Abstract
Objectives
To assess survival after liver resection and transplantation in patients with HCC beyond Milan criteria.
Summary Background Data
The role of liver resection and transplantation remains controversial for patients with hepatocellular carcinoma (HCC) beyond Milan criteria. Resection of advanced tumors and transplantation using extended-criteria are pursued at select high-volume center
Methods
Patients from 5 liver cancer centers in the United States who had liver resection or transplantation for HCC beyond Milan criteria between 1990 and 2011 were included in the study. Multivariable and propensity-matching analyses estimated the effects of clinical factors and operative selection on survival.
Results
Of 608 patients beyond Milan without vascular invasion, 480 (79%) underwent resection and 128 (21%) underwent transplantation. Clinicopathologic profiles between resection and transplant patients differed significantly. Hepatitis C and cirrhosis were more prevalent in transplantation group (p<0.001). Resection patients had larger tumors (median 9 cm, IQR: 6.5-12.9 cm versus median 4.1, IQR: 3.4-5.3 cm, p<0.001); transplant patients were more likely to have multiple tumors (78% versus 28%, p<0.001). Overall (OS) and disease-free survival (DFS) were both greater after tumor downstaging and transplantation than resection (all p<0.001). OS did not differ between liver transplant recipients who were not pretreated or pretreated and failed to downstage compared to propensity-matched liver resection patients (p≥0.176); DFS in this propensity matched cohort was greater after liver transplantation (p≤0.017).
Conclusions
Liver resection and transplantation provide curative options for patients with HCC beyond Milan criteria. Further treatment strategies aimed at the efficiency and durability of tumor downstaging and expansion of the role of transplantation among suitable candidates could improve outcomes in patients with large or multifocal HCC.
Graphical abstract
Liver resection and transplantation provide curative treatment options for patients with HCC beyond Milan criteria. Patients selected for each treatment are different. Further treatment strategies aimed at the efficiency and durability of tumor downstaging and expansion of the role of transplantation among suitable candidates could improve outcomes in patients with large or multifocal HCC.
Introduction
Selection of patients with hepatocellular carcinoma (HCC) for liver resection and liver transplantation remains complex. Currently the most widely recommended paradigm for operative treatment with curative intent is limited to patients with very early and early stages of HCC based on the Barcelona Clinic Liver Cancer (BCLC) staging system. These patients are considered within Milan criteria and are selected for treatment based on presence and/or severity of underlying chronic liver disease, patient-specific socioeconomic factors, clinical performance status and variations in available regional and national resources, including most importantly availability of liver transplantation.
Non-operative therapy is the recommended treatment of choice for most patients beyond Milan criteria with BCLC B or intermediate HCC.1, 2 Liver resection and transplantation for patients with HCC beyond Milan criteria remains controversial. Recent multi-institutional study from the HCC East-West study group which included data from 9 centers in Europe, Japan, Argentina, and United States, reported 57% 5-year overall survival (OS) and 27% 5-year disease-free survival (DFS) for selected patients beyond Milan criteria but without major vascular invasion treated with liver resection.3 Expansion of candidacy for liver transplantation in patients with HCC beyond Milan criteria has also been explored at multiple centers with several proposed expanded criteria.4-7
Direct comparisons of liver resection to liver transplantation in patients with HCC beyond Milan criteria have been sparse. Moreover, assessment of outcomes is confounded by the clinicopathological heterogeneity of patients with hepatocellular carcinoma that affects selection of operative approach. The classic 1993 Bismuth et al. study described equivalently poor 3-year overall and disease-free survival after resection and transplantation in patients with HCC > 5 centimeters.8 A more recent study suggested nearly equivalent overall survival among patients within extended criteria (tumors < 6.5 centimeters) selected for resection and transplantation.9 Multiple groups have advocated liver resection as a bridge to transplantation or salvage transplantation for patients with recurrent HCC after resection.10, 11 This study assesses the survival of patients with HCC beyond Milan criteria treated with liver resection or liver transplantation at five high-volume referral centers. Given previously published data, we hypothesized no difference between liver resection and liver transplantation in patients beyond Milan criteria selected for operation.
Methods
Study design and population
This multi-institutional retrospective cohort study investigated associations between choice of operative treatment and survival. Patients with pathologically confirmed hepatocellular carcinoma treated with liver resection or transplantation between January 1990 and August 2011 at one of the following referral institutions: Baylor University Medical Center, Mayo Clinic (Rochester), Memorial-Sloan Kettering Cancer Center, University of Florida (Gainesville), and Washington University School of Medicine were included. Individual data collection and management were reviewed and approved by Institutional Review Board at each center; de-identified data were used for merger and statistical analyses. All patients with HCC beyond Milan criteria without vascular invasion were included in this study; patients within Milan criteria were examined previously.12 Patients beyond Milan criteria were defined based on tumor size and number using preoperative imaging. All patients met one of the following criteria at the time of diagnosis: 1) single tumor > 5 centimeters, 2) 2 or 3 tumors with at least one tumor exceeding 3 centimeters, or 3) more than 3 tumors of any size. Patient with major vascular invasion were excluded.
