Abstract
Objective
Sexual partner concurrency is common among men who have sex with men (MSM) and may increase the probability of HIV transmission during recent (acute or early) infection. We examined the relationship between concurrency and HIV transmission network characteristics (proxies for HIV transmission) among MSM with recent HIV infection.
Design
Observational study integrating behavioral, clinical, and molecular epidemiology.
Methods
We inferred a partial HIV transmission network using 986 HIV-1 pol sequences obtained from HIV-infected individuals in San Diego, California (1996-2015). We further analyzed data from 285 recently HIV-infected MSM in the network who provided information on up to three sexual partners in the past three months, including the timing of intercourse with each partner. Concurrency was defined as sexual partners overlapping in time. Logistic and negative binomial regression were used to investigate the link between concurrency and HIV transmission network characteristics (i.e., clustering and degree or number of connections to others in the network) among these MSM.
Results
Of recently HIV-infected MSM (N=285), 54% reported concurrent partnerships and 54% were connected by ≥1 putative transmission link to others (i.e., clustered) in the network (median degree=1.0; interquartile range: 0.0-3.0). Concurrency was positively associated with HIV transmission network clustering (adjusted odds ratio=1.83, 95% confidence interval [CI]: 1.08-3.10) and degree (adjusted incidence rate ratio=1.48, 95% CI: 1.02-2.15)
Conclusions
Our findings provide empirical evidence consistent with the hypothesis that concurrency facilitates HIV transmission during recent infection. Interventions to mitigate the impact of concurrency on HIV transmission may help curb the HIV epidemic among MSM.
Keywords: concurrency, partial HIV transmission network, acute and early HIV infection, men who have sex with men, molecular epidemiology
INTRODUCTION
Acute and early HIV infection, characterized by a high viral load and increased infectiousness [1], contribute disproportionately to HIV transmission [2]. One factor hypothesized to enhance HIV transmission during acute and early infection is sexual partner concurrency [3], where “sexual intercourse with one partner occurs between two acts of intercourse with another partner” [4]. Concurrency reduces the time between sexual partner acquisition, and thus may facilitate HIV transmission by increasing the probability of exposing an uninfected partner during the highly infectious period following HIV acquisition from an infected concurrent partner [4].
Simulation studies have demonstrated the impact of concurrency on the spread of HIV during acute and early infection [5-7]; however, most epidemiologic studies to date have not been appropriately designed to empirically evaluate the role of concurrency [8, 9]. Advances in molecular epidemiologic methods support HIV transmission network inference through the identification of HIV-infected individuals with genetically related viruses [10-12]. As such, research that combines behavioral, clinical, and molecular epidemiology to assess the relationship between concurrency and HIV transmission network characteristics (i.e., clustering and degree [number of connections to others in the network]) could provide empirical evidence to better evaluate the hypothesis that concurrency facilitates HIV transmission.
Men who have sex with men (MSM) continue to bear the greatest burden of HIV infection in the United States, and account for the majority (68%) of HIV infections diagnosed among adults and adolescents [13]. Previous research suggests concurrency is common among MSM in the United States, with 63% of MSM surveyed in New York City reporting concurrent partners in the past three months [14] and 45% of MSM evaluated in a national, web-based survey reporting concurrent partners in the past six months [15]. Thus, even a small increased risk of HIV transmission due to concurrency during acute or early infection could have a substantial impact on the HIV epidemic among MSM.
To investigate the influence of concurrency on HIV transmission, we examined the association between concurrency and HIV transmission network characteristics using behavioral, clinical, and molecular epidemiologic data from MSM with recent (i.e., acute or early) HIV infection.
METHODS
Study Population
Our study population was drawn from two cohorts of HIV-infected individuals in San Diego, California. The first cohort consists of newly HIV-diagnosed and antiretroviral therapy (ART) naïve adults and adolescents identified at University of California, San Diego (UCSD) HIV screening centers in central San Diego between January 1996 and June 2015. An estimated date of infection (EDI) was calculated using a previously reported algorithm [16] and persons ≥16 years of age with recent infection were subsequently enrolled in the San Diego Primary Infection Resource Consortium (SD-PIRC). As previously described [17], between 1996 and 2006, individuals reporting recent exposure or symptoms consistent with acute HIV infection were classified as having recent infection in the presence of one of the following: (1) negative HIV antibody (Ab) test (enzyme-linked immunoassay [EIA] or rapid test) followed by positive HIV Ab test in the past 12 months, (2) negative HIV Ab test and positive HIV RNA, or (3) positive HIV Ab test and detuned EIA results consistent with early infection (Vironostika LS EIA, bioMerieux; Durham, NC). Beginning in 2007, individuals seeking HIV testing were staged according to the UCSD Early Test screening algorithm, which includes rapid HIV Ab testing followed by detuned EIA testing for those with positive rapid test results and HIV nucleic acid amplification testing (NAAT) for those with negative rapid test results [18]. Individuals with detuned results consistent with early infection or positive NAAT results were classified as having recent HIV infection. Partner services were offered to recently HIV-infected individuals to facilitate HIV testing of their recent sexual and needle-sharing contacts. The second cohort consists of HIV-infected individuals who initiated care at the UCSD HIV clinic and contributed blood samples to the Center for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS) between July 2007 and August 2013. Clinical data needed to determine the stage of HIV infection (recent vs. chronic) at the time of sample collection were not available for this cohort. UCSD's Institutional Review Board approved the study protocols, and all study participants provided written informed consent.
