Abstract
The Oxford Classification of IgA nephropathy does not account for glomerular crescents. However, studies that reported no independent predictive role of crescents on renal outcomes excluded individuals with severe renal insufficiency. In a large IgA nephropathy cohort pooled from four retrospective studies, we addressed crescents as a predictor of renal outcomes and determined whether the fraction of crescent-containing glomeruli associates with survival from either a ≥50% decline in eGFR or ESRD (combined event) adjusting for covariates used in the original Oxford study. The 3096 subjects studied had an initial mean±SD eGFR of 78±29 ml/min per 1.73 m2 and median (interquartile range) proteinuria of 1.2 (0.7–2.3) g/d, and 36% of subjects had cellular or fibrocellular crescents. Overall, crescents predicted a higher risk of a combined event, although this remained significant only in patients not receiving immunosuppression. Having crescents in at least one sixth or one fourth of glomeruli associated with a hazard ratio (95% confidence interval) for a combined event of 1.63 (1.10 to 2.43) or 2.29 (1.35 to 3.91), respectively, in all individuals. Furthermore, having crescents in at least one fourth of glomeruli independently associated with a combined event in patients receiving and not receiving immunosuppression. We propose adding the following crescent scores to the Oxford Classification: C0 (no crescents); C1 (crescents in less than one fourth of glomeruli), identifying patients at increased risk of poor outcome without immunosuppression; and C2 (crescents in over one fourth of glomeruli), identifying patients at even greater risk of progression, even with immunosuppression.
Keywords: IgA nephropathy, glomerulonephritis, Renal pathology, Oxford classification, crescents
The Oxford Classification for IgA nephropathy1,2 established mesangial hypercellularity (M), segmental glomerulosclerosis (S), and moderate to severe interstitial fibrosis and tubular atrophy (T) as independent risk factors for a poor renal outcome, including a combined event of either ESRD or a ≥50% decrease in eGFR, an increase in the rate of eGFR decline, or both. Endocapillary hypercellularity (E), although not predictive of poor outcome in the Oxford cohort, was associated with a significantly reduced rate of eGFR decline in patients treated with immunosuppressive therapy compared with those who were not. The Oxford Classification is, thus, composed of scores for M, E, S, and T. However, the presence or absence of cellular/fibrocellular crescents was not a significant predictor of these outcomes in the original Oxford cohort1 or a number of validation studies3–6 with similar entry criteria, which excluded patients with eGFR of <30 ml/min per 1.73 m2 at the time of biopsy and/or progression to ESRD within 12 months of the biopsy.
However, other studies with less restrictive entry criteria found crescents to be a prognostic indicator of a poor outcome.7–10 Katafuchi et al.7 found similar results to those in the original Oxford study in patients meeting entry criteria for the latter, although in their entire cohort of 702 patients, cellular/fibrocellular crescents and T score (but not M or S) independently predicted development of ESRD. Crescents also predicted ESRD in those 286 patients not meeting the entry criteria of the original Oxford study. The median value for percentage of crescents in patients with eGFR of <30 ml/min per 1.73 m2 was only 10%, and as such, these were not patients with rapidly progressive lesions, to whom the Oxford Classification does not apply. Walsh et al.,8 in a retrospective study of 146 patients with primary IgA nephropathy without limiting entry criteria, found that the presence of any crescents (including fibrous crescents) was a significant, independent predictor of a composite end point of doubling of serum creatinine (SCr), ESRD, or death in a multivariate model adjusted for age, sex, proteinuria, systolic BP, and baseline SCr. Two studies of pediatric patients with IgA nephropathy from Japan9 and Sweden10 without restrictive entry criteria also found cellular or fibrocellular crescents to be predictive of a poor outcome (eGFR<60 ml/min per 1.73 m2 and ESRD or eGFR reduction >50%, respectively) by univariate analysis and by multivariate analysis in the Japanese cohort. Interestingly, these studies also found by univariate analysis that Oxford M, E, and T scores (but not S) were predictive of poor outcomes, suggesting that, in children, active lesions (E and crescents) may have a greater effect on outcomes than chronic lesions (particularly S).
These findings suggest that, when considering the full range of patients with IgA nephropathy, including both adults and children as well as patients with a low eGFR at the time of biopsy and/or a rapidly progressive clinical course, inclusion of a crescent score in addition to the Oxford M, E, S, and T scores may help in prediction of renal outcomes. However, the above studies do not directly compare outcomes in treated versus untreated patients, although repeat biopsy studies suggest that crescents may represent a lesion reversible by immunosuppressive therapy.11–13 Furthermore, the effect of different fractions of crescents was not determined, except in the study by Katafuchi et al.,8 which established an optimal cutoff value of 7% crescents in predicting development of ESRD.
In this study, we addressed active (cellular or fibrocellular) crescents as potential predictors of renal outcomes in IgA nephropathy in a large cohort pooled from four retrospective studies: Oxford,1,2 the validation IgA (VALIGA) Study,14 and two large Asian databases,5,7 including both adults and children as well as patients with a low GFR (<30 ml/min per 1.73 m2) and/or a rapidly progressive clinical course. We also studied how different fractions of crescents were associated with the rate of renal function decline and survival from a combined outcome defined as either a ≥50% decline in renal function or ESRD adjusting for covariates used in the original Oxford study and stratifying patients on the basis of whether they received immunosuppressive therapy.
Results
Cohort Description
The 3096 patients had initial eGFR of 78±29 ml/min per 1.73 m2. The cohort was 42% women and predominantly of Asian and white ethnicities (Table 1). Initial proteinuria was 1.2 (0.7–2.3) g/d. Patients were followed for a median of 4.7 (2.9–7.0) years, during which 37% received immunosuppression and 74% received blockers of the renin-angiotensin system (RASB). Mean arterial pressure (MAP) was well controlled, and the follow-up proteinuria was 0.8 (0.4–1.4) g/d. The rate of renal function decline was just under 2 ml/min per 1.73 m2 per year; 13% experienced a combined event, and 11% experienced ESRD.
Table 1.
