Abstract
Antisocial behavior (AB), including aggression, violence, and theft, is thought be underpinned by abnormal functioning in networks of the brain critical to emotion processing, behavioral control, and reward-related learning. To better understand the abnormal functioning of these networks, research has begun to investigate the structural connections between brain regions implicated in AB using diffusion tensor imaging (DTI), which assesses white-matter tract microstructure. This systematic review integrates findings from 22 studies that examined the relationship between white-matter microstructure and AB across development. In contrast to a prior hypothesis that AB is associated with greater diffusivity specifically in the uncinate fasciculus, findings suggest that adult AB is associated with greater diffusivity across a range of white-matter tracts, including the uncinate fasciculus, inferior fronto-occipital fasciculus, cingulum, corticospinal tract, thalamic radiations, and corpus callosum. The pattern of findings among youth studies was inconclusive with both higher and lower diffusivity found across association, commissural, and projection and thalamic tracts.
Abbreviations: AB, antisocial behavior; AD, axial diffusivity; APD, antisocial personality disorder; CD, conduct disorder; CU, callous-unemotional; DMN, default mode network; DTI, diffusion tensor imaging; FA, fractional anisotropy; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; MD, mean diffusivity; RD, radial diffusivity; SLF, superior longitudinal fasciculus; UF, uncinate fasciculus
Keywords: Antisocial behavior, Callous-unemotional traits, Diffusion tensor imaging, Neuroimaging, Psychopathy, Systematic review
Highlights
-
•
Antisocial behavior (AB) is characterized by impaired emotion and reward processing.
-
•
These behaviors may develop from microstructural abnormalities of white-matter tracts.
-
•
We provide a systematic review of 22 diffusion tensor imaging studies of AB.
-
•
Adult AB was linked to greater diffusivity (“poorer integrity”) across a range of white-matter tracts.
-
•
For youth AB, there were mixed findings.
1. Introduction
Antisocial behavior (AB), a complex behavioral phenotype that includes violence, aggression, and rule-breaking, is central to the psychiatric diagnoses of Antisocial Personality Disorder (APD) in adults and Conduct Disorder (CD) in youth. AB encapsulates a striking failure to respect the rights of others and conform to societal laws, and predicts a host of negative sequelae, including crime, substance use, and poor physical and mental health (Fergusson et al., 2005, Shaw and Gross, 2008). Moreover, AB is harmful and financially costly to victims, families, and communities (Scott et al., 2001). To improve the efficacy of prevention and treatment efforts, neuroimaging techniques have increasingly been applied to better understand the neural mechanisms underlying the etiology of AB.
1.1. Neural correlates of antisocial behavior
AB is characterized by impairments in affective processing, reduced capacity to learn from reward and punishment, heightened impulsivity, emotional dysregulation, and risk-taking (Blair, 2001, Byrd et al., 2014). AB is theorized to arise from dysfunction in brain areas linked to inhibition and reward, including the orbitofrontal and ventromedial prefrontal cortices, as well as dysfunction in areas that process emotion and threat, including the amygdala and anterior cingulate (Blair, 2001, Hyde et al., 2013). Functional magnetic resonance imaging studies support these theories by demonstrating decreased prefrontal cortex functioning during reward-based and reinforcement learning tasks among adults and youth with AB (Finger et al., 2011, Yang and Raine, 2009). Abnormal amygdala reactivity to threatening stimuli has also been established as a neural marker of AB (Coccaro et al., 2007, Hyde et al., 2014, Kiehl et al., 2001, Viding et al., 2012).
Together, these findings suggest that AB arises from dysfunction in a network of regions critical to emotion processing, reward, and learning (Blair et al., 2014, Kiehl et al., 2001). Thus, a better understanding of the neural underpinnings of AB may be gained by moving beyond a focus on regionally-specific activation to examine regional connections (Insel et al., 2010). In support of this idea, studies have reported reduced functional connectivity between prefrontal and limbic structures among adults and youth high on AB (Finger et al., 2012, Marsh et al., 2008, Motzkin et al., 2011). However, impairments in the functioning of networks may be the result of disruptions to the physical structures connecting different brain regions (Hyde et al., 2013). By investigating microstructural abnormalities in the white-matter tracts that connect brain regions, we may gain better understanding of the neurobiological basis of AB.
1.2. Diffusion tensor imaging (DTI)
Diffusion tension imaging (DTI) is a non-invasive method that assesses water diffusion to index microstructural properties of white-matter tracts (Beaulieu, 2009, Winston, 2012). Specifically, DTI measures how freely water molecules can move within white-matter tracts based on microstructural features (e.g., fiber diameter, fiber density, and myelination) that restrict perpendicular diffusion and constrain water movement to certain directions. Tracts that force water molecules to move in the direction of the main orientation of fibers are often described as having higher microstructural “integrity” (Thomason and Thompson, 2011). To measure the diffusivity within white-matter tracts, studies compute fractional anisotropy (FA), which represents the ratio of diffusivity across different directions of the tract, ranging from 0 to 1 (Beaulieu, 2009, Winston, 2012) with higher FA values indexing lower diffusivity within tracts, which is interpreted as “greater integrity” (Basser et al., 1994, Thomason and Thompson, 2011). A second value of interest is mean diffusivity (MD), which represents the mean diffusion value across all directions of movement. Both FA and MD values are informed by axial diffusivity (AD) and radial diffusivity (RD) values. AD values represent longitudinal diffusion along the main fiber direction, with higher values thought to index lower diffusion, described as “greater integrity” (Budde et al., 2009). Radial diffusivity (RD) values represent perpendicular diffusion of water, with higher values thought to index “reduced integrity” (Klawiter et al., 2011). Examining AD and RD in addition to FA or MD values may help to precisely pinpoint the nature of diffusion differences and thus microstructural white-matter differences.
1.3. DTI and antisocial behavior
To examine whether there are white-matter microstructural abnormalities related to AB, studies have typically employed region-of-interest (ROI) analyses that identify specific tracts a priori. In an ROI approach, DTI indices are extracted and averaged from regions defined using automated atlases (Mori et al., 2005) or via manual dissection of specific tracts, allowing values to be compared across subjects. Based on the findings of functional and structural imaging studies, DTI studies adopting a ROI approach have focused on the uncinate fasciculus (UF), an association tract that connects the amygdala and prefrontal regions (Pandya et al., 2015). However, the link between AB and altered white-matter microstructure of the UF has not been consistently replicated, with studies reporting lower FA, no difference in FA, or higher FA among individuals high on AB relative to healthy controls (e.g., Finger et al., 2012, Motzkin et al., 2011, Passamonti et al., 2012).
One possible explanation for this mixed picture in the direction of findings centers on the type of sample examined. In particular, the presence of psychopathic traits represents a potential moderator of effects. Psychopathic traits identify adults with more severe and violent forms of AB, as well as deficits in empathy and guilt, and a callous interpersonal style (Patrick, 2007). Similarly, callous-unemotional (CU) traits identify children with AB who show empathy and guilt deficits, reduced concern for others, and particularly severe AB across the lifespan (Frick and White, 2008). Previous research has established divergent patterns of amygdala reactivity and affective processing contingent on the level of psychopathic or CU traits, such that individuals high on AB and psychopathic/CU traits exhibit reduced threat responsivity, whereas those high on AB without concurrent psychopathic/CU traits show exaggerated threat-related responses (Blair et al., 2014, Hyde et al., 2014). These functional differences suggest there could also be divergence in white-matter microstructural abnormalities in tracts connecting the amygdala with prefrontal regions. Among forensic samples, there are also high rates of psychopathy, which could account for the mixed pattern of findings noted between AB and FA in the UF. However, this question has yet to be systematically explored within a review. A second factor that could affect the direction of findings across DTI studies of AB is age, especially given increases in FA within white-matter tracts that become evident during early adulthood (Westlye et al., 2009). Thus, it is crucial to consider the influence of age when evaluating DTI studies of AB. A third factor that could explain mixed findings is analytic approach. In contrast to the ROI-based approaches described above, whole-brain voxel-based approach methods, including tract-based spatial statistics (TBSS), register DTI values into a common space and use voxel-wise statistical analytic methods to examine differences across the whole brain (e.g., Bach et al., 2014). Interestingly, when studies have adopted whole-brain approaches, they typically report white-matter microstructural differences in many other tracts beyond the UF in both adult (Sundram et al., 2012) and youth samples (Haney-Caron et al., 2014). Given the prevailing view in the field that the UF is specifically critical to the development of AB (e.g., Craig et al., 2009), a review is needed to evaluate whether the mixed findings arise because of differences in methodology (i.e., ROI vs. whole-brain approach) and whether or not AB is marked by specific deficits in the microstructure of the UF.
1.4. Current review
This systematic review evaluated findings from DTI studies of AB among both youth and adult samples. Our goal was to examine links between AB and the microstructure of association pathways (i.e., tracts connecting structures within the cerebral cortex within the same hemisphere), with a focus on tracts connecting frontal and temporal lobe regions implicated in functional and structural imaging studies of AB. We also evaluated evidence from studies examining white-matter microstructural abnormalities in commissural pathways (i.e., connecting similar regions across hemispheres) and projection and thalamic pathways (i.e., connecting cortical and subcortical structures). Our second aim was to examine the influence of sample age on findings and whether abnormalities in white-matter were specific to individuals high on psychopathy or CU traits. Finally, we evaluated whether method (ROI vs. whole-brain) coincided with the identification of specific versus widespread white-matter microstructural differences in tracts. Finally, because research demonstrates that other DTI indices beyond FA capture more specific microstructural abnormalities, we included results for AD, RD, and MD values when reported by studies to aid in the interpretation of findings.
2. Methods
2.1. Study selection
We included peer-reviewed, empirical studies published in English that examined associations between AB, psychopathy, and/or CU traits, and DTI measures of brain white-matter tracts. To capture a narrow behavioral phenotype, we excluded studies where participants were recruited based on criteria that did not specifically assess AB (i.e., excluding studies that assessed aggression arising in the context of another disorder). Thus, we excluded studies where the primary inclusion criterion was a diagnosis of ADHD (but see van Ewijk et al., 2012 for a systematic review of white-matter tract abnormalities in ADHD), schizophrenia (Hoptman et al., 2002), alcohol/substance use and dependence (Thayer et al., 2013), and bipolar disorder (Saxena et al., 2012). Online searches were performed in the databases OVID (Medline), EMBASE, EBSCO (PsycINFO, PsycARTICLES, Child Development & Adolescent studies, Criminal Justice Abstracts, and Violence & Abuse Abstract), and NCBI (PubMed) on April 4th 2016. We used search terms relating to AB, focusing on a wide range of terms to capture similar constructs across domains, including criminology (e.g., delinquency; crime; violence), psychiatry (e.g., APD; CD; psychopathy), and developmental psychopathology (e.g., aggression; CU traits). Thus, the search was sensitive to studies that assessed clinical diagnoses of childhood CD and adult APD, as well as continuous behavior scales. We combined terms assessing AB or CU traits/psychopathy with those assessing DTI (see Supplemental Table 1 for search terms used).
3. Results
3.1. Retrieved studies
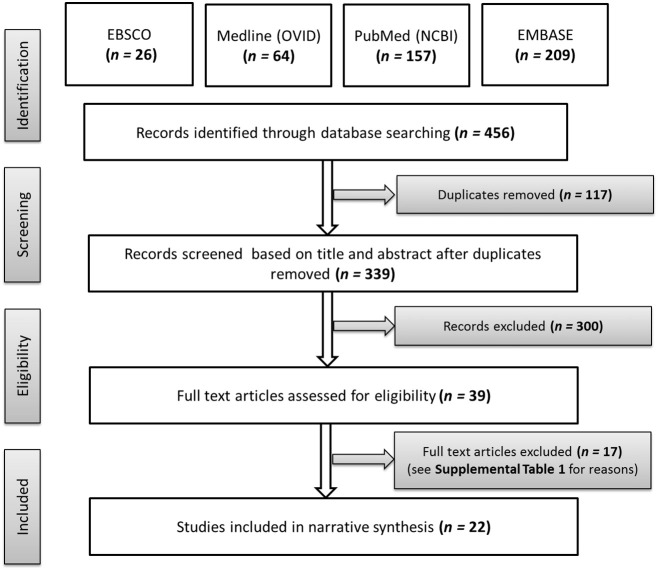
The flow of studies through the screening is summarized in Fig. 1. We identified 456 publications, of which 117 were duplicates. After removing duplicates, we screened 339 reports, 300 of which were excluded based on title or abstract. Full texts of the remaining 39 reports were screened in detail; 17 were excluded, most commonly for the following reasons: (1) no measure of AB, (2) structural MRI only (i.e., no DTI), and/or (3) not published in a peer-reviewed journal (i.e., conference proceedings) (Supplemental Table 2 summarizes why studies were excluded at this stage). Twenty-two publications that met inclusion criteria were included in the review (Fig. 1).
