Abstract
Objective
Fatty acid oxidation in macrophages is thought to regulate inflammatory status and insulin-sensitivity. An important unanswered question in this field is whether carnitine acetyl-transferase (CrAT) that regulates fatty acid oxidation and mitochondrial acetyl-CoA balance is required to integrate nutrient stress sensing to inflammatory response in macrophages.
Methods
Mice with myeloid lineage-specific Crat deletion were subjected to several metabolic stressors, including high-fat diet-induced obesity, fasting, and LPS-induced endotoxemia. Their metabolic homeostasis was compared to that of Crat-sufficient littermate controls. Inflammatory potential of Crat-deficient and Crat-sufficient macrophages were measured both in vitro and in vivo.
Results
Our studies revealed that ablation of CrAT in myeloid lineage cells did not impact glucose homeostasis, insulin-action, adipose tissue leukocytosis, and inflammation when animals were confronted with a variety of metabolic stressors, including high-fat diet, fasting, or LPS-induced acute endotoxemia.
Conclusions
These findings demonstrate that unlike muscle cells, substrate switch mechanisms that control macrophage energy metabolism and mitochondrial short-chain acyl-CoA pools during nutrient stress are controlled by pathways that are not solely reliant on CrAT.
Keywords: Macrophage, Adipose tissue, Inflammation, Carnitine acyltransferase
Highlights
-
•
Role of CrAT in macrophage inflammation was tested in vivo.
-
•
Myeloid CrAT does not regulate inflammation during HFD-induced obesity.
-
•
Fasting-induced metabolic stress is not regulated by myeloid CrAT expression.
-
•
CrAT expression in myeloid cells does not regulate LPS-induced acute endotoxemia.
-
•
Mitochondrial acetyl-CoA efflux via CrAT does not regulate macrophage inflammation.
1. Introduction
Within the last decade, our understanding of the importance of hematopoietic cells in obese adipose tissue and how this regulates inflammation has grown tremendously [1]. In lean animals and humans, resident adipose tissue macrophages (ATM) help maintain tissue homeostasis. But macrophages are also particularly important in regulation of whole-body inflammation [2], [3], [4], [5]. During metabolic insult, including obesity or fasting, the resident ATMs become outnumbered by macrophages recruited for tissue maintenance and repair [6], [7]. The significance of metabolism in macrophage differentiation is emphasized by recent findings that anti-inflammatory macrophages require fatty acid oxidation, whereas pro-inflammatory macrophages switch to glycolytic metabolism [8], [9], [10], [11], [12]. Importantly, much of what we know about macrophage substrate switches that are required for cellular function, metabolism, and inflammatory status has relied on in vitro experiments with specific cytokine skewing of macrophages; whether these paradigms persist in vivo is less clear.
Fatty acid oxidation in macrophages is reliant on the transport of fatty acids across mitochondrial membrane through conjugation of acyl groups to carnitine, which is regulated by carnitine acyltransferases [13]. Carnitine palmitoyltransferase 1 and 2 (CPT1, CPT2) conjugate carnitine to long-chain fatty acids and are required for fatty acid oxidation [13]. Carnitine O-octanoyltransferase (CrOT) and carnitine acetyltransferase (CrAT) conjugate medium-chain and short-chain acyl-CoA to carnitine, respectively [13]. CrAT is localized primarily within the mitochondrial matrix and catalyzes both the addition and the removal of carnitine from acetyl-CoA [14], facilitating the efflux of mitochondrial acetyl-CoA and buffering the intracellular pools of acetyl-CoA and carnitine. Consistent with an important role of fatty acid oxidation in macrophages, CPT1, CPT2, Crat and Crot are abundantly expressed in macrophages [15]. Interestingly, the CrAT activity is reduced during obesity and aging, leading to impaired glycemic control [16], [17]. Notably, muscle-specific deletion of CrAT was shown to reduce exercise performance [18] and exacerbated metabolic dysregulation in HFD mice [19]. Moreover, CrAT-deficient muscle accumulates long-chain acyl-carnitines, an indicator of incomplete β-oxidation [19]. CrAT-mediated acetylcarnitine production and efflux regulates glucose homeostasis and alleviates product inhibition of pyruvate dehydrogenase (PDH) that controls glycolysis and glucose oxidation [16]. Given the critical role of each of these mechanisms in macrophage function and inflammatory status [9], [10], we hypothesized that ablation of CrAT from macrophages would promote macrophage-mediated inflammation during nutrient stress. Surprisingly, we found that loss of CrAT expression in myeloid-lineage cells had no impact on total body glucose metabolism or adipose tissue inflammation in conditions of high-fat diet mediated nutrient overload. Deficiency of CrAT-mediated nutrient stress sensing in macrophages did not impact NLRP3 inflammasome activation or differentiation into M1/M2-like polarization. Furthermore, macrophage expression of CrAT was also not required to mount a successful fasting response nor impacted LPS-induced inflammation, which is reliant on increased lipolysis as well as increased glycolysis. Our findings reveal that, unlike muscle cells, macrophages have unique metabolic substrate requirement machinery where CrAT expression in is dispensable for regulating adipose tissue inflammation and whole body glucose metabolism under conditions of metabolic stress.
