Abstract
Background
Calcium-based and non-calcium-based phosphate binders have similar efficacy in the treatment of hyperphosphatemia; however, calcium-based binders may be associated with hypercalcemia, vascular calcification, and adynamic bone disease.
Scope
A post hoc analysis was carried out of data from a 16-week, Phase IV study of patients with end-stage renal disease (ESRD) who switched to lanthanum carbonate monotherapy from baseline calcium acetate/calcium carbonate monotherapy. Of the intent-to-treat population (N=2520), 752 patients with recorded dose data for calcium acetate (n=551)/calcium carbonate (n=201) at baseline and lanthanum carbonate at week 16 were studied. Elemental calcium intake, serum phosphate, corrected serum calcium, and serum intact parathyroid hormone levels were analyzed.
Findings
Of the 551 patients with calcium acetate dose data, 271 (49.2%) had an elemental calcium intake of at least 1.5 g/day at baseline, and 142 (25.8%) had an intake of at least 2.0 g/day. Mean (95% confidence interval [CI]) serum phosphate levels were 6.1 (5.89, 6.21) mg/dL at baseline and 6.2 (6.04, 6.38) mg/dL at 16 weeks; mean (95% CI) corrected serum calcium levels were 9.3 (9.16, 9.44) mg/dL and 9.2 (9.06, 9.34) mg/dL, respectively. Of the 201 patients with calcium carbonate dose data, 117 (58.2%) had an elemental calcium intake of at least 1.5 g/day, and 76 (37.8%) had an intake of at least 2.0 g/day. Mean (95% CI) serum phosphate levels were 5.8 (5.52, 6.06) mg/dL at baseline and 5.8 (5.53, 6.05) mg/dL at week 16; mean (95% CI) corrected serum calcium levels were 9.7 (9.15, 10.25) mg/dL and 9.2 (9.06, 9.34) mg/dL, respectively.
Conclusion
Calcium acetate/calcium carbonate phosphate binders, taken to control serum phosphate levels, may result in high levels of elemental calcium intake. This may lead to complications related to calcium balance.
Keywords: calcium acetate, calcium carbonate, calcium phosphates, chronic kidney failure, elemental calcium intake, hyperphosphatemia, lanthanum carbonate
Introduction
Hyperphosphatemia (serum phosphate >5.5 mg/dL) is a highly prevalent condition of end-stage renal disease (ESRD) [1,2]. Observational studies have established that hyperphosphatemia is associated with adverse clinical outcomes, including all-cause and cardiovascular mortality, in patients with ESRD [3–9]. The management of hyperphosphatemia in patients with ESRD is challenging and typically requires a combination of dialysis, dietary phosphorus restriction, and oral phosphate binder therapy [10,11]. The phosphate binders currently used in clinical practice can be broadly classified into calcium-based (calcium acetate/calcium carbonate) and non-calcium-based (sevelamer hydrochloride/sevelamer carbonate, lanthanum carbonate and iron-based) binders [12]. The binder types have similar efficacy in reducing serum phosphate levels [13]; however, calcium-based binders have been associated with hypercalcemia, vascular calcification, and adynamic bone disease (low bone turnover) [13–16]. The impact of calcium-based binders compared with non-calcium-based ones on mortality was evaluated by Jamal et al. [17] in an updated meta-analysis of 11 randomized controlled trials. Patients assigned to non-calcium-based binders were shown to have a 22% reduction in all-cause mortality compared with those assigned to calcium-based binders (risk ratio, 0.78; 95% confidence interval [CI]: 0.61, 0.98).
To address the potential adverse effects of excess calcium load associated with calcium-based binders, the Kidney Disease Outcomes Quality Initiative (KDOQI, 2003) has made a number of guideline recommendations [10]. These include proposals that the maximum daily intake of elemental calcium from calcium-based phosphate binders should be 1.5 g, and that the total daily intake of calcium from both binders and dietary sources should not exceed 2.0 g. In addition, a maximum dialysate calcium concentration of 1.25 mmol/L is recommended to prevent intradialytic calcium loading. It is further suggested that calcium-based phosphate binders should be avoided in patients undergoing dialysis who also have vascular calcification, hypercalcemia, or plasma parathyroid hormone (PTH) levels lower than 150 pg/mL. Thus, the KDOQI guidelines emphasize the importance of minimizing calcium exposure while normalizing serum phosphate levels in patients with ESRD.
There is a need for data to understand the effects of calcium loading associated with calcium-based phosphate binders in patients with ESRD. Here, we describe a post hoc analysis of data from a clinical study of US patients with ESRD [18]. The primary objective was to evaluate elemental calcium intake in patients with ESRD receiving calcium acetate/calcium carbonate monotherapy. The secondary objectives were to evaluate changes in serum phosphate levels, serum calcium levels, and serum PTH levels in patients who switched from calcium acetate/calcium carbonate to lanthanum carbonate monotherapy.
