Abstract
AKI leads to tubular injury and interstitial inflammation that must be controlled to avoid the development of fibrosis. We hypothesized that microRNAs are involved in the regulation of the balance between lesion formation and adaptive repair. We found that, under proinflammatory conditions, microRNA-146a (miR-146a) is transcriptionally upregulated by ligands of IL-1 receptor/Toll–like receptor family members via the activation of NF-κB in cultured renal proximal tubular cells. In vivo, more severe renal ischemia-reperfusion injury (IRI) associated with increased expression of miR-146a in both allografts and urine of human kidney transplant recipients, and unilateral IRI in mice induced miR-146a expression in injured kidneys. After unilateral IRI, miR-146a−/− mice exhibited more extensive tubular injury, inflammatory infiltrates, and fibrosis than wild-type mice. In vitro, overexpression or downregulation of miR-146a diminished or enhanced, respectively, IL-1 receptor–associated kinase 1 expression and induced similar effects on C-X-C motif ligand 8 (CXCL8)/CXCL1 expression by injured tubular cells. Moreover, inhibition of CXCL8/CXCL1 signaling prevented the development of inflammation and fibrosis after IRI in miR-146a−/− mice. In conclusion, these results indicate that miR-146a is a key mediator of the renal tubular response to IRI that limits the consequences of inflammation, a key process in the development of AKI and CKD.
Keywords: ischemia-reperfusion, Cell Signaling, delayed graft function, fibrosis, renal epithelial cell, mRNA
AKI is a widespread pathologic condition that is increasing alarmingly in incidence.1 AKI is estimated to occur in one in five adults and one in three children who are hospitalized with acute illness.2 AKI has been associated with increased short– and long–term mortality and prolonged hospital stays, and compelling evidence has shown associations of AKI with the incidence of CKD and acceleration to ESRD. The economic consequences of AKI are substantial; with costs of $4.7 billion for 498,000 hospital stays, acute renal failure was one of the most expensive conditions treated in United States hospitals in 2011.3
Regardless of its cause, AKI leads to tubular injury and interstitial inflammation.4 Because the tubular epithelium has the capacity to proliferate and replace lost cells, the kidney can recover after acute injury5; however, this repair must be controlled to avoid the development of fibrosis, leading to CKD. Therefore, the balance between postinjury lesion formation and adaptive repair is a key determinant of the fate of the injured kidney. Inflammation is a major process in the renal response to injury. Inflammation is necessary for the induction of tissue repair, but inflammatory activity is a double-edged sword, because uncontrolled inflammation leads to the development of progressive kidney fibrosis with consequent loss of function.6 Identifying the endogenous factors involved in the downregulation of inflammation would be a major step in improving AKI treatment, but unfortunately, these factors remain poorly understood.
MicroRNAs (miRs) are short, noncoding RNAs that regulate gene expression at the post-transcriptional level by targeting the 3′ untranslated regions (UTRs) of specific mRNAs.7 miRs act as key regulators of many biologic processes by fine tuning the concentrations of their targets. Many renal diseases with significant inflammation, such as ischemia-reperfusion injury (IRI), GN, or acute kidney allograft rejection,8–10 have been associated with alterations in miR expression profiles. However, the roles of miRs in the pathophysiology of and the renal response to injury are only beginning to be recognized. Although the tubular epithelium is an active player in the development of inflammation-induced injury and although inflammation profoundly alters epithelial cells, the miR expression profiles of tubular epithelial cells exposed to inflammatory injury have not been studied. After the binding of specific ligands to IL-1 receptors and Toll-like receptors (TLRs) expressed by epithelial cells, activation of the NF-κB pathway and other signaling pathways leads to tubular expression of numerous components of the innate immune response, such as proinflammatory cytokines, complement factors, chemokines, and adhesion molecules. These components promote the attraction and activation of neutrophils, macrophages, and lymphocytes, leading to enhancement of the inflammatory response.11 Because an amplified inflammatory response can be deleterious, negative cellular feedback loops should exist to limit the duration and intensity of the inflammatory response.12 We hypothesized that miRs are involved in the tubular response to inflammation.
Using in vitro and in vivo experiments, we identified miR-146a as a mediator of the renal response to injury that limits the consequences of inflammation. These findings indicate that miR-146a performs a key function in the development of acute kidney diseases and CKDs.
Results
miR-146a Is Induced in Renal Tubular Epithelial Cells under Proinflammatory Conditions
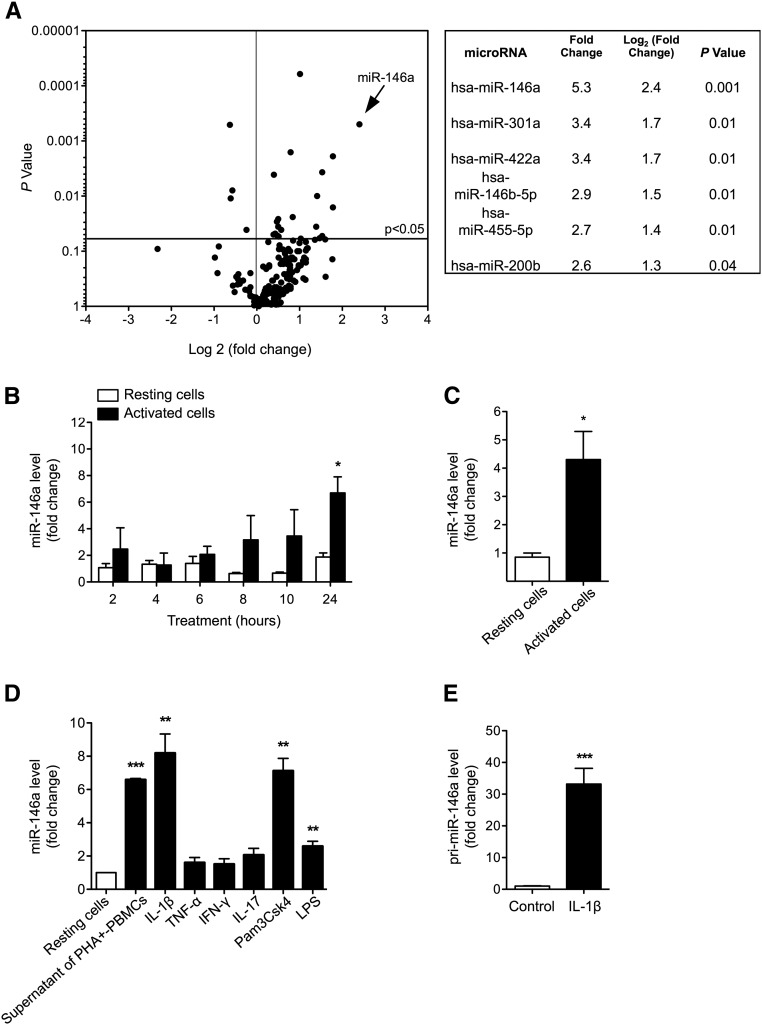
To investigate whether miRs are involved in the adaptation of tubular epithelial cells to inflammation, we first aimed to characterize the miR expression profiles of renal tubular epithelial cells exposed to proinflammatory cytokines. In a first discovery approach, we exposed proximal tubular epithelial HK-2 cells to the supernatant of phytohemagglutinin (PHA)-activated PBMCs. This treatment strongly induced the expression of the chemokines C-X-C motif ligand 10 (CXCL10) and CCL5 within 2 hours, thus confirming the activated status of the PBMCs (Supplemental Figure 1A). Next, we examined the miR profiles of HK-2 cells treated with supernatants of PHA-activated PBMCs for 24 hours and resting HK-2 cells (Figure 1A, Supplemental Table 1). We found that a limited number of miRs is regulated by the supernatant of PHA-activated PBMCs (Figure 1A).
Figure 1.
miR-146a is induced in renal tubular epithelial cells under proinflammatory conditions. (A) Volcano plot showing changes in miR levels as detected by TaqMan low–density arrays of RNA from HK-2 cells incubated (n=3) or not incubated (n=3) for 24 hours with the cellfree supernatant of PBMCs activated with PHA. The volcano plot graphs the log2 of the fold change in the expression of each gene between the samples versus the P values from the t test. The table provides fold changes and P values of miRs with fold differences in expression greater than two. (B) Time-dependent induction of miR-146a in HK-2 cells incubated (black bars) or not incubated (white bars) for 2–24 hours with supernatants of PHA-activated PBMCs (means±SEM of three independent experiments). (C) miR-146a levels in primary cultures of human renal epithelial cells activated (black bar) or not activated (white bar) with supernatants of PHA-activated PBMCs (mean±SEM of three independent experiments). (D) miR-146a expression in HK-2 cells incubated for 24 hours with cytokines and TLR ligands (mean±SEM of three independent experiments). (E) The levels of pri–miR-146a in HK-2 cells incubated for 24 hours with 50 ng/ml IL-1β. Target miR levels were normalized to the RNU48 snRNA level. *P<0.05; **P<0.01; ***P<0.001.
