Abstract
Primary membranous nephropathy (MN) is an autoimmune disease mainly caused by autoantibodies against the recently discovered podocyte antigens: the M-type phospholipase A2 receptor 1 (PLA2R) and thrombospondin type 1 domain-containing 7A (THSD7A). Assays for quantitative assessment of anti-PLA2R antibodies are commercially available, but a semiquantitative test to detect anti-THSD7A antibodies has been only recently developed. The presence or absence of anti-PLA2R and anti-THSD7A antibodies adds important information to clinical and immunopathologic data in discriminating between primary and secondary MN. Levels of anti-PLA2R antibodies and possibly, anti-THSD7A antibodies tightly correlate with disease activity. Low baseline and decreasing anti-PLA2R antibody levels strongly predict spontaneous remission, thus favoring conservative therapy. Conversely, high baseline or increasing anti-PLA2R antibody levels associate with nephrotic syndrome and progressive loss of kidney function, thereby encouraging prompt initiation of immunosuppressive therapy. Serum anti-PLA2R antibody profiles reliably predict response to therapy, and levels at completion of therapy may forecast long-term outcome. Re-emergence of or increase in antibody titers precedes a clinical relapse. Persistence or reappearance of anti-PLA2R antibodies after kidney transplant predicts development of recurrent disease. We propose that an individualized serology-based approach to MN, used to complement and refine the traditional proteinuria-driven approach, will improve the outcome in this disease.
Keywords: membranous nephropathy, clinical nephrology, Immunology and pathology, PLA2R, THSD7A
Membranous nephropathy (MN) is a morphologic pattern of injury characterized by thickening of the glomerular capillary wall because of subepithelial deposition of immune complexes and complement components and attendant new basement membrane synthesis (Figure 1). MN is caused by an array of conditions with differing etiologies and pathogeneses.
Figure 1.
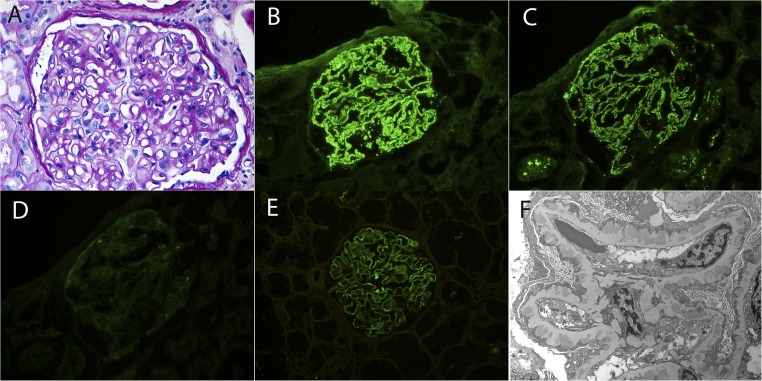
Primary PLA2R–associated MN. (A) Light microscopy shows thickened GBMs without mesangial or endocapillary hypercellularity (periodic acid-Schiff stain). Original magnification, ×40. (B–E) Immunofluorescence microscopy reveals granular staining for (B) IgG, (C) C3, and (E) PLA2R, with negative staining for (D) C1q. Original magnification, ×40. (F) Electron microscopy shows subepithelial deposits separated from each other by basement membrane spikes. No subendothelial or mesangial electron dense deposits are present. Tubuloreticular structures are absent in endothelial cells. Original magnification, ×4800.
Primary MN, responsible for approximately 80% of cases, is an organ-specific autoimmune disease, in which circulating autoantibodies bind to an autoantigen on the surface of the podocytes. Two major target antigens are now firmly recognized: the M-type phospholipase A2 receptor 1 (PLA2R)1 and the thrombospondin type 1 domain-containing 7A (THSD7A).2,3 Together, PLA2R-associated MN and THSD7A-associated MN account for the large majority (about 80% or more) of primary MN. Although the presence of anti-PLA2R antibodies (Abs) and anti-THSD7A Abs was initially considered to be mutually exclusive, patients with dual positivity have recently been described.4 The remaining cases of primary MN may either be caused by autoantibodies against as yet unidentified intrinsic antigens or reflect misclassified PLA2R-associated MN, THSD7A-associated MN, or secondary MN. Thus, the term idiopathic MN may no longer be appropriate.
In approximately 20% of cases in adults, the MN lesion is secondary to various disorders, including infection (hepatitis B), systemic disease (SLE and sarcoidosis), drugs (nonsteroidal anti-inflammatory drugs), thyroiditis, and malignancy. Foreign antigens may travel through the glomerular basement membrane (GBM), become planted under the podocytes, and subsequently, be bound by circulating Abs.5 Alternatively, circulating immune complexes may dissociate and reform in the subepithelial space.6,7
Traditionally, the differential diagnosis and management of MN is on the basis of the integration of clinical and biopsy findings.8 In this article, we will explore how the discovery of the target podocyte antigens and the development of commercial assays for PLA2R and THSD7A Abs may guide diagnosis and management and complement traditional algorithms.9 The following questions will be addressed. (1) Can PLA2R and THSD7A Abs be used to diagnose MN and thereby, avoid the need for a kidney biopsy? (2) Can PLA2R and THSD7A Abs adequately discriminate between primary and secondary MN and thus, obviate the need to search for the presence of a secondary underlying condition? (3) Can quantification of PLA2R and THSD7A Abs guide prognostication and the decision to start, tailor, or withdraw immunosuppressive treatment for primary MN? (4) Can PLA2R and THSD7A Ab levels aid the prediction, detection, and treatment of post-transplant recurrence? Evidence for the clinical usefulness of determining plasma PLA2R Ab levels has mushroomed over the past 2 years and will be the main focus of this review. Because the discovery of THSD7A was more recent, only limited data address the role of THSD7A Ab in diagnosis, prognosis, and management of MN. For each question, we will present the available evidence, followed by an opinion-based proposal to integrate this evidence in the clinical approach to the patient.
