Abstract
Introduction
The influence of financial ties to pharmaceutical companies remains controversial. We aimed to assess a potential relationship between pharmaceutical payments and prescription patterns for degarelix and denosumab.
Materials and Methods
Medicare Provider Utilization and Payment Data: Physician and Other Supplier Public Use File (Medicare B) data containing 2012 claims compared to OpenPayments (Sunshine Act) data for the second half of 2013. Urologists and medical oncologists who billed Medicare for degarelix or denosumab were cross referenced in both databases and payments were aggregated into a consolidated dataset. Adjusted beneficiary count and total Medicare reimbursement were compared according to receipt of Sunshine payment, and an association between Sunshine payment amount and total Medicare reimbursement was also assessed.
Results
Of the 160 prescribers of degarelix and 1,507 prescribers of denosumab, 91 (57%) and 854 (57%) received Sunshine payment, respectively. Degarelix prescribers who received Sunshine payment had higher median total Medicare reimbursement ($13,257 vs. $9,554, p = 0.01). Denosumab prescribers who received Sunshine payment had both higher median adjusted beneficiary count (55 vs. 50, p < 0.001) and median total Medicare reimbursement ($69,620 vs. $60,732, p < 0.001). On multivariable analysis, both receipt of Sunshine payment (adjusted median difference $5,844, 95% CI $937 - $10,749) and oncology specialty (adjusted median difference $34,380, 95% CI $26,715 - $42,045) were independently associated with total Medicare reimbursement for denosumab.
Conclusions
In the case of degarelix and denosumab, there is a weak association between pharmaceutical company payments on prescribers' prescription behavior patterns.
Keywords: degarelix, denosumab, prescriber payments, sunshine act, medicare
Introduction
Financial ties between pharmaceuticals and physician prescribers have long been scrutinized in the medical literature.1-3 While limiting criminal behavior has historically been a focus of regulators, raising awareness of potential conflicts of interest has only recently entered the jurisdiction of the United States government. Whether biases exist in prescriber patterns is a matter of ongoing debate, with varying opinions on the appropriateness of prescriber-pharmaceutical interactions,4-6 but the lack of large-scale national databases on prescriber patterns and pharmaceutical payments have limited formal study design. In April 2014 the Centers for Medicare and Medicaid Services (CMS) released 10 million billing records for 880,000 healthcare providers, accounting for over $77 billion of Medicare dollars distributed in 2012. CMS later released payment data from drug manufacturers with 68,000 payment records amounting to over $3.7 billion. To our knowledge, these are the largest national databases on physician reimbursements and pharmaceutical payments to date.
Heavily marketed drugs may be particularly susceptible to prescriber bias. Two examples are degarelix and denosumab, two new treatments for prostate cancer. Degarelix, a gonadotropin-releasing hormone (GnRH) antagonist manufactured by Ferring Pharmaceuticals, was U.S. Food and Drug Administration (FDA) approved in 2008 for the treatment of advanced prostate cancer.7 Denosumab, a receptor activator of nuclear factor kappa-B ligand (RANK-L) inhibitor manufactured by Amgen, was FDA approved in 2011 for bone loss in prostate cancer patients undergoing hormone ablation for metastatic prostate cancer.8 Disclosures of advertising spending for these two drugs are limited, as Ferring is a private company and Amgen provides figures on a consolidated basis. However, in the case of denosumab, sales have increased at a compounded rate of 60% per year, from $554 million to $2.25 billion, since obtaining FDA approval.9, 10 Whether prescriber adoption is influenced by payments from pharmaceuticals is unclear. A combination of the two CMS databases enables an opportunity for focused study of these two drugs.
Our study sought to identify whether there was an association between pharmaceutical payments and prescription patterns of degarelix and denosumab within the CMS databases. Medicare B prescribers were stratified according to whether they received Sunshine Act payments or not as a basis for comparison.
Materials and Methods
Data and Study Population
We used the 2012 Medicare Provider Utilization and Payment Data: Physician and Other Supplier Public Use File (Medicare B) database files that was provided by CMS to identify all urologists and oncologists who prescribed degarelix and denosumab.11 Medicare B contains over 10 million records of prescriber reimbursement data extracted from the National Claims History Standard Analytic Files. It includes information on services and procedures provided to Medicare beneficiaries by physicians and other healthcare professionals. Data include physician national provider identifier, name, address, city, state, Healthcare Common Procedure Coding System (HCPCS) code, specialty, service count, beneficiary per day service count (adjusted beneficiary count), and billing and reimbursement amounts. Each line item represents a separate physician's billing data for each drug. The database contains fee-for-service data and does not include indications for listed services or procedures. Prescribing urologists and oncologists were extracted from the database. These prescribers were linked by name and address with the second half of 2013 CMS Open Payments (Sunshine Act) database to determine whether they received payments from Ferring Pharmaceuticals or Amgen.12 The Sunshine Act database contains payment data and includes physician name, address, city, state, specialty, manufacturer name and identifiers, payment amounts, and payment characteristics such as date and form of payment. Each line item represents a single payment to a physician.
