 19.4 | ND | Adalimumab | 8.3 | ND |
| 69 | Disease activity | 483 | ND | 9.4 | None, colectomy | NA | NA |
| 100 | Disease activity | 1644 | ND | ND | Tacrolimus | NA | NA |
| 139 | Disease activity | 742 | 13.2 | ND | Adalimumab | ND | 8.45 |
| 140 | Disease activity | 722 | 7.9 | ND | Adalimumab | ND | ND |
| 147 | Disease activity | 84 | ND | ND | Salicylates | NA | NA |
| 150 | Disease activity | 702 | 18.7 | ND | Adalimumab | ND | 12.18 |
| 165 | Disease activity | 497 | ND | ND | Prednisone | NA | NA |
| 178 | Disease activity | 1401 | 11.3 | ND | Adalimumab | NA | NA |
| Mean ± SDe | 832 ± 565 | 15.9 ± 8.5 | 11.75 ± 3.3 | 8.3 | 9.5 ± 1.8 | ||
Medications at the time of ATI and IFXL.
Human antichimeric antibody.
ND = not detected.
NA = no previous measurement available.
Mean ± SD for all 18 subjects.
Baseline Characteristics of Subjects With and Without Loss of Response
Fifty-seven percent (77 of 134) subjects had loss of response. Of 77 subjects who had loss of response, 72 (94%) had at least one GI physician initiated intervention, including increase in infliximab infusion dose (n = 45, 63%); increase in infusion frequency (n = 57, 79%); or addition of an immunomodulator (n = 28, 39%). Additionally, 10 (7%) had GI surgery since beginning infliximab, and 6 (48%) had infusion reactions (all were successfully rechallenged). Of the 77 subjects who lost response, 61 (79%) had CD and 16 (21%) had UC. Subjects with UC were not more likely to have loss of response compared with those with CD. Age, sex, CRP, combination therapy use, or ATI or IFXL level in all 134 study subjects did not differ between subjects with (n = 77) or without (n= 57) loss of response. We also analyzed loss of response by disease subtype: CD or UC. CD subjects with loss of response were on infliximab longer than those without loss of response (median, 33.1; IQR, 19.6–52.0) months compared with 19.8 months (IQR, 9.6–45.9; P = 0.049). Otherwise, there were no significant differences in demographics, Paris classification, CRP, ATI, or IFXL level or use of combination therapy regarding loss of response by disease subtype.
Prevalence of ATI in Patients Currently Receiving Infliximab Infusions
Because there is no established a priori definition of what constitutes a clinical cutoff by HMSA in pediatric patients, we used 4 different cut-points for our prevalence analysis. Of the 134 patients currently receiving infliximab, 27 (20%) had ATI ≥5 U/mL, 18 (13%) had ATI levels ≥10 U/mL, 14 (10%) had ATI levels ≥12 U/mL, and 13 (10%) had ATI levels ≥15 U/mL. We compared age, sex, disease phenotype, activity, and length of treatment between subjects with an ATI <5 U/mL (n = 107) and those with ATI ≥5 U/mL (n = 27) (Table 3). Subjects with an ATI <5 U/mL had a longer duration of infliximab therapy compared with those with ATI ≥5 U/mL (median 27.6; [IQR, 14.2–57.2]) versus 19.2 months (IQR, 8.4–30.3), respectively (P = 0.01). Combination therapy (at the time of serum sampling), disease severity, remission, phenotype, and CRP did not differ significantly between subjects with ATI <5 U/mL compared with those with ATI ≥5 U/mL.
TABLE 3.
