Abstract
Primary hyperoxaluria type 1 (PH1), an inherited rare disease of glyoxylate metabolism, arises from mutations in the enzyme alanine-glyoxylate aminotransferase. The resulting deficiency in this enzyme leads to abnormally high oxalate production resulting in calcium oxalate crystal formation and deposition in the kidney and many other tissues, with systemic oxalosis and ESRD being a common outcome. Although a small subset of patients manages the disease with vitamin B6 treatments, the only effective treatment for most is a combined liver-kidney transplant, which requires life-long immune suppression and carries significant mortality risk. In this report, we discuss the development of ALN-GO1, an investigational RNA interference (RNAi) therapeutic targeting glycolate oxidase, to deplete the substrate for oxalate synthesis. Subcutaneous administration of ALN-GO1 resulted in potent, dose-dependent, and durable silencing of the mRNA encoding glycolate oxidase and increased serum glycolate concentrations in wild-type mice, rats, and nonhuman primates. ALN-GO1 also increased urinary glycolate concentrations in normal nonhuman primates and in a genetic mouse model of PH1. Notably, ALN-GO1 reduced urinary oxalate concentration up to 50% after a single dose in the genetic mouse model of PH1, and up to 98% after multiple doses in a rat model of hyperoxaluria. These data demonstrate the ability of ALN-GO1 to reduce oxalate production in preclinical models of PH1 across multiple species and provide a clear rationale for clinical trials with this compound.
Keywords: end-stage renal disease, RNAi therapeutics, oxalate, primary hyperoxaluria type I, siRNA
Primary hyperoxaluria type 1 (PH1) is a severe, rare, autosomal-recessive inborn error of metabolism resulting in increased endogenous production of oxalate due to a deficiency of the liver peroxisomal enzyme alanine-glyoxylate aminotransferase (AGT).1 The prevalence of PH1 may be as high as one in 150,000 in Western populations, based upon a recent genetic analysis,2 and even greater in regions where consanguineous marriages are common.3–5 PH1 presents primarily as a pediatric disease, with symptoms first appearing before 4 years of age in nearly half of the patients in the Rare Kidney Stone Consortium (RKSC) PH Registry.6 Individuals with a defect in AGT fail to properly detoxify glyoxylate through its conversion to glycine. This results in a build-up of glyoxylate, which is then oxidized to oxalate by lactate dehydrogenase. Oxalate is then excreted by the kidney. In PH1, elevated excretion of this relatively insoluble metabolite results in the formation of calcium oxalate crystals in the kidney and urinary tract, manifested as recurrent nephrolithiasis and/or nephrocalcinosis and progressive kidney disease leading to kidney failure, often at a very young age.7 A retrospective analysis of patients in the RKSC PH registry emphasized the role of urinary oxalate in the pathology of PH1, showing that oxalate levels both at diagnosis and during follow-up were the major predictor of outcome for these patients.8 As renal function declines below 30–45 ml/min per 1.73 m2, oxalate elimination is further compromised; plasma levels rise and exceed solubility limits such that calcium oxalate deposits form in the vasculature, skin, retina, heart, and bone, resulting in severe end-organ damage (including blindness, anemia, cardiac failure, and pathologic fractures) and associated morbidity and mortality.7
Currently, no approved therapeutics exist for the treatment of PH1. Aside from vitamin B6 supplementation for a subset of responders, approaches for disease management range from conservative measures (hyperhydration, citrate) that delay but do not prevent the decline in renal function,9 to dialysis. Since kidney dialysis does not adequately remove oxalate despite intensive schedules, it continues to accumulate in the body. In these circumstances, combined liver-kidney transplantation provides the only effective treatment. While the underlying metabolic defect is corrected by liver transplantation, this is associated with significant mortality and morbidity, and is not viable for infants with severe PH1 or for many patients in resource-poor countries.7 Thus, there is a significant unmet need for an efficacious and durable treatment to stop liver oxalate production and prevent disease progression without the need for liver transplant in PH1.
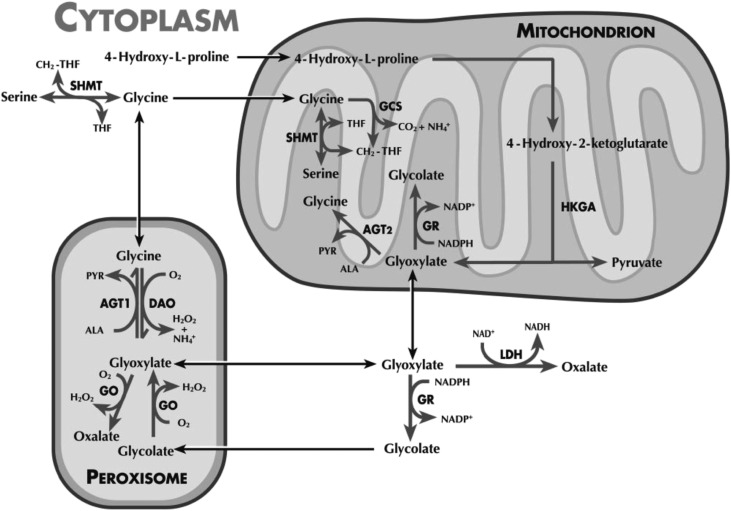
Inhibiting glycolate oxidase (GO), a hepatic, peroxisomal enzyme upstream of AGT, is one possible mechanism for depleting diseased livers of substrate for oxalate synthesis, to potentially prevent the pathology that develops in PH1.10–13 GO, encoded by the hydroxyacid oxidase (HAO1) gene, catalyzes the oxidation of glycolate to glyoxylate, the immediate precursor to oxalate synthesis in hepatocytes (Figure 1). Suppression of GO activity should inhibit oxalate production while causing an accumulation of glycolate. Unlike oxalate, glycolate is soluble and readily excreted in the urine.
Figure 1.
Hepatic oxalate synthesis pathway. ALN-GO1 targets hepatic GO.27 Copyright clearance center license number 3863190482947.
