Despite improved clinical care in patients with diabetes, diabetic complications remain a major healthcare burden. The management of cardiovascular risk factors, such as hyperlipidemia, has improved through the availability of effective treatments, and with a greater proportion of patients reaching lipid, BP, and glycemic targets, the rates of incident diabetic cardiovascular complications have declined over recent decades, although there seems to be less effect on the burden of renal complications.1 Recent analysis of data from NHANES 1998–2014 also highlighted the changing pattern of diabetic kidney complications, whereby a decline in the prevalence of albuminuria, the classic feature of diabetic nephropathy, was observed, but it was accompanied by a rise in the prevalence of reduced renal function as measured by eGFR.2 Indeed, whereas diabetic nephropathy is traditionally defined as the presence of proteinuria or progression to ESRD, there is now increasing utilization of decreased renal function, as reflected by declined eGFR, in the definition of diabetic kidney complications.2 Review of available global renal registry data reveals that most countries are witnessing an increasing proportion of ESRD related to diabetes.3 The marked increase in the prevalence of type 2 diabetes (T2D); a change in epidemiology, with an increasing proportion of young patients being affected; the improved survival from cardiovascular complications; and the rather limited number of renoprotective interventions currently available have all contributed to an increasing global burden of diabetic kidney disease (DKD).4,5
Given the great healthcare burden associated with diabetic renal complications, there has been much interest in the search of genetic factors for diabetic kidney complications. Earlier efforts involved linkage studies and candidate gene–based studies, and the advent of hypothesis–free genome–wide approaches is now providing additional motivation for genetic studies that may help unravel underlying pathophysiologic pathways, identify novel drug targets or drug indications, and explore the causal role of biomarkers and the opportunity to project the long-term safety of drugs.6,7
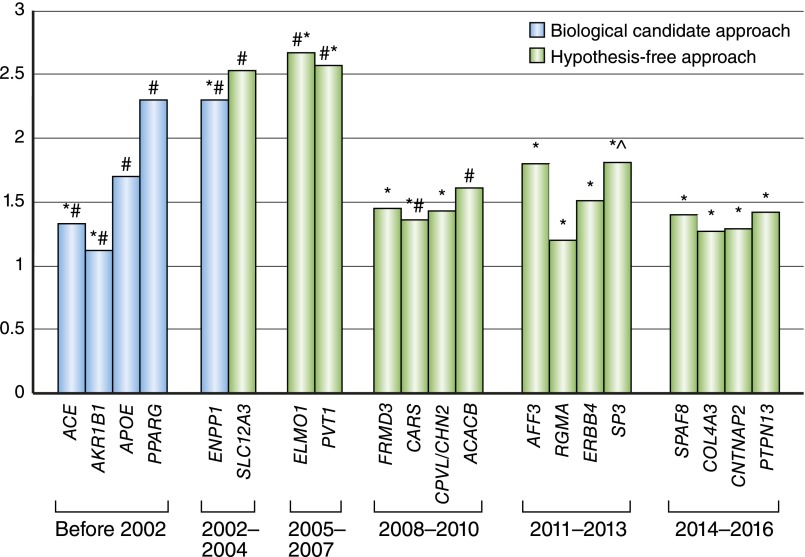
The last decade has witnessed tremendous progress in the identification of genetic factors for T1D and T2D, with now >100 variants identified for T2D.8 Despite these advances, the genetics of diabetes are still considered a geneticist’s nightmare, and much of the heritability remains unexplained.9 For DKD, the search for genetic factors has been even more challenging, with decades of research yielding only a limited number of genetic variants consistently found to be associated, and so far, only very few variants have been identified through genome–wide association studies (GWASs) achieving genome-wide significance10–12 (Figure 1). Some of the obstacles impeding progress include the limited sample size in studies so far conducted, the heterogeneity of the renal disease phenotype being studied due to the different definitions of diabetic nephropathy being used in studies, and the restriction of genetic variants being investigated to focus mainly on common genetic variants. Furthermore, the presence of other pathologies (for example, hypertensive glomerulosclerosis or other glomerulopathy) and changes secondary to obesity, hypertension, and hyperlipidemia present added challenges, especially in the case of kidney disease complicating T2D. Given these challenges, our current understanding of the genetic architecture of DKD lags far behind that of many other common diseases.
Figure 1.
Progress in the identification of genetic loci for DKD. The list of loci and effect estimates are derived mainly from ref. 10 and the original studies cited. Loci with consistent association with the different definitions of DKD are included, with the majority of studies on the basis of DKD being defined as ESRD. Most variants listed, with a few exceptions, have not achieved association at the genome–wide significance threshold. Although the nearest gene to each variant has been indicated, the direct role of the gene listed has not been established at most of the loci. *Discovered/replicated in studies in subjects with T1D. #Discovered/replicated in studies in subjects with T2D. ^Evidence of sex difference in the association signal, with significant association detected only for women.
