Abstract
Antibodies that are specific to organ donor HLA have been involved in the majority of cases of antibody-mediated rejection in solid organ transplant recipients. However, recent data show that production of non-HLA autoantibodies can occur before transplant in the form of natural autoantibodies. In contrast to HLAs, which are constitutively expressed on the cell surface of the allograft endothelium, autoantigens are usually cryptic. Tissue damage associated with ischemia-reperfusion, vascular injury, and/or rejection creates permissive conditions for the expression of cryptic autoantigens, allowing these autoantibodies to bind antigenic targets and further enhance vascular inflammation and renal dysfunction. Antiperlecan/LG3 antibodies and antiangiotensin II type 1 receptor antibodies have been found before transplant in patients with de novo transplants and portend negative long–term outcome in patients with renal transplants. Here, we review mounting evidence suggesting an important role for autoantibodies to cryptic antigens as novel accelerators of kidney dysfunction and acute or chronic allograft rejection.
Keywords: acute allograft rejection, acute renal failure, apoptosis, clinical immunology, endothelial cells
Successful management of ESRD through kidney transplantation (KT) is one of the most important advances in medical care achieved in the second half of the 20th century. KT improves both life expectancy and quality of life compared with dialysis.1,2 With current immunosuppressive regimens, the incidence of acute rejection is approximately 15%–20% in kidney transplant recipients.3 Although this figure is inferior to the rates reported two decades ago, rejection still represents a challenge for transplant physicians. Treatment with increased immunosuppression leads to excellent recovery of graft function in many patients, but some patients experience treatment failure and subsequent progression to graft loss. Among the factors relevant to the prognosis of rejection, the presence of antibody-mediated damage to the microcirculation is associated with adverse long–term graft outcomes.4–6
Although circulating, anti–HLA, donor–specific antibodies (DSAs) have been involved in the majority of patients with antibody-mediated rejection (ABMR), this type of rejection can also occur in patients who are DSA negative.7,8 Although adsorption of DSA within the allograft has been proposed to explain the occurrence of ABMR in DSA-negative patients,9 mounting evidence has pointed to the role of non-HLA antibodies as important contributors to ABMR. Non-HLA autoantibodies have been associated with rejection in kidney, heart, and lung transplant recipients.7 In contrast to HLAs, which are constitutively expressed on the cell surface of the allograft endothelium, autoantigens are usually cryptic and become exposed after tissue damage prompted by ischemia-reperfusion or allograft rejection. Tissue damage seems to play an important role in both fueling the production of these autoantibodies and if persistent, allowing these autoantibodies to react with their antigenic target, therefore enhancing inflammation at sites of injury.10,11 Although some autoantibodies have been described in patients with classic autoimmune conditions, such as SLE, others have been reported in absence of autoimmune diseases or sensitizing events.12,13 In this work, we review the mechanistic role of autoantibodies in accentuating renal damage and dysfunction and discuss recent evidence pointing to vascular injury as an important contributor to both their production and effect.
Alloimmune Graft Injury Leads to the Production of Autoantibodies
The concept of renal damage–mediated autoantibody production leading to enhanced renal injury is not novel. In the 1960s, Milgrom and coworkers14 reported that rabbits that had rejected mismatched kidney transplants developed antibody-mediated lesions in their native kidneys. This early work showed that alloimmune attack to a kidney graft can lead to the development of autoantibodies, possibly through the release or increased immunogenicity of kidney-specific autoantigens. Although these autoantibodies were able to induce disease in the rabbits’ native kidneys, whether this occurred through complement-dependent mechanisms was not studied at the time.
The involvement of acute and chronic rejection processes in the development of autoantibodies has also been examined in patients with transplants. For instance, polyreactive, natural IgG autoantibodies against apoptotic Jurkat cells were isolated from the sera of kidney transplant recipients with ABMR.15 Whether these autoantibodies participated in accelerated rejection was not addressed in this cross-sectional study, but the purified IgGs led to C4d deposition at the surface of targeted apoptotic cells in the presence of complement. The capacity of these autoantibodies to activate complement through the classical pathway suggested a potential role in enhancing allograft damage. In another study, kidney transplant recipients with transplant glomerulopathy, a key feature of chronic ABMR, were shown to have increased levels of autoantibodies to agrin, a component of the vascular basement membrane.16 The number of previous acute rejection episodes was higher in patients with antiagrin antibodies, again suggesting that alloimmune graft damage may have fueled the production of autoantibodies. Lastly, vimentin, an intracellular intermediate filament protein, can be expressed at the surface of apoptotic T cells and neutrophils as well as endothelial cells.17 Increased antivimentin antibodies have been reported in patients with chronic rejection and failed kidney allografts,18 whereas the levels observed in transplant-naïve patients with end stage CKD were similar to those observed in blood donors.19
Autoantibodies Aggravate Acute or Chronic Rejection
Although allograft injury can lead to the production of autoantibodies, both human and animal data have shown that autoantibodies can, in turn, accelerate and/or enhance renal allograft damage. In the seminal work published a decade ago, Dragun et al.12 showed that agonistic autoantibodies to angiotensin II type 1 receptors (AT1R-Abs) were associated with a severe form of acute vascular rejection with refractory hypertension in patients with renal transplants who were DSA negative. Passive transfer of AT1R-Abs in a rat model of KT accentuated allograft vascular injury and hypertension, therefore replicating the salient clinical features observed in patients with kidney transplants and showing the pathogenic effect of these autoantibodies.