Variables
HCPCS codes J9155 and J0897 were used to identify instances of degarelix and denosumab injections, respectively, in the Medicare B dataset. Specialty, service count, beneficiary per day service count (adjusted beneficiary count), and average payment per service were listed for each prescribing provider. Service count represents a standardized Medicare-defined billing unit. The adjusted beneficiary count represents the number of doses prescribed by each provider. The primary outcome, total Medicare reimbursement, was calculated by multiplying the average payment per service by total service count. Prescribers were linked to the Sunshine Act database via first and last name. In the event of duplicate name entries, matching was performed with the prescriber's state, city, and address. Sunshine Act payments were calculated by summing all listed reimbursements.
Statistical Analysis
For each drug, prescriber specialty, adjusted beneficiary count, and total Medicare reimbursement were compared between those prescribers who received Sunshine payments and those who did not. Wilcoxon rank-sum tests and Chi-square tests were used for continuous and categorical variables, respectively. For degarelix, comparison of specialty could not be performed between groups for degarelix because there were no oncology prescribers who received Sunshine payments.
Median Sunshine payment was calculated for each drug. Spearman rank correlation was used to assess the relationship between and total Sunshine payments and total Medicare reimbursement for each drug.
For denosumab, the association between receipt of Sunshine payment and total Medicare reimbursement was assessed using median regression. The estimated regression coefficient describes the predicted median difference in Medicare reimbursement between groups. Prescriber specialty was included in the adjusted model. A test for interaction was performed to identify whether the association between receipt of Sunshine payment and total Medicare reimbursement differed between urologists and oncologists. Adjusted analyses were not performed for degarelix because no oncology prescribers received Sunshine payment.
All analyses were performed using STATA, version 14 (StataCorp, College Station, TX). This study utilized public databases and was IRB exempt.
Results
Of the 160 prescribers of degarelix and 1,507 prescribers of denosumab, 91 (57%) and 854 (57%) received Sunshine payment, respectively. Characteristics of the study population are summarized in Table 1. No oncology prescribers of degarelix received Sunshine payment. For denosumab, oncologists were more likely than urologists to have received Sunshine payment (58% vs. 46%, p = 0.002). For degarelix, those prescribers who received Sunshine payment had higher median total Medicare reimbursement ($13,257 vs. $9,554, p = 0.01). For denosumab, those prescribers who received Sunshine payment had both higher median adjusted beneficiary count (55 vs. 50, p < 0.001) and median total Medicare reimbursement ($69,620 vs. $60,732, p < 0.001).
Table 1.
Characteristics of the study population stratified by receipt of sunshine payment for degarelix and denosumab.
| Degarelix | Denosumab | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristics | No Sunshine Payment (N = 69) | Sunshine Payment (N = 91) | P value* | No Sunshine Payment (N = 653) | Sunshine Payment (N = 854) | P value |
|
| ||||||
| Specialy (%) | -- | 0.002 | ||||
| Urology | 62 (90) | 91 (100) | 93 (14) | 78 (9) | ||
| Oncology | 7 (10) | 0 (0) | 560 (86) | 776 (89) | ||
|
| ||||||
| Adjusted beneficiary count, median (IQR) | 36 (23-62) | 52 (29-79) | 0.051 | 50 (31-74) | 55 (38-79) | <0.001 |
|
| ||||||
| Total Medicare reimbursement, USD, median (IQR) | 9,554 (7,507-14,291) | 13,257 (9,398-17,772) | 0.01 | 60,732 (37,182-92,577) | 69,620 (44,281-99,272) | <0.001 |
Abbreviations: IQR, interquartile range; USD, United States dollars
P values determined using Chi-Square test for categorical variables and Wilcoxon rank-sum tests for continuous variables.
To degarelix prescribers who received Sunshine payment(s), the median total Sunshine payment amount was $28.15 (IQR $16.25-$61.96). There was no correlation between total Sunshine payment amount and total Medicare reimbursement (Figure 1, ρ = -0.07, p = 0.51). To denosumab prescribers who received Sunshine payment(s), the median total Sunshine payment amount was $47.13 (IQR $21.08-$107.16). There was a correlation between total Sunshine payment amount and total Medicare reimbursement (ρ = .10, p = 0.003).