Among the differentially expressed miRs, miR-146a, also referred to as miR-146a-5p, was the most strongly upregulated (Figure 1A). Time course experiments confirmed early and progressive miR-146a upregulation in HK-2 cells in response to inflammatory mediators (Figure 1B), and this regulation was confirmed in primary cultures of human renal epithelial cells (Figure 1C).
To determine the inflammatory mediators that induce miR-146a expression, we exposed HK-2 cells to the proinflammatory cytokines IL-1β, TNF-α, IFN-γ, and IL-17 as well as to TLR ligands for 24 hours. IL-1β, the TLR-2 ligand Pam3CSK4, and the TLR-4 ligand LPS significantly induced miR-146a expression (Figure 1D). In additional in vitro experiments, IL-1β was used to induce miR-146a expression in tubular cells. Time course concentration dependence analyses of IL-1β–induced miR-146a expression showed that the optimal experimental conditions were 24 hours of treatment with 50 ng/ml IL-1β (Supplemental Figure 1, B and C).
IL-1β treatment induced the expression of the primary miR-146a transcript primiR-146a in HK-2 cells. This result confirmed the transcriptional aspect of inflammation–induced miR-146a expression (Figure 1E).
IL-1β–Induced NF-κB Signaling Upregulates miR-146a in Renal Tubular Epithelial Cells
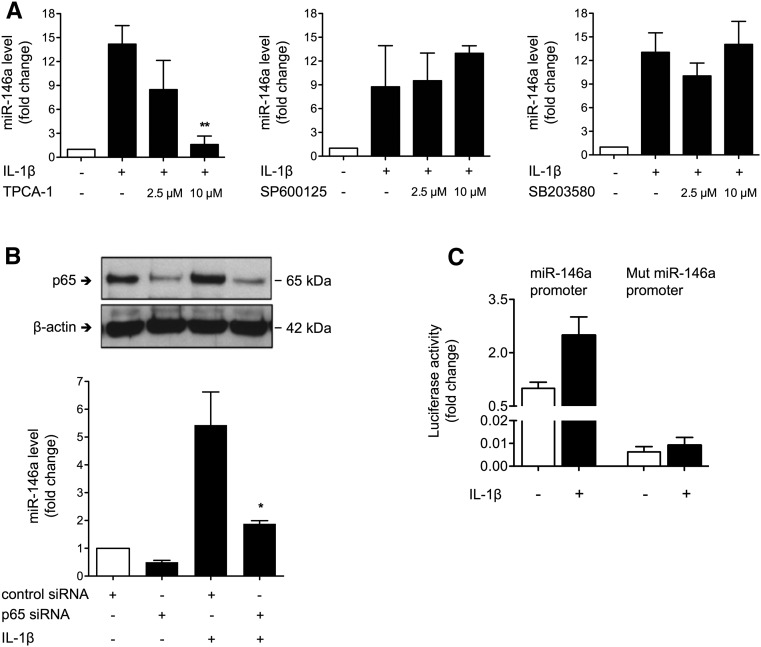
To further study the molecular pathway involved in IL-1β–mediated miR-146a induction, we used pharmacologic inhibitors to block the downstream pathways involved in IL-1R/TLR signaling. IL-1β–mediated miR-146a induction was abolished by the NF-κB pathway inhibitor TPCA-1, whereas chemical inhibition of the c-Jun N–terminal kinase pathway or the p38 MAP kinase pathway did not alter IL-1β–induced miR-146a expression (Figure 2A). To provide further evidence that NF-κB is the critical intermediate between IL-1R/TLR activation and miR-146a, we evaluated the effect of p65 suppression on IL-1β–mediated miR-146a regulation. We found that IL-1β–mediated induction of miR-146a was significantly attenuated by knockdown of the p65 NF-κB subunit using siRNA (Figure 2B). The involvement of NF-κB in IL-1β–induced miR-146a expression was further confirmed by the activity of a luciferase reporter vector carrying the miR-146a promoter and two NF-κB binding sites. Conversely, IL-1β–induced luciferase activity was abolished in the luciferase reporter vector containing mismatches in the two NF-κB binding sites (Figure 2C).
Figure 2.
The levels of miR-146a induced by IL-1β in renal tubular epithelial cells are NF-κB dependent. (A) miR-146a expression after incubation of untreated (white bars) HK-2 cells and cells treated for 24 hours with 50 ng/ml IL-1β (black bars) or 2.5 or 10 μmol/L pharmacologic inhibitor SP600125, SB203580, or TPCA-1. (B) Western blot of p65 NF-κB subunit and β-actin in HK-2 cells after transfection with p65 siRNA or scrambled siRNA (upper panel) and miR-146a expression in transfected untreated HK-2 cells and cells treated with 50 ng/ml IL-1β (lower panel). (C) Activity of the luciferase reporter vector carrying the 600-bp segment of the WT promoter of miR-146a and two NF-κB binding sites compared with a vector containing mismatches in the two NF-κB binding sites (Mut) in HK-2 cells incubated for 24 hours with 50 ng/ml IL-1β. Data are shown as means±SEM of two to three independent experiments. *P<0.05; **P<0.01.
miR-146a Is Induced in Response to Renal IRI in Humans and Mice
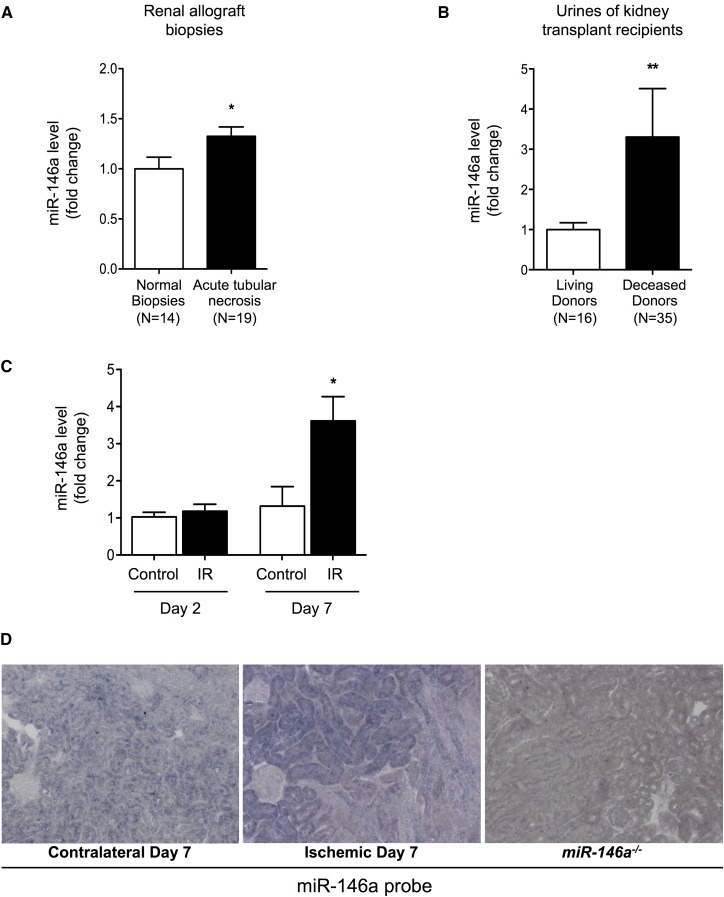
Our in vitro experiments showed that miR-146a was induced in an NF-κB–dependent manner in tubular epithelial cells under proinflammatory conditions. We next aimed to confirm our in vitro observations in the context of human pathology. IRI is a prototypical condition in which inflammatory mediators, including innate immune effectors and reactive oxygen species, lead to phenotypic changes in tubular cells. Under clinical conditions, the renal consequences of ischemia-reperfusion are characterized by acute tubular injury occurring immediately after kidney transplantation. Interestingly, miR-146a expression was slightly but significantly increased in renal allografts of patients who displayed acute tubular necrosis early after transplantation compared with those of patients who displayed normal allograft biopsy results (Figure 3A). We exploited variable severities of IRI observed early after transplantation in recipients of living (minimal IRI) or deceased donor (strong IRI) kidneys to study the miR-146a expression level in urine samples collected 10 days after renal transplantation. We found an increased urinary level of miR-146a in recipients of deceased donor kidneys compared with recipients of living donor kidneys. These results suggest that miR-146a expression correlates with IRI severity (Figure 3B).
