Abstract
Individuals age >65 years old are the fastest expanding population demographic throughout the developed world. Consequently, more aged patients than before are receiving diagnoses of impaired renal function and nephrosclerosis—age–associated histologic changes in the kidneys. Recent studies have shown that the aged kidney undergoes a range of structural changes and has altered transcriptomic, hemodynamic, and physiologic behavior at rest and in response to renal insults. These changes impair the ability of the kidney to withstand and recover from injury, contributing to the high susceptibility of the aged population to AKI and their increased propensity to develop subsequent progressive CKD. In this review, we examine these features of the aged kidney and explore the various validated and putative pathways contributing to the changes observed with aging in both experimental animal models and humans. We also discuss the potential for additional study to increase understanding of the aged kidney and lead to novel therapeutic strategies.
Keywords: Aging, glomerulosclerosis, kidney dysfunction, metabolism, molecular biology, progression of renal failure
The Centers for Disease Control and Prevention predicts that 72 million Americans will be ages 65 years old or older by 2030, accounting for approximately 20% of the United States population.1 Eurostat predicts that 28% of Europeans will be ages over 65 years old by 2060.2 These increasing numbers of elderly individuals will inevitably lead to increasing diagnoses of age–related kidney impairment.
In renal aging, a complex interplay of genetics, environmental change, and cellular dysfunction leads to characteristic structural and functional changes.3 This review summarizes our current understanding of the factors driving age-associated changes in the kidney.
Clinical Features of Renal Aging in Humans
Structural Changes of Aging
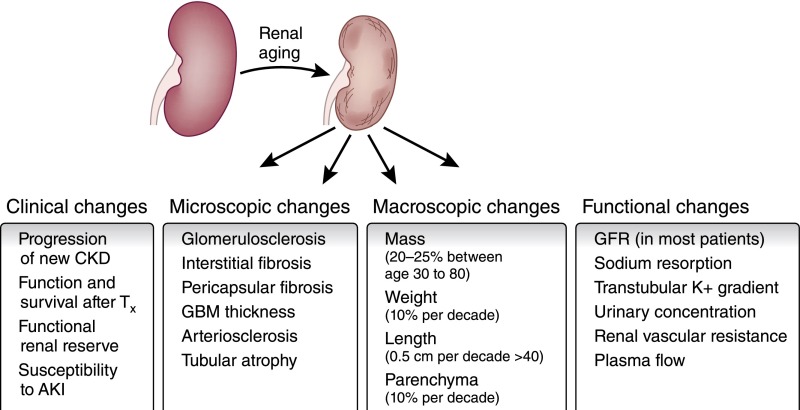
With age, there is a decline in total nephron size and number, tubulointerstitial changes, glomerular basement membrane thickening, and increased glomerulosclerosis (Figure 1).4,5 This age–related histologic appearance is frequently described as nephrosclerosis, and it describes a combination of two or more histologic features: any global glomerulosclerosis, tubular atrophy, interstitial fibrosis >5%, and any arteriosclerosis. A study of healthy kidney donors showed nephrosclerosis in only 2.7% of biopsies from donors <30 years old, 58% from donors 60–69 years old, and 73% from donors >70 years old.6 Cadaver studies estimate that the upper limit of normal glomerulosclerosis in aging exceeds 10%.7
Figure 1.
Functional and structural changes in the aging kidney. With increasing age, there are alterations in the function of the kidney. These are accompanied by both macroscopic and microscopic changes and result in an alteration in the response of the kidney to diverse insults. GBM, glomerular basement membrane; Tx, transplant.
Nephrosclerosis remains a poorly understood observation, and its importance within an aging kidney is far from clear. We know that nephrosclerosis correlates with aging and mild hypertension in healthy living donor kidneys.8 Importantly, however, age-related decline in measured GFR does not correlate with the presence or absence of nephrosclerosis.9 In fact, nephrosclerosis does not correlate with urine albumin excretion, family history of ESRD, body mass index, serum cholesterol, glucose, or uric acid.6 It remains unclear then whether nephrosclerotic changes have any contribution to the functional changes seen in aging or are perhaps distinct and unrelated.10,11
The Aging-CKD Spectrum
Our understanding of the pathways underlying renal aging is incomplete and derived from studies of healthy aging kidneys and extrapolation from experimental and clinical studies of CKD.
It is important to note the distinction between these conditions, with the mechanisms of progressive genetic–, immune–, or toxin–mediated injury seen in CKD distinct from the gradual, prevalent changes seen in the aging kidney. Throughout this review, we will focus on the changes seen in the healthy aged kidney, although due to the paucity of experimental and clinical data available in aging kidneys, at times, reference will be made to mechanisms in progressive CKD, which may also be of relevance to the uninjured but aged kidney. Processes discussed below, such as cellular senescence, fibrosis, vascular rarefaction, and glomerular loss, are common to both aging and CKD, despite differences in causation and natural history. Similarities are also seen in the behavior of the chronically damaged kidney and the aged kidney, including their heightened susceptibility to additional injury and deficient repair.12
Declining GFR
Population GFR declines with age, with longitudinal studies differing in their reported rates of decline.13,14 Although the Modification of Diet in Renal Disease Study suggested renal function declined at a rate of 3.8 ml/min per year per 1.73 m2, rates as low as 0.4 ml/min per year per 1.73 m2 in The Netherlands have been described.15–18 A Japanese cohort study suggests that the rate of GFR decline increases with advancing age.19
Studies of robustly phenotyped Kuna Indians with minimal prevalence of hypertension and cardiovascular disease show comparable declines in renal function over time, suggesting that there is a true age–related decline rather than the cumulative effects of cardiovascular disease.20 How a significant minority of individuals apparently remains free of nephrosclerosis and GFR loss remains poorly understood and merits additional study.
Decreased Tubular Function
Aging is characterized by progressive tubular dysfunction, decreased sodium reabsorption, potassium excretion, and urine concentrating capacity potentially contributing to an increased susceptibility to AKI.21–23 Elderly patients show decreased transtubular potassium gradients and fail to increase distal tubule potassium excretion when hyperkalemic or in response to fludrocortisone.24 Decreased potassium excretion correlates with decreasing GFR and may reflect a degree of reduced sodium and chloride delivery to the distal convoluted tubule.25
Vascular Changes
There are important changes to blood vessel structure and function in the aging kidney. There is increased extracellular matrix (ECM) deposition, increased intimal cell proliferation in preglomerular arterioles, and increased intrarenal shunting and capillary bypassing predominantly affecting the cortex.26
Increased renal sympathetic tone increases vasoconstriction, whereas aortic baroreceptor attenuation of sympathetic tone decreases with age.27,28 Renal vasodilators, such as atrial natriuretic peptide, nitric oxide (NO), and amino acids, become less effective.29–31 Human studies show decreased NO production and platelet responsiveness,32 with accumulation of the NO synthase inhibitor asymmetric dimethylarginine in elderly individuals.33 In particular, aging men become increasingly NO dependent to maintain renal plasma flow.34
Biologic Processes and Mediators Implicated in Experimental Aging
Most rodent experimental models of renal disease are undertaken in young animals, potentially affecting their relevance to the aging kidney. There are limited or no data available regarding the response of the aged rodent kidney to experimental GN, AKI, ureteric obstruction, diabetic nephropathy, 5/6th nephrectomy, adriamycin nephropathy, or renal transplantation. Some aspects of renal aging may be studied in vitro, but others require study in vivo in aged mice or other experimental animals (Table 1).
Table 1.
Studies of putative aging pathways in vitro, in vivo, and in human
| Aging Factor | In Vitro Studies | Experimental Studies | Human Studies |
|---|---|---|---|
| Telomere shortening | Shown in cells to reduce with length of passage. Critical shortening leads to senescence105 | Reduced in mice with age.107 Impaired regeneration after IRI108 | Reduced with age, oxidative stress, CKD, and HD.149,151 Risk factor for CVD150 |
| Klotho signaling | Klotho opposes signaling of IGF1 and insulin42 in cell lines in vitro | Klotho deficiency decreases lifespan.44 Overexpression reduces IGF1 and Wnt signaling and increases lifespan42 | Reduced with age.131 Reduction associated with calcification and vascular disease135 |
| Wnt signaling | Promotes profibrotic genes (e.g., Snail, PAI1, and MMP7)51 | Levels increase with injury and in response to falling Klotho with aging.52 Mediates renal RAAS signaling57 | Increases seen in CKD and linked to organ fibrosis196 |
| PPARγ levels | Reduces oxidative stress/senescence in human fibroblasts63 | Reduced activity with age.58,59 Agonists reduce renal inflammation/injury64 | Studies of PPARγ agonists suggest reduction in rates of proteinuria in patients with diabetes137 |
| Antioxidant capacity | Aged rats have reduced renal antioxidant capacity and enhanced renal injury.78 Reduced oxidative stress lessens renal injury197 | Higher levels of oxidative stress in human aging and CKD.73 AGE accumulates with age141 | |
| Fibrosis | AT2 promotes fibrosis of glomerular cells and promotes reduction of SIRT-389 | Collagens I and III and TGF-β are upregulated in aging mice50 and rats.65 G2/M arrest is implicated in postinjury renal fibrosis92 | Nephrosclerosis is a feature of aging and of hypertensive renal disease.10,11 Fibrosis and AT2 hypersensitivity seen in aged kidneys140 |
| Senescence/G1 arrest | Human and animal cells undergo senescence in vitro in response to stress or prolonged culture.94 p16INK4a KO epithelial cells convert to mesenchyme more readily101 | p16INK4a and SA-β-galactosidase are markers for senescent cells and increased in aged animals and postinjury. G2/M arrest seen in scarred kidneys in response to injury92 | Increased numbers of senescent renal cells correlate with increased injury and reduced transplant function145,146 |
| Vascular changes | Aged mice aortas have increased G2/M–phase cell cycle arrest in vitro198 | Reduced renal capillary density in aged mice124 and in response to severe IRI114 | Increased renal vascular tone and vascular stiffening with age.199 Loss of efficacy of vasodilators200 |
| Pericyte behavior | Pericytes (but not myofibroblasts) stabilize endothelial cell cultures in vitro173 | Reduction of interstitial pericytes with aging.124 Increased myofibroblasts in response to UUO and IRI201 | Comparative studies in aged humans (with or without CKD) have not been undertaken |
Changes in activity of various signaling pathways and mechanisms implicated in the response of kidney to increasing age. Column 2 indicates cellular changes observed in vitro, column 3 reports effects seen in experimental models of renal aging and injury, and column 4 shows any reported effects in human aging and renal disease. HD, hemodialysis; CVD, cardiovascular disease; PAI1, plasminogen activator inhibitor 1, RAAS, renin-angiotensin-aldosterone system; SIRT-3, sirtuin-3; KO, knockout; SA, senescence associated; UUO, unilateral ureteral obstruction.
