Abstract
Lysozyme amyloidosis (ALys) is a rare form of hereditary amyloidosis that typically manifests with renal impairment, gastrointestinal (GI) symptoms, and sicca syndrome, whereas cardiac involvement is exceedingly rare and neuropathy has not been reported. Here, we describe a 40-year-old man with renal impairment, cardiac and GI symptoms, and peripheral neuropathy. Renal biopsy specimen analysis revealed amyloidosis with extensive involvement of glomeruli, vessels, and medulla. Amyloid was also detected in the GI tract. Echocardiographic and electrocardiographic findings were consistent with cardiac involvement. Proteomic analysis of Congo red–positive renal and GI amyloid deposits detected abundant lysozyme C protein. DNA sequencing of the lysozyme gene in the patient and his mother detected a heterozygous c.305T>C alteration in exon 3, which causes a leucine to serine substitution at codon 102 (Human Genome Variation Society nomenclature: p.Leu102Ser; legacy designation: L84S). We also detected the mutant peptide in the proband’s renal and GI amyloid deposits. PolyPhen analysis predicted that the mutation damages the encoded protein. Molecular dynamics simulations suggested that the pathogenesis of ALys p.Leu102Ser is mediated by shifting the position of the central β-hairpin coordinated with an antiparallel motion of the C-terminal helix, which may alter the native-state structural ensemble of the molecule, leading to aggregation-prone intermediates.
Keywords: amyloidosis, lysozyme, hereditary amyloid, malfolding proteins
Hereditary amyloidoses are a rare group of diseases characterized by extracellular deposition of misfolded mutant proteins in the form of insoluble fibrils. Approximately 2% of the renal amyloidoses are hereditary. They are due to mutations in proteins, including fibrinogen α-chain (AFib),1 transthyretin (mutATTR),1 gelsolin (AGel),1 lysozyme (lysozyme amyloidosis [ALys]),1 apo A-I (AApoAI),1 apo A-II (AApoAII),1 apo C-III (AApoCIII),2 and apo C-II (AApoCII).3 Depending on the mutant protein, hereditary amyloidoses can affect a variety of organs and present with a wide spectrum of clinical manifestations. ALys is a very rare autosomal dominant hereditary amyloidosis associated with a number of lysozyme mutations (Table 1).4–11 Renal failure, gastrointestinal (GI) manifestations, and sicca syndrome are the most common clinical manifestations in patients with ALys. Cardiac involvement is rare,8 and peripheral neuropathy due to ALys has not been previously reported. Here, we report on a patient with hereditary amyloidosis with a novel lysozyme variant (HGVS nomenclature: p.Leu102Ser; legacy designation that does not consider the signal peptide: L84S) that is associated with a unique phenotype characterized by nephropathy, neuropathy, GI manifestations, and probable cardiac involvement.
Table 1.
Genotype and phenotype characteristics of the previously reported amyloidogenic lysozyme variants
| Protein Variant | Sequence Variant (mRNA) | Location | Ethnicity | Phenotype | Representative Reference |
| p.Ile74Thr | c.221T>C | Exon 2 | British | Renal dysfunction, Petechiae | 4 |
| p.Asp85His | c.253G>C | Exon 2 | British | Renal dysfunction, GI tract manifestations, liver hematoma and rupture, sicca syndrome | 4,11 |
| p.Tyr72Asn | c.214T>A | Exon 2 | Swedish | GI tract manifestations, hereditary hemorrhagic telangiectasia, sicca syndrome, cardiac manifestations | 10 |
| p.Phe75Ile | c.223T>A | Exon 2 | Italian Canadian | Renal dysfunction | 5 |
| p.Trp82Arg | c.244T>C | Exon 2 | French | Renal dysfunction, GI tract manifestations, sicca syndrome | 7 |
| p.Trp82Arg | c.244T>A | Exon 2 | Italian | Spontaneous hepatic hemorrhage, GI tract manifestations | 9 |
| p.Asp85Gly | c.254A>G | Exon 2 | Romanian | Renal dysfunction, sicca syndrome | 6 |