Fig. 1.
Summary of the flow of identified studies through screening, eligibility, and inclusion stages of review.
3.2. Sample, methodological, and analytic features of included studies
3.2.1. Sample types
Of the ten adult studies, the majority compared forensic samples to community samples of men only. Exceptions were two studies of community and high-risk males (Beyer et al., 2014, Sobhani et al., 2015), one study of a community sample with equal numbers of men and women (Karlsgodt et al., 2015), and one study of clinic-referred women who were compared to controls drawn from the community (Lindner et al., 2016) (Table 1). Of the 12 studies of youth, 11 assessed community samples or children at risk for AB, and 1 assessed a forensic sample. Youth were almost exclusively assessed during mid-to-late adolescence (e.g., 14–18), with the exception of one study that assessed 10-year-olds (Decety et al., 2015). Four studies included roughly equal numbers of boy and girls (40–60% girls); five studies had a majority of boys (10–39% girls), and three studies assessed boys only (Table 2).
Table 1.
Summary of sample characteristics and main findings for studies examining AB and white-matter tract abnormalities in adults measured by DTI.
| Study | N | Type | % ♀ | Exclusion criteria or covariates | Behavioral measures: dimensional or categorical analysis | Method | Key findings |
|---|---|---|---|---|---|---|---|
| Beyer et al., 2014 | 93 | 1 | 0 | Exclusions: other psychiatric or neurologic disorder, Covariates: |
AB: Buss Perry Aggression (S) & Taylor Aggression Paradigm (O) | ROI-A: (DT) |
Dimensional: no significant associations Categorical: AB + vs. HC: no significant differences |
| Craig et al., 2009 | 18 | 1 & 4 | 0 | Exclusions: other psychiatric or neurologic disorder, psychotropic medication Covariates: IQ & age-matched HC |
AB: male offenders from specialist forensic inpatient units Psychopathic traits: PCL-R (I) |
ROI-M (DT) | Categorical: AB + P + vs. HC: ↓ FA right UF. No significant differences in left UF, ILF, or IFOF. AB + P + vs. SA +: ↓ FA bilateral UF |
| Hoppenbrouwers et al., 2013 | 22 | 1 & 4 | 0 | Exclusions: other psychiatric or substance use disorders, head trauma, seizures Covariates: age-matched male HC | AB: male offenders convicted for assault, homicide, human trafficking, & kidnap Psychopathic traits: PCL-R (I) |
Whole-brain (TBSS) |
Dimensional: interpersonal-affective: ↓ FA left UF, IFOF, & ATR; Lifestyle-antisocial: ↓ FA right ATR, UF, & IFOF Categorical: AB + P + vs. HC: ↓ FA bilateral UF, IFOF, ATR, anterior CG |
| Karlsgodt et al., 2015 | 45 | 1 | 46 | Exclusions: Axis I disorder Covariates: age & gender |
AB: Buss Perry Aggression (S) | Whole-brain (TBSS) | Dimensional: aggressive acts: ↓ FA right SLF; ↑ RD right SLF |
| Lindner et al., 2016 | 67 | 1 & 3 | 100 | Exclusions: bipolar disorder, psychosis, neurologic disorder Covariates: Axis I disorders, physical & sexual abuse |
AB: SCID-I | Whole-brain (TBSS) | Categorical: AB + vs. HC: ↓ AD bilateral corpus callosum, CG, Fmin, and IFOF, and left CR (controlling for depression, anxiety, substance dependence and experience of abuse) AB + vs. clinical comparison: ↓ FA bilateral corpus callosum, forceps minor |
| Motzkin et al., 2011 | 27 | 4 | 0 | Exclusions: age > 45, IQ < 70, history of psychosis & psychotropic medication Covariates: | AB: Adult male inmates at a medium-security correctional facility Psychopathic traits: PCL-R (I) |
ROI-A (VBA & DT) | Categorical: AB + P + vs. AB + P −: ↓ FA right UF (relative to overall reduction in whole brain FA). No significant differences in SLF, ILF, IFOF |
| Sethi et al., 2015 | 26 | 3 | 0 | Exclusions: other psychiatric or substance use disorders, head trauma, reading age < 10 Covariates: alcohol & cocaine dependency |
AB: Offenders incarcerated for violent crime Psychopathic traits: PCL-R (I) |
ROI-M (DT) |
Dimensional: interpersonal-affective: ↓ FA left & right dorsal CG Categorical: AB + P + vs. HC: ↓ FA left dorsal CG |
| Sobhani et al., 2015 | 24 | 1 & 2 | 0 | Exclusions: other psychiatric or neurologic disorder Covariates: IQ, age |
Psychopathic traits: Concurrent (age 24): Psychopathic Personality Index (PPI). Prior (age 14–15): CPS, APSD, PCL:YV to form composite score | ROI-M (DT) | Dimensional: current PPI score psychopathy scores and prior psychopathy composite score: ↓ FA right UF |
| Sundram et al., 2012 | 30 | 1 & 4 | 0 | Exclusions: other psychiatric, neurologic or substance use disorder Covariates: age & IQ-matched HC |
AB: ICD-10 ASPD diagnosis (I) Psychopathic traits: PCL-R (I & case notes) |
Whole-brain (VBA) |
Dimensional: Total PCL-R & Lifestyle-Antisocial factor: ↓ FA in frontal lobe. Lifestyle-Antisocial factor: ↑ MD in frontal lobe. Categorical: AB + P + vs. HC: ↓ FA bilateral CC, IC, IFOF, right ACR, right UF, left ILF, & left PTR. ↑ MD right IFOF, UF, CC, & ACR |
| Wolf et al., 2015 | 147 | 1 & 4 | 0 | Exclusions: IQ < 70; psychotropic medication; psychiatric disorder; head trauma Covariates: age, race, substance use disorder |
AB: Adult male inmates at a medium-security correctional facility Psychopathic traits: PCL-R (I) |
ROI-A (TBSS) | Dimensional: total psychopathy scores: ↓ FA right UF (no association with left UF & unrelated to comparison tracts, CG, IC, TR, & CC & whole-brain FA). Interpersonal-affective factor: ↓ FA right UF (controlling for Factor 2). Interpersonal Facet: ↓ FA right UF |
Note that we report findings from the most stringent analyses carried out by included studies, including models controlling for multiple comparisons or overlap of outcome variables.
Type of sample: 1 = community; 2 = high-risk; 3 = clinic; 4 = forensic.
DTI acronyms: ACR = anterior corona radiata; AD = axial diffusivity; ATR = anterior thalamic radiation; CC = corpus callosum; CG = cingulum/cingulate gyrus; CT = corticospinal tract; Fmaj = forceps major; Fmin = forceps minor; FA = fractional anisotropy; IC = internal capsule; IFOF = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; PTR = posterior thalamic radiation; RD = radial diffusivity; SCR = superior corona radiata; SLF = superior longitudinal fasciculus; UF = uncinate fasciculus;
DTI acronyms: ACR = anterior corona radiata; AD = axial diffusivity; ATR = anterior thalamic radiation; CC = corpus callosum; CG = cingulum/cingulate gyrus; CT = corticospinal tract; Fmaj = forceps major; Fmin = forceps minor; FA = fractional anisotropy; IC = internal capsule; IFOF = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; PTR = posterior thalamic radiation; RD = radial diffusivity; SCR = superior corona radiata; SLF = superior longitudinal fasciculus; UF = uncinate fasciculus.
Behavior acronyms: AB = antisocial behavior; CU = callous-unemotional; P = psychopathy; S = self-reported; P = parent-reported; I = structured interview; O = observed.
Behavior acronyms: AB = antisocial behavior; CU = callous-unemotional; P = psychopathy; S = self-reported; P = parent-reported; I = structured interview; O = observed.
Behavior groupings: AB + P + = high on AB and psychopathy; AB + P − = high on AB and low on psychopathy; AB + CU + = high on AB and callous-unemotional traits; HC = healthy controls.
DTI method acronyms: DT = deterministic tractography; ROI-A = region of interest automatic generation; ROI-M = region of interest, manually drawn; TBSS = tract-based spatial statistics; VBA = voxel based analyses.
Table 2.
Summary of sample characteristics and main findings for studies examining AB and white-matter tract abnormalities in youth measured by DTI.
| Study | N | Type & age | % ♀ | Exclusion criteria or covariates | Behavioral measures | Method | Key findings |
|---|---|---|---|---|---|---|---|
| Breeden et al., 2015 | 47 | 1 M (SD) = 14 (3) |
47 | Exclusions: IQ < 80 Covariates: age &IQ |
AB: SDQ & CBCL (P) Psychopathic/CU traits: ICU (PC) |
ROI-A: (TBSS) |
Dimensional: CU traits: ↓ FA bilateral UF, & right fornix, controlling for overlapping externalizing and similar when examining only within AB + group AB: No associations found controlling for CU traits Categorical: AB + CU- vs. HC: ↓ FA right UF, right fornix; AB + CU + vs. HC: ↓ FA bilateral UF, bilateral fornix; AB + CU + vs. AB + CU −: no significant differences |
| Decety et al., 2015 | 110 | 1 & 2 M (SD) = 10 (1) |
52 | Exclusions: developmental disorder, head trauma Covariates: age, gender & race (also explored as moderators) |
AB: DISC (P&S) | Whole brain (TBSS) |
Dimensional: CD symptoms: ↑ AD in left CT, right SCR, & right Fmin but ↑ RD in right SLF – findings particularly pronounced for females & African–American participants relative to Caucasian/Hispanic. Categorical: AB + vs. subAB: no significant differences subAB + vs. HC: no significant differences AB + vs. HC: no significant differences but gender & race interactions (AD & RD) - regions comparable to above |
| Finger et al., 2012 | 31 | 1 & 3 M (SD) = 14 (2) |
10 | Exclusions: psychiatric or neurological disorder, head trauma, IQ < 80 Covariates: age, IQ |
AB: KSADS DSM-IV (I) Psychopathic/CU traits: ASPD (P) & PCL:YV (I) |
Whole brain (TBSS) & ROI-A: (DT) | Categorical: AB + P + vs. HC: no significant differences |
| Haney-Caron et al., 2014 | 41 | 1 & 2 M (SD) = 16 (1) |
39 | Exclusions: any other psychiatric disorder Covariates: gender, CD age of onset, & IQ |
AB: KSADS DSM IV (I) | Whole brain: (TBSS) |
Dimensional: CD symptoms: ↓ FA right ACR, bilateral SCR, IFOF, ILF, & left CC & PCR. ↑ FA bilateral ATR (similar controlling for CD onset age, gender, & IQ) Categorical: AB + vs. HC: ↓ FA bilateral SCR, ACR, IFOF, SLF, ILF, IC, & ped. ↓ AD bilateral SCR, ACR, IFOF, SLF, ILF, IC, ped, UF, CC, CG, Fmin, & right PCR |
| Hummer et al., 2015 | 66 | 1 M (SD) = 14 (1) |
27 | Covariates: Age (also examined as moderator) | AB: KSADS DSM-IV (I) | ROI-A: (TBSS) | Categorical: AB + ADHD − vs. HC: no significant differences AB + ADHD + vs. HC: no significant differences AB + vs. HC interaction with age: ↑ FA with increasing age only in HC vs. AB groups in CC & left SLF |
| Li et al., 2005 | 76 | 1 M (SD) = 14 (1) |
39 | Exclusions: HC: no psychiatric disorder Covariates: age |
AB: KSADS DSM-IV (I) | Whole brain: (VBA) | Categorical: AB + vs. HC: ↓ FA bilateral SLF |
| Pape et al., 2015 | 145 | 4 M (SD) = 18 (2) |
14 | Covariates: age & IQ | Psychopathic/CU traits: YPI (S) | Whole brain (TBSS) | Dimensional: CU traits: ↑ AD bilateral CT Grandiose-manipulative traits: ↑ FA and ↓ RD bilateral ATR, CT, Fmin, IFOF, UF, CC; ↓ RD bilateral CG, Fmaj, ILF, SLF |
| Passamonti et al., 2012 | 26 | 1 & 2 M (SD) = 18 (1) |
0 | Exclusions: developmental disorder, physical illness, IQ < 85; HC with IQ > 115 Covariates: ADHD |
AB: KSADS DSM IV (I) Psychopathic/CU traits: YPI (S) |
Whole brain (VBA) & ROI-M (DT) | Categorical: AB + vs. HC: ↑ FA, ↑ AD, & ↓ RD bilateral external capsule; (whole-brain); ↑ FA, ↑ AD, & ↓ RD bilateral UF (ROI). Similar accounting for effects of ADHD symptoms & hemispheres. |
| Peper et al., 2015 | 258 | 1 M (SD) = 14 (3) |
52 | Exclusions: neurological, psychiatric or endocrine illness. Covariates: age |
AB: Buss Perry Aggression (S) | ROI-A (DT) |
Dimensional: Age: ↑ FA overall Verbal aggression: ↑ MD temporal–temporal Hostility: ↓ MD in subcortical-temporal & temporal-temporal |
| Sarkar et al., 2013 | 43 | 1 & 2 M (SD) = 16 (2) |
0 | Exclusions: IQ < 80 Covariates: age |
AB: KSADS DSM-IV (I), SDQ (P&S) Psychopathic/CU traits: PCL:YV (I) &APSD (P&S) |
ROI-M: (DT) |
Dimensional: SDQ (total & conduct problem scales): ↑ FA bilateral UF; APSD (total & CU traits scales): ↑ FA bilateral UF Categorical: AB + P + vs. HC: ↑ FA left UF |
| Zhang et al., 2014b | 69 | 1 & 3 M (SD) = 15 (1) |
0 | Exclusions: psychiatric disorder, head trauma, past year substance abuse, IQ ≤ 80 | AB: SCID DSM-IV (I), SDQ & BIS (S) Psychopathic/CU traits: APSD (S) |
Whole brain (TBSS) & ROI-A (DT) |
Dimensional: no significant associations Categorical: AB + vs. HC: ↑ FA bilateral CC, left ACR, right SCR; ↓ RD bilateral CC, left ACR |
| Zhang et al., 2014a | 56 | 1 & 3 M (SD) = 14 (1) |
46 | Exclusions: psychiatric disorder, substance use, head trauma, IQ ≤ 80 Covariates: gender tested as moderator |
AB: SCID DSM-IV (I) & SDQ (S) CU traits: APSD (S) |
ROI-A: (VBA & DT) |
Dimensional: CU traits: ↓ RD & ↓ MD right UF (boys with CD only; uncorrected) Categorical: AB + vs. HC: no significant differences |
Note that we report findings from the most stringent analyses carried out by included studies, including models controlling for multiple comparisons or overlap of outcome variables.