RNA interference (RNAi) is a naturally occurring cellular mechanism for regulating gene expression mediated by small interfering RNAs (siRNAs). Synthetic siRNAs can be designed to target the endogenous mRNA transcript of a given gene, leading to its cleavage and the subsequent suppression of synthesis of the encoded protein.14–17 Clinical trials of investigational RNAi therapeutics suggest they have substantial potential to treat a variety of different diseases.18,19 Efficient delivery of RNAi-based therapies to the liver is now accomplished by conjugation of N-acetylgalactosamine to the siRNA to direct uptake via the asialoglycoprotein receptor on hepatocytes.20–22 Such drug candidates show potent silencing of the targeted gene across species, with significant effects on disease demonstrated in both animal models and humans after subcutaneous dosing.23–26 Here, we report preclinical results for ALN-GO1, an RNAi therapeutic targeting hepatic GO, as a potential treatment for PH1. ALN-GO1 demonstrated potent, durable HAO1 mRNA silencing in normal rodents and primates and profound lowering of urinary oxalate in mouse and rat disease models. Taken together, these results emphasize the potential for ALN-GO1 to decrease oxalate synthesis in patients with PH1 and provide a potential therapeutic alternative to liver transplantation.
Results
Liver HAO1 mRNA Silencing Leads to Increased Serum Glycolate in Healthy Rodents
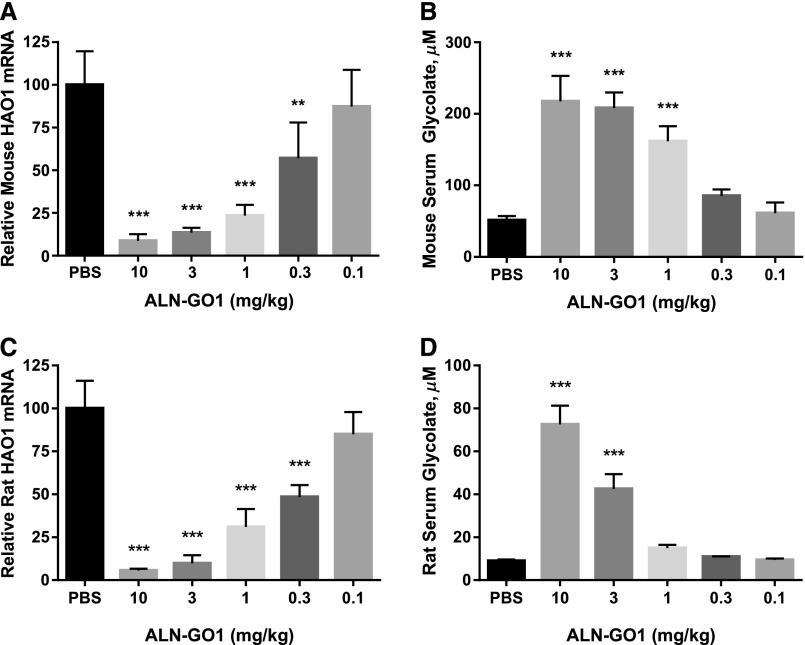
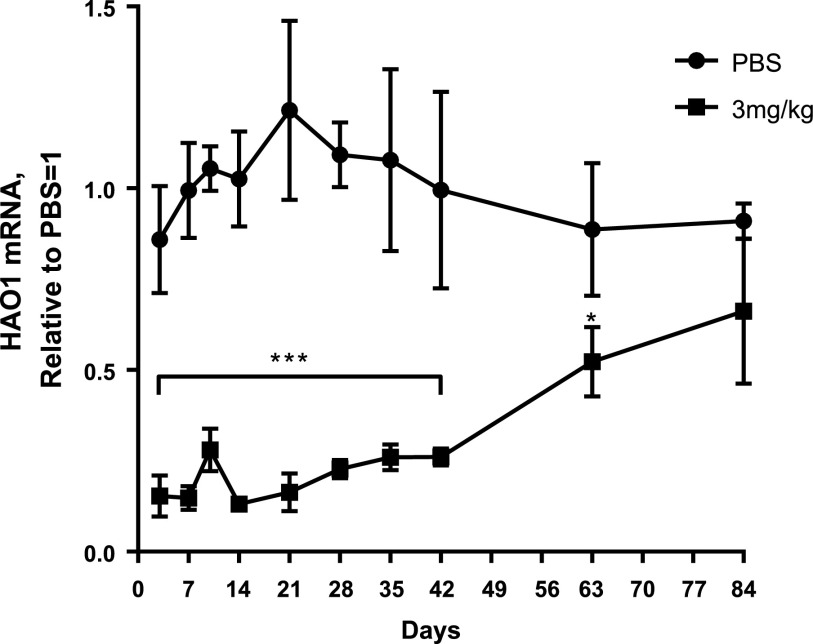
ALN-GO1, an N-acetylgalactosamine–conjugated siRNA, binds to the asialoglycoprotein receptor on the surface of hepatocytes to mediate cellular uptake and intracellular silencing of HAO1 mRNA.20–22 As shown in Figure 2, A and C, a single subcutaneous dose of ALN-GO1 in wild-type mice and rats, respectively, led to potent, dose-dependent silencing of HAO1 mRNA with an ED50 of approximately 0.3 mg/kg and maximal silencing >90% at 10 mg/kg in the livers of both species. As predicted, silencing of HAO1 in rodents led to commensurate dose-dependent increases in the upstream substrate glycolate. Serum glycolate levels increased over four- and sevenfold in mice and rats, respectively (Figure 2, B and D). Additionally, a single 3 mg/kg dose of ALN-GO1 in mice mediated rapid, durable, and robust HAO1 mRNA lowering of >80% within 72 hours that was maintained for several weeks before recovering toward baseline (Figure 3).
Figure 2.
Silencing liver HAO1 mRNA results in increased serum glycolate in healthy rodents. Levels of (A) liver HAO1 mRNA and (B) serum glycolate 10 days after a single subcutaneous dose of ALN-GO1 in C57BL/6 mice. Levels of (C) liver HAO1 mRNA and (D) serum glycolate 10 days after a single subcutaneous dose of ALN-GO1 in Sprague–Dawley rats. Bars represent the mean of three or four animals and error bars depict the SD. Stars indicate significance of Dunnett multiple comparison tests of each dose level versus PBS (after one-way ANOVA; all treatment effects significant at P<0.001): **P<0.01; ***P<0.001.
Figure 3.
A single dose of ALN-GO1 results in sustained liver HAO1 mRNA silencing. Liver HAO1 mRNA at various time points following a single subcutaneous dose of PBS or 3 mg/kg ALN-GO1 in C57BL/6 mice. Points represent the mean of three animals and error bars depict the SD. Overall difference in HAO1 mRNA over time between PBS and 3 mg/kg groups was assessed via repeated ANOVA measures; treatment and treatment×time interactions were both significant at P<0.001 and P<0.001, respectively. Stars indicate significance level of Tukey post hoc comparisons of mean HAO1 mRNA in PBS versus 3 mg/kg groups at each day: *P<0.05; ***P<0.001. Bracket below stars indicates that all points in span share the noted significance level.