Autoantibody Assays and Antigen Staining
Western blotting, as used in the seminal studies revealing the PLA2R Ab1 and THSD7A Ab,2 is unsuitable for routine clinical use. The first commercially available assay for PLA2R Ab was a cell-based assay using indirect immunofluorescence (CBA-IFA; Euroimmun, Luebeck, Germany). Its semiquantitative nature makes it less suitable for monitoring disease progression and therapeutic response. A subsequently developed ELISA (Euroimmun) is not as sensitive as Western blotting and CBA-IFA but quantitates plasma levels of PLA2R Ab and is currently used routinely in many clinical laboratories. The most recent diagnostic acquisition is a laser bead immunoassay (ALBIA; Mitogen Advanced Diagnostics Laboratory, Calgary, Canada), providing a sensitive and quantitative assay of PLA2R Ab. In addition, the technology is designed to simultaneously measure multiple targets in a single sample and thus, can probe for the presence of other conditions (SLE, ANCA-associated vasculitis, anti-GBM disease, etc.).10
In clinical practice, the ELISA test is the most suitable for follow-up PLA2R Ab measurements, whereas the CBA-IFA is a more sensitive technique for the detection of very low PLA2R Ab levels, in the diagnosis of PLA2R-associated MN, when low PLA2R Ab levels are suspected, or when the ELISA is not conclusive. The manufacturer’s definition for PLA2R Ab positivity of the commercial ELISA assay is >14 RU/ml. However, it has been suggested that any titer >2 RU/ml may be considered positive; this threshold improves sensitivity but will obviously lead to false positives.11 A semiquantitative indirect immunofluorescence test to detect THSD7A Ab was recently developed12 but is not yet commercially available.
PLA2R-associated MN and THSD7A-associated MN may also be diagnosed by showing the antigen in the immune deposits, using immunofluorescence or immunoperoxidase methods on pronase-digested sections of paraffin-embedded biopsy material, and using a polyclonal anti-PLA2R or THSD7A Ab. Most patients with primary MN and positive PLA2R Ab have an intense granular PLA2R staining along the glomerular capillary walls (Figure 1E). This pattern of staining has been called enhanced PLA2R staining.13 In normal kidneys or other glomerular diseases, the antigen is only weakly detectable on the podocyte surface. A negative PLA2R staining shows no staining or only a minimal blush representing the intrinsic PLA2R staining of the epithelial cells (Figure 2). Generally, a strong association between glomerular PLA2R staining and circulating PLA2R Ab is found,14–16 in particular when the Ab levels are measured in close proximity to the time of biopsy.13 Likewise, granular staining for THSD7A is enhanced in biopsies of patients with MN and positive THSD7A Ab.2,4,12 However, its distinction from the linear THSD7A staining pattern in normal kidneys or other glomerular diseases requires an experienced renal pathologist.
Figure 2.
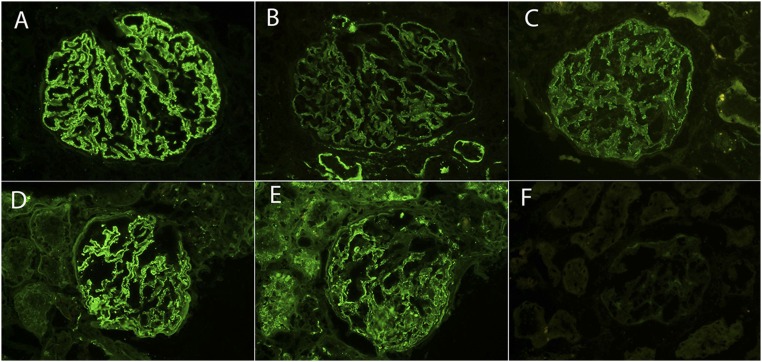
PLA2R staining. A–C show a biopsy of MN with positive PLA2R staining, and D–F show a biopsy of MN with negative PLA2R staining. (A and D) IgG, (B and E) C3, and (C and F) PLA2R. Original magnification, ×40.
Serology-Based Diagnosis
Traditional Approach
The kidney biopsy is the gold standard in recognizing the pattern of injury lesion of MN. However, standard light, immunofluorescence, and electron microscopic examination may not be sufficient to establish the true nature of the disorder. The main challenge in the diagnosis of MN is the differentiation between primary and secondary MN, in particular malignancy-associated MN in elderly patients. The concurrence of MN and secondary disease does not necessarily prove causality but may be coincidental. Conversely, the absence of a secondary cause at diagnosis does not rule out its development later in the disease course. It is current common practice to exclude secondary causes of the lesion of MN on the basis of a detailed medical history, physical examination, laboratory analysis, and imaging. There is no consensus on how aggressive the search should be, especially as regards an occult malignancy. A careful evaluation of the immunopathologic characteristics of the biopsy specimen may provide additional discriminative information.17 Subepithelial, intramembranous, and mesangial deposits suggest secondary MN (Figure 3); an exclusive subepithelial location of the deposits is more typical for primary MN (Figure 1). C1q deposition is rarely seen in primary MN but may be seen in other secondary causes, particularly in SLE. IgG subclass staining may further help to classify MN. IgG1, IgG2, and IgG3 generally dominate in the deposits of secondary MN, whereas a preponderance of IgG4 is characteristic for primary MN, reflecting the fact that PLA2R Ab and THSD7A Ab are mainly of the IgG4 subclass.
Figure 3.
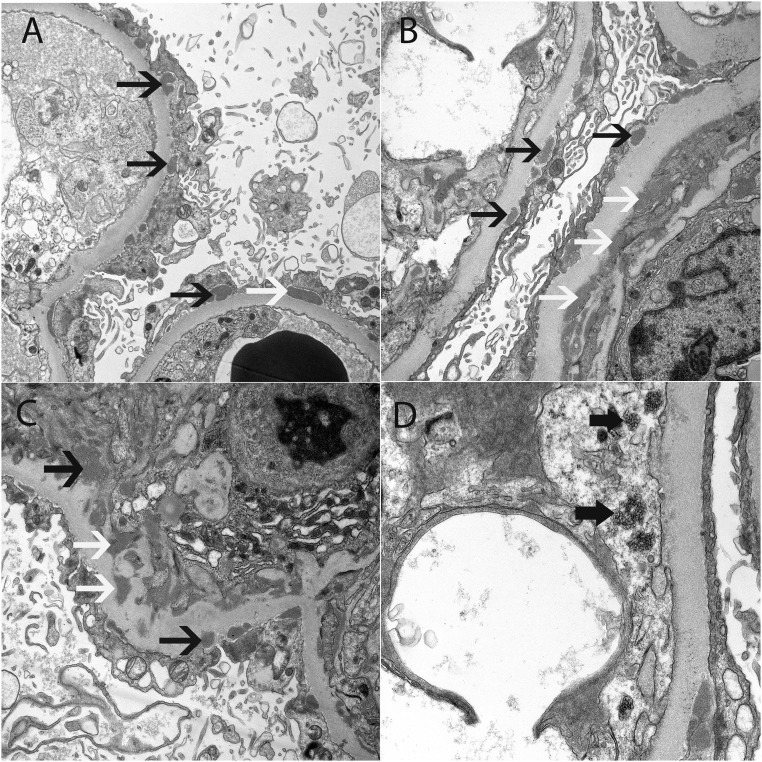
Early secondary MN due to an autoimmune disease. Electron microscopy shows (A) subepithelial deposits along the capillary walls, (B) subepithelial and subendothelial deposits along the capillary walls, (C) subepithelial and mesangial electron dense deposits, and (D) tubuloreticular inclusions in the endothelial cells. Thin black arrows point to subepithelial deposits, white arrows point to subendothelial deposits in B and mesangial deposits in C, and thick black arrows point to tubuloreticular inclusions. Original magnification, ×6800 in A; ×9300 in B; ×9300 in C; ×18,500.