Studies have shown increased susceptibility of the aged kidney to ischemia-reperfusion injury (IRI) or toxic AKI.35,36 Aged mice exhibit increased mortality, AKI severity, and chemokine/cytokine responses in a model of uterine sepsis.37 Furthermore, aged mice exhibited increased mortality, prolonged injury, reduced regeneration, increased scarring, and microvascular rarefaction after renal IRI compared with young mice.38
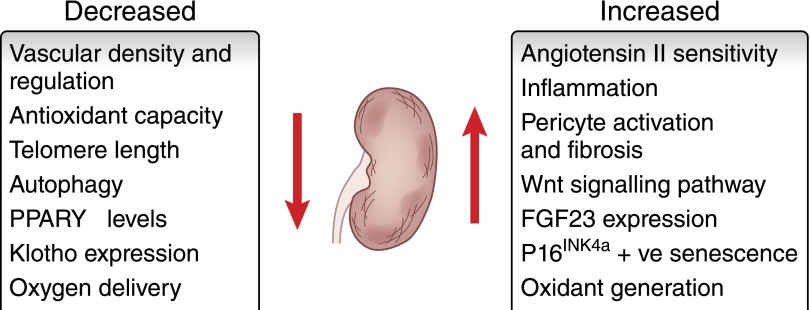
The biology of aging is complex, involving diverse changes to cells, tissues, organs, and the surrounding microenvironment (Figure 2). Many of these processes and mediators are discussed below, but the reader should appreciate that this list is not exhaustive.
Figure 2.
Alterations in cellular and physiologic pathways in the aging kidney. Diverse physiologic, cellular, and gene expression alterations occur in the aging kidney, affecting homeostasis, function, and the response to renal injury.
Signaling Pathways and Oxidative Stress in the Aging Kidney
Falling Klotho Levels
Klotho is a transmembrane protein strongly expressed in the kidney and a coreceptor for fibroblast growth factor-23 (FGF-23). Although its exact physiologic role in aging remains incompletely understood, Klotho has a role in modulating diverse aging–associated pathways. These include calcium and phosphate metabolism, with implications for vascular calcification, hypoxia, cellular regeneration, and senescence. Indeed, homozygous transgenic Klotho knockout mice show arteriosclerosis and vascular changes as part of their aging phenotype.39 Similarly, FGF-23 knockout mice display high serum phosphate and increased renal phosphate reabsorption in addition to their aging-like phenotypes.40,41 It may be that these vascular changes contribute directly to the aging phenotype that we observe.
Klotho’s effects on tissue function, autophagy, and fibrosis could contribute to abnormal healing and possibly, nephrosclerosis.42,43 Importantly, Klotho-deficient mice exhibit reduced lifespan, skin and muscle atrophy, osteoporosis, and ectopic calcification.44 Conversely, mice overexpressing Klotho have a longer mean lifespan.42
Klotho decreases epithelial senescence in response to oxidative stress, reduces binding of NFκB, and increases cell survival in experimental uremia.45 Klotho also represses insulin and IGF1 signaling, likely contributing to reduced oxidative stress in mice and in vitro models using Klotho overexpression.42,44,46 Importantly, Klotho supplementation in a rat UUO model attenuated renal fibrosis.47
Increasing Wnt Activation
Mechanisms for the antifibrotic effects of Klotho include suppression of FGF and modulation of Wnt signaling.48–50 Wnt is a conserved signaling pathway activated postinjury that promotes profibrotic gene expression.51 As Klotho levels fall during aging, Wnt signaling increases, promoting fibrosis and vascular calcification,52 although additional experiments are required to clarify causality. Wnt activation promotes renal fibrosis in murine models and is a target for inhibition,53,54 with antagonism of Wnt and its downstream targets ameliorating experimental renal fibrosis.55,56 The interplay between potentially causative pathways is illustrated by studies showing that renin-angiotensin-aldosterone signaling is Wnt mediated, with experimental blockade protecting mice from postinjury fibrosis and proteinuria.57
Declining Peroxisome Proliferator–Activated Receptor-γ Levels
Peroxisome proliferator–activated receptor-γ (PPARγ) is a nuclear receptor with activity that decreases with age in experimental rodent models, whereas PPARγ agonists increase Klotho expression.58,59 The PPARγ pathway protects against oxidative stress and improves vascular function in vitro and in aging rats,60–62 with PPARγ agonists protecting human fibroblasts against features of aging and oxidative stress in vitro.63 PPARγ agonism by pioglitazone or baicalin improves age–related vascular oxidative stress or renal inflammation, respectively, providing a potential therapeutic strategy for elderly patients with reduced PPARγ activity.59,64
Angiotensin II
Angiotensin II (AT2) is increased in aged rats compared with young controls,65 driving increased fibrosis, glomerular cell growth and ECM accumulation,66 altered mitochondrial redox function, and cytoplasmic oxidative stress in the aging kidney.65,67,68 Angiotensin I receptor activation simulates the profibrotic β-catenin/Wnt pathway mentioned above.69 Treating aging rats with captopril reduces TGF-β activity and attenuates renal fibrosis.70,71 AT2 antagonism via ACEi/ARB improves mitochondrial number and function in rats, and additional studies are warranted.72
Oxidative Stress
A balance exists in tissues between reactive oxygen species (ROS) generation and oxidant scavenging and defense mechanisms. When this balance is disturbed by increased generation of ROS, decreased detoxification, or both, then oxidative stress may occur. It has been hypothesized that oxidative stress leads to tissue damage and contributes to the aging phenotype. Certainly, there is evidence in murine and human studies of both increased ROS generation and altered oxidant removal in aging.73–75
There is a continuous generation of oxidative species through various mechanisms, including mitochondrial oxidative phosphorylation, which increases within the aging kidney.75,76 Studies in aged rat kidneys support the theory that there is also reduced oxidant defense showing decreased antioxidative capacity and reduced levels of Cu/Zn-SOD, catalase, and GSH reductase.77,78 This overall increased oxidative load may contribute to chronic cellular stress and mitochondrial injury76 as well as apoptosis and possibly, may induce tubular cell damage.79,80
Contributing to this increased oxidative stress, it has been noted that sirtuins (important antioxidant molecules) are diminished with age. Sirtuins protect against renal inflammation, fibrosis, and apoptosis while improving autophagy.81,82 Thus, defective ability to respond to cell stress in aged kidneys may contribute to the aged phenotype.83 Mouse models of reduced SIRT-1 expression show increased apoptosis and fibrosis after UUO.84 Additional Sirtuin functions include histone deacetylation and regulation of transcription factors controlling cellular stress and survival.85,86 Altered Sirtuin levels in aging may contribute to aging phenotypes by altering the kidneys capacity to respond to oxidative stress and thus, suffering increased oxidative DNA damage.87,88 Interestingly, AT2 downregulates SIRT-3 in vitro, suggesting that the damaging effects of raised AT2 levels and low Sirtuin levels may be related in the aging kidney.89
Cell Cycle Progression in the Aged Kidney
Aged animals have reduced proliferative responses after experimental IRI. Tubular epithelial cells in aged mice express higher levels of zinc-α-(2)-glycoprotein (AZGP1), limiting proliferation after IRI.90 Although reduced proliferation might be expected to delay recovery, AZGP1 knockout mice displayed worsened fibrosis after IRI, with AZGP1 administration being protective, implicating control of proliferation as a mechanism-limiting fibrosis with aging.91 Studies in several CKD models show that G2/M arrest in tubular epithelial cells promotes renal fibrosis, but no studies have examined G2/M arrest in aging kidneys.92
Cellular senescence, defined as a state of permanent cell cycle arrest, is a key antiproliferative response to aging and injury. This crucial process shuts down damaged cells, protects against malignant transformation, and limits excess fibrosis at both baseline and after injury.93
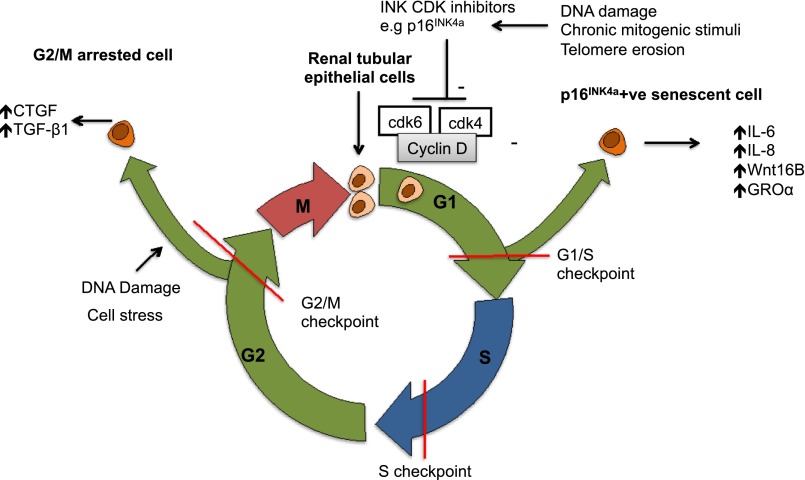
Senescence may occur as a result of repeated cell division and telomere shortening (replicative senescence) or after factors, such as oxidative stress or genotoxic injury (stress–induced premature senescence) (Figure 3).94 Increased numbers of senescent cells accumulate in multiple organs, including the kidney with advancing age (identified by p16INK4a or senescence–associated β-galactosidase expression).
Figure 3.
Cell cycle progression in the aged kidney. Cell cycle arrest in G1/S phase becomes more prevalent with age and results in p16INK4a–positive senescent cells expressing multiple cytokines and promoting autocrine and paracrine changes in aged kidneys. Although studies are lacking in aged animals, increased G2/M cell cycle arrest in response to injury promotes maladaptive repair in murine kidney injury, with raised G2/M counts correlating with fibrosis.92,194 G2/M cell cycle arrest may have variable effects in different cell types, being profibrotic in renal tubular cells but preventing intimal hyperplasia in young smooth muscle cells.195 CTGF, connective tissue growth factor β; GROa, human growth regulated oncogene-α; CDK, cyclin-dependent kinase.
Cell senescence limits fibroblast proliferation in tissue wounds; however, there is increasing interest in the role of the Senescence–Associated Secretory Phenotype (SASP) in promoting fibrosis.93 SASP promotes fibrosis and organ dysfunction in aging via release of factors, including IL-6, IL-8, Wnt16B, and GROα.95–97 Studies in murine renal transplantation showed that renal p16INK4a deletion reduced pathologic changes and interstitial fibrosis post-IRI, supporting clinical findings that cellular senescence contributes to adverse long–term allograft outcomes.98 Cell stress is known to induce stress–induced premature senescence, and consistent with this, porcine models have shown that renal p16INK4a expression increases after IRI.99 Interestingly, p16INK4a knockout mice exposed to experimental renal injury show improved recovery after IRI but worsened fibrosis after UUO.100,101 These superficially inconsistent findings may reflect the different pathologic processes at play, with p16INK4a deficiency leading to less cell death and enhanced regenerative proliferation in AKI but the lack of p16INK4a-induced senescence inducing an exaggerated, maladaptive fibroblast response to ongoing injury in UUO.