Liver HAO1 mRNA Silencing Leads to Increased Serum Glycolate in Healthy Primates
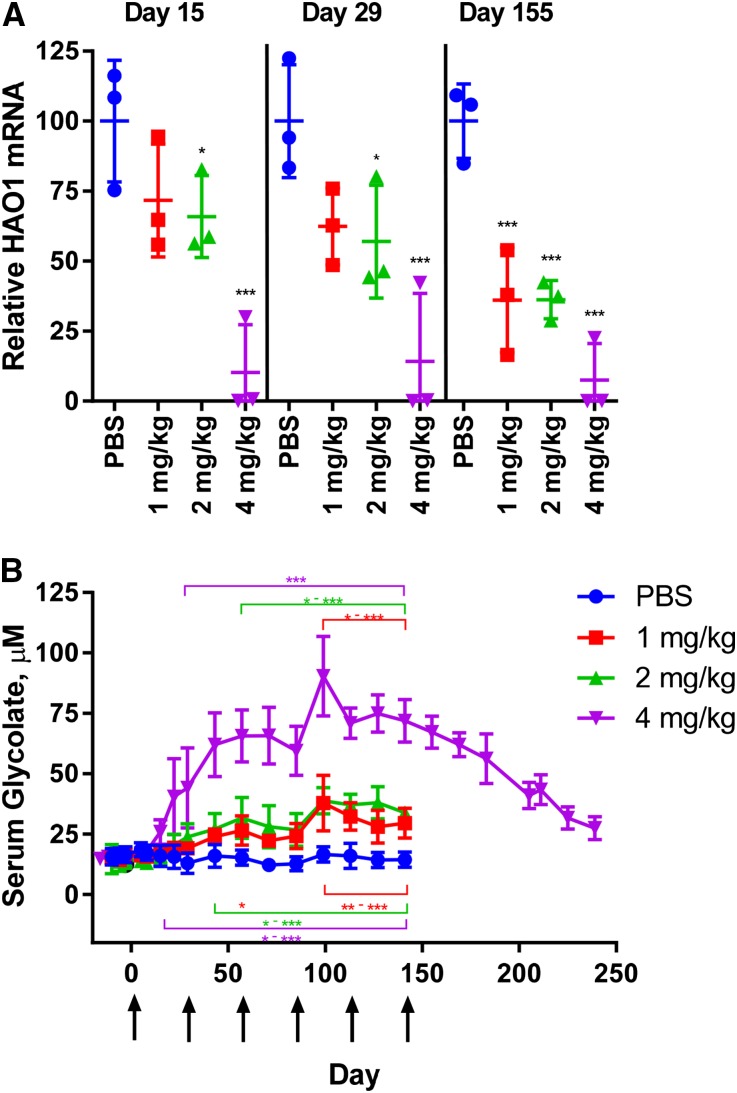
To explore this therapeutic approach in healthy primates, male cynomolgus monkeys were treated monthly with ALN-GO1 and evaluated for onset and duration of liver HAO1 mRNA silencing and subsequent increases in serum glycolate levels (Figure 4, A and B). These results demonstrate up to 99% HAO1 mRNA silencing in monkey livers by day 15 after a single dose of 4 mg/kg ALN-GO1, maintained with a monthly dosing schedule. Lower dose levels of 1 and 2 mg/kg demonstrated intermediate liver HAO1 mRNA silencing, which was slightly enhanced with repeat dosing each month. As in rodents, HAO1 mRNA silencing led to dose-dependent increases in serum glycolate in monkeys, with a six-fold increase over baseline seen at the top dose of 4 mg/kg monthly. The effects of ALN-GO1 were potent and durable as evidenced by the maintenance of both HAO1 mRNA silencing and serum glycolate elevation with monthly dosing. Remarkably, even 100 days after the last dose in the 4 mg/kg treatment group, glycolate levels had not yet returned to baseline. As expected, urinary glycolate levels were also elevated in monkeys treated with ALN-GO1 (up to three-fold in the 4 mg/kg group), while urinary oxalate levels remained unchanged from the low starting levels in these healthy animals (Supplemental Figure 1). These results are similar to those in healthy rats treated with ALN-GO1, where no effects on the low levels of urinary oxalate were seen (Supplemental Figure 2).
Figure 4.
Silencing liver HAO1 mRNA leads to increased serum glycolate in cynomolgus monkeys. Levels of (A) liver HAO1 mRNA and (B) serum glycolate in healthy nonhuman primates treated monthly with PBS or ALN-GO1 at 1, 2, or 4 mg/kg. Points represent individual animals (mRNA) or group averages (glycolate) of n=3 animals/group and error bars depict the SD. Arrows indicate dosing days. For HAO1 mRNA (A), stars indicate significance of Tukey post hoc tests of each group daily mean versus corresponding PBS daily mean (after repeated ANOVA measures; treatment, time, and treatment×time interaction all significant at P<0.001): *P<0.05; **P<0.01; ***P<0.001; ****P<0.001. For glycolate (B), stars indicate significance of Tukey post hoc tests after repeated ANOVA measures (treatment, time, and treatment×time interaction all significant at P<0.001): the stars above the data points indicate pairwise comparisons of each group daily mean versus corresponding PBS daily mean; the stars below the data points indicate pairwise comparisons of each group daily mean versus baseline levels (average of predose values). Colors indicating 4, 2, and 1 mg/kg as in legend: *P<0.05; **P<0.01; ***P<0.001. Bracket above/below stars indicates that all points in span share the noted significance level(s).
Liver HAO1 mRNA Silencing Leads to Decreased Oxalate in PH1 Mice
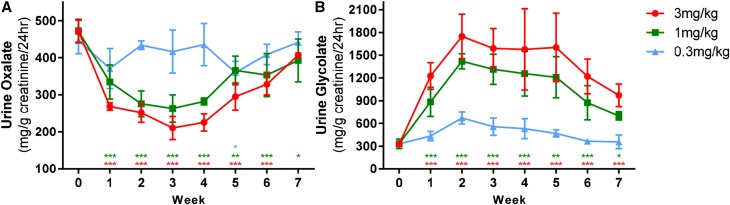
To test the therapeutic hypothesis, ALN-GO1 was next evaluated in animal models of primary hyperoxaluria. In mice deficient in AGXT (a genetic model of PH1), a single 3 mg/kg injection of ALN-GO1 reduced urinary oxalate approximately 50% by week 2–3, and this effect was sustained for ≥3 weeks before recovering to baseline levels 7 weeks postdose. Consistent with the therapeutic mechanism, urinary glycolate levels increased in these same animals up to five-fold above predose concentrations, were maintained for approximately 4 weeks, and had not yet returned to baseline levels after 7 weeks. Smaller reductions in urinary oxalate coupled with smaller increases in urinary glycolate were seen following a single subcutaneous injection of 1 or 0.3 mg/kg ALN-GO1 in these mice (Figure 5).