Baseline and follow-up characteristics (n=3096)
| At renal biopsy | Values at renal biopsy |
| Cohorts: Oxford, the VALIGA Study, Japan, China, na | 234, 1145, 700, 1017 |
| Women, % | 42 |
| Ethnicity: white, Asian, other, % | 41, 58, 1 |
| Age, yr | 35±14 |
| eGFR, ml/min per 1.73 m2 | 78±29 |
| MAP, mmHg | 97±15 |
| Proteinuria, g/d | 1.2 (0.7–2.3) |
| Pathology findings | % of total biopsies |
| M1 | 33 |
| E1 | 20 |
| S1 | 77 |
| T0, T1, T2 | 75, 20, 5 |
| Crescents (cellular or fibrocellular) | 36 |
| Follow-up parameters | Values during follow-up |
| Length of follow-up, yr | 4.7 (2.9–7.0) |
| Any immunosuppression, % | 37 |
| RASB, % | 74 |
| MAP, mmHgb | 95±10 |
| Proteinuria, g/db | 0.8 (0.4–1.4) |
| Rate of renal function decline, ml/min per 1.73 m2 per yr | −1.9±6.3 |
| ESRD, % | 11 |
| Combined event, % | 13 |
Values are expressed as means±SD, medians (interquartile ranges), or percentages.
Excluding 44 individuals with no information on crescents or immunosuppressive therapy during follow-up.
Time averaged.
Renal Biopsy Findings
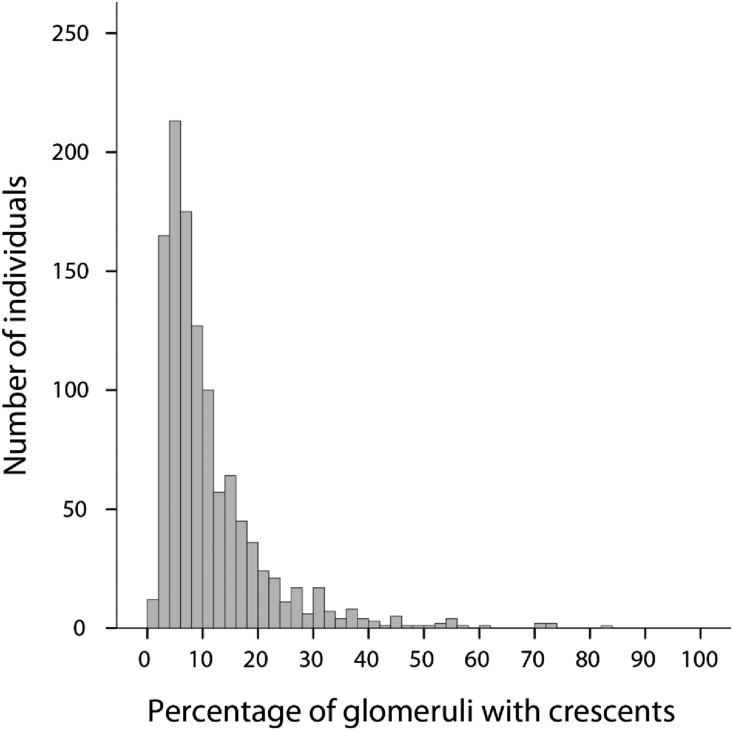
Pathology findings are detailed in Table 1. Cellular and/or fibrocellular crescents were present in 36% of individuals. The distribution of the percentage of crescents observed in each biopsy is shown in Figure 1. Of the 1118 with any cellular/fibrocellular crescents, 61% had crescents in <10% of glomeruli, whereas 39% had a fraction of glomeruli with crescents one tenth or more, 20% had a fraction of glomeruli with crescents one sixth or more, and only 9% had a fraction of glomeruli with crescents one fourth or more. When applied to the entire cohort, 14%, 7%, and 3% had crescents in greater than or equal to one tenth, greater than or equal to one sixth, and greater than or equal to one fourth of glomeruli, respectively. The presence of crescents correlated with each component of the MEST score, with the strongest association found with the E lesion (odds ratio of having E concurrent with any crescents, 5.98; 95% confidence interval [95% CI], 4.94 to 7.24; P<0.001).
Figure 1.
Distribution of the percentage of glomeruli with crescents in biopsies with any crescents. Crescents were present in 1118 (36%) of 3096 total biopsies.
Immunosuppression
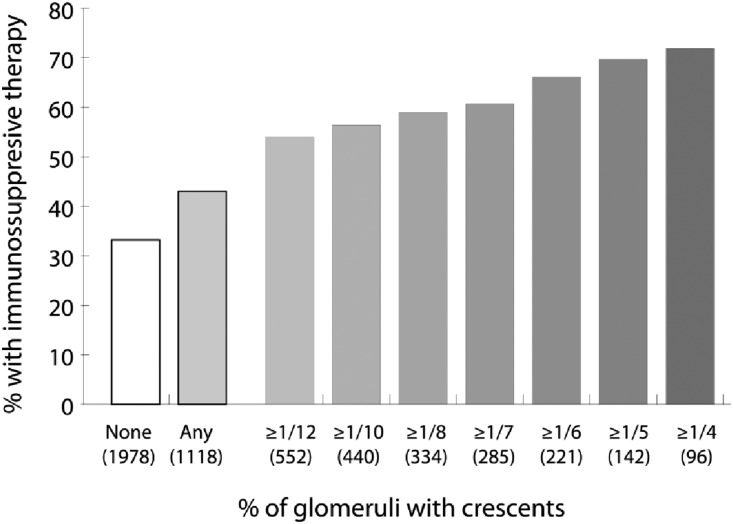
Thirty-three percent of patients without any crescents received immunosuppression compared with 43% of patients with crescents (P<0.001). Stepwise examination of increasing fractions of glomeruli with crescents revealed a steady increase in the percentage of patients treated with immunosuppression (Figure 2). Patients with crescents who were not treated had greater eGFR, lower proteinuria, fewer crescents, less E, and less severe T compared with those who received immunosuppression (Table 2).
Figure 2.
Associations between the presence and fraction of glomeruli with crescents and the subsequent use of immunosuppression. Total numbers of biopsies in each category of crescents are given in parentheses; the total number of biopsies was 3096.
Table 2.