| p.Trp130Arg | c.388T>C | Exon 4 | German | Renal dysfunction, GI tract manifestations, liver dysfunction, splenomegaly, sicca syndrome, heart failure | 8 |
| p.Leu102Ser | c.305T>C | Exon 3 | American with mixed heritage | Renal dysfunction, GI tract manifestations, peripheral neuropathy, possible cardiac involvement | This report |
Results
Patient History
A 40-year-old white man was admitted for chest pain, lightheadedness, and vasovagal syncope and noted to have renal insufficiency with a serum creatinine of 2.4 mg/dl. He had a history of apical hypertrophic cardiomyopathy (diagnosed 6 years prior on the basis of echocardiographic and stress testing), gout, chronic indomethacin use, and anemia. The patient complained of incapacitating peripheral neuropathy involving the legs up to the knees characterized by bilateral severe stabbing-like pain associated with decreased skin sensation and mild burning sensation, which improved substantially on gabapentin. He had GI symptoms, including early satiety, nausea after meals, and vomiting in addition to abdominal bloating and dysphagia. The patient had no prior history of hypertension, hypotension, diabetes, autoimmune disease, or kidney disease. There was no history of postural hypotension, incontinence or urinary retention, heat intolerance, erectile dysfunction, or ophthalmologic symptoms. Physical examination was negative for edema, skin rash, and organomegaly. Neurologic examination revealed evidence of length-dependent sensory-predominant polyneuropathy with a decreased perception of pinprick stocking distribution to ankles bilaterally and mildly decreased vibratory sensation of large toes and reflexes of 2/4 bilaterally. The rest of the neurologic examination, including that of upper extremities and cranial nerves, was unrevealing. Electromyography and nerve biopsy were not performed. An echocardiogram showed a moderate to severe concentric increase in left ventricular wall thickness and hypertrophied anterolateral papillary muscle with a left ventricular ejection fraction of 68%. There was systolic left ventricular cavity obliteration with a midventricular maximal instantaneous Doppler gradient at rest of 6 mmHg, increasing to 25 mmHg during the strain phase of the Valsalva maneuver. The global average left ventricular longitudinal peak systolic strain was abnormal at −14%. There was some regional heterogeneity in strain, with the apical segments showing the most preserved strain values. The electrocardiogram showed sinus rhythm, normal voltage QRS complexes, and no Q waves, but t-wave inversion was noted in leads I and aVL, with mild biphasic t waves in leads V5 and V6.
The patient had an eGFR of 27 ml/min, potassium of 5.5 mmol/L, sodium of 136 mmol/L, hemoglobin of 10 g/dl, serum troponin of 0.03 ng/ml, ProBNP of 234 pg/ml (normal), positive stool occult blood test, normal serum C3 and C4 complement levels, negative serum and urine protein electrophoresis with immunofixation for monoclonal protein, and normal serum free light chain ratio. Urinalysis showed 1+ protein, 1+ blood, 0 WBC/hpf, and 0 RBC/hpf. Despite discontinuation of indomethacin, serum creatinine gradually increased to 2.8 mg/dl 2 months later, prompting a kidney biopsy that showed ALys amyloidosis (see Proteomics Findings). GI endoscopic biopsies detected amyloid in the gastric, duodenal, and rectal mucosa. A pyrophosphate scan ([99mTc]-PYP) did not detect myocardium tracer uptake. Cardiac gadolinium magnetic resonance imaging could not be performed due to the patient’s renal failure. Five months postrenal biopsy, he had a mildly elevated troponin T level of 0.12 ng/ml, an elevated NT-proBNP level of 4890 pg/ml, a serum creatinine level of 2.3 mg/dl, and a 24-hour urine protein of 0.4 g. He continued to have limited activity due to symptoms of fatigue, lack of energy, and peripheral neuropathy.