Demographics, Disease Phenotype/Severity, and Length of Treatment in Subjects with ATI ≥5 or ATI <5 (n = 134)
| Characteristic | ATI (U/mL)
|
P | |
|---|---|---|---|
| <5 (n = 107) | ≥5 (n = 27) | ||
| Most recent diagnosis | |||
| CD | 91 (85%) | 23 (85%) | 1.00 |
| UC | 16 (15%) | 4 (15%) | |
| Most recent disease severity | |||
| CD (PCDAI) | 5.0 (0.0–10.0) | 0.0 (0.0–10.0) | 0.57 |
| UC (PUCAI) | 10.0 (0.0–15.0) | 2.5 (0.0–15.0) | 0.53 |
| Disease remission | |||
| CD (PCDAI 0–10) | 76 (84%) | 19 (83%) | 1.00 |
| UC (PUCAI 0–9) | 7 (44%) | 3 (75%) | 0.58 |
| Months on infliximab | 27.6 (14.2–57.2)a | 19.2 (8.4–30.3) | 0.01 |
| Combination therapy | 42 (39%) | 8 (30%) | 0.36 |
| CRP obtained | 105 (98%) | 27 (100%) | 1.00 |
| CRP | 0.10 (0.10–0.23) | 0.11 (0.10–0.46) | 0.34 |
| CRP >0.5 | 11 (10%) | 5 (19%) | 0.32 |
Before discontinuation.
ATI Correlate with Reduced Infliximab Trough Levels
Patients with ATI ≥5 U/mL had significantly lower IFXL (Fig. 1). In patients with ATI ≥5 U/mL (n = 27), the median IFXL was 1.0 μg/mL (IQR, 1.0–9.3). In contrast, for patients with ATI <5 U/mL (n = 107), the median IFXL was 12.2 μg/mL (IQR, 7.6–25.0; P < 0.001). Among patients with ATI ≥5 U/mL, 59% had IFXL <5 μg/mL; in contrast, only 14% of patients with ATI <5 U/mL had low IFXL (P < 0.0001). The negative correlation between ATI and IFXL persisted irrespective of which cut-point was used to define a “positive” ATI (Fig. 1).
FIGURE 1.
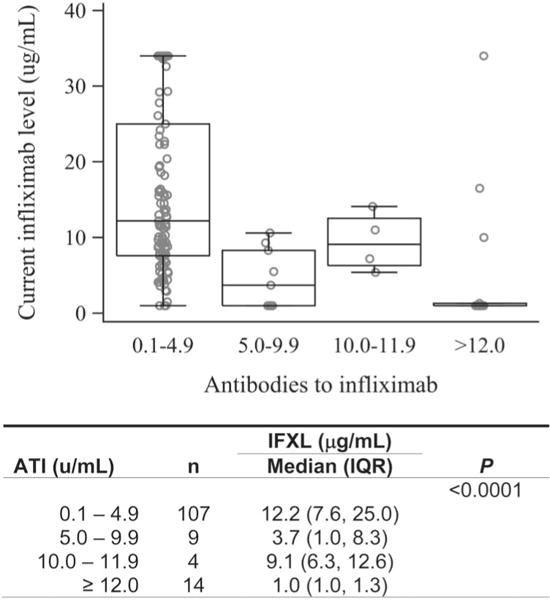
IFXL by ATI. ATI at 4 different ranges show significant decreases in IFXL (P < 0.0001).
Higher Cumulative Dose of Infliximab Correlates with Higher Infliximab Trough Level
Patients receiving more than 5 mg/kg of infliximab every 2 months (whether due to increased dose or increased frequency of infusions) had higher IFXL. Specifically, patients receiving the higher doses (>5 mg/kg) (n = 65) had median IFXL of 14.5 μg/mL (IQR, 8.7–29) compared with patients on the standard dose of 5 mg/kg (n = 69; median IFXL, 7.9 μg/mL; IQR, 3.6–12.7) (P = 0.004). There were no clinical differences noted in the patient populations regarding disease activity or the need for surgery.