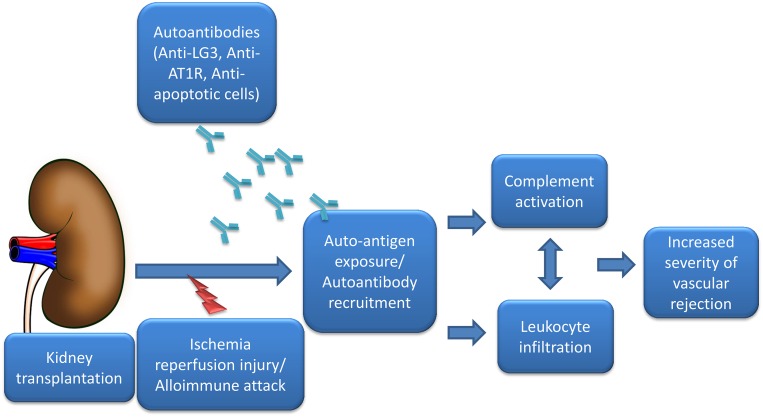
Previous work from our group showed that endothelial apoptosis leads to the release of LG3, a fragment of perlecan, a large proteoglycan normally present within the vascular and to a lesser extent, the tubular basement membrane.20,21 Because endothelial apoptosis is a prominent feature of acute vascular rejection in KT,22 we measured LG3 in patients with acute vascular rejection and observed elevated circulating levels compared with those in patients with kidney transplants with normal function and tubulointerstitial rejection.23 We hypothesized that LG3, a C–terminal laminin G motif normally embedded within perlecan but released in the circulation in association with vascular apoptosis, could behave as a neoantigen and fuel the development of autoantibodies. In other work, we showed that patients with kidney transplants and acute vascular rejection have significantly higher anti–LG3 antibody titers at the time of rejection compared with controls.24 The anti-LG3 antibodies that we identified were of complement–activating IgG isotypes. To characterize the functional effect of anti-LG3 antibodies in acceleration of rejection, we turned to a murine model of vascular rejection on the basis of aorta transplantation between two fully mismatched donors and recipients. Passive transfer of anti-LG3 antibodies accelerated vascular inflammation, obliterative remodeling, and C4d deposition, showing their mechanistic effect in complement activation and aggravation of vascular injury (Figure 1). In patients with kidney transplants, the acute vascular rejection episodes associated with high anti–LG3 titers were early events, occurring at a median of 12 days after transplantation. Because this was unexpectedly early for a de novo antibody response, we evaluated whether anti-LG3 antibodies were present before transplantation. We observed that anti-LG3 titers were significantly higher before transplantation in patients with kidney transplants who went on to develop acute vascular rejection,24 therefore identifying pretransplant anti–LG3 titers as predictors of rejection.
Figure 1.
