Abstract
Objective
Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) possess multiple bioactive isoforms that are rendered non-insulinotropic by the enzyme dipeptidyl peptidase-4 (DPP-4). Recently, some ELISA kits have been developed to specifically measure “active” GIP and GLP-1, but it is unclear if these kits can accurately quantify all bioactive forms. Therefore, it remains uncertain to what extent treatment with a DPP-4 inhibitor boosts levels of biologically active GIP and GLP-1. Thus, we evaluated our novel receptor-mediated incretin bioassays in comparison to commercially available ELISA kits using plasma samples from healthy subjects before and after DPP-4 inhibitor administration.
Methods
We utilized cell lines stably co-transfected with human GIP or GLP-1 receptors and a cAMP-inducible luciferase expression construct for the bioassays and commercially available ELISA kits. Assays were tested with synthetic GIP and GLP-1 receptor agonists and plasma samples collected from subjects during a 75 g oral glucose tolerance test (OGTT) performed before or following 3-day administration of a DPP-4 inhibitor.
Results
A GIP isoform GIP(1–30)NH2 increased luciferase activity similarly to GIP(1–42) in the GIP bioassay but was not detectable by either a total or active GIP ELISA kit. During an OGTT, total GIP levels measured by ELISA rapidly increased from 0 min to 15 min, subsequently reaching a peak of 59.2 ± 8.3 pmol/l at 120 min. In contrast, active GIP levels measured by the bioassay peaked at 15 min (43.4 ± 6.4 pmol/l) and then progressively diminished at all subsequent time points. Strikingly, at 15 min, active GIP levels as determined by the bioassay reached levels approximately 20-fold higher after the DPP-4 inhibitor treatment, while total and active GIP levels determined by ELISA were increased just 1.5 and 2.1-fold, respectively. In the absence of DPP-4 inhibition, total GLP-1 levels measured by ELISA gradually increased up to 90 min, reaching 23.5 ± 2.4 pmol/l, and active GLP-1 levels determined by the bioassay did not show any apparent peak. Following administration of a DPP-4 inhibitor there was an observable peak of active GLP-1 levels as determined by the bioassay at 15 min after oral glucose load, reaching 11.0 ± 0.62 pmol/l, 1.4-fold greater than levels obtained without DPP-4 inhibitor treatment. In contrast, total GLP-1 levels determined by ELISA were decreased after DPP-4 inhibitor treatment.
Conclusion
Our results using bioassays indicate that there is a greater increase in plasma levels of bioactive GIP than GLP-1 in subjects treated with DPP-4 inhibitors, which may be unappreciated using conventional ELISAs.
Keywords: Receptor-mediated incretin bioassays, Glucose-dependent insulinotropic polypeptide, Glucagon-like peptide-1, Dipeptidyl peptidase-4
Highlights
-
•
Receptor-mediated bioassays were used to measure GIP and GLP-1 in humans.
-
•
The GIP bioassay, but not two ELISAs, detected both GIP(1–42) and GIP(1–30)NH2.
-
•
Active GIP levels were increased more than GLP-1 after DPP-4 inhibitor treatment.
Abbreviations
- BSA
bovine serum albumin
- DPP-4
dipeptidyl peptidase-4
- DMEM
Dulbecco's Modified Eagle's Medium
- FBS
fetal bovine serum
- GIP
glucose-dependent insulinotropic polypeptide
- GLP-1
glucagon-like peptide-1
- KRB
Krebs Ringer Buffer
- OGTT
oral glucose tolerance test
- PBS
phosphate buffered saline
- PC
prohormone convertase
- T2DM
type 2 diabetes mellitus
1. Introduction
Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) are incretins released from the gut that promote insulin secretion from pancreatic beta cells in a meal dependent manner [1]. Additionally, GIP and GLP-1 increase insulin biosynthesis, promote beta cell proliferation and reduce beta cell apoptosis [1]. Pro-GIP and proglucagon are processed to GIP and GLP-1, respectively, in the gut primarily by prohormone convertase (PC) 1/3 [2]. Mature GIP predominantly consists of 42 amino acids and is secreted from K-cells concentrated in the upper small intestine [1]. The major insulinotropic forms of GLP-1 are GLP-1(7–36)NH2 and GLP-1(7–37), which are liberated from proglucagon via the action of PC 1/3 and released from L-cells mainly distributed in the lower small intestine and colon [1]. Both incretin hormones are rapidly cleaved after secretion by dipeptidyl peptidase-4 (DPP-4) into truncated forms that are no longer insulinotropic [1]. Although recently developed ELISA kits may be able to detect active forms of GIP and GLP-1, it is unclear if these ELISAs can accurately quantify biologically active forms of the hormones because they are antibody based measurements, and immunoreactivity may not always coincide with bioactivity of peptide hormones. Moreover, recent reports suggest that a shorter form GIP(1–30)NH2 is secreted from the pancreas and the gut [2], [3], and this form has insulinotropic activity almost equivalent to GIP(1–42) [2]. It was unclear, however, if this form is detectable by active GIP ELISAs.