Recent seminal studies used transgenic animals to induce specific depletion of p16INK4a expressing senescent cells and showed reduced markers of aging in multiple organs, including the kidney, and increased overall lifespan.102 Other work has used Bcl2/xL inhibitors to deplete senescent cells in nontransgenic animals.103 Although these findings open up exciting new therapeutic avenues for the selective targeting of senescent cells to prolong healthy lifespan, additional studies focusing on the aging kidney are required.
Telomere Shortening
Telomeres are nucleotide sequences that act as a defensive cap, limiting activation of DNA repair pathways, protecting genetic material, and minimizing background cellular stress response.104,105 Although telomere length declines with age, it remains controversial whether this is a primary process or a byproduct of aging.104,106 As telomeres shorten with aging and oxidative stress, chromosome instability ensues, leading to cellular instability, senescence, and subsequent apoptosis.107
Increased telomere shortening in telomerase-deficient mice is associated with increased tubular injury and reduced tubular proliferation after renal IRI, with reduced tubular cell autophagy implicated in the limited regenerative response.108,109 This implies a potential causal role for telomere shortening in some of the vulnerability of aging kidneys to injury, and it is noteworthy that experimental elongation of shortened telomeres resulted in partial reversal of aged organ degeneration.110
Hypoxic Damage and Disordered Repair
Under physiologic conditions, the kidney is supported by a network of resident mononuclear phagocytes and pericytes contributing to tissue homeostasis and vascular stability. Renal oxygen delivery and the functional status of resident and recruited cells in the kidney have been shown to alter in aged and injured experimental animals.
Hypoxia
Although the healthy kidney has areas of low oxygen tension, reduced capillary density and increased hypoxia are recognized as potential drivers of CKD, and the role in normal aging is being explored. In experimental CKD, the expected angiogenic response to hypoxia fails, instead resulting in fibrosis.111 Increased renal hypoxia has also been shown throughout aged rat kidneys, most prominently in the cortical zones, as detected by use of the hypoxia–sensitive marker pimonidazole.112 Aged rat kidneys show decreased VEGF globally and increased antiangiogenic thrombospondin-1, resulting in capillary loss with increased glomerular sclerosis.113 Recently reported techniques to quantify subtle changes in the renal vasculature have potential to yield new information on the evolution of renal circulatory changes and hypoxia with advancing age.114
Leukocytes
Changes in leukocyte function promoting inflammatory activation occur with aging, although whether this is a cause or effect of aging remains unclear.115 Increased inflammatory signaling and macrophage infiltration116 with alterations in inflammasome components, such as NOD–like receptor P3, NLRC4, procaspase-1, NFκB, and cytokines, including IL-1β and IL-18, occur in aging.117 Aged murine macrophages show impaired autophagy and reduced nitrate release and phagocytosis.118 Healthy aged mice have increased glomerular macrophage numbers with increased macrophage infiltration evident postinjury, with renal IRI models showing an increased influx of macrophage and T lymphocytes.38,119 Additionally, aged mice show defective upregulation of the cytoprotective enzyme hemoxygenase-1 after IRI, with pharmacologic macrophage hemoxygenase-1 induction protecting against subsequent IRI.35 Finally, aged macrophages express reduced anti–inflammatory IL-10 during tissue repair in nonrenal injury models.120 Given the importance of IL-10 and the negative prognostic role of macrophage infiltrates in human renal disease, these aging-associated changes potentially contribute to the increased rates of injury and maladaptive repair seen in aged kidneys.
Additional evidence for the importance of the aging immune system in renal aging comes from young-old bone marrow transplant studies showing that aged animals receiving bone marrow transplants from young mice exhibited reduced renal fibrosis and cellular senescence.121
Pericytes
Although important for microvascular health, pericytes are also recognized as key cells in renal fibrosis.122,123 In aged mice, renal pericytes decline in number and adopt a profibrotic phenotype,124 implicating them in aging–related fibrotic changes. Pericyte-endothelial detachment under pathologic conditions and their differentiation into myofibroblasts promote microvascular rarefaction, hypoxia, and fibrosis.125,126 Proposed mediators of this pericyte-endothelial crosstalk include VEGF and PDGF,127 and blocking this pericyte-endothelial interaction attenuates microvascular damage and interstitial fibrosis.128,129
Disordered Repair
The normal enzymatic equilibrium is disturbed in aging, and the balance of metalloproteinases (MMPs) shifts toward fibrosis, potentially via upregulation of tissue inhibitor of MMP1 and increased leukocyte recruitment,50 a pattern likely to result in increased collagen deposition. Longitudinal studies of aging mice show increased Collagen I, Collagen III, and TGF-β1,50 whereas aging rat kidneys exhibit increased ECM deposition and TGF-β3 expression and decreased MMP1 activity, suggesting altered collagen production and processing.130 Additional noninflammatory pathways may contribute to the histologic changes seen, including pathways driven by Wnt and AT2 as mentioned.54
The Aging Human Kidney
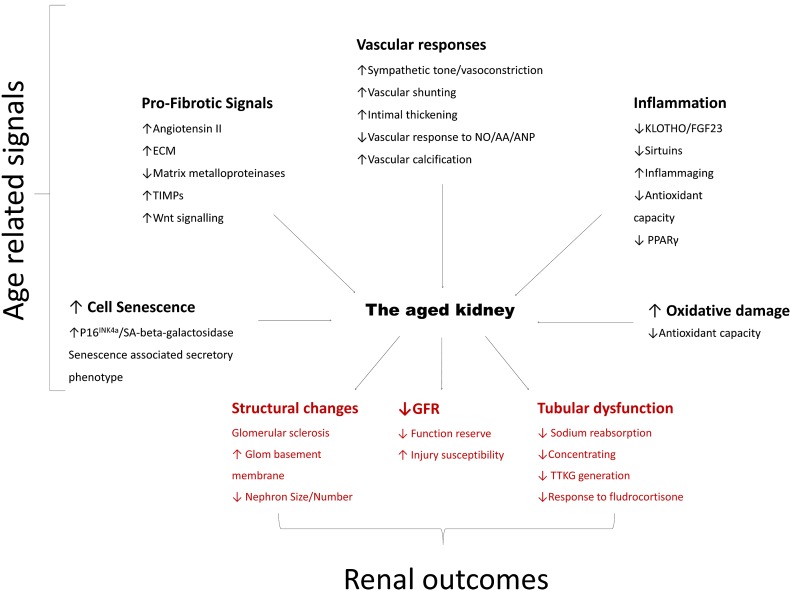
The clinical implications of renal aging in humans extend beyond changes in glomerular and tubular function. Although data generated by animal studies implicate multiple pathways of potential importance for human renal aging (Figure 4), data supporting their involvement in humans are currently sparse, with additional studies required.
Figure 4.
Age-related pathways contributing to altered renal outcomes in the elderly. Multiple pathways interact to produce the changes of renal aging and ↓GFR. Black text indicates implicated upstream effectors of aging, whereas red text reports the functional and histologic changes found in the aged kidney. NO/AA/ANP, nitric oxide/amino acids/atrial natriuretic peptide; SA, senescence associated; TIMP, tissue inhibitors of metalloproteinases; TTKG, trans-tubular potassium gradient.
Signaling Pathways and Oxidative Stress in the Aging Kidney
Falling Klotho Levels
Klotho and FGF-23 are present in human kidneys.131 Klotho levels decline with age and are implicated in accelerated age–related CKD and atherosclerosis.132,133 Conversely, patients with increased functional Klotho expression are reported to have increased lifespan.134 As Klotho falls, FGF-23 levels increase and alter phosphate and calcium homeostasis. Clinical studies in patients on dialysis and patients with CKD show that higher FGF-23 levels associate with increased mortality.135
Increasing Wnt Activation
Although direct evidence of Wnt activation in human aging is lacking, several Wnt antagonists are now undergoing phase 1 clinical trials for cancer therapy in humans.136 If effective, these agents offer new therapeutic options for aging–associated or fibrotic renal disease.
Declining PPARγ Levels
Agonists of PPARγ are used clinically as antidiabetic agents. Retrospective reviews of renal outcomes in clinical practice suggest that augmented PPARγ activity opposes proteinuria in these patients.137 A meta-analysis of PPARγ use has also shown that they associate with reduced rates of cerebrovascular disease, supporting a role in delaying age–associated pathology.138 There is a need for prospective trials assessing their effects on renal function.
AT2
Despite decreased plasma renin activity in the elderly, serum AT2 levels do not fall, and hypersensitivity to AT2 develops in the renal vasculature.139,140 Although ACEi and ARB drugs are in widespread use, there is a lack of human data on the effect of AT2 blocking treatments on normal renal aging and outcomes at present.
Oxidative Stress
As discussed, oxidative stress represents a disruption of the balance of oxidant handling in tissues. In humans, longitudinal studies show increased oxidative stress in normal aging and CKD.73,141 Research has focused on advanced glycation end products as drivers of oxidative stress in aging. These molecules accumulate with age and are associated with increased arterial stiffness, inflammation, oxidative stress, and declining renal function.142 One pharmacologic attempt to modify antioxidant status in patients with diabetic nephropathy showed no effect on proteinuria, despite increased circulating antioxidant levels.143 Whether an alternative, longer–term treatment approach in the healthy aged population might have efficacy remains untested.
Cell Cycle Progression in the Aged Kidney
The presence of increased numbers of senescent cells has been noted in chronic allograft nephropathy and proposed as drivers of the progressive fibrosis seen.144 Recent advances in our understanding of the roles of aging and stress in inducing the detrimental SASP phenotype add to the importance of senescence cells found in both aged and disease–affected human renal biopsies.145–147 In humans, senescence is maximal in the medulla, potentially reflecting increased oxidative and cellular stress and relative hypoxia resulting from the vascular changes discussed previously.148
Telomeres
Telomeres shorten in human kidneys at a rate of 0.25% length per year.149 Although telomere shortening provides an elegant explanation of cellular aging, currently, no data exist to link shorter telomeres to any histologic or functional measure of renal aging. Shorter telomeres associate with CKD and worse cardiovascular outcomes and are shorter in diabetic nephropathy, where they associate with rates of disease progression.150,151 Furthermore, studies of patients on hemodialysis show increased rates of telomere attrition, suggesting that they shorten in response to the physiologic stress.152 Although intriguing, the importance of telomere shortening in human aging remains to be elucidated.
Hypoxia, Inflammation, and Nephrosclerosis in the Aged Kidney
Because of the inherent risks of renal biopsy, samples of healthy aged kidney are seldom available for assessment of levels of nephrosclerosis, and there are no time course studies available to chart the temporal relationships of the histologic findings in the aged kidney. Ongoing progress in imaging technology should enable serial noninvasive assessment of renal perfusion, vascular resistance, hypoxia, inflammation, and atrophy in healthy young and aged volunteers.