Methods
Primary study design
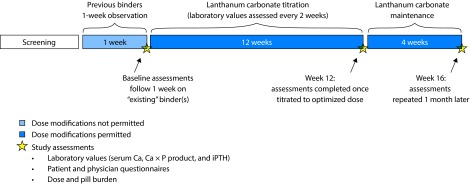
The study design was described in detail in Vemuri et al. [18] In brief, this was a 16-week, open-label, Phase IV, multicenter study of adult US patients with ESRD and hyperphosphatemia who switched to lanthanum carbonate monotherapy from other phosphate binder regimens, including calcium-based binders, sevelamer hydrochloride, and combination therapies (ClinicalTrials.gov: NCT00160121). The analysis in this article did not involve any new studies of human or animal subjects. There was an initial screening visit and a 1-week observation period during which patients remained on their previous phosphate binder therapy with no dose modifications permitted (Figure 1). After the observation period, patients began a 12-week lanthanum carbonate titration period (starting dose, 1500 mg/day; target maximum dose, 3750 mg/day) without washout to achieve serum phosphate levels within the KDOQI target range of 3.5–5.5 mg/dL [10]. Patients then continued lanthanum carbonate treatment for an additional 4-week maintenance period. Serum phosphate concentration was measured at four time points: screening, baseline (end of the 1-week observation period on previous phosphate binder therapy [week 0]), end of the lanthanum carbonate titration (week 12), and maintenance (week 16). The intent-to-treat (ITT) population (N=2520) included all patients who received at least one dose of lanthanum carbonate and were assessed for at least one efficacy evaluation. Safety assessments for the overall population are described in the primary study [18]; these data were not summarized for the set analyzed in this post hoc analysis.
Figure 1. Design of the Vemuri et al. [18] study.
The Vemuri study evaluated the efficacy of conversion to lanthanum carbonate monotherapy from previous binder regimens. The present study is a post hoc analysis of the specific subset of patients who received previous calcium acetate/calcium carbonate monotherapy before conversion to lanthanum carbonate monotherapy. With kind permission from Springer Science+Business Media: The real-world dose-relativity of sevelamer hydrochloride and lanthanum carbonate monotherapy in patients with end-stage renal disease, Adv Ther 2013 Dec; 30(12);1100–10, Wilson RJ, Keith MS, Preston P, Copley JB, Figure 1. Ca × P, calcium phosphate product; iPTH, intact parathyroid hormone.
The study protocol was approved by an Institutional Review Board, and written, informed consent was obtained from all patients before study participation.
Post hoc analysis
Analysis population
The post hoc analysis population comprised patients in the ITT population who had received previous calcium acetate/calcium carbonate monotherapy, and had recorded dose data for calcium acetate/calcium carbonate at baseline and lanthanum carbonate at week 16.
Phosphate binder dose formulations
Calcium acetate was taken in just one formulation (PhosLo*), comprising 667 mg tablets/capsules (elemental calcium, 169 mg), whereas calcium carbonate was taken in a variety of formulations, including generic calcium carbonate, Tums†, and Os-Cal‡ (Table 1). Lanthanum carbonate (Fosrenol§) was taken as 250 mg or 500 mg tablets [18].
Table 1.
Calcium acetate/carbonate dose formulations.
| Formulation, dose | Elemental calcium dose | Number of tablets that provide elemental calcium ≥1.5 g/day | Number of tablets that provide elemental calcium ≥2.0 g/day | |
|---|---|---|---|---|
| Calcium acetate | PhosLo 667 mg | 169 mg | 9 | 12 |
|
| ||||
| Calcium carbonate | Calci-Mix powder 1250 mg | 500 mg | 3 | 4 |
|
| ||||
| Calcium carbonate (generic) | ||||
| 400 mg | 160 mg | 10 | 13 | |
| 500 mg | 200 mg | 8 | 10 | |
| 600 mg | 240 mg | 7 | 9 | |
| 650 mg | 260 mg | 6 | 8 | |
|
| ||||
| Caltrate 600 Plus 1500 mg | 600 mg | 3 | 4 | |
|
| ||||
| Os-Cal 1250 mg | 500 mg | 3 | 4 | |
|
| ||||
| Tums | ||||
| Regular, 500 mg | 200 mg | 8 | 10 | |
| Extra strength, 750 mg | 300 mg | 5 | 7 | |
Tablet numbers were rounded up to the nearest whole tablet.
Statistical analysis
Patient-level daily phosphate binder doses, serum phosphate, corrected (albumin-adjusted) serum calcium levels, and serum intact PTH levels recorded at baseline and at week 16 were analyzed in the overall analysis population and by daily elemental calcium stratum (<1.0, 1.0 to <1.5, 1.5 to <2.0, 2.0 to <2.5, ≥2.5 g/day). Summary statistics were produced for all measurements. As a result of the post hoc nature of this evaluation, no formal statistical analyses were performed.
Results
Post hoc analysis population
A total of 1045 patients in the ITT population switched from calcium acetate/calcium carbonate monotherapy to lanthanum carbonate monotherapy and had recorded baseline dose data for calcium acetate/calcium carbonate. The post hoc analysis population comprised a subset of 752 patients with recorded dose data for calcium acetate (n=551)/calcium carbonate (n=201) at baseline and lanthanum carbonate at week 16. Exclusion of patients from the post hoc analysis population was mainly due to study discontinuation before week 16 (Table 2). The most commonly reported reason for study discontinuation was adverse events (n=252 [14.3%]). Baseline demographic and clinical characteristics were similar in the post hoc analysis population and in the excluded patients (Table 3).
Table 2.
Disposition of the post hoc analysis population.
| Total ITT population (N=2520) | Excluded population (n=1768) | Post hoc populationa (n=752) | ||
|---|---|---|---|---|
| Calcium acetate monotherapy (n=551) | Calcium carbonate monotherapy (n=201) | |||
| Completed, n (%) | 1751 (69.5) | 1052 (59.5) | 515 (93.5) | 184 (91.5) |
| Unknown, n (%) | 22 (0.9) | 21 (1.2) | 1 (0.2) | 0 |
| Withdrew from primary study, n (%) | 747 (29.6) | 695 (39.3) | 35 (6.4) | 17 (8.5) |
| Adverse event, n (%) | 272 (10.8) | 252 (14.3) | 11 (2.0) | 9 (4.5) |
| Withdrew consent, n (%) | 177 (7.0) | 161 (9.1) | 11 (2.0) | 5 (2.5) |
| Investigator decision, n (%) | 142 (5.6) | 134 (7.6) | 7 (1.3) | 1 (0.5) |
| Alternative phosphate binder, n (%) | 69 (2.7) | 66 (3.7) | 3 (0.5) | 0 |
| Death, n (%) | 38 (1.5) | 38 (2.1) | 0 | 0 |
| Lost to follow-up, n (%) | 49 (1.9) | 44 (2.5) | 3 (0.5) | 2 (1.0) |
The post hoc analysis population comprised patients with recorded dose data for calcium acetate/calcium carbonate at baseline and lanthanum carbonate at week 16.