Figure 1.
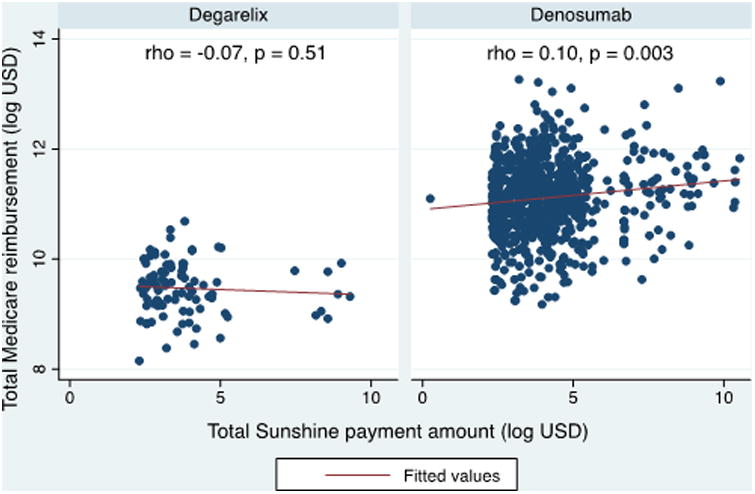
Relationship between total Sunshine payment amount and total Medicare reimbursement for degarelix and denosumab. Scatter plot of total Sunshine payment amount vs. total Medicare reimbursement in the log scale. Red line represents fitted linear prediction. P values generated using Spearman correlation.
Univariable and multivariable analyses examining predictors of total Medicare reimbursement for denosumab are shown in Table 2. On multivariable analysis, both receipt of Sunshine payment (adjusted median difference $5,844, 95% CI $937 - $10,749) and oncology specialty (adjusted median difference $34,380, 95% CI $26,715 - $42,045) were independently associated with total Medicare reimbursement. The interaction between receipt of Sunshine payment and prescriber specialty is not associated with total Medicare payment (p = 0.25).
Table 2.
Median regression analysis examining predictors of total Medicare payment.
| Univariable Analysis | Multivariable analysis* | |||
|---|---|---|---|---|
| Variable | Predicted Median Difference (95% CI) | P value* | Adjusted Median Difference (95% CI) | P value |
| Sunshine | 0.001 | 0.02 | ||
| No | Reference | Reference | ||
| Yes | 8,912 (3,702-14,121) | 5,844 (937-10,749) | ||
| Specialty | <0.001 | <0.001 | ||
| Urologist | Reference | Reference | ||
| Oncologist | 36,649 (29,131-44,167) | 34,380 (26,715-42,045) | ||
P values are computed using the Wald test. The multivariable model included receipt of Sunshine payment and prescriber specialty
Discussion
To our knowledge, this study is the first to utilize the Medicare reimbursements and Sunshine Act databases to study the association between industry payments and prescriber patterns. We found a modest difference in physician total Medicare reimbursement between those who did and did not receive Sunshine Act payments for both degarelix and denosumab. This is despite the fact that the majority of total Sunshine payments to prescribers were less than $50. In the case of denosumab, there was a weak correlation between total Sunshine payment and total Medicare reimbursement. Furthermore, the association between receipt of Sunshine payment and Medicare reimbursement of denosumab remained after adjusting for type of specialty.
Conflicts of interest in medicine have been studied extensively, and there is much literature to suggest a positive association between pharmaceutical interaction and professional behavior.1-3, 13 This is in contrast to prescriber perceptions, as there is an apparent association between number of attended drug lunches and the belief that discussions with pharmaceutical representatives had no effect on prescription behavior.14 Chren and Landefeld demonstrated that physicians who received money to attend symposia, speak at symposia, or perform research were more likely to request a drug be added to a hospital formulary (odds ratio 5.7).1 Likewise, a multi-institutional study at seven Midwest teaching hospitals reported brief conversations with pharmaceutical representatives and honoraria or research support as predictors of drug addition requests to a hospital formulary.15 The same study noted 25% of faculty and 32% of residents changed their practices at least once based on pharmaceutical representative contact. Despite being well reported in the literature, historical study methodology has been limited by lack of objective measurements and a reliance on survey data which is subject to recall bias. Furthermore, the scarcity of negative studies in this field may include a component of publication bias.16 Our study, in contrast to prior studies, possesses the advantage of utilizing quantitative payment data, and is not as susceptible to recall bias.