Renal Biopsy Findings
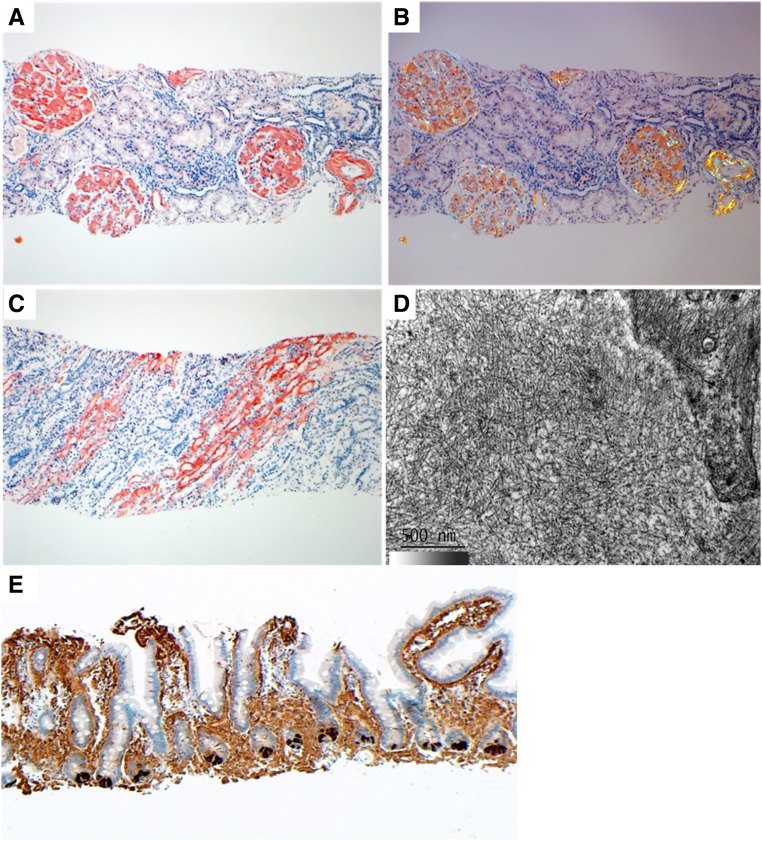
The renal biopsy specimen contained renal cortex and medulla. A total of 22 glomeruli were sampled, two of which were globally sclerotic. Extensive acellular eosinophilic material that stained strongly positive with Congo red (CR), which exhibited apple-green birefringence under polarized light, was present in glomerular mesangium and basement membranes, arterial and arteriolar walls, and the basement membranes of medullary collecting ducts and vasa recta (Figure 1, A–C). There was moderate tubular atrophy, interstitial fibrosis, and arteriosclerosis. Electron microscopy of the deposits showed randomly oriented fibrils ranging from 8 to 14 nm (mean =10 nm) in diameter typical of amyloid (Figure 1D). Amyloid deposits were negative for IgG, IgA, κ, and λ by immunofluorescence on frozen tissue.
Figure 1.
Renal and GI biopsy findings. (A) Abundant glomerular mesangial, glomerular capillary wall, and arterial congophilic amyloid deposits are seen. (B) Renal CR-positive amyloid deposits display apple-green birefringence under polarized light. (C) Extensive congophilic amyloid deposits are seen in the medulla involving collecting ducts and vasa recta basement membranes. (D) On electron microscopy, amyloid deposits are composed of randomly oriented straight fibrils. (E) The intestinal mucosal amyloid deposits stain strongly for lysozyme using immunohistochemistry. Paneth cells at the bases of the glands serve as an internal positive control for lysozyme. Proteomic data confirming the diagnosis of ALys with the p.Leu102Ser abnormality from this specimen are presented in Supplemental Figure 1. Magnification, ×100 in A–C and E; ×50,000 in D.
Proteomics Findings
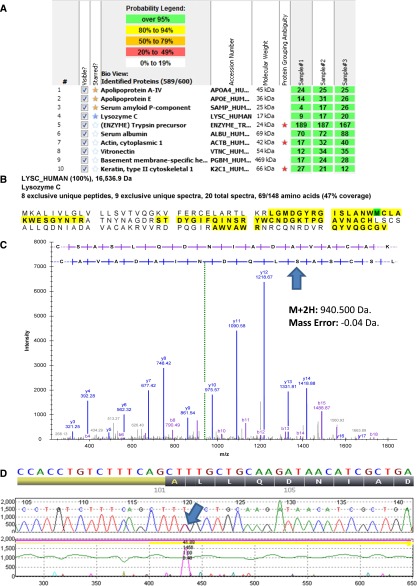
We used a previously published laser microdissection–assisted liquid chromatography–based tandem mass spectrometry method for typing the glomerular and medullary amyloid deposits of the patient.12 We detected Lysozyme C (LYZ) protein along with universal amyloid tissue biomarkers (APOE, APOAIV, and SAP) (Figure 2A).13 Protein sequence coverage analysis detected peptides from both N- and C-terminal portions of LYZ protein, suggesting that the protein was deposited in its entirety (Figure 2B). We used a previously published bioinformatics protocol14 to detect a novel mutation in LYZ protein, in which the leucine at position 102 was substituted by serine (p.Leu102Ser) (Figure 2C). Because of the GI symptoms, the patient also had GI biopsies, which showed extensive mucosal CR-positive amyloid deposits. Proteomic typing of intestinal amyloid deposits showed LYZ protein with the p.Leu102Ser mutation along with the common amyloid tissue biomarkers (Supplemental Figure 1). We did not detect the wild-type (WT) form of the mutant peptide in either renal or GI amyloid deposits. The intestinal amyloid was also positive for lysozyme by immunohistochemistry (Figure 1E).