Autoantibodies aggravate rejection. Ischemia-reperfusion injury at or near the time of transplantation or alloimmune attack to the graft creates permissive conditions for the enhanced availability of cryptic antigens, such as LG3, and increased interactions with antigens present on apoptotic cells. Preexisting circulating autoantibodies (anti-LG3, AT1R-Abs, or antibodies directed toward apoptotic cells) bind to their target and increase vasoconstriction41 and also, allograft vascular inflammation at least in part through complement-mediated mechanisms, leading to increased severity of rejection.12,13,15,24
The effect of pretransplant autoantibodies on post–transplant graft outcomes has also been documented by others. Elevated pretransplant levels of AT1R-Abs have been associated with an increased risk of post–transplant hypertensive emergencies and severe rejection episodes targeting the vascular compartment.12 Similarly, pretransplant levels of polyspecific, natural autoantibodies reactive to apoptotic cells have been associated with increased risk of rejection and reduced allograft survival in kidney transplant recipients.13 Additionally, both de novo formation and pretransplant levels of antifibronectin and anticollagen type 4 autoantibodies were linked with transplant glomerulopathy in patients with kidney transplants.25 Autoantibodies have also been linked to acute and chronic allograft rejection in patients with heart and lung transplants.26–28 Despite the ample correlational evidence, the clinical factors that influence the levels of these autoantibodies before transplantation remain unclear. AT1R-Abs have been associated with scleroderma,29,30 lupus,31 and preeclampsia.32 However, no correlations between allosensitizing events, classic autoimmune diseases before transplantation, and anti–LG3 or antiapoptotic cell autoantibodies have been observed.13,24,33
Ischemia-Reperfusion Creates a Permissive Environment for Autoantibodies to Enhance Tissue Injury
The concept of “innate autoimmunity,” as conceived by Carroll and coworkers,10,34–36 refers to natural autoantibodies that synergize with ischemia-reperfusion injury to enhance complement–mediated organ damage. It was initially reported in murine models of intestinal and skeletal ischemia-reperfusion injury, where naturally occurring IgM antibodies were found to bind to a cryptic self-antigen that was unmasked by tissue injury.10,34–36 This led to complement activation through the classic pathway and enhanced tissue injury. This natural antibody was polyspecific, because it was shown to bind nonmuscle myosin heavy chain in ischemic intestinal tissue35 and glycogen phosphorylase in skeletal muscle36,37 exposed to ischemia-reperfusion injury. Blockade of this autoantibody diminished tissue damage in a model of cardiac ischemia-reperfusion injury.38
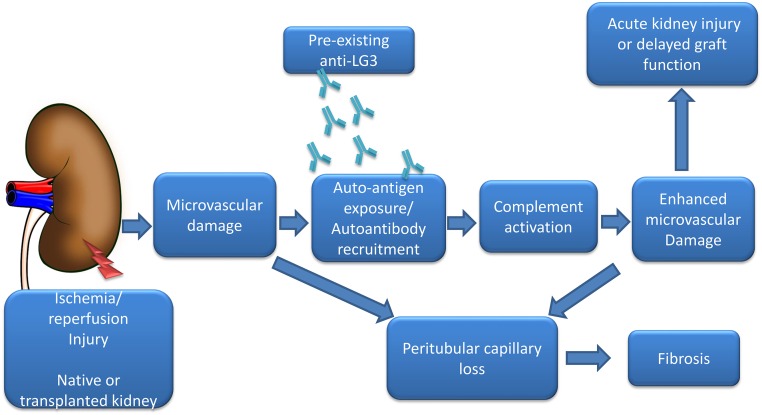
We also found an association between ischemia-reperfusion and the capacity of anti-LG3 antibodies to aggravate vascular damage. Using a murine model of HLA–mismatched aortic transplantation, we noted that passive transfer of anti-LG3 accelerated vascular inflammation and complement deposition but only when the allograft was made ischemic before transplantation.24 More recently, we reported that, in patients with ESRD awaiting a kidney transplant, pretransplant anti–LG3 levels were associated with an increased risk of delayed graft function. Using a murine model of renal ischemia-reperfusion injury and passive transfer of anti-LG3 antibodies, we explored the mechanisms supporting the association between anti-LG3 and aggravation of renal dysfunction. We found that anti-LG3 antibodies aggravate AKI at least in part through increased complement activation within peritubular capillaries, leading to microvascular rarefaction and increased tubulointerstitial fibrosis (Figure 2).39
Figure 2.
Anti-LG3 autoantibodies enhance renal microvascular injury postischemia-reperfusion in native and transplanted kidneys. Renal ischemia-reperfusion leads to initial microvascular damage, which enhances the expression/availability of cryptic antigens, such as LG3. Circulating anti-LG3 reaches these antigenic targets and promotes further microvascular injury, at least in part through complement-dependent mechanisms. Microvascular damage leads to peritubular capillary dropout and enhanced renal fibrosis.39
Along the same lines, lung transplant recipients with elevated pretransplant titers of autoantibodies against type 5 collagen and K-α1 tubulin were shown to be at increased risk of severe ischemia-reperfusion injury during lung transplantation.28 The bronchoalveolar lavage fluid from patients with primary graft dysfunction contained higher levels of C4d, suggesting that these autoantibodies activate complement through the classic complement pathway. In a murine model of syngeneic lung transplantation, passive transfer of autoantibodies to collagen type 5 and K-α1 tubulin induced graft inflammation and fibrosis in the transplanted lung but not in native noninjured lungs.11 Collectively, these results suggest that ischemia-reperfusion, independent of alloimmunity, can create permissive conditions needed for autoantibodies to enhance graft damage.