Diagnostic Value of PLA2R Ab
The specificity of PLA2R Ab for the lesion of MN nears 100%.18 PLA2R Abs have not been detected in either patients with other kidney or systemic diseases or healthy individuals. This has led to the suggestion that a kidney biopsy in patients with positive PLA2R Ab may not be required, especially when there are relative contraindications to perform a biopsy.17 Approximately 50%–80% of patients with primary MN test positive for PLA2R Ab.19 The large variability among published studies reflects, in part, the type of test used (Western blot, CBA-IFA, and ALBIA are more sensitive than ELISA) or the ethnic origin of the study population (the prevalence of PLA2R Ab is lower in Japanese cohorts). However, the most influential contributor to the observed variability is the timing of measurement in relation to the disease course. There are three scenarios accounting for the inability to detect PLA2R Ab in patients with primary MN. In the first scenario, serum samples may be collected when the patient has already entered immunologic remission either spontaneously or by virtue of immunosuppressive therapy. Proteinuria may persist because of the time lag between immunologic and clinical remission or because of irreversible podocyte damage in the absence of active disease.20 In these patients, PLA2R Ab may be low or undetectable, but glomerular PLA2R staining may serve as a footprint of PLA2R-associated MN. As an example, only 22% of serum samples obtained long after the histologic diagnosis were PLA2R Ab positive, whereas 59% of the corresponding biopsies were positive on PLA2R staining.15 In the second scenario, serum PLA2R Ab may be falsely negative early in the disease course, and seroconversion may occur later on.21,22 This phenomenon has been explained by “kidney as a sink”21: PLA2R Abs enter the circulation, bind to the target antigens on the podocytes, and are rapidly cleared from the blood. Only when the rate of production exceeds the buffering capacity of the kidney do patients exhibit seropositivity. Serial assessment of PLA2R Ab for at least 3 months should, therefore, be performed in patients with positive glomerular PLA2R staining who are initially seronegative but have persistent disease activity. These two mechanisms explain why up to 30% of patients with PLA2R-associated MN may be seronegative; they can be properly categorized by performing a PLA2R staining on the kidney biopsy.14,15,22 The inverse (i.e., positive PLA2R Ab and negative glomerular PLA2R staining) is distinctly uncommon14,15,22 and in all likelihood, reflects a technical artifact. A third scenario is when a non-PLA2R Ab mechanism (THSD7A or other not yet defined Ab) is involved.
A clinically highly relevant question is whether a positive PLA2R serology reliably excludes secondary MN and obviates further diagnostic workup. No patients with positive PLA2R Ab1,23–26 or glomerular PLA2R13 were reported in a number of studies of well defined groups of secondary MN. In particular, class 5 SLE seems to be almost uniformly PLA2R negative, consistent with the pathophysiology of immune complex deposition in SLE. Others studies found small numbers of positive patients in various groups of secondary MN.15,27–33 The question thus arises whether these patients represent true secondary MN or rather, PLA2R-associated MN with coincident secondary disease. The latter possibility is supported by the following findings in such studies. First, in some patients, the glomerular histologic features of secondary MN were absent, and the predominant IgG subclass was IgG4.27,30,32 Second, patients are described who entered remission without treatment of the secondary disease,32 and conversely, other patients exhibited persistent or recurrent proteinuria, despite successful treatment of the secondary disease.27 However, in certain subgroups of secondary MN, positive glomerular PLA2R staining has been observed in a substantial number of patients. In a study of 39 patients with hepatitis B-related MN, 25 (64%) had a positive PLA2R staining, overlapping with hepatitis B antigen deposits.31 Furthermore, the immunopathologic characteristics were typical for secondary MN. Similarly high proportions of positive PLA2R staining were seen in hepatitis C (seven of 11; 64%)28 and active sarcoidosis (three of four; 75%28 and five of nine; 55%34), thus suggesting that these disorders may induce an immune response to PLA2R.
Diagnostic Value of THSD7A Ab
Thus far, the THSD7A Ab has not been detected in healthy controls or patients with other renal and systemic diseases,2,32 yielding a 100% specificity for the lesion of MN. The percentages of primary MN that are THSD7A associated range from 3% in Europe and the United States to 9% in Japan (Table 1). Notably, in a large number of patients with THSD7A-associated MN (eight of 38; 21%), a malignant tumor was detected within a median time of 3 months from diagnosis of MN.12,35 Analysis of endometrial carcinoma12 and gallbladder carcinoma35 cells revealed elevated THSD7A protein expression. Remarkably, the initiation of chemotherapy resulted in a decline THSD7A Ab followed by a decrease in proteinuria.35 These findings have led to the thesis that the immune system may recognize tumor THSD7A as a foreign antigen leading to the production of THSD7A Ab, the latter then binding to podocyte THSD7A in situ. If confirmed, the concept of primary versus secondary MN may require revision.
Table 1.
Prevalence of THSD7A-associated MN in different cohorts
| Ref. | Origin | Positive THSD7A Ab | Positive THSD7A Staining |
|---|---|---|---|
| Tomas et al.2 | Europe/Boston | 15 of 483 Prevalent primary MN (3.1%), two of 67 prevalent secondary MN (3.0%) | Five of five THSD7A Ab positive, zero of 50 THSD7A Ab negative |
| Iwakura et al.32 | Japan | Five of 55 incident primary MN (9.1%), zero of 37 incident secondary MN (0%) | |
| Hoxha et al.12 | Hamburg/Boston | Nine of 345 incident primary MN (2.6%), 29 of 931 prevalent primary MN (3.1%) | 18 of 18 THSD7A Ab positive, one THSD7A Ab negative |
| Larsen et al.4 | United States | Nine of 258 incident MNa (3.5%; two of nine dual positive for THSD7A/PLA2R) |
All native kidney biopsies with MN, except membranous lupus nephritis (International Society of Nephrology/Renal Pathological Society class 5).