Figure 2.
Proteomic and genetic detection of amyloidosis derived from lysozyme Leu102Ser variant. (A) Proteins highlighted with yellow stars are the universal amyloid tissue biomarkers. LYZ protein is highlighted with a blue star. Numbers in the boxes represent the total numbers of spectra matched to the protein in a sample. Multiple dissections are presented independently. (B) Portions of the LYZ sequence detected in the sample are highlighted with bold black letters on yellow background. (C) Tandem mass spectrometry spectrum of the mutant peptide detected in the CR-positive renal amyloid deposits of the patient. An arrow marks the p.Leu102Ser amino acid sequence change. (D) LYZ gene mutation in the patient. An arrow marks the detected c.305T→C (p.Leu102Ser) mutation.
Germline DNA Sequencing Results
We performed Sanger sequencing of the LYZ gene from the proband and found a heterozygous c.305T>C alteration in exon 3, which results in a leucine to serine substitution at codon 102 (p.Leu102Ser) (Figure 2D) that corresponds to the mutant peptide detected in the renal amyloid deposits. The same germline DNA alteration was also detected in proband’s mother. This mutation was not reported in dbSNP (build 147; 34.7 million variants), Exome Aggregation Consortium (60,706 individuals), Exome Sequencing Project (version ESP6500; 6503 individuals), or 1000 Genomes (phase 3; 84.4 million variants) data, which suggests that this variant is not a common polymorphism. PolyPhen15 (version 2; HumDiv algorithm) analysis predicted the mutation as damaging with a score of 1.000 (meaning near certainty).
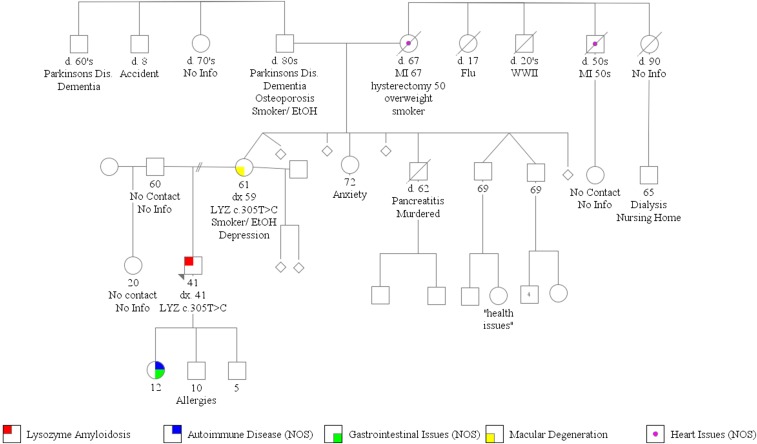
A full family pedigree analysis was conducted (Figure 3). Proband was not in contact with his father (60 years old) or half-sister (20 years old). His mother (61 years old) had macular degeneration at the age of 59 years old, and one of proband’s three children (all minors) had GI symptoms and undefined autoimmune disease. Proband’s grandmother and one of her brothers had heart disease, and a distant cousin had ESRD of unclear etiology and had been on dialysis. The patient has French Canadian, Ashkenazi Jewish, Irish, Polish, Baltic Jewish, and English heritage.
Figure 3.
Family tree of the affected kindred. The proband is indicated in red. Dead individuals are indicated by a diagonal line through the symbol. Miscarriages are indicated with diamonds.