Renal Hypoxia
The clinical use of BOLD MRI imaging has shown a lower Po2 in older kidneys compared with younger subjects.153 Because intrarenal vascular disease contributes to increased glomerular sclerosis in aged biopsies, it is possible that subclinical disease leads to hypoxia before marked macroscopic changes occur.154
Inflammation
Inflammation is increased within the aging kidney in humans, with proinflammatory cytokines detectable in the serum correlating with age–related renal disease.155,156
Future Research
Reviewing the current evidence base in clinical and experimental renal aging, it is clear that more work is required to understand which pathways are dispensable and which represent master regulators of the aging phenotype. Studies in aged animals should allow characterization of both the importance and interdependence of factors predisposing aged kidneys to injury, fibrosis, and maladaptive repair, with subsequent validation in humans. Because of the time and cost constraints inherent in using aged animals, establishing whether models of genetically accelerated aging, such as the BubR1 progeroid mouse, represent useful models of renal aging will be of value.157 BubR1 mice have a shortened lifespan and exhibit a variety of age-related phenotypes, including sarcopenia, cataracts, fat loss, cardiac arrhythmias, arterial wall stiffening, and impaired wound healing. Specific to kidney research, BubR1-deficient mice also show higher senescence–associated β-galactosidase activity in kidney sections than aged matched controls.158 Whether they truly manifest a renal aging phenotype has yet to be determined.
Circulating Factors
Heterochronic parabiosis with aged and young mice sharing a common circulation has provided evidence in nonrenal models that circulating factors may modulate features of aging, including impaired regeneration and increased fibrosis.159–161 Proposed factors include β2-microglobulin and growth differentiation factor 11, and reversal of changes in the brain, cardiac, and skeletal muscle has been shown.162–164 Debate continues as to the significance of individual factors.165–169 Whether such factors affect the function of the aged kidney remains completely unknown.
Novel Experimental Species
Undertaking studies of experimental renal disease in aged mice is challenging, and other organisms may be of use. Zebrafish have been used as a model for AKI and nephron regeneration and exhibit aging-associated abnormalities.170–172 Thus, the use of genetically manipulated zebrafish in renal aging studies may be informative.
Novel Therapeutic Strategies
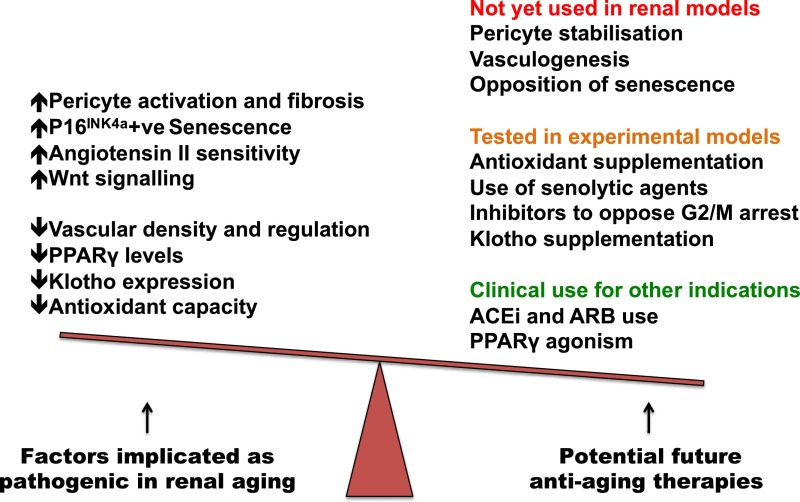
Many pathways implicated in the aging process are the target of interventions to improve the aging phenotype in experimental mice (Figure 5). Klotho agonists are under investigation via repurposing of established agents, including PPARγ agonists and ACEi and ARB drugs. The importance of maintaining a normal renal microvasculature and pericyte pool is increasingly understood,173 and developing strategies to quantify microvascular function and promote endothelial and pericyte health are a pressing clinical need.114
Figure 5.
Potential pathways and therapeutic targets for the treatment of renal aging. Proposed aging–associated pathways (left), and potential interventions to address them (right) coded by current use in patients (green), experimental use in models of renal disease/aging (orange), and potential for future study in the kidney (red). ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Drugs targeting cellular senescence (senolytics) include siRNA therapies, the experimental agent navitoclax, and the licensed drugs dasatinib and quercetin.174 In experiments, these agents show selective toxicity to senescent cells, and their potential utility in animal models and humans merits additional study.
Genetics
Genome–wide association studies (GWASs) have identified upregulation of several genes with aging. Although cumulative damage may well influence much of the elderly genetic milieu, candidate genes have declared themselves as being consistently highly expressed in aged kidneys.175–177 Despite the utility of GWAS in identifying disease-specific pathways, it has proved difficult to discover any canonical aging pathways with GWAS.178
The most promising genes encode for modulators of the glomerular filtration barrier, fibrosis, and inflammatory mediators, although difficulty arises when identified candidate genes do not match the experimental observations or models.179,180 Transcriptomic analysis identified 427 genes strongly associated with renal aging, including mortalin-2, a heat shock protein that may counteract cell senescence, and IGF receptor, a target of Klotho.181–183
GWAS remains, however, a promising tool, because whole-genome analyses of GWAS data suggest that over 80% of the heritability of aging is explained by common genetic variants.184 Future GWASs will continue to generate meaningful results as more advanced statistical techniques develop and researchers increase statistical power by increasing samples number, combining studies using meta-analytic techniques, using multicenter collaborations, and including more extreme phenotypes in the data.184–187
Epigenetics
Epigenetics is the study of genome changes that do not alter DNA sequence. Epigenetic changes in aging include methylation and deacetylation of histone lysine residues, chromatin changes, and increased transcriptional noise.178,188 Interestingly, similar changes in DNA methylation and histones are associated with CKD disease progression.189–191 The role of microRNA expression in modifying gene expression and nephrosclerosis is of interest,192 with data in other organs suggesting an influence on aging.193
Conclusion
Renal aging is complex and remains incompletely understood. Decreased protective factors, hypoxia, and microenvironmental stress drive increasingly disordered inflammation and renal fibrosis. The resulting fibrosis, senescence, and microvascular rarefaction exacerbate damage and promote progression. The future of treating renal aging is likely in understanding the key initiating events and the common downstream pathways present in kidney aging that may be shared with CKD. This knowledge should allow the development of therapies capable of arresting the key mechanisms early to preserve kidney function throughout life.
Disclosures
None.
Acknowledgments
J.H. is supported by Cunningham Trust CT13/16, the Mrs. A.E. Hogg Charitable Trust, and Kidney Research UK. D.A.F. is supported by Intermediate Clinical Fellowship WT100171MA from the Wellcome Trust.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Centers for Disease Control and Prevention : The State of Aging and Healthy in America, Atlanta, GA, Createspace Independent Publisher, 2013 [Google Scholar]
- 2.European Commission : The 2015 ageing report. Eur Econ 3: 424, 2015 [Google Scholar]
- 3.Zhou XJ, Rakheja D, Yu X, Saxena R, Vaziri ND, Silva FG: The aging kidney. Kidney Int 74: 710–720, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Newbold KM, Sandison A, Howie AJ: Comparison of size of juxtamedullary and outer cortical glomeruli in normal adult kidney. Virchows Arch A Pathol Anat Histopathol 420: 127–129, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Nyengaard JR, Bendtsen TF: Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232: 194–201, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, Textor SC, Stegall MD: The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 152: 561–567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan KW, Leung CY, Chan CW: Age-related glomerular sclerosis: Baseline values in Hong Kong. Pathology 22: 177–180, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Denic A, Alexander MP, Kaushik V, Lerman LO, Lieske JC, Stegall MD, Larson JJ, Kremers WK, Vrtiska TJ, Chakkera HA, Poggio ED, Rule AD: Detection and clinical patterns of nephron hypertrophy and nephrosclerosis among apparently healthy adults. Am J Kidney Dis 68: 58–67, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rule AD, Cornell LD, Poggio ED: Senile nephrosclerosis–does it explain the decline in glomerular filtration rate with aging? Nephron Physiol 119[Suppl l]: 6–11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyrier A: Nephrosclerosis: A term in quest of a disease. Nephron 129: 276–282, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Meyrier A: Nephrosclerosis: Update on a centenarian. Nephrol Dial Transplant 30: 1833–1841, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Ferenbach DA, Bonventre JV: Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 11: 264–276, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowe JW, Andres R, Tobin JD, Norris AH, Shock NW: The effect of age on creatinine clearance in men: A cross-sectional and longitudinal study. J Gerontol 31: 155–163, 1976 [DOI] [PubMed] [Google Scholar]
- 14.Lindeman RD, Tobin J, Shock NW: Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33: 278–285, 1985 [DOI] [PubMed] [Google Scholar]
- 15.Baba M, Shimbo T, Horio M, Ando M, Yasuda Y, Komatsu Y, Masuda K, Matsuo S, Maruyama S: Longitudinal study of the decline in renal function in healthy subjects. PLoS One 10: e0129036, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen E, Nardi Y, Krause I, Goldberg E, Milo G, Garty M, Krause I: A longitudinal assessment of the natural rate of decline in renal function with age. J Nephrol 27: 635–641, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Wetzels JFM, Kiemeney LALM, Swinkels DW, Willems HL, den Heijer M: Age- and gender-specific reference values of estimated GFR in Caucasians: The Nijmegen biomedical study. Kidney Int 72: 632–637, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Sarnak MJ, Poindexter A, Wang S-R, Beck GJ, Kusek JW, Marcovina SM, Greene T, Levey AS: Serum C-reactive protein and leptin as predictors of kidney disease progression in the modification of diet in renal disease study. Kidney Int 62: 2208–2215, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Imai E, Horio M, Yamagata K, Iseki K, Hara S, Ura N, Kiyohara Y, Makino H, Hishida A, Matsuo S: Slower decline of glomerular filtration rate in the Japanese general population: A longitudinal 10-year follow-up study. Hypertens Res 31: 433–441, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Hollenberg NK, Rivera A, Meinking T, Martinez G, McCullough M, Passan D, Preston M, Taplin D, Vicaria-Clement M: Age, renal perfusion and function in island-dwelling indigenous Kuna Amerinds of Panama. Nephron 82: 131–138, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Sands JM: Urine concentrating and diluting ability during aging. J Gerontol A Biol Sci Med Sci 67: 1352–1357, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mimran A, Ribstein J, Jover B: Aging and sodium homeostasis. Kidney Int Suppl 37: S107–S113, 1992 [PubMed] [Google Scholar]
