Abstract
Peritoneal dialysis (PD) remains limited by dialysis failure due to peritoneal membrane fibrosis driven by inflammation caused by infections or sterile cellular stress. Given the fundamental role of Toll-like receptors (TLRs) and complement in inflammation, we assessed the potential of peritoneal TLR2, TLR4 and C5a receptors, C5aR and C5L2, as therapeutic targets in PD-associated fibrosis. We detected TLR2–, TLR4–, and C5aR–mediated proinflammatory and fibrotic responses to bacteria that were consistent with the expression of these receptors in peritoneal macrophages (TLR2/4, C5aR) and mesothelial cells (TLR2, C5aR). Experiments in knockout mice revealed a major role for TLR2, a lesser role for TLR4, a supplementary role for C5aR, and no apparent activity of C5L2 in infection–induced peritoneal fibrosis. Similarly, antibody blockade of TLR2, TLR4, or C5aR differentially inhibited bacteria–induced profibrotic and inflammatory mediator production by peritoneal leukocytes isolated from the peritoneal dialysis effluent (PDE) of noninfected uremic patients. Additionally, antibodies against TLR2, TLR4, or the coreceptor CD14 reduced the profibrotic responses of uremic leukocytes to endogenous components present in the PDE of noninfected patients. Enhancing TLR2-mediated inflammation increased fibrosis in vivo. Furthermore, soluble TLR2 (sTLR2), a negative modulator of TLRs that we detected in PDE, inhibited PDE–induced, TLR2– or TLR4–mediated profibrotic responses. Notably, sTLR2 treatment markedly reduced Gram–positive and –negative bacteria–induced fibrosis in vivo, inhibiting proinflammatory and fibrotic genes without affecting infection clearance. These findings reveal the influence of peritoneal TLR2 and TLR4 on PD-associated fibrosis and describe a therapeutic strategy against fibrosis.
Keywords: peritoneal dialysis, immunology and pathology, fibrosis, clinical immunology
Peritoneal dialysis (PD) depends on the structural and functional integrity of the peritoneal membrane. A major factor limiting PD remains peritoneal membrane failure, which is directly related to progressive thickening of the submesothelial compact zone, termed fibrosis, resulting in altered solute transport and dialysis failure. This process is driven by peritoneal inflammation caused by recurrent infections or ongoing cellular stress and tissue injury induced by the dialysis process (sterile inflammation).1 The mechanisms linking inflammation with the genesis and regulation of fibrosis remain to be fully elucidated,2,3 and effective therapies to prevent this PD-associated pathology are still to be developed.
Toll-like receptors (TLRs) are critical to triggering a rapid inflammatory response to clear infections.4 They are expressed in various cell types, including peritoneal mesothelial cells,5 and recognize microorganisms and their components as well as host endogenous molecules released after cellular stress (e.g., High Mobility Group Box 1 [HMGB-1] and heat shock proteins) or generated as a consequence of matrix degradation during tissue injury (e.g., hyaluronan and fibronectin).6,7 TLR triggering leads to the release of proinflammatory and fibrotic mediators (e.g., IL-6, TGF-β, TNF-α, and IL-8).3,8
The complement system also plays a fundamental role in inflammation. Complement activation leads to generation of C5a, a potent proinflammatory peptide with activities including leukocyte chemoattraction and proinflammatory cytokine and chemokine induction. C5a induces responses through the C5a receptor (C5aR). A second C5a receptor, C5L2, can inhibit C5aR.9–11 We and others have shown crosstalk between TLRs and C5aR, by which cell exposure to a combination of TLR ligands and C5a results in synergistically enhanced proinflammatory mediator production.12–15
Given the serious inflammatory conditions resulting from TLR and/or C5aR overactivation, TLRs and C5a receptors are attractive therapeutic targets for the treatment and/or prevention of inflammation-associated pathologies.16–19 In this study, we assessed the potential of peritoneal TLRs, C5aR, and C5L2 as therapeutic targets against peritoneal fibrosis development during PD by investigating their influence on proinflammatory and fibrotic responses in vitro, ex vivo, and in vivo. The study reveals the major influence that TLR2 and TLR4 and to a lesser extent, C5aR exert on infection–associated and sterile peritoneal inflammation and fibrosis development. It shows the therapeutic potential of a TLR targeting strategy to prevent PD-associated fibrosis that is on the basis of the modulatory capacity of the soluble form of TLR2.
Results
Differential Expression and Response of TLR2, TLR4, and C5aR in Human Resident Peritoneal Leukocytes and Mesothelial Cells
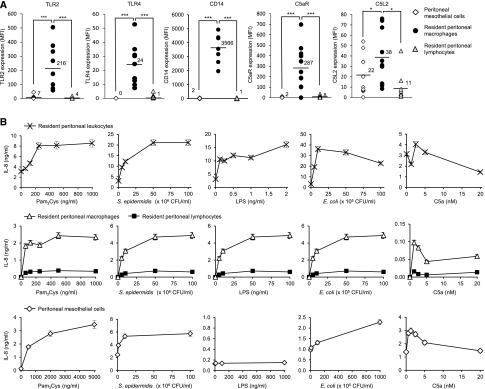
We first examined the expression and response of TLR2, TLR4—the TLRs recognizing the widest range of microbial components involved in PD-associated infections—and C5aR in the main peritoneal cell types involved in the initial response to danger: resident macrophages, lymphocytes, and mesothelial cells (Figure 1A). Peritoneal dialysis effluent (PDE)–isolated resident macrophages showed high levels of TLR2 and C5aR expression and moderate levels of TLR4, whereas mesothelial cells (from greater omentum) and PDE lymphocytes showed very modest levels of TLR2 and C5aR and undetectable (mesothelial cells) or barely detectable (lymphocytes) levels of TLR4. The TLR coreceptor CD14, which enhances TLR-mediated responses,20 was strongly expressed in peritoneal macrophages but barely detectable in lymphocytes and mesothelial cells. The C5aR modulatory receptor, C5L2, was expressed in all of the cell types tested at moderate (macrophages and mesothelial cells) or low (lymphocytes) levels.
Figure 1.
Peritoneal leukocytes and mesothelial cells differentially express TLR2, TLR4, and C5aR and respond to TLR agonists or C5a. (A) Expression levels of TLR2, TLR4, C5aR, the TLR coreceptor CD14, and the C5aR negative regulator C5L2 in PDE–isolated resident peritoneal macrophages, lymphocytes, and peritoneal mesothelial cells (from omentum) as determined by flow cytometry. Results are from the analysis of cells isolated from PDE or omentum of 7–10 different donors. MFI, mean fluorescence intensity. *P<0.05; ***P<0.01. (B) IL-8 levels in culture supernatants of resident peritoneal leukocytes, macrophages, lymphocytes, and peritoneal mesothelial cells stimulated overnight with the indicated concentrations of various TLR agonists or C5a. Leukocytes, macrophages, and lymphocytes were from the same patient sample. Results shown are the mean (±SD) from one experiment representative of three performed with cells from different donors.
Consistent with the expression of the corresponding receptors, TLR2, TLR4, and C5aR agonists triggered proinflammatory responses in PDE leukocytes as judged by the release of the PMN chemoattractant CXCL8/IL-8 induced by Pam3-Cys-Ser-(Lys)4 (Pam3Cys; a synthetic bacterial lipopeptide), the Gram–positive bacterium Staphylococcus epidermidis (both TLR2 agonists21,22), the Gram–negative bacterial cell wall component LPS, and Escherichia coli (both TLR4 agonists23,24) as well as by the C5aR ligand, C5a (Figure 1B). The C5a stimulation profile observed here was similar to that shown by PBMCs,15 with reduced stimulation as the C5a concentration increases and the negative regulator C5L2 becomes engaged. Leukocyte responses were mainly driven by macrophages (Figure 1B) as expected given their high receptor expression levels compared with lymphocytes (Figure 1A). Mesothelial cells responded to Pam3Cys, S. epidermidis, E. coli, and C5a but not to LPS (Figure 1B). Their lack of LPS-induced response confirmed our previous findings,5 because human mesothelial cells did not express TLR4 (Figure 1A). However, they responded to E. coli, probably at least partly through the TLR2-mediated recognition of bacterial lipopeptides25 and the expression of TLR5,5 which recognizes flagellin, the protein component of the flagellum found in Gram-negative bacteria.26
Synergism between TLRs and C5aR in the Pro- and Anti-Inflammatory and Fibrotic Responses of Peritoneal Leukocytes but Not of Mesothelial Cells
We tested whether crosstalk between TLRs and C5aR operates in peritoneal cells, which is the case in PBMCs, resulting in the synergistic enhancement of responses (Supplemental Figure 1).15 Costimulation of PDE-isolated leukocytes with Pam3Cys and C5a resulted in a marked enhancement of IL-8 release compared with the estimated additive effect of each ligand (Figure 2A). A much lower but significant synergistic effect was observed on LPS+C5a stimulation (Figure 2A). By contrast, peritoneal mesothelial cells costimulated with varying concentrations of Pam3Cys+C5a, LPS+C5a, or E. coli+C5a did not show a synergistic response (Figure 2B). Of note and similar to PBMCs,15 the TLR-C5aR crosstalk in peritoneal leukocytes seems to be negatively regulated by C5L2, because blocking C5L2 with a specific mAb substantially increased the synergistic response to Pam3Cys and C5a (Figure 2C). Together, these findings indicated the existence of a C5L2–regulated proinflammatory TLR-C5aR crosstalk in peritoneal leukocytes but not in mesothelial cells, by which concerted stimulation via TLR2—or to a lesser extent, via TLR4—and C5aR imparts hypersensitivity to leukocytes to the cognate ligands.
Figure 2.