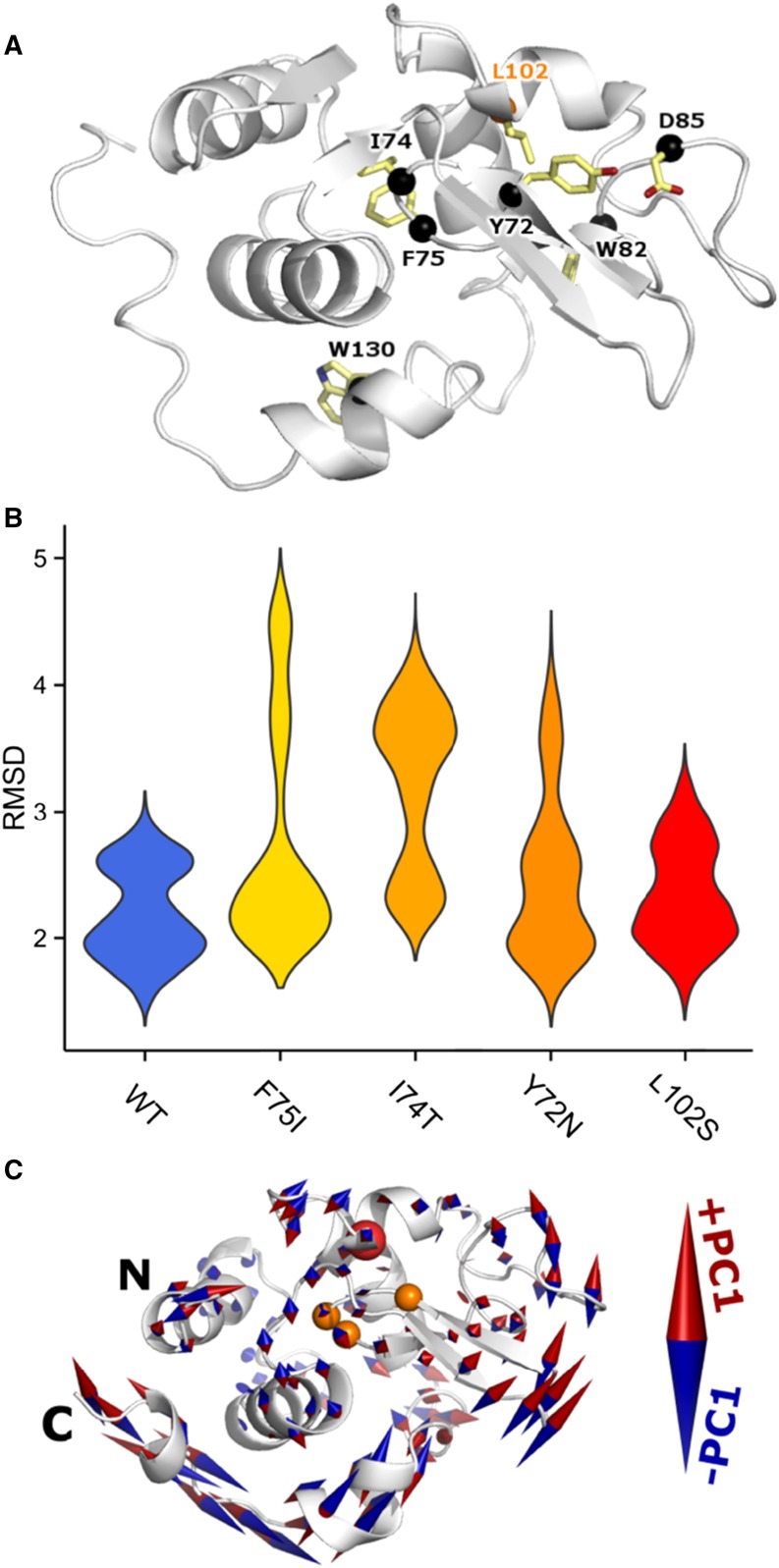
Molecular Dynamics Simulation Findings
The novel p.Leu102Ser mutation is in close three-dimensional spatial proximity to other known ALys variants (Figure 4A, Table 1). Furthermore, we detected the p.Leu102Ser mutant peptide in the deposit (Figure 2C), and the mutation was predicted to be deleterious to the LYZ protein function. These observations supported the hypothesis that the new mutation could destabilize the native LYZ protein structure in ways that are similar to those of other nearby ALys variants. Hence, we generated molecular dynamics (MD) trajectories for the WT, p.Leu102Ser, and three previously observed ALys variants (p.Tyr72Asn [Y72N], p.Ile74Thr [I74T], and p.Phe75Ile [F75I]) that are in close spatial proximity to the novel variant (Figure 4A). Simulation of each variant was prepared independently, and 25 ns MD trajectory was generated in triplicate and used to compare and contrast simulated molecular motions from each context. From a global perspective, the combined 390 ns MD indicated that amyloidosis variants sampled more expanded states as measured by root mean squared deviation (Figure 4B). Comparing across variants using principal component (PC) analysis, we identified the dominant motions (PCs) of each molecular system. The first PC indicates a shift in the position of the central β-hairpin coordinated with an antiparallel motion of the C-terminal helix (Figure 4C). The WT structure displays this motion, but simulations of multiple amyloidosis variants exhibit more extensive sampling of it. This increased structural motion may be a part of the early molecular events that alter the native-state structural ensemble, leading to aggregation-prone intermediates.
Figure 4.