2. Research design and methods
2.1. Animals
Cratfl/fl mice were generously provided by Dr. Randall Mynatt (Pennington Biomedical Research Center [19]). Cratfl/fl were bred to LysM-Cre (B6.129P2-Lyz2tm1(cre)/fo/J, Jackson Labs) to ablate Crat in all myeloid lineage cells, henceforth referred to as CratMϕ−/−. Cre-negative Cratfl/fl littermate controls were used in all experiments. All experiments were performed in compliance with the Yale Institutional Animal Care and Use Committee.
2.2. In vitro assays
Bone marrow-derived macrophages (BMDM) were generated as previously described [20]. For NLRP3 inflammasome activation, BMDM were primed with LPS (1 μg/mL) for 4 h, followed by treatment with ATP (5 mM, 1 h), sphingosine (50 μM, 1 h), monosodium urate crystals (MSU, 250 μg/mL, 5 h), silica (200 μg/mL, 5 h), ceramide (C6, 80 μg/mL, 6 h), or palmitate (200 μM, 18 h). For macrophage polarization, BMDM were skewed towards M1 (LPS + IFNγ 20 ng/mL), M2 (IL-4 10 ng/mL), or left untreated (M0) for 24 h. Real-time metabolism was measured in M2-skewed BMDM using a Seahorse metabolic flux analyzer (Seahorse, Agilent). BMDM were polarized for 24 h prior to mitochondrial stress test (etomoxir 40 μM, oligomycin 1.5 μM, FCCP 0.75 μM, Rotenone 2 μM, Antimycin A 2 μM). Fatty acid oxidation was calculated by dividing OCR after etomoxir injection by baseline OCR. Spare respiratory capacity was calculated by subtracting baseline OCR from maximum OCR after FCCP injection.
2.3. Gene expression
mRNA was isolated in Trizol using the Qiagen RNeasy kit. cDNA was transcribed using iScript cDNA synthesis kit (Bio-Rad). Gene expression was measured by RT-PCR by ΔΔCt method and expressed relative to Gapdh.
2.4. Protein expression
Protein expression was evaluated by SDS-PAGE western blot. IL-1β (Genetex), Caspase-1 (generously provided by Genentech), CRAT (Proteintech) and β-Actin (Cell Signaling) were visualized by chemiluminescence.
2.5. In vivo metabolic assays
High-fat diet (HFD, 60%) feeding was initiated at 6 weeks old, and mice were fed ad libitum for 12 weeks. ATM were isolated by magnetic F4/80-positive selection (LifeTech). Mice were fasted for 12 h (glucose tolerance test, 0.4 g/kg bw glucose i.p.) or 4 h (insulin tolerance test, 0.8 U/kg bw i.p.). For fasting experiments, mice were fasted 24 h, beginning at 10am. For endotoxemia experiments, mice were challenged with LPS (25 μg i.p.) and euthanized 4 h later for analysis of inflammation.
2.6. Flow cytometry
Visceral and subcutaneous adipose tissue were digested in Collagenase I as previously described [21] to isolate the stromal vascular fraction (SVF). SVF was stained with live/dead viability dye (Invitrogen), CD3, B220, CD11b, F4/80, CD11c (all from eBioscience), and CD206 (Biolegend) to gate T cells, B cells, and macrophage subsets. Data were acquired on a custom LSR II (BD Bioscience) and analyzed in FlowJo (Treestar).
