Abstract
An increasing number of elderly patients (≥65 years) receive a donor kidney from elderly donors after brain death (DBD) or after circulatory death (DCD). These organs are allocated within the Eurotransplant Senior Program, but outcomes must be evaluated. From the Dutch Organ Transplantation Registry, we selected 3597 recipients (≥18 years) who received a first DBD or DCD kidney during 2002–2012, and categorized them as young or elderly recipients receiving a graft from either a young or elderly donor, stratified by donor type. In multiple logistic regression analysis, elderly recipients of elderly DCD kidneys experienced more delayed graft function and acute rejection than did elderly recipients of young DBD kidneys (odds ratios 10.43 [95% confidence interval (95% CI), 5.75 to 18.91] and 2.78 [95% CI, 1.35 to 5.73], respectively). In Cox regression analysis, elderly recipients of elderly DCD kidneys had a 5-year mortality risk higher than that of elderly recipients of young DBD kidneys (hazard ratio, 1.86; 95% CI, 1.15 to 3.02). Elderly recipients of elderly kidneys had a 5-year mortality rate comparable to that of waitlisted elderly patients remaining on dialysis. Among elderly recipients, 63.8% of those who received elderly DCD kidneys, 45.5% of those who received elderly DBD kidneys, and approximately 26% of those who received young DBD or DCD kidneys had an eGFR<30 ml/min per 1.73 m2 (including primary nonfunction) after 1 year. In conclusion, improving donor selection and preservation is warranted if the allocation of elderly DCD grafts to elderly recipients is to be expanded.
Keywords: kidney transplantation, Donation after circulatory death, Donation after brain death, Elderly donors, Elderly recipients, Eurotransplant Senior Program
Studies of kidney transplantation in elderly recipients have shown favorable results, demonstrating improved survival compared with waitlisted patients remaining on dialysis.1–3 Combined with an expanding geriatric population and a persistent shortage of donor organs, this has led to an upward trend in the acceptance of grafts from older donors fulfilling expanded donor criteria.4–8
Between 2011 and 2013, the majority (54%) of deceased donor kidney transplants in The Netherlands were from donations after circulatory death (DCD),8 and DCD programs are emerging in many other countries.9,10 DCD kidneys offer a valuable extension of the donor pool, but at the expense of increasing the risk of delayed graft function (DGF) compared with kidneys from donation after brain death (DBD).11–13 A recent analysis of the use of DCD donors older than 60 years showed no difference in graft survival between kidneys from DCD or DBD donors.13 However, the situation in The Netherlands may be different owing to specific allocation of organs from older DCD donors to recipients >65 years within the Eurotransplant Senior Program (ESP).
By allocating donors aged ≥65 years to recipients aged ≥65 years, the ESP intends to improve the match between life expectancy of donor organs and recipients. The key components are allocation without prospective matching for human leukocyte antigen (HLA) in favor of local allocation, in order to reduce cold ischemia time (CIT) and thus the risk of injury.14 Whereas all elderly transplant candidates are conventionally listed for regular kidney allocation within both Eurotransplant and the ESP, only non-HLA immunized elderly candidates are additionally eligible for elderly kidney allocation in ESP. Allocation within the ESP is based on urgency points derived solely from waiting time, starting from the first day of maintenance dialysis treatment. A counterbalance system prevents one country in the Eurotransplant region from donating more or fewer grafts to another country, and therefore waiting times differ between countries.15 The ESP increases availability of elderly donors, thus reducing time on the waiting list.16 However, the effect of this policy on long-term graft and patient survival in elderly recipients has not been evaluated in a program including DCD kidneys. If elderly DCD kidneys are damaged by the DCD retrieval procedure, this may result in unfavorable outcomes in vulnerable elderly recipients.
We used data from the Dutch Organ Transplantation Registry to analyze patient and graft survival among elderly (≥65 years) recipients of elderly (≥65 years) DBD or DCD kidneys, and compared outcomes to those of elderly recipients of young (<65 years) DBD kidneys, i.e., the optimal deceased donor for elderly recipients. We tested the hypothesis that kidney transplantation in elderly recipients using either young or elderly DBD and DCD kidneys leads to similar transplant outcomes. Furthermore, we compared these results to (1) mortality of elderly waitlisted patients on dialysis, and (2) the outcome of young (<65 years) recipients transplanted with either young (<65 years) DBD or DCD kidneys.
Results
Baseline Differences across Age Groups
Table 1 shows the characteristics of recipients and donors. The median age of young recipients was 48 years, and the median age of elderly recipients was 68 years. DCD donors were more likely to be male, especially in the elderly DCD group. The elderly DBD and DCD donors were less likely to have a history of smoking compared with young donors (P<0.001). DCD donors received inotropic medication less frequently than DBD donors (P<0.001). The median CIT was shortest in elderly recipients of elderly DBD kidneys (14.9 hours). However, this was 17.5 hours in elderly recipients receiving a DCD kidney from an elderly donor (P=0.01). Within the ESP, 30.2% and 6.1% of transplantations were performed after a CIT in the range 18–24 hours and >24 hours, respectively. Recipient dialysis vintage and final eGFR before donation were lower within the ESP versus outside the ESP (P<0.001 and P<0.001, respectively).
Table 1.