Figure 3.
miR-146a is induced in vivo after renal IRI. (A) miR-146a levels in kidney transplant biopsies from patients with delayed graft function after kidney transplantation (n=19) compared with those from patients in stable condition at 3 months post-transplantation (n=14). (B) miR-146a levels at 10 days post-transplantation in urine samples from kidney transplant recipients who received a kidney from a deceased (n=35) or a living donor (n=16). (C) Ischemia-induced upregulation of miR-146a expression in C57BL/6 mouse kidneys (n=5–8) on days 2 and 7 after unilateral IRI (black bars) compared with in contralateral kidneys (white bars). (D) In situ hybridization for miR-146a in sections of the contralateral kidney (left panel) and the postischemic kidney (center panel) 7 days after IRI. Kidney sections from miR-146a−/− mice were used as controls (right panel). The results of quantitative PCR have been normalized to the RNU48 snRNA levels for human samples or the U6 snRNA levels for mouse samples. Data are presented as means±SEM. Original magnification, ×400. *P<0.05; **P<0.01.
To confirm that renal ischemia induces tubular miR-146a expression, we studied miR-146a expression in mouse kidneys after unilateral IRI. The miR-146a level was increased in injured kidneys 7 days after ischemia-reperfusion compared with contralateral kidneys (Figure 3C); this finding confirmed previous observations.8 In situ hybridization revealed that miR-146a was predominantly overexpressed in tubular cells. In contrast, very little interstitial staining for miR-146a was observed (Figure 3D, center panel).
miR-146a−/− Mice Are Susceptible to Renal IRI
To further evaluate the instrumental role of miR-146a in the renal tubular response to IRI in vivo, we studied miR-146a−/− mouse13 phenotypes in response to IRI. For this purpose, wild–type miR-146a+/+ (WT) and miR-146a−/− mice were subjected to unilateral IRI.
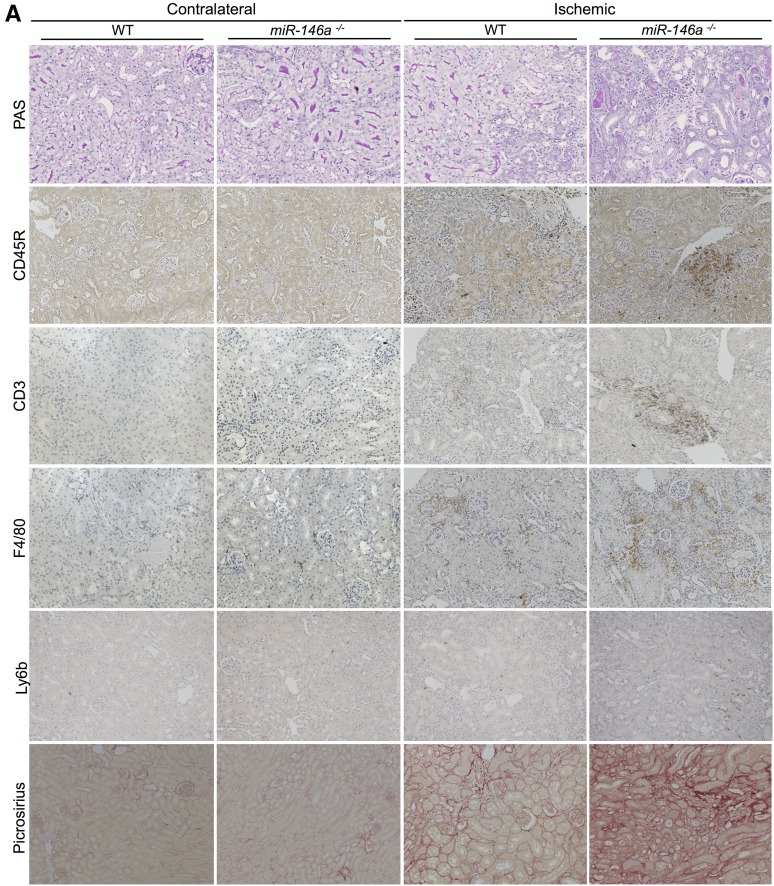
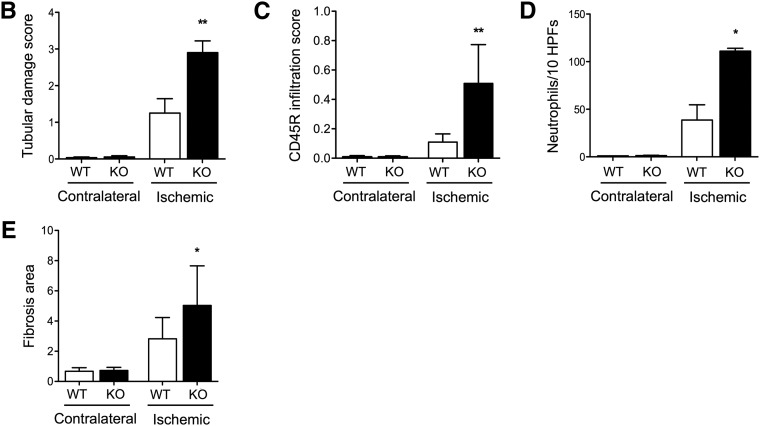
Seven days after IRI, although miR-146a was significantly induced in response to IRI in WT mice, we did not observe any difference in tubular lesions between WT and miR-146a−/− mice (data not shown). Fourteen days after IRI, the WT mice had minimal tubular lesions on the experimental kidneys compared with their contralateral kidneys. miR-146a−/− mice displayed significantly more severe tubular damage than WT mice, with more tubular dilations and vacuolizations, loss of the brush border, tubular casts, and tubular necrosis at the corticomedullary junction (Figure 4A). The contralateral kidneys of WT and miR-146a−/− mice did not display tubular injury. Semiquantitative analysis of tubular lesions confirmed greater tubular lesions in miR-146a−/− mice (Figure 4B). Ischemic kidneys from miR-146a−/− mice showed extensive renal infiltration of CD45R+ leukocytes (Figure 4, A and C), primarily including CD3+ T lymphocytes, F4/80+ monocytes-macrophages, and a few PMN cells (Figure 4, A and D). Inflammatory cell infiltration predominated in the outer medullary rays and extended nearly to the cortex. There were few inflammatory cells in ischemic kidneys from WT mice, and the contralateral kidneys did not display leukocyte infiltration.
Figure 4.
miR-146a deficiency increases tubular injury, interstitial inflammation, and fibrosis after renal IRI. (A) Representative sections of outer medullae from WT and miR-146a−/− mice at 14 days after reperfusion stained with periodic acid–Schiff (PAS) or immunostained for CD45R, CD3, F4/80, and Ly6b. Alternatively, collagen deposits were visualized by picrosirius red staining. Original magnification, ×200. (B) Semiquantitative analysis of tubular damage in injured kidneys of WT (n=6) and miR-146a−/− (n=6) mouse kidneys at day 14 post-IRI. (C) Semiquantitative analysis of CD45R+ leukocyte infiltration into the injured kidneys of WT and miR-146a−/− mice at day 14 post-IRI. (D) Quantification of infiltrating Ly6b+ neutrophils into the injured kidneys of WT and miR-146a−/− mice at day 14 post-IRI. (E) Quantification of WT and miR-146a−/− mouse kidneys stained with picrosirius red for visualization of collagen deposits. Data are shown as means±SEM; n=6–10 mice per group. Asterisks indicate comparisons of injured kidneys between WT and miR-146a−/− mouse. *P<0.05; **P<0.01.
Finally, we investigated whether the increased tubular injury and interstitial inflammation observed in miR-146a−/− mice 14 days after IRI translated into increased chronic lesions. As shown in Figure 4A, the ischemic kidneys from miR-146a−/− mice showed increased fibrosis as confirmed by quantification of picrosirius staining (Figure 4E).
miR-146a Targets the NF-κB Pathway and CXCL8
We next wondered which targets of miR-146a are involved in the greater susceptibility of miR-146a−/− mouse kidneys to IRI. To this end, in vitro gain and loss of function experiments were performed on HK-2 cells (Supplemental Figure 1D).