Synergism between TLRs and C5aR in peritoneal macrophages but not in peritoneal mesothelial cells enhances pro– and anti–inflammatory and fibrotic mediator release. Levels of proinflammatory cytokines and fibrotic markers in the culture supernatants of (A, C, and D) PDE–isolated resident peritoneal leukocytes or (B) peritoneal mesothelial cells (from omentum) stimulated overnight with the indicated concentrations of Pam3Cys (or 250 ng/ml), LPS (or 1 ng/ml), or heat–killed E. coli in the presence or absence of increasing concentrations of C5a. In C, cells were preincubated with an anti–C5L2 blocking mAb (1D9; 5 μg/ml). IL-8, IL-13, TGF-β, and TIMP-1 levels were determined by ELISA, and IL-6, IL-10, TNF-α, MMP-1, MMP-3, and MMP-9 levels were determined by multiplex ELISA (Meso Scale Discovery). Results are from one experiment (±SD) representative of (A and B) 10 or (C and D) five performed with cells from different donors. The P values indicate statistical significance for the comparison between the additive response to a TLR agonist alone and C5a alone and the response to the combination of TLR agonist and C5a. *P<0.05; **P<0.01; ***P<0.01.
The potential effect of peritoneal leukocyte TLR2-C5aR crosstalk on fibrosis development was evaluated by testing for synergism in the release of pro- and anti-inflammatory and fibrotic mediators (Figure 2D). Pam3Cys+C5a induced a marked synergistic enhancement in the release of the profibrotic cytokines TGF-β and IL-13.27–29 By contrast, the release of other profibrotic and proinflammatory cytokines (i.e., IL-6 and TNF-α) and the anti–inflammatory and antifibrotic cytokine IL-10 showed either a very modest enhancement (IL-6 and TNF-α) or only an additive effect (IL-10) (Figure 2D). Matrix metalloproteinases (MMPs), which are involved in both augmenting and attenuating fibrosis,30 were only slightly affected by the TLR2-C5aR crosstalk (Figure 2D), with a very modest increase on Pam3Cys stimulation at low C5a concentrations (MMP-1 and MMP-9) and a stronger synergistic enhancement at higher C5a concentration (MMP-1, MMP-3, and MMP-9). By contrast, the effect of TLR2-C5aR crosstalk on a negative regulator of MMPs, tissue inhibitor of metalloproteinases-1 (TIMP-1), was marked and at low C5a concentrations (Figure 2D). These data indicated synergism between TLR2 and C5aR in the release of pro- and anti-inflammatory and fibrotic mediators, with a differential effect depending on the mediator.
Contribution of TLR2, TLR4, and C5aR but Not C5L2 to Peritoneal Fibrosis Development in Bacteria–Induced Mouse Peritonitis Models
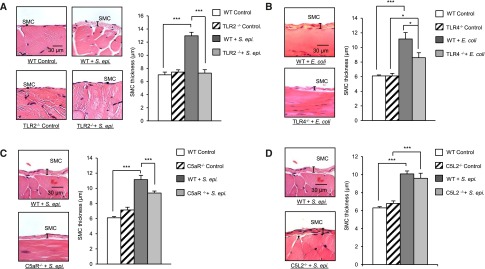
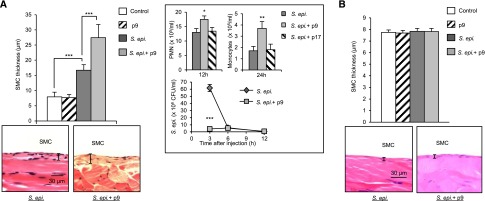
We next evaluated the relevance of peritoneal TLR– and C5aR–mediated responses to PD–associated bacteria–induced peritoneal fibrosis development in vivo. We used our established3 murine model of recurrent bacteria–induced peritoneal inflammation leading to fibrosis. In this model, the key features of repeated clinical bacterial peritonitis episodes with the eventual development of peritoneal fibrosis typically observed in patients on PD are mimicked by the weekly peritoneal injection (four injections) of a previously defined dose of whole (heat–killed or live) S. epidermidis (TLR2 agonist; a main causative pathogen of PD-associated peritonitis) or E. coli (TLR4 agonist) (Concise Methods, Supplemental Figure 2), both reported to trigger complement activation and C5a generation.31,32 Four weeks after the fourth infection/inflammatory episode, peritoneal fibrosis is measured in sections of parietal membrane. Repeated intraperitoneal injection of heat–killed S. epidermidis in wild-type (WT) mice resulted in substantial peritoneal fibrosis (Figure 3A). Notably, fibrosis was not observed after S. epidermidis injection in TLR2-deficient (TLR2−/−) mice (Figure 3A). By contrast, TLR4−/− mice injected with E. coli showed a partial (approximately 45%) reduction in fibrosis compared with WT mice (Figure 3B), consistent with the possibility that, in addition to TLR4, other receptors (e.g., TLR2 and TLR5) may be involved in E. coli–induced profibrotic responses.
Figure 3.
TLR2, TLR4, C5aR, and C5L2 differentially contribute to bacteria–induced peritoneal fibrosis development in mouse peritonitis models. WT and (A) TLR2−/−, (B) TLR4−/−, (C) C5aR−/−, or (D) C5L2−/− mice (n=5 per group) were inoculated intraperitoneally four times at weekly intervals with heat–killed S. epidermidis (5×108 CFU per mouse) or E. coli (2×107 CFU per mouse) or left untreated (control). Four weeks after the last injection, histologic analysis of the peritoneal membrane was conducted. Sections of peritoneal membrane (5 μm) were stained with hematoxylin and eosin, and the thickness of the submesothelial compact zone (SMC; layer between the muscle and membrane surface) was determined using the Leica Qwin imaging software. Representative fields (×40 magnification) are shown. Bar plots show the mean (±SEM) of SMC thickness in each experimental group. The mean of SMC was determined as described in Concise Methods. *P<0.05; ***P<0.01.
The role of C5aR and C5aR-TLR2 crosstalk in fibrosis development in mice was also investigated. The repeated injection of heat–killed S. epidermidis in C5aR−/− mice resulted in moderate (approximately 40%) fibrosis reduction compared with WT mice (Figure 3C). This contrasted with the dramatic effect of TLR2 deficiency, which completely prevented fibrosis development (Figure 3A). These findings indicated a prominent role of TLR2 in S. epidermidis–induced peritoneal fibrosis development. Furthermore, the absence of fibrosis in the S. epidermidis–injected TLR2−/− mice suggested that the locally generated C5a may induce fibrosis mainly indirectly by enhancing TLR2-mediated responses through the C5aR-TLR2 crosstalk. This putative enhancement may explain the approximately 40% reduction in fibrosis observed in the S. epidermidis–injected C5aR−/− mice (Figure 3C). We, therefore, tested whether C5L2, which controls peritoneal C5aR-TLR2 crosstalk in vitro (Figure 2C), also modulates fibrosis development in vivo. Fibrosis was comparable between S. epidermidis–injected WT and C5L2−/− mice (Figure 3D), suggesting that the TLR2-C5aR crosstalk signal intermediates involved in profibrotic responses are not controlled by C5L2 or most likely, that C5L2 controls equally both the pro- and antifibrotic signaling shown to be positively affected by TLR2-C5aR crosstalk (Figure 2D). Collectively, these findings indicated a major role for TLR2, a lesser role for TLR4, and only a supplementary role for C5aR in bacteria–induced peritoneal fibrosis but no apparent net influence of C5L2.
Differential Contribution of TLR2, TLR4, and C5aR to Bacteria–Induced Profibrotic and Inflammatory Mediator Release by Peritoneal Leukocytes from Uremic Patients on PD
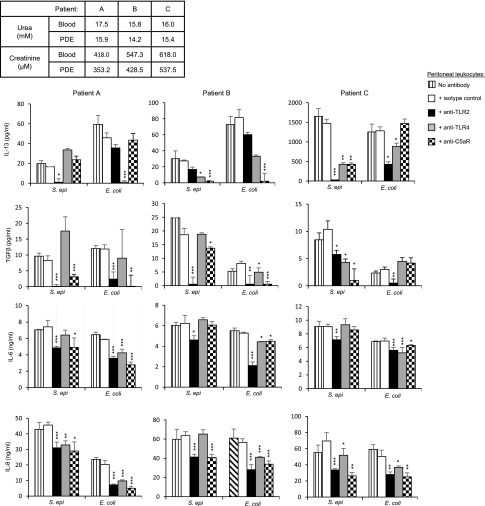
The clinical relevance of the findings described above was evaluated by testing whether the uremic milieu, present in patients on PD, has an effect on the observed role of TLR2, TLR4, and C5aR in bacteria–induced peritoneal fibrosis development. To this end, the involvement of these receptors was evaluated in uremic peritoneal leukocytes isolated from noninfected PDE of patients whose uremic status was confirmed (urea and creatinine levels in PDE and blood). Consistent with the findings obtained by using the mouse peritonitis model (Figure 3), blocking TLR2, TLR4, and C5aR with specific mAbs significantly reduced S. epidermidis– and E. coli–induced profibrotic (IL-13, TGF-β, and IL-6) and inflammatory (IL-6 and IL-8) cytokine release by uremic leukocytes. The extent of the blocking effect depended on the individual, the bacterium, and the cytokine tested (Figure 4), with TLR2 blockade consistently showing a significant effect on profibrotic cytokine release.
Figure 4.