Characteristics of transplant donors and recipients (n=3597)
| Characteristics | All Renal Allografts | Recipients <65 yr | Recipients ≥65 yr | ||||
|---|---|---|---|---|---|---|---|
| Young Donor (<65 yr) DBD | Young Donor (<65 yr) DCD | Young Donor (<65 yr) DBD | Young Donor (<65 yr) DCD | Elderly Donor (≥65 yr) DBD | Elderly Donor (≥65 yr) DCD | ||
| N (%) | 3597 (100%) | 1730 (48.1%) | 1153 (32.1%) | 188 (5.2%) | 144 (4.0%) | 245 (6.8%) | 137 (3.8%) |
| Donor age, yra | 50.0 (40.0–59.0) | 48.0 (38.0–55.0) | 48.0 (38.0–57.0) | 51.0 (43.0–58.0) | 48.0 (35.3–57.0) | 68.0 (66.0–71.0) | 67.0 (66.0–69.0) |
| Donor sex malea | 1844 (51.3%) | 840 (48.6%) | 641 (55.6%) | 82 (43.6%) | 80 (55.6%) | 109 (44.5%) | 92 (67.2%) |
| Donor smoking status (yes)a,b | 1593 (47.2%) | 807 (50.2%) | 503 (46.0%) | 87 (52.1%) | 69 (49.3%) | 89 (37.2%) | 38 (29.2%) |
| Donor weight, kga,c | 75.0 (65.0–85.0) | 75.0 (65.0–84.0) | 75.0 (65.0–85.0) | 75.0 (69.0–85.0) | 75.0 (70.0–90.0) | 75.0 (68.5–85.0) | 78.0 (70.0–85.0) |
| Donor height, ma | 1.75 (1.68–1.80) | 1.74 (1.68–1.80) | 1.75 (1.68–1.80) | 1.72 (1.67–1.78) | 1.75 (1.70–180) | 1.70 (1.65–1.76) | 1.75 (1.68–1.80) |
| Inotropes before donation (yes)a | 1084 (30.1%) | 604 (34.9%) | 249 (21.6%) | 63 (33.5%) | 42 (29.2%) | 106 (43.3%) | 20 (14.6%) |
| Hypotensive period (yes)a | 972 (27.0%) | 506 (29.2%) | 274 (23.8%) | 51 (27.1%) | 35 (24.3%) | 82 (33.5%) | 24 (17.5%) |
| Terminal MDRD donora,d | 93.1 (72.3–119.2) | 90.7 (69.3–120.4) | 99.1 (79.6–122.8) | 90.4 (72.9–124.0) | 94.7 (77.5–122.0) | 81.3 (68.5–100.8) | 83.9 (67.7–108.6) |
| CIT, ha,e | 17.0 (13.7–21.2) | 16.8 (13.0–21.8) | 17.7 (14.8–21.1) | 17.3 (14.1–21.8) | 17.6 (14.0–22.2) | 14.9 (12.0–18.8) | 17.5 (14.2–20.0) |
| Recipient age, yra | 54.0 (43.0–62.0) | 49.0 (40.0–57.0) | 51.0 (41.0–58.0) | 67.5 (66.0–70.0) | 68.0 (66.0–70.0) | 69.0 (66.0–71.0) | 68.0 (66.0–71.0) |
| Recipient sex malea | 2134 (59.3%) | 964 (55.7%) | 715 (62.0%) | 114 (60.6%) | 85 (59.0%) | 166 (67.8%) | 90 (65.7%) |
| Pre-emptive (yes)a,f | 161 (4.6%) | 111 (6.8%) | 31 (2.7%) | 6 (3.2%) | 1 (0.7%) | 8 (3.3%) | 4 (2.9%) |
| Recipient dialysis vintage, yra,g | 4.1 (2.8–5.5) | 4.2 (2.9–5.8) | 4.3 (3.1–5.6) | 3.6 (2.2–5.5) | 3.5 (2.4–5.1) | 3.4 (2.5–4.6) | 3.1 (2.2–4.0) |
| HLA-A mismatcha,h | |||||||
| 0 Mismatch | 1245 (36.6%) | 695 (42.2%) | 388 (35.4%) | 81 (44.0%) | 45 (33.1%) | 28 (12.7%) | 8 (6.5%) |
| 1 Mismatch | 1735 (51.0%) | 769 (46.7%) | 612 (55.8%) | 88 (47.8%) | 76 (55.9%) | 125 (56.8%) | 65 (52.8%) |
| 2 Mismatches | 425 (12.5%) | 181 (11.0%) | 97 (8.8%) | 15 (8.2%) | 15 (11.0%) | 67 (30.5%) | 50 (40.7%) |
| HLA-B mismatcha,i | |||||||
| 0 Mismatch | 824 (24.2) | 486 (29.5%) | 232 (21.1) | 64 (34.8) | 35 (25.7%) | 5 (2.3%) | 2 (1.6%) |
| 1 Mismatch | 1794 (52.7) | 816 (49.6%) | 708 (64.5) | 92 (50.0) | 76 (55.9%) | 67 (30.5%) | 35 (28.5%) |
| 2 Mismatches | 787 (23.1) | 343 (20.9%) | 157 (14.3) | 28 (15.2) | 25 (18.4%) | 148 (67.3%) | 86 (69.9%) |
| HLA-Dr mismatcha,j | |||||||
| 0 Mismatch | 1315 (38.8) | 710 (43.2%) | 417 (38.4%) | 107 (58.2%) | 44 (32.6%) | 31 (14.1%) | 6 (4.9%) |
| 1 Mismatch | 1778 (52.4) | 806 (49.0%) | 650 (59.9%) | 74 (40.2%) | 86 (63.7%) | 99 (45.0%) | 63 (51.2%) |
| 2 Mismatches | 298 (8.8) | 128 (7.8%) | 18 (1.7%) | 3 (1.6%) | 5 (3.7%) | 90 (40.9%) | 54 (43.9%) |
| Cause of renal failurea | |||||||
| Polycystic kidney disease | 509 (14.2%) | 241 (13.9%) | 188 (16.3%) | 17 (9.0%) | 21 (14.6%) | 29 (11.8%) | 13 (9.5%) |
| GN | 791 (22.0%) | 374 (21.6%) | 305 (26.5%) | 31 (16.5%) | 24 (16.7%) | 30 (12.2%) | 27 (19.7%) |
| Renal vascular disease | 586 (16.3%) | 252 (14.6%) | 161 (14.0%) | 41 (21.8%) | 28 (19.4%) | 73 (29.8%) | 31 (22.6%) |
| Diabetes | 478 (13.3%) | 270 (15.6%) | 11 (9.5%) | 19 (10.1%) | 21 (14.6%) | 34 (13.9%) | 24 (17.5%) |
| Chronic renal failure, etiology unknown | 542 (15.1%) | 246 (14.2%) | 163 (14.1%) | 43 (22.9%) | 24 (16.7%) | 46 (18.8%) | 20 (14.6%) |
| Pyelonephritis | 255 (7.1%) | 138 (8.0%) | 80 (6.9%) | 15 (8.0%) | 9 (6.3%) | 7 (2.9%) | 6 (4.4%) |
| Other | 436 (12.1%) | 209 (12.1%) | 146 (12.7%) | 22 (11.7%) | 17 (11.8%) | 26 (10.6%) | 16 (11.7%) |
Results presented are the median (interquartile range) or N (%). For the group differences test, continuous variables are tested with the Kruskal–Wallis, categoric variables are tested with the Fisher exact test.
Group differences test P<0.01. MDRD, Modification of Diet in Renal Disease.
219 missing values.
2 missing values.
20 missing values.
303 missing values.
115 missing values.
115 missing values and excluding all pre-emptive transplantations (n=161).
192 missing values.
192 missing values.
206 missing values.
Comparison of Transplant Outcomes
Table 2 shows the observed incidence of transplant outcomes for each patient group. Corresponding multivariate analyses are shown in Table 3. In elderly recipients, the primary nonfunction (PNF) rate was 12.4% for elderly DCD kidneys, 9.8% for young DCD kidneys, and 8.0% for young DBD kidneys, with a notably small incidence of 5.7% for elderly DBD kidneys. Using the elderly recipients of young DBD kidneys as the reference group, adjusted logistic regression indicated no differences in PNF between the subpopulations of elderly transplant recipients.