It is presently unclear whether patients with dual PLA2R and THSD7A Ab positivity, as recently described,4 behave phenotypically as PLA2R-associated MN, THSD7A-associated MN, or an entirely distinct form of MN.
A Proposed Diagnostic Algorithm
In patients in whom MN is suspected, we propose a serology-based diagnostic approach (Figure 4). When PLA2R Abs are detected in plasma and there is no evidence for secondary disease, one may consider to not perform a kidney biopsy, provided that patients have normal or only mildly decreased kidney function. In patients with impaired kidney function, a biopsy may aid in excluding a crescentic form of MN36 or superimposed disease and assessing the degree of chronic damage.
Figure 4.
Diagnostic algorithm. The diagnostic evaluation starts with a quantification of PLA2R Ab (ELISA; potentially superseded by ALBIA in the near future) and a screening for secondary disease, including antinuclear Abs, viral hepatitis serology, thyroiditis autoimmunity, chest x-ray (or computed tomography in patients at high risk for malignancy), and an evaluation for sarcoidosis in selected patients. The presence of PLA2R Ab and the absence of evidence for secondary disease confirm the diagnosis of a PLA2R Ab–associated MN, even without additional pathologic evidence. In patients with negative PLA2R Ab, staining for the PLA2R antigen on the biopsy identifies additional patients as PLA2R-associated MN. When both PLA2R Ab and antigen are negative, traditional immunopathologic characteristics, as delineated above, along with measurement of THSD7A Ab should be used to categorize MN as either primary or secondary. Patients without clear evidence of secondary disease but with immunopathologic characteristics suggestive of secondary MN may have an occult neoplasm and should be comprehensively screened for cancer. In view of the high prevalence of malignancy in THSD7A-associated MN, these patients should also undergo a thorough evaluation for occult malignancy. This algorithm will likely change with the availability of new information. Recent data12 indicate that a substantial proportion of THSD7A-associated MN was IgG4 negative on biopsy, irrespective of whether a malignancy was present, suggesting that all patients who are PLA2R Ab/antigen negative, independent of the dominant IgG subtype in their renal biopsy, may need to be tested for THSD7A. We recognize that the diagnostic tools in this algorithm, in particular the measurement of THSD7A Ab and the specialized immunopathology studies, may not be widely or routinely available at present.
Serology-Based Assessment of Prognosis
Traditional Approach
The natural course of MN is unpredictable. A sizeable proportion (20%–30%) of patients, even those with nephrotic syndrome at presentation, achieves spontaneous long-term remission. Others have chronic, persistent low-grade proteinuria and preserved renal function. Both groups do not require immunosuppression (IS). A third group consists of patients with persistent high-grade proteinuria at high risk for progression to ESRD. Clinical predictors of poor renal outcome are persistent high-grade proteinuria (>8 g/24 h) over the course of 6 months, rapid rate of decline of renal function over this 6-month interval, and elevated serum creatinine at the time of diagnosis.37 Scientific guidelines recommend initiation of IS in such patients.8 However, the prolonged observation period required to assess the risk of progression may either delay treatment beyond the point at which such treatment may prove effective or incur significant residual kidney damage. Conversely, patients may be judged to be at high risk and exposed to potentially toxic IS but still develop a spontaneous remission.38 The intrinsic weakness of the “wait and see” clinical approach is that indices such as proteinuria and serum creatinine may not promptly or reliably reflect disease activity in MN for the following reasons: (1) a prolonged delay (extending over several months) may exist between changes in immunologic and clinical activity, and (2) proteinuria and elevated serum creatinine do not discriminate between immunologically active disease and irreversible structural damage to podocytes and the basement membrane.
Prognostic Value of PLA2R Ab
Although some studies found an association between the degree of proteinuria and PLA2R Ab titer at a defined time point,30,33,39,40 others found only a weak or no association.13,22,41 Such variability in association likely reflects the time lag between immunologic and clinical activity, and indeed, a latency period as long as 8 months has been observed between the presence of PLA2R Ab in serum and the first clinical manifestations of MN.42 Low baseline PLA2R Ab levels predict subsequent spontaneous remission,40,43 whereas high baseline PLA2R Ab levels are associated with development of nephrotic syndrome in patients with initial non-nephrotic proteinuria44 and with progressive loss of kidney function.44–46 Serial measurements of PLA2R Ab levels provide even better prognostic information. Decreasing levels strongly predict remission of proteinuria.39,47,48 A recent study suggested that PLA2R Ab epitope-specific assays with analysis of the epitope profile and intramolecular epitope spreading may be developed and refined as a prognostic test.49 Serum Ab activity restricted to the cysteine-rich epitope of the PLA2R molecule is associated with a high rate of spontaneous remission, whereas epitope spreading during follow-up associates with worsening of the disease.49 The prognostic utility of PLA2R Ab assays will most likely improve as epitope-specific assays are developed for clinical use, akin to what occurred in anti-GBM disease.
Prognostic Value of THSD7A Ab
Limited data suggest that THSD7A Ab levels are associated with disease activity in THSD7A-associated MN,2,12 but confirmation in larger cohorts with serial Ab measurements is required.
A Proposed Prognostic Algorithm
In patients with PLA2R-associated MN, we propose a serology-based assessment of prognosis (Figure 5). The fate of patients who are Ab negative is more controversial, likely reflecting the heterogeneous nature of this subgroup, as detailed in the previous section. A high proportion of patients who are PLA2R and THSD7A Ab negative develops spontaneous remission,50 thereby avoiding the need for IS: delaying such therapy may thus be warranted.
Figure 5.
Prognostic algorithm. Although the temporal relationship between PLA2R Ab levels and disease activity is well established, a time lag of several months between immunologic and clinical response should be taken into account. Measurement of PLA2R Ab may obviate the need for a “wait and see” period of 6 months, as recommended by the Kidney Disease Improving Global Outcomes guidelines, and allow more rapid treatment decisions. Serial PLA2R Ab measurements may guide the decision to start IS. Patients with initial high PLA2R Ab titers should be followed monthly, whereas those with moderate to low PLA2R Abs should be re-evaluated bimonthly. This recommendation does not apply to patients with rapidly declining renal function, in whom prompt initiation of IS may be warranted. PLA2R Ab ELISA (Euroimmun): low =14–86 U/ml; moderate =87–204 U/ml; and high ≥204 U/ml.53 SCreat, serum creatinine.