Preoperative demographic and clinical variables included in data abstraction were age, sex, race, BMI (kg/m2), hepatitis B and C infection, history of alcohol abuse, fibrosis (International Association for Study of the Liver grades 1-3), cirrhosis, laboratory studies (alpha-fetoprotein (AFP), albumin, total bilirubin, creatinine, INR, platelet count), clinical evidence of portal hypertension during imaging or operative findings (ascites, varices, and/or splenomegaly), Child-Pugh-Turcotte (CPT) score, and Model for End-Stage Liver Disease (MELD) score. Pathological characteristics of the HCC were abstracted from pathologic records of the liver resection or explant specimens. Preoperative tumor directed treatment including transarterial chemoembolization (TACE), bland embolization, radioembolization, external beam radiation, radiofrequency ablation (RFA), and combination embolization with RFA were abstracted. All transplant recipients underwent deceased-donor transplantation.
Outcome measures
Outcome measures included overall survival (OS) and disease-free survival (DFS). Survival was estimated from the time of operation (resection or transplantation) to death or recurrence (as applicable).
Analyses
Distributional characteristics of data for continuous variables are reported as medians with interquartile range (IQR), and as percentages for categorical data. Bivariable analyses were used to compare demographic and clinical data between patients who had liver resection and transplantation using Pearson’s Chi-square test statistic for categorical variables and Wilcoxon rank-sum test for continuous variables. Kaplan-Meier survival analysis, with the log-rank test for between-group comparisons, was used to test the bivariable effect of operative treatment on survival. Cox proportional hazards regression was used to develop a multivariable model adjusting for the combined effects of age, multiple tumors, tumor size, MELD > 12, BMI>30, and operative treatment on survival. Patient age was stratified into 5 groups during multivariable survival analyses (≤ 40 years; 41-50 years; 51-60 years, 61-70 years, and ≥ 71 years). Tumor characteristics of liver resection patients who had 5 year DFS were compared to patients who had disease recurrence within 5 years of resection.
A series of sub-group comparisons were used to test the effect of tumor downstaging among liver transplant recipients to within Milan criteria on survival. Characteristics of tumor downstaging were abstracted from explant pathology reports. Liver transplant recipients who were preoperatively treated with TACE were stratified as 1) untreated, 2) treated and failed to downstage, and 3) treated and downstaged. In addition, liver transplant recipients who were preoperatively treated with any treatment (TACE, bland embolization, radioembolization, external beam radiation, RFA, and combination embolization with RFA) were stratified as 1) untreated, 2) treated and failed to downstage, and 3) treated and downstaged. Untreated and failed to downstage patient groups were combined during survival analyses. Propensity-matched 1:1 (liver transplantation to liver resection) analyses were performed. Liver transplant recipients who were untreated prior to transplantation or pretreated and failed to downstage were matched to liver resection patients by age group, tumor size, and tumor number. One liver transplant recipient died on the day of the operation and was not matched with a resection patient. Liver resection patients who were pretreated prior to resection were matched 1:1 to liver transplant recipients by down-staging group, age group, tumor number and tumor size.
The statistical significance of observed differences in group means was assessed at the of p < 0.05 threshold. IBM SPSS Statistics version 22 and GraphPad Prism version 5 software were used to perform both data management and statistical analysis.
Results
Six hundred and forty one patients with HCC with imaging characteristics of tumors beyond Milan criteria had liver resection or transplantation during the study period. From this group, 33 patients with major vascular invasion were excluded. Of the 608 patients in the final study cohort, 480 patients had liver resection and 128 underwent liver transplantation. Demographic and clinical characteristics are summarized in Table 1. Patient-specific and HCC tumor characteristics differed significantly. Patient selected for transplantation were younger and more frequently had chronic liver disease, history of alcohol abuse and hepatitis C (all p<0.001). Liver transplant patients more frequently had sequelae of significant underlying chronic liver disease including portal hypertension, and higher CPT and MELD scores (all p<0.001). Liver resection patients had larger tumors (median 9 cm, IQR: 6.5-12.9 cm versus median 4.1 cm, IQR: 3.4-5.3 cm, p<0.001). Liver transplant recipients more frequently had multiple tumors (78% versus 28%, p<0.001). Among patients with solitary tumors, tumor size was larger in liver resection patients (median 9.5 cm, IQR: 7-13cm) than liver transplant recipients (median 5.9 cm, IQR: 5.3-6 cm), p<0.001.
Table 1.