DPP-4 inhibitors are widely used to improve glycemic control in patients with type 2 diabetes mellitus (T2DM), and they are a particularly effective treatment for non-obese diabetes patients in East Asia. More than 70% of Japanese patients treated with anti-diabetic drugs receive DPP4 inhibitors or GLP-1 mimetics and approximately 60% receive a DPP-4 inhibitor as a first-line therapy [4]. We wished to evaluate how DPP-4 inhibitors alter the levels of GIP and GLP-1, using both conventional commercially available assays and novel cell-based, receptor-mediated bioassays. Our results using the bioassays indicate that active GIP levels increase dramatically following DPP-4 inhibitor treatment, much greater than that of GLP-1, and this finding is not revealed by the ELISAs we tested.
2. Material and methods
2.1. Subjects and study protocol
We recruited 10 non-diabetic subjects with informed consent for a 75 g OGTT male, age 32.3 ± 5.6 years, BMI 23.3 ± 5.6 kg/m2, HbA1c 5.1 ± 0.28% (31.5 ± 2.7 mmol/mol); average ± SD. We performed a second OGTT after DPP-4 inhibitor treatment (sitagliptin: 100 mg/day for 3 days) in 5 subjects following the first OGTT without DPP-4 inhibitor treatment (male, age 35.2 ± 6.3 years, BMI 23.3 ± 3.0 kg/m2, HbA1c 5.1 ± 0.31% (31.8 ± 3.1 mmol/mol); average ± SD). The study was performed in accordance with the Declaration of Helsinki, and the research protocol was approved by the Research Ethics Committee of Asahikawa Medical University).
2.2. Study protocol
All subjects were fasted for 10–12 h before the OGTTs. Blood glucose levels were measured at 0, 15, 30, 60, 90, and 120 min, and blood samples were collected at the same time points using blood collection tubes containing DPP-4 inhibitor (P800; BD, Tokyo, Japan). The plasma samples were separated by centrifugation (3000 RPM for 15 min) at 4 °C, aliquoted, and stored at −80 °C until the assays.
2.3. Assays
We measured blood glucose and plasma insulin levels (ELISA # 10-1113-01, Mercodia, Uppsala, Sweden) at 0, 15, 30, 60, 90, and 120 min of the OGTT. We measured plasma total GIP (EZHGIP-54K, Millipore, Tokyo, Japan) and total GLP-1 (EZGLP1T-36K, Millipore, Tokyo, Japan) levels by ELISA according to the manufacturer's instructions, while active GIP and active GLP-1 levels by our bioassays employing HEK293 cell lines stably co-transfected with human forms of either the GIP receptor (GIPR) or GLP-1 receptor (GLP-1R), and a cyclic AMP-inducible luciferase expression construct. Each assay was performed on previously unthawed plasma aliquots. We also utilized a commercial active GIP ELISA kit (# 27201, IBL, Fujioka, Japan) to assay the samples from 5 subjects collected during the OGTT at 0 and 15 min with or without the sitagliptin administration.
For further details for the bioassays, stably selected co-transfected HEK293 cell lines were cultured in Dulbecco's Modified Eagle's Medium (DMEM) (25 mmol/l glucose) with 10% (v/v) fetal bovine serum (FBS) (GIBCO, Tokyo, Japan), 100 IU/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Tokyo, Japan) at 37 °C in 5% CO2. Cells were incubated overnight in 96-well plates (100,000 cells/well). Media was then replaced with samples or synthetic peptide standards (GIP bioassay: GIP(1–42), GIP(1–30)NH2, GLP-1(7–36)NH2, glucagon: 10−13–10−6 mol/l, GLP-1 bioassay: GLP-1(7–36)NH2, GIP(1–42), GIP(1–30)NH2 and glucagon: 10−13–10−9 mol/l) that were diluted in Krebs Ringer Buffer (KRB) (pH 7.4) containing 0.5% (w/v) bovine serum albumin (BSA) (Sigma–Aldrich, Tokyo, Japan) and incubated for 5 h at 37 °C in 5% CO2. We diluted plasma samples with KRB before active GIP and GLP-1 measurement by bioassay (GIP: 20-fold dilution, GLP-1: 50-fold dilution). After incubation, we measured luciferase activity with the Bright-Glo Assay Kit (Promega, Madison, WI, USA) using a Thermo Scientific Appliskan (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions and calculated the hormone concentrations as previously described [5].