2.7. Statistical analysis
Statistical analyses as described in the figure legends were performed in Prism (GraphPad). P < 0.05 was considered significant for all experiments.
3. Results
3.1. CrAT-deficient macrophages have normal in vitro polarization
ATM are a major source of adipose tissue inflammation, driven in part by a macrophage metabolic switch from fatty acid oxidation to glycolysis [1], [6]. CrAT was previously identified to regulate metabolic flexibility in muscle and whole body glucose homeostasis [17], [18], [19]. To understand the role of CrAT in macrophage function, we tested whether genetic ablation of CrAT from bone marrow derived macrophages (BMDM) altered their activation status and polarization in vitro. Crat gene deletion was verified by qPCR (Figure 1A) and western blot (Figure 1B) and did not alter expression of acyl-transferase family member Cpt1a (Figure 1C) or plasma membrane lipid scavenger CD36 (Figure 1D), both of which facilitate macrophage fatty acid oxidation and M2-like alternative activation [8]. Crat-deficient BMDM were polarized in vitro towards M1 or M2 fates and expression of canonical lineage-specific genes, iNOS (Nos2) and Arginase 1 (Arg1) were measured. CratMϕ−/− and Cratfl/fl cells had equal expression of iNOS (Figure 1E) and Arg1 (Figure 1F). In addition to M1/M2 polarization, activation of the NLRP3 inflammasome is a critical driver of adipose tissue inflammation and metabolic dysregulation [22]. Importantly, equal caspase-1 activation and IL-1β secretion were observed in response to a variety of NLRP3 activators regardless of CrAT expression (Figure 1G). Because CRAT belongs to the acyltransferase enzyme family that controls fatty acid oxidation we also tested whether Crat deletion induced a functional deficiency in macrophage metabolism. We examined mitochondrial metabolism in M2-polarized BMDM because these cells are well known to upregulate and rely on fatty acid oxidation and mitochondrial metabolism [8]. Although Crat-deficient BMDM had slightly elevated basal OXPHOS (Figure 1H), their reliance on fatty acid oxidation, probed by treatment with etomoxir, was equal to Crat-sufficient cells (Figure 1I). Spare respiratory capacity was also unaffected by Crat deletion (Figure 1J). We conclude that CrAT-deficient macrophages retain both pro-inflammatory and anti-inflammatory potential.
Figure 1.
Crat-deficient BMDM retain normal inflammatory potential. Bone marrow-derived macrophages were stimulated in vitro to test the requirement of Crat expression for inflammatory activation. (A) Cre-mediated Crat deletion was verified by RT-PCR and (B) western blot. (C) Cpt1a and (D) CD36 gene expression were measured in unstimulated BMDM to validate Crat-specific deletion. BMDM were polarized towards M1-like or M2-like and (E) iNOS and (F) Arginase 1 gene expression were measured by RT-PCR. (G) Caspase-1 activation and IL-1β secretion were assessed culture supernatants and whole cell lysates by western blot. (H–J) Mitochondria oxidative phosphorylation metabolism in M2 BMDM was measured by Seahorse assay. (H) Mitochondrial stress test. (I) Relative fatty acid oxidation and (J) Spare respiratory capacity were calculated as described in methods. Statistical differences were calculated by unpaired two-tailed student's t-tests or 2-way ANOVA between genotypes. Data are pooled from 3 independent experiments and are expressed as mean ± SEM. Each symbol represents a sample pooled from at least 2 mice of indicated genotype. (F) Data are representative of 3 independent experiments, each pooled from at least n = 3 mice per genotype.