Patient demographics and clinical characteristics
| Liver Resection (n=480) |
Liver Transplant (n=128) |
p-value | |
|---|---|---|---|
| Age (years) | 66 (53-74) | 56 (50-62) | <0.001 |
| Male | 304 (63) | 99 (77) | 0.002 |
| White race | 392 (82) | 90 (70) | 0.013 |
| BMI (kg/m2) | 26 (23-30) | 27 (25-31) | 0.031 |
| Hepatitis B | 81 (17) | 9 (7) | 0.004 |
| Hepatitis C | 48 (10) | 87 (68) | <0.001 |
| History of alcohol abuse | 39 (8) | 36 (28) | <0.001 |
| Fibrosis | 108 (23) | n/a | |
| Cirrhosis | 94 (20) | 128 (100) | <0.001 |
| Alpha-fetoprotein | 13 (4-278) | 101 (18-167) | 0.208 |
| Albumin | 4.1 (3.7-4.3) | 3.3 (2.9-3.7) | <0.001 |
| Total bilirubin | 0.6 (0.5-0.9) | 1.4 (0.7-2.4) | <0.001 |
| Creatinine | 1.0 (0.9-1.2) | 0.9 (0.7-1.1) | 0.522 |
| INR | 1.0 (1.0-1.1) | 1.3 (1.1-1.5) | <0.001 |
| Platelets | 243 (183-333) | 77 (53-152) | <0.001 |
| Portal hypertension | 39 (8) | 128 (100) | <0.001 |
| CPT score | 5 (5-5) | 7 (6-9) | <0.001 |
| MELD score | 8 (6-9) | 13 (11-17) | <0.001 |
| Largest tumor diameter (cm) | 9.0 (6.5-12.9) | 4.1 (3.4-5.3) | <0.001 |
| Single tumor | 345 (72) | 28(22) | <0.001 |
| Size single tumor (cm) | 9.5 (7-13) | 5.9(5.3-6.0) | <0.001 |
| ≥ 2 tumors | 135 (28) | 100 (78) | <0.001 |
| Size largest tumor (cm) | 8.1 (5.5-12) | 3.9 (3-3-4.6) | <0.001 |
| Any pre-treatment | 26 (5.4) | 104 (81.3) | <0.001 |
| Preoperative TACE only | 25 (5.2) | 88 (68.8) | <0.001 |
| Preoperative therapy (other) | 1 (0.2) | 16 (12.5) | <0.001 |
Data reported as n (%) or as median (interquartile range)
Preoperative therapy other:
Resection: TACE and RFA (1 patient)
Transplant: RFA (5 patients); TACE and RFA (4 patients); radioembolization (3 patients); TACE and radioembolization (1 patient); bland embolization (1 patient); external beam radiation (1 patient).
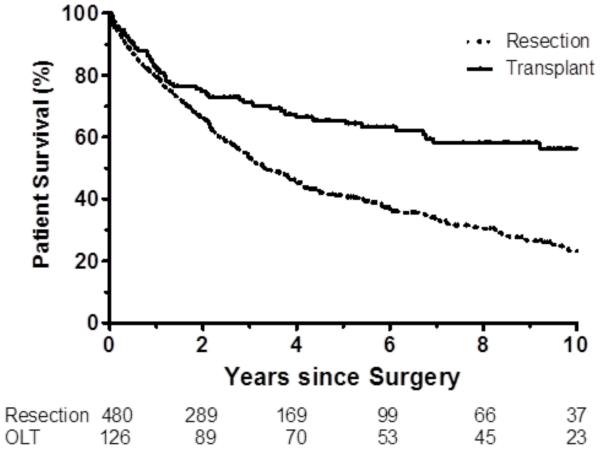
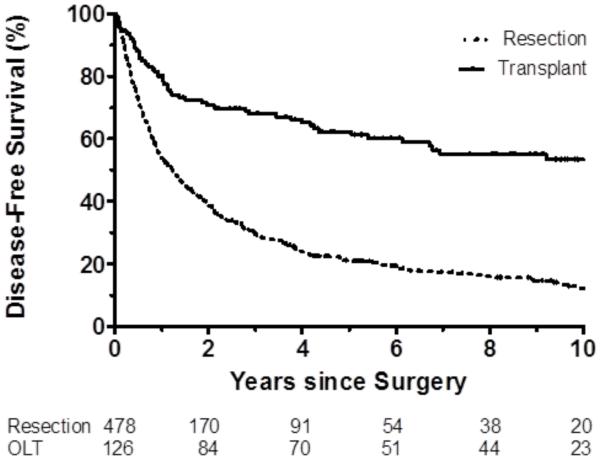
Overall 1-year, 5-year, and 10-year survival was significantly greater after liver transplantation (82%, 65%, and 56% respectively) than liver resection (79%, 41%, and 23% respectively), log-rank p<0.001 (Figure 1A). Disease-free 1-year, 5-year, and 10-year survival was also greater after liver transplantation (80%, 62%, and 53%, respectively) than liver resection (54%, 21%, and 12% respectively), log-rank p<0.001 (Figure 1B). Overall and disease-free survival were significantly greater among liver transplant recipients than liver resection patients with underlying cirrhosis (all p<0.001).
Figure 1.
a. Overall survival in full cohort transplant compared with resection (p<0.001).
b. Disease-free survival in full cohort transplant compared with resection (p<0.001).