Serology-Based Monitoring of Treatment
Traditional Approach
A comprehensive discussion of IS regimens for MN51 is beyond the scope of this perspective. Generally, patients are given a specified course of IS for a fixed period of time, regardless of the response to treatment. Response to therapy is traditionally assessed using standard laboratory parameters (Table 2) and highly unpredictable. If a patient responds to IS, a drop in proteinuria usually occurs within a few months. However, complete or partial remission may be delayed for up to 18 months or longer after completion of IS. The latter course is characteristic of extensive immune damage that requires prolonged podocyte remodeling to restore the architecture and function of the glomerular filtration barrier. Certain patients may not respond to the initial type of IS therapy but may respond to an alternative IS regimen. More than 20% of patients treated with various IS regimens and followed for 10 years never achieve remission, with 75% of these patients progressing to ESRD.52 One third of patients who respond to IS eventually relapse, which unfavorably affects long-term prognosis.52 Notably, none of the clinical variables at presentation predict a relapse.52 In addition, refractory or relapsing immune injury is difficult to distinguish clinically from incomplete filtration barrier repair or the development of glomerulosclerosis. A repeat kidney biopsy may be helpful in deciding whether to restart IS because of the presence of fresh immune deposits or conversely, eschew IS because of widespread chronic and irreversible histologic damage.
Table 2.
Traditional assessment of treatment response
| Complete remission | Proteinuria <0.3 g/24 h |
| Partial remission | Proteinuria >50% reduction from baseline and ≥0.3 and <3.5 g/24 h |
| No remission | Proteinuria <50% decrease from baseline level or ≥3.5 g/24 h |
| Relapse | Recurrence of proteinuria ≥3.5 g/24 h |
| ESRD | eGFR<15 ml/min per 1.73 m2 or requirement of RRT |
Therapeutic Monitoring with PLA2R Ab
Whether baseline PLA2R Ab titer predicts the response to IS is controversial. Patients with low PLA2R Ab before the start of IS have a higher probability of remission and attain remission of proteinuria sooner than patients with high PLA2R Ab, as observed in some studies22,44,53 but not in others.54,55 In contrast, the plasma profiles of PLA2R Ab reliably predict outcome. A decline in PLA2R Ab levels consistently heralds a decline in proteinuria.22,33,44,47,53–56 Although the rate of Ab reduction varies among studies, the general pattern that emerges is that Abs decrease dramatically in the first 3 months of treatment and disappear over 6–9 months followed by a remission of proteinuria over 12–24 months. Importantly, PLA2R Ab levels at the completion of therapy may forecast the nature of the long-term outcome.54 Patients with undetectable levels after therapy have a high likelihood of remaining in remission for 5 years.54 Conversely, patients with <50% reduction in Ab titers are likely to have resistant disease. None of the patients with positive Ab after completion of treatment remained in remission at 2 years.54 Re-emergence of or an increase in Ab titers precedes a clinical relapse by approximately 3 months.53
In patients with similar baseline clinical characteristics and Ab levels, the relative Ab reduction was greater in those treated with the combination of tacrolimus and rituximab compared with those treated with cyclophosphamide,55 and a greater reduction was also observed in patients treated with cyclophosphamide compared with those treated with mycophenolate mofetil.54 In contrast, in other studies, the decline of Ab levels was not affected by the type of IS (alkylating agents, calcineurin inhibitors, and different dosing regimens of rituximab).44,47,56
The recognition that patients with therapy-resistant disease have high Ab levels may engender interest in plasma exchange as a rescue therapy in these patients, as recently supported in a pilot study.57
A Proposed Therapeutic Algorithm
In subjects with detectable PLA2R Ab, serial measurement of Ab titer serves as a guide to IS therapy (Figure 6).
Figure 6.
Therapeutic algorithm. Patients with a prompt and robust immunologic response may receive shorter than usual courses of IS. It is important to recognize that, for not only rituximab67 but also, cyclophosphamide,68 the stoppage of such therapies leaves sustained effects on B cell depletion for a variable period of time. A conversion to an alternative therapy should be considered in those who do not show a significant reduction in Ab titers. Patients with high baseline PLA2R Ab may require a longer duration of therapy to achieve immunologic remission and may need to be treated with more intensive and prolonged IS, possibly including plasma exchange.
Serology-Based Monitoring of Post-Transplant Recurrence
Traditional Approach
MN frequently recurs after kidney transplantation. In studies using systematic protocol biopsies, which enable diagnosis of MN at a subclinical stage, recurrence rates of up to 50% have been described.58,59 Some patients develop de novo MN after transplantation, with a histologic pattern indistinguishable from recurrent MN. Although the etiology of de novo MN is uncertain, it may reflect an alloimmune disease caused by donor-specific anti-HLA Abs.60,61
The risk of recurrence is highest during the first year after transplantation, with cases detected as early as 1 week after surgery.62 A second wave of recurrence occurs after 4–5 years, possibly due to tapering of transplant IS. About one third of patients with recurrent MN have no progression and do not require additional IS.58 The remainder exhibits advancing disease with a high risk of graft loss. In several recent series,58,62,63 treatment with rituximab achieved a high rate of clinical response, even attended by histologic resolution. Clinical parameters do not predict which patients will develop recurrent disease. In addition, histologic findings correlate poorly with clinical manifestations,58 possibly reflecting the superimposed effect of maintenance IS, and should not be used alone to guide therapy.
Recurrence Monitoring with PLA2R Ab
Although several case series reported that high PLA2R Ab titers at the time of transplantation strongly predicted subsequent recurrence,62–64 others found only a low positive predictive value.60,65 There are several possible explanations for the inconsistent recurrence in patients with positive PLA2R Ab at the time of transplantation. The induction therapy and transplant IS may be sufficient to induce immunologic remission. Furthermore, the PLA2R epitopes of the transplant may differ from those of the native kidney and may not be recognized by the Ab. Finally, a subclinical recurrence with low-grade proteinuria may be masked by treatment with angiotensin converting enzyme inhibitors or calcineurin inhibitors. Conversely, several series show a high recurrence rate in patients with negative PLA2R Ab at the time of transplantation.58,64 Obviously, some of these patients may have non-PLA2R–associated MN. Performing a PLA2R antigen staining on archived native biopsies addresses this possibility. Additionally, recurrences detected by protocol biopsies in the absence of overt proteinuria may reflect low levels of Ab that are below the threshold of detection by serologic tests.