- 23.Michelis MF: Hyperkalemia in the elderly. Am J Kidney Dis 16: 296–299, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Mc Greevy C, Horan J, Jones D, Biswas K, O’Meara YM, Mulkerrin EC: A study of tubular potassium secretory capacity in older patients with hyperkalaemia. J Nutr Health Aging 12: 152–155, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Musso C, Liakopoulos V, Stefanidis I, De Miguel R, Imperiali N, Algranati L: Correlation between creatinine clearance and transtubular potassium concentration gradient in old people and chronic renal disease patients. Saudi J Kidney Dis Transpl 18: 551–555, 2007 [PubMed] [Google Scholar]
- 26.Takazakura E, Sawabu N, Handa A, Takada A, Shinoda A, Takeuchi J: Intrarenal vascular changes with age and disease. Kidney Int 2: 224–230, 1972 [DOI] [PubMed] [Google Scholar]
- 27.Hajduczok G, Chapleau MW, Johnson SL, Abboud FM: Increase in sympathetic activity with age. I. Role of impairment of arterial baroreflexes. Am J Physiol 260: H1113–H1120, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Jerkić M, Vojvodić S, López-Novoa JM: The mechanism of increased renal susceptibility to toxic substances in the elderly. Part I. The role of increased vasoconstriction. Int Urol Nephrol 32: 539–547, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Mulkerrin EC, Brain A, Hampton D, Penney MD, Sykes DA, Williams JD, Coles GA, Woodhouse KW: Reduced renal hemodynamic response to atrial natriuretic peptide in elderly volunteers. Am J Kidney Dis 22: 538–544, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Fuiano G, Sund S, Mazza G, Rosa M, Caglioti A, Gallo G, Natale G, Andreucci M, Memoli B, De Nicola L, Conte G: Renal hemodynamic response to maximal vasodilating stimulus in healthy older subjects. Kidney Int 59: 1052–1058, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Fliser D, Zeier M, Nowack R, Ritz E: Renal functional reserve in healthy elderly subjects. J Am Soc Nephrol 3: 1371–1377, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Sverdlov AL, Ngo DTM, Chan WPA, Chirkov YY, Horowitz JD: Aging of the nitric oxide system: Are we as old as our NO? J Am Heart Assoc 3: e000973, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kielstein JT, Bode-Böger SM, Frölich JC, Ritz E, Haller H, Fliser D: Asymmetric dimethylarginine, blood pressure, and renal perfusion in elderly subjects. Circulation 107: 1891–1895, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Ahmed SB, Fisher NDL, Hollenberg NK: Gender and the renal nitric oxide synthase system in healthy humans. Clin J Am Soc Nephrol 2: 926–931, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Ferenbach DA, Nkejabega NCJ, McKay J, Choudhary AK, Vernon MA, Beesley MF, Clay S, Conway BC, Marson LP, Kluth DC, Hughes J: The induction of macrophage hemeoxygenase-1 is protective during acute kidney injury in aging mice. Kidney Int 79: 966–976, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Nath KA, Grande JP, Farrugia G, Croatt AJ, Belcher JD, Hebbel RP, Vercellotti GM, Katusic ZS: Age sensitizes the kidney to heme protein-induced acute kidney injury. Am J Physiol Renal Physiol 304: F317–F325, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maddens B, Vandendriessche B, Demon D, Vanholder R, Chiers K, Cauwels A, Meyer E: Severity of sepsis-induced acute kidney injury in a novel mouse model is age dependent. Crit Care Med 40: 2638–2646, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Clements ME, Chaber CJ, Ledbetter SR, Zuk A: Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS One 8: e70464, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T: Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113: 561–568, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Razzaque MS, Sitara D, Taguchi T, St-Arnaud R, Lanske B: Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D mediated process. FASEB J 20: 720–722, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M: Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.John GB, Cheng C-Y, Kuro-o M: Role of Klotho in aging, phosphate metabolism, and CKD. Am J Kidney Dis 58: 127–134, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuro-o M: Klotho as a regulator of oxidative stress and senescence. Biol Chem 389: 233–241, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Sopjani M, Rinnerthaler M, Kruja J, Dermaku-Sopjani M: Intracellular signaling of the aging suppressor protein Klotho. Curr Mol Med 15: 27–37, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Yang H, Fogo AB: Cell senescence in the aging kidney. J Am Soc Nephrol 21: 1436–1439, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Liu Q-F, Ye J-M, Deng Z-Y, Yu L-X, Sun Q, Li S-S: Ameliorating effect of Klotho on endoplasmic reticulum stress and renal fibrosis induced by unilateral ureteral obstruction. Iran J Kidney Dis 9: 291–297, 2015 [PubMed] [Google Scholar]
- 48.Kuro-o M: Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr Opin Nephrol Hypertens 15: 437–441, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Zhou L, Li Y, Zhou D, Tan RJ, Liu Y: Loss of Klotho contributes to kidney injury by derepression of Wnt/β-catenin signaling. J Am Soc Nephrol 24: 771–785, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Chen X, Hong Q, Lin H, Zhu H, Liu Q, Wang J, Xie Y, Shang X, Shi S, Lu Y, Yin Z: TIMP-1 promotes age-related renal fibrosis through upregulating ICAM-1 in human TIMP-1 transgenic mice. J Gerontol A Biol Sci Med Sci 61: 1130–1143, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Tan RJ, Zhou D, Zhou L, Liu Y: Wnt/β-catenin signaling and kidney fibrosis. Kidney Int Suppl (2011) 4: 84–90, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bian A, Neyra JA, Zhan M, Hu MC: Klotho, stem cells, and aging. Clin Interv Aging 10: 1233–1243, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA: Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807–810, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Maarouf OH, Aravamudhan A, Rangarajan D, Kusaba T, Zhang V, Welborn J, Gauvin D, Hou X, Kramann R, Humphreys BD: Paracrine Wnt1 drives interstitial fibrosis without inflammation by tubulointerstitial cross-talk. J Am Soc Nephrol 27: 781–790, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hao S, He W, Li Y, Ding H, Hou Y, Nie J, Hou FF, Kahn M, Liu Y: Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol 22: 1642–1653, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He W, Kang YS, Dai C, Liu Y: Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol 22: 90–103, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou L, Li Y, Hao S, Zhou D, Tan RJ, Nie J, Hou FF, Kahn M, Liu Y: Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J Am Soc Nephrol 26: 107–120, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iemitsu M, Miyauchi T, Maeda S, Tanabe T, Takanashi M, Irukayama-Tomobe Y, Sakai S, Ohmori H, Matsuda M, Yamaguchi I: Aging-induced decrease in the PPAR-alpha level in hearts is improved by exercise training. Am J Physiol Heart Circ Physiol 283: H1750–H1760, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Wang P, Li B, Cai G, Huang M, Jiang L, Pu J, Li L, Wu Q, Zuo L, Wang Q, Zhou P: Activation of PPAR-γ by pioglitazone attenuates oxidative stress in aging rat cerebral arteries through upregulating UCP2. J Cardiovasc Pharmacol 64: 497–506, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Zhang H, Li Y, Fan Y, Wu J, Zhao B, Guan Y, Chien S, Wang N: Klotho is a target gene of PPAR-gamma. Kidney Int 74: 732–739, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Zhang R, Zheng F: PPAR-gamma and aging: One link through klotho? Kidney Int 74: 702–704, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Sung B, Park S, Yu BP, Chung HY: Modulation of PPAR in aging, inflammation, and calorie restriction. J Gerontol A Biol Sci Med Sci 59: 997–1006, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Briganti S, Flori E, Bellei B, Picardo M: Modulation of PPARγ provides new insights in a stress induced premature senescence model. PLoS One 9: e104045, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim HA, Lee EK, Kim JM, Park MH, Kim DH, Choi YJ, Ha YM, Yoon J-H, Choi JS, Yu BP, Chung HY: PPARγ activation by baicalin suppresses NF-κB-mediated inflammation in aged rat kidney. Biogerontology 13: 133–145, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Sangaralingham SJ, Wang BH, Huang L, Kumfu S, Ichiki T, Krum H, Burnett JC Jr. : Cardiorenal fibrosis and dysfunction in aging: Imbalance in mediators and regulators of collagen. Peptides 76: 108–114, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fogo AB: The role of angiotensin II and plasminogen activator inhibitor-1 in progressive glomerulosclerosis. Am J Kidney Dis 35: 179–188, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Vajapey R, Rini D, Walston J, Abadir P: The impact of age-related dysregulation of the angiotensin system on mitochondrial redox balance. Front Physiol 5: 439, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benigni A, Cassis P, Remuzzi G: Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol Med 2: 247–257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cuevas CA, Gonzalez AA, Inestrosa NC, Vio CP, Prieto MC: Angiotensin II increases fibronectin and collagen I through the β-catenin-dependent signaling in mouse collecting duct cells. Am J Physiol Renal Physiol 308: F358–F365, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruiz-Torres MP, Bosch RJ, O’Valle F, Del Moral RG, Ramírez C, Masseroli M, Pérez-Caballero C, Iglesias MC, Rodríguez-Puyol M, Rodríguez-Puyol D: Age-related increase in expression of TGF-beta1 in the rat kidney: Relationship to morphologic changes. J Am Soc Nephrol 9: 782–791, 1998 [DOI] [PubMed] [Google Scholar]
- 71.Cruz CI, Ruiz-Torres P, del Moral RG, Rodríguez-Puyol M, Rodríguez-Puyol D: Age-related progressive renal fibrosis in rats and its prevention with ACE inhibitors and taurine. Am J Physiol Renal Physiol 278: F122–F129, 2000 [DOI] [PubMed] [Google Scholar]
- 72.de Cavanagh EMV, Piotrkowski B, Basso N, Stella I, Inserra F, Ferder L, Fraga CG: Enalapril and losartan attenuate mitochondrial dysfunction in aged rats. FASEB J 17: 1096–1098, 2003 [DOI] [PubMed] [Google Scholar]
- 73.Vlassara H, Torreggiani M, Post JB, Zheng F, Uribarri J, Striker GE: Role of oxidants/inflammation in declining renal function in chronic kidney disease and normal aging. Kidney Int Suppl 114: S3–S11, 2009 [DOI] [PubMed] [Google Scholar]
