Abstract
Delirium is associated with circadian rhythm disruption. In this study we have explored whether circadian variation of melatonin is an indicator for delirium. Melatonin levels were determined from the first day of hospitalization and up to three days after the onset of delirium. Patients who did not developed delirium exhibited a daily melatonin rhythm, while in patients that developed delirium, the melatonin rhythm was lost and mean melatonin levels were found decreased as early as three days before the diagnosis of delirium, indicating that on arrival to the hospital circadian melatonin disruption can be used as an indicator of delirium.
Keywords: Human, Circadian rhythm, Sleep, Dementia, Geriatric
1. Introduction
Delirium is a neuropsychiatric syndrome characterized by a fluctuating mental and behavioral condition. It can include reduced alertness, global cognitive decline, delusional-hallucinatory phenomena, mood swings and gross disturbances of behavior [1]. Delirium has a higher prevalence among older patients, with a proportion of 11–42% [2]. Symptoms of delirium are tightly related with circadian rhythm and sleep disruption. In patients developing delirium, the sleep/wake cycle is reversed and patients are drowsy and take naps during the day, while nighttime sleep is short and fragmented [3]. Overall, 73% of delirious patients have at least moderate alterations of sleep/wake cycle [4]; while in older patients these alterations reach up to 98% [5].
The integrity of the sleep/wake cycle and the temporal order for a variety of physiological and behavioral functions, including melatonin, are driven by the circadian system. The synthesis and release of melatonin is dependent on the adequate internal synchronization between the suprachiasmatic nuclei and the pineal gland. Moreover, as melatonin receptors are present in components of the circadian system including the suprachiasmatic nucleus, the reproductive system, renal and immune systems, among others [6], [7], melatonin is suggested to function as an internal signal of circadian synchronization [8], [9]. This feature points out the relevance of studying melatonin as a reliable indicator of the timing in the circadian system.
Several studies have suggested that delirium is accompanied by a loss of temporal variation in plasma melatonin. In patients that underwent major abdominal surgery and developed postoperative delirium, plasma levels of melatonin were reduced and suffered sleep disturbances, including delayed sleep phase disorder or reversal of the sleep-wake cycle [10]. A different study reported cases of delirium in ICUs that presented a loss of melatonin circadian rhythms [11]. Other indicators of melatonin secretion are also altered in delirious patients, like urinary levels of the metabolite of melatonin; 6-sulphatoxymelatonin (6-SMT), which are mainly elevated in hypoactive patients and decreased in hyperactive patients [12].
In order to further determine whether the loss of melatonin rhythmicity can be used as an indicator for the onset of delirium, patients' melatonin levels were assessed from their first day of hospital admission up to delirium onset or until hospital discharge. Data reported here indicate that melatonin levels are modified before delirium onset and that the loss of melatonin rhythms can be used as a strategy to predict risk to develop delirium. This simple strategy may give insight of the patient’s prognosis.
2. Subjects and methods
This study was conducted in the inpatient service of the Department of Internal Medicine at general hospital “Dr. Manuel Gea González” in Mexico City. All patients (>65 years) admitted between 2012 December to 2013 February who signed informed consent forms were included; patients who were taking drugs that entrain circadian rhythms were excluded. All participants underwent a daily evaluation of delirium risk based on the DSM-IV-TR diagnostic criteria, and provided daytime and nighttime melatonin samples. Due to budget constraints, two investigators that were trained for the collection of samples of salivary melatonin and diagnosis of delirium and blinded to the patients' condition, analyzed the samples of 7 patients who developed delirium (D), from 3 days prior to 3 days after its setting. The mean day of delirium onset in these patients was the fourth day of hospitalization, therefore, 6 patients who didn't develop delirium (ND) until discharges were randomly selected, and their melatonin levels analyzed from days 1 through 7 of hospitalization. The study was approved by the ethical committee of the “Dr. Manuel Gea González” Hospital; No.14-82-2012. Patients received their food at 8:00 and 19:00 h.
2.1. Lighting conditions
Lighting conditions in the Internal Medicine service was 131±17.8 lx, during the day with light provided by overhead fluorescent white lamps from 07:00 to 19:00, and of 40.38±11 lx during the night (19:00 – 07:00).
2.2. Melatonin determination
In all patients, saliva samples were obtained in the morning (07:00 h) and at night (21:00 h), from the day of admission until hospital discharge. Salivary samples were taken by suction in the oral cavity and stored under refrigeration, until analysis. Concentration of salivary melatonin was determined with a kit for ELISA method (enzyme immunoassay for direct quantitative determination of melatonin in human saliva; of IBL International; No. Cat. RE54041).