The NF-κB pathway is a known target of miR-146a14 and plays a critical role during IRI.15 We explored the consequences of miR-146a overexpression in renal tubular cells on 84 genes of the NF-κB pathway under proinflammatory conditions using a PCR array.
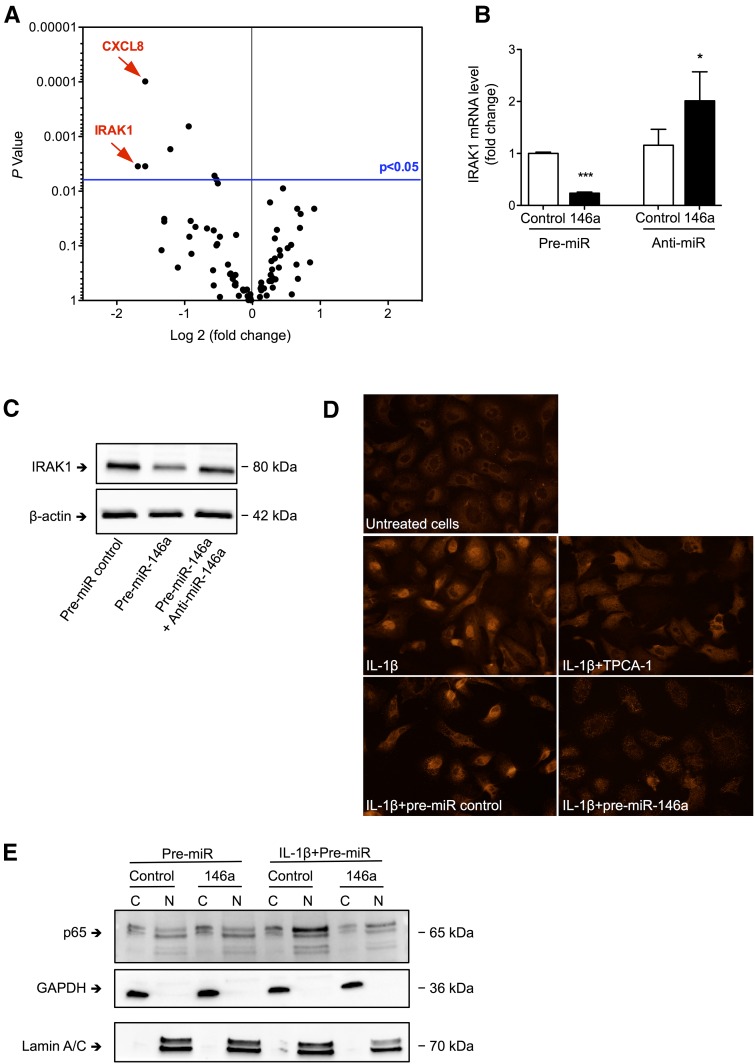
miR-146a overexpression resulted in significant downregulation of a limited number of mRNAs, including IL-1 receptor–associated kinase 1 (IRAK1) and CXCL8 mRNAs (Supplemental Table 2). PCR array screening confirmed the effect of miR-146a on the IL-1 signal transducer IRAK1, a known miR-146a target14 that was among the most significantly downregulated mRNAs in miR-146a–overexpressing cells (Figure 5A). We confirmed by conventional quantitative RT-PCR that IRAK1 mRNA expression was downregulated by miR-146a overexpression and conversely, upregulated by miR-146a knockdown (Figure 5B). At the protein level, transfection of pre–miR-146a reduced the levels of IRAK1, which were restored in the presence of anti–miR-146a (Figure 5C). Supporting a functional role of miR-146a in regulating the NF-κB pathway in renal tubular cells, pre–miR-146a led to abolition of IL-1β–induced p65 nuclear translocation (Figure 5D). Furthermore, nuclear extracts of IL-1β–treated cells showed no nuclear enrichment of p65 after transfection with pre–miR-146a in contrast to the nuclear enrichment of p65 observed after transfection with pre–miR-control (Figure 5E).
Figure 5.
miR-146a inhibits nuclear translocation of the p65 NF-κB subunit in response to IL-1β. (A) Volcano plot showing changes in the levels of 84 mRNAs related to the NF-κB pathway as detected by PCR arrays analyzing RNA from HK-2 cells transfected with pre–miR-146a or a control and incubated for 24 hours with 50 ng/ml IL-1β. (B) IRAK1 mRNA levels 24 hours after transfection of HK-2 cells with pre–miR-146a or anti–miR-146a (50 nM). Target mRNA levels were normalized to HPRT levels. (C) Western blot of IRAK1 in HK-2 cells 24 hours after transfection of HK-2 cells with pre–miR-control, pre–miR-146a, or both pre–miR-146a and anti–miR-146a (50 nM). (D) p65 NF-κB subunit immunofluorescence in control HK-2 cells incubated for 1 hour with 50 ng/ml IL-1β and 10 μM TPCA-1 or n HK-2 cells transfected with pre–miR-control or pre–miR-146a; untreated cells were used as a negative control. (E) Western blot of the p65 NF-κB subunit on nuclear and cytoplasmic extracts from HK-2 cells transfected with pre–miR-control or pre–miR-146a in the presence or absence of IL-1β; Western blots of GAPDH and lamin A/C were used to confirm the purity of the cytoplasmic and nuclear fractions, respectively. Data are shown as means±SEM. *P<0.05.
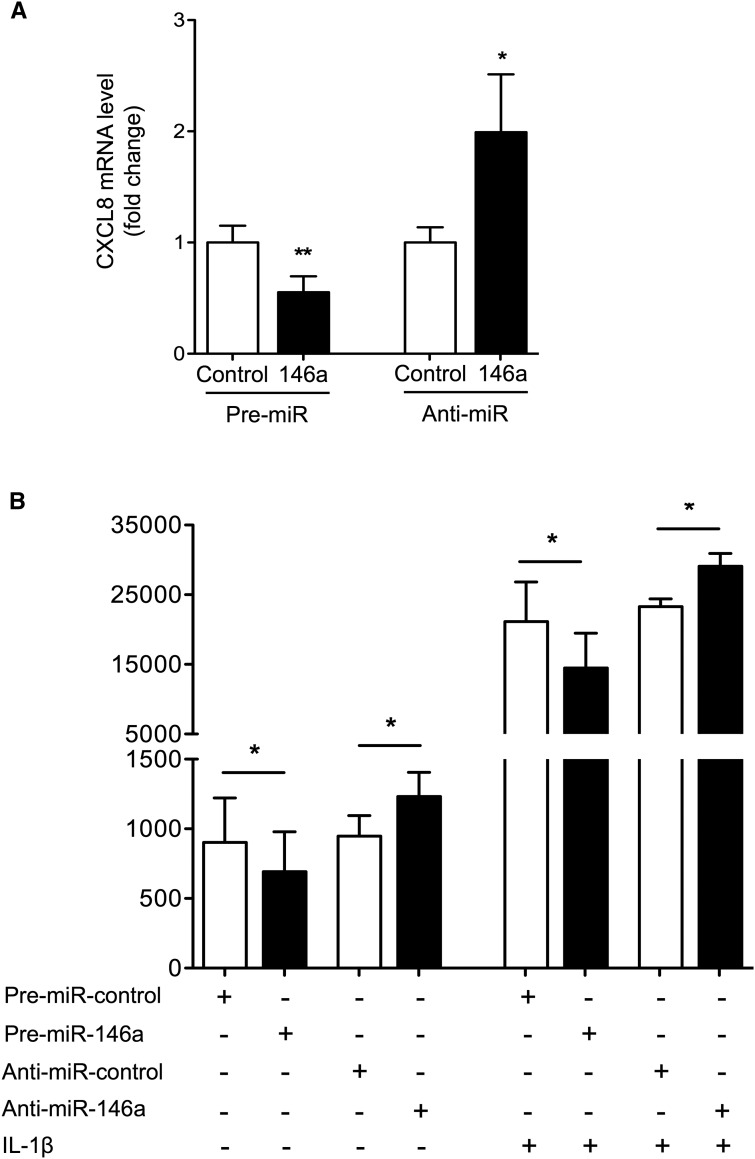
In addition to its effect on IRAK1, overexpression of miR-146a in tubular cells led to highly significant downregulation of the proinflammatory chemokine CXCL8 (Figure 5A). miR-146a overexpression and inhibition reduced and enhanced CXCL8 mRNA expression, respectively (Figure 6A). At the protein level, CXCL8 secretion was reduced after miR-146a overexpression and enhanced after miR-146a inhibition, and these effects were maintained in IL-1β–treated cells (Figure 6B). In silico analysis revealed the lack of miR-146a target sites in the 3′ UTR of CXCL8 mRNA. Consequently, we concluded that miR-146a acts in vitro as a negative regulator of inflammation in renal tubular epithelial cells by downregulating NF-κB pathway activation induced by IL-1β via direct targeting of IRAK1, thus facilitating indirect downstream modulation of CXCL8 secretion.