Table 2.
Clinical outcomes of kidney transplantations
| Clinical Outcomes | All Renal Allografts | Recipients <65 yr | Recipients ≥65 yr | ||||
|---|---|---|---|---|---|---|---|
| Young Donor (<65 yr) DBD | Young Donor (<65 yr) DCD | Young Donor (<65 yr) DBD | Young Donor (<65 yr) DCD | Elderly Donor (≥65 yr) DBD | Elderly Donor (≥65 yr) DCD | ||
| PNF (Yes)a | 259 (7.2%) | 91 (5.3%) | 108 (9.4%) | 15 (8.0%) | 14 (9.8%) | 14 (5.7%) | 17 (12.4%) |
| DGF (Yes)b | 1188 (37.0%) | 306 (19.4%) | 619 (61.7%) | 29 (17.1%) | 87 (69.6%) | 64 (29.2%) | 83 (74.1%) |
| Acute rejection within 3 mo (Yes)c,d | 618 (17.5%) | 287 (17.9%) | 206 (17.9%) | 17 (9.0%) | 13 (9.0%) | 33 (13.5%) | 31 (22.6%) |
| 1-Yr graft survival (%)e,f | 85.8 (84.6 to 87.0) | 88.7 (87.1 to 90.1) | 85.6 (83.4 to 87.5) | 81.9 (75.5 to 86.8) | 79.6 (71.9 to 85.3) | 79.5 (73.7 to 84.1) | 75.3 (67.1 to 81.8) |
| 1-Yr death-censored graft survival (%)e,f | 90.1 (89.1 to 91.1) | 91.9 (90.6 to 93.2) | 88.6 (86.7 to 90.4) | 91.3 (86.7 to 94.8) | 85.3 (79.0 to 90.5) | 89.1 (84.8 to 92.7) | 85.3 (78.7 to 90.6) |
| 1-Yr patient survival (%)e,g | 94.3 (93.4 to 95.0) | 95.8 (94.7 to 96.7) | 95.9 (94.5 to 96.9) | 88.9 (84.4 to 93.5) | 87.7 (81.1 to 92.2) | 88.3 (83.4 to 91.7) | 85.5 (78.2 to 90.5) |
| 5-Yr graft survival (%)e,f | 70.6 (68.9 to 72.2) | 75.4 (73.1 to 77.6) | 72.6 (69.6 to 75.3) | 61.5 (53.3 to 68.7) | 61.9 (52.3 to 70.1) | 52.7 (44.4 to 60.3) | 39.7 (30.0 to 49.3) |
| 5-Yr death-censored graft survival (%)e,f | 84.3 (82.9 to 85.5) | 85.9 (84.1 to 87.6) | 82.6 (80.2 to 84.9) | 87.7 (82.2 to 92.2) | 82.3 (75.4 to 88.3) | 83.8 (78.2 to 88.6) | 75.3 (66.9 to 83.0) |
| 5-Yr patient survival (%)e,g | 79.8 (78.2 to 81.2) | 84.4 (82.3 to 86.2) | 84.2 (81.5 to 86.4) | 68.7 (60.6 to 75.4) | 68.6 (59.2 to 76.3) | 55.0 (46.9 to 62.4) | 50.9 (40.3 to 60.6) |
95% CIs are shown in parentheses.
Data available for 3573 patients, after excluding 24 missing values.
Data available for 3209 patients, after excluding PNF (n=259) and missing values (n=129).
Defined as: no=0 acute rejections; yes= ≥1 acute rejection within 3 months.
Data available for 3539 patients (n=58).
Data available for 3575 patients, after excluding 22 missing values.
Cumulative proportional survival percentages with 95% CIs are shown from cumulative incidence competing risks estimates.
Cumulative proportional survival percentages with 95% CIs are shown from the Kaplan–Meier estimates.
Table 3.
Comparison analyses of transplant outcomes
| Transplant Outcomes | Recipients <65 yr | Recipients ≥65 yr | ||||
|---|---|---|---|---|---|---|
| Young Donor (<65 yr) DBD | Young Donor (<65 yr) DCD | Young Donor (<65 yr) DBD | Young Donor (<65 yr) DCD | Elderly Donor (≥65 yr) DBD | Elderly Donor (≥65 yr) DCD | |
| PNFa | 0.68 (0.34 to 1.36) | 1.34 (0.68 to 2.64) | 1 (Ref) | 1.12 (0.44 to 2.84) | 0.76 (0.31 to 1.87) | 1.93 (0.81 to 4.58) |
| Adjusted model | 0.61 (0.30 to 1.23) | 1.28 (0.64 to 2.56) | 1 (Ref) | 0.98 (0.38 to 2.54) | 0.48 (0.18 to 1.28) | 1.18 (0.45 to 3.06) |
| DGFb | 1.16 (0.75 to 1.80) | 6.94 (4.47 to 10.77)c | 1 (Ref) | 10.45 (5.86 to 18.64)c | 1.77 (1.05 to 2.98)c | 13.94 (7.44 to 26.12)c |
| Adjusted model | 1.08 (0.69 to 1.69) | 7.09 (4.50 to 11.17)c | 1 (Ref) | 10.43 (5.75 to 18.91)c | 1.84 (1.04 to 3.26)c | 14.87 (7.47 to 29.61)c |
| Acute rejection within 3 mod | 1.52 (0.89 to 2.57) | 1.94 (1.14 to 3.30)c | 1 (Ref) | 0.86 (0.39 to 1.89) | 1.35 (0.71 to 2.56) | 3.19 (1.64 to 6.21)c |
| Adjusted model | 1.43 (0.84 to 2.44) | 1.79 (1.04 to 3.08)c | 1 (Ref) | 0.80 (0.36 to 1.76) | 1.24 (0.62 to 2.46) | 2.78 (1.35 to 5.73)c |
| 1-Yr graft failuree | 0.65 (0.43 to 1.00) | 0.73 (0.46 to 1.15) | 1 (Ref) | 1.23 (0.70 to 2.16) | 1.18 (0.71 to 1.95) | 1.52 (0.88 to 2.63) |
| Adjusted model | 0.62 (0.40 to 0.95)c | 0.77 (0.50 to 1.19) | 1 (Ref) | 1.14 (0.65 to 2.00) | 0.98 (0.56 to 1.70) | 1.26 (0.69 to 2.31) |
| 1-Yr death-censored graft failuree | 1.08 (0.58 to 2.01) | 1.44 (0.77 to 2.68) | 1 (Ref) | 1.85 (0.86 to 3.98) | 1.33 (0.63 to 2.80) | 1.98 (0.91 to 4.31) |
| Adjusted model | 0.99 (0.53 to 1.86) | 1.40 (0.75 to 2.63) | 1 (Ref) | 1.70 (0.79 to 3.68) | 1.01 (0.46 to 2.24) | 1.48 (0.64 to 3.42) |
| 1-Yr mortalityf | 0.47 (0.32 to 0.67)c | 0.52 (0.37 to 0.74)c | 1 (Ref) | 1.10 (0.70 to 1.74) | 1.34 (0.91 to 1.99) | 1.64 (1.07 to 2.53)c |
| Adjusted model | 0.49 (0.34 to 0.71)c | 0.52 (0.37 to 0.74)c | 1 (Ref) | 1.11 (0.70 to 1.76) | 1.26 (0.82 to 1.96) | 1.77 (1.09 to 2.86)c |
| 5-Yr graft failuree | 0.70 (0.51 to 0.95)c | 0.66 (0.49 to 0.89)c | 1 (Ref) | 1.08 (0.72 to 1.64) | 1.26 (0.88 to 1.82) | 1.84 (1.25 to 2.71)c |