2.3. Statistical analysis
The age difference between groups was compared using independent samples T-test. The melatonin concentration, was classified as repeated measures and was compared using a two-way analysis of variance (ANOVA), followed by a Tukey post hoc test with significant values set at P<0.05. Statistical analysis was performed with the program STATISTICA version 4.5 and graphs were elaborated with Sigma Plot version 12.
3. Results
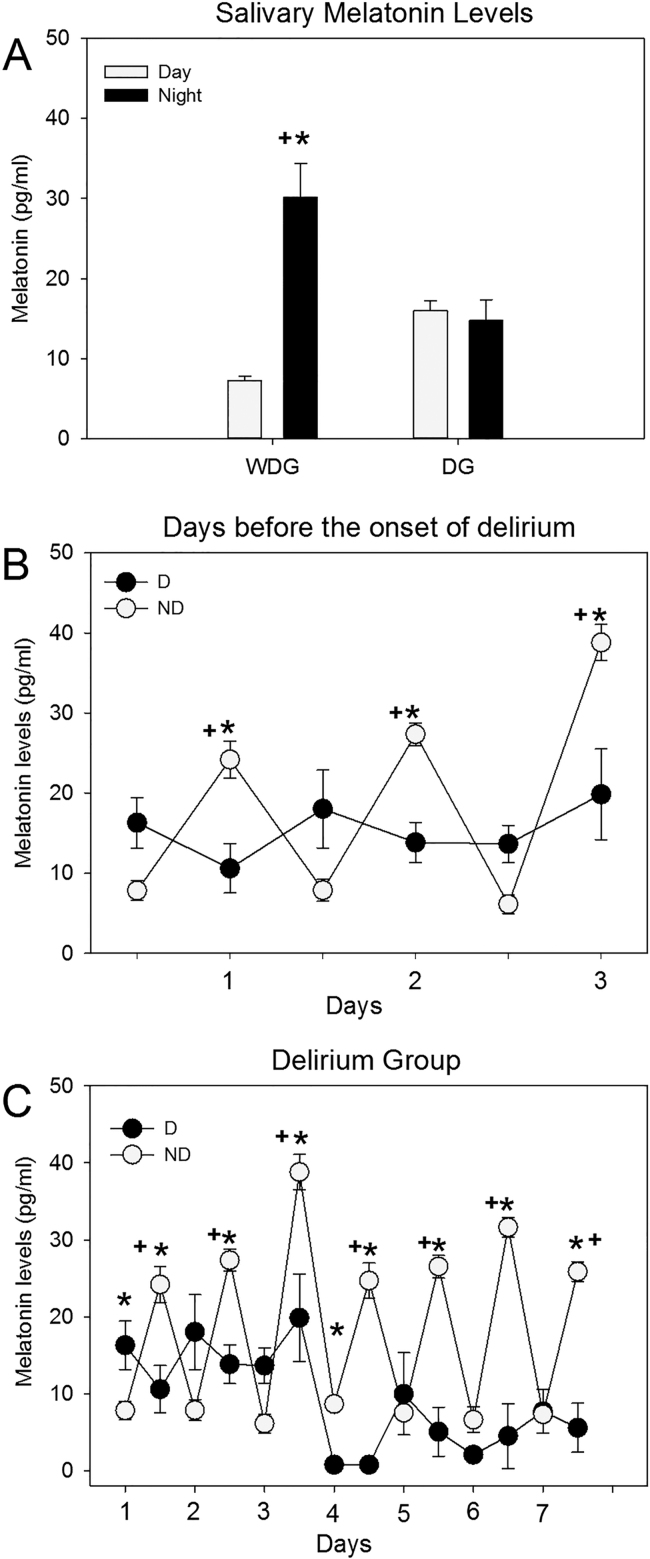
The mean age for the ND group was 72±5.32, and 78.5±5.61 for the D group; there was no statistical difference between them (p=0.696). The underlying disease, for which the patients were admitted to the hospital did not influence the difference in the results since admission diagnoses were counterbalanced (Table 1). In ND patients (n=6), saliva melatonin exhibited low levels in the day and high levels in the night, resulting in a daily rhythm in melatonin concentration (see Fig. 1A). The ANOVA indicated statistical differences between light and dark phases (F (1,10)=174.48; p<0.0001). In the delirium group, melatonin levels remained low in the morning and in the evening, thus resulting in no daily rhythm. Analyzing the seven days (the day of onset of delirium, three days before and three days after), the ANOVA did not show statistical differences between the day and night values (F(1,12)=1.051; p=N.S; see Fig. 1A).
Table 1.