2.4. Peptides
GIP(1–42), GLP-1(7–36)NH2 and glucagon were purchased from Peptide Institute (Osaka, Japan). GIP(1–30)NH2 was purchased from Phoenix Pharmaceuticals (Burlingame, CA, USA).
2.5. Statistical analysis
Data are expressed as mean ± SEM unless otherwise stated. Statistical analysis was performed by two-way repeated ANOVA followed by the Bonferroni post hoc test. Data were analyzed using commercial software (Prism 5; GraphPad, San Diego, CA, USA) and p < 0.05 was considered significant.
3. Results
3.1. The receptor-mediated GIP bioassay detected both GIP(1–42) and GIP(1–30)NH2 whereas the GIP ELISA kits did not detect GIP(1–30)NH2
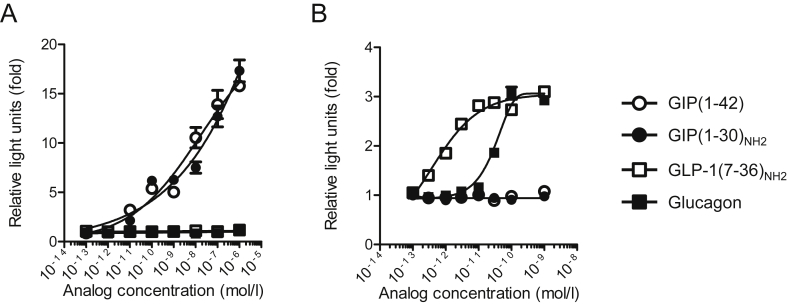
To evaluate the specificity and the characteristics of the bioassays, we examined their responsiveness and specificity with several synthetic glucagon-related peptides. In the GIP bioassay, GIP(1–42) and GIP(1–30)NH2 almost equivalently increased luciferase activity in a concentration dependent manner. GLP-1(7–36)NH2 and glucagon did not increase luciferase activity at any concentration tested (Figure 1A). In contrast, total or active GIP ELISA kits did not detect GIP(1–30)NH2 (Figure S1A and B). In the GLP-1 bioassay, GLP-1(7–36)NH2 increased luciferase activity in a concentration dependent manner. Glucagon induced luciferase activity at concentrations greater than approximately 10−11 mol/l, consistent with prior studies demonstrating relatively low affinity binding of glucagon to GLP-1 receptors [6]. In contrast, GIP(1–42) did not increase luciferase activity in the GLP-1 bioassay (Figure 1B). The DPP-4 inhibitor sitagliptin did not significantly alter luciferase activity in the GIP bioassay (Figure S2).
Figure 1.
The receptor-mediated GIP bioassay detects both GIP(1–42) and GIP(1–30)NH2. The responsiveness and specificity of (A) GIP and (B) GLP-1 receptor-mediated bioassays with GIP, GLP-1, and glucagon peptides. White triangles, GIP(1–42); white inverted triangles, GIP(1–30)NH2; black triangles, GLP-1(7–36)NH2; black inverted triangles, glucagon. Data are presented as mean ± SEM.