In patients with confirmed PLA2R-associated MN, serial monitoring of the Ab titer is informative, because persistence or reappearance of PLA2R Ab is tightly associated with recurrent disease.62,64,65 Finally, PLA2R Ab staining and PLA2R antigen staining are useful in differentiating recurrent MN from de novo MN when the etiology of the native kidney disease is unknown, because they are invariably negative in de novo MN.60,61,66
Recurrence Monitoring with THSD7A Ab
Preliminary data suggest that, in patients with THSD7A-associated MN, high THSD7A Ab titers at the time of transplantation are associated with recurrent disease.3
A Proposed Post-Transplant Algorithm
MN in the native kidney should be categorized as PLA2R or non-PLA2R associated, if necessary by performing PLA2R antigen staining on archived native biopsies. In patients with PLA2R-associated MN and high pretransplant Ab levels, preemptive rituximab therapy before transplantation may be considered. In deceased donor transplantation with high Ab levels at the time of transplantation, aggressive induction therapy and more intense maintenance IS may be required. Serial PLA2R Ab levels (bimonthly in the first year after transplantation, yearly thereafter, and whenever proteinuria increases) may guide the decision to administer rituximab.
Conclusion
Traditionally, the approach to MN is on the basis of conventional indices of renal disease, namely proteinuria and serum creatinine. However, proteinuria does not always accurately reflect disease activity. A change in proteinuria typically lags several months behind a change in immunologic activity. Additionally, proteinuria may reflect irreversible damage to the glomerular filtration barrier in the absence of active disease. The discovery of PLA2R and THSD7A as target antigens provides new diagnostic tools that complement and refine existing ones. A growing body of evidence has documented, and convincingly so, that PLA2R Ab titer tightly correlates with disease activity. Because THSD7A was only recently discovered as a target antigen in MN, there is relatively limited evidence supporting the association of THSD7A Ab with disease activity. However, there is an emerging sense that the association is analogous to what is described for PLA2R Ab and disease activity.
A change from a proteinuria-based approach to a serology-based approach offers two major advantages. First, therapeutic decisions may be made more swiftly, because Ab levels provide a prompt and reliable readout on immunologic status. Second, therapeutic decisions can be made more accurately when proteinuria and Ab levels are discordant. Specifically, persistent proteinuria in the face of low Ab levels may be more easily identified as resulting from chronic damage, whereas high Ab levels in the absence of clinical symptoms may portend an impending relapse.
We propose that a serology-based individualized approach to MN will increase diagnostic and prognostic accuracy, limit unnecessary exposure to IS, optimize efficacy of treatment, and minimize the risk or severity of recurrent disease in allotransplants. A randomized, controlled trial comparing the serology-based with the traditional approach is required to verify that our proposals are valid, safe, effective, and applicable in clinical practice.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Beck LH Jr., Bonegio RG , Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomas NM, Beck LH Jr., Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay AS, Debayle D, Merchant M, Klein J, Salant DJ, Stahl RA, Lambeau G: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371: 2277–2287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomas NM, Hoxha E, Reinicke AT, Fester L, Helmchen U, Gerth J, Bachmann F, Budde K, Koch-Nolte F, Zahner G, Rune G, Lambeau G, Meyer-Schwesinger C, Stahl RA: Autoantibodies against thrombospondin type 1 domain-containing 7A induce membranous nephropathy. J Clin Invest 126: 2519–2532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larsen CP, Cossey LN, Beck LH: THSD7A staining of membranous glomerulopathy in clinical practice reveals cases with dual autoantibody positivity. Mod Pathol 29: 421–426, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Debiec H, Lefeu F, Kemper MJ, Niaudet P, Deschênes G, Remuzzi G, Ulinski T, Ronco P: Early-childhood membranous nephropathy due to cationic bovine serum albumin. N Engl J Med 364: 2101–2110, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Glassock RJ: Human idiopathic membranous nephropathy--a mystery solved? N Engl J Med 361: 81–83, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Beck LH Jr., Salant DJ: Membranous nephropathy: From models to man. J Clin Invest 124: 2307–2314, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidney disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group : KDIGO clinical practice guideline for glomerulonephritis. Kidney Int 2: 139–274, 2012 [Google Scholar]
- 9.Glassock RJ: Antiphospholipase A2 receptor autoantibody guided diagnosis and treatment of membranous nephropathy: A new personalized medical approach. Clin J Am Soc Nephrol 9: 1341–1343, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behnert A, Schiffer M, Müller-Deile J, Beck LH Jr., Mahler M, Fritzler MJ: Antiphospholipase A2 receptor autoantibodies: A comparison of three different immunoassays for the diagnosis of idiopathic membranous nephropathy. J Immunol Res 2014: 143274, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timmermans SA, Damoiseaux JG, Heerings-Rewinkel PT, Ayalon R, Beck LH Jr., Schlumberger W, Salant DJ, van Paassen P, Tervaert JW; Limburg Renal Registry : Evaluation of anti-PLA2R1 as measured by a novel ELISA in patients with idiopathic membranous nephropathy: A cohort study. Am J Clin Pathol 142: 29–34, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Hoxha E, Beck LH Jr., Wiech T, Tomas NM, Probst C, Mindorf S, Meyer-Schwesinger C, Zahner G, Stahl PR, Schöpper R, Panzer U, Harendza S, Helmchen U, Salant DJ, Stahl RA: An indirect immunofluorescence method facilitates detection of thrombospondin type 1 domain-containing 7A-specific antibodies in membranous nephropathy [published online ahead of print July 19, 2016]. J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoxha E, Kneißler U, Stege G, Zahner G, Thiele I, Panzer U, Harendza S, Helmchen UM, Stahl RA: Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int 82: 797–804, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Debiec H, Ronco P: PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med 364: 689–690, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Svobodova B, Honsova E, Ronco P, Tesar V, Debiec H: Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol Dial Transplant 28: 1839–1844, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran R, Kumar V, Kumar A, Yadav AK, Nada R, Kumar H, Kumar V, Rathi M, Kohli HS, Gupta KL, Sakhuja V, Jha V: PLA2R antibodies, glomerular PLA2R deposits and