ATI Correlates with Progression to Surgery
Ten (7%) patients (9 CD, 1 UC) underwent bowel resections after beginning infliximab infusions. Of these 10 patients, 6 patients (60%) had high antibody levels (ATI ≥12 U/mL). In contrast, of the 124 patients who did not undergo surgery, only 10 patients (8%) had ATI ≥12 U/mL (P = 0.01). We did not identify any other significant association between ATI and other clinical outcomes. Specifically, we could not demonstrate that ATI was associated with an increase in dose, a decrease in interval, an infusion reaction, or higher CRP.
ATI and IFXL with Combination Therapy
We also assessed the potential effect of combination therapy at the time serum was drawn for ATI and IFXL. Thirty-nine percent (42 of 107) of patients with ATI <5 U/mL were on combination therapy compared with 30% (8 of 27) with ATI ≥5 U/mL (P = 0.36). Combination therapy was used for 29% (6 of 21) of subjects with serum IFXL <3 μg/mL compared with 39% (44 of 113) in those with IFXL ≥3 μg/mL (P = 0.37). Thus, combination therapy (immunomodulator and infliximab) at the time when levels and antibodies were sampled did not correlate with either increase in level or reduction in antibody compared with monotherapy. However, when we examined if a patient had taken immunomodulators at any time since diagnosis, 95% (100 of 105) of patients with an ATI of <5 U/mL had been on immunomodulators compared with 76% (19 of 25) of patients with ATI ≥5 U/mL (P = 0.007). Seventy-nine percent (15 of 19) of subjects with IFXL <3 μg/mL compared with 94% (104 of 111) with a level of ≥3 μg/mL had taken immunomodulators since diagnosis, which was a trend that did not reach statistical significance (P = 0.06).
Patients Who Change to Other Therapies May Have Persistent ATI
As a comparison group to assess the prevalence of ATI, we obtained ATI and IFXL on 18 patients who had discontinued infliximab therapy. The time between discontinuation of infliximab and serum acquisition ranged from 0 to 64 months (mean, 25.9; SD = 19.1). ATI ≥5 U/mL were identified in 4 (22%) patients, similar to the proportion seen among the 134 patients currently receiving infliximab (27/134, 20%). We hypothesized that this prevalence may be falsely low because of the long lag time between discontinuation of IFX and acquisition of study serum. Therefore, we also conducted a retrospective chart review to see if any prior clinical evaluation for ATI (using a different assay) had been performed by the practicing physician at the time infliximab was stopped. Of our 18 patients, 10 had prior evaluation for ATI, previously termed human antichimeric antibody. Of these 10 patients, 8 had positive ATI at the time of medication discontinuation, with levels ranging from 5.74 to 12.18 U/mL (≤1.69 U/mL is the level of detection). Previous data indicates that an ATI level of 8 μg/mL leads to poor clinical outcomes.5 Of note, the clinical antibody assay performed on the patients by their physicians was an older assay developed by the same company. This assay has since been replaced by the high sensitivity assay used in this study. Of those 8 patients who had antibodies at the time infliximab was discontinued, 3 had persistent antibodies during this re-evaluation (Table 2).
Adalimumab Therapy Yields a Measurable IFXL with Current Assay
Of the 18 patients in whom infliximab was discontinued, 10 were receiving adalimumab and 1 was receiving certolizumab at the time the study serum sample was collected. All 10 patients had detectable IFXL (>1 μg/dL, mean = 10.9 μg/dL, SD = 10) despite having had infliximab discontinued previously (mean time between discontinuation of infliximab and assay blood = 760 d; range, 52–1987 d). No detectable IFX levels (>1 μg/dL) were seen in patients receiving other IBD therapies (e.g., tacrolimus, methotrexate, thalidomide, natalizumab). This finding confirms the knowledge that assays that use TNF-α as a detection modality will detect all proteins that bind TNF-α.14,22
DISCUSSION
In this cross-sectional study, we determined that 20% of pediatric and young adult patients currently receiving infliximab have detectable ATI (≥5 U/mL). We also demonstrate that ATI correlate with a reduction in infliximab level and a higher risk of surgery in patients with IBD. We were unable to clearly show an association with loss of response, perhaps in part because a large proportion of our patients met our a priori definition of loss of response (e.g., dose adjustment). Combination therapy (6-mercaptopurine, azathioprine, or methotrexate with infliximab) at the time of sampling was not associated with lower ATI or higher IFXL. However, previous immunomodulator use correlated with lower levels of ATI.