Figure 5.
Urinary oxalate lowering and serum glycolate increases following ALN-GO1 dosing in PH1 mice. (A) Twenty-four-hour urine oxalate and (B) 24-hour urine glycolate levels in male Agxt1−/− mice after a single subcutaneous dose of ALN-GO1 at the concentrations shown. Points represent the mean of three animals, error bars depict the SD. Differences among treatments over time were assessed via repeated ANOVA measures (one model for each analyte). For both oxalate and glycolate, treatment, time, and treatment×time interactions were significant at P<0.01, P<0.001, and P<0.001, respectively. Stars indicate significance of Tukey post hoc tests of each weekly mean versus week 0 mean, with colors indicating 3, 1, and 0.3 mg/kg according to legend: *P<0.05; **P<0.01; ***P<0.001.
Silencing Liver HAO1 mRNA Leads to Decreased Oxalate in a Novel Rat Model of Hyperoxaluria
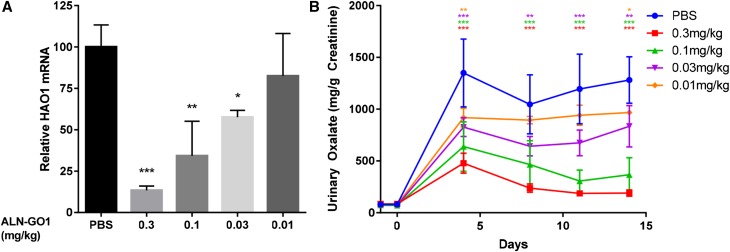
In the absence of a genetic model of PH1 in rats, an induced model of disease was developed using an AGXT targeting siRNA formulated in a lipid nanoparticle to decrease liver AGXT levels by 90%–95%. Preliminary experiments showed only a modest increase in urinary oxalate after AGXT silencing in rats (data not shown). Consequently, 1% v/v ethylene glycol was added to the drinking water to enhance urinary oxalate excretion (see Concise Methods and Supplemental Figure 3C for details) and to demonstrate more clearly the efficacy of GO knockdown in reducing oxalate synthesis in this model. Although rats fed ethylene glycol without AGXT knockdown also develop hyperoxaluria that would be expected to be sensitive to GO silencing, these were not explored because of the desire to mimic the phenotype of PH1 as closely as possible. Consequently, rats with induced hyperoxaluria (through AGXT knockdown and ethylene glycol feeding) received four weekly subcutaneous injections of ALN-GO1 at 3, 1, or 0.3 mg/kg, leading to ≥95% HAO1 mRNA silencing at all doses and nearly complete suppression of urinary oxalate production (98% reduction at the top dose) (Supplemental Figure 3, B and C). Thus, in this induced rat model of hyperoxaluria, multiple doses of ALN-GO1 rapidly and potently reduce urinary oxalate to near baseline levels.
To titrate the relationship of HAO1 silencing to oxalate production, hyperoxaluric rats received a single subcutaneous dose of ALN-GO1 at 0.3, 0.1, 0.03, or 0.01 mg/kg, resulting in dose-dependent suppression of HAO1 mRNA and reductions in urinary oxalate on day 14 postdose (Figure 6).
Figure 6.
Silencing liver HAO1 mRNA reduces urinary oxalate in a rat model of hyperoxaluria. Levels of (A) liver HAO1 mRNA on day 14 and (B) 24-hour urinary oxalate levels at multiple time points after a single subcutaneous dose of ALN-GO1 in hyperoxaluric rats that received 1 mg/kg AGXT targeting siRNA and 1% ethylene glycol in drinking water. Points and bars represent the mean of three animals and error bars depict the SD. For HAO1 mRNA (A), stars indicate significance of Dunnett multiple comparison tests of each dose level versus PBS (after one-way ANOVA; overall treatment effect significant at P<0.001): *P<0.05; **P<0.01; ***P<0.001. For oxalate (B), stars indicate significance of Tukey post hoc tests of each group daily mean versus corresponding PBS daily mean (after repeated measures ANOVA; treatment, time, and treatment×time interaction all significant at P<0.001), with colors indicating 0.3, 0.1, 0.03, and 0.01 mg/kg as in legend: *P<0.05; **P<0.01; ***P<0.001.
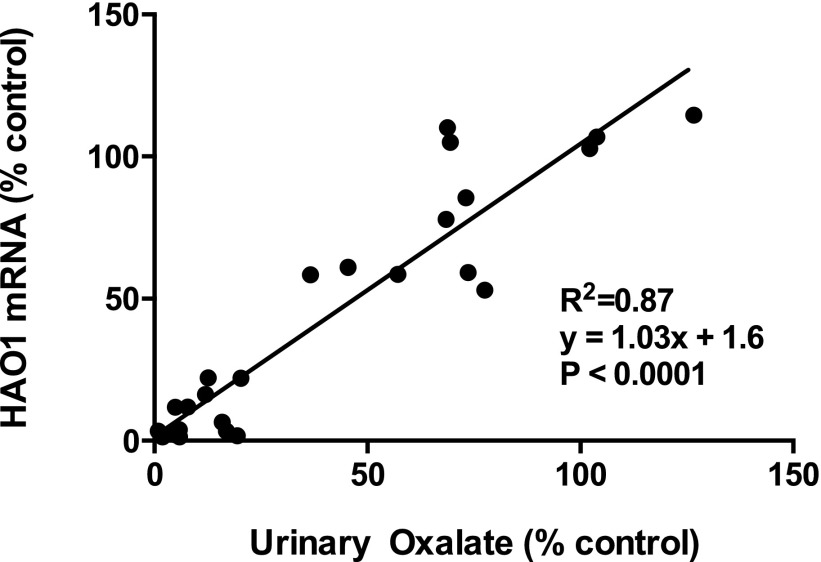
These results, along with the results from the multidose experiment in hyperoxaluric rats, permit analysis of the relationship of HAO1 mRNA levels to oxalate lowering in these animals. When both variables were normalized to vehicle-treated hyperoxaluric animals, the data indicated a 1:1 relationship between percent mRNA silencing and percent urinary oxalate lowering (Figure 7).