variations in PLA2R1 and HLA-DQA1 genes in primary membranous nephropathy in South Asians. Nephrol Dial Transplant 31: 1486–1493, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Ronco P, Debiec H: Pathophysiological advances in membranous nephropathy: Time for a shift in patient’s care. Lancet 385: 1983–1992, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Du Y, Li J, He F, Lv Y, Liu W, Wu P, Huang J, Wei S, Gao H: The diagnosis accuracy of PLA2R-AB in the diagnosis of idiopathic membranous nephropathy: A meta-analysis. PLoS One 9: e104936, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai H, Zhang H, He Y: Diagnostic accuracy of PLA2R autoantibodies and glomerular staining for the differentiation of idiopathic and secondary membranous nephropathy: An updated meta-analysis. Sci Rep 5: 8803, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fervenza FC, Glassock RJ, Bleyer AJ: American Society of Nephrology quiz and questionnaire 2012: Glomerulonephritis. Clin J Am Soc Nephrol 8: 1460–1465, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Logt AE, Hofstra JM, Wetzels JF: Serum anti-PLA2R antibodies can be initially absent in idiopathic membranous nephropathy: Seroconversion after prolonged follow-up. Kidney Int 87: 1263–1264, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Ramachandran R, Kumar V, Nada R, Jha V: Serial monitoring of anti-PLA2R in initial PLA2R-negative patients with primary membranous nephropathy. Kidney Int 88: 1198–1199, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Hoxha E, Harendza S, Zahner G, Panzer U, Steinmetz O, Fechner K, Helmchen U, Stahl RA: An immunofluorescence test for phospholipase-A₂-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant 26: 2526–2532, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Gunnarsson I, Schlumberger W, Rönnelid J: Antibodies to M-type phospholipase A2 receptor (PLA2R) and membranous lupus nephritis. Am J Kidney Dis 59: 585–586, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Akiyama S, Akiyama M, Imai E, Ozaki T, Matsuo S, Maruyama S: Prevalence of anti-phospholipase A2 receptor antibodies in Japanese patients with membranous nephropathy. Clin Exp Nephrol 19: 653–660, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim YG, Choi YW, Kim SY, Moon JY, Ihm CG, Lee TW, Jeong KH, Yang SH, Kim YS, Oh YJ, Lee SH: Anti-phospholipase A2 receptor antibody as prognostic indicator in idiopathic membranous nephropathy. Am J Nephrol 42: 250–257, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Qin W, Beck LH Jr., Zeng C, Chen Z, Li S, Zuo K, Salant DJ, Liu Z: Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol 22: 1137–1143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsen CP, Messias NC, Silva FG, Messias E, Walker PD: Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol 26: 709–715, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Timmermans SA, Ayalon R, van Paassen P, Beck LH Jr., van Rie H, Wirtz JJ, Verseput GH, Frenken LA, Salant DJ, Cohen Tervaert JW; Limburg Renal Registry : Anti-phospholipase A2 receptor antibodies and malignancy in membranous nephropathy. Am J Kidney Dis 62: 1223–1225, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Oh YJ, Yang SH, Kim DK, Kang SW, Kim YS: Autoantibodies against phospholipase A2 receptor in Korean patients with membranous nephropathy. PLoS One 8: e62151, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Q, Li Y, Xue J, Xiong Z, Wang L, Sun Z, Ren Y, Zhu X, Hao CM: Renal phospholipase A2 receptor in hepatitis B virus-associated membranous nephropathy. Am J Nephrol 41: 345–353, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Iwakura T, Ohashi N, Kato A, Baba S, Yasuda H: Prevalence of enhanced granular expression of thrombospondin type-1 domain-containing 7A in the glomeruli of Japanese patients with idiopathic membranous nephropathy. PLoS One 10: e0138841, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei SY, Wang YX, Li JS, Zhao SL, Diao TT, Wang Y, Wang C, Qin Y, Cao Y, Wei Q, Li B: Serum anti-PLA2R antibody predicts treatment outcome in idiopathic membranous nephropathy. Am J Nephrol 43: 129–140, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Stehlé T, Audard V, Ronco P, Debiec H: Phospholipase A2 receptor and sarcoidosis-associated membranous nephropathy. Nephrol Dial Transplant 30: 1047–1050, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Hoxha E, Wiech T, Stahl PR, Zahner G, Tomas NM, Meyer-Schwesinger C, Wenzel U, Janneck M, Steinmetz OM, Panzer U, Harendza S, Stahl RA: A mechanism for cancer-associated membranous nephropathy. N Engl J Med 374: 1995–1996, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez EF, Nasr SH, Larsen CP, Sethi S, Fidler ME, Cornell LD: Membranous nephropathy with crescents: A series of 19 cases. Am J Kidney Dis 64: 66–73, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Cattran DC, Pei Y, Greenwood CM, Ponticelli C, Passerini P, Honkanen E: Validation of a predictive model of idiopathic membranous nephropathy: Its clinical and research implications. Kidney Int 51: 901–907, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Polanco N, Gutiérrez E, Covarsí A, Ariza F, Carreño A, Vigil A, Baltar J, Fernández-Fresnedo G, Martín C, Pons S, Lorenzo D, Bernis C, Arrizabalaga P, Fernández-Juárez G, Barrio V, Sierra M, Castellanos I, Espinosa M, Rivera F, Oliet A, Fernández-Vega F, Praga M; Grupo de Estudio de las Enfermedades Glomerulares de la Sociedad Española de Nefrología : Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol 21: 697–704, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hofstra JM, Beck LH Jr., Beck DM, Wetzels JF, Salant DJ: Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 6: 1286–1291, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hofstra JM, Debiec H, Short CD, Pellé T, Kleta R, Mathieson PW, Ronco P, Brenchley PE, Wetzels JF: Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1735–1743, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murtas C, Bruschi M, Candiano G, Moroni G, Magistroni R, Magnano A, Bruno F, Radice A, Furci L, Argentiero L, Carnevali ML, Messa P, Scolari F, Sinico RA, Gesualdo L, Fervenza FC, Allegri L, Ravani P, Ghiggeri GM: Coexistence of different circulating anti-podocyte antibodies in membranous nephropathy. Clin J Am Soc Nephrol 7: 1394–1400, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guerry MJ, Vanhille P, Ronco P, Debiec H: Serum anti-PLA2R antibodies may be present before clinical manifestations of membranous nephropathy. Kidney Int 89: 1399, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Timmermans SA, Abdul Hamid MA, Cohen Tervaert JW, Damoiseaux JG, van Paassen P; Limburg Renal Registry : Anti-PLA2R antibodies as a prognostic factor in PLA2R-related membranous nephropathy. Am J Nephrol 42: 70–77, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Hoxha E, Harendza S, Pinnschmidt H, Panzer U, Stahl RA: PLA2R antibody levels and clinical outcome in patients with membranous nephropathy and