ITT, intent-to-treat.
Table 3.
Baseline demographics and clinical characteristics of the post hoc analysis population.
| Total ITT population (N=2520) | Excluded patients (n=1768) | Post hoc analysis population (n=752)a | ||
|---|---|---|---|---|
|
| ||||
| Calcium acetate monotherapy (n=551) | Calcium carbonate monotherapy (n=201) | |||
| Age (years) | 57 (14) | 56 (14) | 57 (14) | 58 (14) |
|
| ||||
| Sex, n (%) | ||||
| Male | 1480 (58.7) | 996 (56.3) | 344 (62.4) | 140 (69.7) |
| Female | 1040 (41.3) | 772 (43.7) | 207 (37.6) | 61 (30.3) |
|
| ||||
| Race, n (%) | ||||
| Caucasian | 1355 (53.8) | 941 (53.2) | 302 (54.8) | 112 (55.7) |
| African-American | 1006 (39.9) | 702 (39.7) | 225 (40.8) | 79 (39.3) |
| Asian/Pacific Islander | 55 (2.2) | 43 (2.4) | 10 (1.8) | 2 (1.0) |
| Native American | 13 (0.5) | 11 (0.6) | 1 (0.2) | 1 (0.5) |
| Other | 90 (3.6) | 71 (4.0) | 12 (2.2) | 7 (3.5) |
|
| ||||
| Albumin, g/dL | 3.9 (0.5) | 3.9 (0.5) | 3.9 (0.4) | 3.8 (0.5) |
|
| ||||
| Serum phosphate, mg/dL | 6.0 (1.8) | 6.0 (1.8) | 6.0 (1.9) | 5.8 (1.9) |
|
| ||||
| Corrected serum calcium, mg/dL | 9.5 (2.6) | 9.5 (2.6) | 9.3 (1.6) | 9.7 (3.9) |
|
| ||||
| Intact serum PTH, pg/mL | 327.0 (340.1) | 348.7 (355.7) | 279.6 (303.7) | 286.5 (291.1) |
|
| ||||
| Diabetes, n (%) | 1244 (49.4) | 858 (48.5) | 289 (52.5) | 97 (48.3) |
|
| ||||
| Time on dialysis, years | 3.7 (3.7) | 3.8 (3.8) | 3.3 (3.5) | 3.2 (3.9) |
Data are mean (standard deviation), unless otherwise indicated.
The post hoc analysis population comprised patients with recorded dose data for calcium acetate/calcium carbonate at baseline and lanthanum carbonate at week 16.
ITT, intent-to-treat; PTH, parathyroid hormone.
Calcium carbonate usage
An assessment of calcium carbonate usage revealed that 89.1% of patients on calcium carbonate monotherapy took Tums regular or extra-strength formulations (Table 4).
Table 4.
Calcium carbonate usage.
| Calcium carbonate formulation, dose | Patients, n (%) |
|---|---|
| Calci-Mix powder 1250 mg | 1 (0.5) |
| Calcium carbonate (generic)a | 6 (3.0) |
| Caltrate 600 Plus | 1 (0.5) |
| Os-Cal | 14 (7.0) |
| Tumsb | 179 (89.1) |
| Total | 201 (100) |
Calcium carbonate 400, 500, 600, and 650 mg formulations.
Tums regular or extra strength formulations.
Conversion from calcium acetate monotherapy to lanthanum carbonate monotherapy
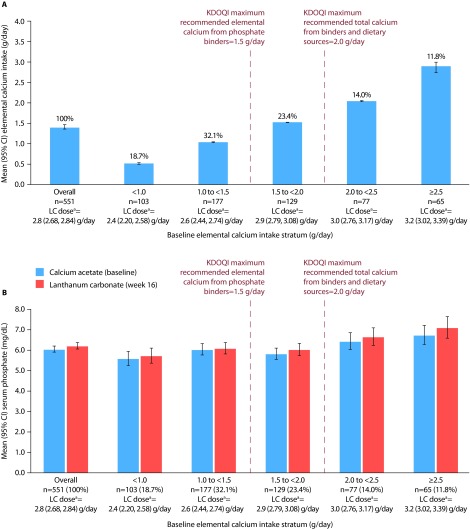
Of the 551 patients with recorded doses of calcium acetate at baseline and lanthanum carbonate at week 16, 271 (49.2%) had an elemental calcium intake of at least 1.5 g/day, and 142 (25.8%) had an elemental calcium intake of at least 2.0 g/day at baseline. Overall mean (95% CI) elemental calcium intake at baseline was 1.4 (1.35, 1.47) g/day (Figure 2A). Overall mean (95% CI) serum phosphate levels were 6.1 (5.89, 6.21) mg/dL on baseline calcium acetate monotherapy and 6.2 (6.04, 6.38) mg/dL after 16 weeks of lanthanum carbonate monotherapy (Figure 2B).
Figure 2. Elemental calcium intake and serum phosphate levels in patients who switched from calcium acetate to lanthanum carbonate monotherapy.