Differences in baseline characteristics (time of biopsy) between patients with crescents given immunosuppression (n=481) or not given immunosuppression (n=637)
| Immunosuppression | P Value | ||
|---|---|---|---|
| No | Yes | ||
| Baseline parameter | |||
| Women, % | 51 | 50 | 0.78 |
| Age, yr | 34±12 | 33±14 | 0.19 |
| eGFR, ml/min per 1.73 m2 | 82±28 | 76±29 | <0.001 |
| MAP, mmHg | 95±15 | 96±14 | 0.53 |
| Proteinuria, g/d | 1.1 (0.6–2.0) | 2.1 (1.1–3.7) | <0.001 |
| Pathology, % | |||
| M | 33 | 38 | 0.09 |
| E | 33 | 47 | <0.001 |
| S | 90 | 92 | 0.20 |
| Ta | 28 | 34 | 0.03 |
| Crescents, % of glomeruli | 7 (4–11) | 10 (6–18) | <0.001 |
Results are presented as means±SD or medians (interquartile ranges).
T score defined by T1 or T2 in reference to T0.
Identification of the Optimal Categorization of the Fraction of Crescents
The presence of crescents was associated with a faster rate of renal function decline compared with its absence (−2.4±7.1 versus −1.6±5.8 ml/min per 1.73 m2; P=0.004) and a lower survival from a combined event (hazard ratio [HR], 1.32; 95% CI, 1.06 to 1.65; P=0.01).
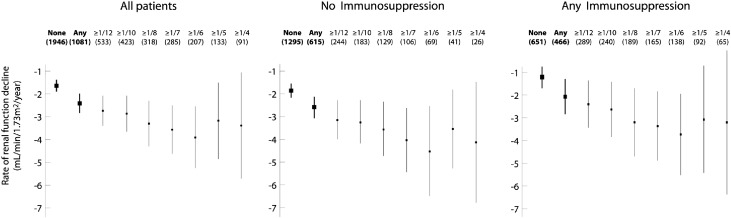
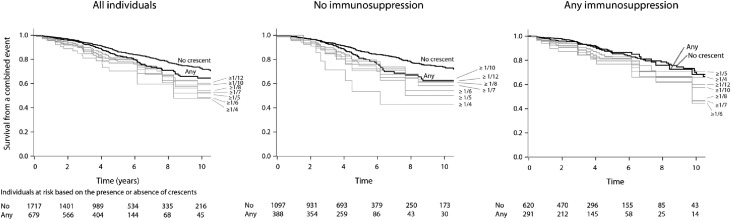
We repeated these univariate analyses to examine the rate of renal function decline and survival from a combined event in individuals having greater than or equal to one twelfth, greater than or equal to one tenth, greater than or equal to one eighth, greater than or equal to one seventh, greater than or equal to one sixth, greater than or equal to one fifth, and greater than or equal to one fourth of glomeruli with crescents. In Figure 3, a proportion-dependent relationship can be appreciated between the fraction of glomeruli with crescents and the rate of renal function decline up until one sixth of glomeruli in all patients, those with no immunosuppression, and those receiving immunosuppression. Survival from a combined event progressively declined up to greater than or equal to one fourth of glomeruli with crescents in the total cohort and those not receiving immunosuppression (Figure 4). The proportion-dependent association between crescents and survival from the combined event was lost in patients receiving immunosuppression (Figure 4). Also, note in both Figures 3 and 4 that, overall, treated patients did better than untreated patients with respect to both outcome variables.
Figure 3.
Univariate effect of the fraction of glomeruli with crescents on the rate of renal function decline in all patients, patients not receiving immunosuppression, and those receiving immunosuppression. Data shown are means with 95% CIs; 2% of individuals had no reported rate of renal function decline and are not included in the analysis.
Figure 4.
Univariate effect of the presence and fraction of glomeruli with crescents on survival from a combined event in all patients, patients not receiving immunosuppression, and those receiving immunosuppression. Each curve represents patients with biopsies showing the indicated fraction of crescents. Numbers of patients without and with any crescents are listed at baseline and after 2, 4, 6, 8 and 10 years of follow-up.
Adjusted Predictive Value of Crescents
We performed multivariate analyses in all patients as well as separately in those who did and did not receive immunosuppression. We used covariates from the original Oxford study: the MEST scores, the initial eGFR, and follow-up MAP and proteinuria. On the basis of findings by univariate analysis, including the plateauing of the rate of eGFR decline above one sixth of crescents (Figure 3) but the continued increase in the risk of the combined event beyond this level, at least in untreated patients (Figure 4), we considered three different categorizations of crescents. These were (1) present or absent only; (2) absent, present in less than one sixth of glomeruli, and present in one sixth or more of glomeruli; and (3) absent, present in less than one fourth of glomeruli, and present in one fourth or more of glomeruli.
We first studied the association of crescents with the rate of decline of eGFR. Using linear regressions, the presence or absence of crescents was not independently predictive of the slope of eGFR. However, using models with two cutoffs of crescents, untreated individuals with a higher fraction of crescents tended to progress more rapidly than those without crescents. Compared with those with no crescents, untreated patients with crescents in greater than or equal to one sixth and greater than or equal to one fourth of glomeruli had adjusted increases in the rate of eGFR decline of 1.7 ml/min per 1.73 m2 per year (95% CI, 0.07 to 3.23; P=0.03) and 2.0 ml/min per 1.73 m2 per year (95% CI, −0.34 to 4.34; P=0.10), respectively.