3.2. Myeloid CrAT ablation has no effect on adipose tissue inflammation during obesity
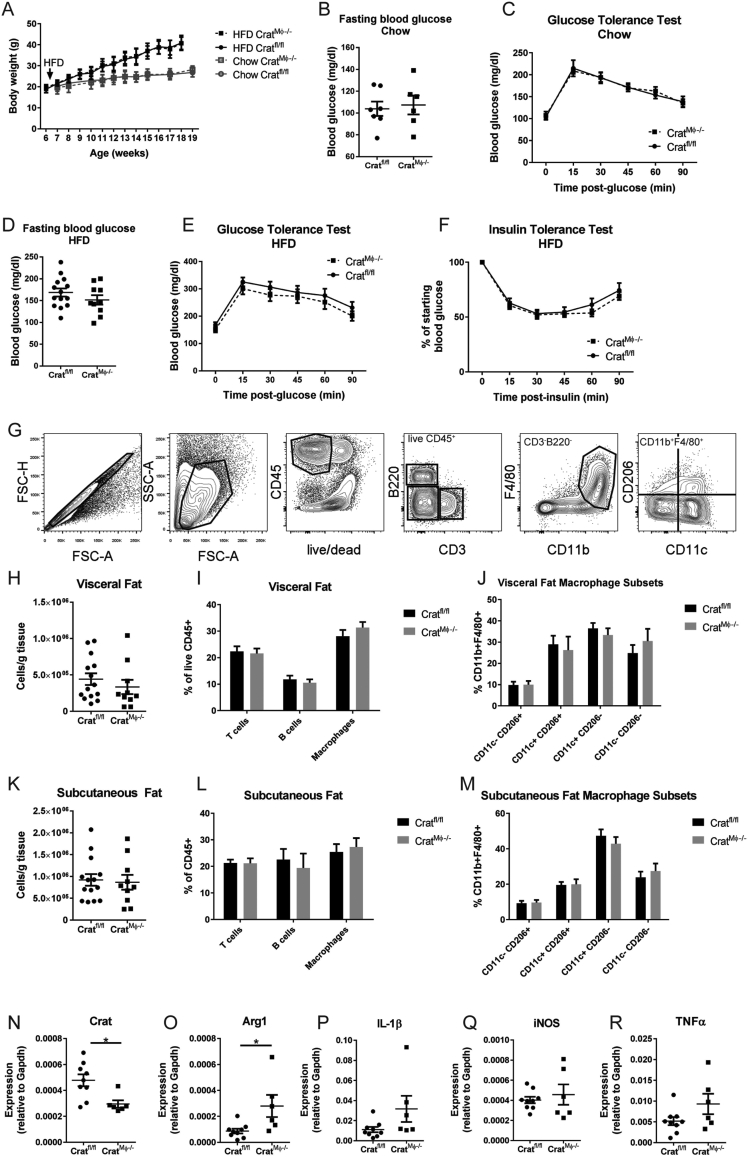
So far, the data showed CrAT expression is dispensable for inflammatory differentiation in vitro. However, the local macrophage phenotype within adipose tissue environment in vivo is far more complicated than can be modeled in vitro. Therefore, CratMϕ−/− mice were fed HFD to induce obesity and metabolic dysregulation and were compared to Cratfl/fl littermate controls. Only male mice were tested because females are not susceptible to HFD-induced metabolic dysregulation [23]. Mice gained weight equally during HFD feeding regardless of myeloid Crat expression (Figure 2A). Chow-fed CratMϕ−/− mice exhibited normal fasting blood glucose levels (Figure 2B) and glucose tolerance (Figure 2C). Obese mice fed HFD exhibited typical elevated fasting blood glucose (Figure 2D) and neither glucose tolerance (Figure 2E) nor insulin sensitivity (Figure 2F) were altered by Crat ablation. To determine whether Crat expression regulates adipose tissue leukocytosis, we analyzed T cells, B cells, and macrophage subsets within the white adipose tissue after 12 weeks of HFD feeding (representative gating strategy shown in Figure 2G). Myeloid Crat expression was dispensable for immune cell infiltration into white adipose tissue (Figure 2H–M). As expected, macrophages, particularly pro-inflammatory CD11c+ subsets, increased in visceral and subcutaneous adipose tissue during HFD (Figure 2J,M), and this was not regulated by Crat. We further assessed M1/M2-like gene expression within isolated ATM from visceral fat. While gene expression analysis confirmed Crat ablation in ATM in vivo (Figure 2N), Arg1 expression was increased in ATM (Figure 2O) while pro-inflammatory cytokines IL-1β, iNOS, and TNFα were somewhat elevated, although these differences did not reach statistical significance (Figure 2P–R). The modest variations in ATM compared to BMDM (Figure 1E,F) probably reflect the more complicated and lipid-rich adipose tissue environment compared to culture media. Overall, these data show that in conditions of diet-induced obesity, Crat expression within the myeloid compartment does not regulate inflammation or glycemic control.
Figure 2.