Whether autoantibodies, such as anti-LG3 antibodies or AT1R-Abs, can enhance the severity of AKI in patients with native kidney disease exposed to ischemia-reperfusion remains to be determined. LG3, the antigenic target of anti-LG3 antibodies, seems to be one of the earliest biomarkers rising in patients with AKI,40 which could create a favorable setting for anti-LG3 to bind to its target, activate complement, and enhance tissue damage. As mentioned above, passive transfer of anti-LG3 antibodies in association with ischemia-reperfusion of native kidneys in mice also accentuates renal dysfunction and aggravates microvascular rarefaction and fibrosis.39 Ischemia increases the contractile activity of AT1R-Abs agonistic autoantibodies in isolated renal artery rings,41 which in turn, could potentially enhance renal vasoconstriction and distal ischemia. These observations suggest that, in nontransplanted patients with AKI, a potential aggravating role of autoantibodies deserves additional investigation.
Mechanisms of Autoantibody Formation in Health and Disease: A Paradigm Shift
In contrast to natural IgM autoantibodies, autoreactive IgGs have traditionally been linked to a break in self-tolerance and pathogenicity.42 In recent years, however, microarray techniques have revealed that both polyspecific and monospecific autoreactive IgG antibodies directed at multiple proteins expressed in human tissues are abundant and ubiquitous in normal human sera.43 Similar to their IgM counterparts, they are thought to be important players in the clearance of danger–associated molecular patterns and restraining the inflammatory response prompted by dying cells and tissue injury.44,45 Although the types and levels of natural IgG autoantibodies are somewhat influenced by age, sex, and disease states, the natural autoantibody profile of a given individual is considered to be relatively stable over time,43 and the factors that can lead to changes in autoantibody profiles remain to be fully characterized. Whether some of the autoantibodies associated with acceleration of rejection represent natural autoantibodies is not entirely clear, although many clues point in that direction. Autoantibodies reactive to apoptotic cells before transplantation in patients with de novo transplants are likely to represent natural antibodies implicated in the clearance of dying cells.13,15 Indeed, the LG3 autoantigen is released by apoptotic vascular and tubular cells,20,21,46,47 suggesting that anti-LG3 antibodies represent natural antibodies produced in response to tissue injury.
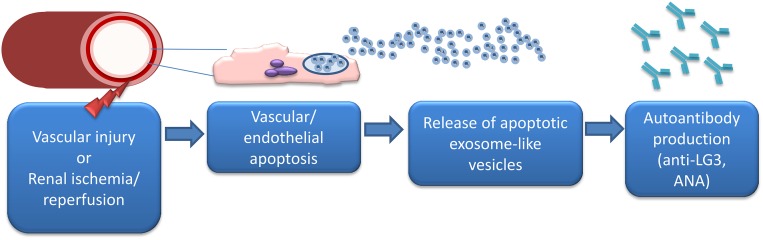
In further support of this hypothesis, we recently described a novel mechanism implicated in the production of anti-LG3 antibodies. We showed that, in endothelial and renal epithelial cells, activation of caspase-3, a cysteine protease central to the effector phase of apoptosis, leads to the release of small membrane–bound vesicles, called exosome–like apoptotic vesicles. These extracellular vesicles carry LG3 and an active 20S proteasome core complex.47 Immunization with exosome–like apoptotic vesicles triggers the production of anti-LG3 antibodies in naïve and transplanted mice.47 Exosome–like apoptotic vesicles induce aggravation of vascular rejection with complement deposition, increased T and B cell infiltration, and increased anti–LG3 antibodies without enhanced production of anti-HLA antibodies. Inhibition of proteasome activity specifically within exosome–like apoptotic vesicles reduces their capacity to trigger anti-LG3 production and aggravate vascular rejection, showing the key role for proteasome activity within these vesicles for controlling their immunogenicity and anti-LG3 production.47 In further support for an important role of vascular injury in triggering the production of exosome–like apoptotic vesicles that lead to anti-LG3 production, we showed that acute vascular injury in mice, in the form of hind limb ischemia or renal artery clamping, increases circulating levels of exosome-like vesicles followed by a surge of anti-LG3 production (Figure 3).47 Endothelial cells, vascular smooth muscle cells, and tubular epithelial cells all release proteasome-active vesicles when injured in vitro. These cell types are targets of injury during ischemia-reperfusion and could all be contributing to the release of immunogenic, apoptotic, exosome–like vesicles in vivo.47 However, additional studies are needed to assess whether episodes of vascular injury in patients awaiting transplantation also lead to the production of immunogenic, exososome–like apoptotic vesicles that can break tolerance to self and induce the production of autoantibodies. Various lines of evidence suggest that ischemia-reperfusion injury occurring either at the time of or before transplantation represents a clinical setting prone to autoantibody production. Indeed, production of autoreactive IgGs directed against kidney-specific antigens has been associated with renal ischemia-reperfusion injury occurring at the time of transplantation.48 The most abundant autoreactive IgGs were directed against antigens present in the renal pelvis, a zone that is particularly sensitive to ischemia-reperfusion injury. Collectively, these results also raise the intriguing possibility that at least some of these autoantibodies could potentially contribute to renal dysfunction and progressive renal loss in patients without transplants as well. Anti-LG3, AT1R-Abs, antivimentin, and antibodies reactive to apoptotic cells have all been described in patients awaiting transplantation. Considering the role of AKI as a powerful predictor of progressive renal failure in patients with native kidney disease,49–51 it remains to be determined whether episodes of ischemia-reperfusion injury in native kidneys can prompt the production of autoantibodies that, in turn, can accelerate renal failure.