Whether the magnitude of payment affects prescriber behavior is an area of ongoing investigation. In a national survey published in 2007, 94% of physicians reported a relationship with the pharmaceutical industry.3 The majority of interactions were in the form of food or drug samples, which is likely the case in our cohort given the modest median payments received. One would expect a higher degree of influence on prescriber patterns at the high end of the distribution. Our study produced mixed results in this regard, as we identified a relationship between total Medicare reimbursement and total Sunshine payments for denosumab but not degarelix. Still, even amongst prescribers of denosumab, the magnitude of the association was weak (ρ = 0.10) and may not be as important as once perceived.
The observations in this study pose an important question for pharmaceutical companies: if the correlation between magnitude of payment and prescriber behavior is weak, what is the return on investment? It is possible that the investment has indirect effects on the prescriber community as a whole; that is, increased adoption by those who receive Sunshine payments would result in increased adoption by those who do not. Funding events geared towards prescribers could result in increasing general awareness of the drug: first, directly through the participants, and thereafter indirectly through education, discussion, and collaboration. Our study was designed to detect a difference between recipients of Sunshine payments to non-recipients, and would not detect indirect effects on the community as a whole. Specifically, our study did not measure the relative attractiveness of denosumab and degarelix against peer drugs to gauge market share of prescriber behavior.
The limited scope of our study precludes any suggestion that professional societies should modify their policies.2, 17-19 There is a general lack of awareness regarding the potential for conflict of interest,20-22 and guidelines have served an important role in informing healthcare professionals.20 Instead, this paper highlights the importance of quantifiable metrics as a basis for conclusions. Recall bias is a well-documented limitation in survey data,23 and although entries into the CMS database may include an element of recall bias, its effect is likely less than in surveys. Payments, in contrast to physician recollection, can be immediately quantified and corroborated with transaction data. Our study thus defines an effective alternative to surveys, and should serve as a basis for subsequent studies in conflict of interest.
Our report should be considered in in the context of several limitations. First, the Medicare B database contains data from 2012 and Sunshine Act database contains data from the second half of 2013. If the effects of Sunshine payments on Medicare reimbursements are temporally dependent, our study would need concurrent databases for analysis. However, it is unlikely that the small time difference between the two databases would significantly affect prescriber behavior. Secondly, our conclusions are confined to denosumab and degarelix, and therefore its generalizability is limited. It is possible there are differential effects of Sunshine payments on prescription patterns for other classes of drugs. However, denosumab and degarelix are two commonly prescribed drugs with favorable reimbursement and are heavily marketed to urologists and oncologists. At the outset of this study, we felt these drugs were appropriate candidates to test whether prescribers of these drugs may be influenced by industry payments. Thirdly, the CMS has jurisdiction over only federally sponsored programs such as Medicare and Medicaid, and prescription and payment data outside their jurisdiction would not be reported. The quality of the Medicare B database as a basis for drawing conclusions on general practices have been criticized in prior literature.24, 25 Since indications for prescriptions are not listed, it is impossible to determine the quality of care delivered on the basis of billing data alone. The notion that lower billing amounts equates to better care cannot be concluded. Furthermore, Sunshine Act data are reported by the manufacturer, rather than the recipient, and can contain inaccuracies. Listed recipients have the option of contesting entries but lack of awareness may cause errors to persist. Nevertheless, despite these limitations, the CMS is the largest payer of health care in the United States, covering over 90 million Americans,26 making Medicare B and Sunshine Act databases two of the most robust databases available.
Conclusions
Recipients of payments from industry contained within the Sunshine Act database tend to receive more total Medicare reimbursements with regards to degarelix and denosumab. Whether magnitude of Sunshine payment correlates with prescriber behavior continues to be controversial and is a subject of ongoing study.