A, mean elemental calcium intake; B, mean serum phosphate levels. Bars show mean (95% CI). Patients were stratified based on their elemental calcium intake from calcium acetate monotherapy (proportions in each stratum are indicated as percentages in A above each relevant bar).
aMean (95% CI) lanthanum carbonate dose at week 16.
CI, confidence interval; KDOQI, Kidney Disease Outcomes Quality Initiative; LC, lanthanum carbonate.
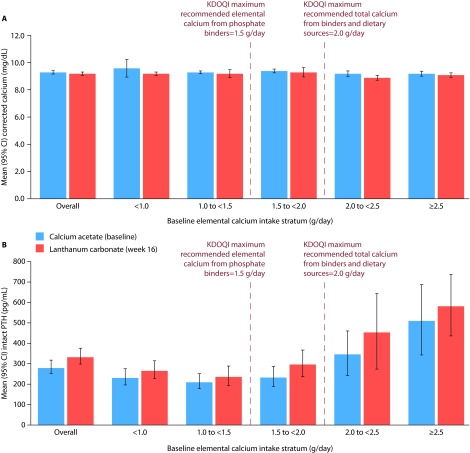
Overall mean (95% CI) corrected serum calcium levels were similar at baseline (9.3 [9.16, 9.44] mg/dL) and at week 16 (9.2 [9.06, 9.34] mg/dL; Figure 3A). However, overall mean (95% CI) serum PTH levels increased slightly (approximately +19%) from baseline (279.6 [244.86, 314.34] pg/mL) to week 16 (332.6 [292.42, 372.78] pg/mL; Figure 3B). In patients in the highest elemental calcium stratum (≥2.5 g/day), mean (95% CI) corrected serum calcium levels showed minimal change between baseline (9.2 [9.00, 9.40] mg/dL) and week 16 (9.1 [8.92, 9.28] mg/dL); however, mean (95% CI) PTH levels were noticeably high at both time points (511.0 [337.13, 684.87] pg/mL and 582.7 [430.67, 734.73] pg/mL, respectively).
Figure 3. Mean corrected serum calcium and intact PTH levels in patients who switched from calcium acetate to lanthanum carbonate monotherapy.
A, mean corrected serum calcium levels; B, mean PTH levels. Bars show mean (95% CI). Patients were stratified based on their elemental calcium intake from calcium acetate monotherapy.
CI, confidence interval; KDOQI, Kidney Disease Outcomes Quality Initiative; PTH, parathyroid hormone.
Conversion from calcium carbonate monotherapy to lanthanum carbonate monotherapy
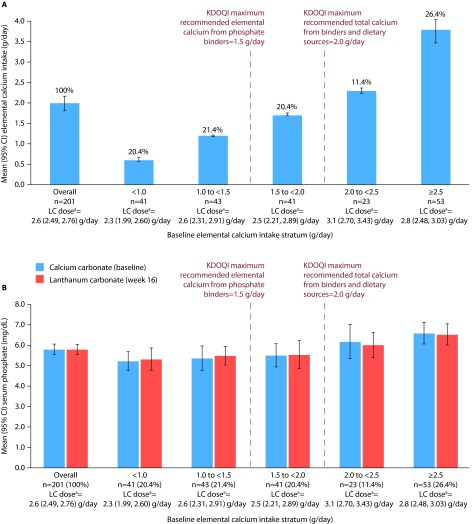
Of the 201 patients with recorded doses of calcium carbonate at baseline and lanthanum carbonate at week 16, 117 (58.2%) had an elemental calcium intake of at least 1.5 g/day and 76 (37.8%) had an elemental calcium intake of at least 2.0 g/day. Overall mean (95% CI) elemental calcium intake at baseline was 2.0 (1.80, 2.17) g/day (Figure 4A).
Figure 4. Elemental calcium intake and serum phosphate levels in patients who switched from calcium carbonate to lanthanum carbonate monotherapy.
A, mean elemental calcium intake; B, mean serum phosphate levels. Bars show mean (95% CI). Patients were stratified based on their elemental calcium intake from calcium carbonate monotherapy (proportions in each stratum are indicated as percentages in A above each relevant bar).
aMean (95% CI) lanthanum carbonate dose at week 16.
CI, confidence interval; KDOQI, Kidney Disease Outcomes Quality Initiative; LC, lanthanum carbonate.
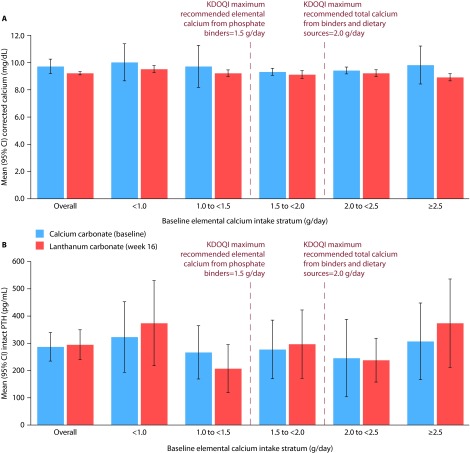
Overall mean (95% CI) serum phosphate levels with baseline calcium carbonate monotherapy (5.8 [5.52, 6.06] mg/dL) were maintained after 16 weeks of lanthanum carbonate monotherapy (5.8 [5.53, 6.05] mg/dL) (Figure 4B).