| Adjusted model | 0.65 (0.46 to 0.91)c | 0.72 (0.51 to 1.01) | 1 (Ref) | 1.03 (0.68 to 1.57) | 1.00 (0.67 to 1.49) | 1.49 (0.97 to 2.29) |
| 5-Yr death-censored graft failuree | 1.41 (0.84 to 2.34) | 1.22 (0.73 to 2.04) | 1 (Ref) | 1.52 (0.78 to 2.96) | 1.44 (0.78 to 2.68) | 2.67 (1.20 to 4.30)c |
| Adjusted model | 1.38 (0.82 to 2.32) | 1.14 (0.68 to 1.91) | 1 (Ref) | 1.37 (0.70 to 2.67) | 1.03 (0.54 to 2.00) | 1.57 (0.79 to 3.11) |
| 5-Yr mortalityf | 0.45 (0.32 to 0.65)c | 0.50 (0.35 to 0.71)c | 1 (Ref) | 1.13 (0.72 to 1.78) | 1.48 (1.00 to 2.19)c | 1.80 (1.17 to 2.76)c |
| Adjusted model | 0.48 (0.33 to 0.69)c | 0.49 (0.35 to 0.70)c | 1 (Ref) | 1.15 (0.73 to 1.83) | 1.35 (0.87 to 2.09) | 1.86 (1.15 to 3.02)c |
In adjusted models, the following variables were taken into account: donor sex, recipient sex, donor hypotensive period, donor terminal Modification of Diet in Renal Disease, donor smoking, CIT, HLA-A mismatch, HLA-B mismatch, HLA-Dr mismatch, recipient original disease, and recipient dialysis vintage.
Data available for 2850 patients.
Data available for 2668 patients.
ORs or HRs with P<0.05.
Data available for 2667 patients.
Data available for 2850 patients.
Data available for 2824 patients.
Presented are ORs (95% CI).
Presented are HRs (95% CI).
Elderly recipients of elderly DCD kidneys experienced the highest rate of DGF (74.1%), followed by 69.6% among elderly recipients of young DCD kidneys. Logistic regression analysis showed an adjusted odds ratio (OR) for DGF of 10.43 (95% confidence interval [95% CI], 5.75 to 18.97; P<0.001) and 14.87 (95% CI, 7.47 to 29.61; P<0.001) for elderly recipients receiving DCD kidneys from young or elderly donors, respectively, using elderly recipients of young DBD kidneys as the reference group.
Elderly recipients of elderly DCD donor grafts had the highest rejection rate (23.9%), whereas elderly recipients of young DBD kidneys had the lowest rejection rate (10.0%). Logistic regression analysis of the two groups showed an adjusted OR of 2.78 (95% CI, 1.35 to 5.73; P=0.01). Interaction analyses revealed that within elderly recipients, the combined effect of an elderly donor with DCD on the risk of acute rejection was 2.8 times higher (adjusted OR, 2.83; 95% CI, 1.05 to 7.59; P=0.04) than the sum of separate effects of elderly donation and DCD donation on acute rejection.
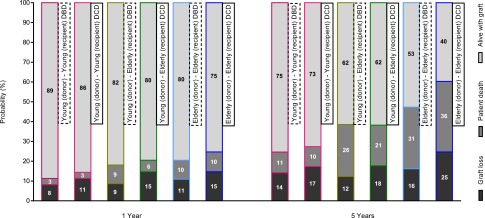
Figure 1 illustrates 1-year and 5-year graft survival according to donor-recipient group, including the proportion of graft failures attributable to graft loss and return to dialysis, or to patient death (see Supplemental Material for stacked survival curves within each group). Only 39.7% of elderly recipients of elderly DCD kidneys were alive with a functioning graft 5 years after transplantation compared with 52.7% of elderly recipients of elderly DBD kidneys. Among elderly recipients of grafts from young donors, 61.9% with a DCD donor and 61.5% with a DBD donor were alive with a functioning graft at 5 years. After adjusting for confounders, Cox regression analysis showed that graft failure at 5 years did not differ significantly between elderly recipients of elderly DCD kidneys compared with elderly recipients of young DBD kidneys (hazard ratio [HR], 1.49; 95% CI, 0.97 to 2.29; P=0.07). Similarly, the adjusted risk for death-censored graft loss at 5 years was not significantly different between elderly recipients of elderly DCD kidneys and young DBD kidneys (HR, 1.57; 95% CI, 0.79 to 3.11; P=0.20).
Figure 1.
One-year and 5-year graft survival according to recipient-donor group. Graft events are shown as (A) graft loss and return to dialysis, (B) patient death, or (C) alive with functioning graft.
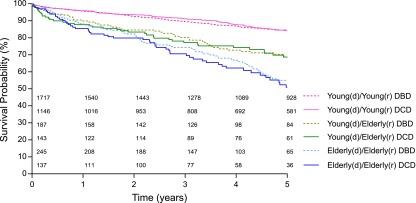
Figure 2 presents 5-year patient survival rates in the different recipient-donor groups. Among elderly patients, survival at 5 years was lowest for recipients of elderly DCD kidneys (50.9%), compared with elderly DBD (55.0%), young DCD (68.6%), or young DBD (68.7%) kidneys. The adjusted HR for 5-year mortality was significantly higher for elderly recipients of elderly DCD kidneys versus young DBD kidneys (HR, 1.86; 95% CI, 1.15 to 3.02; P=0.01). This increased risk was already evident by 1 year post-transplant, at which point the adjusted HR was 1.77 (95% CI, 1.09 to 2.86; P=0.02). Left-truncated survival analysis indicated that the unadjusted median survival age for elderly recipients of elderly DCD kidneys was 3.7 years shorter than that of elderly recipients of young DBD kidneys (P=0.001) (see Supplemental Material).
Figure 2.
Five-year patient survival according to recipient-donor group.