Figure 3.
Vascular injury triggers the release of exosome–like apoptotic vesicles that prompt anti-LG3 production. Acute vascular injury in mice (hind limb ischemia or renal artery clamping) leads to increased circulating levels of exosome–like apoptotic vesicles containing an active 20S proteasome complex. Proteasome activity in exosome–like apoptotic vesicles prompts the production of anti-LG3 antibodies and antinuclear antibodies (ANAs).47 The mechanism underlying anti-LG3 production before transplantation in humans is unclear at this time. The role of acute vascular injuries (vascular access creation/manipulation and acute coronary and peripheral vascular events) in promoting anti-LG3 formation is currently being investigated.
Current and Possible Future Treatment and Prevention Strategies
Although clinical studies have yet to be performed, the various autoantibodies discussed above could serve as biomarkers to improve risk stratification for rejection or delayed graft function or as potential therapeutic targets. Pretransplant autoantibody levels could be added to the current clinical and laboratory variables used to assess the risk of rejection or delayed graft function, which in turn, could help transplant physicians select the most appropriate induction therapy. Current experimental data support the concept of a synergy between ischemia and autoantibodies in enhancing graft damage.24,39,41 If this is confirmed in larger cohort studies, pretransplant autoantibody titers could have implications in terms of organ allocation. For instance, an organ with expected long cold ischemic time or coming from a donor after cardiocirculatory arrest may not be best suited for patients with elevated pretransplant autoantibody titers. After transplantation, autoantibody titers could eventually serve as noninvasive biomarkers for the activity of antibody-mediated and/or vascular rejection.
Because in some patients, autoantibodies actively participate in accelerating graft damage, they could also serve as potential therapeutic targets. Plasmapheresis could be used pre- and/or post-transplant to decrease circulating levels, and intravenous Igs may also be beneficial to reduce their deleterious effect and their production. Angiotensin II receptor blockers represent an interesting therapeutic avenue in patients with AT1R-Ab–mediated rejection in combination with plasmapheresis.12,52 Finally, the recent observation that bortezomib can block the production of anti-LG3 autoantibodies triggered by exosome-like vesicles47 may prove useful to help define therapeutic options for preventing autoantibody production before transplantation.
Conclusion
For more than five decades, kidney transplant rejection has been considered the sole expression of donor-recipient MHC discrepancy that initiates allospecific immune responses. However, the notion needs revisiting in light of mounting evidence clearly delineating the contribution of autoimmune responses to the severity of allograft inflammation and damage. The production of autoantibodies in association with renal damage that may occur post-transplantation, at the time of rejection, at the time of transplantation, or before transplantation and in association with ischemia-reperfusion injury is emerging as an important contributor to the risk of renal dysfunction. A triad of alloimmunity, autoimmunity, and tissue injury can likely trigger the perfect storm for allograft dysfunction and reduced survival. Additional work will allow for better characterization of the molecular pathways that control contributing autoimmune responses and the role of these autoantibodies in promoting the severity of AKI in native kidneys as well as the identification of novel pharmacologic targets of intervention.
Disclosures
None.
Acknowledgments
We thank the J.-L. Levesque Foundation for renewed support.