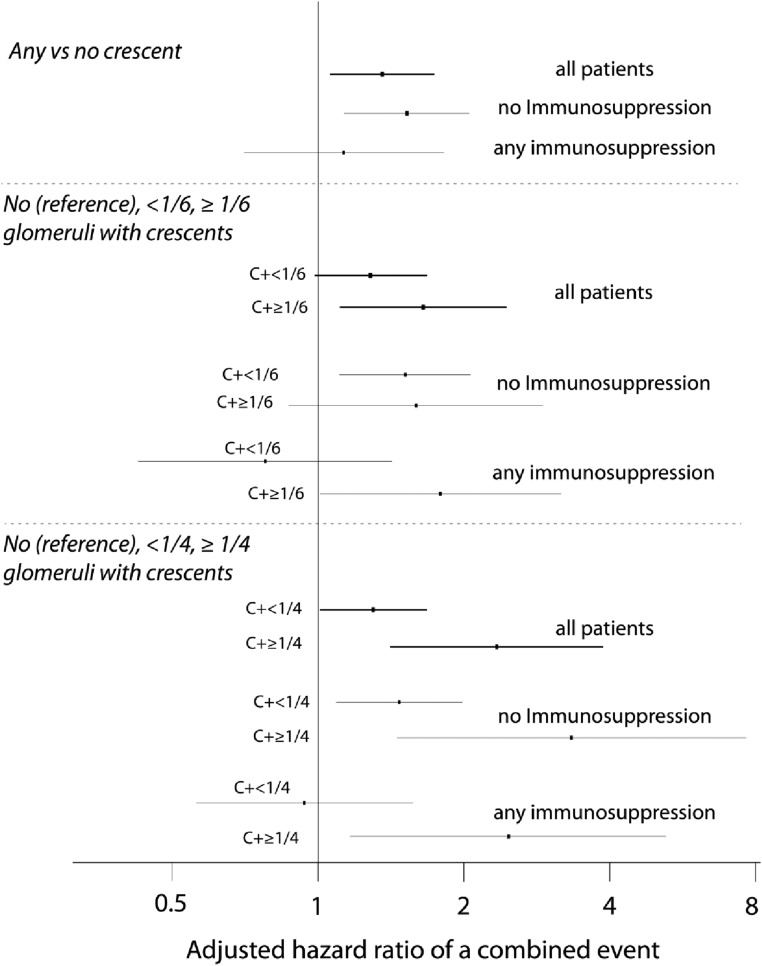
The presence of crescents was predictive of survival from the combined event with an HR of 1.37 (95% CI, 1.07 to 1.75; P=0.01) (Table 3). This risk was greater in untreated individuals but was not statistically significant in those who received immunosuppression (Figure 5, Table 3). Similar results were obtained when we separately analyzed those patients meeting the inclusion criteria of the original Oxford cohort (no immunosuppression: HR of any crescents for combined event, 1.37; 95% CI, 1.01 to 1.85; P=0.04; with immunosuppression: HR, 1.02; 95% CI, 0.60 to 1.74; P=0.93) and those not meeting these criteria (HR, 5.26; 95% CI, 2.01 to 13.74; P=0.001 versus HR, 0.79; 95% CI, 0.21 to 2.98; P=0.79). When we used absent, present in less than one sixth of glomeruli, and present in greater than or equal to one sixth of glomeruli as cutoffs in all patients, a gradation appeared, with adjusted HRs of 1.29 (95% CI, 0.99 to 1.69; P=0.06) and 1.63 (95% CI, 1.10 to 2.43; P=0.02) (Figure 5) for less than one sixth and greater than or equal to one sixth, respectively, in reference to no crescents. A similar gradation existed when using absent, present in less than one fourth of glomeruli, and present in greater than or equal to one fourth of glomeruli as cutoffs in all patients with an HR of 2.29 (95% CI, 1.35 to 3.91) for crescents in one fourth or more of glomeruli (Figure 5).
Table 3.
Multivariate determinants of survival from a combined event
| Parameter | All Patients | No Immunosuppression | Any Immunosuppression | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| eGFR at biopsy | 0.99 | 0.98 to 0.99 | <0.001 | 0.98 | 0.97 to 0.99 | <0.001 | 0.99 | 0.99 to 1.01 | 0.59 |
| MAP, mmHga | 1.02 | 1.01 to 1.03 | 0.001 | 1.00 | 1.00 to 1.02 | 0.17 | 1.05 | 1.02 to 1.07 | <0.001 |
| Proteinuria, g/da | 1.60 | 1.51 to 1.68 | <0.001 | 1.56 | 1.45 to 1.66 | <0.001 | 1.71 | 1.57 to 1.87 | <0.001 |
| M | 1.37 | 1.11 to 1.70 | 0.003 | 1.39 | 1.07 to 1.81 | 0.01 | 1.44 | 0.99 to 2.09 | 0.05 |
| E | 0.76 | 0.56 to 1.04 | 0.08 | 0.88 | 0.61 to 1.28 | 0.51 | 0.72 | 0.41 to 1.26 | 0.25 |
| S | 1.45 | 1.04 to 2.01 | 0.03 | 1.32 | 0.90 to 1.95 | 0.16 | 2.14 | 1.11 to 4.15 | 0.24 |
| Tb | 2.85 | 2.23 to 3.63 | <0.001 | 2.43 | 1.80 to 3.27 | <0.001 | 3.48 | 2.27 to 5.34 | <0.001 |
| Crescents | 1.37 | 1.07 to 1.75 | 0.01 | 1.51 | 1.13 to 2.02 | <0.01 | 1.13 | 0.71 to 1.80 | 0.62 |
Time averaged.
T score defined by T1 or T2 in reference to T0.
Figure 5.
Adjusted HRs of a combined event using different fractions of glomeruli with crescents in all patients, patients not receiving immunosuppression, and those receiving immunosuppression. HRs are in reference to no crescents and adjusted for the MEST scores, initial eGFR, and time-averaged MAP and proteinuria. C+, with crescents.
We then compared the predictive value of crescents in treated and untreated individuals separately (Figure 5). In individuals receiving immunosuppression, predictive values of any crescents and crescents in less than one sixth and less than one fourth of glomeruli were not statistically significant after adjustment for covariates. This was not the case in untreated subjects. However, the predictive value of greater than or equal to one fourth crescents remained significant in both untreated and treated individuals, reaching HRs for the combined event of 3.26 (95% CI, 1.47 to 7.34; P=0.004) and 2.43 (95% CI, 1.17 to 5.06) for untreated and treated patients, respectively (Figure 5).
Added Value of Crescents
To study the discrimination performance of the presence of crescents for the combined event, we used the continuous net reclassification improvement (cNRI). We compared Cox proportional hazards model using eGFR at baseline, time-averaged proteinuria and MAP, and MEST scores with and without crescents (Table 4). In patients not treated with immunosuppression, addition of crescents (any or greater than or equal to one sixth or one fourth of glomeruli) improved the ability of the model to discriminate between patients who did or did not experience the combined event 10 years after biopsy (P<0.05). By contrast, there was no improvement to the discrimination performance by adding crescents to the model in patients receiving immunosuppressive therapy.
Table 4.