Data Collection
HIV-1 pol sequences
CNICS participants and individuals screened by the SD-PIRC provided blood samples to detect genotypic drug resistance via bulk sequencing (GeneSeq HIV-1; LabCorp, Burlington, NC or Viroseq v.2.0; Celera Diagnostics, Alameda, CA) of the partial HIV-1 pol coding region, which included the protease gene and between 305 and 335 5’ amino acids of the reverse transcriptase gene. LabCorp and the UCSD Antiviral Research Center employed standard methods combined with investigator review when necessary to rule out contamination during RNA isolation, amplification, and sequencing.
Computer-assisted self-interviews (CASIs)
CNICS participants did not complete CASIs. SD-PIRC participants completed CASIs during one of three data collection waves (Wave 1: 2002 to 2008; Wave 2: 2009 to May 2011; Wave 3: June 2011 to 2015) to ascertain:
Individual-level characteristics
Socio-demographics (age; race/ethnicity; education), sexual behaviors (past three months) (number of male sexual partners), and illicit drug use (past three months) (marijuana; methamphetamine; ecstasy; amyl nitrite; cocaine; gamma-hydroxybutyric acid; ketamine; and heroin). Illicit drug use was only collected from 2003 to 2008 during Wave 1.
Partner-level characteristics
Wave 1 CASIs collected partner-specific data on up to three sexual partners in the past 12 months, Wave 2 CASIs collected partner-specific data on up to two sexual partners in the past three months, and Wave 3 CASIs collected partner-specific data on up to three sexual partners in the past three months. CASIs collected each partner's age, gender, HIV status, as well as the relationship type (main [primary sex partner] vs. non-main [regular partner, friend, acquaintance, one-time partner, unknown partner, trade partner]) and sexual behaviors practiced with each partner (i.e., any condomless anal intercourse [CAI] [Waves 1 and 2] and frequency of CAI [Wave 3]). CASIs also elicited information on the timing of sexual partnerships: “How long ago did you first have sex with [sex partner]?” and “How long ago did you last have sex with [sex partner]?” in days, weeks, months, or years.
HIV Transmission Network Inference
HIV-1 pol sequences from 986 HIV-infected individuals (800 screened by the SD-PIRC and 186 CNICS participants) were used to infer a partial HIV transmission network, using HIV-TRACE (www.hivtrace.org) following the protocol outlined in Wertheim et al. [12] and Oster et al. [19]. Intra-host HIV-1 pol evolution is slow resulting in <1% divergence from baseline sequences even up to nine years following infection [20], thus this approach can be used to infer HIV transmission networks among HIV-infected individuals of any infection stage.Sequences were codon-aligned and processed using a modified Smith-Waterman algorithm [21] and subtyped according to Subtype Classification Using Evolutionary Algorithms (SCUEAL) [22]. Possible contaminants closely related to common laboratory controls (e.g., HXB2) were resequenced. Tamura-Nei 93 (TN93) genetic distances [23] were measured between all pairs of sequences to identify individuals with highly genetically similar HIV-1 strains. Individuals whose sequences had a TN93 genetic distance ≤1.5% were connected by putative transmission links [12, 24]. In previous work, the mean genetic distance between two randomly selected sequences from SD-PIRC participants was 5.83% (SD=1.46%) and only 0.25% of pairwise distances were less than 1.5% [25]. In a sensitivity analysis, we excluded codons associated with drug resistance mutations [26] to examine their impact on the structure of the network.
Available data on the EDI, date of diagnosis, and date of sample collection were then examined for putative transmission links involving ≥1 individual or node with recent infection to infer (1) the putative source of infection for nodes with recent infection and (2) whether the putative transmission event occurred during the source's period of recent infection. For links connecting two nodes with recent infection, the putative direction of transmission was inferred if the EDI of the putative recipient was >30 days after that of the putative source. If the recipient's EDI was within 180 days of that of the source, the putative transmission event was inferred to have occurred during the source's period of recent infection. For links connecting one node with recent infection and another with chronic infection, the putative direction of transmission was inferred if the EDI of the node with recent infection (i.e., putative recipient) occurred after the date of diagnosis or sample collection of the node with chronic infection (i.e., putative source). For links connecting one node with recent infection and another with an unknown stage of infection, the putative direction of transmission was inferred if the EDI of the node with recent infection (i.e., putative recipient) was >30 days after the date of diagnosis or sample collection of the node with an unknown stage of infection (i.e., putative source). For directed putative transmission links involving ≥1 node with chronic or an unknown stage of infection, transmission events were inferred to have occurred during the source's period of chronic infection.