This work was supported by grants from the Canadian Institutes of Health Research (MOP 15447 and MOP 123436; to H.C. and M.-J.H.) and the Kidney Foundation of Canada (to H.C. and M.-J.H.). H.C. is a research scholar of the Fonds de Recherche du Québec santé and a Kidney Research Scientist Core Education and National Training Program New Investigator. H.C. and M.-J.H. are Canadian National Transplant Research Program (CNTRP) investigators. M.D. is the scientific integration manager of the CNTRP. M.-J.H. is the holder of the Shire Chair in Nephrology, Transplantation and Renal Regeneration of l’Université de Montréal.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Laupacis A, Keown P, Pus N, Krueger H, Ferguson B, Wong C, Muirhead N: A study of the quality of life and cost-utility of renal transplantation. Kidney Int 50: 235–242, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, Stewart DE, Cherikh WS, Wainright JL, Snyder JJ, Israni AK, Kasiske BL: OPTN/SRTR 2012 annual data report: Kidney. Am J Transplant 14[Suppl 1]: 11–44, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Lefaucheur C, Loupy A, Vernerey D, Duong-Van-Huyen JP, Suberbielle C, Anglicheau D, Vérine J, Beuscart T, Nochy D, Bruneval P, Charron D, Delahousse M, Empana JP, Hill GS, Glotz D, Legendre C, Jouven X: Antibody-mediated vascular rejection of kidney allografts: A population-based study. Lancet 381: 313–319, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Witt CA, Gaut JP, Yusen RD, Byers DE, Iuppa JA, Bennett Bain K, Alexander Patterson G, Mohanakumar T, Trulock EP, Hachem RR: Acute antibody-mediated rejection after lung transplantation. J Heart Lung Transplant 32: 1034–1040, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan MA, Nicolls MR: Complement-mediated microvascular injury leads to chronic rejection. Adv Exp Med Biol 735: 233–246, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dragun D, Catar R, Philippe A: Non-HLA antibodies in solid organ transplantation: Recent concepts and clinical relevance. Curr Opin Organ Transplant 18: 430–435, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Amico P, Hönger G, Bielmann D, Lutz D, Garzoni D, Steiger J, Mihatsch MJ, Dragun D, Schaub S: Incidence and prediction of early antibody-mediated rejection due to non-human leukocyte antigen-antibodies. Transplantation 85: 1557–1563, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Martin L, Guignier F, Mousson C, Rageot D, Justrabo E, Rifle G: Detection of donor-specific anti-HLA antibodies with flow cytometry in eluates and sera from renal transplant recipients with chronic allograft nephropathy. Transplantation 76: 395–400, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, Carroll MC: Natural IgM-mediated innate autoimmunity: A new target for early intervention of ischemia-reperfusion injury. Expert Opin Biol Ther 7: 1575–1582, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Subramanian V, Ramachandran S, Banan B, Bharat A, Wang X, Benshoff N, Kreisel D, Gelman AE, Mohanakumar T: Immune response to tissue-restricted self-antigens induces airway inflammation and fibrosis following murine lung transplantation. Am J Transplant 14: 2359–2366, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dragun D, Müller DN, Bräsen JH, Fritsche L, Nieminen-Kelhä M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schönemann C, Unger T, Budde K, Neumayer HH, Luft FC, Wallukat G: Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med 352: 558–569, 2005 [DOI] [PubMed]
- 13.Gao B, Moore C, Porcheray F, Rong C, Abidoglu C, DeVito J, Paine R, Girouard TC, Saidman SL, Schoenfeld D, Levin B, Wong W, Elias N, Schuetz C, Rosales I, Fu Y, Zorn E: Pretransplant IgG reactivity to apoptotic cells correlates with late kidney allograft loss. Am J Transplant 14: 1581–1591, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klassen J, Milgrom FM, McCluskey RT: Studies of the antigens involved in an immunologic renal tubular lesion in rabbits. Am J Pathol 88: 135–144, 1977 [PMC free article] [PubMed] [Google Scholar]