TLR2, TLR4, and C5aR are involved in bacteria–induced profibrotic and inflammatory responses of peritoneal leukocytes from uremic patients on PD. Levels of profibrotic and inflammatory cytokines released by PDE–isolated uremic leukocytes cultured overnight with or without heat–killed S. epidermidis (107 CFU/ml) or E. coli (106 CFU/ml) in the presence of the indicated blocking mAbs or isotype-matched control (5 μg/ml). To maintain the uremic milieu throughout the experiments, the culture medium was supplemented with the patients’ own PDE (1/2 dilution). The uremic status of the patients was confirmed by urea and creatinine measurements in the blood and PDE (table). Results are the mean (±SD) of triplicates after background subtraction (cells cultured in the absence of bacteria but in the presence of the blocking mAbs or isotype control, typically IL-8<1 ng/ml, IL-6<100 pg/ml, IL-13<25 pg/ml, and TGF-β<10 pg/ml). Specific mAb versus isotype control; t test. *P<0.05; **P<0.01; ***P<0.001.
We also analyzed fibrosis–related gene expression in uremic leukocytes after bacterial stimulation and TLR2 blockade. An inhibitory effect of TLR2 blockade on the capacity of S. epidermidis to induce mRNA coding for a number of fibrosis markers was observed (Table 1). Twenty-three of the 84 genes tested were significantly induced (P≤0.05; fold change ≥2) by S. epidermidis (a full list of genes is in Supplemental Table 1). Notably, among the transcripts markedly reduced by blocking TLR2 was that coding for Snail, a transcription factor master regulator of the epithelial-mesenchymal transition, a process that plays a critical role in fibrosis development.33 TLR2 blockade also inhibited a number of Mmps transcripts. Tgf-β transcription was not induced by S. epidermidis at the time point tested (Supplemental Table 1). However, the release of this profibrotic cytokine was induced by S. epidermidis after 72 hours, indicating slower transcription kinetics for this cytokine, and TLR2 blockade reduced this effect markedly (Figure 4). Together, these findings obtained using patients’ uremic peritoneal leukocytes tested in a uremic milieu provided proof of concept for the clinical relevance of the differential involvement of peritoneal TLR2, TLR4, and C5aR in profibrotic and inflammatory responses induced by bacteria.
Table 1.
Effect of blocking TLR2 in uremic peritoneal leukocytes on S. epidermidis–induced, fibrosis–related gene expression
| Gene Symbol | Description | S. epidermidisa | S. epidermidis+Anti-TLR2 | Anti–TLR2 Modulatory Effect, %c | ||
|---|---|---|---|---|---|---|
| Fold Changeb | P Valueb | Fold Changeb | P Valueb | |||
| Ccl11 | Chemokine (C-C motif) ligand 11 | 0.5 | 0.02 | 0.7 | 0.14 | −34.7 |
| Ccl2 | Chemokine (C-C motif) ligand 2 | 12.0 | 0.001 | 14.5 | 0.001 | 22.5 |
| Ccl3 | Chemokine (C-C motif) ligand 3, MIP1a | 16.5 | 0.001 | 3.2 | 0.07 | −86.0 |
| Ccr2 | Chemokine (C-C motif) receptor 2 | 0.3 | 0.01 | 0.4 | 0.02 | −11.2 |
| Ctgf | Connective tissue growth factor | 0.4 | 0.05 | 0.4 | 0.04 | — |
| Edn1 | Endothelin 1 | 14.9 | 0.001 | 11.0 | <0.01 | −28.2 |
| Grem1 | Gremlin 1 | 0.2 | 0.001 | 0.4 | 0.001 | −13.6 |
| Ifng | IFN-γ | 55.2 | 0.001 | 63.5 | 0.001 | 15.4 |
| Il10 | IL-10 | 240.8 | 0.001 | 188.9 | 0.001 | −21.7 |
| Il13 | IL-13 | 10.1 | 0.03 | 9.5 | 0.05 | −5.6 |
| Il1a | IL-1α | 19.7 | 0.001 | 18.9 | 0.001 | −4.0 |
| Il1b | IL-1β | 31.8 | 0.01 | 30.1 | 0.004 | −5.5 |
| Itga1 | Integrin-α1 | 9.5 | 0.001 | 9.4 | 0.001 | −1.9 |
| ItgaV | Integrin-αV | 2.6 | 0.004 | 3.3 | 0.002 | 40.7 |
| Itgb3 | Integrin-β3 | 5.7 | 0.001 | 6.6 | 0.002 | 17.6 |
| Itgb5 | Integrin-β5 | 0.3 | 0.001 | 0.3 | 0.004 | — |
| Itgb8 | Integrin-β8 | 12.6 | 0.004 | 13.7 | 0.01 | 9.4 |
| Mmp1 | MMP-1 | 67.1 | 0.001 | 61.1 | 0.001 | −9.0 |
| Mmp14 | MMP-14 | 3.6 | 0.01 | 2.6 | 0.20 | −38.9 |
| Mmp3 | MMP-3 | 5.5 | 0.001 | 3.6 | 0.001 | −41.7 |
| Mmp8 | MMP-8 | 3.5 | 0.001 | 4.7 | 0.002 | 44.8 |
| Plg | Plasminogen | 2.8 | 0.03 | 1.0 | 0.90 | −100.0 |
| Serpina1 | Serpin peptidase inhibitor, clade A | 9.7 | 0.001 | 8.8 | 0.001 | −10.3 |
| Snai1 | Snail homolog 1 | 7.2 | 0.05 | 5.3 | 0.06 | −31.2 |
| Stat1 | STAT-1, 91 kD | 2.5 | 0.01 | 2.6 | 0.01 | 10.9 |
| Tgif1 | TGFB–induced factor homeobox 1 | 2.3 | 0.03 | 2.3 | 0.04 | — |
| Timp3 | TIMP metallopeptidase inhibitor 3 | 0.3 | 0.001 | 0.3 | 0.001 | — |
| Timp4 | TIMP metallopeptidase inhibitor 4 | 0.4 | 0.02 | 0.4 | 0.02 | — |
| Tnf | TNF | 4.9 | 0.002 | 4.7 | 0.001 | −6.5 |
| Vegfa | Vascular endothelial growth factor A | 6.8 | 0.001 | 6.8 | 0.001 | — |
—, no effect.
Only statistically significant (P<0.05) S. epidermidis–induced ≤0.5 or ≥2-fold changes were considered.
Compared with the control group.
Compared with the effect of S. epidermidis treatment.
Involvement of TLR2, TLR4, CD14, and C5aR in PD–Induced Sterile Peritoneal Inflammation
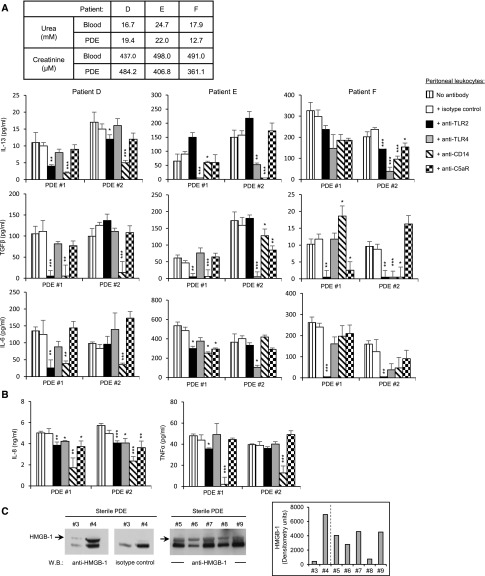
To further assess the clinical relevance of peritoneal TLRs and C5aR to PD-associated fibrosis, their involvement in PD–induced sterile inflammation was investigated. Uremic peritoneal leukocytes isolated from noninfected PDE were cultured overnight with noninfected cellfree PDE in the absence or presence of TLR2–, TLR4–, or C5aR–specific blocking mAbs. The PDE used for stimulation also served to maintain uremic conditions throughout the experiment (Figure 5, A and B). PDE from noninfected patients on PD induced profibrotic (IL-13, TGF-β, and IL-6) and inflammatory (IL-6, IL-8, and TNF-α) responses in the uremic leukocytes (Figure 5, A and B). These responses were reduced by anti-TLR2, anti-TLR4, or both mAbs to a varying extent depending on the individual and the PDE tested. TLR2 blockade showed, however, an overall more consistent effect on profibrotic cytokine release. Additional blocking experiments showed that TLR–mediated peritoneal leukocyte responses to PDE were heavily dependent on the TLR coreceptor CD14, irrespective of the TLR (TLR2 or TLR4) involved (Figure 5, A and B). By contrast, blocking C5aR did not, or very modestly did, affect leukocyte responses (Figure 5, A and B). Notably, production of IL-6 and TNF-α—proinflammatory and fibrotic mediators reported to be important determinants of peritoneal inflammation and peritoneal solute transport in the uninfected state1—was mostly not affected by C5aR blocking (Figure 5, A and B). Similarly, the levels of the profibrotic cytokines TGF-β and IL-13 were not, or were very modestly, affected depending on the patient by C5aR blockade (Figure 5A). These findings indicated that, although PD may induce complement production and its activation in the uninfected state as was reported,34–37 the direct or indirect (via TLR crosstalk) involvement of complement in proinflammatory and fibrotic processes in noninfected patients is modest at best and depends on the patient. In addition, the peritoneal C5a levels in most patients may be extremely low because of dilution in the peritoneum during dialysis, resulting in minimal C5aR activation.
Figure 5.