MD of ALys p.Leu102Ser. (A) Previously reported ALys coding variants are marked by black spheres. Newly observed p.Leu102Ser variant site is indicated in orange text. Previously reported variants are either within the same sequence-based region of LYZ or closely interact with them in three dimensions. p.Leu102Ser is distant in sequence but close in space to previously reported amyloidosis variants. (B) The root mean squared deviation (RMSD) across replicates and for each variant is shown by a smoothed density plot, where the width of each shape is proportional to the number of observations. The WT is summarized in blue, the novel L102S is in red, and three previously identified amyloidosis variants are in shades of orange. (C) PCs were computed across all 15 simulations. The first PC is visualized on the LYZ structure. The final WT conformation is shown in cartoon representation. Amino acid 102 is marked by a red sphere, whereas 72, 74, and 75 are marked by orange spheres. Two terminal amino acids exhibited large motions and are not shown. The first PC indicated anticorrelated motion between the β-hairpin and C-terminal α-helices. Both known and novel ALys variants destabilize LYZ protein by introducing flexibility in the C terminus of the protein.
Discussion
A total of eight hereditary ALys mutations have been reported in literature (Table 1), and all of these amyloidogenic mutations, except one, are located in exon 2 of the LYZ gene.4–11 The phenotypes of patients with ALys are quite variable between families, even in patients with the same variant, and there is no good correlation between the detected mutation and clinical manifestations.16 However, kidney involvement manifesting as renal failure and proteinuria is frequently observed in patients with ALys,4–7,11 generally in association with extrarenal manifestations. In a previous review of 16 patients with hereditary ALys, median proteinuria was 0.3 g/d.16 Remarkably, our patient with the p.Leu102Ser variant also had minimal proteinuria (0.4 g/d), despite the extensive glomerular involvement (Figure 1, A and B). Regardless, renal prognosis in ALys is poor.7,11,16
GI tract involvement observed in our patient is common in most patients with ALys. Biopsy-proven heart involvement by ALys was reported in abstract form in only two families.8 Our patient has cardiac manifestations that are likely to be due to involvement by amyloidosis. Although there was a preceding diagnosis of apical hypertrophic cardiomyopathy, the echocardiographic findings of a concentric increase in left ventricular wall thickness and reduced global longitudinal peak systolic strain with relative sparing of the apex in association with the electrocardiographic findings do not suggest the presence of apical hypertrophic cardiomyopathy but fit better with a unifying diagnosis of cardiac amyloidosis. Remarkably, our patient with the novel p.Leu102Ser variant had peripheral neuropathy. Peripheral neuropathy is most common in patients with AL or mutATTR. To our knowledge, this is the first report of peripheral neuropathy due to ALys and only the third reported patient with presumed cardiac involvement.
The patient’s 61-year-old mother is also a carrier of the LYZ p.Leu102Ser variant but without clinical manifestations of amyloidosis (Figure 3). Only longer-term follow-up will determine if she will remain an unaffected carrier or subsequently develop symptomatic amyloidosis. Additionally, absence of an established history of amyloidosis in this family (although multiple members had heart, kidney, or GI symptoms that could be due to amyloidosis) suggests incomplete penetrance of p.Leu102Ser ALys. This is similar to another reported patient; a patient with p.Phe75Ile ALys had an older sister with the same variant but without clinical signs or symptoms of amyloidosis.5 Variability in disease penetrance, both inter- and intrafamilial, has also been observed in other types of hereditary amyloidosis, such as p.Val50Met ATTR and p.Trp74Arg AApoA1.17
This patient’s case highlights the successful clinical application of amyloid proteomic typing and its ability to detect novel pathologic mutations in hereditary amyloidosis.14 Proteomic detection of the LYZ p.Leu102Ser variant peptide in the amyloid deposit suggested that this novel variant is likely to be pathogenic. MD simulations suggested that the pathogenesis of the mutant protein is mediated by two factors: (1) increased sampling of expanded (partially folded) states that is a hallmark feature of in vivo dynamics of other known ALys mutations18 and (2) shifting the position of the central β-hairpin coordinated with an antiparallel motion of the C-terminal helix, a central structural motion underlying the pathogenesis of other known ALys variants.19
Concise Methods
Renal Histology
Evaluation of the renal biopsy included staining with hematoxylin and eosin, periodic acid–Schiff, Masson trichrome, Jones methenamine silver, and CR. Standard methods were used for immunofluorescence for IgG, IgM, IgA, C3, C1q, albumin, fibrinogen, κ, and λ and for transmission electron microscopy.