Figure 6.
miR-146a targets CXCL8 in renal tubular cells. (A) CXCL8 mRNA levels quantitated by quantitative PCR in HK-2 cells after transfection with pre–miR-146a and anti–miR-146a or corresponding negative controls. (B) CXCL8 protein secretion after transfection with pre–miR-146a and anti–miR-146a or corresponding negative controls in untreated HK2 cells (right panel) or cells incubated with 50 ng/ml IL-1β for 24 hours (left panel). Target mRNA expression was normalized to HPRT expression. Data are shown as means±SEM of four independent experiments. *P<0.05; **P<0.01.
Blocking CXCL8/CXCL1 Biologic Activity Attenuates Renal Fibrosis in miR-146a−/− Mice after Ischemia-Reperfusion
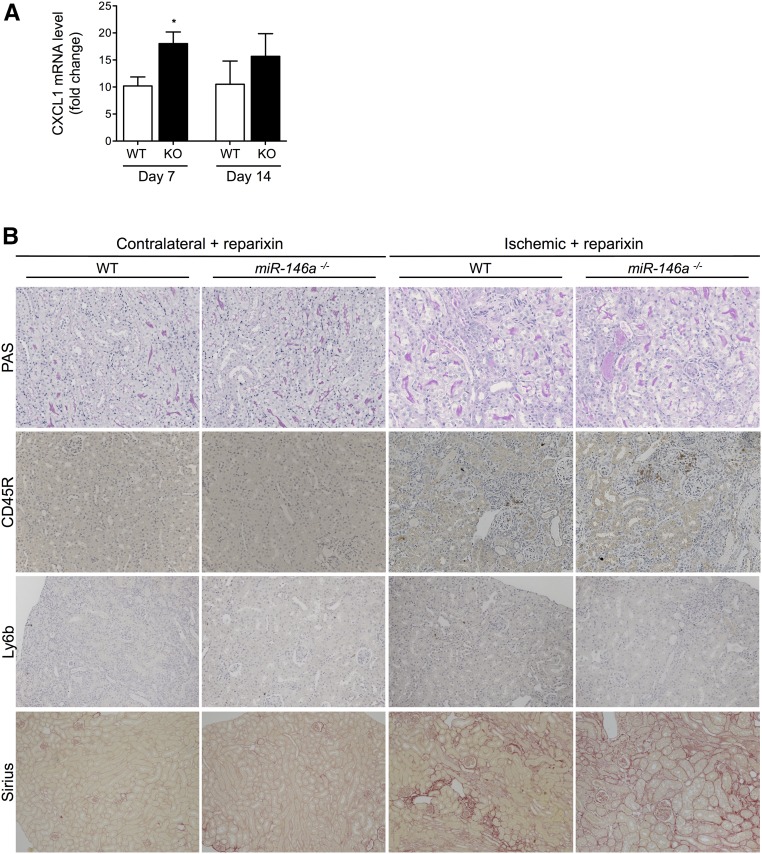
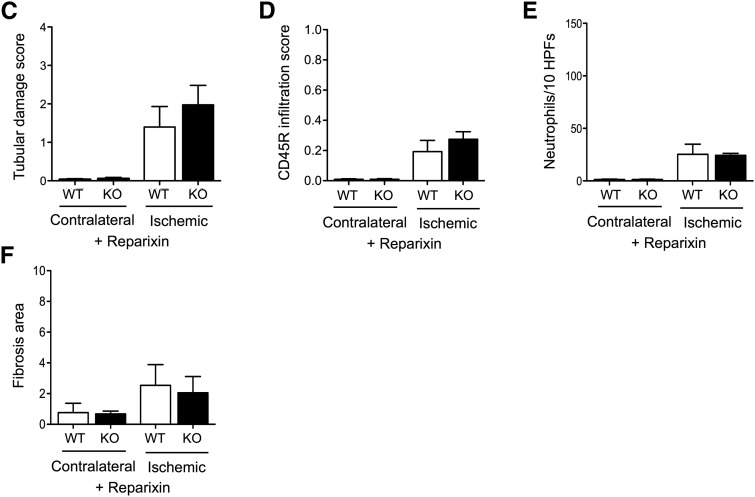
The effect of miR-146a on CXCL8 secretion under proinflammatory conditions prompted us to investigate whether the enhanced tubular lesions and fibrosis observed in miR-146a−/− mice could be driven, at least in part, by the loss of negative control of the NF-κB/CXCL8 pathway. In humans, CXCL8 signaling occurs through its receptors CXCR1 and CXCR2, and in rodents, signaling of the CXCL8 homolog CXCL1 occurs through the CXCR2 receptor. CXCL1 mRNA was increased in miR-146a−/− mouse kidneys 7 and 14 days after IRI (Figure 7A) compared with in WT mouse kidneys. Interestingly, the CXCL1/CXCR2 signaling inhibitor reparixin attenuated post–IRI tubular damage in miR-146a−/− mice (Figure 7B) and abrogated the difference between WT and miR-146a−/− mice with regard to tubular damage severity (Figure 7C), interstitial infiltration (Figure 7, B, D, and E), and collagen deposits (Figure 7, B and F).
Figure 7.
Reparixin prevents renal fibrosis in miR-146a−/− mice after IRI. (A) CXCL1 mRNA levels in WT and miR-146a−/− mouse kidneys 7 and 14 days post-IRI. Target mRNA levels were normalized to HPRT levels. (B) Representative sections of outer medullae from WT and miR-146a−/− mice at 14 days after reperfusion, with reparixin treatment from day 7 to day 14. Original magnification, ×200 for periodic acid–Schiff (PAS), picrosirius red staining, CD45R, and Ly6b immunostaining. (C) Semiquantitative analysis of tubular damage in reparixin–treated postischemic kidneys from WT (n=4) and miR-146a−/− mice (n=4). (D) Semiquantitative analysis of CD45R+ leukocyte infiltration into the injured kidneys of reparixin–treated WT and miR-146a−/− mice at day 14 post-IRI. (E) Quantification of infiltrating Ly6b+ neutrophils into the injured kidneys of reparixin–treated WT and miR-146a−/− mice at day 14 post-IRI. (F) Analysis of picrosirius staining in the renal interstitium of miR-146a−/− mice compared with WT mice. Data are shown as means±SEM; n=6 mice per group. Asterisk indicates comparisons of injured kidneys between WT and miR-146a−/− mice. *P<0.05.
Discussion
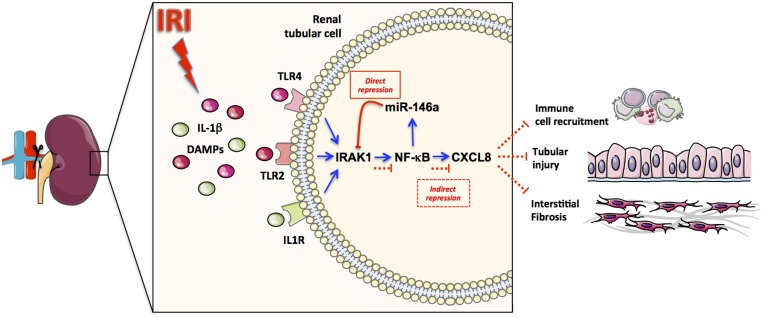
Our study delineated an miR–mediated signaling pathway that counteracts innate immune system activation after renal IRI. We identified miR-146a as a major miR that is induced in tubular cells under proinflammatory conditions and in response to IRI in mouse and human kidneys. In vivo, miR-146a affects the response of tubular epithelial cells to renal IRI. Specifically, miR-146a was found to target the NF-κB pathway in tubular cells and consequently, regulate CXCL8 secretion. Deletion of miR-146a in vivo enhanced the formation of tubular lesions after unilateral IRI, the infiltration of immune cells, and the development of renal fibrosis. These results confirmed the instrumental role of miR-146a in limiting prolonged inflammation (Figure 8).