Exposure of Interest: Sexual Partner Concurrency
Concurrency was defined as overlapping sexual partnerships in the past three months. Participant responses to questions about the timing of sexual partnerships were converted into days to determine the dates of first and last sex with each partner. To identify concurrent partners, we compared the dates of first and last sex for each pair of partners reported by a participant. If periods of sexual activity with each partner in a pair overlapped, the partners were considered “concurrent”. If periods of sexual activity with each partner in a pair did not overlap, the partners were considered “non-concurrent”. If periods of sexual activity with each partner in a pair only overlapped by a single day, the partners were also considered “non-concurrent” because we could not determine whether the partnerships truly overlapped or whether sex with one partner only began after the sexual partnership with the other partner had ended. From this partner-level data, we classified participants with at least one pair of concurrent partners as having concurrent partners in the past three months and participants with only non-concurrent partners as having no concurrent partners in the past three months.
Outcomes of Interest: HIV Transmission Network Characteristics
Outcomes of interest were obtained from the inferred HIV transmission network, and included clustering and degree. Clusters were defined as groups of ≥2 individuals connected by putative transmission links (directed or undirected) within the network, such that HIV-1 pol sequences from clustered individuals were ≤1.5% genetically distant from the sequence of at least one other individual in the cluster. Degree was defined as an individual's number of putative transmission links (directed or undirected) to other individuals in the network.
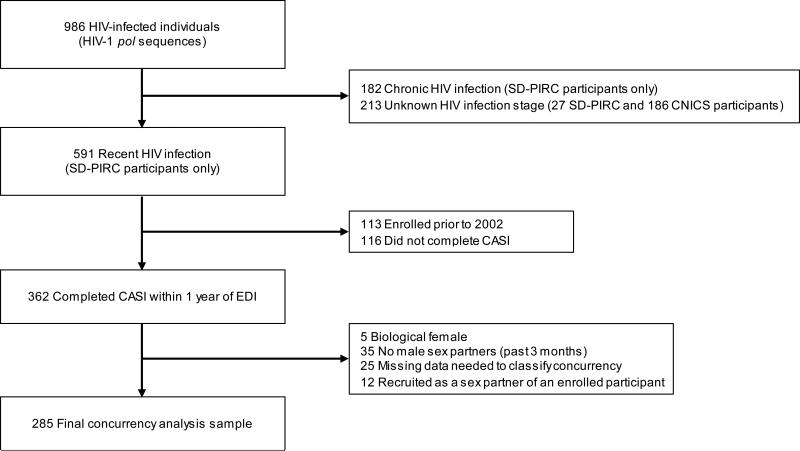
Statistical Analysis
To examine the relationship between sexual partner concurrency and HIV transmission network characteristics among recently HIV-infected MSM, we restricted our analysis to 285 SD-PIRC participants with recent HIV infection who met the following criteria: completed CASI within one year of EDI, biologically male, reported sex with a man in the past three months, and provided data needed to classify concurrency (Figure 1). Participants recruited as sexual contacts of previously enrolled participants were excluded due to the potential for correlated outcomes. First, we calculated descriptive statistics overall and by CASI wave. Next, we used logistic regression to examine the effect of concurrency on clustering within the HIV transmission network. We then used negative binomial regression (due to evidence of over-dispersion) to examine the effect of concurrency on HIV transmission network degree. Final models were adjusted for potential confounders at the individual- (age, race/ethnicity, education, and number of male sexual partners) and partnership-level (relationship type and CAI with recent sexual partners – based on partner-specific data). Models were also adjusted for enrollment year to control for potential differences over time or by CASI wave. In sensitivity analyses, we examined the effect of concurrency on clustering and degree measures derived from the HIV transmission network inferred in the absence of codons associated with drug resistance mutations. Analyses were performed using SAS 9.3 (SAS Institute, Inc.; Cary, NC).
Figure 1.
Flowchart of recently HIV-infected MSM SD-PIRC participants who contributed to the analysis examining the relationship between sexual partner concurrency and HIV transmission network characteristics (MSM=men who have sex with men; SD-PIRC=San Diego Primary Infection Resource Consortium; CASI=computer-assisted self-interview; EDI=estimated data of infection).