Overall mean (95% CI) corrected serum calcium levels were comparable at baseline (9.7 [9.15, 10.25] mg/dL) and at week 16 (9.2 [9.06, 9.34] mg/dL; Figure 5A), as were overall mean (95% CI) serum PTH levels (286.5 [233.20, 339.80] pg/mL and 294.2 [238.63, 349.77] pg/mL, respectively; Figure 5B). In patients in the 2.5 g/day or above elemental calcium stratum, mean (95% CI) corrected serum calcium levels decreased slightly from baseline (9.8 [8.39, 11.21] mg/dL) to week 16 (8.9 [8.62, 9.18] mg/dL), while mean (95% CI) PTH levels increased from baseline (306.5 [164.98, 448.02] pg/mL) to week 16 (373.1 [210.13, 536.07] pg/mL).
Figure 5. Mean corrected serum calcium and intact PTH levels in patients who switched from calcium carbonate to lanthanum carbonate monotherapy.
A, mean corrected serum calcium levels; B, mean PTH levels. Bars show mean (95% CI). Patients were stratified based on their elemental calcium intake from calcium carbonate monotherapy.
CI, confidence interval; KDOQI, Kidney Disease Outcomes Quality Initiative; PTH, parathyroid hormone.
Discussion
Herein we have presented the results of a post hoc analysis of elemental calcium intake in US patients with ESRD taking calcium acetate/calcium carbonate monotherapy. Our main findings were that 49.2% of patients on calcium acetate monotherapy and 58.2% of patients on calcium carbonate monotherapy had an elemental calcium intake of at least 1.5 g/day, which is the recommended maximum daily intake of calcium from calcium-based phosphate binders. Furthermore, 25.8% of patients on calcium acetate monotherapy and 37.8% of patients on calcium carbonate monotherapy had an elemental calcium intake of 2.0 g/day or more, the recommended maximum daily intake of calcium from all sources (phosphate binders, diet, dialysate) [10]. These figures do not take into account the contribution of dietary calcium, and although patients with ESRD typically have a restricted intake of dietary calcium (mean, 0.5–0.6 g/day) [19–21], it is reasonable to suggest that the percentage of patients exceeding the KDOQI recommendation of 2.0 g/day would have been higher if we had been able to calculate the total calcium exposure from all sources. Our results are conservative compared with those from a previous study by Sigrist and McIntyre [21], who reported that 73% (19/26) of patients undergoing dialysis who were prescribed calcium-based binders had an elemental calcium intake from calcium-based binders in excess of 2.0 g/day.
The results of the current analysis raise important questions regarding the impact on calcium balance (net intake minus net excretion) of oral calcium from calcium-based phosphate binders. Several studies have investigated calcium balance in patients with ESRD. Hill et al. demonstrated in a study of eight patients with chronic kidney disease that intake of calcium carbonate was associated with a positive calcium balance compared with patients receiving placebo [22]. Sigrist and McIntyre [21] reported that all of their study patients taking calcium-based binders (n=26) had a positive calcium balance. Here, it should be noted that serum calcium is not a good proxy for calcium balance because more than 99% of total body calcium is stored dynamically in bone; less than 1% exists in the extracellular space. Thus, excess calcium intake does not manifest as hypercalcemia in healthy individuals [23] or in patients with ESRD [21,24]. This is supported by research from Byrne et al., who reported that an excessive calcium intake may coexist with a normal serum calcium level in patients undergoing hemodialysis [25]. Our results are consistent with the concept of calcium homeostasis: serum calcium levels were similar in patients on baseline calcium acetate monotherapy and after switching to lanthanum carbonate monotherapy for 16 weeks. Even in the highest elemental calcium stratum (≥2.5 g/day), serum calcium levels showed little change between baseline and week 16. There was a slight reduction in serum calcium levels when switching from calcium carbonate to lanthanum carbonate. This is consistent with the fact that calcium carbonate doses result in higher overall elemental calcium levels than calcium acetate doses.
A large number of studies have reported that calcium-based binder use is associated with vascular calcification [26–36]. Furthermore, significantly higher all-cause mortality has been reported in patients receiving calcium-based versus non-calcium-based phosphate binders [17]. This is of particular interest, considering that calcium-based binders are more commonly prescribed than non-calcium-based binders [37]. However, not all studies have found an association between calcium-based binders and vascular calcification [38,39]. In one study, in which the majority of participants were prescribed calcium-based binders, KDOQI targets for serum calcium levels were achieved by 57.8% of patients [37]. Nevertheless, cardiovascular events were reported in 76.0% of patients, and calcification was reported in 41.8%, 34.1% of which was caused by vascular calcification [37]. Furthermore, vascular calcification typically occurs in ~70% of patients with stage 3–4 chronic kidney disease [40], for whom calcium-based binders or calcium supplements are rarely prescribed, arguing against the idea that exogenous calcium plays a major role in the pathogenesis of vascular calcification. Thus, a clear etiological pathway linking calcium-based binder use and a positive calcium balance to vascular calcification and mortality has yet to be established.
PTH levels increased slightly after switching from calcium acetate/calcium carbonate to lanthanum carbonate. These results are consistent with the principles of calcium homeostasis, because a daily dose of calcium acetate/calcium carbonate would be expected to suppress PTH secretion [23]. Interestingly, in the subset of patients who received the highest doses of calcium acetate (≥2.5 g/day elemental calcium), mean PTH levels were higher than 500 pg/mL at both baseline and week 16. These high PTH levels are probably related to the high phosphate levels in these patients [41]; mean phosphate levels were 6.7 mg/dL at baseline and 7.1 mg/dL at week 16.