Type of sample: 1 = community; 2 = high-risk; 3 = clinic; 4 = forensic.
DTI acronyms: ACR = anterior corona radiata; AD = axial diffusivity; ATR = anterior thalamic radiation; CC = corpus callosum; CG = cingulum/cingulate gyrus; CT = corticospinal tract; Fmaj = forceps major; Fmin = forceps minor; FA = fractional anisotropy; IC = internal capsule; IFOF = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; PTR = posterior thalamic radiation; RD = radial diffusivity; SCR = superior corona radiata; SLF = superior longitudinal fasciculus; UF = uncinate fasciculus.
Behavior acronyms: AB = antisocial behavior; CU = callous-unemotional; P = psychopathy; S = self-reported; P = parent-reported; I = structured interview.
Behavior groupings: AB + P + = high on AB and psychopathy; AB + P- = high on AB and low on psychopathy; AB + CU + = high on AB and callous-unemotional traits; HC = healthy controls.
DTI method acronyms: DT = deterministic tractography; ROI-A = region of interest automatic generation; ROI-M = region of interest, manually drawn; TBSS = tract-based spatial statistics; VBA = voxel based analyses.
3.2.2. DTI approach
DTI measures can be extracted using tractography or voxel-wise whole-brain analysis. First, tractography involves the construction of fiber trajectories and connection patterns based on the successive orientations associated with adjacent voxels. Defining the points or “seeds” to begin fiber construction (e.g., seeding) can be determined using an ROI approach (placing of seeds in ROIs that are manually drawn or extracted automatically) or using a whole-brain approach (e.g., automatic seeding for the whole brain) (Cercignani, 2010, Jones et al., 2013, Soares et al., 2013). Tractography itself is divided into deterministic versus probabilistic tractography, with the former tracking fibers based on the assumption that every voxel can only be part of one fiber, and the latter estimating probability maps based on the possibility of multiple fibers passing through one voxel. Second, DTI can be analyzed using voxel-wise whole brain analysis, such as voxel-based analysis (VBA) or TBSS, which involve the registration of DTI maps into standard space to allow for the comparison of diffusion parameters of voxels between subjects by ensuring anatomical correspondence among subjects (for fuller descriptions of these different DTI methodologies, see Cercignani, 2010, Jones et al., 2013, Soares et al., 2013).
Across included studies, six of the 10 adult studies and 5 of 12 youth studies adopted an ROI-only approach (Table 1, Table 2). Of the six adult studies that used an ROI-only approach, three studies utilized automated methods to identify ROIs, while three studies analyzed manually-dissected tracts. Four of the adult ROI studies used deterministic tractography, one used TBSS, and one used both deterministic tractography and a voxel-based approach. Of the five youth studies that used an ROI-only approach, four used automated methods, while one study analyzed manually-dissected tracts. Two of the ROI studies in youth used deterministic tractography, two used TBSS, and one used both deterministic tractography and an alternate voxel-based approach. In addition, four of 10 adult studies and 4 of 12 youth studies adopted whole-brain approaches. Of the four whole-brain adult studies, three used TBSS, while one used an alternate voxel-based approach. Of the four youth studies that used a whole-brain only approach, three used TBSS, and one study used an alternate voxel-based approach. Finally, three youth studies combined both ROI- and whole-brain approaches (Table 1, Table 2).
3.2.3. Measurement approach
Three adult studies examined associations using only a case-control approach (e.g., classifying individuals as being high AB/high psychopathy vs. healthy controls). Three studies examined associations dimensionally (i.e., the extent to which individual differences in AB covaried with differences in DTI measures) and four studies adopted both dimensional and case-control approaches. Adult studies examined self-reported AB and interview-assessed or self-reported psychopathy. Items assessing psychopathy were often summed to create a total psychopathy score, but were also examined as subscales reflecting separable factors within the psychopathy construct (Hare, 2006; Table 1). Thus, studies distinguished between subscales assessing deficits in affective and interpersonal traits (e.g., callousness, low interpersonal affiliation; “Factor 1”) versus the harmful, antisocial behaviors associated with psychopathy (e.g., impulsivity, deviant lifestyle, violence; “Factor 2”) (Hare, 2006). Five youth studies examined associations with DTI measures using a case-control approach only; two youth studies examined associations using a dimensional approach only. Five youth studies used both case-control and dimensional approaches. Youth studies most commonly employed self-reported, parent-reported, or teacher-reported measures of AB, aggression, or other forms of externalizing behavior problems, as well as measures of psychopathic or CU traits (Table 2).
3.3. Aim 1: is AB related to brain white-matter tract abnormalities measured by DTI?
To aid in the interpretation of findings, we present the results of studies within three broad tract classifications: association, commissural, and projection and thalamic pathways. Within these classifications, we separate by adult versus youth findings. Because of heterogeneity in methods, sample types, and analytic approaches across studies, we were only able to combine studies in a narrative synthesis and not a meta-analysis. Where relevant, we note in the text if there were different findings based on studies adopting a case-control versus dimensional approach, although these distinctions are summarized in full in Table 1, Table 2, Table 3.
Table 3.
Summary of DTI findings by tract across studies youth and adults.
| Tract and Description | Diagram of tracts | Adult findings | Youth findings |
|---|---|---|---|
| Association pathways | |||
| Cingulum (CG): Connects frontal & temporal lobes, cingulate & medial gyri of frontal, parietal, occipital, & temporal lobes | 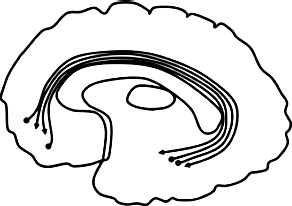 |
Greater diffusivity ↓ FA bilateral CG: interpersonal-affective traits (Sethi et al., 2015), AB + P + vs. HC (Hoppenbrouwers et al., 2013) ↓FA left CG: AB + P + vs. HC (Sethi et al., 2015) ↓AD bilateral CG: AB + vs. HC (Lindner et al., 2016) |
Greater diffusivity ↓ AD bilateral CG: AB + vs. HC (Haney-Caron et al., 2014) Lower diffusivity ↓ RD bilateral CG: psychopathic traits (Pape et al., 2015) |
| Inferior fronto-occipital fasciculus (IFOF): Connects temporal lobe (medially) & frontal lobe (inferiorally). | 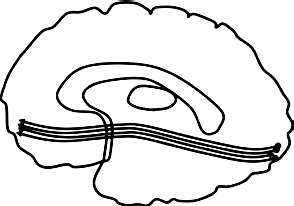 |
Greater diffusivity ↓ FA bilateral IFOF: AB + P + vs. HC (Hoppenbrouwers et al., 2013, Sundram et al., 2012) ↓FA left IFOF: Interpersonal-affective traits (Hoppenbrouwers et al., 2013) ↓FA right IFOF: Lifestyle-antisocial traits (Hoppenbrouwers et al., 2013) ↑MD right IFOF: AB + P + vs. HC (Sundram et al., 2012) ↓AD bilateral IFOF: AB + vs. HC (Lindner et al., 2016) |
Greater diffusivity ↓ FA bilateral IFOF: CD symptoms, AB + vs. HC (Haney-Caron et al., 2014) ↓AD bilateral IFOF: AB + vs. HC: (Haney-Caron et al., 2014) Lower diffusivity ↑ FA & ↓ RD bilateral IFOF: psychopathic traits (Pape et al., 2015) |
| Inferior longitudinal fasciculus (ILF): connects temporal pole & occipital pole. | 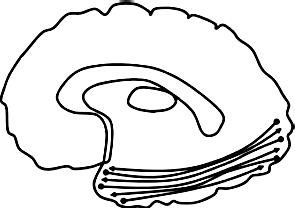 |
Greater diffusivity ↓ FA left ILF: AB + P + vs. HC (Sundram et al., 2012) |
Greater diffusivity ↓ FA bilateral ILF: CD symptoms, AB + vs. HC: (Haney-Caron et al., 2014 ↓ AD bilateral ILF: AB + vs. HC (Haney-Caron et al., 2014) Lower diffusivity ↓ RD bilateral ILF: psychopathic traits (Pape et al., 2015) |
| Superior longitudinal fasciculus (SLF): connects frontal lobe & parietal, occipital, & temporal lobes. | 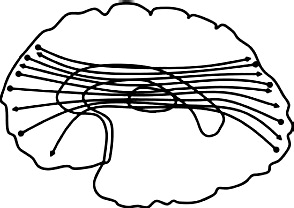 |
Greater diffusivity ↓ FA & ↑ RD right SLF: aggressive acts (Karlsgodt et al., 2015) |
Greater diffusivity ↑ RD right SLF: CD symptoms (Decety et al., 2015) ↓FA bilateral SLF: AB + vs. HC (Haney-Caron et al., 2014, Li et al., 2005); ↓AD bilateral SLF: AB + vs. HC (Haney-Caron et al., 2014) Lower diffusivity ↑ AD right SLF: CD symptoms (Decety et al., 2015) ↓RD bilateral SLF: psychopathic traits (Pape et al., 2015) |
| UF = uncinate fasciculus (UF): connects temporal pole & orbitofrontal cortex. | 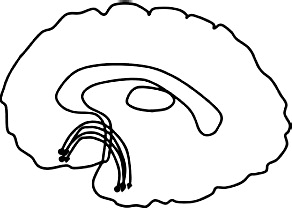 |
Greater diffusivity ↓ FA bilateral UF: AB + P + vs. HC (Hoppenbrouwers et al., 2013) ↓FA right UF: psychopathy (Wolf et al., 2015, Sobhani et al., 2015), interpersonal-affective traits (Wolf et al., 2015) & lifestyle-antisocial (Hoppenbrouwers et al., 2013), AB + P + vs. HC (Craig et al., 2009, Sundram et al., 2012), AB + P + vs. AB + P- (Motzkin et al., 2011) ↓FA left UF: lifestyle-antisocial (Hoppenbrouwers et al., 2013) AB + P + vs. HC: ↓ FA right UF (Craig et al., 2009, Sundram et al., 2012), ↑MD right UF: AB + P + vs. HC (Sundram et al., 2012) |
Greater diffusivity ↓ FA bilateral UF: CU traits, AB + CU + vs. HC (Breeden et al., 2015) ↓FA right UF: AB + CU- vs. HC: (Breeden et al., 2015) ↓AD bilateral UF: AB + vs. HC (Haney-Caron et al., 2014) Lower diffusivity ↑ FA bilateral UF: behavior problems: (Sarkar et al., 2013), psychopathic traits (Sarkar et al., 2013) ↑FA &↓RD bilateral UF: psychopathic traits (Pape et al., 2015) ↑FA left UF: AB + P + vs. HC: (Sarkar et al., 2013); ↑ FA, ↑ AD, & ↓ RD bilateral UF: AB + vs. HC (Passamonti et al., 2012) ↓RD & ↓ MD right UF: CU traits (Zhang et al., 2014a) |
| Commissural pathways | |||
| Corpus callosum (CC), forceps major (Fmaj) & forceps minor (Fmin): Connects cerebral hemispheres: anterior (Fmin) & posterior (Fmaj) | 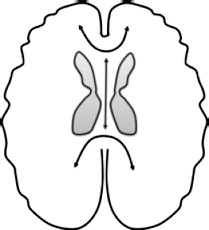 |
Greater diffusivity ↓ FA bilateral CC: AB + P + vs. HC (Sundram et al., 2012) ↑MD right CC: AB + P + vs. HC: (Sundram et al., 2012) ↓AD bilateral CC & Fmin: AB + vs HC & clinical comparison (Lindner et al., 2016) |