- 74.Simão S, Gomes P, Pinto V, Silva E, Amaral JS, Igreja B, Afonso J, Serrão MP, Pinho MJ, Soares-da-Silva P: Age-related changes in renal expression of oxidant and antioxidant enzymes and oxidative stress markers in male SHR and WKY rats. Exp Gerontol 46: 468–474, 2011 [DOI] [PubMed] [Google Scholar]
- 75.Miyazawa M, Ishii T, Yasuda K, Noda S, Onouchi H, Hartman PS, Ishii N: The role of mitochondrial superoxide anion (O2(-)) on physiological aging in C57BL/6J mice. J Radiat Res (Tokyo) 50: 73–83, 2009 [DOI] [PubMed] [Google Scholar]
- 76.Nath KA: The role of Sirt1 in renal rejuvenation and resistance to stress. J Clin Invest 120: 1026–1028, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akçetin Z, Erdemli G, Brömme HJ: Experimental study showing a diminished cytosolic antioxidative capacity in kidneys of aged rats. Urol Int 64: 70–73, 2000 [DOI] [PubMed] [Google Scholar]
- 78.Martin R, Fitzl G, Mozet C, Martin H, Welt K, Wieland E: Effect of age and hypoxia/reoxygenation on mRNA expression of antioxidative enzymes in rat liver and kidneys. Exp Gerontol 37: 1481–1487, 2002 [DOI] [PubMed] [Google Scholar]
- 79.Qiao X, Chen X, Wu D, Ding R, Wang J, Hong Q, Shi S, Li J, Xie Y, Lu Y, Wang Z: Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/reperfusion injury. J Gerontol A Biol Sci Med Sci 60: 830–839, 2005 [DOI] [PubMed] [Google Scholar]
- 80.Small DM, Bennett NC, Roy S, Gabrielli BG, Johnson DW, Gobe GC: Oxidative stress and cell senescence combine to cause maximal renal tubular epithelial cell dysfunction and loss in an in vitro model of kidney disease. Nephron Exp Nephrol 122: 123–130, 2012 [DOI] [PubMed] [Google Scholar]
- 81.Kitada M, Kume S, Takeda-Watanabe A, Kanasaki K, Koya D: Sirtuins and renal diseases: Relationship with aging and diabetic nephropathy. Clin Sci (Lond) 124: 153–164, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW: Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23: 2369–2380, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A, Koya D: Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest 120: 1043–1055, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He W, Wang Y, Zhang M-Z, You L, Davis LS, Fan H, Yang H-C, Fogo AB, Zent R, Harris RC, Breyer MD, Hao C-M: Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest 120: 1056–1068, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sauve AA, Wolberger C, Schramm VL, Boeke JD: The biochemistry of sirtuins. Annu Rev Biochem 75: 435–465, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Longo VD, Kennedy BK: Sirtuins in aging and age-related disease. Cell 126: 257–268, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Radak Z, Koltai E, Taylor AW, Higuchi M, Kumagai S, Ohno H, Goto S, Boldogh I: Redox-regulating sirtuins in aging, caloric restriction, and exercise. Free Radic Biol Med 58: 87–97, 2013 [DOI] [PubMed] [Google Scholar]
- 88.Park S, Mori R, Shimokawa I: Do sirtuins promote mammalian longevity? A critical review on its relevance to the longevity effect induced by calorie restriction. Mol Cells 35: 474–480, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morigi M, Coffman TM, Remuzzi G: Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest 119: 524–530, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schmitt R, Marlier A, Cantley LG: Zag expression during aging suppresses proliferation after kidney injury. J Am Soc Nephrol 19: 2375–2383, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sörensen-Zender I, Bhayana S, Susnik N, Rolli V, Batkai S, Baisantry A, Bahram S, Sen P, Teng B, Lindner R, Schiffer M, Thum T, Melk A, Haller H, Schmitt R: Zinc-α2-Glycoprotein exerts antifibrotic effects in kidney and heart. J Am Soc Nephrol 26: 2659–2668, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV: Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16: 535–543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jun J-I, Lau LF: The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12: 676–685, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Deursen JM: The role of senescent cells in ageing. Nature 509: 439–446, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang G, Rosen DG, Zhang Z, Bast RC Jr., Mills GB, Colacino JA, Mercado-Uribe I, Liu J: The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc Natl Acad Sci USA 103: 16472–16477, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuilman T, Michaloglou C, Vredeveld LCW, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS: Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133: 1019–1031, 2008 [DOI] [PubMed] [Google Scholar]
- 97.Binet R, Ythier D, Robles AI, Collado M, Larrieu D, Fonti C, Brambilla E, Brambilla C, Serrano M, Harris CC, Pedeux R: WNT16B is a new marker of cellular senescence that regulates p53 activity and the phosphoinositide 3-kinase/AKT pathway. Cancer Res 69: 9183–9191, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Braun H, Schmidt BMW, Raiss M, Baisantry A, Mircea-Constantin D, Wang S, Gross M-L, Serrano M, Schmitt R, Melk A: Cellular senescence limits regenerative capacity and allograft survival. J Am Soc Nephrol 23: 1467–1473, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chkhotua AB, Abendroth D, Froeba G, Schelzig H: Up-regulation of cell cycle regulatory genes after renal ischemia/reperfusion: Differential expression of p16(INK4a), p21(WAF1/CIP1) and p27(Kip1) cyclin-dependent kinase inhibitor genes depending on reperfusion time. Transpl Int 19: 72–77, 2006 [DOI] [PubMed] [Google Scholar]
- 100.Lee DH, Wolstein JM, Pudasaini B, Plotkin M: INK4a deletion results in improved kidney regeneration and decreased capillary rarefaction after ischemia-reperfusion injury. Am J Physiol Renal Physiol 302: F183–F191, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wolstein JM, Lee DH, Michaud J, Buot V, Stefanchik B, Plotkin MD: INK4a knockout mice exhibit increased fibrosis under normal conditions and in response to unilateral ureteral obstruction. Am J Physiol Renal Physiol 299: F1486–F1495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM: Naturally occurring p16-positive cells shorten healthy lifespan. Nature 530: 184–189, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W, Luo Y, Wang X, Aykin-Burns N, Krager K, Ponnappan U, Hauer-Jensen M, Meng A, Zhou D: Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22: 78–83, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aubert G, Lansdorp PM: Telomeres and aging. Physiol Rev 88: 557–579, 2008 [DOI] [PubMed] [Google Scholar]
- 105.d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP: A DNA damage checkpoint response in telomere-initiated senescence. Nature 426: 194–198, 2003 [DOI] [PubMed] [Google Scholar]
- 106.Mikhelson VM, Gamaley IA: Telomere shortening is a sole mechanism of aging in mammals. Curr Aging Sci 5: 203–208, 2012 [DOI] [PubMed] [Google Scholar]
- 107.Wills LP, Schnellmann RG: Telomeres and telomerase in renal health. J Am Soc Nephrol 22: 39–41, 2011 [DOI] [PubMed] [Google Scholar]
- 108.Westhoff JH, Schildhorn C, Jacobi C, Hömme M, Hartner A, Braun H, Kryzer C, Wang C, von Zglinicki T, Kränzlin B, Gretz N, Melk A: Telomere shortening reduces regenerative capacity after acute kidney injury. J Am Soc Nephrol 21: 327–336, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cheng H, Fan X, Lawson WE, Paueksakon P, Harris RC: Telomerase deficiency delays renal recovery in mice after ischemia-reperfusion injury by impairing autophagy. Kidney Int 88: 85–94, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jaskelioff M, Muller FL, Paik J-H, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadiñanos J, Horner JW, Maratos-Flier E, Depinho RA: Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469: 102–106, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ballermann BJ, Obeidat M: Tipping the balance from angiogenesis to fibrosis in CKD. Kidney Int Suppl (2011) 4: 45–52, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tanaka T, Kato H, Kojima I, Ohse T, Son D, Tawakami T, Yatagawa T, Inagi R, Fujita T, Nangaku M: Hypoxia and expression of hypoxia-inducible factor in the aging kidney. J Gerontol A Biol Sci Med Sci 61: 795–805, 2006 [DOI] [PubMed] [Google Scholar]
- 113.Kang DH, Anderson S, Kim YG, Mazzalli M, Suga S, Jefferson JA, Gordon KL, Oyama TT, Hughes J, Hugo C, Kerjaschki D, Schreiner GF, Johnson RJ: Impaired angiogenesis in the aging kidney: Vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis 37: 601–611, 2001 [DOI] [PubMed] [Google Scholar]
- 114.Kramann R, Tanaka M, Humphreys BD: Fluorescence microangiography for quantitative assessment of peritubular capillary changes after AKI in mice. J Am Soc Nephrol 25: 1924–1931, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sebastián C, Espia M, Serra M, Celada A, Lloberas J: MacrophAging: A cellular and molecular review. Immunobiology 210: 121–126, 2005 [DOI] [PubMed] [Google Scholar]
- 116.Costa E, Fernandes J, Ribeiro S, Sereno J, Garrido P, Rocha-Pereira P, Coimbra S, Catarino C, Belo L, Bronze-da-Rocha E, Vala H, Alves R, Reis F, Santos-Silva A: Aging is associated with impaired renal function, INF-gamma induced inflammation and with alterations in iron regulatory proteins gene expression. Aging Dis 5: 356–365, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Song F, Ma Y, Bai X-Y, Chen X: The expression changes of inflammasomes in the aging rat kidneys. J Gerontol A Biol Sci Med Sci 71: 747–756, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stranks AJ, Hansen AL, Panse I, Mortensen M, Ferguson DJP, Puleston DJ, Shenderov K, Watson AS, Veldhoen M, Phadwal K, Cerundolo V, Simon AK: Autophagy controls acquisition of aging features in macrophages. J Innate Immun 7: 375–391, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zheng F, Cheng Q-L, Plati A-R, Ye SQ, Berho M, Banerjee A, Potier M, Jaimes EA, Yu H, Guan Y-F, Hao C-M, Striker LJ, Striker GE: The glomerulosclerosis of aging in females: Contribution of the proinflammatory mesangial cell phenotype to macrophage infiltration. Am J Pathol 165: 1789–1798, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang B, Bailey WM, Braun KJ, Gensel JC: Age decreases macrophage IL-10 expression: Implications for functional recovery and tissue repair in spinal cord injury. Exp Neurol 273: 83–91, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang H-C, Rossini M, Ma L-J, Zuo Y, Ma J, Fogo AB: Cells derived from young bone marrow alleviate renal aging. J Am Soc Nephrol 22: 2028–2036, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD: Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16: 51–66, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Humphreys BD, Lin S-L, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS: Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176: 85–97, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stefanska A, Eng D, Kaverina N, Duffield JS, Pippin JW, Rabinovitch P, Shankland SJ: Interstitial pericytes decrease in aged mouse kidneys. Aging (Albany, NY) 7: 370–382, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gomez IG, Duffield JS: The FOXD1 lineage of kidney perivascular cells and myofibroblasts: Functions and responses to injury. Kidney Int Suppl (2011) 4: 26–33, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schrimpf C, Teebken OE, Wilhelmi M, Duffield JS: The role of pericyte detachment in vascular rarefaction. J Vasc Res 51: 247–258, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rojas A, Chang F-C, Lin S-L, Duffield JS: The role played by perivascular cells in kidney interstitial injury. Clin Nephrol 77: 400–408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin S-L, Chang F-C, Schrimpf C, Chen Y-T, Wu C-F, Wu V-C, Chiang W-C, Kuhnert F, Kuo CJ, Chen Y-M, Wu K-D, Tsai T-J, Duffield JS: Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol 178: 911–923, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen Y-T, Chang F-C, Wu C-F, Chou Y-H, Hsu H-L, Chiang W-C, Shen J, Chen Y-M, Wu K-D, Tsai T-J, Duffield JS, Lin S-L: Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int 80: 1170–1181, 2011 [DOI] [PubMed] [Google Scholar]