Clinical characteristic of patients at admission.
| Groups and patients | Age | Diagnosis on admission | |||
|---|---|---|---|---|---|
| Control | Delirium | Control | Delirium | Control | Delirium |
| 1 | 1 | 66 | 85 | Scleroderma | Sacral decubitus ulcer |
| 2 | 2 | 71 | 76 | Metabolic syndrome | Type 2 diabetes |
| 3 | 3 | 72 | 82 | Heart failure, cirrhosis hepatic | Hart failure |
| 4 | 4 | 70 | 69 | Heart failure and hypertension | Heart failure and hypertension |
| 5 | 5 | 82 | 81 | Metabolic syndrome | Metabolic syndrome |
| 6 | 6 | 71 | 78 | Acute kidney injury | Acute kidney injury |
| 7 | 70 | Gastrointestinal hemorrhage | |||
| N=6 | N=7 | 72±2.22 | 77.28±2.32 | ||
Fig. 1.
A) Levels of salivary melatonin during the day and night, in the ND and D. B) Circadian pattern of the three days prior to hospital discharge in ND group and three days before the onset of delirium in the D group. C) Daily pattern of melatonin levels in the group of patients who developed delirium and ND for 7 consecutive days. Values are Means±SE. (*) Significant Difference between ND and D; P<0.05; (+) Significant difference between Day and night; P<0.05.
In patients that developed delirium, melatonin levels turned out constant three days before the diagnosis of delirium was given, while melatonin levels in the ND remained rhythmic even three days prior to discharge. The ANOVA indicated significant differences between groups (F(1,66)=48.90; p<0.00001), due to the factor time (F(5,66)=8.977; p<0.0001), and in the interaction of groups X time (F (5,66)=17.447; p<0.00001) (see Fig. 1B).
Finally, we observed that melatonin levels in the group of patients with delirium, show a loss of rhythmicity after and before of delirium (Fig. 1C).
4. Discussion
Delirium is a common syndrome that increases morbidity and mortality in the hospitalized population, which can be a frightening and distressing experience for patients, their families and caregivers [13]. The need for early diagnosis is crucial to improve the prognosis of the sufferer. This study suggests that there is a relationship between the syndrome of delirium and melatonin daily rhythms. This relationship was previously demonstrated in a group of patients who developed delirium syndrome after alcohol withdrawal [14]. The incidence of delirium is also related to the photoperiod, with a higher incidence of delirium in winter when days are short and melatonin production is increased [15]. This relationship was also previously reported by Wahlund et al. [16], who found that melatonin levels were associated with the level of psychomotor activity in subtypes of affective disorders.
The sleep-wake cycle and psychomotor activity are both under significant circadian regulation and appear to be disturbed in patients developing delirium, suggesting that the integrity of the circadian system is affected preceding or during the onset of delirium [17]. Moreover there is evidence that exogenous melatonin at low doses administered every night to elderly patients admitted to intensive care may be a potential protective agent against the development of delirium [18]. However, once an initial episode of delirium is registered, melatonin does not reduce the severity of delirium, duration of hospital stay, the restraint or sedative use, or mortality [18], [19], which further supports the preventive role of melatonin administration in the environment clinic [19] and in the surgical setting [20].
Delirium is a syndrome that requires an early diagnosis to improve patients' prognosis [21]. This study suggests a possible relationship between delirium and loss of melatonin rhythmicity. The limitations of our study are the reduced sample size, and lack of control for other variables besides age.
5. Conclusions
There is a close relationship between the disruption of the circadian system and the development of delirium. Such findings together with present data indicate a strong association between melatonin daily rhythms and delirium onset thus determining melatonin daily rhythm may be a useful predictive strategy for developing delirium. This strategy is not invasive and does not interfere with the general condition of the patient.
Conflict of interest
The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgments
This study was partially supported by grant DGAPA-PAPIIT IN217315 Universidad Nacional Autónoma de México.
Footnotes
Peer review under responsibility of Brazilian Association of Sleep.