The discrimination performance of models predicting the survival from a combined event at 10 years postbiopsy
| Patient Group and Model | cNRI |
|---|---|
| All patients | |
| Baseline model (without crescents): initial eGFR; time-averaged MAP and proteinuria; and M, E, S, T (0 versus 1–2) | — |
| Baseline model and any crescents | 0.142 (−0.019; 0.222); P=0.08 |
| Baseline model and no, less than one sixth, and greater than or equal to one sixth crescents | 0.132 (−0.085; 0.216); P=0.16 |
| Baseline model and no, less than one fourth, and greater than or equal to one fourth crescents | 0.111 (−0.131; 0.210); P=0.24 |
| Patients with no immunosuppression | |
| Baseline model (without crescents): initial eGFR; time-averaged MAP and proteinuria; and M, E, S, T (0 versus 1–2) | — |
| Baseline model and any crescents | 0.200 (0.053; 0.281); P=0.02 |
| Baseline model and no, less than one sixth, and greater than or equal to one sixth crescents | 0.191 (0.000; 0.276); P=0.05 |
| Baseline model and no, less than one fourth, and greater than or equal to one fourth crescents | 0.205 (0.007; 0.285); P=0.04 |
| Patients with any immunosuppression | |
| Baseline model (without crescents): initial eGFR; time-averaged MAP and proteinuria; and M, E, S, T (0 versus 1–2) | — |
| Baseline model and any crescents | 0.037 (−0.410; 0.591); P=0.78 |
| Baseline model and no, less than one sixth, and greater than or equal to one sixth crescents | 0.030 (−0.505; 0.617); P=0.58 |
| Baseline model and no, less than one fourth, and greater than or equal to one fourth crescents | 0.056 (−0.550; 0.749); P=0.66 |
Results are presented as continuous net reclassification improvement (cNRI) with 95% confidence interval in parentheses. cNRI values significantly greater than zero suggest improvement in discrimination by adding crescents to the model.
Discussion
The original Oxford study1 and several subsequent validation studies3–6,15 with similar entry criteria, excluding patients with eGFR<30 ml/min per 1.73 m2 at the time of biopsy and/or rapid progression to ESRD, did not find crescents to be an independent predictor of poor renal outcomes in patients with IgA nephropathy. However, some other studies with less restrictive entry criteria7–10 did find a significant association between crescents and a composite outcome including either ESRD or doubling of SCr/≥50% reduction in eGFR (in one instance, also including death). Furthermore, in a meta-analysis of 16 retrospective studies of 3893 total patients with IgA nephropathy, Lv et al.16 found in a multivariate model that Oxford M, S, and T scores (but not E) and extracapillary proliferation were significantly associated with development of renal failure events. Some of this association of crescents with poor outcomes could be related to crescentic IgA nephropathy defined by crescents in >50% of glomeruli, the latter being associated with a poor prognosis for renal survival, despite immunosuppressive therapy.17 However, in the study by Katafuchi et al.,7 in which crescents were predictive of development of ESRD in 286 patients not meeting the entry criteria of the original Oxford study, the median value for percentage of crescents in patients with eGFR of <30 ml/min per 1.73 m2 was only 10%. The demographics of the study population may also influence whether crescents are predictive of renal outcomes. For example, Walsh et al.8 studied adults with a relatively high (40 years) mean age and a relatively high (nearly 40%) fraction of women, and unlike most other studies, they included fibrous as well as cellular/fibrocellular crescents in their analysis. The two pediatric studies9,10 without restrictive entry criteria found by univariate analysis that cellular or fibrocellular crescents as well as Oxford M, E, and T scores (but not S) were predictive of poor outcomes, suggesting that, in children, active lesions (E and crescents) may have a greater effect on outcomes than chronic lesions (particularly S).
This study was, therefore, undertaken with three aims: (1) to determine if the presence or absence of cellular/fibrocellular crescents is, indeed, predictive of poor renal outcomes (composite outcome of either ESRD or ≥50% reduction in eGFR; increased rate of eGFR decline) in a large cohort of patients with IgA nephropathy, including both adults and children as well as patients with a low eGFR (<30 ml/min per 1.73 m2) at the time of biopsy; (2) to determine if there is a certain fraction of glomeruli with crescents that is associated with one or both of these outcomes; and (3) to determine if any identified association between any crescents and/or a certain fraction of glomeruli with crescents with poor renal outcome(s) is affected by immunosuppressive therapy. To accomplish this and in particular, allow for evaluation of the latter two questions, we analyzed data from >3000 patients pooled from four previously studied, well defined cohorts: one from Europe,14 two from Asia,5,7 and the original Oxford cohort1,2 that included patients from four continents.
Our findings indicate that the presence of any cellular or fibrocellular crescents negatively affects renal outcomes in patients with IgA nephropathy independent from clinical parameters (including eGFR at biopsy and time-averaged proteinuria and MAP) and Oxford MEST scores. We also confirmed the results of the original Oxford2 study and the VALIGA14 Study that M, S, and T (but not E) scores are predictive of survival from a combined event of ESRD or a ≥50% reduction in eGFR. As in the VALIGA Study,14 the significance of the M and S scores was seen in the full cohort and patients not receiving immunosuppressive therapy but not seen in patients receiving immunosuppression. Likewise, the significance of any crescents as an independent predictor of the combined event was lost in patients receiving immunosuppressive therapy; in the latter, only time-averaged proteinuria and MAP as well as T score were predictors of the combined event. Furthermore, addition of crescents improved the ability of a Cox model to discriminate between patients who did or did not experience the combined event 10 years after the biopsy in patients not receiving immunosuppression but not in patients receiving such treatment. Interestingly, similar findings were found in those patients who did and did not meet the entry criteria for the original Oxford study,1,2 suggesting that differences between studies with respect to the prognostic value of crescents may be related more to factors (e.g., the size and age of the study population and median fraction of glomeruli with crescents in biopsies showing crescents) other than whether patients with low eGFR and/or rapidly progressive clinical course were included.
Stepwise examination of increasing fractions of crescents revealed a steady increase in the percentage of patients treated with immunosuppression. Despite this, by univariate analysis, the rate of eGFR decline progressively increased with an increasing fraction of crescents until one sixth in patients with and without immunosuppression. Survival from a combined renal event decreased with an increasing fraction of crescents from one twelfth to one fourth in patients not receiving immunosuppression but not in those receiving such therapy.
Using a multivariate model with two separate cutoffs for crescents, in less than one sixth and greater than or equal to one sixth of glomeruli and in less than one fourth and greater than or equal to one fourth of glomeruli, revealed additional differences between low and higher levels of crescents as independent predictors of the combined event. Crescents in less than one fourth of glomeruli were an independent predictor of the combined event in all patients and those not receiving immunosuppression but not in patients receiving immunosuppression. By contrast, crescents in greater than or equal to one fourth (≥25%) of glomeruli remained a predictor of the combined event, even in patients receiving immunosuppressive therapy.