TLR2, TLR4, CD14, and C5aR are differentially involved in PD–induced sterile peritoneal inflammation. Levels of (A) profibrotic and (B) inflammatory (B) cytokines released by PDE–isolated uremic leukocytes stimulated (17 hours) with noninfected PDE (PDE#1: urea, 16.6 mM; creatinine, 286.4 μM; PDE#2: urea, 15.8 mM; creatinine, 415.5 μM) in the presence of the indicated blocking mAbs or isotype-matched control (5 μg/ml). The noninfected PDEs used for stimulation also served to maintain uremic conditions throughout the experiment. The uremic status of the patients was confirmed by urea and creatinine measurements in the blood and PDE (table in A). Results are the mean (±SD) of triplicates after background subtraction (cells cultured in the absence of PDE but in the presence of the mAbs, typically IL-8<1 ng/ml, IL-6<100 pg/ml, IL-13<25 pg/ml, and TGF-β<10 pg/ml). Specific mAb versus isotype control; t test. *P<0.05; **P<0.01; ***P<0.001. (C) Western blot (W.B.) analysis and densitometric scanning of HMGB-1 detected in sterile PDE samples (diluted 20 times) from seven donors. Arrows point at the HMGB-1 protein specifically detected. The dashed line in the inset indicates separate gels.
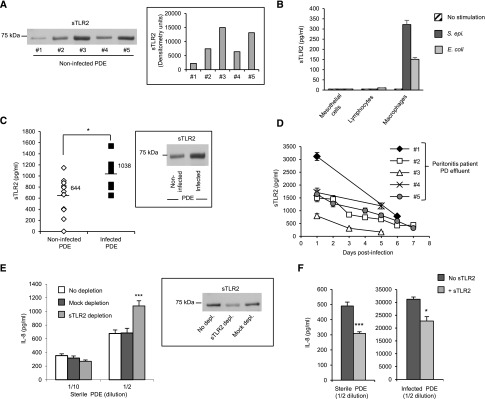
Analysis of fibrosis–related gene expression in uremic leukocytes after noninfected cellfree PDE stimulation and TLR2 blockade further confirmed the involvement of TLR2 in fibrosis processes (Table 2). Only seven of the 84 genes tested were found affected by PDE stimulation at this time point (a full list of genes is in Supplemental Table 2), with TLR2 blockade markedly inhibiting Ccl2 (MCP-1), Egf, and Mmp13 (Table 2). Tgf-β transcription was not induced by PDE at this time point (Supplemental Table 2). However, TGF-β release was induced by PDE after 72 hours, indicating slower transcription kinetics for this cytokine, and blocking TLR2 reduced its release markedly (Figure 5A). Collectively, these findings indicated that peritoneal TLR2, TLR4, and their main coreceptor, CD14, drive profibrotic and inflammatory responses to not only microbial components but also, host endogenous components that may be present in the uremic peritoneum as a consequence of cellular stress or tissue injury induced during PD.1 Consistent with this possibility, we found HMGB-1 (TLR2/TLR4 ligand38,39) in PDE of noninfected patients on PD, confirming a previous finding,40 and its expression varied between patients (Figure 5C).
Table 2.
Effect of blocking TLR2 in uremic peritoneal leukocytes on noninfected PDE–induced, fibrosis–related gene expression
| Gene Symbol | Description | Noninfected PDEa | Noninfected PDE+Anti-TLR2 | Anti–TLR2 Modulatory Effect, %c | ||
|---|---|---|---|---|---|---|
| Fold Changeb | P Valueb | Fold Changeb | P Valueb | |||
| Ccl2 | Chemokine (C-C motif) ligand 2 | 2.2 | 0.02 | 1.1 | 0.88 | −91.0 |
| Egf | EGF | 6.5 | 0.001 | 1.1 | 0.84 | −98.9 |
| Il13 | IL-13 | 3.5 | 0.001 | 3.9 | 0.01 | 16.8 |
| Il13ra2 | IL-3 receptor-α2 | 7.3 | 0.002 | 6.7 | 0.004 | −8.3 |
| Il4 | IL-4 | 4.2 | 0.001 | 3.8 | 0.01 | −11.2 |
| Mmp13 | MMP-13 | 4.1 | 0.001 | 1.1 | 0.84 | −98.0 |
| Smda3 | SMAD family member 3 | 0.4 | 0.02 | 0.2 | <0.01 | 31.0 |
Only statistically significant (P<0.05) PDE#1–induced ≤0.5- or ≥2-fold changes were considered.
Compared with the control group.
Compared with the effect of noninfected PDE treatment.
Effect of TLR2–Mediated Inflammation Intensity on Bacterial Infection–Induced Peritoneal Fibrosis Development
To evaluate the importance of peritoneal TLR–mediated inflammation intensity in infection–induced fibrosis development, we used a TLR2-derived peptide—peptide 9—which we showed amplifies TLR2-mediated inflammation by targeting the TLR coreceptor CD14, enhancing its activity.20 By using the in vivo peritoneal fibrosis model described previously, we found that live S. epidermidis infection in the presence of peptide 9 substantially increased peritoneal fibrosis compared with in mice infected with bacteria in the absence of peptide (Figure 6A). The marked increase in fibrosis in the S. epidermidis+peptide 9 mice correlated with higher peritoneal inflammation (higher numbers of PMN and monocytes recruited to the peritoneum) (Figure 6A, inset). Of note, the TLR2–derived control peptide 17 did not affect inflammatory cell recruitment (Figure 6A, inset). Consistent with the higher number of recruited phagocytes, peptide 9 accelerated bacterial clearance (Figure 6A, inset). Notably, despite the accelerated infection resolution in the presence of the proinflammatory peptide, fibrosis was more severe—probably as a consequence of the enhanced inflammation.
Figure 6.
The intensity of the TLR–mediated inflammatory episodes in addition to their number affects peritoneal fibrosis development. C57BL/6J mice (n=5 per group) were inoculated intraperitoneally (A) four or (B) two times at weekly intervals with (A) live or (B) heat–killed S. epidermidis (5×108 CFU per mouse) or PBS (control) in the presence or absence of a TLR2-derived peptide (p9 or control p17; 20 μg per mouse). Four weeks after the last injection, histologic analysis of the peritoneal membrane was conducted as described in Figure 3. Representative fields (×40 magnification) are shown, and bar plots show the mean (±SEM) of the submesothelial compact zone (SMC) thickness for each group. (A, inset) At the indicated time points after the first injection, peritoneal lavages were obtained. PMN and monocyte numbers were determined by flow cytometry using anti-Ly6G– and anti-7/4–specific mAbs and bacterial titers by overnight culture on microbiologic agar plates. S. epidermidis and p9 versus S. epidermidis alone. *P<0.05; **P<0.01; ***P<0.01.
To evaluate the importance of intensity of inflammation over number of infections in peritoneal fibrosis development, mice were injected only twice (once per week) with S. epidermidis with or without the TLR2-boosting peptide. Fibrosis was not detected after two infection episodes, even in the presence of the proinflammatory peptide (Figure 6B). Together, these findings indicated that, in addition to the number of infection episodes but irrespective of their duration, the intensity of the resulting inflammation seriously affects fibrosis development. This pointed at controlling the intensity of the CD14/TLR-mediated inflammation as a therapeutic strategy against peritoneal fibrosis.
Peritoneal Expression and Inflammation Regulatory Capacity of the TLR Inhibitor Soluble TLR2
To test whether the intensity of the TLR–mediated peritoneal inflammation is naturally controlled, we tested for the peritoneal presence of the natural inhibitor of TLRs, soluble Toll–like receptor 2 (sTLR2), which we previously detected in plasma41 and showed to reduce TLR-mediated inflammation by acting as a decoy receptor and also, interfering with CD14’s coreceptor activity.42 We detected sTLR2 in PDE from noninfected patients, and its level varied between patients (Figure 7A). The source of sTLR2 was mainly the TLR2-expressing macrophages (Figures 1A and 7B), because PDE-isolated macrophages released sTLR2 on activation, whereas sTLR2 was barely detectable or undetectable in the culture supernatants of peritoneal lymphocytes and mesothelial cells (Figure 7B). sTLR2 concentrations were significantly increased in PDE from patients with ongoing bacterial peritonitis (Figure 7C), and the levels declined over time as the infection resolved (Figure 7D).
Figure 7.
sTLR2 is present in PDE from noninfected patients and can reduce PD–induced proinflammatory responses ex vivo. (A) Western blot analysis and densitometric scanning of sTLR2 detected in sterile PDE from different donors. (B) Levels of sTLR2 in the culture supernatants of peritoneal mesothelial cells, sterile PDE–isolated lymphocytes, and macrophages stimulated (16 hours) or not with heat–killed S. epidermidis (5×108 CFU/ml) or E. coli (5×107 CFU/ml). Results are from one experiment representative of three performed with cells from different donors. (C and D) Levels of sTLR2 in PDE from patients without or with ongoing peritoneal infection tested at (C) day 1 or (D) the indicated times postinfection. C, inset shows a representative sTLR2 Western blot profile in the PDE of one patient before and on day 1 of infection. In D, results are expressed as means (±SD) for each time point. *P<0.05. (E and F) Levels of IL-8 in the culture supernatant of (E) PBMC or (F) PDE leukocytes cultured overnight with the indicated dilutions of sterile or infected cellfree PDE (E) depleted of sTLR2, mock depleted, or not depleted or (F) supplemented with 500 ng/ml human recombinant sTLR2. Depletion in E was approximately 80% as estimated by Western blot. Results are from one experiment (±SD) representative of three performed with cellfree PDE from different donors. sTLR2 depleted versus mock depleted or and sTLR2 versus no sTLR2. *P<0.05; ***P<0.01.
We next evaluated the capacity of endogenous peritoneal sTLR2 to control inflammatory responses. Depleting sTLR2 from cellfree noninfected PDE significantly increased PDE’s stimulatory capacity (Figure 7E), suggesting that the proinflammatory capacity of endogenous peritoneal TLR ligands is inhibited by peritoneal sTLR2. Of note, inhibition by sTLR2 was observed in PDE activating cells via TLR2 and TLR4 or mostly, via TLR4 (PDE#2 stimulating patient E’s leukocytes in Figure 5, A and B), suggesting that not only the TLR ligand but also, the coreceptor CD14 was targeted for modulation. Importantly, supplementing cellfree PDE from noninfected or infected patients with purified sTLR2 reduced PDE’s stimulatory capacity (Figure 7F), suggesting that, in addition to its natural activity, sTLR2 may be of therapeutic value against infection–induced and sterile peritoneal inflammation and fibrosis development in PD.