There is an association between serum phosphate levels above the KDOQI-recommended range and poor outcomes, including mortality and cardiovascular complications, in patients with ESRD [10]. One study demonstrated that phosphate levels over 6.2 mg/dL were associated with cardiovascular complications [42]; two further studies demonstrated that the relative risk of mortality increased with serum phosphate levels over 6.5 mg/dL [43] and 7.0 mg/dL [44]. Overall, mean serum phosphate levels tended to remain stable after switching from calcium carbonate or calcium acetate to lanthanum carbonate. Nevertheless, levels were elevated (>5.5 mg/dL) at baseline and after switching, particularly in those with a baseline elemental calcium intake of 2.0 g/day to <2.5 g/day and ≥2.5 g/day. This suggests that very high doses of calcium-based binders were being used in an unsuccessful attempt to control serum phosphate levels. Thus, despite the use of calcium-based binders in doses that frequently exceed the recommended amount of elemental calcium, achieving phosphorus control remains challenging in real-world practice. This is supported by an observational study of 8265 patients in Japan, Europe, and the United States receiving hemodialysis, which demonstrated that 52% had serum phosphate concentrations above the upper target of 5.5 mg/dL, despite the fact that 81% of all patients were taking phosphate binders (of those, ~80% were taking calcium-based binders) [9].
Limitations of the current analysis include those inherent to post hoc analyses [45]. The lack of dietary calcium data meant that the total calcium exposure could not be calculated. Furthermore, the possibility of bias due to unidirectional switching from calcium acetate/calcium carbonate to lanthanum carbonate, in line with the primary study design, cannot be ruled out.
Additional information on dialysis adequacy, dietary phosphorus intake, urinary phosphorus excretion, and residual renal function would be useful for further interpretation of the results; however, these data were not collected in the original study. Furthermore, in some respects, patients served as their own controls because the same patients were studied before and after switching treatment. As such, it is less likely that dialysis adequacy, dietary phosphorus intake, residual renal function, and urinary phosphorus excretion changed before and after switching.
Conclusions
This post hoc analysis of real-world clinical data shows that a large proportion of patients with ESRD taking calcium acetate/calcium carbonate monotherapy to treat hyperphosphatemia ingest elemental calcium at levels above the KDOQI-recommended daily limits. These patients may be at risk of developing vascular calcification and adynamic bone disease. It is important to consider these findings when decisions are made regarding the comparative utility of calcium-based phosphate binders over non-calcium-based phosphate binders in patients with ESRD.
Acknowledgments
The authors would like to thank Peter Preston for his contributions to the analyses in the manuscript. Writing and editorial support was provided by Fernando Gibson, PhD, and Noëlle L O’Regan, PhD, of PharmaGenesis London, London, UK with funding from Shire Development LLC.
Abbreviations
- Ca × P
calcium phosphate product
- CI
confidence interval
- ESRD
end-stage renal disease
- iPTH
intact parathyroid hormone
- ITT
intent-to-treat
- KDOQI
Kidney Disease Outcomes Quality Initiative
- PTH
parathyroid hormone
- SD
standard deviation
Footnotes
Disclosure and potential conflicts of interest: J Brian Copley is an employee of Shire. Rosamund J Wilson is a consultant to Shire. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests forms for the authors are available for download at: http://www.drugsincontext.com/wp-content/uploads/2017/01/dic.212302-COI.pdf.
Contributions: All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. All authors contributed equally to the study design, the collection, analysis, and interpretation of data, and the writing of the manuscript.
Funding declaration: This analysis was funded by Shire Development LLC. The study sponsor had no direct involvement in the study design, in the collection, analysis, and interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Clinical trial registration number: ClinicalTrials.gov: NCT00160121
Correct attribution: Copyright © 2017 Wilson RJ, Copley JB. https://doi.org/10.7573/dic.212302. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 3.0.
Provenance: Submitted, externally peer reviewed
Peer review comments to author: 24 October 2016
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 14 Weller Street, London, SE1 1QU, UK
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252772009
For all manuscript and submissions enquiries, contact Dr Gordon Mallarkey, Editor-in-Chief gordon.mallarkey@bioexcelpublishing.com
For all permissions, rights and reprints, contact Peter Clarke peter.clarke@bioexcelpublishing.com
PhosLo is a registered trademark of Fresenius Medical Care, Bad Homburg, Germany.
Tums is a registered trademark of GlaxoSmithKline, Brentford, UK.
Os-Cal is a registered trademark of GlaxoSmithKline, Brentford, UK.
Fosrenol is a registered trademark of Shire Pharmaceutical Contracts Ltd, Basingstoke, UK.