RESULTS
HIV Transmission Network
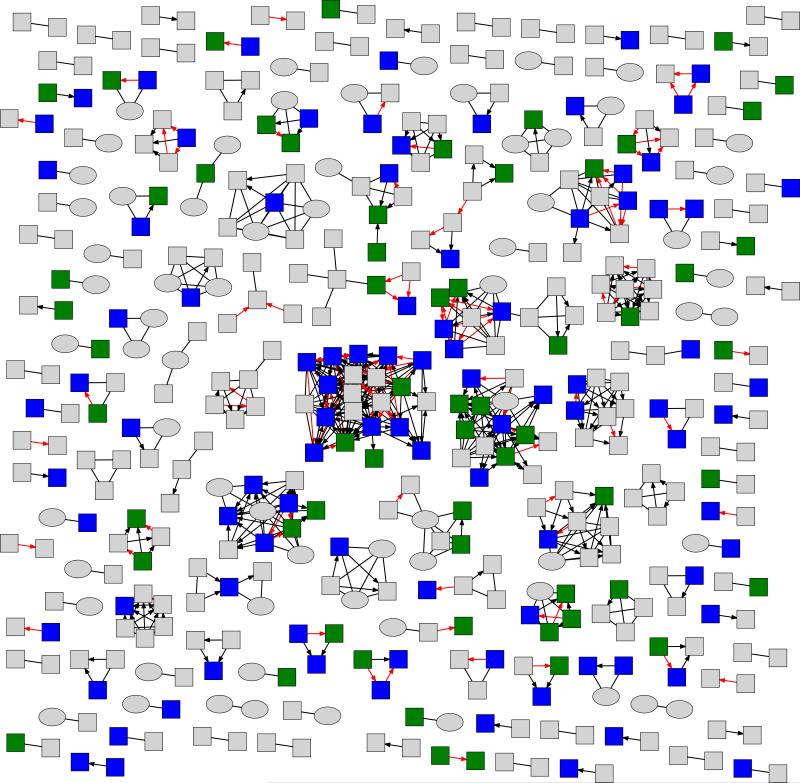
HIV-1 pol sequences from 986 HIV-infected individuals (591 recent; 182 chronic; 213 unknown stage) were used to infer a partial HIV transmission network (Figure 2). The network consisted of 730 putative transmission links involving 47% (462/986) of individuals overall, and 50% (295/591), 51% (93/182), and 35% (74/213) of individuals with recent infection, chronic infection, and an unknown stage of infection, respectively. It was possible to infer the putative direction of transmission for 454 links (62%) and the putative direction and timing (recent vs. chronic) of transmission for 453 links (62%), of which 18% (80/453) were inferred to have been established during the putative source’s period of recent infection. Mean cluster size was 3.2 (SD=2.7; min=2; max=21), with 62% of clusters consisting of dyads. Consistent with previous research [12, 19, 27], findings from our sensitivity analysis excluding codons associated with drug resistance mutations had little impact on the structure of our inferred network (5 linked individuals became unlinked; 1 unlinked individuals became linked).
Figure 2.
Partial HIV transmission network inferred among 186 Center for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS) and 800 San Diego Primary Infection Resource Consortium (SD-PIRC) participants. Only the 462 participants who clustered (i.e., had a putative transmission link to ≥1 other individual) in the network are shown.
Characteristics of Recently HIV-infected MSM SD-PIRC Participants who Completed CASIs
Recently HIV-infected MSM participants (N=285) were predominantly White (60%), well educated (90% completed some college), had a median age of 33 years (interquartile range [IQR]: 26, 40), and had been HIV-infected for a median of 84 days (IQR: 40, 103) (Table 1). In the past three months, 60% reported illicit drug use (excluding marijuana); 41% used amyl nitrite, 29% used methamphetamine, and 21% used ecstasy. When asked about sexual partners (up to three in Wave 1 and 3; up to two in Wave 2) in the past three months, 37% reported ≥1 main sexual partner and 75% reported CAI with ≥1 sexual partner. Fifty-four percent reported concurrent sexual partners and 54% were connected by ≥1 putative transmission link to others (i.e., clustered) in the network (median degree=1.0; IQR: 0.0, 3.0).
Table 1.
Characteristics of recently HIV-infected MSM enrolled in the SD-PIRC (2002 - 2015).