3.2. The administration of a DPP-4 inhibitor markedly increased bioactive GIP levels after glucose load in non-diabetic subjects
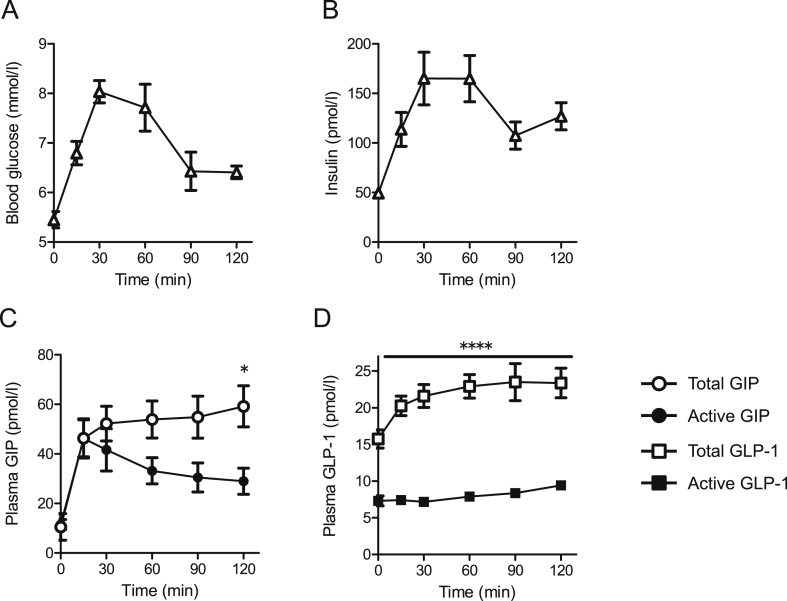
During the OGTTs, blood glucose levels peaked at 30 min and insulin levels reached a peak at 30–60 min (Figure 2A and B). Total GIP levels in the samples measured by ELISA rapidly increased from 0 to 15 min and subsequently gradually increased up to a peak at 120 min (59.2 ± 8.3 pmol/l; Figure 2C). In contrast, active GIP levels determined by the bioassay peaked at 15 min (43.4 ± 6.4 pmol/l; Figure 2C). Total GLP-1 levels as measured by ELISA increased to 30 min and remained at similar levels up to 120 min (23.4 ± 2.0 pmol/l; Figure 2D). Active GLP-1 levels by the bioassay did not show an apparent peak, averaging 7.9 ± 0.35 pmol/l (Figure 2D).
Figure 2.
Active GIP levels by bioassay showed an apparent peak at 15 min during a 75 g OGTT. Blood glucose (A), plasma insulin (B), plasma active (bioassay), and total (ELISA) GIP (C) and GLP-1 (D) levels in samples collected at the indicated times during a 75 g OGTT (n = 10 in each group). For (C) and (D), white circles, total GIP; black circles, active GIP; white squares, total GLP-1; black squares, active GLP-1. Data are presented as mean ± SEM. *p < 0.05, ****p < 0.0001.
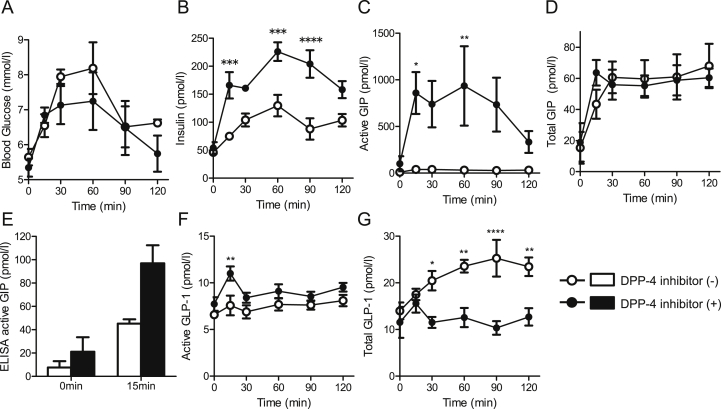
The three-day administration of a DPP-4 inhibitor tended to lower blood glucose levels during an OGTT but without statistical difference (Figure 3A). Postprandial plasma insulin levels were increased approximately 2-fold at several time points compared to before DPP-4 inhibitor administration (Figure 3B). The active GIP levels were markedly elevated at all time points in all 5 subjects following treatment with the DPP-4 inhibitor, but, given the variability, statistical significance was only achieved at two time points (Figure 3C). Active GIP levels measured using the bioassay at 15 min were approximately 20-fold greater than values at the same time point before DPP-4 inhibitor treatment (Figure 3C). In contrast, total GIP levels by ELISA at 15 min were 1.5-fold greater (Figure 3D) while active GIP levels determined by ELISA were 2.1-fold greater than values at the same time point before DPP-4 inhibitor treatment (Figure 3E). Administration of other DPP-4 inhibitors (linagliptin: 5 mg/day and vildagliptin: 100 mg/day) increased active GIP as determined by the bioassay during OGTT similarly to sitagliptin (data not shown).
Figure 3.
The administration of a DPP-4 inhibitor markedly increased bioactive GIP and plasma insulin levels after glucose load in non-diabetic subjects. Blood glucose, plasma insulin, plasma active, and total GIP and GLP-1 levels before and after DPP-4 inhibitor treatment (n = 5 in each group). (A) Blood glucose. (B) Plasma insulin levels. (C) Active GIP levels by bioassay. (D) Total GIP levels by ELISA. (E) Active GIP levels by ELISA at 0 and 15 min. (F) Active GLP-1 levels by bioassay. (G) Total GLP-1 levels by ELISA. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. White circles and white bars, before DPP-4 inhibitor treatment; black circles and black bars, after DPP-4 inhibitor treatment.