- 15.Porcheray F, Fraser JW, Gao B, McColl A, DeVito J, Dargon I, Helou Y, Wong W, Girouard TC, Saidman SL, Colvin RB, Palmisano A, Maggiore U, Vaglio A, Smith RN, Zorn E: Polyreactive antibodies developing amidst humoral rejection of human kidney grafts bind apoptotic cells and activate complement. Am J Transplant 13: 2590–2600, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joosten SA, Sijpkens YW, van Ham V, Trouw LA, van der Vlag J, van den Heuvel B, van Kooten C, Paul LC: Antibody response against the glomerular basement membrane protein agrin in patients with transplant glomerulopathy. Am J Transplant 5: 383–393, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Rose ML: Role of anti-vimentin antibodies in allograft rejection. Hum Immunol 74: 1459–1462, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter V, Shenton BK, Jaques B, Turner D, Talbot D, Gupta A, Chapman CE, Matthews CJ, Cavanagh G: Vimentin antibodies: A non-HLA antibody as a potential risk factor in renal transplantation. Transplant Proc 37: 654–657, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Carter V, Howell WM: Vimentin antibody production in transplant patients and immunomodulatory effects of vimentin in-vitro. Hum Immunol 74: 1463–1469, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Raymond MA, Désormeaux A, Laplante P, Vigneault N, Filep JG, Landry K, Pshezhetsky AV, Hébert MJ: Apoptosis of endothelial cells triggers a caspase-dependent anti-apoptotic paracrine loop active on VSMC. FASEB J 18: 705–707, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Laplante P, Raymond MA, Gagnon G, Vigneault N, Sasseville AM, Langelier Y, Bernard M, Raymond Y, Hébert MJ: Novel fibrogenic pathways are activated in response to endothelial apoptosis: Implications in the pathophysiology of systemic sclerosis. J Immunol 174: 5740–5749, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Ishii Y, Sawada T, Kubota K, Fuchinoue S, Teraoka S, Shimizu A: Injury and progressive loss of peritubular capillaries in the development of chronic allograft nephropathy. Kidney Int 67: 321–332, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Soulez M, Pilon EA, Dieudé M, Cardinal H, Brassard N, Qi S, Wu SJ, Durocher Y, Madore F, Perreault C, Hébert MJ: The perlecan fragment LG3 is a novel regulator of obliterative remodeling associated with allograft vascular rejection. Circ Res 110: 94–104, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Cardinal H, Dieudé M, Brassard N, Qi S, Patey N, Soulez M, Beillevaire D, Echeverry F, Daniel C, Durocher Y, Madore F, Hébert MJ: Antiperlecan antibodies are novel accelerators of immune-mediated vascular injury. Am J Transplant 13: 861–874, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Angaswamy N, Klein C, Tiriveedhi V, Gaut J, Anwar S, Rossi A, Phelan D, Wellen JR, Shenoy S, Chapman WC, Mohanakumar T: Immune responses to collagen-IV and fibronectin in renal transplant recipients with transplant glomerulopathy. Am J Transplant 14: 685–693, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Jurcevic S, Ainsworth ME, Pomerance A, Smith JD, Robinson DR, Dunn MJ, Yacoub MH, Rose ML: Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation 71: 886–892, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Mahesh B, Leong HS, McCormack A, Sarathchandra P, Holder A, Rose ML: Autoantibodies to vimentin cause accelerated rejection of cardiac allografts. Am J Pathol 170: 1415–1427, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bharat A, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, Meyers BF, Mohanakumar T: Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg 90: 1094–1101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker MO, Kill A, Kutsche M, Guenther J, Rose A, Tabeling C, Witzenrath M, Kühl AA, Heidecke H, Ghofrani HA, Tiede H, Schermuly RT, Nickel N, Hoeper MM, Lukitsch I, Gollasch M, Kuebler WM, Bock S, Burmester GR, Dragun D, Riemekasten G: Vascular receptor autoantibodies in pulmonary arterial hypertension associated with systemic sclerosis. Am J Respir Crit Care Med 190: 808–817, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Kill A, Tabeling C, Undeutsch R, Kühl AA, Günther J, Radic M, Becker MO, Heidecke H, Worm M, Witzenrath M, Burmester GR, Dragun D, Riemekasten G: Autoantibodies to angiotensin and endothelin receptors in systemic sclerosis induce cellular and systemic events associated with disease pathogenesis. Arthritis Res Ther 16: R29, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong J, Liang Y, Yang H, Zhu F, Wang Y: The role of angiotensin II type 1 receptor-activating antibodies in patients with lupus nephritis. Int J Clin Pract 67: 1066–1067, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Thway TM, Shlykov SG, Day MC, Sanborn BM, Gilstrap LC III , Xia Y, Kellems RE: Antibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activation. Circulation 110: 1612–1619, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Hönger G, Cardinal H, Dieudé M, Buser A, Hösli I, Dragun D, Hébert MJ, Schaub S: Human pregnancy and generation of anti-angiotensin receptor and anti-perlecan antibodies. Transpl Int 27: 467–474, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Zhang M, Alicot EM, Carroll MC: Human natural IgM can induce ischemia/reperfusion injury in a murine intestinal model. Mol Immunol 45: 4036–4039, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, Kessler