| Wave 1 (N=167) | Wave 2 (N=50) | Wave 3 (N=68) | Total (N=285) | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Median days since EDI | 91 | IQR=78, 143 | 44 | IQR=27, 85 | 70 | IQR=32, 86 | 84 | IQR=40, 103 |
| Median days since HIV diagnosis | 21 | IQR=14, 28 | 21 | IQR=15, 32 | 15 | IQR=11, 28 | 20 | IQR=14, 28 |
| Age (in years) | ||||||||
| 18-29 | 64 | 38.3 | 27 | 54.0 | 27 | 39.7 | 118 | 41.4 |
| 30-39 | 58 | 34.7 | 13 | 26.0 | 23 | 33.8 | 94 | 33.0 |
| 40-49 | 35 | 21.0 | 6 | 12.0 | 9 | 13.2 | 50 | 17.5 |
| ≥50 | 10 | 6.0 | 4 | 8.0 | 9 | 13.2 | 23 | 8.1 |
| Race/ethnicity | ||||||||
| White, non-Hispanic | 112 | 67.1 | 25 | 50.0 | 35 | 51.5 | 172 | 60.4 |
| Black, non-Hispanic | 6 | 3.6 | 3 | 6.0 | 7 | 10.3 | 16 | 5.6 |
| Hispanic | 37 | 22.2 | 17 | 34.0 | 15 | 22.1 | 69 | 24.2 |
| Other | 12 | 7.2 | 5 | 10.0 | 11 | 16.2 | 28 | 9.8 |
| Education | ||||||||
| Less than high school | 2 | 1.2 | 1 | 2.0 | 0 | 0.0 | 3 | 1.1 |
| Completed high school | 14 | 8.4 | 5 | 10.0 | 7 | 10.3 | 26 | 9.1 |
| Some College | 71 | 42.5 | 24 | 48.0 | 29 | 42.7 | 124 | 43.5 |
| Completed college or more | 80 | 47.9 | 20 | 40.0 | 32 | 47.1 | 132 | 46.3 |
| Illicit drug use (past 3 months)* | 61 | 70.1 | 22 | 45.8 | 38 | 55.9 | 121 | 59.6 |
| Marijuana | 49 | 56.3 | 22 | 45.8 | 24 | 35.3 | 95 | 46.8 |
| Methamphetamine | 38 | 43.7 | 8 | 16.7 | 13 | 19.1 | 59 | 29.1 |
| Ecstasy | 22 | 25.3 | 10 | 20.8 | 10 | 14.7 | 42 | 20.7 |
| Amyl nitrite (poppers) | 44 | 50.6 | 11 | 22.9 | 28 | 41.2 | 83 | 40.9 |
| Cocaine | 16 | 18.4 | 8 | 16.7 | 7 | 10.3 | 31 | 15.3 |
| Gamma-hydroxybutyric acid (GHB) | 24 | 27.6 | 7 | 14.6 | 9 | 13.2 | 40 | 19.7 |
| Ketamine | 9 | 10.3 | 1 | 2.1 | 1 | 1.5 | 11 | 5.4 |
| Heroin | 1 | 1.2 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 |
| Median # male sexual partners (past 3 months) | 6 | IQR=3, 14 | 6 | IQR=3, 11 | 5 | IQR=1, 10 | 6 | IQR=2, 12 |
| Recent sexual partners (past 3 months)^ | ||||||||
| ≥1 main partner~ | 49 | 33.1 | 22 | 45.8 | 27 | 40.3 | 98 | 37.3 |
| ≥1 HIV+/unknown status partner | 131 | 79.4 | 31 | 64.6 | 53 | 77.9 | 215 | 76.5 |
| CAI with ≥1 recent partner | 120 | 71.9 | 33 | 70.2 | 59 | 86.8 | 212 | 75.2 |
| Any concurrent partners | 97 | 58.1 | 18 | 36.0 | 38 | 55.9 | 153 | 53.7 |
| HIV transmission network characteristics | ||||||||
| Network clustering | 93 | 55.7 | 30 | 60.0 | 31 | 45.6 | 154 | 54.0 |
| Median network degree | 1 | IQR=0, 3 | 1 | IQR=0, 3 | 0 | IQR=0, 2 | 1 | IQR=0, 3 |
| Source of ≥1 transmission link established at any point during follow-up | 50 | 36.0 | 18 | 40.0 | 10 | 16.4 | 78 | 31.8 |
| Source of ≥1 transmission link established during recent infection | 23 | 19.5 | 5 | 13.9 | 6 | 10.0 | 34 | 15.9 |
Abbreviations: SD-PIRC=San Diego Primary Infection Resource Consortium; MSM=men who have sex with men; EDI=estimated date of infection; IQR=interquartile range; CAI=condomless anal intercourse.
Excluding marijuana.
Partner-specific data collected for most recent (past 3 months) sexual partners: Wave 1 up to 3 partners; Wave 2 up to 2 partners; Wave 3 up to 3 partners.
Relationship type: main (primary sex partner) vs. non-main (regular partner, friend, acquaintance, one-time partner, unknown partner, trade partner).
Among putative transmission links involving ≥1 recently HIV-infected MSM participant (486 links), it was possible to infer the putative direction of transmission for 351 (72%) links and the putative direction and timing (recent vs. chronic) of transmission for 350 (72%) links. A participant was inferred to be the putative source of HIV in 57% (199/351) of putative transmission links established at any point during follow-up, and of those links 23% (45/199) were inferred to have been established during the participant’s period of recent infection. Thirty-two percent of participants were inferred to be the putative source of HIV in ≥1 putative transmission link established at any point during follow-up and 16% were inferred to be the putative source of HIV in ≥1 putative transmission link established during their period of recent infection.