Unlike active GIP, active GLP-1 levels by the bioassay were only modestly elevated after the DPP-4 inhibitor treatment from 0 to 120 min and increased by 1.4-fold at 15 min after oral glucose load (Figure 3F). In contrast, total GLP-1 levels as determined by ELISA were decreased after DPP-4 inhibitor treatment (Figure 3G).
4. Discussion
Plasma GIP and GLP-1 levels are typically measured by ELISA, a highly sensitive immunological method to detect specific antigens. However, it can be difficult for these assays to distinguish antigens with similar epitopes. Indeed, it has been demonstrated that commercially available glucagon ELISA kits have cross-reactivity with glicentin and other proglucagon-derived peptides [7], [8], [9]. The specificity and sensitivity of commercially available kits for the analysis of GLP-1 levels also vary considerably [10]. Additionally, the immunological values obtained with these assays may not accurately reflect the total biological activity as some immunoreactive variants may not be bioactive, and some bioactive forms may not be immunoreactive. The receptor-mediated method we employed here reflects incretin receptor binding ability and intracellular signaling through cyclic AMP production. Therefore, the receptor-mediated bioassays may better reflect biological activity of intact incretin hormones than conventional immunological based methods. For example, we found that GLP-1, glucagon, GLP-2, oxyntomodulin, secretin and mini-glucagon (glucagon(19–29)) did not cross-react in the GIP bioassay, even at concentrations as high as 100 nM (Figure 1 and data not shown). Nevertheless, we cannot discount the possibility that there are fragments of GIP and GLP-1, such as the DPP-4 products GIP(3–42) and GLP-1(9–36)NH2, that may have biological actions mediated by non-cyclic AMP intracellular signaling pathways and thus not detected by our bioassays.
GIP is localized in gut K-cells with PC 1/3, which liberates the 42 amino acid mature form of GIP from Pro-GIP [3]. Recently, it was reported that Pro-GIP is processed to GIP(1–30)NH2 in pancreatic alpha cells and in the gut by PC2 [2], [3] and demonstrated that GIP(1–30)NH2 possesses insulinotropic activity similar to GIP(1–42) [2]. DPP-4 exists as a soluble form in circulation [11] or a membrane bound form in many tissues including endocrine cells of the pancreatic islets [12]. Moreover, recent studies indicate that GIP is particularly susceptible to DPP-4 activity in hematopoietic and endothelial cells [13]. Therefore, circulating GIP(1–30)NH2 concentrations are likely very low in the absence of DPP-4 inhibitor treatment, because GIP(1–30)NH2 is anticipated to be rapidly cleaved by DPP-4 like GIP(1–42) [14]. In the absence of DPP-4 inhibitor treatment, there may be relatively large amounts of circulating GIP(3–30)NH2 with presently unappreciated biological actions. The administration of DPP-4 inhibitor may reduce GIP(1–30)NH2 inactivation, and thus GIP(1–30)NH2 may have contributed to the markedly elevated bioactive GIP levels we measured in subjects following administration of the DPP-4 inhibitor. While our GIP bioassay characterization excludes the possibility of significant contributions from major glucagon-related peptides, some of which are DPP-4 substrates, we cannot exclude the possibility that there are other peptides activating the GIP receptor once stabilized by 3 days of DPP-4 inhibitor treatment.
In this study, active GLP-1 levels by the bioassay were modestly elevated after the DPP-4 inhibitor treatment from 0 to 120 min. In contrast, total GLP-1 levels as determined by ELISA were decreased after DPP-4 inhibitor treatment. Our findings are similar to that of Nauck et al. who also demonstrated that DPP-4 inhibitor treatment increased active GLP-1 and reduced total GLP-1 levels [15]. It is reported that GLP-1(7–36)NH2 stimulates intestinal somatostatin release [16] and somatostatin inhibits GLP-1 secretion from the gut [17]. Consequently, an increase in active GLP-1 levels by DPP-4 inhibition could promote somatostatin secretion which ultimately reduces intestinal GLP-1 secretion. It is also possible that increased bioactive GIP levels might act to reduce GLP-1 levels, as GIP infusion has been reported to significantly decrease postprandial plasma GLP-1 levels during a mixed meal in humans [18].
During the OGTTs, DPP-4 inhibitor administration enhanced postprandial insulin levels and dramatically increased active GIP levels measured by bioassay. GIP stimulates insulin secretion and is reported to enhance glucagon release especially in the hypoglycemic state [19]. It is possible that in our studies with non-diabetic subjects treated with DPP-4 inhibitor, GIP-stimulated glucagon secretion might partially explain the modest reduction of glucose levels despite the hyperinsulinemia. DPP-4 inhibition may have also augmented the biological activity of glucagon [20], although the biological importance of DPP-4-mediated glucagon cleavage for glucagon action in humans remains to be clarified.