Figure 7.
Linear relationship between HAO1 mRNA silencing and urinary oxalate lowering in hyperoxaluric rats. Percent liver HAO1 mRNA remaining versus percent urinary oxalate remaining on day 14 or 28 in hyperoxaluric rats treated with PBS or doses of ALN-GO1 ranging from 0.01 to 3 mg/kg. Points represent individual hyperoxaluric rats normalized to vehicle control. R2, equation, and P value from linear regression.
Discussion
Given the significant morbidity and mortality of PH1 disease and the burden of dual liver-kidney transplantation, a significant unmet need exists for an efficacious and durable new treatment. ALN-GO1 is being developed as an RNAi therapeutic for the treatment of this disease by inhibiting hepatic production of oxalate and thus reducing buildup of this metabolite in the kidney. Suppressing GO via ALN-GO1 inhibits glyoxylate production, the major precursor for oxalate formation. Such inhibition of oxalate synthesis results in an increase in plasma glycolate levels; however, unlike oxalate, glycolate is soluble and readily excreted in the urine. Our data demonstrate the ability of ALN-GO1 to potently, durably, and dose-responsively silence HAO1 mRNA in both mice and rats with a single dose ED50 of approximately 0.3 mg/kg. Additionally, ALN-GO1 silences HAO1 mRNA up to 99% in nonhuman primates at the highest dose of 4 mg/kg, with maximum associated increases in serum glycolate up to approximately six-fold, as compared with PBS-treated controls.
Having demonstrated the ability of ALN-GO1 to silence HAO1 mRNA in wild-type animals, we conducted studies to investigate the functional effects of HAO1 silencing using rodent models of PH1. Consistent with its intended mechanism of action, HAO1 mRNA silencing in mice deficient in AGXT (a genetic model of PH1), led to dose-responsive reductions in urinary oxalate of up to 50%, and accompanying five-fold increases in urinary glycolate. Similarly, in a newly developed rat model with reduced AGXT mRNA levels and ethylene glycol induction (an induced model of hyperoxaluria), treatment with ALN-GO1 reduced urinary oxalate levels in a dose-dependent manner up to 98% and demonstrated a dose-dependent 1:1 correlation of HAO1 mRNA silencing with oxalate reduction. These data illustrate the relationship between HAO1 mRNA silencing and urinary oxalate lowering in two rodent models of hyperoxaluria, and further support the therapeutic hypothesis that silencing HAO1 mRNA could provide a therapeutic benefit by reducing oxalate production.
Our results highlight several potential differences between mice and rats in the models studied. Despite equivalent and near total HAO1 mRNA silencing in these species, maximal urinary oxalate lowering in the mouse model was approximately 50%, whereas nearly complete normalization of oxalate levels (98%) occurred in hyperoxaluric rats. Although this may reflect a number of species-specific differences related to glyoxylate metabolism and renal handling of glycolate,28,29 it is not likely due to a decreased role for GO in hepatocyte-mediated oxalate production in PH1 mice. In fact, as recently described, crossing GO-deficient mice (Hao1−/−) into the AGXT deficient background nearly resolved disease, with oxalate levels near normal, indicating the near total dependence of this pathway in mice on GO.13 Rodents have evolved with AGT expressed in both mitochondria and peroxisomes in contrast to only a peroxisomal localization in humans, suggesting that glyoxylate production in rodent mitochondria may be significant. As a result, mice lacking mitochondrial AGT will excrete greater amounts of glyoxylate produced in the mitochondria into the cytosol compared with wild-type mice. The failure to normalize oxalate excretion in AGXT deficient mice after ALN-GO1 treatment may reflect the importance of mitochondrial AGT in limiting oxalate production in the mouse, as compared with other species. It is also possible that the significant increase in serum glycolate levels in mice (approximately 0.2 mM) after treatment with ALN-GO1 could provide additional substrate for production of oxalate in other tissues, by oxidation in the kidneys due to the activity of HAO2, for example.
The potential efficacy of ALN-GO1 in humans can be gauged in part from the recent report of HAO1 deficiency in an 8-year-old male who excreted 2000 mmol of glycolate/mole creatinine.10 These data suggest that liver HAO1 may metabolize at least 1000 mg of glycolate to glyoxylate per day in an adult. Thus, silencing of HAO1 could be expected to substantially reduce this synthesis of glyoxylate and any resultant conversion to oxalate.
The rodent disease models of hyperoxaluria described in the current work do not demonstrate renal insufficiency under the conditions studied. Although they display hyperoxaluria and crystalluria, rodents can eliminate relatively high oxalate concentrations without renal damage.13,30 However, it is known that PH1 mice fed ethylene glycol (to further enhance oxalate synthesis) do develop even more profound hyperoxaluria, severe nephrocalcinosis, and renal failure whereas healthy mice fed ethylene glycol display much lower oxalate levels and maintain kidney function.30 Consequently, future studies can investigate whether inhibition of oxalate production by ALN-GO1 results in the expected prevention of renal disease when PH1 mice are fed ethylene glycol.
Recent results reported by Martin-Higueras et al.13 support the therapeutic potential for ALN-GO1 to profoundly lower oxalate levels in patients by silencing HAO1 mRNA. In these studies, GO-deficient mice (Hao1−/−) exhibit asymptomatic glycolate elevation, but no phenotypic differences in urine sediment, crystal deposition, histology, or breeding. Additionally, as mentioned above, when bred with hyperoxaluric mice deficient in AGXT (Agxt1−/−) to create double-knockout mice (Agxt1−/− Hao1−/−), urinary oxalate levels returned to wild-type levels.13 Further support for the potential safety of HAO1 silencing in patients comes from the clinical report by Frishberg et al. regarding the child mentioned above with an incidentally discovered homozygous defect in HAO1 who exhibited marked elevations of urine glycolate but no associated metabolic abnormalities including normal oxalate levels, and normal renal and hepatic function.10 Finally, our own studies in healthy rodents and primates indicated no consequences of near complete HAO1 silencing with ALN-GO1 from treatment-induced increases in glycolate levels in the plasma and urine, as its high solubility allows ready excretion. Importantly, although there is no known, or anticipated, toxicity from increased glycolate levels, one cannot be certain that prolonged elevations in glycolate might have some unforeseen effects, and these will be carefully examined in future studies. However, in total, these results point to the safety of silencing HAO1 in support of clinical development of ALN-GO1. Furthermore, these data strongly suggest the potential for decreasing GO activity to starve the disrupted pathway of substrate and reduce the oxalate burden in patients with PH1.