B, Shimaoka M, Chan R, Friend D, Mahmood U, Weissleder R, Moore FD, Carroll MC: Identification of the target self-antigens in reperfusion injury. J Exp Med 203: 141–152, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M, Austen WG Jr. , Chiu I, Alicot EM, Hung R, Ma M, Verna N, Xu M, Hechtman HB, Moore FD Jr. , Carroll MC: Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A 101: 3886–3891, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan RK, Verna N, Afnan J, Zhang M, Ibrahim S, Carroll MC, Moore FD Jr. : Attenuation of skeletal muscle reperfusion injury with intravenous 12 amino acid peptides that bind to pathogenic IgM. Surgery 139: 236–243, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Haas MS, Alicot EM, Schuerpf F, Chiu I, Li J, Moore FD, Carroll MC: Blockade of self-reactive IgM significantly reduces injury in a murine model of acute myocardial infarction. Cardiovasc Res 87: 618–627, 2010 [DOI] [PMC free article] [PubMed]
- 39.Yang B, Dieudé M, Hamelin K, Hénault-Rondeau M, Patey N, Turgeon J, Lan S, Pomerleau L, Quesnel M, Peng J, Tremblay J, Shi Y, Chan JS, Hébert MJ, Cardinal H: Anti-lG3 antibodies aggravate renal ischemia reperfusion injury and long-term renal allograft dysfunction [published online ahead of print May 12, 2016]. Am J Transplant doi: 10.1111/ajt.13866 [DOI] [PubMed] [Google Scholar]
- 40.Haase M, Bellomo R, Albert C, Vanpoucke G, Thomas G, Laroy W, Verleysen K, Kropf S, Kuppe H, Hetzer R, Haase-Fielitz A: The identification of three novel biomarkers of major adverse kidney events. Biomarkers Med 8: 1207–1217, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Lukitsch I, Kehr J, Chaykovska L, Wallukat G, Nieminen-Kelhä M, Batuman V, Dragun D, Gollasch M: Renal ischemia and transplantation predispose to vascular constriction mediated by angiotensin II type 1 receptor-activating antibodies. Transplantation 94: 8–13, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Tarlinton DM, McLean M, Nossal GJ: B1 and B2 cells differ in their potential to switch immunoglobulin isotype. Eur J Immunol 25: 3388–3393, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Nagele EP, Han M, Acharya NK, DeMarshall C, Kosciuk MC, Nagele RG: Natural IgG autoantibodies are abundant and ubiquitous in human sera, and their number is influenced by age, gender, and disease. PLoS One 8: e60726, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avrameas S, Selmi C: Natural autoantibodies in the physiology and pathophysiology of the immune system. J Autoimmun 41: 46–49, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Bäckhed F, Miller YI, Hörkkö S, Corr M, Witztum JL, Binder CJ: Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest 119: 1335–1349, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pilon EA, Dieudé M, Qi S, Hamelin K, Pomerleau L, Beillevaire D, Durocher Y, Zutter M, Coutu D, Perreault C, Hébert MJ: The perlecan fragment LG3 regulates homing of mesenchymal stem cells and neointima formation during vascular rejection. Am J Transplant 15: 1205–1218, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Dieudé M, Bell C, Turgeon J, Beillevaire D, Pomerleau L, Yang B, Hamelin K, Qi S, Pallet N, Béland C, Dhahri W, Cailhier JF, Rousseau M, Duchez AC, Lévesque T, Lau A, Rondeau C, Gingras D, Muruve D, Rivard A, Cardinal H, Perreault C, Desjardins M, Boilard É, Thibault P, Hébert MJ: The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci Transl Med 7: 318ra200, 2015 [DOI] [PubMed] [Google Scholar]
- 48.Li L, Wadia P, Chen R, Kambham N, Naesens M, Sigdel TK, Miklos DB, Sarwal MM, Butte AJ: Identifying compartment-specific non-HLA targets after renal transplantation by integrating transcriptome and “antibodyome” measures. Proc Natl Acad Sci U S A 106: 4148–4153, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basile DP, Donohoe D, Roethe K, Osborn JL: Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Basile DP, Friedrich JL, Spahic J, Knipe N, Mang H, Leonard EC, Changizi-Ashtiyani S, Bacallao RL, Molitoris BA, Sutton TA: Impaired endothelial proliferation and mesenchymal transition contribute to vascular rarefaction following acute kidney injury. Am J Physiol Renal Physiol 300: F721–F733, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molitoris BA: Therapeutic translation in acute kidney injury: The epithelial/endothelial axis. J Clin Invest 124: 2355–2363, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jobert A, Rao N, Deayton S, Bennett GD, Brealey J, Nolan J, Carroll RP, Dragun D, Coates PT: Angiotensin II type 1 receptor antibody precipitating acute vascular rejection in kidney transplantation. Nephrology (Carlton) 20[Suppl 1]: 10–12, 2015 [DOI] [PubMed] [Google Scholar]