References
- 1.Albaaj F, Hutchison A. Hyperphosphataemia in renal failure: causes, consequences and current management. Drugs. 2003;63:577–96. doi: 10.2165/00003495-200363060-00005. http://dx.doi.org/10.2165/00003495-200363060-00005. [DOI] [PubMed] [Google Scholar]
- 2.Hruska KA, Mathew S, Lund R, Qiu P, Pratt R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008;74:148–57. doi: 10.1038/ki.2008.130. http://dx.doi.org/10.1038/ki.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–80. doi: 10.1038/sj.ki.5001514. http://dx.doi.org/10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 4.Menon V, Greene T, Pereira AA, Wang X, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ. Relationship of phosphorus and calcium-phosphorus product with mortality in CKD. Am J Kidney Dis. 2005;46:455–63. doi: 10.1053/j.ajkd.2005.05.025. http://dx.doi.org/10.1053/j.ajkd.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 5.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–18. doi: 10.1097/01.ASN.0000133041.27682.A2. http://dx.doi.org/10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 6.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12:2131–8. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 7.Melamed ML, Eustace JA, Plantinga L, Jaar BG, Fink NE, Coresh J, Klag MJ, Powe NR. Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006;70:351–7. doi: 10.1038/sj.ki.5001542. http://dx.doi.org/10.1038/sj.ki.5001542. [DOI] [PubMed] [Google Scholar]
- 8.Stevens LA, Djurdjev O, Cardew S, Cameron EC, Levin A. Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: evidence for the complexity of the association between mineral metabolism and outcomes. J Am Soc Nephrol. 2004;15:770–9. doi: 10.1097/01.asn.0000113243.24155.2f. http://dx.doi.org/10.1097/01.ASN.0000113243.24155.2F. [DOI] [PubMed] [Google Scholar]
- 9.Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005;67:1179–87. doi: 10.1111/j.1523-1755.2005.00185.x. http://dx.doi.org/10.1111/j.1523-1755.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 10.National Kidney Foundation. KDOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–201. http://dx.doi.org/10.1016/S0272-6386(03)00905-3. [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int. 2009;76(Suppl 113):S1–S130. doi: 10.1038/ki.2009.188. http://dx.doi.org/10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 12.Hutchison AJ, Smith CP, Brenchley PE. Pharmacology, efficacy and safety of oral phosphate binders. Nat Rev Nephrol. 2011;7:578–89. doi: 10.1038/nrneph.2011.112. http://dx.doi.org/10.1038/nrneph.2011.112. [DOI] [PubMed] [Google Scholar]
- 13.Navaneethan SD, Palmer SC, Craig JC, Elder GJ, Strippoli GF. Benefits and harms of phosphate binders in CKD: a systematic review of randomized controlled trials. Am J Kidney Dis. 2009;54:619–37. doi: 10.1053/j.ajkd.2009.06.004. http://dx.doi.org/10.1053/j.ajkd.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Raggi P, James G, Burke SK, Bommer J, Chasan-Taber S, Holzer H, Braun J, Chertow GM. Decrease in thoracic vertebral bone attenuation with calcium-based phosphate binders in hemodialysis. J Bone Miner Res. 2005;20:764–72. doi: 10.1359/JBMR.041221. http://dx.doi.org/10.1359/JBMR.041221. [DOI] [PubMed] [Google Scholar]
- 15.Hutchison AJ, Maes B, Vanwalleghem J, Asmus G, Mohamed E, Schmieder R, Backs W, Jamar R, Vosskuhler A. Efficacy, tolerability, and safety of lanthanum carbonate in hyperphosphatemia: a 6-month, randomized, comparative trial versus calcium carbonate. Nephron Clin Pract. 2005;100:c8–19. doi: 10.1159/000084653. http://dx.doi.org/10.1159/000084653. [DOI] [PubMed] [Google Scholar]
- 16.D’Haese PC, Spasovski GB, Sikole A, Hutchison A, Freemont TJ, Sulkova S, Swanepoel C, Pejanovic S, Djukanovic L, Balducci A, Coen G, Sulowicz W, Ferreira A, Torres A, Curic S, Popovic M, Dimkovic N, De Broe ME. A multicenter study on the effects of lanthanum carbonate (Fosrenol) and calcium carbonate on renal bone disease in dialysis patients. Kidney Int Suppl. 2003:S73–8. doi: 10.1046/j.1523-1755.63.s85.18.x. http://dx.doi.org/10.1046/j.1523-1755.63.s85.18.x. [DOI] [PubMed] [Google Scholar]
- 17.Jamal SA, Vandermeer B, Raggi P, Mendelssohn DC, Chatterley T, Dorgan M, Lok CE, Fitchett D, Tsuyuki RT. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet. 2013;382:1268–77. doi: 10.1016/S0140-6736(13)60897-1. http://dx.doi.org/10.1016/S0140-6736(13)60897-1. [DOI] [PubMed] [Google Scholar]
- 18.Vemuri N, Michelis MF, Matalon A. Conversion to lanthanum carbonate monotherapy effectively controls serum phosphorus with a reduced tablet burden: a multicenter open-label study. BMC Nephrol. 2011;12:49. doi: 10.1186/1471-2369-12-49. http://dx.doi.org/10.1186/1471-2369-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coburn JW, Koppel MH, Brickman AS, Massry SG. Study of intestinal absorption of calcium in patients with renal failure. Kidney Int. 1973;3:264–72. doi: 10.1038/ki.1973.40. http://dx.doi.org/10.1038/ki.1973.40. [DOI] [PubMed] [Google Scholar]
- 20.Taal MW, Masud T, Green D, Cassidy MJ. Risk factors for reduced bone density in haemodialysis patients. Nephrol Dial Transplant. 1999;14:1922–8. doi: 10.1093/ndt/14.8.1922. http://dx.doi.org/10.1093/ndt/14.8.1922. [DOI] [PubMed] [Google Scholar]
- 21.Sigrist M, McIntyre CW. Calcium exposure and removal in chronic hemodialysis patients. J Ren Nutr. 2006;16:41–6. doi: 10.1053/j.jrn.2005.10.006. http://dx.doi.org/10.1053/j.jrn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Hill KM, Martin BR, Wastney ME, McCabe GP, Moe SM, Weaver CM, Peacock M. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3–4 chronic kidney disease. Kidney Int. 2013;83:959–66. doi: 10.1038/ki.2012.403. http://dx.doi.org/10.1038/ki.2012.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol. 2010;(5 Suppl):S23–30. doi: 10.2215/CJN.05910809. http://dx.doi.org/10.2215/CJN.05910809. [DOI] [PubMed] [Google Scholar]