Reduction of Bacterial–Induced Peritoneal Fibrosis by Therapeutic Administration of sTLR2
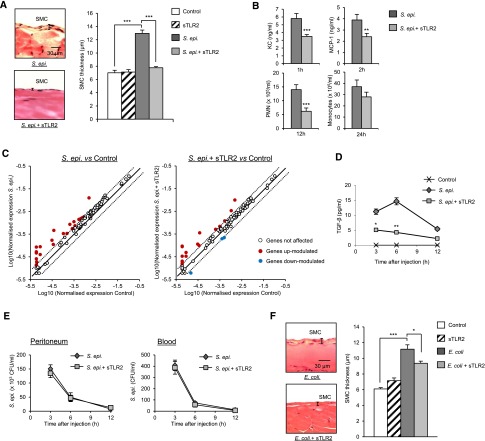
We next tested the therapeutic potential of sTLR2 against PD-induced fibrosis in vivo. Administration of sTLR2 together with the repeated injection of live S. epidermidis prevented fibrosis development (Figure 8A). This was accompanied by a marked reduction in the peritoneal levels of the proinflammatory mediators KC (murine functional counterpart of human IL-8) and MCP-1, prototypical PMN, and monocyte chemoattractants (Figure 8B). This, in turn, resulted in a reduced number of PMN and monocytes recruited to the peritoneum at the peak time of their influx (Figure 8B).
Figure 8.
sTLR2 inhibits infection–induced peritoneal fibrosis development in vivo. C57BL/6J mice (n=5 per group) were inoculated intraperitoneally (A, C, and F) four times at weekly intervals or (B, D, and E) once with (A–E) live S. epidermidis (5×108 CFU per mouse) or (F) heat–killed E. coli (5×107 CFU per mouse) in the presence or absence of sTLR2 (250 ng per mouse). In A and F, 4 weeks after the last injection (day 49), histologic analysis of the peritoneal membrane was conducted as described in Figure 3. Representative fields (×40 magnification) are shown; bar plots show the mean (±SEM) of the submesothelial compact zone (SMC) thickness for each group. In B, D, and E, the animals were euthanized at the indicated time points after the injection, and peritoneal lavages and blood were obtained. Cytokine levels were determined by ELISA, PMN, and monocyte numbers by flow cytometry using anti-Ly6G– and anti-7/4–specific mAbs and bacterial titers by overnight culture on microbiologic agar plate. In C, scatterplots show the effect of sTLR2 on the S. epidermidis–induced modulation of fibrosis-related genes as assessed by quantitative RT-PCR on RNA extracted from peritoneal membranes 1 week after the fourth injection (day 28). S. epidermidis and sTLR2 versus S. epidermidis alone. *P<0.05; **P<0.01; ***P<0.01.
Analysis of fibrosis–related gene transcripts conducted 1 week after the last injection (day 28; before fibrosis becomes detectable) (Figure 8C, Table 3) showed a marked inhibitory effect of sTLR2 on the capacity of S. epidermidis to induce mRNA coding for a number of proinflammatory mediators and fibrosis markers (a full list of genes is in Supplemental Table 3). Twenty-one of the 85 genes tested were significantly induced (P≤0.05; fold change ≥2) by S. epidermidis at this time point, and sTLR2 reduced this effect in 18 of them. Among the transcripts affected by sTLR2 was Fasl. FasL is central to apoptosis, a cell death mechanism that impairs bacterial clearance during PD, induces peritoneal macrophage death, and increases peritoneal inflammation and the production of PMN chemoattractants.43,44 The negative effect on Fasl was prolonged, because it was also observed at 4 weeks after the last infection (day 49) (Table 4). sTLR2 administration also inhibited the S. epidermidis–induced transcription of signal transducer and activator of transcription 1 (STAT-1) (Table 3), a critical signal intermediate for fibrosis development in this model.3 Together with STAT-1, IFN-γ was shown to mediate peritoneal fibrosis3; however, sTLR2 did not significantly affect the strong S. epidermidis–induced transcription of IFN-γ (Table 3). sTLR2 may, nevertheless, affect IFN-γ’s profibrotic effect indirectly through its inhibitory effect on STAT-1—a main signal intermediate downstream of the IFN-γ receptor. sTLR2 also inhibited the S. epidermidis–induced transcription of IL-6 (Table 3), a major driver of peritoneal fibrosis.3 Notably, sTLR2 counteracted the negative effect of S. epidermidis on Mmp-1, Mmp-3, and Mmp-9 observed relatively late (day 49) (Table 4) and the relatively early (day 28) S. epidermidis positive effect on Mmp-13 and the MMP inhibitor Timp-1 (Table 3). sTLR2’s effect on Timp-1 was maintained until day 49 (Table 4). sTLR2 showed an apparent strong positive effect on Tnf-α transcription at day 28 (Table 3); however, this effect was not noticeable at day 49, probably indicating delayed transcription kinetics rather than an increase in transcript level in the presence of sTLR2. Similarly, an effect on transcription kinetics may explain sTLR2’s apparent positive effect on Ccl3/Mip-1α (Table 3). Transcription of the profibrotic cytokine TGF-β was not induced by S. epidermidis at either day 28 or 49 (Supplemental Table 3), whereas its receptor was induced by S. epidermidis and inhibited by sTLR2 at day 28 (Table 3). However, analysis of TGF-β levels in peritoneal lavages of mice at 3, 6, and 12 h postinfection showed induction of TGF-β by S. epidermidis and a marked reduction after administration of sTLR2 and S. epidermidis (Figure 8D).
Table 3.
Changes in fibrosis–related peritoneal gene expression at day 28 in mice infected with S. epidermidis or S. epidermidis and sTLR2
| Gene Symbol | Description | S. epidermidisa | S. epidermidis+sTLR2 | sTLR2 Modulatory Effect, %c | ||
|---|---|---|---|---|---|---|
| Fold Changeb | P Valueb | Fold Changeb | P Valueb | |||
| Ccl12 | Chemokine (C-C motif) ligand 12, MCP5 | 12.4 | <0.01 | 9.4 | 0.02 | −26.0 |
| Ccl3 | Chemokine (C-C motif) ligand 3, MIP1a | 4.3 | 0.03 | 5.2 | <0.01 | 25.8 |
| Ccr2 | Chemokine (C-C motif) receptor 2 | 4.2 | 0.04 | 2.5 | 0.08 | −53.7 |
| Cxcr4 | Chemokine (C-X-C motif) receptor 4 | 2.1 | 0.04 | 1.7 | 0.11 | −31.4 |
| Fasl | Fas ligand (TNF superfamily, member 6) | 8.0 | 0.05 | 3.2 | 0.21 | −68.1 |
| Hgf | Hepatocyte growth factor | 9.8 | 0.05 | 3.7 | 0.05 | −69.6 |
| Ifng | IFN-γ | 14.1 | 0.02 | 15.2 | 0.004 | 8.4 |
| Il10 | IL-10 | 8.7 | 0.01 | 2.5 | 0.001 | −80.2 |
| Il1b | IL-1β | 7.8 | 0.05 | 5.5 | 0.05 | −34.0 |
| Il6 | IL-6 | 3.1 | 0.001 | 1.4 | 0.22 | −79.3 |
| Itga2 | Integrin-α2 | 5.4 | 0.02 | 3.6 | 0.08 | −41.9 |
| Itgb3 | Integrin-β3 | 2.2 | 0.001 | 1.6 | 0.001 | −47.9 |
| Mmp13 | MMP-13 | 4.4 | 0.04 | 1.5 | 0.34 | −84.0 |
| Myc | Myelocytomatosis oncogene | 2.1 | <0.01 | 1.7 | 0.08 | −41.7 |
| Serpine1 | Serine (or cysteine) peptidase inhibitor, clade E, member 1 | 3.5 | 0.02 | 2.1 | 0.002 | −55.2 |
| Smad2 | MAD homolog 2 (Drosophila) | 2.0 | 0.02 | 1.4 | 0.04 | −58.8 |
| Sp1 | Trans-acting transcription factor 1 | 2.2 | 0.002 | 1.5 | 0.05 | −58.8 |
| Stat1 | STAT-1 | 8.1 | 0.05 | 6.4 | 0.04 | −24.0 |
| Tgfbr1 | TGF-β receptor I | 2.3 | 0.001 | 1.6 | 0.10 | −53.1 |
| Timp1 | TIMP-1 | 3.2 | 0.03 | 1.5 | 0.23 | −76.3 |
| Tnf | TNF | 8.1 | 0.04 | 14.3 | 0.04 | 88.5 |
Only statistically significant (P<0.05) S. epidermidis–induced ≤0.5- or ≥2-fold changes were considered.
Compared with the control group.
Compared with the effect of S. epidermidis treatment.
Table 4.
Changes in fibrosis–related peritoneal gene expression at day 49 in mice infected with S. epidermidis or S. epidermidis and sTLR2
| Gene Symbol | Description | S. epidermidisa | S. epidermidis+sTLR2 | sTLR2 Modulatory Effect, %c | ||
|---|---|---|---|---|---|---|
| Fold Changeb | P Valueb | Fold Changeb | P Valueb | |||
| Fasl | Fas ligand (TNF superfamily, member 6) | 2.6 | 0.24 | 0.9 | 0.91 | −105.2 |
| Mmp1a | MMP-1a (interstitial collagenase) | 0.5 | 0.001 | 0.7 | 0.21 | −48.1 |
| Mmp3 | MMP-3 | 0.4 | 0.01 | 0.9 | 0.51 | −87.7 |
| Mmp9 | MMP-9 | 0.4 | 0.02 | 0.5 | 0.02 | −16.6 |
| Timp1 | TIMP-1 | 0.6 | 0.22 | 0.3 | 0.04 | N/A |
N/A, not applicable.