Figure 8.
miR-146a plays a critical role in limiting the inflammatory response to ischemia-reperfusion. After IRI, the induction of TLR/IL-1R signaling leads to the activation of the NF-κB pathway, promoting CXCL8 secretion by tubular cells. This process facilitates immune cell recruitment, leading to tubular injury and interstitial fibrosis. NF-κB activation also induces miR-146a expression, which indirectly suppresses CXCL8 secretion by targeting IRAK1, thus balancing the inflammatory consequences of renal IRI.
TLR/IL-1R signaling plays a major role in renal IRI.16 IRI results in NF-κB activation and phenotypic changes in the epithelium that amplify local inflammation.17 All of these receptors signal through MyD88 and IRAK1 to induce NF-κB activation and produce cytokines and chemokines that attract neutrophils and macrophages, thereby promoting a nonspecific host inflammatory response. To avoid uncontrolled innate immune activation, which can lead to detrimental effects on the function of epithelial cells and organ failure, activation of TLR/IL-1R signaling must be tightly regulated, and endogenous regulatory feedback is needed to promote repair after IRI. Previously published data suggest that limited numbers of miRs are induced in kidney tissue in response to IRI.8 Notably, miR-146a was induced in a relatively late step, consistent with the hypothesis that miR-146a might be involved in a negative feedback loop. Taganov et al.14 first reported that NF-κB–dependent induction of miR-146a activated a negative feedback loop, leading to downregulation of TLR signaling in monocytes by targeting the 3′ UTR of TRAF6 and IRAK1. Our results combined with data from the literature support a role of miR-146a as a general regulator of the innate immune response in not only immune cells but also, cells that are targeted by inflammation. Consistent with this universal role of miR-146a, a recent study of mouse myocardial IRI showed that increasing the expression of miR-146a using a lentivirus expressing miR-146a significantly reduced the myocardial infarct size and prevented the cardiac dysfunction typically induced by IRI via the suppression of IRAK1 and TRAF6 expression.18
miR-146a seems to be a key effector in limiting the intensity and duration of renal epithelial activation after stimulation by proinflammatory cytokines. For instance, we found that the anti-inflammatory activity of miR-146a was at least partially mediated by the regulation of CXCL8 secretion. In silico analyses did not find miR-146a seed matches in the CXCL8 3′ UTR, and this negative result suggested that miR-146a does not directly regulate CXCL8 production but rather, regulates the translation of other proteins affecting CXCL8 production, such as IRAK1. In fibroblasts, in vitro experiments have shown that miR-146a is induced by senescence and acts as a negative regulator of excessive IL-6 and CXCL8 secretion, which is a hallmark of senescence.19 Chemokines and their receptors are involved in the physiopathology of epithelial cells, their responses to the induction of innate immunity, and the regulation of their morphology.20 Human CXCL8, formerly known as IL-8, and its rodent homolog CXCL1 are early proinflammatory chemokines secreted by mononuclear immune cells and epithelial cells to trigger neutrophil chemotaxis,21,22 leading to the recruitment and activation of PMN leukocytes. The expression of CXCL8/CXCL1 is predominantly regulated by NF-κB. CXCL8/CXCL1 has been implicated in a broad range of diseases characterized by PMN cell infiltration, including IRI. If the initial role of the CXCL8/CXCL1 pathway includes the induction of the local innate immune response after activation of the NF-κB pathway, persistent or excessive expression of CXCL8/CXCL1 could perpetuate immune cell recruitment, with deleterious effects on inflamed tissue. It has been shown that administration of a neutralizing anti–CXCL8 mAb limited PMN cell infiltration and tissue injury in a rabbit model of lung reperfusion injury.23
Our results suggest that miR-146a is induced in the kidneys after IRI in mice. In humans, miR-146a is enriched in the renal tissue of kidney recipients with acute tubular necrosis and the urine of deceased donor kidney recipients; these observations suggest a role of miR-146a in human IRI. After renal transplantation, IRI, also referred to as delayed graft function, is unavoidable and negatively affects both short– and long–term graft survival.24 IRI affects 20%–30% of deceased donor kidneys, and this rate could be higher if considering the increasing number of organs from marginal donors. A recent study identified upregulation of miR-24 in the kidneys of renal transplant recipients with a prolonged cold ischemia time and mice after experimental IRI.25 These authors showed that miR-24 inhibition induces regulation of tubular epithelial and endothelial cell apoptosis, leading to the modulation of kidney function and inflammation.25
Taken together, these results guide innovative miR–based therapeutics as well as new miR–based biomarkers of IRI in humans. In a murine model of intestinal IRI, local administration of miR-146a or its inducing agent 3,30-diinodolylmethane prevented the cellular accumulation of IRAK1 and the development of IRI–induced tissue damage.26 Interestingly, targeting the TLR/IL-1R pathway seemed to be more efficient than targeting NF-κB directly, because complete blockade of NF-κB is deleterious and exacerbates epithelial IRI–mediated tissue damage.27 Using a mouse model of renal fibrosis induced by unilateral ureteral obstruction, Morishita et al.28 recently reported the beneficial effects of miR-146a overexpression through miR-146a delivery using polyethylenimine nanoparticles in renal fibrosis, thus providing a preclinical demonstration of the great potential of this approach.
Our results suggest a role of tubular miR-146a in regulating the renal response to inflammation and IRI; however, our in vivo results obtained in mice systemically lacking miR-146a do not exclude the involvement of immune cells in this process. Notably, miR-146a is also highly expressed in macrophages and involved in the macrophage response to TLR engagement.14 Macrophages of the M1 phenotype are known to play critical roles in the development of renal lesions induced by IRI, whereas the M2 phenotype has been associated with renal repair.29 Bhatt et al.30 recently showed increased expression of M1 activation markers and suppression of M2 macrophage markers in miR-146a−/− mice under diabetic conditions. These results suggest that miR-146a deficiency skews macrophage polarization toward a more inflammatory phenotype.30 Tubular cell–specific miR-146a knockout will be required to determine the respective roles of deregulated immune cells and tubular cells in the renal response to IRI.
In conclusion, we showed that miR-146a contributes to the renal response to IRI in vivo by influencing the tubular epithelial response to inflammation via NF-κB pathway regulation, CXCL8 secretion, and postischemic renal fibrosis. The upregulation of miR-146a in the kidney tissue and urine of kidney transplant recipients suggests a role of miR-146a in human renal IRI. Collectively, these results emphasize miR-146a as an important effector of the pathogenesis of the renal response to IRI injury. Thus, miR-146a holds potential as a novel therapeutic target for AKI.
Concise Methods
Cell Culture Experiments and Reagents
Immortalized human proximal tubular cells from the HK-2 cell line were cultured in DMEM containing 1% FCS, 5 μg/ml insulin, 10 μg/ml human apotransferrin, 5 ng/ml selenium, 6.5 ng/ml tri-iodothyronin, 500 ng/ml hydrocortisone, 10 ng/ml EGF, 25 IU/ml penicillin, and 25 μg/ml streptomycin and then, plated at 75% confluence in six-well plates. Primary cultures of renal proximal tubular cells were collected as previously described.31 The cells were treated for 24 hours with various amounts of cellfree supernatants of PBMCs incubated with 2 μg/ml PHA (collected as previously described10), 50 ng/ml IL-1β, 100 ng/ml LPS (Sigma-Aldrich, St. Louis, MO), 50 ng/ml TNF-α, 50 ng/ml IFN-γ, 50 ng/ml IL-17 (R&D Systems, Minneapolis, MN), or 100 ng/ml Pam3CSK4 (InvivoGen, San Diego, CA).
TPCA-1, SP600125, and SB203580 were purchased from Sigma-Aldrich. Cells in six-well plates were cultured for 1 hour in the presence or absence of the indicated concentrations of TPCA-1, SP600125, or SB203580 and then, stimulated with 50 ng/ml IL-1β for 24 hours.
Transfection of HK-2 Cells
HK-2 cells were reverse transfected with pre–miR-control, pre–miR-146a, anti–miR-control, anti–miR-146a (50 nM; Ambion, Foster City, CA), p65 siRNA, or control siRNA (100 nM; Cell Signaling Technology, Danvers, MA) using Lipofectamine (Qiagen, Germantown, MD) according to the manufacturer’s instructions in serum-free medium. After transfection for 24 hours, the cells were washed and exposed to 50 ng/ml IL-1β for an additional 24 hours.