Proteomic Typing of Amyloid Deposits
CR-positive amyloid deposits were microdissected from formalin-fixed, paraffin-embedded tissue sections and analyzed by liquid chromatography–based tandem mass spectrometry as previously described.12 The data were processed using a previously described bioinformatics pipeline.12 A pathologist scrutinized the resulting proteome profile for an amyloid confirming universal molecular signature (APOE, SAP, and APOAIV).13 ALys amyloid type was finalized by correlating the renal pathology observations with the most abundant amyloidogenic protein that was consistently detected across all replicate dissections. A secondary previously published14 bioinformatics protocol was used to screen the peptide tandem mass spectrometry for unexpected amino acid mutations, and the novel LYZ p.Leu102Ser mutation was detected in the sample.
Lysozyme Immunohistochemistry
Immunohistochemistry was performed on paraffin sections of the small bowel biopsy specimen using a Ventana BenchMark XT Automated Immunostainer (Ventana Medical Systems Inc., Tucson, AZ). After deparaffinization, the tissue sections were pretreated for 30 minutes with Cell Conditioning Solution 1 (Ventana) followed by a 32-minute incubation at 37°C in a rabbit polyclonal antilysozyme antibody solution (1:2000; Dako, Carperteria, CA). The reactivity with the primary antibody was visualized using the Vantana ultraView Universal Diaminobenzidine Detection Kit following the manufacturer’s instructions.
Germline DNA Sequencing
Genetic evaluation for germline mutations in the LYZ gene was performed using a peripheral blood sample. All four exons of LYZ gene were amplified using hybrid primers containing 20–22 bases of gene-specific sequence and a universal sequencing primer sequence (19 or 23 bases for the forward and reverse primers) at the 5′ end. Amplified products were sequenced using universal sequencing primers, the ABI Big Dye Terminators (Applied Biosystems, Foster City, CA), and capillary electrophoresis on an ABI 3730 Sequencer. Data were analyzed using Mutation Surveyor (SoftGenetics, College Station, PA) configured to use corresponding reference sequence obtained from GenBank (with accession no. NM_000239.2).
MD Simulations
We followed a previously published protocol3 to perform generalized Born implicit solvent MD simulations with WT, p.Tyr72Asn (Y72N), p.Ile74Thr (I74T), p.Phe75Ile (F75I), and p.Leu102Ser (L102S) variants. Simulations were carried out using NAMD and the CHARMM22 with CMAP force field. Protein Data Bank structure 1LZ1 was used for starting conformation. Initial mutant conformations were generated using Mutator (version 1.3) VMD plugin. We used an interaction cutoff of 12 Å, with strength tapering (or switching) beginning at 10 Å, a simulation time step of 1 fs, and conformations recorded every 2 ps. Each initial conformation was used to generate three replicates, and each was energy minimized for 5000 steps followed by heating to 300 K over 300 ps via a Langevin thermostat. Another 25 ns of simulation trajectory was generated, and the final 20 ns were analyzed. All trajectories were first aligned to the initial WT conformation using Cα atoms. Root mean squared deviation was calculated using only Cα atoms. PC analysis was performed in Cartesian space. Analysis was carried out using custom scripts, leveraging VMD and Bio3D R package. Protein structure visualization was performed in PyMOL (The PyMOL Molecular Graphics System, Version 1.5.0.3; Schrödinger, LLC).
Disclosures
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016090951/-/DCSupplemental.