Only statistically significant (P<0.05) S. epidermidis–induced ≤0.5- or ≥2-fold changes were considered.
Compared with the control group.
Compared with the effect of S. epidermidis treatment.
Of note, the reduced inflammation and phagocyte recruitment in the presence of sTLR2 did not affect the capacity of the mice to clear the infection, because no difference in bacterial load (peritoneum and blood) between sTLR2-treated and nontreated mice was observed (Figure 8E). This is likely because of the fact that reduced PMN recruitment was significant at the peak of their influx (approximately 12 hours), and by this time, the mice had cleared the infection almost completely. In addition, other immune mechanisms (e.g., complement activation) likely contribute to bacterial clearance.
The fibrosis-preventing capacity of sTLR2 extended to fibrosis induced by Gram-negative bacteria. Administration of sTLR2 together with E. coli resulted in reduced peritoneal fibrosis (Figure 8F), although moderate (approximately 35%) compared with that exerted on S. epidermidis–induced fibrosis (Figure 8A). This reflects the fact that sTLR2 does not operate as a TLR decoy receptor for most Gram–negative bacterial components but can still reduce Gram–negative bacteria–induced TLR–mediated fibrotic signaling by inhibiting CD14, a coreceptor for most TLRs.42 Thus, the therapeutic administration of sTLR2 can inhibit peritoneal fibrosis development induced by repeated peritoneal bacterial infections by acting on a number of proinflammatory and fibrotic mediators but critically, without affecting infection resolution.
Discussion
Peritoneal fibrosis (and the concomitant functional alterations in the peritoneal membrane) is a major cause of PD cessation. It is detected, to a variable extent, in approximately 50% and 80% of patients within 1 and 2 years on PD, respectively.45–47 Peritoneal inflammation is a major driver of fibrosis, and treatment options to reduce or prevent it have been suggested.1 They include strategies to improve residual renal function to limit the production and activity of inflammatory cytokines; the use of biocompatible PD solutions to reduce peritoneal membrane injury and cellular stress; the use of catheters resistant to bacterial biofilm formation; the use of twin bags, Y-set systems, and antibiotic administration before PD catheter insertion to prevent peritoneal infection; and improvement in fluid status to prevent endotoxemia. In addition, strategies to inhibit the profibrotic activity of TGF-β have been evaluated.48 However, a limited number of intervention studies have not yet been able to define effective strategies to consistently lower the inflammatory burden in these patients.1 Critically, studies to identify the main peritoneal receptors involved in triggering the initial proinflammatory and fibrotic responses and develop therapeutic strategies to reduce their activation have not been conducted. Here, we identified peritoneal TLR2 and TLR4 as critical proinflammatory and fibrotic receptors and revealed their potential as therapeutic targets against PD–associated fibrosis development. We described a therapeutic strategy that targets TLRs by using a decoy soluble receptor, sTLR2, which also inhibits the activity of the common TLR coreceptor CD14, thus reducing proinflammatory and fibrotic responses to different pathogens and endogenous TLR ligands.
The therapeutic potential of targeting TLRs was highlighted by the pivotal role that TLR2 and TLR4 activation showed in microbial– and sterile inflammation–induced profibrotic responses in vivo and ex vivo in patients’ uremic leukocytes and the conclusive demonstration that the intensity of the TLR–mediated proinflammatory responses dictates the severity of peritoneal fibrosis. Thus, an efficient therapy against peritoneal fibrosis should not only treat recurrent infections but also, reduce the severity of inflammatory episodes. In this regard, the observation that, in our in vivo model of bacteria-induced fibrosis, the transcript levels of many inflammatory and fibrotic mediators remained elevated long after the resolution of the infectious episode (a finding in agreement with previous reports in clinical PD49,50) highlights the need for a therapy capable of maintaining low levels of these mediators, like the one proposed here.
The potential of sTLR2 as an efficient therapy was further shown by its ability to inhibit peritoneal inflammation and fibrosis development in mice without affecting their capacity to clear the infection. This presents a clear advantage over strategies to block TLR activation completely (for example, by using antibodies), because these would be predicted to have a detrimental effect on infection clearance. Preclinical studies have, however, evaluated combining anti-TLR2/TLR4 antibodies with broad spectrum antibiotics to achieve strong anti–inflammatory effects while controlling infection.51,52 Thus, a comparative evaluation of the sTLR2 strategy and these alternative therapies in PD models of infection/fibrosis will be required. The efficacy of sTLR2 either as a preventive or therapeutic treatment also remains to be assessed, because in this in vivo study, it was administered together with the infecting bacteria. Because of its ability to target both the TLR ligand and the common TLR coreceptor, CD14, sTLR2 presents a dual therapeutic benefit as shown here by its capacity to inhibit proinflammatory and fibrotic responses in models of Gram–positive and –negative bacterial peritoneal infections and patients’ sterile PDE activating cells via TLR2 or TLR4. The sTLR2–based antifibrotic strategy described here could, thus, prove to be a valuable adjunct to standard antibiotic therapies during PD infections or more biocompatible PD solutions. sTLR2’s capacity to modulate CD14 activity would also be potentially useful against fungal and viral infections in which TLRs other than TLR2 and TLR4 are involved. It may also be potentially useful in fibrosis-associated conditions of organs, such as the kidney, lung, and liver, and other PD–associated inflammatory conditions (for example, to help reduce the elevated risk of cardiovascular diseases).53
Concise Methods
Cells and PDE
Samples from healthy individuals (omentum and blood) and patients on PD (spent PD dialysate) were obtained in accordance with the institutional review board of Cardiff University and the local National Health Service Research Ethics Committee. Written informed consent was obtained from all donors. Sampling was carried out within the United Kingdom Clinical Research Network under study portfolios identification numbers 11838 (PERITPD) and 11839 (LEUKPD) and adhered to the Declaration of Helsinki. Spent PD dialysates were obtained from continuous ambulatory patients on PD after an overnight dwell. Resident peritoneal leukocytes were obtained by centrifugation (two times at 425×g for 15 minutes at 4°C) of noninfected dialysates and cultured in RPMI 1640 Medium (Invitrogen, Carlsbad, CA ) supplemented with 10% FCS (<0.06 U/ml endotoxin; heat inactivated; 56°C for 30 minutes; HyClone Laboratories, Logan, UT). The cellfree (centrifuged) dialysate supernatants were aliquoted and kept frozen (−85°C) until further use. To confirm the uremic status of the patients, the levels of urea and creatinine in the PDE and blood were tested (Clinical Pathology Laboratory, Cardiff and Vale University Hospital). Uremia was defined as urea >7.8 mM and creatinine >110 μM (United Kingdom–wide criteria defined by the National Health Service).
Peritoneal lymphocytes and macrophages were isolated from freshly prepared PDE-leukocytes after adhesion (2 hours at 37°C) in the absence of serum. The purity of the macrophage (adherent fraction ≥90%) and lymphocyte (nonadherent fraction ≥95%) populations was determined by flow cytometry on the basis of their forward and side scatter properties and CD14 expression levels. Cell viability was assessed using the eFluor Viability Dye (eBioscience, San Diego, CA) and always ≥85%.
Peritoneal mesothelial cells were prepared by tryptic digest of omental biopsies obtained after elective surgery of patients not on PD as previously described5 and cultured in M199 medium (Invitrogen) supplemented with insulin (0.5 μg/ml), transferrin (0.5 μg/ml), and 10% FCS.
Human PBMCs were obtained through Ficoll density gradient centrifugation and cultured in RPMI 1640 Medium supplemented with 10% FCS.
Functional Assays
For activation experiments (Figures 1, 2, and 4), triplicate aliquots of peritoneal leukocytes, lymphocytes, macrophages, and PBMCs (1.5×104 cells per well in 96-well plates) or mesothelial cells (4×104 cells per well in 48 well-plates) were cultured in the presence of the indicated concentrations of ultrapure LPS (E. coli O111:B4 strain; Invivogen), the synthetic bacterial lipopeptide Pam3Cys HCl (EMC Microcollections), heat–killed E. coli (O111:B4 strain; Invivogen), or heat–killed (20 minutes at 100°C) S. epidermidis (ATCC 12228; American Type Culture Collection, Manassas, VA) in the absence or presence of the indicated concentrations of purified human C5a (Comptech). For the experiments shown in Figure 4, the medium was supplemented with the patients’ own PDE (1:2 dilution) to maintain the uremic milieu throughout the experiment. Cell culture supernatants were collected for cytokine assays after 16 hours of stimulation. For TGF-β determinations, cells were washed (RPMI 1640) after 48 hours of stimulation and cultured for another 24 hours in the absence of FCS before culture supernatants were collected. Supernatants were tested for IL-8, TGF-β, IL-13, and TIMP-1 by ELISA (R&D Systems, Minneapolis, MN) and IL-6, TNF-α, IL-10, MMP-1, MMP-3, and MMP-9 by using the multiplex ELISA platform Meso Scale Discovery. RNA was extracted, and gene array analyses were performed as described below.