Sexual Partner Concurrency and HIV Transmission Network Characteristics among Recently HIV-infected MSM SD-PIRC Participants who Completed CASIs
Compared to recently HIV-infected MSM participants without concurrent partners, those with concurrent partners were more likely to cluster in the network (61% vs. 46%; p=0.01) and had a higher mean network degree (2.7 [SD=4.0] vs. 1.7 [SD=3.0]; p=0.02) (Table 2). After adjusting for enrollment year, age, race/ethnicity, education, number of male sexual partners, relationship type with recent sexual partners, and CAI with recent sexual partners, concurrency was positively associated with HIV transmission network clustering (adjusted odds ratio=1.83, 95% confidence interval [CI]: 1.08, 3.10) and degree (adjusted incidence rate ratio=1.48, 95% CI: 1.02, 2.15). Findings from sensitivity analyses examining the effect of concurrency on clustering and degree measures derived from the HIV transmission network inferred in the absence of codons associated with drug resistance mutations were nearly identical. A greater proportion of participants with concurrent partners was inferred to be the putative source of HIV in ≥1 putative transmission link established at any point during follow-up (38% vs. 25%; p=0.02) and ≥1 putative transmission link established during their period of recent infection (19% vs. 12%; p=0.17; although not statistically significant).
Table 2.
Sexual partner concurrency and HIV transmission network characteristics among recently HIV-infected MSM enrolled in the SD-PIRC.
| Unadjusted | Adjusted^ | ||||||
|---|---|---|---|---|---|---|---|
| Network clustering | n | (%) | p-value | OR | (95% CI) | OR | 95% CI |
| No concurrent sexual partners (n=132) | 60 | 45.5 | Ref | - | Ref | - | |
| ≥1 concurrent sexual partner (n=153) | 94 | 61.4 | 0.01 | 1.91 | 1.19, 3.07 | 1.83 | 1.08, 3.10 |
| Network degree | mean | SD | p-value | IRR | 95% CI | IRR | 95% CI |
| No concurrent sexual partners (n=132) | 1.7 | 3.0 | Ref | - | Ref | - | |
| ≥1 concurrent sexual partner (n=153) | 2.7 | 4.0 | 0.02 | 1.59 | 1.08, 2.33 | 1.48 | 1.02, 2.15 |
Abbreviations: SD-PIRC=San Diego Primary Infection Resource Consortium; MSM=men who have sex with men; SD=standard deviation; CAI=condomless anal intercourse; OR=odds ratio; IRR=incidence rate ratio; CI=confidence interval.
Adjusted for enrollment year, age, race/ethnicity, education, # of recent (past 3 months) male sexual partners, ≥1 recent main sexual partner, and CAI with ≥1 recent sexual partner.
DISCUSSION
Previous epidemiologic studies have primarily investigated the link between an individual's recent concurrency and prevalent HIV status, and thus have not provided sufficient evidence to evaluate the hypothesis that concurrency during recent infection increases an individual's probability of HIV transmission [9]. In this study, we utilized information from an inferred partial HIV transmission network to gain insight on an individual's possible history of HIV transmission. Our combined use of behavioral, clinical, and molecular epidemiologic data allowed us to examine the relationship between concurrency and HIV transmission network characteristics (proxies for HIV transmission) [28] among MSM with recent HIV infection, and thus better evaluate the concurrency hypothesis.
We found that concurrency during recent infection among MSM in San Diego, California was associated with having ≥1 putative transmission link (i.e., clustering) and the number of putative transmission links (i.e., degree) to others in the inferred HIV transmission network. The proportion of participants inferred to be the putative source of HIV in ≥1 putative transmission link established during their period of recent infection did not differ in a statistically significant manner by concurrency. However, 38% of participants with concurrent partners were inferred to be the putative source of HIV in ≥1 putative transmission link established at any point during follow-up compared to only 24% of participants without concurrent partners. These discrepant findings are likely explained by the fact that 77% of putative transmission links where participants were inferred to be the putative source of HIV were not established during their period of recent infection. However, due to incomplete sampling, particularly during the brief but highly infectious period following HIV acquisition, these links may represent transmission pathways that include unobserved intermediates who acquired HIV from participants during recent infection and subsequently transmitted the virus to linked individuals captured in our partial HIV transmission network. While our findings do not provide direct evidence that concurrency facilitates HIV transmission during recent infection, they are consistent with the concurrency hypothesis and suggest concurrency during recent infection is associated with HIV transmission among MSM.
Our findings should be considered in the context of several limitations. First, social desirability bias and recall bias may have led to inaccurate or incomplete reporting of partner-specific data and HIV transmission risk behaviors. As such, some participants may have been misclassified with respect to concurrency since our definition of concurrency depends on accurate measurement of the timing of reported sexual partnerships. Second, participants reported a median of six sexual partners in the past three months. Because detailed partner-specific data, including the timing of sexual intercourse, were only collected for up to three partners, we may have underestimated the prevalence of concurrency. However, it is unlikely that any potential misclassification of participants’ concurrency status was differential with respect to the HIV transmission network characteristics of interest. Third, CASIs varied slightly by data collection wave, which limited the covariates that could be examined across all waves. Fourth, our inferred HIV transmission network represents an incomplete network given that we based our inference on HIV-1 pol sequences obtained from a convenience sample of HIV-infected individuals. Fifth, although we utilized SD-PIRC participants’ clinical data to calculate EDIs and infer the putative direction and timing of transmission, these data cannot determine with certainty whether or when transmission actually occurred between individuals connected by putative transmission links. The presence of a putative transmission link (directed or undirected) between two individuals indicates that their viruses were genetically related, as such links may represent transmission pathways that include unobserved intermediates or a shared common source of infection (observed or unobserved). Finally, due to our small sample size we were unable to examine the relationship between concurrency and the putative direction and timing of transmission in multivariate analyses.