It has been reported that the insulinotropic action of GIP is severely diminished in patients with T2DM [21], [22], [23]. As a result, it is generally assumed that GIP contributes little to the therapeutic efficacy of DPP-4 inhibitors. Moreover, there was no added therapeutic benefit following the addition of a dipeptidyl peptidase-4 inhibitor to ongoing therapy with a GLP-1 receptor agonist, despite increased plasma levels of immunoreactive intact GIP (and GLP-1) [24]. However, administration of the GLP-1 receptor antagonist exendin(9–39) only blocks ∼50% of the glucose lowering effect of DPP-4 inhibitor treatment in humans [15], [25], suggesting that DPP-4– sensitive factors beyond circulating GLP-1 substantially contribute to the actions of DPP-4 inhibitors. In agreement, DPP-4 inhibitor treatment in mice with GLP-1 receptor knockout still increased plasma insulin levels and improved glucose tolerance similarly as in wildtype mice [26]. GIP is a likely candidate, because DPP-4 therapy is completely ineffective in mice with combined knockout of GIP and GLP-1 receptors, suggesting that extended activity of both GIP and GLP-1 accounts for the full glucose lowering actions of DPP-4 inhibitors [26], [27]. Moreover, a unimolecular GLP-1/GIP co-agonist demonstrated enhanced antihyperglycemic and insulinotropic efficacy relative to selective GLP-1 agonists in diabetic rodents and humans [28]. Our findings of a particularly large increase in bioactive GIP levels in humans treated with DPP-4 inhibitors, combined with observations that administration of a GIP(1–30) analog can improve glycemic control in diabetic rodents [29], supports the notion that increased GIP bioactivity contributes significantly to the therapeutic benefit of DPP-4 therapy.
Our study was limited to assessing glucose-stimulated incretin responses in healthy subjects during an OGTT. However, incretin secretion is also simulated by fat ingestion [1]. Therefore, future studies should examine bioactive incretin responses to other nutrients too. In addition, there are miscellaneous reports with ELISAs indicating that plasma GIP and GLP-1 levels in subjects with T2DM are increased, comparable, or even decreased relative to those in healthy subjects [30], [31]. The bioassays described here may be useful tools to measure bioactive incretin levels to resolve these discrepancies.
In conclusion, our studies suggest that ELISAs may typically underestimate the levels of bioactive incretins, particularly assays that do not detect GIP(1–30)NH2. Moreover, we find that DPP-4 inhibitor treatment has a much greater impact on plasma bioactive GIP levels than bioactive GLP-1 levels in healthy subjects. Therefore, the contribution of GIP to the therapeutic efficacy of DPP-4 inhibitor treatment warrants additional examination.
Acknowledgements
This research was supported in part by research grants from the Japan Diabetes Foundation (Incretin Research), the Japan Society for the Promotion of Science (JSPS; Grant-in-Aid for Scientific Research (C) #23591291), and Insulin Research Foundation (Novo Nordisk Pharma, Japan). YF also received scholarships from Eli Lilly, Pfizer and MSD. TJK gratefully acknowledges fellowship support from JSPS while on sabbatical at Kyoto University.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.12.009.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
TY, YF, Y Takeda, JH, HS, HK, Y Takiyama, YM, AA, TJK, and MH contributed to the study concept and design. TJK produced the HEK293 cell lines co-transfected with human forms of GIPR or GLP-1 receptor, and a cyclic AMP-inducible luciferase expression construct. TY and YF acquired the data. TY, YF, Y Takeda, JH, HS, HK, Y Takiyama, YM, AA, and MH analyzed and interpreted the data. TY, YF, and TJK drafted the manuscript. TY, YF, Y Takeda, JH, HS, HK, Y Takiyama, YM, AA, TJK, and MH reviewed the manuscript for important intellectual content. YF and MH are the guarantors of this work.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Cho Y.M., Merchant C.E., Kieffer T.J. Targeting the glucagon receptor family for diabetes and obesity therapy. Pharmacology & Therapeutics. 2012;135:247–278. doi: 10.1016/j.pharmthera.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Fujita Y., Wideman R.D., Asadi A., Yang G.K., Baker R., Webber T. Glucose-dependent insulinotropic polypeptide is expressed in pancreatic islet α-cells and promotes insulin secretion. Gastroenterology. 2010;138:1966–1975. doi: 10.1053/j.gastro.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 3.Fujita Y., Asadi A., Yang G.K., Kwok Y.N., Kieffer T.J. Differential processing of pro-glucose-dependent insulinotropic polypeptide in gut. The American Journal of Physiology Gastrointestinal and Liver Physiology. 2010;298:G608–G614. doi: 10.1152/ajpgi.00024.2010. [DOI] [PubMed] [Google Scholar]