On the basis of these findings, we propose three individual crescent scores, C0 (no crescents), C1 (crescents in more than zero but less than one fourth of glomeruli), and C2 (crescents in one fourth or more of glomeruli), be added to the MEST scores of the Oxford Classification. Twenty-five percent of glomeruli are easy to assess, and there is precedent within the Oxford Classification for the use of two separate cutoffs for a single parameter, T, indicating different levels of effect of moderate (T1) and severe (T2) degrees of T on the combined renal event and the rate of eGFR decline.2 Likewise, although C1 suggests patients as having increased risk (compared with C0) of a poor renal outcome, C2 identifies patients who are at increased risk of a poor outcome, even if given immunosuppressive therapy. It is important to note, however, that this only applies to well defined cellular or fibrocellular crescents and not to fibrous crescents, the latter being composed of <10% cells and ≥90% matrix as defined in the Oxford Classification2 as well as other morphologic classifications of glomerular diseases.18,19 In the original Oxford study, there was excellent interobserver agreement among pathologists in identifying the fraction of glomeruli showing cellular plus fibrocellular crescents; this was not the case with fibrous crescents, which may be difficult to distinguish from sclerosis of the glomerular tuft with an associated capsular adhesion.2 Furthermore, our findings should not be taken as a specific treatment recommendation; whether and to what extent the benefits of immunosuppressive therapy on renal outcomes in IgA nephropathy are modified by the presence and/or fraction of glomeruli with crescents need to be addressed in a separate study. Presently, decisions regarding immunosuppression need to take into account clinical factors and other pathologic findings in addition to crescents.
The selection of one fourth of glomeruli with crescents as the cutoff for C2 was on the basis of the findings shown in Figure 5 and discussed above. Only 96 of 1118 biopsies (8.8%) with any crescents and 3096 total biopsies (3.1%) met this criterion for C2, although a similar low fraction of patients shows T2 lesions (e.g., 3.6% in the VALIGA Study9). We considered one sixth rather than one fourth as the cutoff for C2, because in univariate analyses, the decrease in eGFR seen with increasing fractions of crescents plateaued at one sixth of glomeruli in patients who did and did not receive immunosuppression. The one sixth cutoff would increase the fraction of patients with C2 to 7.1% of total patients and 19.8% of those with crescents. However, despite the appeal of making the C2 score applicable to a larger fraction of patients, the data, specifically those on the basis of the predictive value of crescents in a multivariate analysis including clinical parameters, Oxford MEST scores, and the effect of immunosuppressive therapy, are more supportive of the higher (greater than or equal to one fourth of glomeruli) cutoff, and the unique value of the Oxford Classification among published morphologic classifications for glomerular diseases is that the former is fully data driven and evidence based.20
As noted above, repeat renal biopsy studies in patients with IgA nephropathy have shown that crescents and necrosis as well as E represent lesions that are reversible with immunosuppressive therapy.11–13 Shen et al.11 studied renal biopsies in patients with active IgA nephropathy that were performed before and after immunosuppressive therapy. They found that crescents on pretreatment biopsies involving <50% of glomeruli in all patients were reversed in 71% of patients, similar to E (77%).11 Thus, as with E, crescents in a minority of glomeruli often represent a lesion reversible by immunosuppressive therapy. These findings are consistent with our findings and suggest that patients whose biopsies show these active lesions be considered for immunosuppressive treatment. Use of corticosteroids and other immunosuppressive agents in IgA nephropathy remains controversial, and a recent multicenter, randomized, controlled trial in patients with IgA nephropathy and persistent proteinuria of ≥0.75 g/d despite supportive care, including RASB, showed no significant effect of adding immunosuppressive therapy to continued supportive care on the change in eGFR after 3 years or development of ESRD.21 However, this study excluded patients with eGFR<30 ml/min per 1.73 m2 or rapid eGFR decline and did not report histologic findings. It is, therefore, not possible to determine if its conclusions apply to patients with crescents and E.
This study has several important limitations. As a retrospective study involving patients from multiple centers, criteria for treatment with immunosuppressive agents were nonuniform as were specific treatment regimens, use of RASB, and length of follow-up. Clinical end points and recording of data during follow-up also differed between groups of patients, most notably the Japanese cohort7 that used only ESRD as an end point and did not record time-averaged proteinuria or MAP. Still, our findings clearly support addition of crescent scores to the Oxford/MEST Classification, indicating lesions that are independently associated with a poor renal outcome in untreated patients (C1) and even those treated with immunosuppressive therapy (C2). Additional studies, preferably in more uniformly treated patient cohorts, are needed to validate these conclusions and further test the optimal cutoff for C2.
Concise Methods
Study Design
This is a post hoc analysis of four retrospective cohorts. The Oxford cohort (n=265) included participants from 16 countries and four continents1,2; the VALIGA9 Study was European (n=1147), and there were two Asian cohorts: one from China (n=1026)5 and one from Japan (n=702).7 The Chinese study only included adults as opposed to the other three, which had both children and adults.
The inclusion criteria were biopsy–proven IgA nephropathy with pathology slides available for review. Inclusion required a minimum of eight glomeruli in the Oxford and the VALIGA Study cohorts and a minimum of 10 glomeruli in the Chinese and Japanese cohorts. Patients with Henoch–Schönlein purpura nephritis, diabetes, IgA nephropathy known to be secondary to liver disease, and IgA nephropathy superimposed on ANCA–associated necrotizing/crescentic GN were excluded. The Oxford and Chinese cohorts excluded those with initial eGFR <30 ml/min per 1.73 m2 and proteinuria <0.5 g/24 h as well as individuals with <1 year of follow-up. The other two studies had no restrictions on initial eGFR or proteinuria, and the length of follow-up was required to be ≥1 year unless ESRD occurred before that time.
Data were collected on patients’ characteristics at the time of renal biopsy and during follow-up, including demographics, BP, eGFR, and proteinuria. We had available follow-up information on the use of RASB and immunosuppressive therapy.
The four cohorts included 3140 individuals. We discarded two subjects without crescent assessment and 42 with no information on immunosuppression, because these parameters were essential to our study. Other variables were missing in ≤2% of patients, except for combined events and follow-up BP and proteinuria measurements in the Japanese cohort; multivariate analyses, therefore, excluded this group of patients. In total, 3096 patients were, thus, included in the study.