Crat expression in ATM does not regulate inflammation during obesity. Obesity was induced in male mice by HFD feeding for 12 weeks. (A) Body weights were measured weekly after initiation of HFD, denoted by arrow. (B) Fasting blood glucose and (C) glucose tolerance were measured in chow-fed male mice after 12 h overnight fasting. (D) Fasting blood glucose, (E) glucose tolerance, and (F) insulin tolerance were measured in HFD-fed male mice. (G) Representative flow cytometry gating strategy of SVF of visceral adipose tissue. (H) Total cellularity, (I) abundance of immune cell populations and (J) macrophage subsets in visceral adipose tissue were analyzed by flow cytometry. (K) Total cellularity, (L) abundance of immune cell populations and (M) macrophage subsets in subcutaneous adipose tissue were analyzed by flow cytometry. (N–R) Gene expression in macrophages isolated from obese visceral adipose tissue was measured by RT-PCR and normalized to Gapdh. For all graphs, data were pooled from 2 independent cohorts, each with at least n = 5 mice/group, and are expressed as mean ± SEM. Each symbol represents an individual mouse. Statistical differences were calculated by two-way ANOVA for time-course analyses or unpaired two-tailed student's t-tests between genotypes.
3.3. CrAT expression in myeloid cells does not regulate fasting-induced lipolysis
In contrast to the detrimental pro-inflammatory functions of ATM during obesity, ATM also have an important protective role in tissue remodeling and homeostasis [24], [25]. During periods of increased lipolysis, macrophages are recruited to adipose tissue and phagocytize excess lipids to protect tissue integrity [26].We next asked whether CrAT might regulate ATM function during fasting-induced negative energy balance that requires increased mitochondrial fatty acid oxidation. After 24 h fasting, a time point previously reported to induce macrophage recruitment to adipose tissue and phagocytosis of lipids [26], all mice had similar weight loss (Figure 3A) and fasting blood glucose (Figure 3B) regardless of sex or genotype. During fasting, limited glucose availability induces a metabolic switch towards fatty acid oxidation to sustain energetic demands. Fatty acid oxidation requires fatty acid transport into the mitochondria, ultimately producing acetyl-CoA, which is then converted into ketone bodies for export to the brain and heart as an alternative energy substrate. Myeloid Crat expression did not regulate production of β-hydroxybutyrate (BHB, Figure 3C), the most abundant ketone body produced during fasting. Glycerol release from adipose tissue explants, a measure of lipolysis, was also unaffected by Crat expression in ATM in both visceral (Figure 3D) and subcutaneous (Figure 3E) adipose depots. Altogether, this suggests that CrAT does not regulate the ATM response to fasting-induced lipolysis.
Figure 3.
Myeloid Crat expression does not regulate fasting-induced lipolysis response. Systemic lipolysis responses were measured in male and female mice after 24 h fasting. (A) Body weights were measured pre- and post-fasting, and the percentage of body weight lost after fasting was calculated. (B) Blood glucose and (C) blood BHB levels were measured after 24 h fasting. Glycerol release from (D) visceral and (E) subcutaneous adipose tissue explants was measured after 24 h fasting to determine relative level of lipolysis. Statistical differences between genotypes were calculated by student's t-test for each sex. For all graphs, data were pooled from 3 independent experiments and are expressed as mean ± SEM. Each symbol represents an individual mouse.
3.4. Myeloid-specific CrAT expression does not regulate inflammatory response during LPS-induced lipolysis
In addition to metabolic homeostasis, macrophages are important mediators of acute inflammation. LPS injections (i.p.) were used to model acute endotoxemia. Of note, LPS causes lethal inflammation that is fueled by glycolysis [27]. Therefore, we examined whether myeloid Crat expression regulated the inflammatory response after LPS administration in vivo. As expected, the LPS treatment reduced blood glucose concentrations (Figure 4A) and induced upregulation of TNFα, IL-1β, and IL-18 in visceral adipose tissue (Figure 4B) and spleen (Figure 4C) 4 h post-injection. Thus, similar to a chronic HFD metabolic challenge, deficiency of Crat in macrophages did not impact the ability of animals to mount acute inflammatory response.
Figure 4.