- 4.Seino Y., Kuwata H., Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. Journal of Diabetes Investigation. 2016;7(Suppl 1):102–109. doi: 10.1111/jdi.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yanagimachi T., Fujita Y., Takeda Y., Honjo J., Atageldiyeva K.K., Takiyama Y. Pancreatic glucose-dependent insulinotropic polypeptide (GIP) (1-30) expression is upregulated in diabetes and PEGylated GIP(1-30) can suppress the progression of low-dose-STZ-induced hyperglycaemia in mice. Diabetologia. 2016;59:533–541. doi: 10.1007/s00125-015-3842-y. [DOI] [PubMed] [Google Scholar]
- 6.Kieffer T.J., Heller R.S., Unson C.G., Weir G.C., Habener J.F. Distribution of glucagon receptors on hormone-specific endocrine cells of rat pancreatic islets. Endocrinology. 1996;137:5119–5125. doi: 10.1210/endo.137.11.8895386. [DOI] [PubMed] [Google Scholar]
- 7.Wewer Albrechtsen N.J., Hartmann B., Veedfald S., Windeløv J.A., Plamboeck A., Bojsen-Møller K.N. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia. 2014;57:1919–1926. doi: 10.1007/s00125-014-3283-z. [DOI] [PubMed] [Google Scholar]
- 8.Bak M.J., Albrechtsen N.W., Pedersen J., Hartmann B., Christensen M., Vilsbøll T. Specificity and sensitivity of commercially available assays for glucagon and oxyntomodulin measurement in humans. European Journal of Endocrinology. 2014;170:529–538. doi: 10.1530/EJE-13-0941. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo T., Miyagawa J., Kusunoki Y., Miuchi M., Ikawa T., Akagami T. Postabsorptive hyperglucagonemia in patients with type 2 diabetes mellitus analyzed with a novel enzyme-linked immunosorbent assay. Journal of Diabetes Investigation. 2015;7:324–331. doi: 10.1111/jdi.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bak M.J., Wewer Albrechtsen N.J., Pedersen J., Knop F.K., Vilsbøll T., Jørgensen N.B. Specificity and sensitivity of commercially available assays for glucagon-like peptide-1 (GLP-1): implications for GLP-1 measurements in clinical studies. Diabetes. Obesity and Metabolism. 2014;11:1155–1164. doi: 10.1111/dom.12352. [DOI] [PubMed] [Google Scholar]
- 11.Mulvihill E.E., Drucker D.J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocrine Reviews. 2014;35:992–1019. doi: 10.1210/er.2014-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omar B.A., Liehua L., Yamada Y., Seino Y., Marchetti P., Ahrén B. Dipeptidyl peptidase 4 (DPP-4) is expressed in mouse and human islets and its activity is decreased in human islets from individuals with type 2 diabetes. Diabetologia. 2014;57:1876–1883. doi: 10.1007/s00125-014-3299-4. [DOI] [PubMed] [Google Scholar]
- 13.Mulvihill E.E., Varin E.M., Gladanac B., Campbell J.E., Ussher J.R., Baggio L.L. Cellular sites and mechanisms linking reduction of dipeptidyl peptidase-4 activity to control of incretin hormone action and glucose homeostasis. Cell Metabolism. 2016 doi: 10.1016/j.cmet.2016.10.007. S1550–4131(16) 30538-1. Epub Nov 4. [DOI] [PubMed] [Google Scholar]
- 14.Kieffer T.J., McIntosh C.H., Pederson R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 15.Nauck M.A., Kind J., Köthe L.D., Holst J.J., Deacon C.F., Broschag M. Quantification of the contribution of GLP-1 to mediating insulinotropic effects of DPP-4 inhibition with vildagliptin in healthy subjects and patients with type 2 diabetes using exendin [9-39] as a GLP-1 receptor antagonist. Diabetes. 2016;65:2440–2447. doi: 10.2337/db16-0107. [DOI] [PubMed] [Google Scholar]
- 16.Brubaker P.L., Efendic S., Greenberg G.R. Truncated and full-length glucagon-like peptide-1 (GLP-1) differentially stimulate intestinal somatostatin release. Endocrine. 1997;1:91–95. doi: 10.1007/BF02738808. [DOI] [PubMed] [Google Scholar]