- 130.Gagliano N, Arosio B, Santambrogio D, Balestrieri MR, Padoani G, Tagliabue J, Masson S, Vergani C, Annoni G: Age-dependent expression of fibrosis-related genes and collagen deposition in rat kidney cortex. J Gerontol A Biol Sci Med Sci 55: B365–B372, 2000 [DOI] [PubMed] [Google Scholar]
- 131.Lim K, Groen A, Molostvov G, Lu T, Lilley KS, Snead D, James S, Wilkinson IB, Ting S, Hsiao L-L, Hiemstra TF, Zehnder D: α-klotho expressison in human tissues. J Clin Endocrinol Metab 100: E1308–E1318, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Keles N, Caliskan M, Dogan B, Keles NN, Kalcik M, Aksu F, Kostek O, Aung SM, Isbilen B, Oguz A: Low serum level of klotho is an early predictor of atherosclerosis. Tohoku J Exp Med 237: 17–23, 2015 [DOI] [PubMed] [Google Scholar]
- 133.Xu Y, Sun Z: Molecular basis of klotho: From gene to function in aging. Endocr Rev 36: 174–193, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arking DE, Krebsova A, Macek M Sr., Macek M Jr., Arking A, Mian IS, Fried L, Hamosh A, Dey S, McIntosh I, Dietz HC: Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA 99: 856–861, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nitta K, Nagano N, Tsuchiya K: Fibroblast growth factor 23/klotho axis in chronic kidney disease. Nephron Clin Pract 128: 1–10, 2014 [DOI] [PubMed] [Google Scholar]
- 136.Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, Yang SX, Ivy SP: Targeting notch, hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nat Rev Clin Oncol 12: 445–464, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Speeckaert MM, Vanfraechem C, Speeckaert R, Delanghe JR: Peroxisome proliferator-activated receptor agonists in a battle against the aging kidney. Ageing Res Rev 14: 1–18, 2014 [DOI] [PubMed] [Google Scholar]
- 138.Liu J, Wang LN: Peroxisome proliferator-activated receptor gamma agonists for preventing recurrent stroke and other vascular events in patients with stroke or transient ischaemic attack. Cochrane Database Syst Rev 1: CD010693, 2014 [DOI] [PubMed] [Google Scholar]
- 139.Duggan J, Kilfeather S, O’Brien E, O’Malley K, Nussberger J: Effects of aging and hypertension on plasma angiotensin II and platelet angiotensin II receptor density. Am J Hypertens 5: 687–693, 1992 [DOI] [PubMed] [Google Scholar]
- 140.Yoon HE, Choi BS: The renin-angiotensin system and aging in the kidney. Korean J Intern Med 29: 291–295, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Panickar KS, Jewell DE: The beneficial role of anti-inflammatory dietary ingredients in attenuating markers of chronic low-grade inflammation in aging. Horm Mol Biol Clin Investig 23: 59–70, 2015 [DOI] [PubMed] [Google Scholar]
- 142.Vlassara H, Uribarri J, Ferrucci L, Cai W, Torreggiani M, Post JB, Zheng F, Striker GE: Identifying advanced glycation end products as a major source of oxidants in aging: Implications for the management and/or prevention of reduced renal function in elderly persons. Semin Nephrol 29: 594–603, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yubero-Serrano EM, Woodward M, Poretsky L, Vlassara H, Striker GE; AGE-less Study Group : Effects of sevelamer carbonate on advanced glycation end products and antioxidant/pro-oxidant status in patients with diabetic kidney disease. Clin J Am Soc Nephrol 10: 759–766, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Halloran PF, Melk A, Barth C: Rethinking chronic allograft nephropathy: The concept of accelerated senescence. J Am Soc Nephrol 10: 167–181, 1999 [DOI] [PubMed] [Google Scholar]
- 145.Melk A, Schmidt BMW, Vongwiwatana A, Rayner DC, Halloran PF: Increased expression of senescence-associated cell cycle inhibitor p16INK4a in deteriorating renal transplants and diseased native kidney. Am J Transplant 5: 1375–1382, 2005 [DOI] [PubMed] [Google Scholar]
- 146.Verzola D, Gandolfo MT, Gaetani G, Ferraris A, Mangerini R, Ferrario F, Villaggio B, Gianiorio F, Tosetti F, Weiss U, Traverso P, Mji M, Deferrari G, Garibotto G: Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am J Physiol Renal Physiol 295: F1563–F1573, 2008 [DOI] [PubMed] [Google Scholar]
- 147.Sis B, Tasanarong A, Khoshjou F, Dadras F, Solez K, Halloran PF: Accelerated expression of senescence associated cell cycle inhibitor p16INK4A in kidneys with glomerular disease. Kidney Int 71: 218–226, 2007 [DOI] [PubMed] [Google Scholar]
- 148.Melk A, Schmidt BMW, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF: Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int 65: 510–520, 2004 [DOI] [PubMed] [Google Scholar]
- 149.Melk A, Ramassar V, Helms LM, Moore R, Rayner D, Solez K, Halloran PF: Telomere shortening in kidneys with age. J Am Soc Nephrol 11: 444–453, 2000 [DOI] [PubMed] [Google Scholar]
- 150.Raschenberger J, Kollerits B, Titze S, Köttgen A, Bärthlein B, Ekici AB, Forer L, Schönherr S, Weissensteiner H, Haun M, Wanner C, Eckardt K-U, Kronenberg F; GCKD study Investigators : Association of relative telomere length with cardiovascular disease in a large chronic kidney disease cohort: The GCKD study. Atherosclerosis 242: 529–534, 2015 [DOI] [PubMed] [Google Scholar]
- 151.Raschenberger J, Kollerits B, Ritchie J, Lane B, Kalra PA, Ritz E, Kronenberg F: Association of relative telomere length with progression of chronic kidney disease in two cohorts: Effect modification by smoking and diabetes. Sci Rep 5: 11887, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Boxall MC, Goodship THJ, Brown AL, Ward MC, von Zglinicki T: Telomere shortening and haemodialysis. Blood Purif 24: 185–189, 2006 [DOI] [PubMed] [Google Scholar]
- 153.Epstein FH, Prasad P: Effects of furosemide on medullary oxygenation in younger and older subjects. Kidney Int 57: 2080–2083, 2000 [DOI] [PubMed] [Google Scholar]
- 154.Kasiske BL: Relationship between vascular disease and age-associated changes in the human kidney. Kidney Int 31: 1153–1159, 1987 [DOI] [PubMed] [Google Scholar]
- 155.Sarkar D, Fisher PB: Molecular mechanisms of aging-associated inflammation. Cancer Lett 236: 13–23, 2006 [DOI] [PubMed] [Google Scholar]
- 156.Costello-White R, Ryff CD, Coe CL: Aging and low-grade inflammation reduce renal function in middle-aged and older adults in Japan and the USA. Age (Dordr) 37: 9808, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM: Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479: 232–236, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A, Kumar R, Jenkins RB, de Groen PC, Roche P, van Deursen JM: BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet 36: 744–749, 2004 [DOI] [PubMed] [Google Scholar]
- 159.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA: Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433: 760–764, 2005 [DOI] [PubMed] [Google Scholar]
- 160.Ruckh JM, Zhao J-W, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJM: Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 10: 96–103, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park J-S, Couillard-Després S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T: The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477: 90–94, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Smith LK, He Y, Park J-S, Bieri G, Snethlage CE, Lin K, Gontier G, Wabl R, Plambeck KE, Udeochu J, Wheatley EG, Bouchard J, Eggel A, Narasimha R, Grant JL, Luo J, Wyss-Coray T, Villeda SA: β2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med 21: 932–937, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL: Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344: 630–634, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim M-J, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ: Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 344: 649–652, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Loffredo FS, Steinhauser ML, Jay SM, Gannon J, Pancoast JR, Yalamanchi P, Sinha M, Dall’Osso C, Khong D, Shadrach JL, Miller CM, Singer BS, Stewart A, Psychogios N, Gerszten RE, Hartigan AJ, Kim M-J, Serwold T, Wagers AJ, Lee RT: Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153: 828–839, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Smith SC, Zhang X, Zhang X, Gross P, Starosta T, Mohsin S, Franti M, Gupta P, Hayes D, Myzithras M, Kahn J, Tanner J, Weldon SM, Khalil A, Guo X, Sabri A, Chen X, MacDonnell S, Houser SR: GDF11 does not rescue aging-related pathological hypertrophy. Circ Res 117: 926–932, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rodgers BD, Eldridge JA: Reduced circulating GDF11 is unlikely responsible for age-dependent changes in mouse heart, muscle, and brain. Endocrinology 156: 3885–3888, 2015 [DOI] [PubMed] [Google Scholar]
- 168.Brun CE, Rudnicki MA: GDF11 and the mythical fountain of youth. Cell Metab 22: 54–56, 2015 [DOI] [PubMed] [Google Scholar]