- 24.Moe SM, Chertow GM. The case against calcium-based phosphate binders. Clin J Am Soc Nephrol. 2006;1:697–703. doi: 10.2215/CJN.00560206. http://dx.doi.org/10.2215/CJN.00560206. [DOI] [PubMed] [Google Scholar]
- 25.Byrne FN, Kinsella S, Murnaghan DJ, Kiely M, Eustace JA. Lack of correlation between calcium intake and serum calcium levels in stable haemodialysis subjects. Nephron Clin Pract. 2009;113:c162–8. doi: 10.1159/000232597. http://dx.doi.org/10.1159/000232597. [DOI] [PubMed] [Google Scholar]
- 26.Braun J, Asmus HG, Holzer H, Brunkhorst R, Krause R, Schulz W, Neumayer HH, Raggi P, Bommer J. Long-term comparison of a calcium-free phosphate binder and calcium carbonate–phosphorus metabolism and cardiovascular calcification. Clin Nephrol. 2004;62:104–15. doi: 10.5414/cnp62104. [DOI] [PubMed] [Google Scholar]
- 27.Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19:213–6. doi: 10.1681/ASN.2007080854. http://dx.doi.org/10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- 28.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–41. doi: 10.1038/sj.ki.5002059. http://dx.doi.org/10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 29.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68:1815–24. doi: 10.1111/j.1523-1755.2005.00600.x. http://dx.doi.org/10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 30.Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–52. doi: 10.1046/j.1523-1755.2002.00434.x. http://dx.doi.org/10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 31.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–83. doi: 10.1056/NEJM200005183422003. http://dx.doi.org/10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 32.Guerin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15:1014–21. doi: 10.1093/ndt/15.7.1014. http://dx.doi.org/10.1093/ndt/15.7.1014. [DOI] [PubMed] [Google Scholar]
- 33.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–40. doi: 10.1093/ndt/gfg414. http://dx.doi.org/10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 34.Qunibi W, Moustafa M, Muenz LR, He DY, Kessler PD, Diaz-Buxo JA, Budoff M. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis. 2008;51:952–65. doi: 10.1053/j.ajkd.2008.02.298. http://dx.doi.org/10.1053/j.ajkd.2008.02.298. [DOI] [PubMed] [Google Scholar]
- 35.Raggi P, Vukicevic S, Moyses RM, Wesseling K, Spiegel DM. Ten-year experience with sevelamer and calcium salts as phosphate binders. Clin J Am Soc Nephrol. 2010;(5 Suppl 1):S31–40. doi: 10.2215/CJN.05880809. http://dx.doi.org/10.2215/CJN.05880809. [DOI] [PubMed] [Google Scholar]
- 36.Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, Russo L, Scafarto A, Andreucci VE. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int. 2007;72:1255–61. doi: 10.1038/sj.ki.5002518. http://dx.doi.org/10.1038/sj.ki.5002518. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Martin JL, Carrero JJ, Benedik M, Bos WJ, Covic A, Ferreira A, Floege J, Goldsmith D, Gorriz JL, Ketteler M, Kramar R, Locatelli F, London G, Martin PY, Memmos D, Nagy J, Naves-Diaz M, Pavlovic D, Rodriguez-Garcia M, Rutkowski B, Teplan V, Tielemans C, Verbeelen D, Wuthrich RP, Martinez-Camblor P, Cabezas-Rodriguez I, Sanchez-Alvarez JE, Cannata-Andia JB. COSMOS: the dialysis scenario of CKD-MBD in Europe. Nephrol Dial Transplant. 2013;28:1922–35. doi: 10.1093/ndt/gfs418. http://dx.doi.org/10.1093/ndt/gfs418. [DOI] [PubMed] [Google Scholar]
- 38.Friedman EA. Calcium-based phosphate binders are appropriate in chronic renal failure. Clin J Am Soc Nephrol. 2006;1:704–9. doi: 10.2215/CJN.01831105. http://dx.doi.org/10.2215/CJN.01831105. [DOI] [PubMed] [Google Scholar]
- 39.McCullough PA, Sandberg KR, Dumler F, Yanez JE. Determinants of coronary vascular calcification in patients with chronic kidney disease and end-stage renal disease: a systematic review. J Nephrol. 2004;17:205–15. [PubMed] [Google Scholar]
- 40.Budoff MJ, Rader DJ, Reilly MP, Mohler ER, 3rd, Lash J, Yang W, Rosen L, Glenn M, Teal V, Feldman HI. Relationship of estimated GFR and coronary artery calcification in the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis. 2011;58:519–26. doi: 10.1053/j.ajkd.2011.04.024. http://dx.doi.org/10.1053/j.ajkd.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silver J, Levi R. Cellular and molecular mechanisms of secondary hyperparathyroidism. Clin Nephrol. 2005;63:119–26. doi: 10.5414/cnp63119. http://dx.doi.org/10.5414/CNP63119. [DOI] [PubMed] [Google Scholar]
- 42.Marchais SJ, Metivier F, Guerin AP, London GM. Association of hyperphosphataemia with haemodynamic disturbances in end-stage renal disease. Nephrol Dial Transplant. 1999;14:2178–83. doi: 10.1093/ndt/14.9.2178. http://dx.doi.org/10.1093/ndt/14.9.2178. [DOI] [PubMed] [Google Scholar]
- 43.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–17. doi: 10.1053/ajkd.1998.v31.pm9531176. http://dx.doi.org/10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 44.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–82. doi: 10.1016/s0272-6386(12)70364-5. http://dx.doi.org/10.1016/S0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 45.Curran-Everett D, Milgrom H. Post-hoc data analysis: benefits and limitations. Curr Opin Allergy Clin Immunol. 2013;13:223–4. doi: 10.1097/ACI.0b013e3283609831. http://dx.doi.org/10.1097/ACI.0b013e3283609831. [DOI] [PubMed] [Google Scholar]