Cox proportional hazard model summarizing effects of covariates on OS is summarized in Table 2. After adjusting for significant effects of age (HR=1.21, 95% CI: 1.10-1.3, p<0.001) and presence of multiple tumors (HR=1.72, 95% CI: 1.34-2.21, p<0.001), liver transplantation (HR=0.51, 95% CI: 0.31-0.83, p=0.007) was associated with significantly greater overall survival. Cox proportional hazard model summarizing effects of covariates on DFS is summarized in Table 3. After adjusting for significant effects of age (HR=1.10, 95% CI: 1.01-1.19, p=0.032), presence of multiple tumors (HR=1.72, 95% CI: 1.37-2.15, p<0.001), and tumor size (HR=1.03; 95% CI: 1.01-1.05, p=0.018), liver transplantation (HR=0.35, 95% CI: 0.01-0.55, p<0.001) was associated with significantly greater disease-free survival.
Table 2.
Cox Proportional Hazards Model (Overall Survival)
| HR | 95% CI | p-value | |
|---|---|---|---|
| Age | 1.21 | 1.10 – 1.33 | <0.001 |
| MELD >12 | 1.51 | 0.99 – 2.29 | 0.052 |
| BMI > 30 | 1.11 | 0.85 – 1.45 | 0.451 |
| Multiple tumors | 1.72 | 1.34 – 2.21 | <0.001 |
| Tumor Size | 1.02 | 0.99 – 1.05 | 0.080 |
| Liver transplantation | 0.51 | 0.31 – 0.83 | 0.007 |
Table 3.
Cox Proportional Hazards Model (Disease-free Survival)
| HR | 95% CI | p-value | |
|---|---|---|---|
| Age | 1.10 | 1.01 – 1.19 | 0.032 |
| MELD >12 | 1.29 | 0.83 – 1.78 | 0.309 |
| BMI > 30 | 1.15 | 0.91 – 1.47 | 0.244 |
| Multiple tumors | 1.72 | 1.37 – 2.15 | <0.001 |
| Tumor Size | 1.03 | 1.01 – 1.05 | 0.018 |
| Liver transplantation | 0.35 | 0.01 – 0.55 | <0.001 |
Among liver resection patients, 73 patients (15%) had DFS greater than 5 years. Tumor characteristics from this patient group were compared to patients who had liver resection and HCC recurrence within 5 years (Table 4). Favorable factors associated with greater DFS included younger age and presence of solitary tumor (both p≤0.034). Associations with lower AFP levels and absence of cirrhosis were statistically marginal (p=0.059 and p=0.054, respectively). Six of 480 resection patients (1%) had salvage liver transplantation for HCC recurrence; median time between resection and transplantation was 5 years (IQR: 2-10 years).
Table 4.
Patient and tumor characteristics among liver resection patients with ≥5 year DFS
| DFS≥5 years (n=73) |
DFS<5 years (n=407) |
p-value | |
|---|---|---|---|
| Age (years) | 63 (49-71) | 66 (53-75) | 0.034 |
| Fibrosis | 9 (12) | 73 (18) | 0.311 |
| Cirrhosis | 8 (11) | 86 (21) | 0.054 |
| Alpha-fetoprotein | 4.4 (3-45) | 16.8 (4-297) | 0.059 |
| MELD | 7 (6, 8) | 8 (6, 9) | 0.078 |
| Largest tumor diameter | 9 (7, 12) | 9 (6.5, 13) | 0.956 |
| Single tumor | 64 (88) | 281 (69) | 0.001 |
| Any pretreatment | 1 (1.4) | 25 (6.1) | 0.155 |
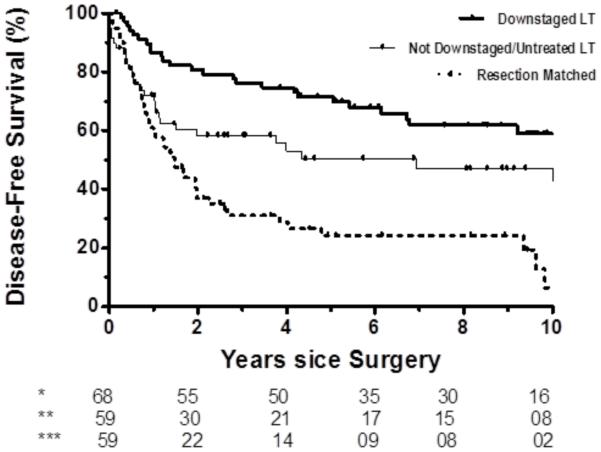
A series of sub-group survival analyses were performed to estimate the effects of tumor downstaging on survival. Twenty-six patients (5.4%) in the liver resection group had pretreatment prior to operation compared to 104 (81.3%) of liver transplant recipients, p<0.001. Pretransplant treatment strategies are summarized in Table 1. Most patients were pretreated with TACE only. Among liver transplant recipients, 63 patients (49%) had TACE with downstaging to within Milan, 33 patients (26%) had TACE and failed to downstage, and 32 patients (25%) were not treated with TACE. Both overall and disease-free survival characteristics of 1:1 matched analyses were statistically different (both p≤0.024) and are represented in Figure 2 (comparisons of matched pairs Supplement Table A). Overall survival was not different between liver transplant recipients who were untreated or pretreated with TACE and failed to downstage compared to propensity-matched 1:1 resection patients (1-year and 5-year OS 79% and 57% versus 87% and 46%, p=0.176). Disease-free survival, however, was significantly greater in liver transplant recipients who were untreated with TACE or were treated with TACE and failed to downstage patients compared to propensity-matched liver resection patients (1-year and 5-year DFS 74% and 53% versus 59% and 24%, p=0.004).