References
- 1.Dember LM: Amyloidosis-associated kidney disease. J Am Soc Nephrol 17: 3458–3471, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Valleix S, Verona G, Jourde-Chiche N, Nédelec B, Mangione PP, Bridoux F, Mangé A, Dogan A, Goujon JM, Lhomme M, Dauteuille C, Chabert M, Porcari R, Waudby CA, Relini A, Talmud PJ, Kovrov O, Olivecrona G, Stoppini M, Christodoulou J, Hawkins PN, Grateau G, Delpech M, Kontush A, Gillmore JD, Kalopissis AD, Bellotti V: D25V apolipoprotein C-III variant causes dominant hereditary systemic amyloidosis and confers cardiovascular protective lipoprotein profile. Nat Commun 7: 10353, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nasr SH, Dasari S, Hasadsri L, Theis JD, Vrana JA, Gertz MA, Muppa P, Zimmermann MT, Grogg KL, Dispenzieri A, Sethi S, Highsmith WE Jr., Merlini G, Leung N, Kurtin PJ: Novel type of renal amyloidosis derived from Apolipoprotein-CII [published online ahead of print June 13, 2016]. J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepys MB, Hawkins PN, Booth DR, Vigushin DM, Tennent GA, Soutar AK, Totty N, Nguyen O, Blake CC, Terry CJ, Feest TG, Zalin AM, Hsuan JJ: Human lysozyme gene mutations cause hereditary systemic amyloidosis. Nature 362: 553–557, 1993 [DOI] [PubMed] [Google Scholar]
- 5.Yazaki M, Farrell SA, Benson MD: A novel lysozyme mutation Phe57Ile associated with hereditary renal amyloidosis. Kidney Int 63: 1652–1657, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Woolliver C, Coriu D, Murphy D, Kestler D, Wang S, Weiss DT, Solomon A: Familial amyloidosis associated with a novel mutation (D67G) in the lysozyme gene. Amyloid 13 (Suppl 1): 69, 2006 [Google Scholar]
- 7.Valleix S, Drunat S, Philit JB, Adoue D, Piette JC, Droz D, MacGregor B, Canet D, Delpech M, Grateau G: Hereditary renal amyloidosis caused by a new variant lysozyme W64R in a French family. Kidney Int 61: 907–912, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Sperry B, Hanna M, Ikram A, Theis J, Leung N, Highsmith WE, Grogan M, Dispenzieri A: Novel mutation in the lysozyme gene leading to hereditary lysozyme amyloidosis with biopsy-proven cardiac involvement. Presented at the XVth International Symposium on Amyloidosis, Upsala, Sweden, July 3–7, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Jean E, Ebbo M, Valleix S, Benarous L, Heyries L, Grados A, Bernit E, Grateau G, Papo T, Granel B, Daniel L, Harlé JR, Schleinitz N: A new family with hereditary lysozyme amyloidosis with gastritis and inflammatory bowel disease as prevailing symptoms. BMC Gastroenterol 14: 159, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girnius S, Skinner M, Spencer B, Prokaeva T, Bartholomew C, O’Hara C, Seldin DC, Connors LH: A new lysozyme tyr54asn mutation causing amyloidosis in a family of Swedish ancestry with gastrointestinal symptoms. Amyloid 19: 182–185, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Gillmore JD, Booth DR, Madhoo S, Pepys MB, Hawkins PN: Hereditary renal amyloidosis associated with variant lysozyme in a large English family. Nephrol Dial Transplant 14: 2639–2644, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR 3rd, Dogan A: Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood 114: 4957–4959, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Vrana JA, Theis JD, Dasari S, Mereuta OM, Dispenzieri A, Zeldenrust SR, Gertz MA, Kurtin PJ, Grogg KL, Dogan A: Clinical diagnosis and typing of systemic amyloidosis in subcutaneous fat aspirates by mass spectrometry-based proteomics. Haematologica 99: 1239–1247, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dasari S, Theis JD, Vrana JA, Zenka RM, Zimmermann MT, Kocher JP, Highsmith WE Jr., Kurtin PJ, Dogan A: Clinical proteome informatics workbench detects pathogenic mutations in hereditary amyloidoses. J Proteome Res 13: 2352–2358, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR: A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sattianayagam PT, Gibbs SD, Rowczenio D, Pinney JH, Wechalekar AD, Gilbertson JA, Hawkins PN, Lachmann HJ, Gillmore JD: Hereditary lysozyme amyloidosis -- phenotypic heterogeneity and the role of solid organ transplantation. J Intern Med 272: 36–44, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Hellman U, Alarcon F, Lundgren HE, Suhr OB, Bonaiti-Pellié C, Planté-Bordeneuve V: Heterogeneity of penetrance in familial amyloid polyneuropathy, ATTR Val30Met, in the Swedish population. Amyloid 15: 181–186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagan CL, Johnson RJ, Dhulesia A, Dumoulin M, Dumont J, De Genst E, Christodoulou J, Robinson CV, Dobson CM, Kumita JR: A non-natural variant of human lysozyme (I59T) mimics the in vitro behaviour of the I56T variant that is responsible for a form of familial amyloidosis. Protein Eng Des Sel 23: 499–506, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumoulin M, Canet D, Last AM, Pardon E, Archer DB, Muyldermans S, Wyns L, Matagne A, Robinson CV, Redfield C, Dobson CM: Reduced global cooperativity is a common feature underlying the amyloidogenicity of pathogenic lysozyme mutations. J Mol Biol 346: 773–788, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.