Evaluating ALN-GO1 pharmacology in initial clinical studies with healthy volunteers presents a challenge as they have normal oxalate levels and GO is not a secreted serum protein; liver biopsies would be the only way to directly monitor HAO1 mRNA knockdown. However, measuring glycolate increases as a biomarker of drug activity removes the need for liver biopsy. Although urinary glycolate has been routinely used as a biomarker in preclinical rodent studies of PH1,13,28,31 serum glycolate elevations reported here across species may be an additional sensitive biomarker to monitor HAO1 mRNA silencing in the absence of a circulating serum protein as it could help reduce potential errors and inconsistencies associated with 24-hour urine collections and still provide a robust signal that target lowering has been achieved.
During manuscript preparation, Dutta et al. published preclinical results, utilizing siRNAs in an lipid nanoparticle (LNP) delivery system targeting HAO1 that largely corroborate the findings presented here.32 In both studies, HAO1 silencing led to glycolate increases in rodents and nonhuman primates and oxalate reductions in PH1 mice. Important distinctions include the subcutaneous delivery and extensive duration of ALN-GO1 pharmacological activity versus the intravenously infused, LNP delivery results reported.32 In addition, we have extended the results to a newly created rat model of hyperoxaluria showing near normalization of urinary oxalate levels after ALN-GO1 administration. These features of ALN-GO1 could impact its eventual clinical application by allowing infrequent, convenient self-administration for this chronic condition.
In summary, the data presented support the continued development of subcutaneously administered ALN-GO1 as a potential therapy for the treatment of PH1. It specifically targets mRNA of the HAO1 gene, which encodes the hepatic peroxisomal enzyme, GO, to reduce the production of glyoxylate and therefore oxalate. ALN-GO1 has shown potent dose-dependent activity resulting in reduced liver HAO1 mRNA levels with the expected increases in glycolate levels in wild-type and diseased animals and subsequent reductions in urinary oxalate in diseased animals. These preclinical results support the potential for ALN-GO1 to significantly reduce, and potentially abrogate, hepatic production of oxalate in PH1 patients where unmet medical need remains high. Taken collectively, the data from the literature on PH1 and AGT deficiency, the robust nonclinical data with ALN-GO1, along with prior and ongoing clinical experience with other investigational siRNA therapeutics in humans,18,19 supports the continued development of ALN-GO1 for the treatment of patients with PH1. Although decreasing liver oxalate synthesis through GO inhibition has specific application to PH1, future studies should investigate whether related approaches to substrate reduction may be possible for additional disorders with a metabolic etiology, including other forms of hyperoxaluria.
Concise Methods
Care and Use of Laboratory Animals
All studies were conducted using protocols consistent with local, state and federal regulations as applicable and with adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All studies were approved by the Institutional Animal Care and Use Committees at Alnylam Pharmaceuticals (University of Alabama at Birmingham, AL) or Covance Laboratories Inc. (Madison, WI), as applicable. All procedures using cynomolgus monkeys were conducted by Covance Laboratories Inc.
Design, Synthesis, and Formulation of siRNAs
The design and synthesis of ALN-GO1 and AGXT siRNAs and the formulation of AGXT siRNA in lipid nanoparticles was done as previously described.20,23,24
RNA Extraction and Quantitative Real-Time PCR
Rat and cynomolgus monkey liver tissues were harvested and snap frozen in liquid nitrogen. Total RNA was isolated from frozen liver tissues using the RNAqueous-4PCR kit (Life Technologies, Guilford, CT; Catalog no. AM1914). RNA was eluted in nuclease-free water and diluted to a stock concentration of 25 ng/µl. Total RNA was reverse transcribed using a High Capacity cDNA synthesis kit (Applied Biosystems, Catalog no. 4374967). Quantitative PCR was performed on cDNA using the Roche (Basel, Switzerland) LightCycler480 Probes Master Mix in the Roche 480 LightCycler Real Time PCR System to determine the relative abundance of HAO1 mRNA normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The Taqman probes used for rat and cynomolgus monkey HAO1 mRNA detection were Life Technologies Catalog nos. Rn01486035_m1 and Mf02872680_m1, and the Taqman probes used for rat and cynomolgus monkey GAPDH mRNA detection were Life Technologies catalog nos. 4351317 and Mf04392546_g1, respectively.
Panomics Quantigene 2.0 bDNA Assay
Mouse liver tissues were harvested and snap frozen in liquid nitrogen and tissue lysates were prepared for liver mRNA quantitation. Tissues were lysed using lysis buffer (Epicenter, Madison, WI; Catalog no. MTC096H) containing proteinase K (Ambion, Foster City, CA; Catalog no. AM2546). Branched DNA analysis was performed using the Quantigene 2.0 kit (Panomics, Fremont, CA) to determine the relative abundance of HAO1 mRNA relative to the housekeeping gene GAPDH. The HAO1 probe set that was used for HAO1 and GAPDH mRNA detection were SB-14142 and SB-10001, respectively.
Mouse, Nonhuman Primate Urine, and Serum Oxalate, Glycolate, and Creatinine
For oxalate determination, part of the urine collection was acidified to pH<1 with hydrochloric acid (HCL) prior to storage at −80°C to prevent any possible oxalate crystallization that could occur with cold storage and/or oxalogenesis associated with alkalinization. The remaining nonacidified urine was frozen at −80°C for the measurement of other urinary parameters. Serum preparations were stored at −80°C and filtered through Nano-sep centrifugal filters (VWR International, Batavia, IL) with a 10,000 nominal molecular weight limit prior to ion chromatographic analysis. Centrifugal filters were washed with 10 mM HCL prior to sample filtration to remove any contaminating trace organic acids trapped in the filter device. Urinary creatinine was measured on a chemical analyzer, and urinary oxalate by ion chromatography, as previously described.28 Ion chromatography coupled with negative electrospray mass spectrometry (Thermo Fisher Scientific, Waltham, MA) was used to measure serum and urinary glycolate as previously described.29
Rat Urine Oxalate and Creatinine
Twenty-four-hour urine samples were collected at room temperature into 50 ml conical tubes containing mineral oil (Catalog no. M8662; Sigma-Aldrich, St. Louis, MO). Samples were centrifuged for 10 minutes at 13,000 rpm in a microcentrifuge. Supernatants were acidified with HCL (Catalog no. 653799; Sigma-Aldrich) then stored at −80°C. Urinary oxalate concentration was determined using the commercially available Trinity Biotech (Jamestown, NY) Oxalate Kit (Catalog no. 591-D) as per manufacturer’s instructions. Urine creatinine was measured using the Olympus (Tokyo, Japan) AU400 Chemistry Analyzer.