Figure 2.
a. Overall survival: TACE pretreatment in transplant patients (p=0.024 for overall comparison). 1) TACE and Downstaged – Transplant (*); 2) TACE and Not Downstaged or Untreated – Transplant (**); 3) Resection Matched (***). Survival between not downstaged / untreated liver transplant recipients and matched resection patients is not different (p=0.176)
b. Disease-free survival: TACE pretreatment in transplant patients (p<0.001 for overall comparison). 1) TACE and Downstaged – Transplant (*); 2) TACE and Not Downstaged or Untreated – Transplant (**); 3) Resection Matched (***). Survival in the downstaged / untreated liver transplant recipients greater than matched resection patients (p=0.004).
Among liver transplant recipients, 68 patients (53%) had any pretransplant treatment with downstaging to within Milan, 36 patients (28%) had any pretransplant treatment and failed to downstage, and 24 patients (19%) were not pretreated prior to transplantation. Both overall and disease-free survival characteristics of 1:1 matched analyses were statistically different (both p≤0.012) among these groups and are represented in Figure 3 (comparisons of matched pairs Supplement Table B). Overall survival was not different between liver transplant recipients who were untreated or had any pretransplant treatment modality and failed to downstage compared to propensity-matched 1:1 resection patients (1-year and 5-year OS 77% and 52% versus 88% and 46%, p=0.509). Disease-free survival, however, was significantly greater in liver transplant recipients who were either untreated or were treated and failed to downstage compared to propensity-matched 1:1 liver resection patients (1-year and 5-year DFS 72% and 50% versus 59% and 24%, p=0.017).
Figure 3.
a. Overall survival: Any pretreatment in transplant patients (p=0.012 for overall comparison). 1) Downstaged - Transplant (*); 2) Not Downstaged or Untreated – Transplant (**); 3) Resection Matched (***). Survival between not downstaged / untreated liver transplant recipients and matched resection patients is not different (p=0.509).
b. Disease-free survival: Any pretreatment in transplant patients (p<0.001 for overall comparison). 1) Downstaged – Transplant (*); 2) Not Downstaged or Untreated - Transplant (**); 3) Resection Matched (***). Survival in the downstaged / untreated liver transplant recipients greater than matched resection patients (p=0.017).
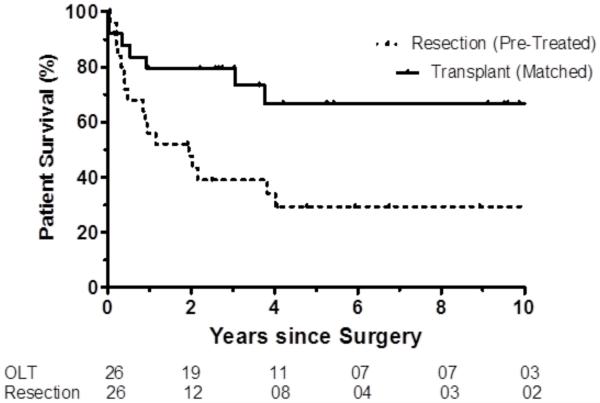
Twenty six liver resection patients who had preresection treatment were matched 1:1 by down-staging group, age group, tumor number and tumor size to patients from the liver transplant cohort. OS and DFS were significantly greater (both p≤0.012) among propensity-matched pretreated patients after liver transplantation than liver resection, Figure 4 (comparisons of matched pairs Supplement Table C).
Figure 4.
a. Overall survival: Pretreatment in resection patients. Resection pretreated and liver transplant matched (p=0.012).
b. Disease-free survival: Pretreatment in resection patients. Resection pretreated and liver transplant matched (p<0.001).
Discussion
Survival after liver resection and transplantation for patients with HCC exceeding Milan criteria is noticeably different. Despite study hypothesis supported by historical studies, both OS and DFS were significantly greater after liver transplantation. Patients treated with resection more frequently had solitary and large tumors and patients managed with transplantation more frequently had multiple smaller tumors. After multivariable adjustment for patient-specific and tumor characteristics, but before propensity-matching for effects of tumor downstaging, liver transplantation remained associated with greater OS and DFS.