References
- 1.Ramirez-Bermudez J., Lopez-Gómez M., Sosa Ana L., Aceves S., Nader-Kawachi J., Nicolini H. Frequency of delirium in a neurological emergency room. J Neuropsychiatry Clin Neurosci. 2006;18:108–112. doi: 10.1176/jnp.18.1.108. [DOI] [PubMed] [Google Scholar]
- 2.Siddiqi N., House A.O., Holmes J.D. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing. 2006;35:350–364. doi: 10.1093/ageing/afl005. [DOI] [PubMed] [Google Scholar]
- 3.Peterson M.J., Benca R.M. Sleep in mood disorders. Psychiatr Clin North Am. 2006;29:1009–10032. doi: 10.1016/j.psc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Meagher D.J., Moran M., Raju B., Gibbons D., Donnelly S., Saunders J., Trzepacz P.T. Phenomenology of delirium. Assessment of 100 adult cases using standardized measures. Br J Psychiatry. 2007;190:135–141. doi: 10.1192/bjp.bp.106.023911. [DOI] [PubMed] [Google Scholar]
- 5.Jabbar F., Leonard M., Meehan K., O’Connor M., Cronin C., Reynolds P., Meaney A.M., Meagher D. Neuropsychiatric and cognitive profile of patients with DSM-IV delirium referred to an old age psychiatry consultation-liaison service. Int Psychogeriatr. 2011;23:1167–1174. doi: 10.1017/S1041610210002383. [DOI] [PubMed] [Google Scholar]
- 6.Ekmekcioglu C. Melatonin receptors in humans: biological role and clinical relevance. Rev Artic Biomed Pharmacother. 2006;60:97–108. doi: 10.1016/j.biopha.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Daan S., Beersma D.G.M., Borbly A. Timing of human sleep: recovery process gated by a circadian pacemaker. Am J Physiol. 1984;246:R161–R178. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- 8.Reiter R.J. Melatonin: clinical relevance. Best Pract Res Clin Endocrinol Metab. 2003;17:273–285. doi: 10.1016/s1521-690x(03)00016-2. [DOI] [PubMed] [Google Scholar]
- 9.Pévet P. The internal time-giver role of melatonin. A key for our health. Rev Neurol. 2014;3787:00951–00955. doi: 10.1016/j.neurol.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Shigeta H., Yasui A., Nimura Y., Machida N., Kageyama M., Miura M., Menjo M., Ikeda K. Postoperative delirium and melatonin levels in elderly patients. Am J Surg. 2001;182:449–454. doi: 10.1016/s0002-9610(01)00761-9. [DOI] [PubMed] [Google Scholar]
- 11.Olofsson K., Alling C., Lundberg D., Malmros C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand. 2004;48:679–684. doi: 10.1111/j.0001-5172.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 12.Balan S., Leibovitz A., Zila S.O., Ruth M., Chana W., Yassica B., Rahel B., Richard G., Neumann E., Blagman B., Habot B. The relation between the clinical subtypes of delirium and the urinary level of 6-SMT. J Neuropsychiatry Clin Neurosci. 2003;15:363–366. doi: 10.1176/jnp.15.3.363. [DOI] [PubMed] [Google Scholar]
- 13.Candy B., Jackson K.C., Jones L., Leurent B., Tookman A., King M. Drug therapy for delirium in terminally ill adult patients. Cochrane Database Syst Rev. 2012;14:11. doi: 10.1002/14651858.CD004770.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Mukai M., Uchimura N., Hirano T., Ohshma H., Ohshima M., Nakamura J. Circadian rhythms of hormone concentrations in alcohol withdrawal. Psychiatry Clin Neurosci. 1998;52:238–240. doi: 10.1111/j.1440-1819.1998.tb01051.x. [DOI] [PubMed] [Google Scholar]
- 15.Balan S., Leibovitz A., Freedman L., Blagman B., Ruth M., Ady S., Habot B. Seasonal variation in the incidence of delirium among the patients of a geriatric hospital. Arch Gerontol Geriatr. 2001;33:287–293. doi: 10.1016/s0167-4943(01)00192-3. [DOI] [PubMed] [Google Scholar]
- 16.Wahlund B., Grahn H., Saaf J., Wetterberg L. Affective disorder subtyped by psychomotor symptoms, monoamine oxidase, melatonin and cortisol: identification of patients with latent bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 1998;248:215–224. doi: 10.1007/s004060050041. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald J.M., Adamis D., Trzepacz P.T., O’Regan N., Timmons S., Dunne C., Meagher D.J. Delirium: a disturbance of circadian integrity? Med Hypotheses. 2013;81:568–576. doi: 10.1016/j.mehy.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 18.Al-Aama T., Brymer C., Gutmanis I., Woolmore-Goodwin S.M., Esbaugh J., Dasgupta M. Melatonin decreases delirium in elderly patients: a randomized, placebo-controlled trialy. Int J Geriatr Psychiatry. 2011;26:687–694. doi: 10.1002/gps.2582. [DOI] [PubMed] [Google Scholar]
- 19.Inouye S.K., Bogardus Jr S.T., ., Charpentier P.A., Leo-Summers L., Acampora D., Holford T.R., Cooney Jr L.M. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 20.Marcantonio E.R., Flacker J.M., Wright R.J., Resnick N.M. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516–522. doi: 10.1046/j.1532-5415.2001.49108.x. [DOI] [PubMed] [Google Scholar]
- 21.Ford A.H., Almeida O.P. Pharmacological interventions for preventing delirium in the elderly. Maturitas. 2015;81(2):287–292. doi: 10.1016/j.maturitas.2015.03.024. [DOI] [PubMed] [Google Scholar]