For testing cell responses to sterile cellfree PDE supernatants (Figure 5), peritoneal leukocytes (1.5×104 cells per well) were stimulated with PDE (1:2 dilution) from patients with no ongoing clinical peritoneal infection and no history of infection in the 6 months before PDE collection. The sterility of the cellfree PDE used was confirmed by negative bacterial growth (on microbiologic agar plates) and bacterial DNA detection by quantitative PCR. Briefly, total DNA was extracted from cellfree PDE of noninfected patients by a chelex-based method as previously described.54 One PDE sample was spiked with heat–killed S. epidermidis before extraction to serve as a positive control. Panbacterial DNA was then amplified by quantitative PCR using the Femto Bacterial DNA Quantification Kit (Zymo Research), and the amount of DNA in PDE was determined using the kit’s internal standard curve as a reference. A Ct of 30 cycles, corresponding to 10 fg bacterial DNA per 1 ml PDE, was set as a cutoff for PDE sterility (Supplemental Figure 3).
sTLR2 depletion from PDE before cell stimulations was performed as previously described41 by sequential immunoprecipitations (four rounds) with the purified polyclonal anti–TLR2 antibody TLR2p (generated in our laboratories41 by immunization with an N–terminal 20-mer TLR2 peptide; SKEESSNGASLSGDRNGIGK) or isotype control (mock depletion; purified rabbit Igs; Sigma-Aldrich, St. Louis, MO). The extent of the sTLR2 depletion was up to 85% depending on the experiment as judged by Western blotting followed by densitometric analysis of the gels. In some experiments (Figure 7), sTLR2 levels in PDE were determined by ELISA (R&D Systems). For the experiment shown in Figure 7F, PDE–induced cell stimulation was conducted in the presence of human recombinant sTLR2 (500 ng/ml; R&D Systems).
For blocking experiments, cells were preincubated (30 minutes at 37°C) with 5 μg/ml functional–grade anti–TLR2 (clone T2.5; eBioscience), anti–TLR4 (clone 3C3; Hycult), anti–CD14 (clone MY4; Beckman Coulter, Inc., Brea CA), or anti–C5aR (clone S5/1; Hycult) blocking mAbs or the corresponding isotype–matched control.
Flow Cytometry
TLR2, TLR4, CD14, and C5aR cell surface expression was determined by flow cytometry as previously described55 using anti-TLR2 (clone T2.5), anti-TLR4 (clone 15C1), anti-CD14 (clone MY4), and anti-C5aR (clone S5/1) mAbs and isotype-matched controls. To determine C5L2 expression levels, staining with a PE–conjugated anti–C5L2–specific mAb (clone 1D9, Biolegend) was performed at room temperature to allow for antibody internalization and detection of intracellular C5L2. Peritoneal lymphocytes and macrophages were identified in the peritoneal leukocyte preparations from PDE by their CD14 staining and forward and side scatter profiles (Supplemental Figure 4). Cell viability (eFluor Viability Dye) was always ≥85%.
In Vivo Experiments
All procedures were carried out under a Home Office Project License. Inbred 8- to 10-week-old WT (C57/BL6J, C57BL/10J, and BALB/cJ), TLR2−/− (B6.129-Tlr2tm1Kir/J), TLR4−/− (C57BL/10ScNJ), and C5aR−/− (C.129S4[B6]-C5ar1tm1Cge/J) mice were obtained from The Jackson Laboratory (Bar Harbor, ME), and C5L2−/− mice (on a BALB/c background) were bred and maintained in the laboratory of J.K. Mice were intraperitoneally inoculated with PBS (500 μl), SES (an S. epidermidis cellfree supernatant prepared as previously described56), heat–killed or live S. epidermidis (5×108 CFU per mouse), or heat–killed E. coli (2×107 CFU per mouse) in the absence or presence of previously defined optimal doses of the TLR2-derived peptide 9 (TNSLIKKFTFRNVKITDESLFQVMKLLN; 20 μg per mouse; >95% pure; Lifetein)20 or human recombinant sTLR242 (250 ng per mouse). Injections were repeated weekly for 4 or 2 weeks as indicated, and mice were then left untreated for a week (day 28) or 4 weeks (day 49) before parietal peritoneal membrane was collected for fibrosis assessment. Through the use of this model (four injections with one per week), fibrosis became detectable at day 49 after repeated injection with SES and whole (heat–killed or live) S. epidermidis or E. coli as well as injection of PBS, albeit to a much lesser extent (Supplemental Figure 2). The latter most likely resulted from sterile inflammation induced by tissue injury/cellular stress. SES, S. epidermidis, and E. coli are known to induce cell responses mainly via TLR2 (SES5 and S. epidermidis21) and TLR4 (E. coli23,24).
For determination of leukocyte numbers, cytokine levels, and bacterial load, mice were euthanized at the indicated time points after the first injection, their peritoneal cavity was lavaged with 2 ml ice-cold PBS, and peripheral blood was obtained by cardiac puncture. Leukocyte numbers in the lavages were determined by Coulter Counting (Coulter Z2; Beckman Coulter, Inc.) and differential double staining (anti–Ly6-PE; Becton Dickinson, San Diego, CA; and anti–7/4-A647; Serotec) followed by flow cytometric analysis. Cytokine levels were determined by ELISA, and bacterial CFU in peritoneal lavages and blood samples was determined by culturing on Mueller–Hinton agar plates (Oxoid) overnight at 37°C.
Peritoneal Membrane Histology
Peritoneal membrane biopsies were fixed (24 hours at 4°C) with a neutral buffered 10% formalin solution (Sigma-Aldrich) saline embedded in paraffin, and sections (5-μm thickness) were stained with hematoxylin and eosin. Slides were analyzed with a Leica DFC49 Microscope (×40 objective; Leica Microsystems, Buffalo Grove, IL) and camera, and the thickness of the submesothelial cell compact zone was measured as previously described.3 Briefly, three different fields of view were examined for each mouse, six measurements were made per field, and the average of the 10 highest measurements was taken as the mean of submesothelial cell compact zone thickness for each animal.
Western Blotting
The Western blot technique was as previously described.57 Cellfree PDE samples were diluted 1:20 in Laemmli reducing sample buffer and subjected to 10% SDS-PAGE before Western blot analysis using the anti–TLR2 polyclonal antibody TLR2p described above (1:2000) or an anti–HMGB-1 polyclonal antibody (1:1000; Ab18256; Abcam, Inc., Cambridge, MA). Densitometric analysis was performed using the ImageJ software.
Fibrosis–Focused Gene Arrays
Total RNA was isolated from peritoneal leukocytes or mice peritoneal membranes using the RNeasy Mini Kit (Qiagen, Germantown, MD). RT-PCR was performed on 200 ng RNA using the RT2 First Strand Kit (including a genomic DNA elimination step; Qiagen). Fibrosis gene array quantitative RT-PCR was performed on the resulting cDNA using the human or mouse fibrosis RT2 Profiler PCR Array (85 genes; Qiagen) and the ABI ViiA7 Thermocycler. The results were analyzed using Qiagen software. Changes in gene expression compared with control were considered statistically significant when P<0.05 and biologically relevant when the fold change was ≤0.5 or ≥2 as recommended by the manufacturer. The complete dataset is shown in Supplemental Table 1.
Statistical Analyses
Statistical analyses of the data were performed by using an unpaired t test. P values of <0.05 were considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank M. López-Cabrera (Centro de Biología Molecular-Severo Ochoa-Consejo Superior de Investigaciones Científicas, Madrid, Spain), C. Fielding (Cardiff University), and J.E. Rey Nores (Cardiff Metropolitan University) for critical comments and reading of the manuscript.
This work was supported by the National Institute of Social Care and Health Research (NISCHR) and Kidney Research UK (KRUK) Fellowships (to A.-C.R.), grant SFB/TR22 from the Deutsche Forschung Germany (to J.K.), grants from the NISCHR, Wales (to M.E. and M.O.L.), and a KRUK project grant (to M.E.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015080923/-/DCSupplemental.