Greater diffusivity ↓ FA left CC: CD symptoms (Haney-Caron et al., 2014) ↓AD bilateral CC & Fmin: AB + vs. HC (Haney-Caron et al., 2014) Lower diffusivity ↑ FA & ↓ RD bilateral CC & Fmin: psychopathic traits (Pape et al., 2015), AB + vs. HC (Zhang et al., 2014b) ↑AD right Fmin: CD symptoms (Decety et al., 2015) ↓RD Fmaj: psychopathic traits (Pape et al., 2015) |
| Projection & thalamic pathways | |||
| Fornix: Connects hippocampus & hypothalamus | 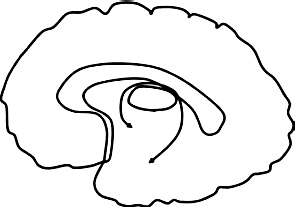 |
Greater diffusivity ↓ FA bilateral fornix: AB + CU + vs. HC: (Breeden et al., 2015) ↓FA right fornix: CU traits, AB + CU- vs. HC (Breeden et al., 2015) |
|
| Cortico-spinal tract (CT), internal capsule (IC), external capsule (EC), peduncle, & corona radiata (ACR, PCR, SCR): Connects spinal cord & cerebral cortex | 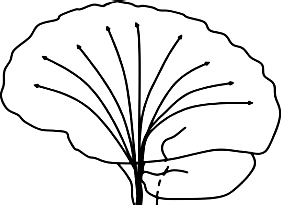 |
Greater diffusivity ↓ FA bilateral IC: AB + P + vs. HC (Sundram et al., 2012) ↓FA & ↑ MD right ACR: AB + P + vs. HC (Sundram et al., 2012). ↓AD left CR: AB + vs. HC (Lindner et al., 2016) |
Greater diffusivity ↓ FA right ACR; bilateral SCR; left PCR: CD symptoms (Haney-Caron et al., 2014); ↓FA & ↓ AD bilateral IC, peduncle, ACR, & SCR (Haney-Caron et al., 2014) ↓AD right PCR: AB + vs. HC (Haney-Caron et al., 2014) Lower diffusivity ↑ FA, ↑ AD, & ↓ RD bilateral CT: CU & psychopathic traits (Pape et al., 2015); right EC: AB + vs. HC: (Passamonti et al., 2012) ↑AD left CT & right SCR: CD symptoms (Decety et al., 2015) ↑FA right SCR: AB + vs. HC (Zhang et al., 2014b) ↑FA & ↓ RD left ACR: AB + vs. HC (Zhang et al., 2014b) |
| Anterior & posterior thalamic radiations (ATR & PTR): Connect the thalamus and visual, somatosensory, auditory, & pre-motor cortices | 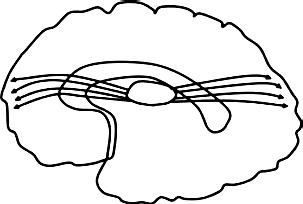 |
Greater diffusivity ↓ FA bilateral ATR: AB + P + vs. HC (Hoppenbrouwers et al., 2013) ↓FA left ATR: Interpersonal-affective (Hoppenbrouwers et al., 2013) ↓FA right ATR: Lifestyle-antisocial traits (Hoppenbrouwers et al., 2013) ↓FA left PTR: AB + P + vs. HC (Sundram et al., 2012) |
Lower diffusivity ↑ FA in bilateral ATR: CD symptoms (Haney-Caron et al., 2014) & psychopathic traits (Pape et al., 2015) |
DTI acronyms: ACR = anterior corona radiata; AD = axial diffusivity; ATR = anterior thalamic radiation; CC = corpus callosum; CG = cingulum/cingulate gyrus; CT = corticospinal tract; Fmaj = forceps major; Fmin = forceps minor; FA = fractional anisotropy; IC = internal capsule; IFOF = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; PTR = posterior thalamic radiation; RD = radial diffusivity; SCR = superior corona radiata; SLF = superior longitudinal fasciculus; UF = uncinate fasciculus. Behavior acronyms: AB = antisocial behavior; CU = callous-unemotional; P = psychopathy. Behavior groupings: AB + P + = high on AB and psychopathy; AB + P- = high on AB and low on psychopathy; AB + CU + = high on AB and callous-unemotional traits; HC = healthy controls.
3.4. Association pathways
3.4.1. Uncinate fasciculus (UF)
The UF reciprocally connects structures in the frontal lobe, including the cingulate gyrus, frontal pole, and orbitofrontal cortex, to structures in the temporal lobe, including the amygdala and temporal pole (Leng et al., 2016).
3.4.1.1. Adults
Six of nine studies of adults reported that AB was related to white-matter microstructural differences in the UF, and specifically to greater diffusivity. Three studies reported non-significant findings. Using a case-control design, adults high on AB and psychopathy had lower FA in the right UF (Craig et al., 2009, Sundram et al., 2012), higher MD in the right UF (Sundram et al., 2012), and lower FA bilaterally in UF (Hoppenbrouwers et al., 2013) compared to healthy controls. Adults with high AB and psychopathy also showed lower FA in the right UF compared to adults with high AB without psychopathy (Motzkin et al., 2011). Dimensionally, lower FA in the right UF was related to higher psychopathy total scores (Sobhani et al., 2015, Wolf et al., 2015), as well as higher subscale scores for interpersonal-affective (Wolf et al., 2015) and lifestyle-antisocial (Hoppenbrouwers et al., 2013) traits.
3.4.1.2. Youth
Six out of 11 studies reported that AB was significantly related to white-matter microstructural abnormalities of the uncinate fasciculus (UF), although in mixed directions. Youth with AB were reported to show higher FA in the UF compared to healthy controls (Passamonti et al., 2012). Higher FA in the UF was also related to more behavior problems (Sarkar et al., 2013), and higher CU and psychopathic traits (Pape et al., 2015, Sarkar et al., 2013). Finally, within a subsample of boys with CD, CU traits were related to lower RD in the right UF (Zhang et al., 2014a). However, two studies reported the opposite pattern in terms of tract diffusivity: within a case-control design, youth with high AB had lower FA in the UF (Breeden et al., 2015). Youth high on AB also showed lower AD in the UF compared to healthy controls (Haney-Caron et al., 2014). Dimensionally, higher CU traits were uniquely related to lower FA bilaterally in the UF, after accounting for AB (Breeden et al., 2015).
3.4.2. Cingulum, inferior fronto-occipital fasciculus (ifof), superior longitudinal fasciculus (SLF), inferior longitudinal fasciculus (ILF)
In addition to the UF, AB was linked to abnormal white-matter microstructure in other association tracts, including the cingulum, inferior fronto-occipital fasciculus (IFOF), superior longitudinal fasciculus (SLF), and inferior longitudinal fasciculus (ILF) (Table 3). The cingulum, SLF, and ILF connect regions within the limbic system, including the posterior cingulate, medial prefrontal cortex, and medial temporal lobe (Teipel et al., 2010). Additionally, the ILF and IFOF share projections at the posterior temporal and occipital lobes and connect visual association areas of the occipital lobe, auditory and visual association areas, and prefrontal cortex (Catani et al., 2013). Disconnection of the ILF has been linked to impaired communication between the occipital and temporal lobes, including the amygdala (Fox et al., 2008)
3.4.2.1. Adults
Eight studies investigated white-matter microstructure within association pathways, with four studies utilizing a whole-brain approach, two studies using ROIs for the SLF, ILF, and IFOF, and two studies using an ROI of the cingulum. In general, AB and psychopathy were associated with greater diffusivity in white-matter tracts, indexed by lower FA in the cingulum (Hoppenbrouwers et al., 2013, Sethi et al., 2014), IFOF (Hoppenbrouwers et al., 2013, Sundram et al., 2012), ILF (Sundram et al., 2012), and SLF (Karlsgodt et al., 2015). Additionally, AB and psychopathy was associated with higher MD in the IFOF (Sundram et al., 2012) and aggression was associated with higher RD in the SLF (Karlsgodt et al., 2015). Finally, women with CD had lower AD in the cingulum and IFOF compared to healthy controls (Lindner et al., 2016) (Table 1, Table 3).
3.4.2.2. Youth
Eight youth studies investigated white-matter microstructural abnormalities in association pathways beyond the UF. Of these studies, only two studies adopting a whole-brain approach identified significant relationships between AB and white-matter microstructure of the cingulum, IFOF, and ILF. However, the direction of the findings of these two studies was contrasting. Haney-Caron et al. (2014) found AB to be associated with lower FA, while Pape et al. (2015) found AB to be associated with higher FA and AD, as well as lower RD. For the SLF specifically, four studies reported white-matter microstructural differences among youth with AB, but again in contrasting directions with two studies finding AB to be associated with higher FA in the SLF (Haney-Caron et al., 2014, Li et al., 2005), but another reporting lower RD in the SLF (Pape et al., 2015). Interestingly, one study found evidence in both directions, reporting higher RD and higher AD of the right SLF (Decety et al., 2015) (Table 2).
3.4.3. Summary of association pathways
The evidence from adult samples was largely consistent, showing AB to be related to disrupted white-matter microstructure across association pathways, including the cingulum, IFOF, ILF, SLF, and UF. The relationships found for the cingulum, IFOF, ILF, and SLF emerged in around half the studies that adopted a whole-brain approach, with the rest reporting null findings (Table 3). The UF was implicated in 6 of 9 studies, although the majority of these studies used an ROI-approach focused on the UF only. Among youth, four studies found evidence for higher FA in the UF, although two studies reported lower FA or AD, and five studies found no significant differences. Only two of eight youth studies identified relationships between AB and white-matter microstructural differences in association pathways beyond the SLF or UF, although effects were in contrasting directions. Overall, while AB was most often associated with white-matter microstructural abnormalities in the UF, there is evidence that other association pathways are implicated in AB. Further, AB appears differentially related to disrupted white-matter microstructure of the UF for adults versus youth – typically by greater diffusivity of the UF in adults with AB but lower diffusivity in youth with AB.
3.5. Commissural pathways
3.5.1. Corpus callosum, forceps minor, and forceps major
The corpus callosum is the largest fiber bundle in the brain and connects the two hemispheres. The forceps minor (anterior) passes through the corpus callosum and connects medial and lateral frontal cortices. The forceps major (posterior) curves backwards linking the occipital poles of the hemispheres. Studies of callosotomies and animal work indicate that disruption to commissural tracts can cause imbalance in interhemispheric communication (for a review, see Bloom and Hynd, 2005).