Preclinical Studies in Genetic Agxt1−/− PH1 Mouse Model
Twelve to 15-week-old male AGT-deficient mice (Agxt1−/−), null mutant mice lacking liver AGXT mRNA and protein (created as previously described),30 were housed in metabolic cages and 24-hour urine was collected on mineral oil to prevent evaporation and 2% sodium azide to prevent bacterial growth, as previously described.31 Animals were injected subcutaneously with a single dose of PBS or ALN-GO1, and urine oxalate and glycolate levels were evaluated at multiple time points until 7 weeks postdose.
Preclinical Studies in Rat Model of Hyperoxaluria
Male Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA) weighing approximately 150 g upon arrival were used to create an induced rat model of hyperoxaluria.
Rats were housed continuously in metabolic cages and received weekly intravenous doses of 1 mg/kg AGXT targeting siRNA formulated in an LNP to reduce AGXT levels to more closely resemble the PH1 disease state. Rats received subcutaneous injections of PBS or ALN-GO1. Urinary oxalate increases were further induced with ethylene glycol (Catalog no. BDH1125–1LP; BDH, VWR Analytical, Radnor, PA) provided as 1% v/v in the drinking water daily. Twenty-four-hour urinary oxalate levels were evaluated at multiple time points and liver mRNA levels were evaluated by quantitative PCR in animals euthanized on day 14 or 28.
Rats and mice were fed ad libitum rodent chow (10 mg/g calcium and between 0.5 and 1 mg/g oxalate) and water.
Preclinical Studies in Cynomolgus Monkeys
Nonhuman primate studies were conducted in experimentally naive male Chinese cynomolgus monkeys (Macaca fascicularis) between 2 and 3.5 kg body wt. Subcutaneous ALN-GO1 (2 ml/kg) was administered once a month for 6 months on study days 1, 29, 57, 85, 113, and 141. Where applicable, PBS served as the control article and was administered at a dose volume equal to the respective test article volume. Urine from each animal was collected at ambient temperature overnight; collections for all animals were conducted at approximately the same time each day for approximately the same duration of time. Blood was collected via venipuncture of the femoral vein (or from a saphenous vein) into serum separator tubes. Liver biopsies were collected on days 15, 29, and 155. As applicable, blood and urine samples were collected prior to dose administration, and prior to anesthetic administration for liver biopsy procedures.
Statistical Analyses
All graphing and statistical analyses were conducted in GraphPad Prism v.6 (GraphPad Software Inc., San Diego, CA). Data were not transformed beyond what is indicated in figures. Figures report significance of post hoc tests (Dunnett or Tukey) after simple or repeated ANOVA measures as described in figure legends.
Disclosures
A.L., T.R., J.H., B.R.B., N.N., P.H., K.F., D.E., and W.Q., are employees and shareholders of Alnylam Pharmaceuticals (Cambridge, MA). X.L., R.P.H., and J.K. received research support from Alnylam Pharmaceuticals.
Supplementary Material
Acknowledgments
We thank Zhixin Wang, Song Lian Zhou (The University of Alabama at Birmingham), Jessica Newton, and Matthew Algarin (Alnylam Pharmaceuticals) for their excellent technical assistance.
Work by X.L., R.P.H. and J.K. was in part supported by National Institutes of Health grants DK054468 and DK083527.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016030338/-/DCSupplemental.
References
- 1.Hoppe B: An update on primary hyperoxaluria. Nat Rev Nephrol 8: 467–475, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Hopp K, Cogal AG, Bergstralh EJ, Seide BM, Olson JB, Meek AM, Lieske JC, Milliner DS, Harris PC; Rare Kidney Stone Consortium : Phenotype-Genotype Correlations and Estimated Carrier Frequencies of Primary Hyperoxaluria. J Am Soc Nephrol 26: 2559–2570, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamoun A, Lakhoua R: End-stage renal disease of the Tunisian child: epidemiology, etiologies, and outcome. Pediatr Nephrol 10: 479–482, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Al-Eisa AA, Samhan M, Naseef M: End-stage renal disease in Kuwaiti children: an 8-year experience. Transplant Proc 36: 1788–1791, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Boualla L, Tajir M, Oulahiane N, Lyahyai J, Laarabi FZ, Chafai Elalaoui S, Soulami K, Ait Ouamar H, Sefiani A: AGXT Gene Mutations and Prevalence of Primary Hyperoxaluria Type 1 in Moroccan Population. Genet Test Mol Biomarkers 19: 623–628, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Rare Kidney Stone Consortium: Primary Hyperoxaluria; 25-September-2015. Available at: http://www.rarekidneystones.org/hyperoxaluria. Accessed December 17, 2015
- 7.Cochat P, Rumsby G: Primary hyperoxaluria. N Engl J Med 369: 649–658, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Zhao F, Bergstralh EJ, Mehta RA, Vaughan LE, Olson JB, Seide BM, Meek AM, Cogal AG, Lieske JC, Milliner DS; Investigators of Rare Kidney Stone Consortium : Predictors of Incident ESRD among Patients with Primary Hyperoxaluria Presenting Prior to Kidney Failure. Clin J Am Soc Nephrol 11: 119–126, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandrile G, van Woerden CS, Berchialla P, Beck BB, Acquaviva Bourdain C, Hulton SA, Rumsby G; OxalEurope Consortium : Data from a large European study indicate that the outcome of primary hyperoxaluria type 1 correlates with the AGXT mutation type. Kidney Int 86: 1197–1204, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Frishberg Y, Zeharia A, Lyakhovetsky R, Bargal R, Belostotsky R: Mutations in HAO1 encoding glycolate oxidase cause isolated glycolic aciduria. J Med Genet 51: 526–529, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Murray MS, Holmes RP, Lowther WT: Active site and loop 4 movements within human glycolate oxidase: implications for substrate specificity and drug design. Biochemistry 47: 2439–2449, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen ZW, Vignaud C, Jaafar