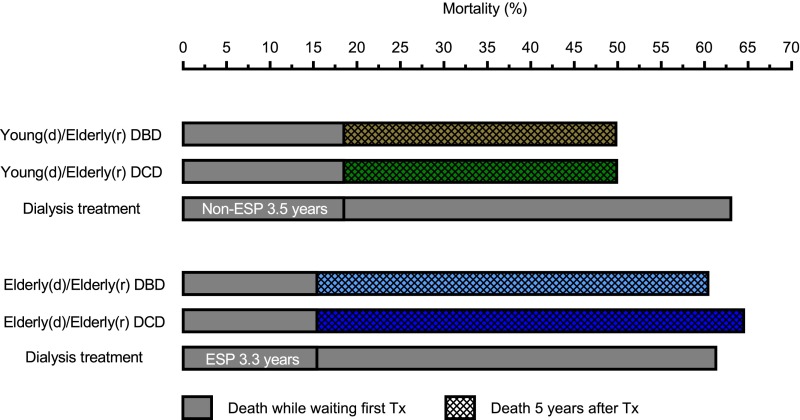
Figure 3 shows mortality rates among elderly patients from the point at which they started dialysis treatment and were waitlisted for first transplantation, with inclusion of the median waiting times for either an ESP or non-ESP donor kidney plus 5 years post-transplant, or during equivalent follow-up times for waitlisted patients remaining on dialysis. From the start of dialysis treatment until 5 years after transplantation, mortality was slightly higher in elderly recipients of elderly DCD kidneys (64.5%) versus elderly DBD kidneys (60.4%). If elderly patients remained on dialysis instead of being transplanted with an ESP donor kidney, mortality was comparable (61.3%). For elderly recipients of young DBD kidneys, mortality from the start of dialysis to 5 years post-transplant was virtually identical to that of elderly recipients of young DCD kidneys (49.8% and 49.9%, respectively). If these patients remained on dialysis instead of being transplanted with a non-ESP donor kidney, mortality was higher (63.0%).
Figure 3.
Mortality of elderly patients from start of dialysis treatment and active registration on the waiting list for first transplantation, with inclusion of the median waiting times for either an ESP or non-ESP donor kidney plus 5 years post-transplant, or during equivalent follow-up times for waitlisted elderly patients remaining on dialysis treatment. Waiting time for transplanted patients was equal to dialysis vintage. Unadjusted mortality rates are shown. d, donor; r, recipient; Tx, transplantation.
Table 4 shows eGFR stages at 1 year after transplantation according to recipient-donor group. Including patients with graft loss and PNF, 63.8% of elderly recipients of an elderly DCD kidney had eGFR<30 ml/min per 1.73 m2 compared with 45.5% of elderly recipients of an elderly DBD graft (P=0.02). Renal function was significantly better in elderly recipients of either young DBD or young DCD kidneys, with 25.8% and 26.4% having eGFR<30 ml/min per 1.73 m2, respectively (P<0.001 for both categories versus elderly recipients of elderly DCD). The incidence of eGFR<30 ml/min per 1.73 m2 in elderly recipients of young DBD or DCD grafts was comparable to that of young recipients of young DBD or DCD kidneys (25.9% and 34.9%, respectively).
Table 4.
Kidney Disease Improving Global Outcomes stages of CKD at 1 year post-transplantation with exclusion and inclusion of PNF and graft loss within timeframe
| Variables | All Renal Allografts | Recipients<65 yr | Recipients≥65 yr | ||||
|---|---|---|---|---|---|---|---|
| Young Donor (<65 yr) DBD | Young Donor (<65 yr) DCD | Young Donor (<65 yr) DBD | Young Donor (<65 yr) DCD | Elderly Donor (≥65 yr) DBD | Elderly Donor (≥65 yr) DCD | ||
| Exclusion of PNF and graft loss within timeframe | |||||||
| eGFR after 1 yra,b | |||||||
| Stage 1 & 2; eGFR≥60 | 741 (23.4%) | 414 (29.4%) | 219 (24.1%) | 48 (33.1%) | 39 (37.1%) | 18 (9.9%) | 3 (3.1%) |
| Stage 3A; eGFR≤59 & eGFR≥45 | 582 (18.4%) | 299 (21.3%) | 190 (20.9%) | 36 (24.8%) | 19 (18.1%) | 26 (14.4%) | 12 (12.4%) |
| Stage 3B; eGFR≤44 & eGFR≥30 | 844 (26.7%) | 419 (29.8%) | 261 (28.8%) | 37 (25.5%) | 34 (32.4%) | 66 (36.5%) | 27 (27.8%) |
| Stage 4; eGFR≤29 & eGFR≥15 | 579 (18.3%) | 239 (17.0%) | 203 (22.4%) | 22 (15.2%) | 8 (7.6%) | 59 (32.6%) | 48 (49.5%) |
| Stage 5; eGFR≤14 | 95 (13.2%) | 35 (2.5%) | 34 (3.7%) | 2 (1.4%) | 5 (4.8%) | 12 (6.6%) | 7 (7.2%) |
| Difference with elderly DBD donor | Na | <0.001 | <0.001 | <0.001 | <0.001 | Na | 0.019 |
| Difference with elderly DCD donor | Na | <0.001 | <0.001 | <0.001 | <0.001 | 0.019 | Na |
| Including PNF and graft loss within timeframe | |||||||
| eGFR after 1 yrb,c | |||||||
| Stage 1 & 2; eGFR≥60 | 741 (26.1%) | 414 (27.1%) | 219 (21.3%) | 48 (29.4%) | 39 (31.2%) | 18 (8.9%) | 3 (2.6%) |
| Stage 3A; eGFR≤59 & eGFR≥45 | 582 (20.5%) | 299 (19.6%) | 190 (18.4%) | 36 (22.1%) | 19 (15.2%) | 26 (12.9%) | 12 (10.3%) |
| Stage 3B; eGFR≤44 & eGFR≥30 | 844 (29.7%) | 419 (27.4%) | 261 (25.3%) | 37 (22.7%) | 34 (27.2%) | 66 (32.7%) | 27 (23.3%) |
| Stage 4; eGFR≤29 & eGFR≥15 | 579 (20.4%) | 239 (15.7%) | 203 (19.7%) | 22 (13.5%) | 8 (6.4%) | 59 (29.2%) | 48 (41.4%) |
| Stage 5; eGFR≤14 | 417 (3.3%) | 156 (10.2%) | 157 (15.2%) | 20 (12.3%) | 25 (20.0%) | 33 (16.3%) | 26 (22.4%) |
| Difference with elderly DBD donor | Na | <0.001 | <0.001 | <0.001 | <0.001 | Na | 0.037 |
| Difference with elderly DCD donor | Na | <0.001 | <0.001 | <0.001 | <0.001 | 0.037 | Na |
When included, patients with PNF are listed in CKD Stage 5. eGFR units shown as ml/min per 1.73 m2. Na, not applicable.