Luciferase Reporter Activity Assay for the miR-146a Promoter
The 600-bp sequences containing the two WT or mutated NF-κB binding sites and cloned into the pGL3 reporter vector were provided by Ingrid Struman.32 HK-2 cells were transfected with 0.5 μg indicated pGL3 vector and 0.01 μg control vector pRenilla-Luc using Lipofectamine (Qiagen). After 24 hours, the cells were treated with 50 nM IL-1β, and 24 hours later, a luciferase assay was performed using a Dual-Luciferase Assay System (Promega, Madison, WI). Firefly luciferase activity was normalized to Renilla luciferase activity.
Western Blotting
Western blot analyses were performed as previously described.33 Briefly, HK-2 cells were lysed in cell lysis buffer (9803; Cell Signaling Technology) containing a complete protease inhibitor cocktail (Roche, Basel, Switzerland). Protein extracts were resolved via SDS-PAGE followed by transfer to the appropriate membrane and incubation of the membrane overnight with antibodies against IRAK1 (1:200; sc-7883; Santa Cruz Biotechnology, Santa Cruz, CA), p65 (1:50; 4764; Cell Signaling Technology), GAPDH (1:500; Mab374; EMD Millipore, Billerica, MA), lamin A/C (1:500; 2032; Cell Signaling Technology), or β-actin (1:10,000; A5316; Sigma-Aldrich). Subsequently, the membranes were incubated with the appropriate horseradish peroxidase–conjugated secondary antibody. Chemiluminescent signals were detected using a Bio-Rad ChemiDoc MP System (Bio-Rad, Hercules, CA).
For nuclear and cytoplasmic extracts, untransfected HK-2 cells and cells transfected with pre–miR-control or pre–miR-146a for 24 hours and then, treated or not treated for 2 hours with 50 ng/ml IL-1β were used. The NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Fisher Scientific, Vernon Hills, IL) was used according to the manufacturer’s instructions. The extracted proteins were used for Western blot analyses.
mRNA and miR Isolation and Quantitative RT-PCR Analyses
Total RNA was isolated from cultured cells, kidney sections, or urine samples using QIAzol Reagent and the miRNeasy Kit (Qiagen) according to the manufacturer’s instructions.
For mRNA analysis, reverse transcription was performed using the High-Capacity Reverse-Transcription Kit (Life Technologies, Carlsbad, CA). Quantitative real–time PCR was performed using the SYBR Green method (Bio-Rad). Levels of expression were determined by normalization to the housekeeping gene HPRT. PCR primers were purchased from Qiagen (RT2 qPCR Primer Assays). For pri–miR-146a expression analysis, real-time PCR was performed using a TaqMan pri-miR Assay (Applied Biosystems, Foster City, CA).
For miR analysis, reverse transcription was performed using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems), and PCR products were amplified from cDNA samples using TaqMan MicroRNA Assays (Applied Biosystems). The small nucleolar RNA molecules U6 (mouse) and RNU48 (human) were used as endogenous controls for normalization of miR-146a expression levels. Fold changes in miR expression were calculated using the ΔΔCt method.
Global miR Profiling
We used the TaqMan Low-Density Array Human MicroRNA Panel, version 2.0 (Applied Biosystems), a 384-well microfluidic card containing primers and probes for 736 human miRs. Briefly, total RNA (800 ng) from HK-2 cells exposed for 24 hours to supernatants of PHA-activated PBMCs10 was reverse transcribed using TaqMan Megaplex RT Primers. Quantitative PCR was performed using an Applied BioSystems 7900HT Thermocycler according to the manufacturer’s instructions. Fold changes in the expression of each miR were normalized to the endogenous control RNU48 small nucleolar RNA. The relative expression levels between samples were calculated using the comparative ΔCt (threshold cycle number) method, and a control sample (normal) was used as the reference point.
NF-κB PCR Array
We used the RT2 Profiler PCR Array (Qiagen) according to the manufacturer’s instructions for the human NF-κB signaling pathway to simultaneously profile 84 related genes. The experiments were conducted using cDNAs from four different transfection experiments of pre–miR-146a or pre–miR-control in the presence of 50 ng/ml IL-1β.
CXCL8/IL-8 ELISA
HK-2 cells transfected with pre–miR-146a or anti–miR-146a or the corresponding controls were washed and exposed to 50 ng/ml IL-1β for 24 hours. The supernatants were harvested, and the levels of CXCL8 were determined using DuoSet ELISA (R&D Systems) according to the manufacturer’s instructions. All of the samples were tested in duplicate.
Patients
We obtained kidney allograft biopsies and urine samples from kidney transplant recipients from the Department of Kidney Transplantation of Necker Hospital. The patients’ characteristics are shown in Supplemental Table 3. This study was approved by the ethics committee of Ile-de-France XI (13016), and all of the participating patients provided written informed consent.
Allograft biopsy samples were placed in RNAlater (Ambion) and stored at −80°C until RNA extraction. Kidney transplant biopsies were obtained from 19 patients who experienced delayed graft function and whose allograft biopsies showed acute tubular necrosis. Samples from 15 patients with stable renal function and normal biopsy results at 3 months post-transplantation were used as controls.
Urine samples were obtained at 10 days post-transplantation from kidney transplant recipients who received a kidney from a deceased (n=35) or a living (n=16) donor. Briefly, the urine was centrifuged at 2000×g for 30 minutes within 4 hours of collection, and RNA was extracted from the pellet using the miRNeasy Mini Kit.34
Animal Studies
Male C57BL/6 WT mice and miR-146a knockout mice were purchased from Charles River Laboratories (Wilmington, MA) and housed under standard conditions. miR-146a−/− mice were backcrossed at least eight times in a C57BL/6 genetic background. Littermates were used as WT controls. Ten- to 12-week-old male mice were used for all experiments. All animal experimental procedures were conducted in accordance with institutional and legislative regulations and approved by the local authorities.
For the IRI protocol, after flank incisions, the left renal pedicles were dissected and clamped using microvascular clamps for 30 minutes at 37°C. After ischemia, the clamps were released for reperfusion. For the reparixin protocol, the mice received intraperitoneal injections (15 mg/kg) daily from day 7 post-IRI until euthanasia. For all experiments, the contralateral kidney served as an internal control for comparison with the injured kidney. At the time of euthanasia 14 days after IRI, the kidneys were removed for morphologic and RNA analyses.
Morphologic Analyses
After kidney extraction, one half of each kidney was fixed immediately in PBS-buffered 4% paraformaldehyde overnight and then, embedded in paraffin. Four-micrometer sections were used for immunostaining and coloration, including periodic acid–Schiff, hematoxylin and eosin, and picrosirius staining, to evaluate histologic damage.
The tissue sections were examined by light microscopy and scored on a semiquantitative scale of 0–5+ according to the percentage of tubules of the outer medulla area that displayed tubular necrosis, tubular dilation, loss of the brush border, and tubular casts as follows: 0, no lesion; 1+, ≤10%; 2+, 11%–25%; 3+, 26%–45%; 4+, 46%–75%; and 5+, ≥76%.35 Ten sections of each tissue were examined at ×200 magnification.
The fibrotic lesions were quantified using NIS Elements AR software, version 3.00. Staining of all kidney samples with picrosirius was performed simultaneously, and red intensity above a defined threshold was defined as fibrosis. Each section was analyzed along the corticomedullary junction before averaging, and the results were expressed as percentages of the total area of the selected fields.
Immunohistochemistry and Immunofluorescence
Immunostaining for inflammatory cell infiltration was performed using the following primary antibodies: monoclonal rat anti–mouse F4/80 (1:100; MCA497; AbD Serotec), monoclonal rat anti–mouse CD45R (1:100; sc-19597; Santa Cruz Biotechnology), monoclonal rabbit anti–mouse CD3 (1:100; ab16669; Abcam, Inc., Cambridge, MA), and monoclonal rat anti–mouse Ly6b (1:100; ab53457; Abcam, Inc.). Briefly, deparaffinized kidney sections were boiled in citrate buffer for antigen retrieval. Kidney sections were blocked with 3% BSA and incubated overnight at 4°C with primary antibodies followed by incubation with the appropriate horseradish peroxidase–conjugated secondary antibodies. The slides were counterstained with Harris hematoxylin. Blinded analysis of CD45R+ cellular infiltrate was performed by calculating the percentage of positively stained cells as follows: 0, no infiltration; 1+, 1%–5%; 2+, 6%–10%; 3+, 11%–15%; 4+, 16%–20%; and 5+, >20%. At least 10 sections of each tissue were examined at ×200 magnification. Blinded analysis of Ly6b+ cellular infiltrate was performed by assessing 20 consecutive HPFs (magnification, ×400); the number of cells staining positively for Ly6b were counted and expressed as cells per 10 HPFs.