Indirect comparisons to previously published multi-institutional data from the HCC East-West study group are important. 1-year and 5-year OS data for liver resection patients in the current study (79% and 41%) are comparable to the overall survival among patients beyond Milan criteria but without major vascular invasion in the East-West study group (71% and 57%). Similarly the 1-year and 5-year DFS for liver resection patients in our study (54% and 21%) are comparable to the disease-free survival in the East-West study group (63% and 27%).3 Liver transplantation was not evaluated in the East-West study cohort, however, both OS and DFS after transplantation in our study was significantly greater.
Undoubtedly, patient selection is an important contributor to survival after operative treatment of patients with HCC beyond Milan criteria. Successful tumor pretreatment and downstaging is arguably the best measure of tumor biology in this study. Overall survival was greater in the successfully pretreated transplant recipients than resection patients. Although many patients in the transplant group were pretreated with TACE, radioembolization, or ablation, disease-free survival of the untreated patients as well as patients who failed to downstage within Milan criteria but underwent transplantation was greater than the propensity matched patients undergoing resection. Importantly, overall survival among liver transplant recipients who were untreated or failed to downstage prior to transplantation and propensity-matched resection patients was similar. Only 5% liver resection patients were pretreated prior to resection and both OS and DFS was significantly worse among these patients than propensity-match liver transplant recipients.
Clearly patient selection parameters for resection and transplantation are disparate. Both resection and transplantation can be appropriate for patients with HCC exceeding radiographic Milan criteria and choice of approach is multifaceted and depends on patient-specific characteristics. Selection for resection focuses on preoperative liver function as well as parenchymal preservation and reserve. In contrast, evaluation for transplantation focuses on indirect measures of tumor biology and patients socioeconomic capacity. To balance the selection process for the two groups in a retrospective study is not possible. The degree of liver dysfunction and future liver remnant are not factors in limiting liver transplantation. Conversely socioeconomic factors rarely exclude resection, and markers of tumor biology, with exception of major vascular invasion, have not been consistently used in selection for resection. Regardless of tumor characteristics, many liver resection patients are not candidates for transplantation; similarly many liver transplant recipients are not candidates for resection. Treatment center, regional, and national variations in practice, donor availability and utilization also influence selection of treatment for patients with large or multifocal HCC.
Our data clearly show that patients selected for resection and transplantation were different despite all harboring radiographic features used to define tumors beyond Milan criteria: 1) single tumor > 5 centimeters, 2) 2 or 3 tumors with at least one tumor exceeding 3 centimeters, or 3) more than 3 tumors of any size. While classically, the Milan criteria are used in transplantation to define patients with cirrhosis who are unresectable, two factors favor clinically utility of these radiographic tumor specific cut-offs: 1) descriptors of tumor size and multifocality are clinically meaningful, and 2) recent studies (such as multi-institutional East-West study group) used similar radiographic parameters to define and analyze treatment options among all patients with hepatocellular carcinoma. Moreover, although many clinicopathological factors have correlated with survival in patients selected for resection, absolute contraindications for resection (with exception of decompensated cirrhosis) in patients with large or multifocal HCC or operative selection consensus guidelines have not been established for this population.
Liver transplant recipients who were successfully downstaged prior to transplantation had significantly greater survival than both non-downstaged and resected patients. While disease-free survival was greater after liver transplantation in untreated patients and those who failed to downstage, overall survival did not differ in propensity-matched cohort. Only 6 patients who were initially resected had salvage transplantation for HCC recurrence. Shortage of donor livers and high recurrence among poorly selected patients precludes liver transplantation as first-line treatment for all patients with large or multifocal HCC. At present approximately 6000-6500 liver transplants are performed yearly in United States, with only 15-25% of donor organs allocated for patients with HCC.13, 14 However, judicious expansion of liver transplantation criteria for patients with HCC has been supported by evidence,4, 6, 7, 15 but not adopted by United Network for Organ Sharing.
Conversely, a neoadjuvant approach to liver resection candidates, adopted in a number of other hepatobiliary, pancreatic, and upper gastrointestinal malignancies,16-18 has not been used in patients large or multifocal HCC. Only 26 patients in liver resection group were pretreated before operation. Neoadjuvant therapy has an established, but debated, role before liver transplantation, in particular to facilitate tumor downstaging and to prevent interval HCC progression until transplantation.19-22 Recent data demonstrate significant improvement in long-term survival in patients with complete pathologic response to pretransplant neoadjuvant therapy.23 In contrast, preoperative TACE before liver resection did not improve either disease-free or overall survival in two recent randomized trials, one of which largely enrolled BCLC B patients beyond Milan criteria.24, 25 Importantly, there is significant heterogeneity in patient enrollment strategies, reported tumor biology, as well as TACE technique and drug/dosage selection within studies evaluating role of TACE in patients with HCC selected for liver resection.26, 27 A recent trial reported greater survival using adjuvant TACE after liver resection with curative intent among patients beyond Milan without major vascular invasion.28 In addition, preoperative radioembolization has been gaining acceptance at select centers for patients with intermediate and advanced HCC prior to selection for resection.29-31
Another strategy to increase the role of transplantation for patients with HCC beyond Milan criteria may be the application of Ab Initio liver transplantation selectively. Although this approach has been previously employed and recently prospectively evaluated in patients with early stage HCC harboring worrisome histologic features such as microvascular invasion and/or satellitosis,32 similar findings maybe applicable after resection of patients with HCC beyond Milan criteria and an interval of observation to exclude early progression.