3.5.1.1. Adults
Of the four adult studies that used whole-brain approaches, two studies reported that AB was related to greater diffusivity in commissural pathways. First, Sundram et al. (2012) reported lower FA bilaterally in the corpus callosum and higher MD in the right corpus callosum among men high on AB and psychopathic traits compared to healthy controls (Sundram et al., 2012). Second, Lindner et al. (2016) reported lower AD in the corpus callosum and forceps minor among women with CD compared to healthy controls, covarying for a range of psychiatric disorders, including substance dependence, and experience of physical and sexual abuse (Lindner et al., 2016). Moreover, lower FA in the corpus callosum and forceps minor differentiated CD women compared to a clinical comparison group (i.e., women without CD, but similar rates of comorbid mental disorders and physical and sexual abuse as CD women).
3.5.1.2. Youth
AB was related to lower diffusivity in commissural pathways in four of seven studies that utilized a whole-brain approach (Table 3). Youth with AB had higher FA and lower RD in the corpus callosum compared to healthy controls (Zhang et al., 2014b); psychopathic traits were dimensionally related to higher FA in the corpus callosum and forceps minor (Pape et al., 2015); and CD symptoms were related to higher AD in the right forceps minor (Decety et al., 2015). In contrast, one study reported that CD symptoms were associated with lower FA and AD in the corpus callosum and forceps minor (Haney-Caron et al., 2014).
3.5.2. Summary of commissural pathways
Among adults, two of four whole-brain studies reported that individuals with high AB showed disrupted white-matter microstructure bilaterally in the corpus callosum. Among youth, findings were mixed with three of seven studies reporting that AB and psychopathic traits were related to higher FA, higher AD, or lower RD in the corpus callosum, but one study reporting that AB was related to lower FA and AD of the corpus callosum, and forceps minor and major (Table 3).
3.6. Projection & thalamic pathways
3.6.1. Fornix
The fornix is one of the main tracts connecting the hippocampus with other subcortical regions, including the anterior nuclei of the thalamus and contralateral hippocampus.
3.6.1.1. Adults
No studies found that AB was linked to white-matter microstructural differences of the fornix.
3.6.1.2. Youth
One youth study adopting an ROI approach reported that AB was related to white-matter microstructural differences in the fornix. Within a case-control design, youth with high AB had lower FA in the fornix when compared to healthy controls. Dimensionally in the same study, higher CU traits were specifically related to lower FA in the right fornix, controlling for AB (Breeden et al., 2015).
3.6.2. Corticospinal tract, internal and external capsules, peduncle, and corona radiata
The corticospinal tract represents the major descending pathway for motor function connecting the motor cortex to the spinal cord, which converges on the internal and external capsules and peduncle, and passes through the corona radiata (Jang, 2011).
3.6.2.1. Adults
Two of four adult studies that used whole-brain approaches reported greater diffusivity in projection pathways originating with the corticospinal tract, while the others reported null findings. First, Sundram et al. (2012) found that men with AB and psychopathy had lower FA in the internal capsule and right anterior corona radiata compared to healthy controls. Second, women with CD had lower AD in the left corona radiata compared to healthy controls, notably at the intersection with the IFOF (Lindner et al., 2016).
3.6.2.2. Youth findings
Five of seven studies of youth that used whole-brain approaches reported abnormal white-matter microstructure in projection pathways originating with the corticospinal tract, with four studies reporting lower diffusivity in these tracts. Using a case-control design, youth with AB showed higher FA, higher AD, and lower RD in the right external capsule (Passamonti et al., 2012), higher FA in the right superior corona radiata (Zhang et al., 2014b), and higher FA and lower RD in the left anterior corona radiata (Zhang et al., 2014b) when compared to healthy controls. Examined dimensionally, CD symptoms were related to higher AD in the left corticospinal tract and right superior corona radiata (Decety et al., 2015), and psychopathic traits were related to higher FA and AD and lower RD in the corticospinal tract (Pape et al., 2015). In contrast, one study reported that CD was related to lower FA in the right anterior corona radiata, bilateral superior corona radiata, and left posterior corona radiata (Haney-Caron et al., 2014) (Table 2, Table 3).
3.6.3. Anterior and posterior thalamic radiations
Thalamic radiations extend from different nuclei of the thalamus and project to visual, somatosensory, and visual cortices via the peduncle and internal capsule. Portions of the posterior thalamic radiation of the internal capsule connect fibers of the optic radiation and are involved in the visual system (Sherbondy et al., 2008). Physical disruption of these networks is thought to affect the detection and processing of facial expressions of emotion, which is compromised in individuals high on AB (Marsh and Blair, 2008).
3.6.3.1. Adults
Two of five studies reported greater diffusivity in white-matter tracts in thalamic radiations, and two studies reported null findings (see Table 3). Within a case-control design, adults high on AB and psychopathy showed lower FA in the anterior thalamic radiation (Hoppenbrouwers et al., 2013) and in the left posterior thalamic radiation (Sundram et al., 2012) relative to healthy controls. Dimensionally, higher interpersonal-affective scores were related to lower FA in the left anterior thalamic radiation, and higher lifestyle-antisocial scores were related to lower FA in the right anterior thalamic radiation (Hoppenbrouwers et al., 2013).
3.6.3.2. Youth
Of seven studies of youth adopting whole-brain approaches, two studies reported that AB was associated with lower diffusivity of the anterior thalamic radiation. CD symptoms were associated with higher FA in the anterior thalamic radiation (Haney-Caron et al., 2014) and psychopathic traits were related to higher FA and lower RD in the anterior thalamic radiation (Pape et al., 2015).
3.6.4. Summary of projections and thalamic pathways
Among adults, there was some consistency across three studies reporting AB to be related to greater diffusivity in projection and thalamic pathways, indexed via lower FA or lower AD. Among youth, there were mixed findings as to the nature of white-matter microstructural differences in projection and thalamic pathways, including reports of both lower and higher FA in the corticospinal tract, internal and external capsules, anterior and superior corona radiata, and anterior thalamic radiations (Table 3).
3.7. Aim 2: are white-matter tract abnormalities specific to AB with psychopathic traits?
3.7.1. Adults
Six of ten studies examined forensic samples of adults with high AB and psychopathy, consistently demonstrating greater diffusivity in tracts among these individuals relative to healthy controls. Thus, there is evidence that the white-matter tract microstructural deficits are more pronounced among antisocial individuals with psychopathy. The most compelling evidence to support this conclusion comes from a study of incarcerated males, among whom the lower FA found in the right UF was specific to those with psychopathy versus those without psychopathy (Motzkin et al., 2011). This case-control approach inherently accounted for AB, enabling microstructural deficits to be uniquely linked to psychopathy. Moreover, Sobhani et al. (2015) demonstrated that psychopathic traits in a high-risk sample were related to lower FA in the UF. In further support of potential specificity of white-matter microstructural deficits in the UF to psychopathy, Beyer and colleagues examined aggression and anger among a community sample, where rates of psychopathy are likely lower, and reported no difference between a high AB versus low AB group (Beyer et al., 2014). At the same time, in another community sample, aggressive acts were related to lower FA and higher RD in the SLF (Karlsgodt et al., 2015). However, without an assessment of psychopathic traits, it is difficult to ascertain whether individual differences in psychopathy would have accounted for this finding.
3.7.2. Youth
Only seven of the 12 studies of youth included measures of CU or psychopathic traits, which limits the extent to which we could evaluate whether these traits accounted for white-matter tract microstructural differences. Of these seven studies, one study that examined a community sample of youth found that CU traits were specifically related to lower FA in the UF and fornix within a dimensional analysis that controlled for concurrent AB (Breeden et al., 2015). However, two studies that investigated CU/psychopathic traits in isolation (i.e., without controlling for concurrent AB) found that they were related to higher FA, lower RD, and higher AD in various commissural, projection, and association tracts (Pape et al., 2015, Sarkar et al., 2013). CU traits were also found to be specifically related to lower RD and MD of the right UF among males with CD (Zhang et al., 2014a). Finally, among a clinic-referred and community sample, no differences were found in white-matter microstructure for youth high on AB and psychopathic traits compared to healthy controls using both whole-brain and ROI approaches (Finger et al., 2012). Moreover, of the five studies that examined AB without assessing concurrent CU or psychopathic traits, the direction of findings was mixed, and two studies reporting no association between AB and white-matter microstructure. Thus, it is difficult to ascertain the extent to which differences noted in these latter studies are specific to AB in general or whether they were accounted for unmeasured levels of CU or psychopathic traits.
4. Discussion
The included DTI studies demonstrate that adult AB is related to greater diffusivity (i.e., “poorer integrity”) within association, commissural, and thalamic and projection pathways, as indexed via lower FA and AD, and higher RD values. By integrating across studies, this review implicates greater diffusivity in a wider range of tracts than previously thought, contradicting the hypothesis that AB is characterized by specific white-matter microstructural deficits of the UF. In contrast to adult findings, DTI studies of youth identified a mixed pattern of white-matter microstructural differences among youth with AB compared to healthy controls. We discuss the DTI findings for AB in relation to abnormalities within specific tracts and attempt to reconcile the conflicting findings from youth studies. We also situate findings within an emerging science that focuses on the organization of the brain into intrinsic functional networks (Menon, 2011).
4.1. What are the white-matter tract abnormalities in adult AB?
Among adults, evidence from ten studies suggests that AB is associated with greater diffusivity within white-matter tracts across commissural, association, and projection and thalamic pathways, including the corpus callosum, cingulum, IFOF, ILF, SLF, UF, corticospinal tract, corona radiata, and thalamic radiations, as indexed via lower FA and AD and higher RD values. These findings contradict the notion that AB arises from a specific abnormality in UF microstructure, suggesting instead that AB reflects dysfunction in white-matter across regions and hemispheres. This widespread dysfunction is consistent with behavioral characteristics associated with AB that encompass deficits in affective, cognitive, attentional, and reward processing. The greater diffusion within white-matter tracts emerged in both dimensional and case-control analyses, suggesting that, rather than characterizing only severe AB within forensic samples, greater diffusivity (i.e., “poorer integrity”) within white-matter tracts is associated with the full dimensional range of AB. However, we did not find evidence that abnormal microstructure was related to any one microstructural component specifically, as findings emerged across FA, AD, and RD values.
It was striking that the majority of adult studies assessed forensic male samples. At the same time, this is a population where rates of APD and psychopathy are well-established as being higher, making them a logical target of investigation given their impact on crime, violence, and harm to society (Hare, 2006). However, one study examined white-matter microstructure in a female-only sample with a novel design that enabled comparison of women with CD, clinical comparison women with similar rates of depression, anxiety, substance use, and experience of physical and sexual abuse, and healthy controls (Lindner et al., 2016). The study found that lower AD of the corpus callosum was specifically associated with CD, independent of other psychiatric disorders or experience of abuse. Given that adults with AB likely experience many comorbid psychosocial problems, the findings of Lindner and colleagues help to more precisely isolate white-matter microstructure abnormalities specific to AB, and provide a useful analytic model for future studies.
However, differences in white-matter microstructure have also been observed in other patients with an array of psychiatric conditions when compared to healthy controls, including reduced FA and increased MD in the CC of those with autistic spectrum disorders (Aoki et al., 2013), lower FA in the CC, SLF, ILF, corticospinal tract and thalamic radiations of patients with early-onset schizophrenia (Tamnes and Agartz, 2016), and alterations in white-matter integrity of the right anterior corona radiata, right forceps minor, and bilateral internal capsule among children and adults with ADHD (van Ewijk et al., 2012). Thus, DTI measures may be uniquely sensitive to potentially transdiagnostic changes to the microstructure of white-matter in the brain (i.e., generally greater diffusivity or “poorer white matter integrity”). Future research is needed to continue to isolate the microstructural alterations that are specific to AB following the analytic approach of Lindner and colleagues.
4.2. What are the white-matter tract abnormalities in youth AB?
Among youth, the evidence from 12 studies was more mixed as to the nature of white-matter abnormalities. While some studies reported reduced diffusivity within commissural, association, and projection and thalamic pathways indexed by higher FA or AD, other studies reported greater diffusivity or no differences in diffusivity in identical tracts. These contrasting findings were not accounted for by differences in sample type or age, ROI versus whole-brain methodology, or DTI measures (i.e., RD vs. AD). It is noteworthy that unlike the adult studies, which comprised a relatively homogenous set of forensic samples with APD, the youth samples were a mix of clinic-referred, high-risk, and community samples, as well as one adjudicated sample and one sample that explicitly excluded individuals with ADHD diagnoses. Almost all youth studies also had relatively wide age ranges.