- 169.Egerman MA, Cadena SM, Gilbert JA, Meyer A, Nelson HN, Swalley SE, Mallozzi C, Jacobi C, Jennings LL, Clay I, Laurent G, Ma S, Brachat S, Lach-Trifilieff E, Shavlakadze T, Trendelenburg A-U, Brack AS, Glass DJ: GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab 22: 164–174, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Diep CQ, Ma D, Deo RC, Holm TM, Naylor RW, Arora N, Wingert RA, Bollig F, Djordjevic G, Lichman B, Zhu H, Ikenaga T, Ono F, Englert C, Cowan CA, Hukriede NA, Handin RI, Davidson AJ: Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature 470: 95–100, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sander V, Davidson AJ: Kidney injury and regeneration in zebrafish. Semin Nephrol 34: 437–444, 2014 [DOI] [PubMed] [Google Scholar]
- 172.McKee RA, Wingert RA: Zebrafish renal pathology: Emerging models of acute kidney injury. Curr Pathobiol Rep 3: 171–181, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schrimpf C, Xin C, Campanholle G, Gill SE, Stallcup W, Lin S-L, Davis GE, Gharib SA, Humphreys BD, Duffield JS: Pericyte TIMP3 and ADAMTS1 modulate vascular stability after kidney injury. J Am Soc Nephrol 23: 868–883, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhu Y, Tchkonia T, Pirtskhalava T, Gower A, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Borden G, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL: The achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 14: 644–658, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pattaro C, Köttgen A, Teumer A, Garnaas M, Böger CA, Fuchsberger C, Olden M, Chen M-H, Tin A, Taliun D, Li M, Gao X, Gorski M, Yang Q, Hundertmark C, Foster MC, O’Seaghdha CM, Glazer N, Isaacs A, Liu C-T, Smith AV, O’Connell JR, Struchalin M, Tanaka T, Li G, Johnson AD, Gierman HJ, Feitosa M, Hwang S-J, Atkinson EJ, Lohman K, Cornelis MC, Johansson Å, Tönjes A, Dehghan A, Chouraki V, Holliday EG, Sorice R, Kutalik Z, Lehtimäki T, Esko T, Deshmukh H, Ulivi S, Chu AY, Murgia F, Trompet S, Imboden M, Kollerits B, Pistis G, Harris TB, Launer LJ, Aspelund T, Eiriksdottir G, Mitchell BD, Boerwinkle E, Schmidt H, Cavalieri M, Rao M, Hu FB, Demirkan A, Oostra BA, de Andrade M, Turner ST, Ding J, Andrews JS, Freedman BI, Koenig W, Illig T, Döring A, Wichmann H-E, Kolcic I, Zemunik T, Boban M, Minelli C, Wheeler HE, Igl W, Zaboli G, Wild SH, Wright AF, Campbell H, Ellinghaus D, Nöthlings U, Jacobs G, Biffar R, Endlich K, Ernst F, Homuth G, Kroemer HK, Nauck M, Stracke S, Völker U, Völzke H, Kovacs P, Stumvoll M, Mägi R, Hofman A, Uitterlinden AG, Rivadeneira F, Aulchenko YS, Polasek O, Hastie N, Vitart V, Helmer C, Wang JJ, Ruggiero D, Bergmann S, Kähönen M, Viikari J, Nikopensius T, Province M, Ketkar S, Colhoun H, Doney A, Robino A, Giulianini F, Krämer BK, Portas L, Ford I, Buckley BM, Adam M, Thun G-A, Paulweber B, Haun M, Sala C, Metzger M, Mitchell P, Ciullo M, Kim SK, Vollenweider P, Raitakari O, Metspalu A, Palmer C, Gasparini P, Pirastu M, Jukema JW, Probst-Hensch NM, Kronenberg F, Toniolo D, Gudnason V, Shuldiner AR, Coresh J, Schmidt R, Ferrucci L, Siscovick DS, van Duijn CM, Borecki I, Kardia SLR, Liu Y, Curhan GC, Rudan I, Gyllensten U, Wilson JF, Franke A, Pramstaller PP, Rettig R, Prokopenko I, Witteman JCM, Hayward C, Ridker P, Parsa A, Bochud M, Heid IM, Goessling W, Chasman DI, Kao WHL, Fox CS; CARDIoGRAM Consortium; ICBP Consortium; CARe Consortium; Wellcome Trust Case Control Consortium 2 (WTCCC2) : Genome-wide association and functional follow-up reveals new loci for kidney function. PLoS Genet 8: e1002584, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Köttgen A, Glazer NL, Dehghan A, Hwang S-J, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, Smith AV, Arking DE, Astor BC, Boerwinkle E, Ehret GB, Ruczinski I, Scharpf RB, Chen Y-DI, de Boer IH, Haritunians T, Lumley T, Sarnak M, Siscovick D, Benjamin EJ, Levy D, Upadhyay A, Aulchenko YS, Hofman A, Rivadeneira F, Uitterlinden AG, van Duijn CM, Chasman DI, Paré G, Ridker PM, Kao WHL, Witteman JC, Coresh J, Shlipak MG, Fox CS: Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41: 712–717, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gorski M, Tin A, Garnaas M, McMahon GM, Chu AY, Tayo BO, Pattaro C, Teumer A, Chasman DI, Chalmers J, Hamet P, Tremblay J, Woodward M, Aspelund T, Eiriksdottir G, Gudnason V, Harris TB, Launer LJ, Smith AV, Mitchell BD, O’Connell JR, Shuldiner AR, Coresh J, Li M, Freudenberger P, Hofer E, Schmidt H, Schmidt R, Holliday EG, Mitchell P, Wang JJ, de Boer IH, Li G, Siscovick DS, Kutalik Z, Corre T, Vollenweider P, Waeber G, Gupta J, Kanetsky PA, Hwang S-J, Olden M, Yang Q, de Andrade M, Atkinson EJ, Kardia SLR, Turner ST, Stafford JM, Ding J, Liu Y, Barlassina C, Cusi D, Salvi E, Staessen JA, Ridker PM, Grallert H, Meisinger C, Müller-Nurasyid M, Krämer BK, Kramer H, Rosas SE, Nolte IM, Penninx BW, Snieder H, Fabiola Del Greco M, Franke A, Nöthlings U, Lieb W, Bakker SJL, Gansevoort RT, van der Harst P, Dehghan A, Franco OH, Hofman A, Rivadeneira F, Sedaghat S, Uitterlinden AG, Coassin S, Haun M, Kollerits B, Kronenberg F, Paulweber B, Aumann N, Endlich K, Pietzner M, Völker U, Rettig R, Chouraki V, Helmer C, Lambert J-C, Metzger M, Stengel B, Lehtimäki T, Lyytikäinen L-P, Raitakari O, Johnson A, Parsa A, Bochud M, Heid IM, Goessling W, Köttgen A, Kao WHL, Fox CS, Böger CA: Genome-wide association study of kidney function decline in individuals of European descent. Kidney Int 87: 1017–1029, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Johnson SC, Dong X, Vijg J, Suh Y: Genetic evidence for common pathways in human age-related diseases. Aging Cell 14: 809–817, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tampe B, Zeisberg M: Contribution of genetics and epigenetics to progression of kidney fibrosis. Nephrol Dial Transplant 29[Suppl 4]: iv72–iv79, 2014 [DOI] [PubMed] [Google Scholar]
- 180.Noordmans GA, Hillebrands J-L, van Goor H, Korstanje R: A roadmap for the genetic analysis of renal aging. Aging Cell 14: 725–733, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP, Rubinek T: Klotho: A tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene 27: 7094–7105, 2008 [DOI] [PubMed] [Google Scholar]
- 182.Bartke A: Long-lived klotho mice: New insights into the roles of IGF-1 and insulin in aging. Trends Endocrinol Metab 17: 33–35, 2006 [DOI] [PubMed] [Google Scholar]
- 183.Rodwell GEJ, Sonu R, Zahn JM, Lund J, Wilhelmy J, Wang L, Xiao W, Mindrinos M, Crane E, Segal E, Myers BD, Brooks JD, Davis RW, Higgins J, Owen AB, Kim SK: A transcriptional profile of aging in the human kidney. PLoS Biol 2: e427, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Broer L, van Duijn CM: GWAS and meta-analysis in aging/longevity. Adv Exp Med Biol 847: 107–125, 2015 [DOI] [PubMed] [Google Scholar]
- 185.Plomin R, Haworth CMA, Davis OSP: Common disorders are quantitative traits. Nat Rev Genet 10: 872–878, 2009 [DOI] [PubMed] [Google Scholar]
- 186.Tan Q, Zhao JH, Li S, Kruse TA, Christensen K: Power assessment for genetic association study of human longevity using offspring of long-lived subjects. Eur J Epidemiol 25: 501–506, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shi H, Belbin O, Medway C, Brown K, Kalsheker N, Carrasquillo M, Proitsi P, Powell J, Lovestone S, Goate A, Younkin S, Passmore P, Morgan K, Craig D, Johnston J, Mcguinness B, Todd S, Kehoe PG, Hooper NM, Vardy ERLC, Mann DM, Smith AD, Wilcock G, Warden D; Genetic and Environmental Risk for Alzheimer’s Disease Consortium; Alzheimer’s Research UK Consortium : Genetic variants influencing human aging from late-onset Alzheimer’s disease (LOAD) genome-wide association studies (GWAS). Neurobiol Aging 33: 1849.e5–1849.e18, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rando TA, Chang HY: Aging, rejuvenation, and epigenetic reprogramming: Resetting the aging clock. Cell 148: 46–57, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dwivedi RS, Herman JG, McCaffrey TA, Raj DSC: Beyond genetics: Epigenetic code in chronic kidney disease. Kidney Int 79: 23–32, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Reddy MA, Natarajan R: Epigenetics in diabetic kidney disease. J Am Soc Nephrol 22: 2182–2185, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Smyth LJ, McKay GJ, Maxwell AP, McKnight AJ: DNA hypermethylation and DNA hypomethylation is present at different loci in chronic kidney disease. Epigenetics 9: 366–376, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Trionfini P, Benigni A, Remuzzi G: MicroRNAs in kidney physiology and disease. Nat Rev Nephrol 11: 23–33, 2015 [DOI] [PubMed] [Google Scholar]
- 193.Ben-Avraham D: Epigenetics of aging. Adv Exp Med Biol 847: 179–191, 2015 [DOI] [PubMed] [Google Scholar]
- 194.Canaud G, Bonventre JV: Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol Dial Transplant 30: 575–583, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hariri RJ, Hajjar DP, Coletti D, Alonso DR, Weksler ME, Rabellino E: Aging and arteriosclerosis. Cell cycle kinetics of young and old arterial smooth muscle cells. Am J Pathol 131: 132–136, 1988 [PMC free article] [PubMed] [Google Scholar]
- 196.Rong S, Zhao X, Jin X, Zhang Z, Chen L, Zhu Y, Yuan W: Vascular calcification in chronic kidney disease is induced by bone morphogenetic protein-2 via a mechanism involving the Wnt/β-catenin pathway. Cell Physiol Biochem 34: 2049–2060, 2014 [DOI] [PubMed] [Google Scholar]
- 197.Yonekura Y, Fujii H, Nakai K, Kono K, Goto S, Shinohara M, Nishi S: Anti-oxidative effect of the β-blocker carvedilol on diabetic nephropathy in non-obese type 2 diabetic rats [published online ahead of print July 14, 2015]. Clin Exp Pharmacol Physiol 10.1111/1440-1681.12447 [DOI] [PubMed] [Google Scholar]
- 198.Bianchessi V, Badi I, Bertolotti M, Nigro P, D’Alessandra Y, Capogrossi MC, Zanobini M, Pompilio G, Raucci A, Lauri A: The mitochondrial lncRNA ASncmtRNA-2 is induced in aging and replicative senescence in Endothelial Cells. J Mol Cell Cardiol 81: 62–70, 2015 [DOI] [PubMed] [Google Scholar]
- 199.London G, Covic A, Goldsmith D, Wiecek A, Suleymanlar G, Ortiz A, Massy Z, Lindholm B, Martinez-Castelao A, Fliser D, Agarwal R, Jager KJ, Dekker FW, Blankestijn PJ, Zoccali C; for EUropean REnal and CArdiovascular Medicine working group of the European Renal Association–European Dialysis and Transplant Association (ERA–EDTA) : Arterial aging and arterial disease: Interplay between central hemodynamics, cardiac work, and organ flow-implications for CKD and cardiovascular disease. Kidney Int Suppl (2011) 1: 10–12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zeng Y, Wang P-H, Zhang M, Du J-R: Aging-related renal injury and inflammation are associated with downregulation of Klotho and induction of RIG-I/NF-κB signaling pathway in senescence-accelerated mice. Aging Clin Exp Res 28: 69–76, 2015 [DOI] [PubMed] [Google Scholar]
- 201.Ren S, Duffield JS: Pericytes in kidney fibrosis. Curr Opin Nephrol Hypertens 22: 471–480, 2013 [DOI] [PubMed] [Google Scholar]