In our population, increased ATI strongly correlate with lower IFXL. In adults, clearance of infliximab is greatly increased in the presence of ATIs and results in low IFX trough levels.23–25 The precise mechanism by which ATI reduces IFXL is unknown. Postulated mechanisms include increased proteolytic catabolism, Fc-γ–mediated internalization into phagocytes, and elimination by the reticuloendothelial system (reviewed in Ref. 26). Whether low drug levels lead to antibody formation or antibody formation lowers drug levels has yet to be clarified. Because this was a cross-sectional study, we could not evaluate the relationship between these two variables over time. Future longitudinal studies will determine the temporal association between antibody development, infliximab level, and loss of response.
Combination therapy with immunomodulators and infliximab has been shown to decrease ATI and increase IFXL compared with infliximab monotherapy.2,5,10,27,28 In our study, only a subset of our patients (39%) were on combination therapy. We did not find differences in the prevalence of ATI with current combination therapy compared with those on infliximab monotherapy. However, we did find a lower prevalence of ATI in subjects who had previously taken immunomodulators. This finding could be explained in a few different ways. Subjects may have had a period of combination therapy before levels were drawn but had their immunomodulator discontinued before serum sampling. Additionally, prior use of immunomodulators, even before transition to infliximab monotherapy, may alter the future immune response resulting in less ATI formation.
Similarly, we did not find significant differences in IFXL with combination therapy. In our study, prior immunomodulator use was associated with higher trough IFXL. This result is similar to that of Cornillie et al,29 who found that that combination therapy with immunomodulators versus monotherapy with infliximab resulted in numerically higher but not significantly different week 14 trough IFXL compared with monotherapy in patients with sustained response to 5 mg/kg maintenance treatment. Feagan et al28 found lower ATI and higher IFXL in subjects who received methotrexate with infliximab. However, these study subjects differ from ours because they were also treated with corticosteroids at the time of induction, which could have influenced antibody formation.
Multiple studies have linked the presence of ATI to inferior outcomes in IBD.5,6,30 Our most significant clinical outcome was the association of elevated ATI with the risk of surgery after starting infliximab. Using the same HMSA used in our study, Vande Casteele et al11 found that ATI levels varied over time, but sustained high levels of ATI lead to permanent loss of response. This study also found that subjects with low infliximab trough levels at week 14 are at risk for ATI formation and infliximab discontinuation.11 We did not detect a correlation between ATI or IFXL and loss of response to infliximab. The variation in ATI over time may have clouded our ability to correlate levels and ATI with loss of response using a cross-sectional study design. Longitudinal monitoring for ATI and IFXL may reveal clinically useful trends, such as persistent elevated ATI predicting loss of response.
Multiple prior studies have shown higher ATI and lower IFXL to be associated with poorer outcomes. Fifty-seven percent of our patients met our a priori definition of loss of response. We defined loss of response by increase in infliximab dose over the standard 5 mg/kg, a decrease in dosing interval (i.e., more often than every 8-wk infusions) to treat IBD disease activity, progression to surgery while on infliximab, or infusion reaction similar to Oussalah et al16 in 2010. We could not clearly show an association between IFXL or ATI and outcomes other than surgery. Our cohort was largely in remission, based on disease activity indices and laboratory markers of inflammation (erythrocyte sedimentation rate and CRP). Other groups have shown using a prior ELISA assay that IFXL predict clinical outcomes. Maser et al8 found that higher IFXL were associated with endoscopic improvement, lower CRP, and remission by Harvey–Bradshaw score in CD. In UC, Seow et al7 found that detectable serum infliximab was associated with higher rates of remission (Mayo score) and endoscopic improvement, and that an undetectable infliximab level was associated with an increased risk of colectomy.