For immunofluorescence, untreated HK-2 cells and cells treated for 2 hours with 50 ng/ml IL-1β alone or after transfection with pre–miR-control or pre–miR-146a or pretreatment with the NF-κB inhibitor TPCA-1 were fixed in 4% paraformaldehyde for 20 minutes, permeabilized in PBS containing 0.2% Triton X-100, and then, incubated with an antibody against p65 (1:50; 4764; Cell Signaling Technology).
In Situ Hybridization
Eight-micrometer sections were used for in situ hybridization. Briefly, we used a 5′–3′ DIG–labeled miR-146a probe (Exiqon, Vedbaek, Denmark) according to the manufacturer’s instructions, with a hybridization temperature of 58°C and a probe concentration of 0.08 μmol/L. The U6 DIG–labeled probe was used as a positive control, and miR-146a−/− mouse kidney sections were used as negative controls.
Statistical Analyses
The results are expressed as the means±SEM. All of the statistical analyses were performed using GraphPad Prism software (GraphPad Software, La Jolla, CA). Two–sided P values <0.05 were considered statistically significant. For statistical comparisons between two groups, we used unpaired two–tailed t tests. For comparisons between three or more groups, we used one-way ANOVA followed by the Tukey post hoc test. Asterisks indicate the levels of significance as follows: *P<0.05, **P<0.01, and ***P<0.001.
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful to Prof. Pierre-Laurent Puig, Prof. Philippe Beaune, and the members of Institut National de la Santé et de la Recherche Médicale (INSERM) U775 for their logistic help and Sophie Berissi and Noemie Gadessaud for technical assistance.
This research was supported, in part, by grants from the Réseau Thématique de Recherche et de Soins Centaure, the Groupe Coopératif des Transplanteurs d'Ile de France, the Agence de la Biomédecine, and the Emmanuel Boussard Foundation. L.A. was supported by INSERM.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016010045/-/DCSupplemental.
References
- 1.Siew ED, Davenport A: The growth of acute kidney injury: A rising tide or just closer attention to detail? Kidney Int 87: 46–61, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rewa O, Bagshaw SM: Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10: 193–207, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Torio CM, Andrews RM: National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2011. HCUP Statistical Brief #160. August 2013. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb160.pdf [PubMed]
- 4.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK: Acute kidney injury: A springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonventre JV: Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol 14[Suppl 1]: S55–S61, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Lee SB, Kalluri R: Mechanistic connection between inflammation and fibrosis. Kidney Int Suppl 119: S22–S26, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartel DP: MicroRNAs: Target recognition and regulatory functions. Cell 136: 215–233, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J: Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci U S A 107: 14339–14344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szeto CC, Li PK: MicroRNAs in IgA nephropathy. Nat Rev Nephrol 10: 249–256, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Anglicheau D, Sharma VK, Ding R, Hummel A, Snopkowski C, Dadhania D, Seshan SV, Suthanthiran M: MicroRNA expression profiles predictive of human renal allograft status. Proc Natl Acad Sci U S A 106: 5330–5335, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonventre JV, Zuk A: Ischemic acute renal failure: An inflammatory disease? Kidney Int 66: 480–485, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Ruland J: Return to homeostasis: Downregulation of NF-κB responses. Nat Immunol 12: 709–714, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, Garcia-Flores Y, Luong M, Devrekanli A, Xu J, Sun G, Tay J, Linsley PS, Baltimore D: miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med 208: 1189–1201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taganov KD, Boldin MP, Chang KJ, Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 103: 12481–12486, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A: NF-kappaB in renal inflammation. J Am Soc Nephrol 21: 1254–1262, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Leventhal JS, Schröppel B: Toll-like receptors in transplantation: Sensing and reacting to injury. Kidney Int 81: 826–832, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Akira S: Toll-like receptor signaling. J Biol Chem 278: 38105–38108, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Ha T, Liu L, Zou J, Zhang X, Kalbfleisch J, Gao X, Williams D, Li C: Increased expression of microRNA-146a decreases myocardial ischaemia/reperfusion injury. Cardiovasc Res 97: 432–442, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Orjalo AV, Rodier F, Lithgow GJ, Campisi J: MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging (Albany, NY) 1: 402–411, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells EK, Yarborough O 3rd, Lifton RP, Cantley LG, Caplan MJ: Epithelial morphogenesis of MDCK cells in three-dimensional collagen culture is modulated by interleukin-8. Am J Physiol Cell Physiol 304: C966–C975, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hébert CA, Baker JB: Interleukin-8: A review. Cancer Invest 11: 743–750, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Cugini D, Azzollini N, Gagliardini E, Cassis P, Bertini R, Colotta F, Noris M, Remuzzi G, Benigni A: Inhibition of the chemokine receptor CXCR2 prevents kidney graft function deterioration due to ischemia/reperfusion. Kidney Int 67: 1753–1761, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Sekido N, Mukaida N, Harada A, Nakanishi I, Watanabe Y, Matsushima K: Prevention of lung reperfusion injury in rabbits by a monoclonal antibody against interleukin-8. Nature 365: 654–657, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Chapman JR, O’Connell PJ, Nankivell BJ: Chronic renal allograft dysfunction. J Am Soc Nephrol 16: 3015–3026, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Lorenzen JM, Kaucsar T, Schauerte C, Schmitt R, Rong S, Hübner A, Scherf K, Fiedler J, Martino F, Kumarswamy R, Kölling M, Sörensen I, Hinz H, Heineke J, van Rooij E, Haller H, Thum T: MicroRNA-24 antagonism prevents renal ischemia reperfusion injury. J Am Soc Nephrol 25: 2717–2729, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chassin C, Hempel C, Stockinger S, Dupont A, Kübler JF, Wedemeyer J, Vandewalle A, Hornef MW: MicroRNA-146a-mediated downregulation of IRAK1 protects mouse and human small intestine against ischemia/reperfusion injury. EMBO Mol Med 4: 1308–1319, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M: The two faces of IKK and NF-kappaB inhibition: Prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med 9: 575–581, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Morishita Y, Imai T, Yoshizawa H, Watanabe M, Ishibashi K, Muto S, Nagata D: Delivery of microRNA-146a with polyethylenimine nanoparticles inhibits renal fibrosis in vivo. Int J Nanomedicine 10: 3475–3488, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferenbach DA, Bonventre JV: Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 11: 264–276, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatt K, Lanting LL, Jia Y, Yadav S, Reddy MA, Magilnick N, Boldin M, Natarajan R: Anti-inflammatory role of microRNA-146a in the pathogenesis of diabetic nephropathy [published online ahead of print December 8, 2015]. J Am Soc Nephrol doi:ASN.2015010111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pallet N, Thervet E, Le Corre D, Knebelmann B, Nusbaum P, Tomkiewicz C, Meria P, Flinois JP, Beaune P, Legendre C, Anglicheau D: Rapamycin inhibits human renal epithelial cell proliferation: Effect on cyclin D3 mRNA expression and stability. Kidney Int 67: 2422–2433, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Halkein J, Tabruyn SP, Ricke-Hoch M, Haghikia A, Nguyen NQ, Scherr M, Castermans K, Malvaux L, Lambert V, Thiry M, Sliwa K, Noel A, Martial JA, Hilfiker-Kleiner D, Struman I: MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J Clin Invest 123: 2143–2154, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viau A, El Karoui K, Laouari D, Burtin M, Nguyen C, Mori K, Pillebout E, Berger T, Mak TW, Knebelmann B, Friedlander G, Barasch J, Terzi F: Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest 120: 4065–4076, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anglicheau D, Muthukumar T, Hummel A, Ding R, Sharma VK, Dadhania D, Seshan SV, Schwartz JE, Suthanthiran M: Discovery and validation of a molecular signature for the noninvasive diagnosis of human renal allograft fibrosis. Transplantation 93: 1136–1146, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, Alexander SI, Sharland AF, Chadban SJ: TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest 117: 2847–2859, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.