Definitions
We estimated the eGFR using the four–variable Modification of Diet in Renal Disease formula in adults22 and the Schwartz formula in children.23 As initially performed in the VALIGA Study,14 the maximum GFR was set at 120 ml/min per 1.73 m2, because the accuracy of high eGFR values is imprecise using these formulas and thus, may bias the estimation of the rate of renal function decline. MAP was calculated as diastolic BP +1/3(systolic BP − diastolic BP). To determine time-averaged MAP and time-averaged proteinuria, we calculated the average of the mean values of each 6-month period; these were not available in the Japanese cohort. Immunosuppressive therapy after kidney biopsy was defined as treatment with any immunosuppressive agent, regardless of duration or dose. RASB was defined as the use of angiotensin–converting enzyme inhibitor and/or angiotensin receptor blocker after biopsy.
The outcomes were the rate of renal function decline (slope of eGFR) and the survival from a combined event defined by either a ≥50% reduction in eGFR or ESRD (eGFR<15 ml/min per 1.73 m2). Combined events were also not available in the Japanese cohort.
Pathology Review
Five pathologists scored renal biopsies from the original Oxford study. We used the assessment of the two central pathologists for patients in the VALIGA Study, excluding the local pathologist’s assessment. Two pathologists scored the biopsies from the Chinese cohort also using the Oxford criteria. In the Japanese cohort, the biopsies were scored by a single pathologist. For the VALIGA Study and the Chinese study, one of the pathologists was also involved in scoring the original Oxford cohort; interclass correlation coefficients among pathologists in the Chinese study for defining MEST scores were all excellent (range =0.66–0.94).5
Scoring was previously described in detail.1,2,14 In summary, in the Oxford, Chinese, and Japanese cohorts, M1 was defined as an M score of >0.5; this score was the mean mesangial cellularity score in all scorable glomeruli on the basis of the number of mesangial cells in the most cellular mesangial area, excluding the central core and region of the vascular pole (less than four cells, score of zero; four or five cells, score of one; six or seven cells, score of two; and eight or more cells, score of three). In the VALIGA Study, M1 corresponded to >50% of glomeruli showing M, the latter defined as four or more cells in one or more mesangial areas, not including the regions specified above. E and S were scored as absent (zero) or present (one). T was initially scored according to the estimated percentage of interstitial fibrosis and tubular atrophy in the cortex: T0 (≤25% of cortex), T1 (26%–50%), and T2 (>50%). Because few biopsies had T2 lesions, multivariate models used T0 versus T1 and T2 to increase statistical power.
For each biopsy, we determined the fraction of glomeruli with cellular or fibrocellular crescents. A crescent was defined as extracapillary proliferation of more than two cell layers of any size; this definition was uniform in all studies. A cellular crescent was defined by >50% of the lesion occupied by cells, and a fibrocellular crescent was defined by ≤50% of the lesion occupied by cells and <90% occupied by matrix. Fibrous crescents (composed of ≥90% matrix2,18,19) were not taken into account.
The initial goal of the Oxford Classification1,2 was to offer a simple and reproducible scoring system that added prognostic value independent of other clinical findings. Similar to the M, E, S, and T scores, a crescent score would have to be categorical and on the basis of one or more cutoffs that are easily determined. Because biopsy findings are usually reported with a number of glomeruli with crescents and a total number of glomeruli, we felt it best to study the predictive values of different fractions of glomeruli with crescents in addition to any versus no crescents. The proportions studied were greater than or equal to one twelfth, greater than or equal to one tenth, greater than or equal to one eighth, greater than or equal to one seventh, greater than or equal to one sixth, greater than or equal to one fifth, and greater than or equal to one fourth affected glomeruli. There were too few individuals to study with higher fractions of crescents.
Statistical Analyses
Normally distributed variables are presented as means±SD and compared using the t test. Nonparametric continuous variables were presented as medians (interquartile ranges) and compared with the Mann–Whitney U test. Categorical variables are presented as percentages and compared using the chi-squared test.
In the Oxford study, the VALIGA Study, and the Chinese study, the rate of renal function decline was determined by fitting a straight line through available data for eGFR using the principle of least squares. This was plotted and visually examined in each patient. Obvious outliers were censored. For the Japanese cohort, the slope was obtained by subtracting the initial from the last eGFR and dividing by the length of follow-up in years.
To identify the optimal cutoffs of the categorization of crescents, we visually inspected the relation between each proposed fraction and outcomes. We used Kaplan–Meier survival curves and Cox regression to test the relation between crescents, expressed in categories, and the survival from a combined event. The multivariate models (linear regression for slope and Cox regression for survival) addressed the predictive value of crescents adjusted for covariates used in the initial Oxford models (initial eGFR; time-averaged MAP and proteinuria; and M, E, S, and T lesions). Because the presence of crescents was strongly associated with the subsequent use of immunosuppression, we performed a priori each model for the entire cohort plus the untreated and treated groups separately.
The net reclassification improvement offers a measure of the prediction increment of adding crescents to existing risk factors. It is calculated by adding the probability of reclassifying individuals into true positive or true negative events and subtracting the probability of reclassifying individuals into false positive or false negative events after a new risk factor is added to a statistical model. Values significantly greater than zero suggest improvement in discrimination. The net reclassification improvement is useful when using large sample size models where a significant P value may be misleading and an incremental change in c statistic is difficult to show after multiple important risk factors are already included.24 The cNRI is preferred for survival data, which contain not only events and nonevents but also, subjects lost to follow-up.25 We estimated cNRIs using different categorizations of crescentic lesions in reference to a baseline model without crescents. Finally, we internally validated our models using a bootstrap of 1000 generated samples.
All P values were two tailed, and values <0.05 were considered statistically significant. Analyses were carried out using SPSS (version 13; IBM SPSS, Chicago, IL) and the R software (version 3.1.2; Free Software Foundation).