The prevalence of concurrency (54%) in our sample of recently HIV-infected MSM is similar to that documented in previous studies (45% to 63%) [14, 15]. Our findings combined with those from simulation studies [5-7], suggest this high prevalence of concurrency may contribute to the disproportionate burden of HIV among MSM in the United States and point to the need for HIV prevention strategies that mitigate the effect of concurrency on HIV transmission within this population. One strategy might include the expansion of indications for pre-exposure prophylaxis (PrEP) use among MSM in the United States to include sexual partner concurrency. PrEP use among MSM who report concurrent sexual partners could reduce their risk of HIV acquisition from HIV-infected sexual partners, and thus their subsequent probability of transmitting HIV to uninfected concurrent sexual partners. Future research should explore the impact of PrEP use in the context of concurrency on HIV incidence among MSM, as well as factors associated with sexual partner concurrency at the individual- and partnership-level to inform PrEP delivery strategies.
Acknowledgments
The authors thank the Center for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS) for contributing data to the analysis. This work was supported by the National Institute on Drug Abuse [grant number K01 DA040543 to H.A.P.]; the National Institute of Allergy and Infectious Diseases [grant numbers U01 AI43638, R01 HD083042, R01 MH100974, R24 AI106039, R21 MH100974, P01 AI074621, P30 AI035214, and K01 AI110181 to J.O.W.]; the California HIV Research Program [grant number RN07-SD-702]; and the California Collaborative Treatment Group [grant number El11-SD-005].
Footnotes
Author Contributions
Study design: H.A.P., J.O.W., L.L., R.S.G., S.J.L., M.Y.K.; cohort study design and sample collection: S.J.L.; sequence analysis: J.O.W.; statistical analysis: H.A.P.; draft manuscript preparation: H.A.P., M.Y.K.; final manuscript edit and approval: H.A.P., J.O.W., L.L., R.S.G., S.J.L., M.Y.K.
Conflicts of Interest
J.O.W. provides scientific consulting services to the Centers for Disease Control and Prevention. For the remaining authors, no conflicts of interest were declared.
REFERENCES
- 1.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–93. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 2.Miller WC, Rosenberg NE, Rutstein SE, Powers KA. Role of acute and early HIV infection in the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5:277–82. doi: 10.1097/COH.0b013e32833a0d3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris M, Goodreau SM, Moody J. Sexual networks, concurrency, and STD/HIV. In: Holmes KK, Sparling PF, Stamm WE, Piot P, Wasserheit J, editors. Sexually Transmitted Diseases. 4 ed. McGraw Hill; New York: 2008. [Google Scholar]
- 4.UNAIDS. [10 November 2015];Consultation on concurrent sexual partnerships: recommendations from a meeting of the UNAIDS reference group on estimates, modelling and projecion held in Nairobi Kenya. Available at: http://www.epidem.org/sites/default/files/reports/Concurrency_meeting_recommendations_Updated_Nov_2009.pdf.
- 5.Eaton JW, Hallett TB, Garnett GP. Concurrent sexual partnerships and primary HIV infection: a critical interaction. AIDS Behav. 2011;15:687–92. doi: 10.1007/s10461-010-9787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodreau SM, Cassels S, Kasprzyk D, Montano DE, Greek A, Morris M. Concurrent partnerships, acute infection and HIV epidemic dynamics among young adults in Zimbabwe. AIDS Behav. 2012;16:312–22. doi: 10.1007/s10461-010-9858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS. 1997;11:641–8. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Knopf A, Morris M. Lack of association between concurrency and HIV infection: an artifact of study design. J Acquir Immune Defic Syndr. 2012;60:e20–1. doi: 10.1097/QAI.0b013e3182460b79. author reply e1. [DOI] [PubMed] [Google Scholar]
- 9.Morris M. Barking up the wrong evidence tree. Comment on Lurie & Rosenthal, “Concurrent partnerships as a driver of the HIV epidemic in sub-Saharan Africa? The evidence is limited”. AIDS Behav. 2010;14:31–3. doi: 10.1007/s10461-009-9639-6. discussion 4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hue S, Clewley JP, Cane PA, Pillay D. HIV-1 pol gene variation is sufficient for reconstruction of transmissions in the era of antiretroviral therapy. AIDS. 2004;18:719–28. doi: 10.1097/00002030-200403260-00002. [DOI] [PubMed] [Google Scholar]
- 11.Smith DM, May SJ, Tweeten S, Drumright L, Pacold ME, Kosakovsky Pond SL, et al. A public health model for the molecular surveillance of HIV transmission in San Diego, California. AIDS. 2009;23:225–32. doi: 10.1097/QAD.0b013e32831d2a81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wertheim JO, Leigh Brown AJ, Hepler NL, Mehta SR, Richman DD, Smith DM, et al. The global transmission network of HIV-1. J Infect Dis. 2014;209:304–13. doi: 10.1093/infdis/jit524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention [8 March 2015];HIV Surveillance Report: Diagnoses of HIV Infection in the United States and Dependent Areas. 2013 Available at: http://www.cdc.gov/hiv/library/reports/surveillance/2013/surveillance_Report_vol_25.html.