Myeloid Crat expression does not regulate inflammatory response to acute endotoxemia. Mice were injected with LPS i.p. and acute inflammatory response was assessed 4 h later. (A) Blood glucose was measured before and after LPS injection. (B, C) Induction of inflammatory genes was measured in visceral fat and spleen in sham and LPS-injected mice. Data are representative of 3 independent experiments. Each symbol represents an individual mouse. Statistical differences were calculated by (A) 2-way ANOVA comparing genotypes pre- and post-LPS, or (B,C) 1-way ANOVA comparing genotypes for each gene.
4. Discussion
CrAT regulates the intracellular acetyl-CoA availability by enabling efflux of short-chain acyl-CoA from the mitochondria [28]. Deletion of CrAT from smooth muscle has revealed important roles for CrAT in mitochondrial energy metabolism [18], maintenance of the mitochondrial acetylated proteome [28], and whole body glucose metabolism during HFD [19]. Metabolic switch from fatty acid oxidation to glycolysis and byproducts of TCA cycle regulate macrophage inflammation [8], [9], [10], [11]. Acetyl-CoA is a critical intracellular metabolite poised to regulate many cellular processes, including transcription, post-translational modifications, and metabolism, but regulators of the acyl-CoA pool in macrophage inflammation has not been studied. In light of these findings, we hypothesized that CrAT deletion in myeloid cells would exaggerate adipose tissue inflammation and metabolic dysregulation. Remarkably, CrAT expression in all myeloid-lineage cells was dispensable for whole body glycemic regulation in response to a variety of metabolic challenges, including high-fat diet, fasting-induced lipolysis, and LPS-induced acute inflammation.
The balance between glycolytic metabolism and fatty acid oxidation regulates macrophage differentiation and inflammatory responses [8]. Unlike CPT1a, CrAT does not directly regulate fatty acid oxidation, but its regulation of carbon efflux from the mitochondria during glucose overload facilitates metabolic flexibility, a characteristic likely important for macrophages given their diverse functions. The lack of any discernable phenotype after myeloid-specific CrAT ablation in obesity, fasting, or endotoxemia suggests macrophages (and other myeloid cells) do not rely on this regulatory pathway during metabolic stress. These results were surprising given the critical role of cellular metabolism in macrophage inflammation. Furthermore, the tissue-specific requirement (smooth muscle versus myeloid cells) of CrAT function under the same metabolic challenge (HFD) is striking. It is not clear why myeloid cells do not rely on CrAT to regulate inflammation and glycemic control during metabolic stress. It is possible that macrophages are equipped with alternative regulatory pathways that either (a) compensate for the function of CrAT, or (b) are prioritized over CrAT, thus leaving CrAT itself dispensable in macrophages. Alternatively, acetyl-CoA might be used or metabolized so rapidly in macrophages that CrAT is simply non-essential. Additionally, these pathways may be temporally regulated, which was not evaluated in our studies.
In vitro studies have revealed that fatty acid metabolism and inflammation are intimately linked in macrophages. However, the in vivo environments in which macrophages reside are far more complex than can be modeled in vitro, emphasizing the need for more thorough analyses of in vivo inflammatory models to fully understand the complicated and dynamic metabolic regulation of inflammation. In conclusion, our data highlight the importance of cell type-specific metabolic differences in whole body glycemic control. Our data also reveal the important finding that Crat-mediated mitochondrial efflux of acetyl-CoA is not intrinsically required by macrophages for their regulation of nutrient stress response and inflammation.
Acknowledgments
We thank Dr. R Mynatt for providing the Crat floxed mice. ELG performed experiments and data analysis, and prepared manuscript. VDD conceived and supervised project, analyzed data, and prepared manuscript. We thank Kim Nguyen and Yun-Hee Youm for expert technical assistance.
Funding: Dixit Lab is supported in part by US National Institutes of Health (NIH) grants AG043608, AI105097, AG051459 and P01AG051459, The Glenn Foundation for Aging Research and Cure for Alzheimers Foundation. ELG is supported in part by postdoctoral fellowship from American Foundation of Aging Research (AFAR) and American Heart Association (AHA).
Conflict of interest
None declared.