4.2.1. Does ADHD explain mixed youth findings?
While we excluded studies that focused solely on ADHD, many of the included studies may have inadvertently included children with varying levels of ADHD symptomatology. In a recent meta-analysis and systematic review, ADHD was related to lower FA, particularly in projection and thalamic radiation tracts, but also to higher FA in other networks (van Ewijk et al., 2012). Thus, given the potential overlap of measures of AB and ADHD among youth within included studies (Thapar et al., 2001), unmeasured ADHD symptomatology may have contributed to the mixed pattern of findings. Even when ADHD was included as a covariate, such statistical covariance methods do not completely control for neurodevelopmental differences, and both structural and functional brain differences have been reported between “pure” samples with ADHD or CD versus healthy controls (Haney-Caron et al., 2014, Rubia et al., 2009). At the same time, one study reported no significant differences in SLF white-matter microstructure between youth with CD/ODD with or without co-occurring ADHD suggesting that ADHD may not solely explain group differences in white-matter microstructure between AB groups and healthy controls (Hummer et al., 2015). Future studies are needed to contrast white-matter differences in samples of healthy control children versus children with “pure” ADHD or CD diagnoses, as well as joint ADHD/CD diagnoses, given high rates of comorbidity among these disorders.
4.2.2. Does age explain mixed youth findings?
In addition to differences relating to unmeasured ADHD symptomatology, there are well-established age-related changes in brain structure and functioning across development, making it challenging to reliably interpret findings of white-matter differences in youth with AB in samples with wide-ranging ages assessed cross-sectionally. Indeed, white-matter volume shows protracted increases across adolescence and the structural connectivity between cortical regions matures at different time points during development (Lenroot and Giedd, 2006), with evidence of increasing FA being associated with age, but not AB, in several included studies of youth (e.g., Hummer et al., 2015, Peper et al., 2015). Even though we did not find effects based on sample age among youth studies, it is possible that divergent associations with AB arose from individual differences in the extent of maturation of white-matter tracts within samples. However, included studies typically examined only modestly-sized samples with wide age ranges, making it is difficult to draw precise conclusions about the influence of age on the pattern of findings. Moreover, associations in white-matter development are influenced by puberty and the release of sex hormones, such as testosterone and luteinizing hormone, which influence brain mechanisms and global white-matter volume (Herting et al., 2012). Although white-matter tracts show increased maturation with increasing chronological age, most white-matter tracts only reach mature levels of integrity after puberty (Asato et al., 2010). Thus, pubertal stage may be a more precise moderator of individual differences in white-matter tracts than age, which has yet to be examined in relation to AB among youth.
To more precisely examine whether age moderates the association of AB and white-matter tract abnormalities, developmentally-informed studies are needed that can extensively replicate findings in samples with narrow age ranges that account for pubertal stage. Prospective longitudinal studies are also needed to examine the developmental course of AB in relation to altered trajectories of white-matter microstructure. For example, AB may be underpinned by developmental delay, with antisocial youth exhibiting abnormally high increases in FA at some developmental points, followed by rapid decreases by adulthood (Passamonti et al., 2012). Alternatively, rather than abnormally high increases, the higher FA found among youth with AB could reflect dysfunction in the normative pruning of axons. Combining DTI approaches with in vivo myelin mapping is one way to examine these questions in future studies (Deoni et al., 2011). Prospective longitudinal studies could help to establish whether abnormalities in white-matter microstructure temporally precede the onset of AB in youth, or develop as a secondary consequence of AB and associated risky behaviors (e.g., substance use).
4.2.3. Does sample type explain mixed youth findings?
The mixed findings for youth may have arisen because of differences in the types of samples examined. Studies of adults were relatively consistent in comparing forensic samples high on AB and psychopathy with healthy controls (7 of 10 studies). In contrast, the majority of youth studies compared adolescents drawn from clinics or high-risk settings to healthy controls from the community, with only one study of forensic youth. While there is likely some homotypic continuity between youth who receive a diagnosis of CD and adults diagnosed with APD in forensic settings, there are likely many important individual-level and experiential differences between these groups. Indeed, most children who exhibit the sorts of behavior problems that precipitate referral to a clinic do not subsequently develop APD. Prospective studies of youth diagnosed with CD using follow-back analyses have established that severe violence, CU traits, substance use, depression, and growing up in low socioeconomic environments are specific risk factors for adult APD beyond an earlier diagnosis of childhood CD (Lahey et al., 2005). Similar follow-back longitudinal approaches are needed within DTI studies to differentiate between white-matter microstructural differences among youth with CD who desist versus persist in AB over time.
4.2.4. Does exposure to environmental risk explain mixed youth findings?
Finally, lower FA has been associated with risky social and emotional experiences, including severe deprivation in early childhood (Eluvathingal et al., 2006) and high levels of parental verbal abuse (Choi et al., 2009) - factors that are also implicated in the etiology of AB beginning early in childhood (Shaw and Gross, 2008). The mixed findings noted in the current review suggest that future studies of white-matter maturation among youth with AB should consider accounting for the influence of environmental and social variables. For example, by adopting the case-control approach of Lindner and colleagues and comparing groups with versus without AB who are matched based on experience of maltreatment, abuse, or other negative exposures, studies could isolate the white-matter deficits are specific to AB, rather than deficits that may be reflective of risky social or emotional experiences.
4.3. Network-based functional implications of white-matter tract abnormalities in AB
Adopting a network approach is useful for integrating evidence across included studies because it helps conceptualize AB as the consequence of abnormalities in multiple brain areas. Indeed, while the majority of neuroimaging research to date has been driven by a focus on regionally-specific neural correlates, the current review provides an opportunity to evaluate several distinct systems, which underpin diverse functional processes. Given that AB and psychopathy are defined by deficits in a range of social, cognitive, and affective processes, we hypothesize three ways in which impaired physical connectivity of the networks contributes to these processes. In relation to commissural pathways, abnormalities in white-matter microstructure among individuals high on AB and CU/psychopathic traits were found in the corpus callosum and forceps minor. In particular, greater diffusivity within white-matter tracts (i.e., lower FA) is thought to cause an imbalance in interhemispheric communication, which has been further hypothesized to promote approach behaviors linked to AB, such as impulsivity and aggression, as well as undermine the ability to integrate information about the thoughts and emotions of others with behavior (Lindner et al., 2016, Schutter and Harmon-Jones, 2013).
Greater diffusivity was also reported among association pathways, including the cingulum, fornix, SLF, UF, and ILF. Interestingly, many of these tracts connect regions within the default mode network (DMN). The DMN is typically more active under conditions of rest (i.e., mind-wandering, day-dreaming), but less active during cognitively-demanding tasks (Fox et al., 2005, Raichle et al., 2001). Of relevance to AB, the DMN is active when participants engage in socio-affective processing (Buckner et al., 2008), moral judgement making (Greene et al., 2001), and evaluations of self- and other- emotional states (Ochsner et al., 2004). Abnormalities in the physical connections between DMN regions as measured by DTI may contribute to the emotional detachment and violence seen in highly antisocial and psychopathic individuals by limiting their capacity for introspective social, moral, and affective processing (Sethi et al., 2014). This assertion is supported by findings of a recent study where reduced DMN functional connectivity was related to higher interpersonal-affective traits scores (i.e., “Factor 1” of psychopathy) among 142 adult male prison inmates (Philippi et al., 2015).
Finally, there were white-matter tract abnormalities among association and thalamic and projection pathways linked to AB, including ILF, IFOF, internal capsules, and thalamic radiations. These pathways connect visual association areas of the occipital lobe, auditory and visual association areas, and the prefrontal cortex (Catani et al., 2013). Physical disruption of this network of tracts is thought to affect the detection and processing of expressions of emotion, which is compromised in individuals high on AB, who show deficits in recognizing fearful and sad facial expressions (Marsh and Blair, 2008) and in individuals high on psychopathy, who show impairments in the processing of both facial and vocal expressions across several emotions (i.e., not only fear and sadness) (Dawel et al., 2012). Moreover, the ILF and IFOF connect regions of the “salience” or cingulo-opercular network and “executive control” or fronto- parietal network, which act together in the integration of salient emotional and cognitive information to facilitate goal-orientated behavior (Dosenbach et al., 2006, Menon, 2011). Diminished physical connectivity of these functional networks could contribute to the behavioral dysfunction seen in AB by impairing detection and mapping of salient external stimuli and internal events, with consequences for cognition, affective processing, and attention.
4.4. The influence of CU/psychopathic traits on white-matter tract abnormalities in AB
It is difficult to draw conclusions about the contribution of CU/psychopathic traits to relationships between white-matter tract abnormalities and AB. A major issue was that most studies investigated relationships between white-matter microstructure and AB or CU/psychopathic traits simultaneously, making it challenging to establish unique links. Importantly, previous research examining task-related reactivity or functional connectivity provides support for unique and divergent neural mechanisms of psychopathic traits, and in some cases, CU traits (Hyde et al., 2014, Viding et al., 2012). Future studies that investigate associations between microstructural abnormalities of white-matter tracts and psychopathic/CU traits while accounting for AB, and vice versa, and either using dimensional (cf. Breeden et al., 2015) or case-control analyses (cf. Motzkin et al., 2011, Viding et al., 2012), are needed to understand differences in underlying neural circuitry for these separate constructs.
A second issue relevant to interpreting the associations between CU traits/psychopathy and white-matter microstructure abnormalities centers on the role of DTI method: ROI versus whole-brain analysis. Early studies provided evidence that psychopathy may be related to more diffuse white-matter microstructure (e.g., lower FA) of the UF, which is borne out in four of the included adult studies. However, all four employed ROI-analysis (Craig et al., 2009, Motzkin et al., 2011, Sobhani et al., 2015, Wolf et al., 2015). Among four studies that employed whole-brain analysis, two failed to find that AB or psychopathy were related to lower FA values of the UF (Karlsgodt et al., 2015, Lindner et al., 2016). Moreover, while the other two studies confirmed UF reduced integrity (Hoppenbrouwers et al., 2013, Sundram et al., 2011), they also reported greater diffusivity within white-matter (i.e., lower FA) across a range of other tracts. Thus, a major finding from this review is that the previously-purported specificity of white-matter abnormalities to the UF may have been contingent on studies using a ROI-focused analysis, which challenges current etiological models of AB. At the same time, the UF is in a region of many intersecting tracts and whole-brain analyses may be limited in power to detect differences. Thus future studies could employ the “hybrid” approach of Motzkin and colleagues, who used an ROI, but contrasted UF findings with those for the SLF, ILF, and IFOF, still finding specificity of abnormalities to the UF (Motzkin et al., 2011; also see Passamonti et al., 2012).
4.5. Other future directions
In addition to contrasting findings from ROI versus whole-brain approaches, future studies of AB more broadly should contrast different techniques within ROI versus whole-brain approaches. In particular, the deterministic tractography methods adopted by many included studies may be limited in terms of the accuracy with which directions and pathways of fibers can be determined, especially in regions of the brain where multiple fiber bundles of different orientations are crossing each other (e.g., SLF and UF). Even when included studies addressed this potential limitation by comparing DTI measures using a voxel-wide approach (i.e., rather than reconstructing fiber tracts), there is the possibility of normalization or skeletonization errors (for a more in-depth discussion of the strengths and weaknesses of these methods, see Cercignani, 2010, Jones et al., 2013, Soares et al., 2013). Both tractography and voxel-wide approach were represented within included studies, but we did not identify a clear pattern of findings related to one or other type of methodology. Importantly, however, very few studies employed the same techniques, particularly within the youth studies (e.g., only three of 12 youth studies used an identical analytic approach). Inconsistencies in methodology thus likely contributed to the mixed findings among the youth studies, highlighting the importance of future studies including a clear rationale for using a specific technique and incorporating a range of different techniques to enable better comparison across studies.