Patient-specific and HCC characteristics that affected patient selection for operation clearly differed in the two groups and cannot be fully addressed retrospectively. The study population was limited to patients only undergoing resection or transplantation. As such, intention-to-treat analyses that have been used to address effects of tumor downstaging and pretransplant dropout among liver transplant recipients,22, 33 were not possible with our data. We do not know the number or characteristics of those patients with tumors beyond Milan criteria who were listed for transplantation but had disease progression that precluded transplantation. Selection for transplantation in patients who did not downstage to within Milan cannot be compared to a group of patients who failed downstaging and did not proceed to transplantation. Moreover there may have been patients who were initially considered transplant candidates but subsequently underwent resection or non-operative treatment. Similarly, selection of patients for resection cannot be compared to patients with HCC who were not selected for operative treatment.
Finally, we did not correlate clinicopathological findings in our patients undergoing resection with survival to potentially use for selection criteria for resection. Preoperative alpha-fetoprotein levels were similar in both patient groups. Microvascular tumor invasion data were not available for a significant proportion of our population and were not included in the study. Not surprisingly, 20% of resection patients with long-term disease-free survival were younger and more likely to have smaller tumors, lower AFP levels, and less likely to have cirrhosis. Considerable research effort has focused on additional biologic markers of HCC, particularly those involved in angiogenesis.34, 35 Recent data suggest applicability of a model score based on preoperative protein induced by vitamin K absence-II and alpha-fetoprotein levels in correlation with explant pathology on prediction of survival among patients with HCC beyond Milan criteria selected for transplantation.36
In summary, both liver resection and liver transplantation provide curative options in selected patients with HCC beyond Milan criteria. Both patient-specific and tumor characteristics in patients selected for resection and transplantation are different. While liver transplant recipients downstaged with preoperative therapy had significantly greater survival; disease-free survival, but not overall survival, was significantly greater in liver transplant recipients who were untreated or failed to downstage compared to propensity-matched liver resection patients. Expansion of liver transplant criteria, introduction and expansion of post-resection liver transplant strategies, and improved tumor directed treatment with either neoadjuvant or adjuvant therapy in addition to liver resection, are warranted to improve outcomes in patients with HCC beyond Milan criteria.
Supplementary Material
Footnotes
Multi-institutional retrospective cohort study
References
- 1.Forner A, Gilabert M, Bruix J, et al. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525–535. doi: 10.1038/nrclinonc.2014.122. [DOI] [PubMed] [Google Scholar]
- 2.Guy J, Kelley RK, Roberts J, et al. Multidisciplinary management of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2012;10:354–362. doi: 10.1016/j.cgh.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Torzilli G, Belghiti J, Kokudo N, et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257:929–937. doi: 10.1097/SLA.0b013e31828329b8. [DOI] [PubMed] [Google Scholar]
- 4.Yao FY, Xiao L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 5.Onaca N, Davis GL, Goldstein RM, et al. Expanded criteria for liver transplantation in patients with hepatocellular carcinoma: a report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl. 2007;13:391–399. doi: 10.1002/lt.21095. [DOI] [PubMed] [Google Scholar]
- 6.Duffy JP, Vardanian A, Benjamin E, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246:502–9. doi: 10.1097/SLA.0b013e318148c704. discussion 509-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 8.Bismuth H, Chiche L, Adam R, et al. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145–151. doi: 10.1097/00000658-199308000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koniaris LG, Levi DM, Pedroso FE, et al. Is surgical resection superior to transplantation in the treatment of hepatocellular carcinoma? Ann Surg. 2011;254:527–37. doi: 10.1097/SLA.0b013e31822ca66f. discussion 537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adam R, Azoulay D, Castaing D, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg. 2003;238:508–18. doi: 10.1097/01.sla.0000090449.87109.44. discussion 518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu F, Wei Y, Wang W, et al. Salvage liver transplantation for recurrent hepatocellular carcinoma within UCSF criteria after liver resection. PLoS One. 2012;7:e48932. doi: 10.1371/journal.pone.0048932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman WC, Klintmalm G, Hemming A, et al. Surgical treatment of hepatocellular carcinoma in North America: can hepatic resection still be justified? J Am Coll Surg. 2015;220:628–637. doi: 10.1016/j.jamcollsurg.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 13.Singal AK, Guturu P, Hmoud B, et al. Evolving frequency and outcomes of liver transplantation based on etiology of liver disease. Transplantation. 2013;95:755–760. doi: 10.1097/TP.0b013e31827afb3a. [DOI] [PubMed] [Google Scholar]