non-nephrotic range proteinuria under treatment with inhibitors of the renin-angiotensin system. PLoS One 9: e110681, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanigicherla D, Gummadova J, McKenzie EA, Roberts SA, Harris S, Nikam M, Poulton K, McWilliam L, Short CD, Venning M, Brenchley PE: Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int 83: 940–948, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Hoxha E, Harendza S, Pinnschmidt H, Panzer U, Stahl RA: M-type phospholipase A2 receptor autoantibodies and renal function in patients with primary membranous nephropathy. Clin J Am Soc Nephrol 9: 1883–1890, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radice A, Trezzi B, Maggiore U, Pregnolato F, Stellato T, Napodano P, Rolla D, Pesce G, D’Amico M, Santoro D, Londrino F, Ravera F, Ortisi G, Sinico RA: Clinical usefulness of autoantibodies to M-type phospholipase A2 receptor (PLA2R) for monitoring disease activity in idiopathic membranous nephropathy (IMN). Autoimmun Rev 15: 146–154, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, Stahl RA: Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol 25: 1357–1366, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seitz-Polski B, Dolla G, Payré C, Girard CA, Polidori J, Zorzi K, Birgy-Barelli E, Jullien P, Courivaud C, Krummel T, Benzaken S, Bernard G, Burtey S, Mariat C, Esnault VL, Lambeau G: Epitope spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy. J Am Soc Nephrol 27: 1517–1533, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoxha E, Harendza S, Pinnschmidt HO, Tomas NM, Helmchen U, Panzer U, Stahl RA: Spontaneous remission of proteinuria is a frequent event in phospholipase A2 receptor antibody-negative patients with membranous nephropathy. Nephrol Dial Transplant 30: 1862–1869, 2015 [DOI] [PubMed] [Google Scholar]
- 51.Hofstra JM, Fervenza FC, Wetzels JF: Treatment of idiopathic membranous nephropathy. Nat Rev Nephrol 9: 443–458, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Kanigicherla DA, Short CD, Roberts SA, Hamilton P, Nikam M, Harris S, Brenchley PE, Venning MC: Long-term outcomes of persistent disease and relapse in primary membranous nephropathy [published online ahead of print January 13, 2016]. Nephrol Dial Transplant [DOI] [PubMed] [Google Scholar]
- 53.Ruggenenti P, Debiec H, Ruggiero B, Chianca A, Pellé T, Gaspari F, Suardi F, Gagliardini E, Orisio S, Benigni A, Ronco P, Remuzzi G: Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol 26: 2545–2558, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bech AP, Hofstra JM, Brenchley PE, Wetzels JF: Association of anti-PLA2R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 9: 1386–1392, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Medrano AS, Escalante EJ, Cáceres CC, Pamplona IA, Allende MT, Terrades NR, Carmeno NV, Roldán EO, Agudelo KV, Vasquez JJ: Prognostic value of the dynamics of M-type phospholipase A2 receptor antibody titers in patients with idiopathic membranous nephropathy treated with two different immunosuppression regimens. Biomarkers 20: 77–83, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Beck LH Jr., Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, Cosio FG, Cattran DC, Salant DJ: Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol 22: 1543–1550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Müller-Deile J, Schiffer L, Hiss M, Haller H, Schiffer M: A new rescue regimen with plasma exchange and rituximab in high-risk membranous glomerulonephritis. Eur J Clin Invest 45: 1260–1269, 2015 [DOI] [PubMed] [Google Scholar]
- 58.Grupper A, Cornell LD, Fervenza FC, Beck LH Jr., Lorenz E, Cosio FG: Recurrent membranous nephropathy after kidney transplantation: Treatment and long-term implications [published online ahead of print December 30, 2015]. Transplantation 2015 [DOI] [PubMed] [Google Scholar]
- 59.Dabade TS, Grande JP, Norby SM, Fervenza FC, Cosio FG: Recurrent idiopathic membranous nephropathy after kidney transplantation: A surveillance biopsy study. Am J Transplant 8: 1318–1322, 2008 [DOI] [PubMed] [Google Scholar]
- 60.Debiec H, Martin L, Jouanneau C, Dautin G, Mesnard L, Rondeau E, Mousson C, Ronco P: Autoantibodies specific for the phospholipase A2 receptor in recurrent and de novo membranous nephropathy. Am J Transplant 11: 2144–2152, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Larsen CP, Walker PD: Phospholipase A2 receptor (PLA2R) staining is useful in the determination of de novo versus recurrent membranous glomerulopathy. Transplantation 95: 1259–1262, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Quintana LF, Blasco M, Seras M, Pérez NS, López-Hoyos M, Villarroel P, Rodrigo E, Viñas O, Ercilla G, Diekmann F, Gómez-Roman JJ, Fernandez-Fresnedo G, Oppenheimer F, Arias M, Campistol JM: Antiphospholipase A2 receptor antibody levels predict the risk of posttransplantation recurrence of membranous nephropathy. Transplantation 99: 1709–1714, 2015 [DOI] [PubMed] [Google Scholar]
- 63.Gupta G, Fattah H, Ayalon R, Kidd J, Gehr T, Quintana LF, Kimball P, Sadruddin S, Massey HD, Kumar D, King AL, Beck LH Jr.: Pre-transplant phospholipase A2 receptor autoantibody concentration is associated with clinically significant recurrence of membranous nephropathy post-kidney transplantation. Clin Transplant 30: 461–469, 2016 [DOI] [PubMed] [Google Scholar]
- 64.Kattah A, Ayalon R, Beck LH Jr., Sethi S, Sandor DG, Cosio FG, Gandhi MJ, Lorenz EC, Salant DJ, Fervenza FC: Anti-phospholipase A2 receptor antibodies in recurrent membranous nephropathy. Am J Transplant 15: 1349–1359, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seitz-Polski B, Payré C, Ambrosetti D, Albano L, Cassuto-Viguier E, Berguignat M, Jeribi A, Thouret MC, Bernard G, Benzaken S, Lambeau G, Esnault VL: Prediction of membranous nephropathy recurrence after transplantation by monitoring of anti-PLA2R1 (M-type phospholipase A2 receptor) autoantibodies: A case series of 15 patients. Nephrol Dial Transplant 29: 2334–2342, 2014 [DOI] [PubMed] [Google Scholar]
- 66.Kattah AG, Alexander MP, Angioi A, De Vriese AS, Sethi S, Cosio FG, Lorenz EC, Cornell LD, Fervenza FC: Temporal IgG Subtype changes in recurrent idiopathic membranous nephropathy. Am J Transplant 16: 2964–72, 2016 [DOI] [PubMed] [Google Scholar]
- 67.Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, Nachman PH, Bergstralh EJ, Leung N, Cosio FG, Hogan MC, Dillon JJ, Hickson LJ, Li X, Cattran DC; Mayo Nephrology Collaborative Group : Rituximab therapy in idiopathic membranous nephropathy: A 2-year study. Clin J Am Soc Nephrol 5: 2188–2198, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Fessler BJ, Ding L, Viviano L, Tchao NK, Phippard DJ, Asare AL, Lim N, Ikle D, Jepson B, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh K, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Mueller M, Sejismundo LP, Mieras K, Stone JH: Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363: 417–427, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]