Data available for 2841 patients.
Indicates P<0.01; tested with the Fisher exact test.
Data available for 3163 patients.
Discussion
To our knowledge, this is the first study to evaluate outcomes after renal transplantation in elderly patients receiving deceased-donor grafts from either young (<65 years) or elderly (≥65 years) donors. In elderly patients, engraftment from elderly DCD donors resulted in an increased mortality risk compared with kidneys from young DBD donors, an effect that was apparent by 1 year after transplantation. The unadjusted median survival age for elderly recipients was 3.7 years longer when receiving a graft from a young DBD donor versus an elderly DCD donor. Our data suggest that elderly recipients of kidneys from elderly donors do not experience a survival benefit compared with remaining waitlisted on dialysis. Renal function was lower in elderly recipients receiving a graft from an elderly donor compared with a young donor, particularly for elderly DCD donors. Strikingly, the incidence of acute rejection in elderly recipients of elderly DBD kidneys was similar to that for young DBD kidneys, but higher in elderly recipients of elderly DCD kidneys.
A number of studies have provided data on graft survival after donation from an older donor, but most exclusively analyzed either DBD16–25 or DCD kidneys.26 Frei and colleagues concluded that allocation of kidneys within the ESP did not negatively affect graft and patient survival when compared with regular allocation of grafts from donors aged ≥60 years to recipients aged 60–64 years.16 However, their study did not compare ESP-allocated grafts with outcomes in elderly recipients of grafts from donors <65 years, because too few of these recipients were available in their analysis, and no DCD kidneys were included. Summers and colleagues compared several transplant outcomes after engraftment of 1768 DCD kidneys versus 4127 DBD kidneys in the UK while investigating the effect of high donor age and increased CIT.13 They concluded that higher donor age was associated with increased graft loss, and that this was equivalent for DCD and DBD transplants within donor age groups. We also observed an increased risk of death in elderly patients receiving kidneys from elderly DBD or DCD donors compared with younger donors, with a particular increase in risk when the kidney was from an elderly DCD donor. Our study design differs in two important aspects. Firstly, in accordance with ESP allocation, senior donors were defined as aged ≥65 years, as opposed to ≥60 years in the UK study.13 Next, our data specifically examine the effect of allocation of kidneys from elderly donors to elderly recipients.
The ESP allocation system aims to achieve short ischemia times through local allocation without HLA matching. DBD kidneys allocated via ESP had the shortest CIT. Interestingly, this was not achieved for DCD grafts, because CIT was not shorter than for kidneys allocated via the regular Eurotransplant Kidney Allocation System. A reluctance by transplant centers to accept DCD kidneys from elderly donors may play a role in delaying their allocation. Prolonged CIT, and a greater number of HLA mismatches, may contribute to the relatively high PNF rate in elderly recipients receiving elderly DCD grafts, and the poorer graft and patient survival with higher risk for DGF and/or acute rejection.27 It is likely that DCD kidneys from elderly donors are less resilient to ischemia-mediated damage.28
Strengths of our study are the national cohort of recipients with large numbers in each of the age and donor categories, a high-quality database with good follow up, and availability of data on mortality after graft loss. Some limitations should be considered. We were not able to account for center-specific differences in demographics, treatment, or outcomes. Patients with panel reactive antibodies are not eligible for the ESP, biasing the distribution of immunized elderly recipients in our cohort. However, this bias would be expected to favor outcomes in ESP recipients and therefore does not explain our results. We could not verify whether comorbidities at the time of transplantation were comparable between subgroups of elderly recipients. However, Dutch transplant centers generally list all eligible elderly patients for both regular allocation and for the ESP program. The outcome of mortality on the waiting list was not proportional over time and is slightly biased (immortal time bias). Because active registration on the waiting list may occur later than initiation of dialysis treatment, mortality may be underestimated. However, to avoid overestimation, we compared transplanted patients with patients on dialysis who were actively waitlisted, and thus considered in a healthy state for transplantation. The cut-off values for donor and recipient age may not apply to the allocation policy in countries outside the Eurotransplant region. Nevertheless, our results may be helpful when considering future changes to organ allocation systems.
ESP allocation has clear merits. It has reduced waiting times for elderly recipients and has increased the availability of grafts from younger donors for recipients <65 years.16 Eurotransplant outcomes compare favorably with those from the United States, as shown in a recent study examining the outcome of DBD kidneys from donors aged ≥65 years.22 Snoeijs and colleagues concluded that there was no clear advantage for kidney transplantation from a DBD or DCD expanded criteria donor (ECD) compared with continuing dialysis treatment and waiting for transplantation from a standard criteria DBD donor.29 However, ECD grafts may still provide a survival advantage in elderly recipients if waiting times were substantially shorter than waiting for a standard criteria donor.1,30 Elderly recipients of deceased-donor kidneys from a younger donor only waited for 3–4 months more than for an ESP donor kidney. This small difference in waiting time is explained by the competitive possibility of an ESP offer for the majority of elderly patients. If elderly patients chose to only be listed for regular allocation, their expected waiting time would be similar to the younger patient group i.e., 12 months longer than that for the elderly recipients of ESP donor kidneys.
Instead of abandoning the allocation of elderly donors and extending waiting times, efforts should be made to increase safe utilization of grafts from elderly donors. Decreasing CIT,13 use of machine perfusion,31 and, possibly, histologic evaluation of allografts before transplantation,32 might improve the quality and selection of elderly donors, contributing to better outcomes while maintaining the donor pool. Additionally, reintroducing HLA matching when allocating elderly DCD donor kidneys may reduce rejection rates.
In conclusion, our study shows that acceptance of elderly DCD kidneys for elderly recipients is associated with an increased mortality risk. Elderly recipients of DCD kidneys from elderly donors experience the additional burdens of increased DGF and acute rejection, with inferior renal function. As survival rates for elderly patients on dialysis improve, there is no apparent survival benefit for transplantation when engrafting kidneys from increasingly elderly donors and ECDs to elderly recipients. Optimizing organ quality might close the gap between survival outcomes after transplantation from elderly deceased donors versus standard criteria donors. Patients should be counseled about their survival prospects when accepting standard or expanded donation criteria grafts and given the opportunity to participate in decision-making.
Concise Methods
Study Population
The Dutch Organ Transplantation Registry records kidney transplantation follow-up data from all eight transplantation centers in The Netherlands, including the date of recipient death after allograft failure. We included recipients (n=3654), aged ≥18 years, of a first kidney from a DBD donor or a DCD category 3 donor (donation after controlled circulatory death)33 between January 5, 2002 and January 5, 2012. We excluded 57 transplantations in which kidneys from donors aged ≥65 years were transplanted to recipients <65 years, because these constituted exceptional rescue allocations and numbers were too low to draw firm conclusions. Patients were followed for at least 6 months, with a final follow-up date of July 5, 2012. Additionally, we used the Dutch Renal Replacement Registry database to compare patient survival of elderly patients (≥65 years; n=504) who were waitlisted between January 1, 2000 and January 1, 2010 for a first kidney transplant. In this survival analysis, patients on dialysis were censored on the transplant date, and the last follow-up date was January 1, 2014.