Finally, an important implication of this review is that future studies need to isolate the neurobiological meaning of white-matter microstructural differences and what the functional consequences are for socioemotional, neurocognitive, or behavioral functions (i.e., do differences in white-matter microstructure predict functional reactivity in regions implicated in youth AB/CU traits?; for an example of a study testing this question, see Breeden et al., 2015). We have also remained cautious in our interpretation of DTI measures, and have focused our presentation of results on specific measures (i.e., focusing on higher versus lower FA) or the nature of diffusivity within tracts. In particular, we note the persuasive arguments of Jones and colleagues who suggest that terms such as “lower white-matter integrity” or “lower connectivity” of tracts, while popular in the literature, are highly problematic (Jones et al., 2013). Indeed, several possible explanations exist for why DTI measures, including FA, may be different in one group relative to another. Importantly, many of these reasons have nothing to do with purported “integrity” of white-matter tracts, such as axon diameter, packing density, branching of tracts, myelination, or other microscopic changes to axonal structure (Beaulieu, 2002, Hoeft et al., 2007, Jones et al., 2013). As outlined by Jones and colleagues, part of the ambiguity surrounding the interpretation of DTI measures arises from the nature of the measures themselves and the fitting of a tensor model. Accordingly, future studies of white-matter microstructural differences in AB could be informed by incorporating approaches that are more sensitive to microstructural changes, including Diffusion Kurtosis Imaging (Jensen and Helpern, 2010) and Magnetization Transfer Imaging, which is highly sensitive to potential demyelination as a cause of altered DTI parameters (Wozniak and Lim, 2006). Combining these alternative approaches with DTI may lead to a better understanding of the specific microstructural alterations that underpin AB.
4.6. Limitations
The emerging literature on structural connectivity in AB suggests that differences in white-matter microstructure contribute to AB. However, several limitations both of included studies and the current review undermine the conclusions that can be drawn about white-matter microstructure abnormalities in AB. First, due to heterogeneity in methodology (e.g., sample, age, measures, analyses), we were unable to perform a meta-analysis and could not determine the magnitude of effect sizes. Second, this review was likely limited by the “file-drawer problem” (Reysen, 2006) in that we could not account for unpublished findings, particularly given that only five included studies found no significant effects (4 in youth; 1 in adults). Third, a major limitation in all but two of the included studies was that samples invariably included individuals who were extreme on both AB and CU/psychopathic traits or among whom the level of CU/psychopathic traits was unknown. Thus, the findings of many included studies may confound severity of AB with the presence of interpersonal-affective deficits associated with CU/psychopathic traits. Future studies need to examine unique associations of psychopathy or CU traits with white-matter microstructure abnormalities, parsing variance in overall AB severity or overlapping constructs of aggression and impulsivity, or by using a case-control approach that compares individuals high on AB with versus without psychopathy (for examples of this type of analytic approach, see Breeden et al., 2015, Motzkin et al., 2011, Philippi et al., 2015). Fourth, only two of the included studies examined demographic moderators (Decety et al., 2015, Zhang et al., 2014a). In terms of moderation by gender, these two youth studies reported findings in opposite directions – one reported increased AD in white-matter tracts that was specific to girls (Decety et al., 2015) and the other reported lower RD in the UF that was specific to boys (Zhang et al., 2014b). It is interesting that in one adult study that examined only women, the direction of the relationship between AB and white-matter abnormalities was consistent with findings from studies of only men (i.e., lower AD; Lindner et al., 2016). Nevertheless, future research is needed to expand our knowledge of sex-specific effects of white-matter abnormalities on AB across development.
Additionally, only one study examined race as a moderator, reporting that white-matter microstructure abnormalities, indexed by higher AD, were more strongly related to AB among African-American children compared to Caucasian/Hispanic children (Decety et al., 2015). Other studies were inconsistent in reporting of the racial and/or ethnic group membership of participants, which is relevant to understanding AB as previous research has found that many of the most consistently-reported relationships between psychopathy and brain reactivity fail to replicate in African-American samples (Baskin-Sommers et al., 2011, Hyde et al., 2015, Lorenz and Newman, 2002). Finally, this review emphasizes the need for future research to consider the context in which participants grow up, given evidence of the influence of exposure to environmental stressors or abuse on relationships with white-matter integrity (Lindner et al., 2016), and to examine samples of individuals from different economic strata, who may have specific neurodevelopmental pathways toward AB (Raine, 2002).
4.7. Conclusion
Despite these limitations, the research presented in the 22 reviewed studies demonstrates significant associations between diffusivity of white-matter tracts and AB. Specifically, lower FA in association, commissural, and projection and thalamic pathways was found among adults with AB and psychopathic traits. Importantly, the review found that AB across the lifespan was associated with widespread white-matter microstructure differences rather than specific deficits to the UF, as previously thought. These findings demonstrate the importance of utilizing both whole-brain and ROI approaches to identify abnormalities in the structural networks underlying AB. Among youth studies, the evidence was mixed with different studies reporting both greater and reduced diffusivity of white-matter tracts, or no differences in white-matter microstructure of youth with AB versus healthy controls. The review supports the existence of an association between AB and altered microstructure of brain white-matter tracts, and guides future research by highlighting the need to focus on a wider range of tracts beyond the UF alone, adopt a more developmentally-focused approach by examining samples with narrower age range, and account for environmental risk and pubertal stage.
Conflict of interest statement
No conflicts declared.
Acknowledgements
This research was supported by Grant L40MH108392 to L.W. Hyde. R. Waller was supported in her efforts by a NIAAA T32 Fellowship in the Addiction Center, Department of Psychiatry, University of Michigan (2T32AA007477-24A1).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.01.014.
Contributor Information
Rebecca Waller, Email: rewaller@umich.edu.
Luke W. Hyde, Email: lukehyde@umich.edu.
Appendix A. Supplementary data
Supplementary Methods and Results Tables.
References
- Aoki Y., Abe O., Nippashi Y., Yamasue H. Comparison of white matter integrity between autism spectrum disorder subjects and typically developing individuals: a meta-analysis of diffusion tensor imaging tractography studies. Mol. Autism. 2013;4:1–17. doi: 10.1186/2040-2392-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato M., Terwilliger R., Woo J., Luna B. White matter development in adolescence: a DTI study. Cereb. Cortex. 2010;20:2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M., Laun F.B., Leemans A., Tax C.M., Biessels G.J., Stieltjes B. Methodological considerations on tract-based spatial statistics (TBSS) NeuroImage. 2014;100:358–369. doi: 10.1016/j.neuroimage.2014.06.021. [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers A.R., Newman J.P., Sathasivam N., Curtin J.J. Evaluating the generalizability of a fear deficit in psychopathic African American offenders. J. Abnorm. Psychol. 2011;120:71–78. doi: 10.1037/a0021225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser P.J., Mattiello J., LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu A. A space for measuring mind and brain: interdisciplinarity and digital tools in the development of brain mapping and functional imaging, 1980–1990. Brain Cogn. 2002;49:13–33. doi: 10.1006/brcg.2001.1461. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. Science. Elsevier; 2009. The biological basis of diffusion anisotropy. [Google Scholar]
- Beyer F., Munte T.F., Wiechert J., Heldmann M., Kramer U.M. Trait aggressiveness is not related to structural connectivity between orbitofrontal cortex and amygdala. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0101105. (Electronic Resource) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J.R. Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. J. Neurol. Neurosurg. Psychiatry. 2001;71:727–731. doi: 10.1136/jnnp.71.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R.J.R., Leibenluft E., Pine D.S. Conduct disorder and callous–unemotional traits in Youth. N. Engl. J. Med. 2014;371:2207–2216. doi: 10.1056/NEJMra1315612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom J.S., Hynd G.W. The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol. Rev. 2005;15:59–71. doi: 10.1007/s11065-005-6252-y. [DOI] [PubMed] [Google Scholar]
- Breeden A.L., Cardinale E.M., Lozier L.M., VanMeter J.W., Marsh A.A. Callous-unemotional traits drive reduced white-matter integrity in youths with conduct problems. Psychol. Med. 2015:1–14. doi: 10.1017/S0033291715000987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Budde M.D., Xie M., Cross A.H., Song S.-K. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J. Neurosci. 2009;29:2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd A.L., Loeber R., Pardini D.A. Antisocial behavior, psychopathic features and abnormalities in reward and punishment processing in youth. Clin. Child. Fam. Psychol. Rev. 2014;17:125–156. doi: 10.1007/s10567-013-0159-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M., Dell'acqua F., Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neurosci. Biobehav. Rev. 2013;37:1724–1737. doi: 10.1016/j.neubiorev.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Cercignani M. In: Strategies for Patient-control Comparison of Diffusion MR Data. Jones D.K., editor. Oxford University Press; Oxford, UK: 2010. [Google Scholar]
- Choi J., Jeong B., Rohan M.L., Polcari A.M., Teicher M.H. Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biol. Psychiatry. 2009;65:227–234. doi: 10.1016/j.biopsych.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro E.F., McCloskey M.S., Fitzgerald D.A., Phan K.L. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol. Psychiatry. 2007;62:168–178. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Craig M.C., Catani M., Deeley Q., Latham R., Daly E., Kanaan R. Altered connections on the road to psychopathy. Mol. Psychiatry. 2009;14(946–953):907. doi: 10.1038/mp.2009.40. [DOI] [PubMed] [Google Scholar]
- Dawel A., O'Kearney R., McKone E., Palermo R. Not just fear and sadness: meta-analytic evidence of pervasive emotion recognition deficits for facial and vocal expressions in psychopathy. Neurosci. Biobehav. Rev. 2012;36:2288–2304. doi: 10.1016/j.neubiorev.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Decety J., Yoder K.J., Lahey B.B. Sex differences in abnormal white matter development associated with conduct disorder in children. Psychiatry Res. 2015;233:269–277. doi: 10.1016/j.pscychresns.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni S.C., Mercure E., Blasi A., Gasston D., Thomson A., Johnson M. Mapping infant brain myelination with magnetic resonance imaging. J. Neurosci. 2011;31:784–791. doi: 10.1523/JNEUROSCI.2106-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N., Visscher K., Palmer E., Miezin F., Wenger K., Kang H. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eluvathingal T.J., Chugani H.T., Behen M.E., Juhász C., Muzik O., Maqbool M. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117:2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Fergusson D.M., Horwood J.L., Ridder E.M. Show me the child at seven: the consequences of conduct problems in childhood for psychosocial functioning in adulthood. J. Child Psychol. Psychiatry. 2005;46:837–849. doi: 10.1111/j.1469-7610.2004.00387.x. [DOI] [PubMed] [Google Scholar]
- Finger E.C., Marsh A.A., Blair K.S., Reid M.E., Sims C., Ng P. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. Am. J. Psychiatr. 2011;168:152–162. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger E.C., Marsh A., Blair K.S., Majestic C., Evangelou I., Gupta K. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res. 2012;202:239–244. doi: 10.1016/j.pscychresns.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C.J., Iaria G., Barton J.J. Disconnection in prosopagnosia and face processing. Cortex. 2008;44:996–1009. doi: 10.1016/j.cortex.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Frick P.J., White S.F. Research review: the importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. J. Child Psychol. Psychiatry. 2008;49:359–375. doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- Greene J.D., Sommerville R.B., Nystrom L.E., Darley J.M., Cohen J.D. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293:2105–2108. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Haney-Caron E., Caprihan A., Stevens M.C. DTI-measured white matter abnormalities in adolescents with conduct disorder. J. Psychiatr. Res. 2014;48:111–120. doi: 10.1016/j.jpsychires.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare R.D. Psychopathy: a clinical and forensic overview. Psychiatr. Clin. N. Am. 2006;29:709–724. doi: 10.1016/j.psc.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Herting M.M., Maxwell E.C., Irvine C., Nagel B.J. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb. Cortex. 2012;22:1979–1992. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F., Barnea-Goraly N., Haas B.W., Golarai G., Ng D., Mills D. More is not always better: increased fractional anisotropy of superior longitudinal fasciculus associated with poor visuospatial abilities in Williams syndrome. J. Neurosci. 2007;27:11960–11965. doi: 10.1523/JNEUROSCI.3591-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppenbrouwers S.S., Nazeri A., de Jesus D.R., Stirpe T., Felsky D., Schutter D.J. White matter deficits in psychopathic offenders and correlation with factor structure. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0072375. (Electronic Resource) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman M.J., Volavka J., Johnson G., Weiss E., Bilder R.M., Lim K.O. Frontal white matter microstructure, aggression, and impulsivity in men with schizophrenia: a preliminary study. Biol. Psychiatry. 2002;52:9–14. doi: 10.1016/s0006-3223(02)01311-2. [DOI] [PubMed] [Google Scholar]