References
- 1.Gregor M.F., Hotamisligil G.S. Inflammatory mechanisms in obesity. Annual Review of Immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 2.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of Clinical Investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagareddy P.R., Kraakman M., Masters S.L., Stirzaker R.A., Gorman D.J., Grant R.W. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metabolism. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uysal K.T., Wiesbrock S.M., Marino M.W., Hotamisligil G.S. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 6.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of Clinical Investigation. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Neill L.A., Pearce E.J. Immunometabolism governs dendritic cell and macrophage function. The Journal of Experimental Medicine. 2016;213:15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang S.C., Everts B., Ivanova Y., O'Sullivan D., Nascimento M., Smith A.M. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nature Immunology. 2014;15:846–855. doi: 10.1038/ni.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lampropoulou V., Sergushichev A., Bambouskova M., Nair S., Vincent E.E., Loginicheva E. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metabolism. 2016;24:158–166. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jha A.K., Huang S.C., Sergushichev A., Lampropoulou V., Ivanova Y., Loginicheva E. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vats D., Mukundan L., Odegaard J.I., Zhang L., Smith K.L., Morel C.R. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metabolism. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Houten S.M., Wanders R.J. A general introduction to the biochemistry of mitochondrial fatty acid beta-oxidation. Journal of Inherited Metabolic Disease. 2010;33:469–477. doi: 10.1007/s10545-010-9061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pieklik J.R., Guynn R.W. Equilibrium constants of the reactions of choline acetyltransferase, carnitine acetyltransferase, and acetylcholinesterase under physiological conditions. The Journal of Biological Chemistry. 1975;250:4445–4450. [PubMed] [Google Scholar]
- 15.Heng T.S., Painter M.W., Immunological Genome Project C The Immunological Genome Project: networks of gene expression in immune cells. Nature Immunology. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 16.Seiler S.E., Martin O.J., Noland R.C., Slentz D.H., DeBalsi K.L., Ilkayeva O.R. Obesity and lipid stress inhibit carnitine acetyltransferase activity. Journal of Lipid Research. 2014;55:635–644. doi: 10.1194/jlr.M043448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noland R.C., Koves T.R., Seiler S.E., Lum H., Lust R.M., Ilkayeva O. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. The Journal of Biological Chemistry. 2009;284:22840–22852. doi: 10.1074/jbc.M109.032888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seiler S.E., Koves T.R., Gooding J.R., Wong K.E., Stevens R.D., Ilkayeva O.R. Carnitine acetyltransferase mitigates metabolic inertia and muscle fatigue during exercise. Cell Metabolism. 2015;22:65–76. doi: 10.1016/j.cmet.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muoio D.M., Noland R.C., Kovalik J.P., Seiler S.E., Davies M.N., DeBalsi K.L. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metabolism. 2012;15:764–777. doi: 10.1016/j.cmet.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Youm Y.H., Nguyen K.Y., Grant R.W., Goldberg E.L., Bodogai M., Kim D. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nature Medicine. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant R., Youm Y.H., Ravussin A., Dixit V.D. Quantification of adipose tissue leukocytosis in obesity. Methods in Molecular Biology. 2013;1040:195–209. doi: 10.1007/978-1-62703-523-1_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandanmagsar B., Youm Y.H., Ravussin A., Galgani J.E., Stadler K., Mynatt R.L. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature Medicine. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singer K., Maley N., Mergian T., DelProposto J., Cho K.W., Zamarron B.F. Differences in hematopoietic stem cells contribute to sexually dimorphic inflammatory responses to high fat diet-induced obesity. The Journal of Biological Chemistry. 2015;290:13250–13262. doi: 10.1074/jbc.M114.634568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odegaard J.I., Chawla A. The immune system as a sensor of the metabolic state. Immunity. 2013;38:644–654. doi: 10.1016/j.immuni.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X., Grijalva A., Skowronski A., van Eijk M., Serlie M.J., Ferrante A.W., Jr. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metabolism. 2013;18:816–830. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosteli A., Sugaru E., Haemmerle G., Martin J.F., Lei J., Zechner R. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. The Journal of Clinical Investigation. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang A., Huen S.C., Luan H.H., Yu S., Zhang C., Gallezot J.D. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell. 2016;166:1512–1525 e1512. doi: 10.1016/j.cell.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies M.N., Kjalarsdottir L., Thompson J.W., Dubois L.G., Stevens R.D., Ilkayeva O.R. The acetyl group buffering action of carnitine acetyltransferase offsets macronutrient-induced lysine acetylation of mitochondrial proteins. Cell Reports. 2016;14:243–254. doi: 10.1016/j.celrep.2015.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]