- 14.Tieu HV, Nandi V, Frye V, Stewart K, Oquendo H, Bush B, et al. Concurrent partnerships and HIV risk among men who have sex with men in New York City. Sex Transm Dis. 2014;41:200–8. doi: 10.1097/OLQ.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg ES, Khosropour CM, Sullivan PS. High prevalence of sexual concurrency and concurrent unprotected anal intercourse across racial/ethnic groups among a national, Web-based study of men who have sex with men in the United States. Sex Transm Dis. 2012;39:741–6. doi: 10.1097/OLQ.0b013e31825ec09b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368:218–30. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drumright LN, Little SJ, Strathdee SA, Slymen DJ, Araneta MR, Malcarne VL, et al. Unprotected anal intercourse and substance use among men who have sex with men with recent HIV infection. J Acquir Immune Defic Syndr. 2006;43:344–50. doi: 10.1097/01.qai.0000230530.02212.86. [DOI] [PubMed] [Google Scholar]
- 18.Morris SR, Little SJ, Cunningham T, Garfein RS, Richman DD, Smith DM. Evaluation of an HIV nucleic acid testing program with automated Internet and voicemail systems to deliver results. Ann Intern Med. 2010;152:778–85. doi: 10.1059/0003-4819-152-12-201006150-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oster AM, Wertheim JO, Hernandez AL, Ocfemia MC, Saduvala N, Hall HI. Using Molecular HIV Surveillance Data to Understand Transmission Between Subpopulations in the United States. J Acquir Immune Defic Syndr. 2015;70:444–51. doi: 10.1097/QAI.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hightower GK, May SJ, Perez-Santiago J, Pacold ME, Wagner GA, Little SJ, et al. HIV-1 clade B pol evolution following primary infection. PLoS One. 2013;8:e68188. doi: 10.1371/journal.pone.0068188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hepler NL, Smith DM, Delport W, Young JA, Poon A, Gutierrez J, et al. A Bioinformatics Pipeline for the Analysis and Interpretation of Ultradeep Sequence Data.. 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA. 2012.pp. D–275. [Google Scholar]
- 22.Kosakovsky Pond SL, Posada D, Stawiski E, Chappey C, Poon AF, Hughes G, et al. An evolutionary model-based algorithm for accurate phylogenetic breakpoint mapping and subtype prediction in HIV-1. PLoS Comput Biol. 2009;5:e1000581. doi: 10.1371/journal.pcbi.1000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–26. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 24.Mehta SR, Murrell B, Anderson CM, Kosakovsky Pond SL, Wertheim JO, Young JA, et al. Using HIV Sequence and Epidemiologic Data to Assess the Effect of Self-referral Testing for Acute HIV Infection on Incident Diagnoses in San Diego, California. Clin Infect Dis. 2016 doi: 10.1093/cid/ciw161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little SJ, Kosakovsky Pond SL, Anderson CM, Young JA, Wertheim JO, Mehta SR, et al. Using HIV networks to inform real time prevention interventions. PLoS One. 2014;9:e98443. doi: 10.1371/journal.pone.0098443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wheeler WH, Ziebell RA, Zabina H, Pieniazek D, Prejean J, Bodnar UR, et al. Prevalence of transmitted drug resistance associated mutations and HIV-1 subtypes in new HIV-1 diagnoses, U.S.-2006. AIDS. 2010;24:1203–12. doi: 10.1097/QAD.0b013e3283388742. [DOI] [PubMed] [Google Scholar]
- 27.Wertheim JO, Oster AM, Hernandez AL, Saduvala N, Banez Ocfemia MC, Hall HI. The International Dimension of the U.S. HIV Transmission Network and Onward Transmission of HIV Recently Imported into the United States. AIDS Res Hum Retroviruses. 2016 doi: 10.1089/aid.2015.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dennis AM, Herbeck JT, Brown AL, Kellam P, de Oliveira T, Pillay D, et al. Phylogenetic studies of transmission dynamics in generalized HIV epidemics: an essential tool where the burden is greatest? J Acquir Immune Defic Syndr. 2014;67:181–95. doi: 10.1097/QAI.0000000000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]