- 14.Zarrinpar A, Busuttil RW. Liver transplantation: past, present and future. Nat Rev Gastroenterol Hepatol. 2013;10:434–440. doi: 10.1038/nrgastro.2013.88. [DOI] [PubMed] [Google Scholar]
- 15.Vitale A, Morales RR, Zanus G, et al. Barcelona Clinic Liver Cancer staging and transplant survival benefit for patients with hepatocellular carcinoma: a multicentre, cohort study. Lancet Oncol. 2011;12:654–662. doi: 10.1016/S1470-2045(11)70144-9. [DOI] [PubMed] [Google Scholar]
- 16.Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451–8. doi: 10.1097/01.sla.0000179678.13285.fa. discussion 458-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 18.Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman WC, Majella Doyle MB, Stuart JE, et al. Outcomes of neoadjuvant transarterial chemoembolization to downstage hepatocellular carcinoma before liver transplantation. Ann Surg. 2008;248:617–625. doi: 10.1097/SLA.0b013e31818a07d4. [DOI] [PubMed] [Google Scholar]
- 20.Otto G, Herber S, Heise M, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260–1267. doi: 10.1002/lt.20837. [DOI] [PubMed] [Google Scholar]
- 21.Belghiti J, Carr BI, Greig PD, et al. Treatment before liver transplantation for HCC. Ann Surg Oncol. 2008;15:993–1000. doi: 10.1245/s10434-007-9787-8. [DOI] [PubMed] [Google Scholar]
- 22.Yao FY, Kerlan RK, Jr, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819–827. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agopian VG, Morshedi MM, McWilliams J, et al. Complete pathologic response to pretransplant locoregional therapy for hepatocellular carcinoma defines cancer cure after liver transplantation: analysis of 501 consecutively treated patients. Ann Surg. 2015;262:536–45. doi: 10.1097/SLA.0000000000001384. discussion 543-5. [DOI] [PubMed] [Google Scholar]
- 24.Zhou WP, Lai EC, Li AJ, et al. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg. 2009;249:195–202. doi: 10.1097/SLA.0b013e3181961c16. [DOI] [PubMed] [Google Scholar]
- 25.Kaibori M, Tanigawa N, Kariya S, et al. A prospective randomized controlled trial of preoperative whole-liver chemolipiodolization for hepatocellular carcinoma. Dig Dis Sci. 2012;57:1404–1412. doi: 10.1007/s10620-012-2029-3. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y, Zhang X, Wu L, et al. Meta-analysis: preoperative transcatheter arterial chemoembolization does not improve prognosis of patients with resectable hepatocellular carcinoma. BMC Gastroenterol. 2013;13:51. doi: 10.1186/1471-230X-13-51. 230X-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng X, Sun P, Hu QG, et al. Transarterial (chemo)embolization for curative resection of hepatocellular carcinoma: a systematic review and meta-analyses. J Cancer Res Clin Oncol. 2014;140:1159–1170. doi: 10.1007/s00432-014-1677-4. [DOI] [PubMed] [Google Scholar]
- 28.Zhong C, Guo RP, Li JQ, et al. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for Stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135:1437–1445. doi: 10.1007/s00432-009-0588-2. [DOI] [PubMed] [Google Scholar]
- 29.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Vouche M, Lewandowski RJ, Atassi R, et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013;59:1029–1036. doi: 10.1016/j.jhep.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salem R, Mazzaferro V, Sangro B. Yttrium 90 radioembolization for the treatment of hepatocellular carcinoma: biological lessons, current challenges, and clinical perspectives. Hepatology. 2013;58:2188–2197. doi: 10.1002/hep.26382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrer-Fabrega J, Forner A, Liccioni A, et al. Prospective validation of ab initio liver transplantation in hepatocellular carcinoma upon detection of risk factors for recurrence after resection. Hepatology. 2016;63:839–849. doi: 10.1002/hep.28339. [DOI] [PubMed] [Google Scholar]
- 33.Cillo U, Vitale A, Grigoletto F, et al. Intention-to-treat analysis of liver transplantation in selected, aggressively treated HCC patients exceeding the Milan criteria. Am J Transplant. 2007;7:972–981. doi: 10.1111/j.1600-6143.2006.01719.x. [DOI] [PubMed] [Google Scholar]
- 34.Pang RW, Joh JW, Johnson PJ, et al. Biology of hepatocellular carcinoma. Ann Surg Oncol. 2008;15:962–971. doi: 10.1245/s10434-007-9730-z. [DOI] [PubMed] [Google Scholar]
- 35.Vitale A, Navaglia F, Ramirez Morales R, et al. Molecular refinement of clinical staging in hepatocellular carcinoma patients evaluated for potentially curative therapies. PLoS One. 2011;6:e23093. doi: 10.1371/journal.pone.0023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JH, Cho Y, Kim HY, et al. Tumor Markers Provide Refined Prognostication in Selecting Liver Transplantation Candidate for Hepatocellular Carcinoma Patients Beyond the Milan Criteria. Ann Surg. 2016;263:842–850. doi: 10.1097/SLA.0000000000001578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.