Outcome Measures
We evaluated 1- and 5-year graft survival, defined as patient death or graft loss leading to dialysis treatment (whichever came first), and death-censored graft survival. Additionally, we included the date of death for patients who experienced graft loss to analyze patient survival. Other outcomes were PNF (defined as a graft that never functioned in a recipient who lived for at least 10 days after transplantation), DGF (defined as the need for dialysis within 7 days after transplantation), 1-year renal function (expressed as eGFR in ml/min per 1.73 m2, categorized according to the Kidney Disease Improving Global Outcomes stages of CKD34), and acute rejection (defined as at least one rejection treatment administered within 3 months after transplantation, excluding PNF).
Data Analyses
In accordance with allocation practice in The Netherlands, we defined donors and recipients below the age of 65 years as young and donors and recipients of 65 years and older as elderly. Donors were also categorized as DCD or DBD. The six categories analyzed were: recipient <65 years, DBD donor <65 years; recipient <65 years, DCD donor <65 years; recipient ≥65 years, DCD donor <65 years; recipient ≥65 years, DBD donor <65 years; recipient ≥65 years, DBD donor ≥65 years; and recipient ≥65 years, DCD donor ≥65 years.
Continuous variables are presented as median±interquartile range. We compared continuous variables across groups using one-way Kruskal–Wallis statistics and two-tailed Fisher exact statistics for categoric variables. Cumulative incidence competing risk functions were used to calculate unadjusted incidences of 5-year graft survival, and to take into account the competing events of patient death and graft loss.35 We used Kaplan–Meier curves to estimate patient survival. To assess the median survival age for elderly patients, including death after graft loss, we plotted Kaplan–Meier curves using age at transplantation of the elderly recipient as a left-truncated point (plotted in R version 3.1.136), which means that subjects enter the risk-set at their age at time of transplantation.35 It uses recipient age as the time-axis and accounts for delayed entry of older recipients (e.g., ≥70 years). We performed multiple logistic regression analysis to take into account possible confounders in the association between the six categories (kidneys transplanted from donors ≥65 years to recipients <65 years were excluded) and PNF, DGF, and acute rejection within 3 months post-transplant. The following were considered as potential confounders: (1) donor gender; (2) recipient gender; (3) donor hypotensive period (no/yes); (4) terminal eGFR (Modification of Diet in Renal Disease) of the donor; (5) donor smoking; (6) CIT; (7) HLA-A, HLA-B, and HLA-DR mismatch levels; (8) dialysis vintage (years); and (9) recipient's original disease. We categorized CIT (<12 hours [reference]; ≥12 hours and <18 hours; ≥18 hours and <24 hours; ≥24 hours) and mismatch levels (0; 1; 2 [reference]) for HLA-A, HLA-B, and HLA-DR separately. Recipient primary disease leading to renal failure was categorized (polycystic kidney disease [reference]; GN; renal vascular disease; diabetes; chronic renal failure [etiology unknown]; pyelonephritis; other). We also adjusted for these factors in cause-specific Cox-regression analysis. The hazards were proportional over time. We tested this by defining the six categories as a function of the time variable, which we divided into two equal periods (first versus last follow-up period).
Results are presented as OR or HR values with 95% CIs, using the most clinically favorable category for recipients aged ≥65 years as the reference category, which corresponds to a DBD donor type and donor age <65 years. The presence of significance of interaction was also evaluated, by adding an interaction term (donor and recipient pair × donor type). An interaction OR or HR of 1 indicates no interaction on a multiplicative scale.
Significance levels were set at the 5% level. Analyses were conducted using R (version 3.1.1)36 with the rms package (version 4.1–1-1) and the cmprsk package (version 2.2–7). Kaplan–Meier curves were plotted using GraphPad Prism (version 5.0; GraphPad Software, La Jolla, CA).
Disclosures
None.
Supplementary Material
Acknowledgments
H.P.S. and F.J.B. had full access to all of the data in the study and take responsibility for the integrity and the accuracy of the data analysis. H.P.S., S.P.B.H., J.J.H.H., and F.J.B. designed the study and all authors contributed to the data interpretation. H.P.S., S.P.B.H., and F.J.B. performed the analyses. H.P.S., S.P.B.H., and F.J.B. drafted the initial report, which all authors critically reviewed. All authors gave approval for publication.
On November 28, 2014, the data were presented in an oral communication session, held in Parma, Italy, at a meeting of the European Renal Association – European Dialysis and Transplant Association working group. The data were presented in an oral presentation at the Joint British Transplantation Society–Dutch Transplantation Society congress in Bournemouth, UK, March 11–13 2015. Furthermore, the data were partially presented as a poster during the American Transplant Congress in Philadelphia, PA, May 2–6, 2015.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015080879/-/DCSupplemental.