References
- 1.Chren MM, Landefeld CS. Physicians' behavior and their interactions with drug companies. A controlled study of physicians who requested additions to a hospital drug formulary. JAMA. 1994;271:684. [PubMed] [Google Scholar]
- 2.Coyle SL Ethics, Human Rights Committee, A. C. o. P.-A. S. o. I. M. Physician-industry relations. Part 1: individual physicians. Ann Intern Med. 2002;136:396. doi: 10.7326/0003-4819-136-5-200203050-00014. [DOI] [PubMed] [Google Scholar]
- 3.Campbell EG, Gruen RL, Mountford J, et al. A national survey of physician-industry relationships. N Engl J Med. 2007;356:1742. doi: 10.1056/NEJMsa064508. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbaum L. Conflicts of interest: part 1: Reconnecting the dots--reinterpreting industry-physician relations. N Engl J Med. 2015;372:1860. doi: 10.1056/NEJMms1502493. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum L. Understanding bias--the case for careful study. N Engl J Med. 2015;372:1959. doi: 10.1056/NEJMms1502497. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum L. Beyond moral outrage--weighing the trade-offs of COI regulation. N Engl J Med. 2015;372:2064. doi: 10.1056/NEJMms1502498. [DOI] [PubMed] [Google Scholar]
- 7.Doehn C, Sommerauer M, Jocham D. Degarelix for prostate cancer. Expert Opin Investig Drugs. 2009;18:851. doi: 10.1517/13543780902954713. [DOI] [PubMed] [Google Scholar]
- 8.El-Amm J, Freeman A, Patel N, et al. Bone-targeted therapies in metastatic castration-resistant prostate cancer: evolving paradigms. Prostate Cancer. 2013;2013:210686. doi: 10.1155/2013/210686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amgen, Inc. 10-K report. 2014 Retrieved from http://www.sec.gov/Archives/edgar/data/318154/000031815415000005/amgn-12312014x10k.htm.
- 10.Amgen, Inc. 10-K report. 2011 Retrieved from http://www.sec.gov/Archives/edgar/data/318154/000119312512086670/d241420d10k.htm.
- 11.Services, C. f. M. a. M., pp. Physician and Other Supplier Data CY 2012. [Accessed April 2014]; https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Physician-and-Other-Supplier2012.html.
- 12.Services, C. f. M. a. M., pp. Open Payments Dataset Downloads. [Accessed April 2014]; https://www.cms.gov/OpenPayments/Explore-the-Data/Dataset-Downloads.html.
- 13.Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283:373. doi: 10.1001/jama.283.3.373. [DOI] [PubMed] [Google Scholar]
- 14.Hodges B. Interactions with the pharmaceutical industry: experiences and attitudes of psychiatry residents, interns and clerks. CMAJ. 1995;153:553. [PMC free article] [PubMed] [Google Scholar]
- 15.Lurie N, Rich EC, Simpson DE, et al. Pharmaceutical representatives in academic medical centers: interaction with faculty and housestaff. J Gen Intern Med. 1990;5:240. doi: 10.1007/BF02600542. [DOI] [PubMed] [Google Scholar]
- 16.Boulesteix AL, Stierle V, Hapfelmeier A. Publication Bias in Methodological Computational Research. Cancer Inform. 2015;14:11. doi: 10.4137/CIN.S30747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canadian Medical, A. Physicians and the pharmaceutical industry (update 2001) CMAJ. 2001;164:1339. [PubMed] [Google Scholar]
- 18.Council on, E., Judicial Affairs of the American Medical, A. Guidelines on gifts to physicians from industry: an update. Food Drug Law J. 2001;56:27. [PubMed] [Google Scholar]
- 19.Guidelines for faculty involvement in commercially supported continuing medical education. AAMC Ad Hoc Committee on Misconduct and Conflict of Interest in Research. AAMC Subcommittee on Conflict of Interest in Continuing Medical Education. Acad Med. 1992;67:615. [PubMed] [Google Scholar]
- 20.Sergeant MD, Hodgetts PG, Godwin M, et al. Interactions with the pharmaceutical industry: a survey of family medicine residents in Ontario. CMAJ. 1996;155:1243. [PMC free article] [PubMed] [Google Scholar]
- 21.Reeder M, Dougherty J, White LJ. Pharmaceutical representatives and emergency medicine residents: a national survey. Ann Emerg Med. 1993;22:1593. doi: 10.1016/s0196-0644(05)81266-1. [DOI] [PubMed] [Google Scholar]
- 22.Gibbons RV, Landry FJ, Blouch DL, et al. A comparison of physicians' and patients' attitudes toward pharmaceutical industry gifts. J Gen Intern Med. 1998;13:151. doi: 10.1046/j.1525-1497.1998.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coughlin SS. Recall bias in epidemiologic studies. J Clin Epidemiol. 1990;43:87. doi: 10.1016/0895-4356(90)90060-3. [DOI] [PubMed] [Google Scholar]
- 24.Ko JS, Chalfin H, Trock BJ, et al. Variability in Medicare utilization and payment among urologists. Urology. 2015;85:1045. doi: 10.1016/j.urology.2014.11.054. [DOI] [PubMed] [Google Scholar]
- 25.Dineen MK. Editorial comment. Urology. 2015;85:1050. doi: 10.1016/j.urology.2014.11.055. [DOI] [PubMed] [Google Scholar]
- 26.Services, C. f. M. a. M. CMS Roadmaps Overview [Google Scholar]