- 17.Hansen L., Hartmann B., Bisgaard T., Mineo H., Jørgensen P.N., Holst J.J. Somatostatin restrains the secretion of glucagon-like peptide-1 and -2 from isolated perfused porcine ileum. American Journal of Physiology Endocrinology and Metabolism. 2000;278:E1010–E1018. doi: 10.1152/ajpendo.2000.278.6.E1010. [DOI] [PubMed] [Google Scholar]
- 18.Chia C.W., Carlson O.D., Kim W., Shin Y.K., Charles C.P., Kim H.S. Exogenous glucose-dependent insulinotropic polypeptide worsens post prandial hyperglycemia in type 2 diabetes. Diabetes. 2009;58:1342–1349. doi: 10.2337/db08-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen M., Vedtofte L., Holst J.J., Vilsbøll T., Knop F.K. Glucose-dependent insulinotropic polypeptide: a bifunctional glucose-dependent regulator of glucagon and insulin secretion in humans. Diabetes. 2011;60:3103–3109. doi: 10.2337/db11-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pospisilik J.A., Hinke S.A., Pederson R.A., Hoffmann T., Rosche F., Schlenzig D. Metabolism of glucagon by dipeptidyl peptidase IV (CD26) Regulatory Peptides. 2001;93:133–141. doi: 10.1016/s0167-0115(00)00170-1. [DOI] [PubMed] [Google Scholar]
- 21.Nauck M.A., Heimesaat M.M., Orskov C., Holst J.J., Ebert R., Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. The Journal of Clinical Investigation. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elahi D., McAloon-Dyke M., Fukagawa N.K., Meneilly G.S., Sclater A.L., Minaker K.L. The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7-37) in normal and diabetic subjects. Regulatory Peptides. 1994;51:63–74. doi: 10.1016/0167-0115(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 23.Vilsbøll T., Krarup T., Madsbad S., Holst J.J. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia. 2002;45:1111–1119. doi: 10.1007/s00125-002-0878-6. [DOI] [PubMed] [Google Scholar]
- 24.Nauck M.A., Kahle M., Baranov O., Deacon C.F., Holst J.J. Addition of a dipeptidyl peptidase-4 inhibitor, sitagliptin, to ongoing therapy with the glucagon-like peptide-1 receptor agonist liraglutide: a randomized controlled trial in patients with type 2 diabetes. Diabetes, Obesity and Metabolism. 2016 doi: 10.1111/dom.12802. Epub Oct 6. [DOI] [PubMed] [Google Scholar]
- 25.Aulinger B.A., Bedorf A., Kutscherauer G., de Heer J., Holst J.J., Göke B. Defining the role of GLP-1 in the enteroinsulinar axis in type 2 diabetes using DPP-4 inhibition and GLP-1 receptor blockade. Diabetes. 2014;63:1079–1092. doi: 10.2337/db13-1455. [DOI] [PubMed] [Google Scholar]
- 26.Hansotia T., Baggio L.L., Delmeire D., Hinke S.A., Yamada Y., Tsukiyama K. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes. 2004;53:1326–1335. doi: 10.2337/diabetes.53.5.1326. [DOI] [PubMed] [Google Scholar]
- 27.Flock G., Baggio L.L., Longuet C., Drucker D.J. Incretin receptors for glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes. 2007;56:3006–3013. doi: 10.2337/db07-0697. [DOI] [PubMed] [Google Scholar]
- 28.Finan B., Ma T., Ottaway N., Müller T.D., Habegger K.M., Heppner K.M. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Science Translational Medicine. 2013;5:209ra151. doi: 10.1126/scitranslmed.3007218. [DOI] [PubMed] [Google Scholar]
- 29.Widenmaier S.B., Kim S.J., Yang G.K., De Los Reyes T., Nian C., Asadi A. A GIP receptor agonist exhibits beta-cell anti-apoptotic actions in rat models of diabetes resulting in improved beta-cell function and glycemic control. PLoS One. 2010;5:e9590. doi: 10.1371/journal.pone.0009590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calanna S., Christensen M., Holst J.J., Laferrère B., Gluud L.L., Vilsbøll T. Secretion of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes: systematic review and meta-analysis of clinical studies. Diabetes Care. 2013;36:3346–3352. doi: 10.2337/dc13-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calanna S., Christensen M., Holst J.J., Laferrère B., Gluud L.L., Vilsbøll T. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: systematic review and meta-analyses of clinical studies. Diabetologia. 2013;56:965–972. doi: 10.1007/s00125-013-2841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.