References
- 1.Rao PS, Merion RM, Ashby VB, Port FK, Wolfe RA, Kayler LK: Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation 83: 1069–1074, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Heldal K, Hartmann A, Grootendorst DC, de Jager DJ, Leivestad T, Foss A, Midtvedt K: Benefit of kidney transplantation beyond 70 years of age. Nephrol Dial Transplant 25: 1680–1687, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK: Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 294: 2726–2733, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM: Expanded criteria donors for kidney transplantation. Am J Transplant 3[Suppl 4]: 114–125, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Cecka JM, Cohen B, Rosendale J, Smith M: Could more effective use of kidneys recovered from older deceased donors result in more kidney transplants for older patients? Transplantation 81: 966–970, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Dutch Transplant Foundation (In Dutch: Nederlandse Transplantatie Stichting). Annex of stats and figures 2011 (In Dutch: Cijferbijlage 2011). Available at: http://www.transplantatiestichting.nl/winkel/jaarverslagen. Accessed: June 4, 2014
- 7.Dutch Transplant Foundation (In Dutch: Nederlandse Transplantatie Stichting). Annex of stats and figures 2012 (In Dutch: Cijferbijlage 2012). Available at: http://www.transplantatiestichting.nl/winkel/jaarverslagen. Accessed June 4, 2014
- 8.Nederlandse Transplantatie Stichting. Annex of stats and figures 2013 (In Dutch: Cijferbijlage 2013). Available at: http://www.transplantatiestichting.nl/winkel/jaarverslagen. Accessed: June 4, 2014
- 9.Domínguez-Gil B, Haase-Kromwijk B, Van Leiden H, Neuberger J, Coene L, Morel P, Corinne A, Muehlbacher F, Brezovsky P, Costa AN, Rozental R, Matesanz R; European Committee (Partial Agreement) on Organ Transplantation. Council of Europe (CD-P-TO) : Current situation of donation after circulatory death in European countries. Transpl Int 24: 676–686, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Morrissey PE, Monaco AP: Donation after circulatory death: current practices, ongoing challenges, and potential improvements. Transplantation 97: 258–264, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Snoeijs MGJ, Winkens B, Heemskerk MBA, Hoitsma AJ, Christiaans MH, Buurman WA, van Heurn LW: Kidney transplantation from donors after cardiac death: a 25-year experience. Transplantation 90: 1106–1112, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Summers DM, Johnson RJ, Allen J, Fuggle SV, Collett D, Watson CJ, Bradley JA: Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet 376: 1303–1311, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Summers DM, Johnson RJ, Hudson A, Collett D, Watson CJ, Bradley JA: Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study. Lancet 381: 727–734, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Eurotransplant International Foundation. Eurotransplant Manual Chapter 4 Kidney (ETKAS and ESP, version 3). Available at: https://www.eurotransplant.org/cms/index.php?page=et_manual. Accessed: April 8, 2015
- 15.Eurotransplant International Foundation. Annual Report 2013. Available at: https://www.eurotransplant.org/cms/mediaobject.php?file=AR20135.pdf. Accessed: May 10, 2014
- 16.Frei U, Noeldeke J, Machold-Fabrizii V, Arbogast H, Margreiter R, Fricke L, Voiculescu A, Kliem V, Ebel H, Albert U, Lopau K, Schnuelle P, Nonnast-Daniel B, Pietruck F, Offermann R, Persijn G, Bernasconi C: Prospective age-matching in elderly kidney transplant recipients--a 5-year analysis of the Eurotransplant Senior Program. Am J Transplant 8: 50–57, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Stratta RJ, Sundberg AK, Rohr MS, Farney AC, Hartmann EL, Roskopf JA, Iskandar SS, Hairston G, Kiger DF, Gautreaux MD, Anderson TK, Adams PL: Optimal use of older donors and recipients in kidney transplantation. Surgery 139: 324–333, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Foss A, Heldal K, Scott H, Foss S, Leivestad T, Jørgensen PF, Scholz T, Midtvedt K: Kidneys from deceased donors more than 75 years perform acceptably after transplantation. Transplantation 87: 1437–1441, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Galeano C, Marcén R, Jimenez S, Fernández Rodríguez A, Sosa H, Villafruela JJ, Teruel JL, Burgos FJ, Quereda C: Utilization of elderly kidney donors (>70 years) does not affect graft survival in the medium term. Transplant Proc 42: 3935–3937, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Lai Q, Nudo F, Levi Sandri GB, Melandro F, Ferretti S, Grieco M, Garofalo M, Poli L, Pretagostini R, Berloco PB: Survival after kidney transplantation does not differ with 50-59- or over 60-year-old expanded-criteria donors. Transplant Proc 43: 1030–1032, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Marconi L, Figueiredo A, Campos L, Nunes P, Roseiro A, Parada B, Mota A: Renal transplantation with donors older than 70 years: does age matter? Transplant Proc 45: 1251–1254, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Rose C, Schaeffner E, Frei U, Gill J, Gill JS: A Lifetime of Allograft Function with Kidneys from Older Donors. J Am Soc Nephrol 26: 2483–2493, 2015 [DOI] [PMC free article] [PubMed]
- 23.Sener A, Schweitzer EJ, Munivenkatappa R, Cooper M, Bartlett ST, Philosophe B, Barth RN: Deceased-donor renal transplantation in the geriatric population demonstrates equal graft survival compared with younger recipients. Transplantation 87: 1549–1554, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Mezrich JD, Pirsch JD, Fernandez LA, Foley DP, Bellingham JM, Odorico JS, Leverson GE, Munoz-Del-Rio A, Sollinger HW, Kaufman DB, D’Alessandro AM: Differential outcomes of expanded-criteria donor renal allografts according to recipient age. Clin J Am Soc Nephrol 7: 1163–1171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chavalitdhamrong D, Gill J, Takemoto S, Madhira BR, Cho YW, Shah T, Bunnapradist S: Patient and graft outcomes from deceased kidney donors age 70 years and older: an analysis of the Organ Procurement Transplant Network/United Network of Organ Sharing database. Transplantation 85: 1573–1579, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Thornton SR, Hamilton N, Evans D, Fleming T, Clarke E, Morgan J, Kadi N: Outcome of kidney transplantation from elderly donors after cardiac death. Transplant Proc 43: 3686–3689, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Debout A, Foucher Y, Trebern-Launay K, Legendre C, Kreis H, Mourad G, Garrigue V, Morelon E, Buron F, Rostaing L, Kamar N, Kessler M, Ladrière M, Poignas A, Blidi A, Soulillou JP, Giral M, Dantan E: Each Additional Hour of Cold Ischemia Time Significantly Increases the Risk of Graft Failure and Mortality After Renal Transplantation. Kidney Int 87: 343–349, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Denecke C, Yuan X, Ge X, Kim IK, Bedi D, Boenisch O, Weiland A, Jurisch A, Kotsch K, Pratschke J, Reutzel-Selke A, Tullius SG: Synergistic effects of prolonged warm ischemia and donor age on the immune response following donation after cardiac death kidney transplantation. Surgery 153: 249–261, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Snoeijs MG, Schaubel DE, Hené R, Hoitsma AJ, Idu MM, Ijzermans JN, Ploeg RJ, Ringers J, Christiaans MH, Buurman WA, van Heurn LW: Kidneys from donors after cardiac death provide survival benefit. J Am Soc Nephrol 21: 1015–1021, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schold JD, Meier-Kriesche HU: Which renal transplant candidates should accept marginal kidneys in exchange for a shorter waiting time on dialysis? Clin J Am Soc Nephrol 1: 532–538, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Jochmans I, Moers C, Smits JM, Leuvenink HG, Treckmann J, Paul A, Rahmel A, Squifflet JP, van Heurn E, Monbaliu D, Ploeg RJ, Pirenne J: Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann Surg 252: 756–764, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Rigotti P, Ekser B, Furian L, Baldan N, Valente ML, Boschiero L, Motterlini N, Perna A, Remuzzi G, Ruggenenti P: Outcome of renal transplantation from very old donors. N Engl J Med 360: 1464–1465, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Kootstra G, Kievit JK, Heineman E: The non heart-beating donor. Br Med Bull 53: 844–853, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Klein JP, Moeschberger MV: Survival Analysis: techniques for Censored and Truncated Data: Statistics for Biology and Health, 2nd Ed., New York, Springer, 2003 [Google Scholar]
- 36.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org. 2014
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.