Using the same HMSA assay as our study and a similar study population to ours with well-controlled subjects, Levesque et al found no effect of ATI on ≥70-point increase of Crohn’s Disease Activity Index but did see an effect on the proportion of patients with CRP >5 mg/L.13 Similar to our findings, they did not find a statistically significant relationship between mean Crohn’s disease activity indices and trough IFXL at 8 weeks.13 However, because this was a prospective study, directional trends were assessed. Trough IFXL 8 weeks after infusion predicted either a ≥70-point increase of Crohn’s Disease Activity Index or a CRP >5 mg/L. A second recent prospective study, which examined restarting infliximab in subjects after a drug holiday and used the HMSA to detect ATI and IFXL showed that trough IFXL >2 μg/mL and undetectable ATI early after restarting infusions were predictive of primary response and long-term efficacy.12 Thus, prospective monitoring of ATI and IFXL in pediatric subjects may help identify goal IFXL levels in this population and identify patients at higher risk of losing response and may find an association of ATI and IFXL with other more sensitive clinical outcomes, such as mucosal healing.
In our cohort of 18 subjects who were no longer receiving infliximab, we found detectible IFXL levels in subjects on other anti-TNF agents and ATI even years after discontinuation. The detectable IFXL in subjects no longer receiving infliximab confirms that the mobility shift assay will detect all proteins that bind TNF-α.13,21 Persistent ATI in subject’s even years after infliximab discontinuation has also been reported.31,32
In conclusion, our pediatric study had similar findings to adult studies regarding the relationship between ATI and IFXL. However, assuming that IFXL correlates with outcome, there may be a lag between antibody development, a decline in infliximab level, and clinical symptoms. Additional prospective large pediatric cohorts will prove valuable in answering these questions.
Acknowledgments
The authors thank the MacInnes, Rasmussen, Ward, Wolfman, Grossman, and Clark families for their support of the project.
Supported by infliximab level and antibody testing were performed by Prometheus Laboratories Inc.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 2.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ, Rutgeerts P, Feagan BG, et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology. 2009;137:1250–1260. doi: 10.1053/j.gastro.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 4.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 5.Baert F, Noman M, Vermeire S, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601–608. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 6.Hanauer SB, Wagner CL, Bala M, et al. Incidence and importance of antibody responses to infliximab after maintenance or episodic treatment in Crohn’s disease. Clin Gastroenterol Hepatol. 2004;2:542–553. doi: 10.1016/s1542-3565(04)00238-1. [DOI] [PubMed] [Google Scholar]
- 7.Seow CH, Newman A, Irwin SP, et al. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010;59:49–54. doi: 10.1136/gut.2009.183095. [DOI] [PubMed] [Google Scholar]
- 8.Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:1248–1254. doi: 10.1016/j.cgh.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Ordas I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2012;10:1079–1087. doi: 10.1016/j.cgh.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 10.Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology. 2007;132:863–873. doi: 10.1053/j.gastro.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Vande Casteele N, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol. 2013;108:962–971. doi: 10.1038/ajg.2013.12. [DOI] [PubMed] [Google Scholar]