Disclosures
We thank the International IgA Nephropathy Network for financial support. The authors have no conflicts of interest to disclose.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Roberts IS, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, Cattran DC, Coppo R, D’Agati V, D’Amico G, Emancipator S, Emma F, Feehally J, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Pathology definitions, correlations, and reproducibility. Kidney Int 76: 546–556, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Herzenberg AM, Fogo AB, Reich HN, Troyanov S, Bavbek N, Massat AE, Hunley TE, Hladunewich MA, Julian BA, Fervenza FC, Cattran DC: Validation of the Oxford classification of IgA nephropathy. Kidney Int 80: 310–317, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Le W, Zeng CH, Liu Z, Liu D, Yang Q, Lin RX, Xia ZK, Fan ZM, Zhu G, Wu Y, Xu H, Zhai Y, Ding Y, Yang X, Liang S, Chen H, Xu F, Huang Q, Shen H, Wang J, Fogo AB, Liu ZH: Validation of the Oxford classification of IgA nephropathy for pediatric patients from China. BMC Nephrol 13: 158, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng CH, Le W, Ni Z, Zhang M, Miao L, Luo P, Wang R, Lv Z, Chen J, Tian J, Chen N, Pan X, Fu P, Hu Z, Wang L, Fan Q, Zheng H, Zhang D, Wang Y, Huo Y, Lin H, Chen S, Sun S, Wang Y, Liu Z, Liu D, Ma L, Pan T, Zhang A, Jiang X, Xing C, Sun B, Zhou Q, Tang W, Liu F, Liu Y, Liang S, Xu F, Huang Q, Shen H, Wang J, Shyr Y, Phillips S, Troyanov S, Fogo A, Liu ZH: A multicenter application and evaluation of the oxford classification of IgA nephropathy in adult Chinese patients. Am J Kidney Dis 60: 812–820, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Shi SF, Wang SX, Jiang L, Lv JC, Liu LJ, Chen YQ, Zhu SN, Liu G, Zou WZ, Zhang H, Wang HY: Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: Validation of the Oxford classification. Clin J Am Soc Nephrol 6: 2175–2184, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katafuchi R, Ninomiya T, Nagata M, Mitsuiki K, Hirakata H: Validation study of oxford classification of IgA nephropathy: The significance of extracapillary proliferation. Clin J Am Soc Nephrol 6: 2806–2813, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh M, Sar A, Lee D, Yilmaz S, Benediktsson H, Manns B, Hemmelgarn B: Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol 5: 425–430, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shima Y, Nakanishi K, Hama T, Mukaiyama H, Togawa H, Hashimura Y, Kaito H, Sako M, Iijima K, Yoshikawa N: Validity of the Oxford classification of IgA nephropathy in children. Pediatr Nephrol 27: 783–792, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Edström Halling S, Söderberg MP, Berg UB: Predictors of outcome in paediatric IgA nephropathy with regard to clinical and histopathological variables (Oxford classification). Nephrol Dial Transplant 27: 715–722, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Shen XH, Liang SS, Chen HM, Le WB, Jiang S, Zeng CH, Zhou ML, Zhang HT, Liu ZH: Reversal of active glomerular lesions after immunosuppressive therapy in patients with IgA nephropathy: A repeat-biopsy based observation. J Nephrol 28: 441–449, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Hotta O, Furuta T, Chiba S, Tomioka S, Taguma Y: Regression of IgA nephropathy: A repeat biopsy study. Am J Kidney Dis 39: 493–502, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Tumlin JA, Lohavichan V, Hennigar R: Crescentic, proliferative IgA nephropathy: Clinical and histological response to methylprednisolone and intravenous cyclophosphamide. Nephrol Dial Transplant 18: 1321–1329, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, Roberts IS, Morando L, Camilla R, Tesar V, Lunberg S, Gesualdo L, Emma F, Rollino C, Amore A, Praga M, Feriozzi S, Segoloni G, Pani A, Cancarini G, Durlik M, Moggia E, Mazzucco G, Giannakakis C, Honsova E, Sundelin BB, Di Palma AM, Ferrario F, Gutierrez E, Asunis AM, Barratt J, Tardanico R, Perkowska-Ptasinska A; VALIGA study of the ERA-EDTA Immunonephrology Working Group : Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int 86: 828–836, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee MJ, Kim SJ, Oh HJ, Ko KI, Koo HM, Kim CH, Doh FM, Yoo TH, Kang SW, Choi KH, Lim BJ, Jeong HJ, Han SH: Clinical implication of crescentic lesions in immunoglobulin A nephropathy. Nephrol Dial Transplant 29: 356–364, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Lv J, Shi S, Xu D, Zhang H, Troyanov S, Cattran DC, Wang H: Evaluation of the Oxford Classification of IgA nephropathy: A systematic review and meta-analysis. Am J Kidney Dis 62: 891–899, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Lv J, Yang Y, Zhang H, Chen W, Pan X, Guo Z, Wang C, Li S, Zhang J, Zhang J, Liu L, Shi S, Wang S, Chen M, Cui Z, Chen N, Yu X, Zhao M, Wang H: Prediction of outcomes in crescentic IgA nephropathy in a multicenter cohort study. J Am Soc Nephrol 24: 2118–2125, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noël LH, Pusey CD, Waldherr R, Bruijn JA, Bajema IM: Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 21: 1628–1636, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Sethi S, Haas M, Markowitz GS, D’Agati VD, Rennke HG, Jennette JC, Bajema IM, Alpers CE, Chang A, Cornell LD, Cosio FG, Fogo AB, Glassock RJ, Hariharan S, Kambham N, Lager DJ, Leung N, Mengel M, Nath KA, Roberts IS, Rovin BH, Seshan SV, Smith RJ, Walker PD, Winearls CG, Appel GB, Alexander MP, Cattran DC, Casado CA, Cook HT, De Vriese AS, Radhakrishnan J, Racusen LC, Ronco P, Fervenza FC: Mayo Clinic/Renal Pathology Society consensus report on pathologic classification, diagnosis, and reporting of GN. J Am Soc Nephrol 27: 1278–1287, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas M, Rastaldi MP, Fervenza FC: Histologic classification of glomerular diseases: Clinicopathologic correlations, limitations exposed by validation studies, and suggestions for modification. Kidney Int 85: 779–793, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, Panzer U, Peters H, Benck U, Mertens PR, Kuhlmann U, Witzke O, Gross O, Vielhauer V, Mann JF, Hilgers RD, Floege J; STOP-IgAN Investigators : Intensive supportive care plus immunosuppression in IgA nephropathy. N Engl J Med 373: 2225–2236, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Miller WG: Estimating glomerular filtration rate. Clin Chem Lab Med 47: 1017–1019, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Pencina MJ, D’Agostino RB Sr., Steyerberg EW: Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 30: 11–21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kerr KF, Wang Z, Janes H, McClelland RL, Psaty BM, Pepe MS: Net reclassification indices for evaluating risk prediction instruments: A critical review. Epidemiology 25: 114–121, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]