References
- 1.Cho Y, Hawley CM, Johnson DW: Clinical causes of inflammation in peritoneal dialysis patients. Int J Nephrol 2014: 909373, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambie M, Chess J, Donovan KL, Kim YL, Do JY, Lee HB, Noh H, Williams PF, Williams AJ, Davison S, Dorval M, Summers A, Williams JD, Bankart J, Davies SJ, Topley N; Global Fluid Study Investigators : Independent effects of systemic and peritoneal inflammation on peritoneal dialysis survival. J Am Soc Nephrol 24: 2071–2080, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fielding CA, Jones GW, McLoughlin RM, McLeod L, Hammond VJ, Uceda J, Williams AS, Lambie M, Foster TL, Liao CT, Rice CM, Greenhill CJ, Colmont CS, Hams E, Coles B, Kift-Morgan A, Newton Z, Craig KJ, Williams JD, Williams GT, Davies SJ, Humphreys IR, O’Donnell VB, Taylor PR, Jenkins BJ, Topley N, Jones SA: Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity 40: 40–50, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S: The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol 11: 373–384, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Colmont CS, Raby AC, Dioszeghy V, Lebouder E, Foster TL, Jones SA, Labéta MO, Fielding CA, Topley N: Human peritoneal mesothelial cells respond to bacterial ligands through a specific subset of Toll-like receptors. Nephrol Dial Transplant 26: 4079–4090, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen GY, Nuñez G: Sterile inflammation: Sensing and reacting to damage. Nat Rev Immunol 10: 826–837, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anders HJ, Schaefer L: Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol 25: 1387–1400, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawasaki T, Kawai T: Toll-like receptor signaling pathways. Front Immunol 5: 461, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, Zetoune FS, Gerard NP, Cianflone K, Köhl J, Gerard C, Sarma JV, Ward PA: Functional roles for C5a receptors in sepsis. Nat Med 14: 551–557, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen NJ, Mirtsos C, Suh D, Lu YC, Lin WJ, McKerlie C, Lee T, Baribault H, Tian H, Yeh WC: C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature 446: 203–207, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Bamberg CE, Mackay CR, Lee H, Zahra D, Jackson J, Lim YS, Whitfeld PL, Craig S, Corsini E, Lu B, Gerard C, Gerard NP: The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem 285: 7633–7644, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riedemann NC, Guo RF, Hollmann TJ, Gao H, Neff TA, Reuben JS, Speyer CL, Sarma JV, Wetsel RA, Zetoune FS, Ward PA: Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB J 18: 370–372, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC: Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood 110: 228–236, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Krauss JL, Domon H, Hosur KB, Liang S, Magotti P, Triantafilou M, Triantafilou K, Lambris JD, Hajishengallis G: Microbial hijacking of complement-toll-like receptor crosstalk. Sci Signal 3: ra11, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raby AC, Holst B, Davies J, Colmont C, Laumonnier Y, Coles B, Shah S, Hall J, Topley N, Köhl J, Morgan BP, Labéta MO: TLR activation enhances C5a-induced pro-inflammatory responses by negatively modulating the second C5a receptor, C5L2. Eur J Immunol 41: 2741–2752, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanzler H, Barrat FJ, Hessel EM, Coffman RL: Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med 13: 552–559, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Dunne A, Marshall NA, Mills KH: TLR based therapeutics. Curr Opin Pharmacol 11: 404–411, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Mollnes TE, Christiansen D, Brekke OL, Espevik T: Hypothesis: Combined inhibition of complement and CD14 as treatment regimen to attenuate the inflammatory response. Adv Exp Med Biol 632: 253–263, 2008 [PubMed] [Google Scholar]
- 19.Riedemann NC, Guo RF, Ward PA: Novel strategies for the treatment of sepsis. Nat Med 9: 517–524, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Raby AC, Holst B, Le Bouder E, Diaz C, Ferran E, Conraux L, Guillemot JC, Coles B, Kift-Morgan A, Colmont CS, Szakmany T, Ferrara P, Hall JE, Topley N, Labéta MO: Targeting the TLR co-receptor CD14 with TLR2-derived peptides modulates immune responses to pathogens. Sci Transl Med 5: 185ra64, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Strunk T, Power Coombs MR, Currie AJ, Richmond P, Golenbock DT, Stoler-Barak L, Gallington LC, Otto M, Burgner D, Levy O: TLR2 mediates recognition of live Staphylococcus epidermidis and clearance of bacteremia. PLoS One 5: e10111, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zähringer U, Lindner B, Inamura S, Heine H, Alexander C: TLR2 - promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology 213: 205–224, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S: Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J Immunol 162: 3749–3752, 1999 [PubMed] [Google Scholar]
- 24.Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D, Burns K, Riederer BM, Akira S, Calandra T: Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci U S A 106: 2348–2352, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elson G, Dunn-Siegrist I, Daubeuf B, Pugin J: Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood 109: 1574–1583, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A: The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410: 1099–1103, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, Wakefield LM, Letterio JJ, Wynn TA: IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J Immunol 173: 4020–4029, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Leask A, Abraham DJ: TGF-beta signaling and the fibrotic response. FASEB J 18: 816–827, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Pohlers D, Brenmoehl J, Löffler I, Müller CK, Leipner C, Schultze-Mosgau S, Stallmach A, Kinne RW, Wolf G: TGF-beta and fibrosis in different organs - molecular pathway imprints. Biochim Biophys Acta 1792: 746–756, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Giannandrea M, Parks WC: Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech 7: 193–203, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verbrugh HA, Van Dijk WC, Peters R, Van Der Tol ME, Verhoef J: The role of Staphylococcus aureus cell-wall peptidoglycan, teichoic acid and protein A in the processes of complement activation and opsonization. Immunology 37: 615–621, 1979 [PMC free article] [PubMed] [Google Scholar]
- 32.Harboe M, Garred P, Lindstad JK, Pharo A, Müller F, Stahl GL, Lambris JD, Mollnes TE: The role of properdin in zymosan- and Escherichia coli-induced complement activation. J Immunol 189: 2606–2613, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamouille S, Xu J, Derynck R: Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15: 178–196, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang S, Leung JC, Chan LY, Tsang AW, Chen CX, Zhou W, Lai KN, Sacks SH: Regulation of complement C3 and C4 synthesis in human peritoneal mesothelial cells by peritoneal dialysis fluid. Clin Exp Immunol 136: 85–94, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young GA, Kendall S, Brownjohn AM: Complement activation during CAPD. Nephrol Dial Transplant 8: 1372–1375, 1993 [PubMed] [Google Scholar]
- 36.Reddingius RE, Schröder CH, Daha MR, Willems HL, Koster AM, Monnens LA: Complement in serum and dialysate in children on continuous ambulatory peritoneal dialysis. Perit Dial Int 15: 49–53, 1995 [PubMed]
- 37.Yewdall VM, Boscoe MJ, Bennett-Jones DN, Cameron JS: Clinical applications of crossed immunoelectrophoresis to the study of complement activation. J Clin Lab Immunol 24: 51–56, 1987 [PubMed] [Google Scholar]
- 38.Kim S, Kim SY, Pribis JP, Lotze M, Mollen KP, Shapiro R, Loughran P, Scott MJ, Billiar TR: Signaling of high mobility group box 1 (HMGB1) through toll-like receptor 4 in macrophages requires CD14. Mol Med 19: 88–98, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huebener P, Schwabe RF: Regulation of wound healing and organ fibrosis by toll-like receptors. Biochim Biophys Acta 1832: 1005–1017, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao S, Li S, Li H, Xiong L, Zhou Y, Fan J, Yu X, Mao H: The potential role of HMGB1 release in peritoneal dialysis-related peritonitis. PLoS One 8: e54647, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LeBouder E, Rey-Nores JE, Rushmere NK, Grigorov M, Lawn SD, Affolter M, Griffin GE, Ferrara P, Schiffrin EJ, Morgan BP, Labéta MO: Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J Immunol 171: 6680–6689, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Raby AC, Le Bouder E, Colmont C, Davies J, Richards P, Coles B, George CH, Jones SA, Brennan P, Topley N, Labéta MO: Soluble TLR2 reduces inflammation without compromising bacterial clearance by disrupting TLR2 triggering. J Immunol 183: 506–517, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Catalan MP, Esteban J, Subirá D, Egido J, Ortiz A; Grupo de Estudios Peritoneales de Madrid-FRIAT/IRSIN: Inhibition of caspases improves bacterial clearance in experimental peritonitis. Perit Dial Int 23: 123–126, 2003 [PubMed] [Google Scholar]
- 44.Hohlbaum AM, Gregory MS, Ju ST, Marshak-Rothstein A: Fas ligand engagement of resident peritoneal macrophages in vivo induces apoptosis and the production of neutrophil chemotactic factors. J Immunol 167: 6217–6224, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Garosi G, Di Paolo N: Morphological aspects of peritoneal sclerosis. J Nephrol 14[Suppl 4]: S30–S38, 2001 [PubMed] [Google Scholar]
- 46.Garosi G, Di Paolo N: Pathophysiology and morphological clinical correlation in experimental and peritoneal dialysis-induced peritoneal sclerosis. Adv Perit Dial 16: 204–207, 2000 [PubMed] [Google Scholar]
- 47.Schneble F, Bonzel KE, Waldherr R, Bachmann S, Roth H, Schärer K: Peritoneal morphology in children treated by continuous ambulatory peritoneal dialysis. Pediatr Nephrol 6: 542–546, 1992 [DOI] [PubMed] [Google Scholar]
- 48.Tomino Y: Mechanisms and interventions in peritoneal fibrosis. Clin Exp Nephrol 16: 109–114, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Lai KN, Lai KB, Lam CW, Chan TM, Li FK, Leung JC: Changes of cytokine profiles during peritonitis in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis 35: 644–652, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Yung S, Chan TM: Pathophysiological changes to the peritoneal membrane during PD-related peritonitis: The role of mesothelial cells. Mediators Inflamm 2012: 484167, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spiller S, Elson G, Ferstl R, Dreher S, Mueller T, Freudenberg M, Daubeuf B, Wagner H, Kirschning CJ: TLR4-induced IFN-gamma production increases TLR2 sensitivity and drives Gram-negative sepsis in mice. J Exp Med 205: 1747–1754, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lima CX, Souza DG, Amaral FA, Fagundes CT, Rodrigues IP, Alves-Filho JC, Kosco-Vilbois M, Ferlin W, Shang L, Elson G, Teixeira MM: Therapeutic effects of treatment with anti-TLR2 and anti-TLR4 monoclonal antibodies in polymicrobial sepsis. PLoS One 10: e0132336, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fassett RG, Driver R, Healy H, Coombes JS: Cardiovascular disease in peritoneal dialysis patients. Panminerva Med 51: 151–161, 2009 [PubMed] [Google Scholar]
- 54.Walsh PS, Metzger DA, Higushi R: Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. BioTechniques 10(4): 506-13 (April 1991). Biotechniques 54: 134–139, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Rey Nores JE, Bensussan A, Vita N, Stelter F, Arias MA, Jones M, Lefort S, Borysiewicz LK, Ferrara P, Labéta MO: Soluble CD14 acts as a negative regulator of human T cell activation and function. Eur J Immunol 29: 265–276, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Hurst SM, Wilkinson TS, McLoughlin RM, Jones S, Horiuchi S, Yamamoto N, Rose-John S, Fuller GM, Topley N, Jones SA: Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 14: 705–714, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Durieux JJ, Vita N, Popescu O, Guette F, Calzada-Wack J, Munker R, Schmidt RE, Lupker J, Ferrara P, Ziegler-Heitbrock HW, Labéta MO: The two soluble forms of the lipopolysaccharide receptor, CD14: Characterization and release by normal human monocytes. Eur J Immunol 24: 2006–2012, 1994 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.