- 12.Baert F, Drobne D, Gils A, et al. Early trough levels and antibodies to infliximab predict safety and success of re-initiation of infliximab therapy. Clin Gastroenterol Hepatol. 2014;12:1474–1481. doi: 10.1016/j.cgh.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Levesque BG, Greenberg GR, Zou G, et al. A prospective cohort study to determine the relationship between serum infliximab concentration and efficacy in patients with luminal Crohn’s disease. Aliment Pharmacol Ther. 2014;39:1126–1135. doi: 10.1111/apt.12733. [DOI] [PubMed] [Google Scholar]
- 14.Wang SL, Ohrmund L, Hauenstein S, et al. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab levels in patient serum. J Immunol Methods. 2012;382:177–188. doi: 10.1016/j.jim.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Van Assche G, Magdelaine-Beuzelin C, D’Haens G, et al. Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology. 2008;134:1861–1868. doi: 10.1053/j.gastro.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Oussalah A, Chevaux JB, Fay R, et al. Predictors of infliximab failure after azathioprine withdrawal in Crohn’s disease treated with combination therapy. Am J Gastroenterol. 2010;105:1142–1149. doi: 10.1038/ajg.2010.158. [DOI] [PubMed] [Google Scholar]
- 17.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 18.Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314–1321. doi: 10.1002/ibd.21493. [DOI] [PubMed] [Google Scholar]
- 19.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–447. [PubMed] [Google Scholar]
- 20.Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. 2007;133:423–432. doi: 10.1053/j.gastro.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang SL, Hauenstein S, Ohrmund L, et al. Monitoring of adalimumab and antibodies-to-adalimumab levels in patient serum by the homogeneous mobility shift assay. J Pharm Biomed Anal. 2013;78–79:39–44. doi: 10.1016/j.jpba.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Horin S, Yavzori M, Katz L, et al. The immunogenic part of infliximab is the F(ab’)2, but measuring antibodies to the intact infliximab molecule is more clinically useful. Gut. 2011;60:41–48. doi: 10.1136/gut.2009.201533. [DOI] [PubMed] [Google Scholar]
- 24.Imaeda H, Andoh A, Fujiyama Y. Development of a new immunoassay for the accurate determination of anti-infliximab antibodies in inflammatory bowel disease. J Gastroenterol. 2012;47:136–143. doi: 10.1007/s00535-011-0474-y. [DOI] [PubMed] [Google Scholar]
- 25.Pariente B, Pineton de Chambrun G, Krzysiek R, et al. Trough levels and antibodies to infliximab may not predict response to intensification of infliximab therapy in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:1199–1206. doi: 10.1002/ibd.21839. [DOI] [PubMed] [Google Scholar]
- 26.Colombel JF, Feagan BG, Sandborn WJ, et al. Therapeutic drug monitoring of biologics for inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:349–358. doi: 10.1002/ibd.21831. [DOI] [PubMed] [Google Scholar]
- 27.Bortlik M, Duricova D, Malickova K, et al. Infliximab trough levels may predict sustained response to infliximab in patients with Crohn’s disease. J Crohns Colitis. 2013;7:736–743. doi: 10.1016/j.crohns.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Feagan BG, McDonald JW, Panaccione R, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology. 2014;146:681 e1–688 e1. doi: 10.1053/j.gastro.2013.11.024. [DOI] [PubMed] [Google Scholar]
- 29.Cornillie F, Hanauer SB, Diamond RH, et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63:1721–1727. doi: 10.1136/gutjnl-2012-304094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vermeire S, Noman M, Van Assche G, et al. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut. 2007;56:1226–1231. doi: 10.1136/gut.2006.099978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben-Horin S, Mazor Y, Yanai H, et al. The decline of anti-drug antibody titres after discontinuation of anti-TNFs: implications for predicting reinduction outcome in IBD. Aliment Pharmacol Ther. 2012;35:714–722. doi: 10.1111/j.1365-2036.2012.04997.x. [DOI] [PubMed] [Google Scholar]
- 32.Steenholdt C, Brynskov J, Bendtzen K. Letter: persistence of anti-infliximab antibodies after discontinuation of infliximab in patients with IBD. Aliment Pharmacol Ther. 2012;36:499–500. doi: 10.1111/j.1365-2036.2012.05204.x. [DOI] [PubMed] [Google Scholar]