A, Lévy B, Guéritte F, Guénard D, Lederer F, Mathews FS: High resolution crystal structure of rat long chain hydroxy acid oxidase in complex with the inhibitor 4-carboxy-5-[(4-chlorophenyl)sulfanyl]-1, 2, 3-thiadiazole. Implications for inhibitor specificity and drug design. Biochimie 94: 1172–1179, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Martin-Higueras C, Luis-Lima S, Salido E: Glycolate Oxidase Is a Safe and Efficient Target for Substrate Reduction Therapy in a Mouse Model of Primary Hyperoxaluria Type I. Mol Ther 24: 719–725, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaishnaw AK, Gollob J, Gamba-Vitalo C, Hutabarat R, Sah D, Meyers R, de Fougerolles T, Maraganore J: A status report on RNAi therapeutics. Silence 1, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T: Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411: 494–498, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Elbashir SM, Lendeckel W, Tuschl T: RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev 15: 188–200, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Röhl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachlan I: RNAi-mediated gene silencing in non-human primates. Nature 441: 111–114, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, Liebow A, Bettencourt BR, Sutherland JE, Hutabarat RM, Clausen VA, Karsten V, Cehelsky J, Nochur SV, Kotelianski V, Horton J, Mant T, Chiesa J, Ritter J, Munisamy M, Vaishnaw AK, Gollob JA, Simon A: Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet 383: 60–68, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, Perez J, Chiesa J, Warrington S, Tranter E, Munisamy M, Falzone R, Harrop J, Cehelsky J, Bettencourt BR, Geissler M, Butler JS, Sehgal A, Meyers RE, Chen Q, Borland T, Hutabarat RM, Clausen VA, Alvarez R, Fitzgerald K, Gamba-Vitalo C, Nochur SV, Vaishnaw AK, Sah DW, Gollob JA, Suhr OB: Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med 369: 819–829, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, Hoekstra M, Kandasamy P, Kel’in AV, Milstein S, Taneja N, O’Shea J, Shaikh S, Zhang L, van der Sluis RJ, Jung ME, Akinc A, Hutabarat R, Kuchimanchi S, Fitzgerald K, Zimmermann T, van Berkel TJ, Maier MA, Rajeev KG, Manoharan M: Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc 136: 16958–16961, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Geffen I, Spiess M: Asialoglycoprotein receptor. Int Rev Cytol 137B: 181–219, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Wu J, Nantz MH, Zern MA: Targeting hepatocytes for drug and gene delivery: emerging novel approaches and applications. Front Biosci 7: d717–d725, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Sehgal A, Barros S, Ivanciu L, Cooley B, Qin J, Racie T, Hettinger J, Carioto M, Jiang Y, Brodsky J, Prabhala H, Zhang X, Attarwala H, Hutabarat R, Foster D, Milstein S, Charisse K, Kuchimanchi S, Maier MA, Nechev L, Kandasamy P, Kel’in AV, Nair JK, Rajeev KG, Manoharan M, Meyers R, Sorensen B, Simon AR, Dargaud Y, Negrier C, Camire RM, Akinc A: An RNAi therapeutic targeting antithrombin to rebalance the coagulation system and promote hemostasis in hemophilia. Nat Med 21: 492–497, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Yasuda M, Gan L, Chen B, Kadirvel S, Yu C, Phillips JD, New MI, Liebow A, Fitzgerald K, Querbes W, Desnick RJ: RNAi-mediated silencing of hepatic Alas1 effectively prevents and treats the induced acute attacks in acute intermittent porphyria mice. Proc Natl Acad Sci U S A 111: 7777–7782, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, Jayaraman M, Rajeev KG, Cantley WL, Dorkin JR, Butler JS, Qin L, Racie T, Sprague A, Fava E, Zeigerer A, Hope MJ, Zerial M, Sah DW, Fitzgerald K, Tracy MA, Manoharan M, Koteliansky V, Fougerolles A, Maier MA: Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther 18: 1357–1364, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jayaraman M, Ansell SM, Mui BL, Tam YK, Chen J, Du X, Butler D, Eltepu L, Matsuda S, Narayanannair JK, Rajeev KG, Hafez IM, Akinc A, Maier MA, Tracy MA, Cullis PR, Madden TD, Manoharan M, Hope MJ: Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem Int Ed Engl 51: 8529–8533, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker PR, Cramer SD, Kennedy M, Assimos DG, Holmes RP: Glycolate and glyoxylate metabolism in HepG2 cells. Am J Physiol Cell Physiol 287: C1359–C1365, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Knight J, Holmes RP, Cramer SD, Takayama T, Salido E: Hydroxyproline metabolism in mouse models of primary hyperoxaluria. Am J Physiol Renal Physiol 302: F688–F693, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Knight J, Todd Lowther W, Holmes RP: Hydroxyproline metabolism in a mouse model of Primary Hyperoxaluria Type 1. Biochim Biophys Acta 1852: 2700–2705, 2015 [DOI] [PMC free article] [PubMed]
- 30.Salido EC, Li XM, Lu Y, Wang X, Santana A, Roy-Chowdhury N, Torres A, Shapiro LJ, Roy-Chowdhury J: Alanine-glyoxylate aminotransferase-deficient mice, a model for primary hyperoxaluria that responds to adenoviral gene transfer. Proc Natl Acad Sci U S A 103: 18249–18254, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Knight J, Fargue S, Buchalski B, Guan Z, Inscho EW, Liebow A, Fitzgerald K, Querbes W, Todd Lowther W, Holmes RP: Metabolism of (13)C5-hydroxyproline in mouse models of Primary Hyperoxaluria and its inhibition by RNAi therapeutics targeting liver glycolate oxidase and hydroxyproline dehydrogenase. Biochim Biophys Acta 1862: 233–239, 2016 [DOI] [PMC free article] [PubMed]
- 32.Dutta C, Avitahl-Curtis N, Pursell N, Larsson Cohen M, Holmes B, Diwanji R, Zhou W, Apponi L, Koser M, Ying B, Chen D, Shui X, Saxena U, Cyr WA, Shah A, Nazef N, Wang W, Abrams M, Dudek H, Salido E, Brown BD, Lai C: Inhibition of Glycolate Oxidase With Dicer-substrate siRNA Reduces Calcium Oxalate Deposition in a Mouse Model of Primary Hyperoxaluria Type 1. Mol Ther 24: 770–778, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.