- Hummer T.A., Wang Y., Kronenberger W.G., Dunn D.W., Mathews V.P. The relationship of brain structure to age and executive functioning in adolescent disruptive behavior disorder. Psychiatry Res. 2015;231:210–217. doi: 10.1016/j.pscychresns.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Hyde L.W., Shaw D.S., Hariri A.R. Understanding youth antisocial behavior using neuroscience through a developmental psychopathology lens: review, integration, and directions for research. Dev. Rev. 2013;33:168–223. doi: 10.1016/j.dr.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Byrd A.L., Votruba-Drzal E., Hariri A.R., Manuck S.B. Amygdala reactivity and negative emotionality: divergent correlates of antisocial personality and psychopathy traits in a community sample. J. Abnorm. Psychol. 2014;123:214–224. doi: 10.1037/a0035467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Shaw D.S., Murray L., Gard A., Hariri A.R., Forbes E.E. Dissecting the role of amygdala reactivity in antisocial behavior in a sample of young, low-income, urban men. Clin. Psychol. Sci. 2015;4:527–544. doi: 10.1177/2167702615614511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatr. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jang S.H. Somatotopic arrangement and location of the corticospinal tract in the brainstem of the human brain. Yonsei Med. J. 2011;52:553–557. doi: 10.3349/ymj.2011.52.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.H., Helpern J.A. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010;23:698–710. doi: 10.1002/nbm.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.K., Knösche T.R., Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Karlsgodt K.H., Bato A.A., Blair M.A., DeRosse P., Szeszko P.R., Malhotra A.K. White matter microstructure in the executive network associated with aggression in healthy adolescents and young adults. Soc. Cogn. Affect. Neurosci. 2015;10(9):1251–1256. doi: 10.1093/scan/nsv015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl K.A., Smith A.M., Hare R.D., Mendrek A., Forster B.B., Brink J. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol. Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]
- Klawiter E.C., Schmidt R.E., Trinkaus K., Liang H.-F., Budde M.D., Naismith R.T. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. NeuroImage. 2011;55:1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey B.B., Loeber R., Burke J.D., Applegate B. Predicting future antisocial personality disorder in males from a clinical assessment in childhood. J. Consult. Clin. Psychol. 2005;73:389–399. doi: 10.1037/0022-006X.73.3.389. [DOI] [PubMed] [Google Scholar]
- Leng B., Han S., Bao Y., Zhang H., Wang Y., Wu Y. The uncinate fasciculus as observed using diffusion spectrum imaging in the human brain. Neuroradiology. 2016;58:595–606. doi: 10.1007/s00234-016-1650-9. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Li T.-Q., Mathews V.P., Wang Y., Dunn D., Kronenberger W. Adolescents with disruptive behavior disorder investigated using an optimized MR diffusion tensor imaging protocol. In: Ulmer J.L., Parsons L., Moseley M., Gabrieli J., Ulmer J.L., Parsons L., Moseley M., Gabrieli J., editors. White Matter in Cognitive Neuroscience: Advances in Diffusion Tensor Imaging and Its Applications. New York Academy of Sciences; New York, NY, US: 2005. pp. 184–192. [DOI] [PubMed] [Google Scholar]
- Lindner P., Savic I., Sitnikov R., Budhiraja M., Liu Y., Jokinen J. Conduct disorder in females is associated with reduced corpus callosum structural integrity independent of comorbid disorders and exposure to maltreatment. Transl. Psychiatry. 2016;6 doi: 10.1038/tp.2015.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz A.R., Newman J.P. Do emotion and information processing deficiencies found in Caucasian psychopaths generalize to African–American psychopaths? Personal. Individ. Differ. 2002;32:1077–1086. [Google Scholar]
- Marsh A.A., Blair R.J.R. Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neurosci. Biobehav. Rev. 2008;32:454–465. doi: 10.1016/j.neubiorev.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh A., Finger E., Mitchell D., Reid M., Sims C., Kosson D. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am. J. Psychiatr. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Mori S., Wakana S., Van Zijl P.C., Nagae-Poetscher L. first ed. Elsevier; Amsterdam: 2005. MRI Atlas of Human White Matter. [Google Scholar]
- Motzkin J.C., Newman J.P., Kiehl K.A., Koenigs M. Reduced prefrontal connectivity in psychopathy. J. Neurosci. 2011;31:17348–17357. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Knierim K., Ludlow D.H., Hanelin J., Ramachandran T., Glover G. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J. Cogn. Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Pandya D.N., Petrides M., Cipolloni P.B. Oxford University Press; Oxford, England: 2015. Cerebral Cortex: Architecture, Connections, and the Dual Origin Concept. [Google Scholar]
- Pape L.E., Cohn M.D., Caan M.W., van Wingen G., van den Brink W., Veltman D.J. Psychopathic traits in adolescents are associated with higher structural connectivity. Psychiatry Res. 2015;233:474–480. doi: 10.1016/j.pscychresns.2015.07.023. [DOI] [PubMed] [Google Scholar]
- Passamonti L., Fairchild G., Fornito A., Goodyer I.M., Nimmo-Smith I., Hagan C.C. Abnormal anatomical connectivity between the amygdala and orbitofrontal cortex in conduct disorder. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0048789. (Electronic Resource) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick C.J. Getting to the heart of psychopathy. In: Herve H., Yuille J.C., editors. Erlbaum; Hillsdale, NJ: 2007. pp. 207–252. (Psychopathy: Theory, research, and social implications). [Google Scholar]
- Peper J.S., de Reus M.A., van den Heuvel M.P., Schutter D.J.L.G. Short fused? Associations between white matter connections, sex steroids, and aggression across adolescence. Hum. Brain Mapp. 2015;36:1043–1052. doi: 10.1002/hbm.22684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi C.L., Pujara M.S., Motzkin J.C., Newman J., Kiehl K.A., Koenigs M. Altered resting-state functional connectivity in cortical networks in psychopathy. J. Neurosci. 2015;35:6068–6078. doi: 10.1523/JNEUROSCI.5010-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: a review. J. Abnorm. Child Psychol. 2002;30:311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Reysen S. Publication of nonsignificant results: a survey of psychologists' opinions. Psychol. Rep. 2006;98:169–175. doi: 10.2466/pr0.98.1.169-175. [DOI] [PubMed] [Google Scholar]
- Rubia K., Halari R., Smith A., Mohammad M., Scott S., Brammer M. Shared and disorder specific prefrontal abnormalities in boys with pure attention deficit/hyperactivity disorder compared to boys with pure CD during interference inhibition and attention allocation. J. Child Psychol. Psychiatry. 2009;50:669–678. doi: 10.1111/j.1469-7610.2008.02022.x. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Craig M.C., Catani M., Dell'Acqua F., Fahy T., Deeley Q. Frontotemporal white-matter microstructural abnormalities in adolescents with conduct disorder: a diffusion tensor imaging study. Psychol. Med. 2013;43:401–411. doi: 10.1017/S003329171200116X. [DOI] [PubMed] [Google Scholar]
- Saxena K., Tamm L., Walley A., Simmons A., Rollins N., Chia J. A preliminary investigation of corpus callosum and anterior commissure aberrations in aggressive youth with bipolar disorders. J. Child Adolesc. Psychopharmacol. 2012;22:112–119. doi: 10.1089/cap.2011.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter D.J., Harmon-Jones E. The corpus callosum: a commissural road to anger and aggression. Neurosci. Biobehav. Rev. 2013;37:2481–2488. doi: 10.1016/j.neubiorev.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Scott S., Knapp M., Henderson J., Maughan B. Financial cost of social exclusion: follow up study of antisocial children into adulthood. BMJ. 2001;323:191–194. doi: 10.1136/bmj.323.7306.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi A., Gregory S., Dell'Acqua F., Periche Thomas E., Simmons A., Murphy D.G.M. Emotional detachment in psychopathy: involvement of dorsal default-mode connections. Cortex. 2014 doi: 10.1016/j.cortex.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Sethi A., Gregory S., Dell'Acqua F., Thomas E.P., Simmons A., Murphy D.G.…Craig M.C. Emotional detachment in psychopathy: involvement of dorsal default-mode connections. Cortex. 2015;62:11–19. doi: 10.1016/j.cortex.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Shaw D.S., Gross H.E. Springer; New York: 2008. What We Have Learned About Early Childhood and the Development of Delinquency. [Google Scholar]
- Sherbondy A.J., Dougherty R.F., Napel S., Wandell B.A. Identifying the human optic radiation using diffusion imaging and fiber tractography. J. Vis. 2008;8:12. doi: 10.1167/8.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J., Marques P., Alves V., Sousa N. A hitchhiker's guide to diffusion tensor imaging. Front. Neurosci. 2013;7:31. doi: 10.3389/fnins.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhani M., Baker L., Martins B., Tuvblad C., Aziz-Zadeh L. Psychopathic traits modulate microstructural integrity of right uncinate fasciculus in a community population. Neuroimage Clin. 2015;8:32–38. doi: 10.1016/j.nicl.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundram F., Deeley Q., Sarkar S., Daly E., Latham R., Barker G.J. White matter microstructural abnormalities in antisocial personality disorder: a pilot diffusion tensor imaging study. Eur. Psychol. 2011;26 [Google Scholar]
- Sundram F., Deeley Q., Sarkar S., Daly E., Latham R., Craig M. White matter microstructural abnormalities in the frontal lobe of adults with antisocial personality disorder. Cortex. 2012;48:216–229. doi: 10.1016/j.cortex.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Tamnes C.K., Agartz I. White matter microstructure in early-onset schizophrenia: a systematic review of diffusion tensor imaging studies. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55:269–279. doi: 10.1016/j.jaac.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Teipel S.J., Bokde A.L., Meindl T., Amaro E., Soldner J., Reiser M.F. White matter microstructure underlying default mode network connectivity in the human brain. NeuroImage. 2010;49:2021–2032. doi: 10.1016/j.neuroimage.2009.10.067. [DOI] [PubMed] [Google Scholar]
- Thapar A., Harrington R., McGuffin P. Examining the comorbidity of ADHD-related behaviours and conduct problems using a twin study design. Br. J. Psychiatry. 2001;179:224–229. doi: 10.1192/bjp.179.3.224. [DOI] [PubMed] [Google Scholar]
- Thayer R.E., Callahan T.J., Weiland B.J., Hutchison K.E., Bryan A.D. Associations between fractional anisotropy and problematic alcohol use in juvenile justice-involved adolescents. Am. J. Drug Alcohol Abuse. 2013;39:365–371. doi: 10.3109/00952990.2013.834909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Thompson P.M. Diffusion imaging, white matter, and psychopathology. Clin. Psychol. 2011;7:63–85. doi: 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- van Ewijk H., Heslenfeld D.J., Zwiers M.P., Buitelaar J.K., Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2012;36:1093–1106. doi: 10.1016/j.neubiorev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Viding E., Sebastian C.L., Dadds M.R., Lockwood P.L., Cecil C.A.M., De Brito S.A. Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. Am. J. Psychiatr. 2012;169:1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- Westlye L.T., Walhovd K.B., Dale A.M., Bjørnerud A., Due-Tønnessen P., Engvig A. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb. Cortex. 2009 doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Winston G.P. The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant. Imaging Med. Surg. 2012;2:254–265. doi: 10.3978/j.issn.2223-4292.2012.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R.C., Pujara M.S., Motzkin J.C., Newman J.P., Kiehl K.A., Decety J. Interpersonal traits of psychopathy linked to reduced integrity of the uncinate fasciculus. Hum. Brain Mapp. 2015;36:4202–4209. doi: 10.1002/hbm.22911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J.R., Lim K.O. Advances in white matter imaging: a review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neurosci. Biobehav. Rev. 2006;30:762–774. doi: 10.1016/j.neubiorev.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Raine A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: a meta-analysis. Psychiatry Res. Neuroimaging. 2009;174:81–88. doi: 10.1016/j.pscychresns.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Gao J., Shi H., Huang B., Wang X., Situ W. Sex differences of uncinate fasciculus structural connectivity in individuals with conduct disorder. Biomed. Res. Int. 2014;2014:673165. doi: 10.1155/2014/673165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Zhu X., Wang X., Gao J., Shi H., Huang B. Increased structural connectivity in corpus callosum in adolescent males with conduct disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53:466–475. doi: 10.1016/j.jaac.2013.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods and Results Tables.