In this issue of the Journal of the American Society of Nephrology, Sandholm et al.13 from the SUMMIT Consortium report findings from one of the largest international collaborative efforts in the search for genetic factors for DKD in T1D to date. This major undertaking included >5000 individuals (2563 patients and 2593 controls) in the discovery GWAS and additional samples in the replication phases. Whole-exome sequencing was performed in 997 subjects to explore the contribution of low-frequency variants, and a wide range of definitions of DKD was applied to examine potential association. Although no genetic variants were identified to have association at genome-wide significance, three variants (rs1989248 near CNTNAP2, rs61277444 in PTPN13, and rs7562121 in AFF3) showed suggestive evidence of association through joint meta-analysis of data from two stages, with additional supporting evidence of association for the AFF3 variant after additional de novo genotyping in phase 3. Notably, variants in AFF3 have already been reported in a previous GWAS meta–analysis, which included substantial overlap of study subjects from this one.14
This study provided the first large–scale estimate of heritability of DKD in T1D using genotyping data (from the FinnDiane Study in this case). The estimates of heritability obtained ranged from 0.35 to 0.59 for the different DKD phenotypes, with an estimate of 0.47 for ESRD heritability in T1D. These estimates using the genetic markers captured on the genotyping arrays to compare genetic similarity between individuals have yielded estimates that are broadly similar to those generated from earlier family studies.10
The study team has also conducted comprehensive evaluation of variants previously reported in candidate gene–based studies and GWASs of other forms of kidney diseases. In this analysis, only a few of the previously reported variants for diabetic nephropathy and CKD were found to show significant association, probably due to a combination of differences in the population being examined (presence or absence of T1D and differences in ethnicity) and the possibility of false positivity in some of the smaller earlier studies.
Interestingly, by constructing a weighted genetic risk score for diabetes, obesity, hypertension, or lipid-related phenotypes on the basis of 10–96 established loci for each phenotype from previous GWAS, the SUMMIT Consortium investigators also examined the relationship between the genetic risk for different cardiometabolic phenotypes and the risk of DKD. A genetic risk score constructed from genetic variants for obesity and body mass index was associated with the risk of DKD, suggesting a possible causal role of obesity for DKD, despite the study population consisting of only individuals with T1D. This finding highlights the potential contribution of metabolic effects beyond hyperglycemia in the pathogenesis of DKD and that a combination of metabolic and hemodynamic factors is likely to be important for kidney complications associated with T1D as well as T2D.15 This finding is consistent with studies on the effects of obesity and related cardiometabolic traits in DKD in T1D12 and the pleiotropic effects of variants associated with diabetes and obesity.8 Interestingly, a similar phenomenon was observed in DKD of T2D, in which variants associated with glucose traits or diabetes were associated with development and progression of DKD in subjects with T2D,16 highlighting the potential overlap between genetic factors for DKD and diabetes. Given the current global epidemic of obesity, this observation has important clinical implications. Patients with T1D are increasingly complicated by coexisting obesity and associated metabolic abnormalities, which may accelerate the development of kidney complications. Notably, multifactorial interventions, including weight loss, or targeting hyperglycemia, hypertension, and hyperlipidemia have been found to be associated with reduced development and progression of DKD in patients with T2D.17–19 Application of LD score regression confirmed high genetic correlation between the different DKD phenotypes examined but also gave support to the epidemiologic observation of a link between cigarette smoking and kidney disease in both T1D and T2D.20–22
Although traditional GWAS approaches have focused on identifying single variants that reach stringent statistical thresholds, it is increasingly appreciated that useful insights can be gained from pathway- and network-based analyses using a larger proportion of variants from these studies.23 Gene set enrichment analyses of GWAS results in this study have revealed the potential role of ascorbate and aldarate metabolism in DKD and provide novel hypotheses for additional investigation.
Despite these insights, much of the heritability of DKD remains unexplained. Low-frequency variants have been postulated to contribute to the missing heritability of common diseases, such as T2D, although a recent large–scale study suggests that the contribution of low-frequency variants to T2D risk is likely to be modest.8 In this study, whole-exome sequencing was performed on 997 subjects with rapid onset of macroalbuminuria or ESRD and controls with normal albumin excretion rate despite long duration of T1D in an exploratory study. This found potential association between variants in ERBB4 and ESRD, although other variants in this gene have already been previously implicated through earlier studies. As noted by the authors, a more comprehensive evaluation of the role of low-frequency variants in DKD will require much larger sample sizes. Epigenetic effects may be another important component of the missing heritability. Epigenetic programming is increasingly recognized as an important mechanism mediating developmental exposures and effects24 and seems to play a key role in the legacy effect of hyperglycemia.25
Where does this study leave us? Together with other international genetics research consortia, this study has highlighted the need for large collaborative efforts to address the issue of sample size. This is exemplified by the recent success from the Juvenile Diabetes Research Foundation Diabetic Nephropathy Collaborative Initiative, another major international collaboration, and with genotype data from >15,000 individuals, the largest study of genetics of DKD in T1D to date.26 Ongoing efforts to develop platforms to facilitate large–scale genetic analyses may help to address this challenge.27,28 The current difficulty in being able to aggregate cohorts of sufficiently large sample sizes and the paucity of functional genetic variants also highlight the need to use additional datasets to gain biologic insights into possible gene candidates and mechanisms. Unfortunately, renal transcriptomic data are not currently available from the Genotype-Phenotype Expression Project.29 The availability of renal transcriptomic or eQTL data would be of much value.30 Likewise, studies have increasingly used data from different omics technologies, and collectively, these different approaches may help yield novel insights.12,31
Although this effort from the SUMMIT Consortium has highlighted some of the challenges that face investigators tackling this problem, the study also presented several approaches that can help piece together this difficult puzzle. Although the genetics of diabetic complications might justifiably be considered the worst of nightmares for geneticists, there does seem to be ground for some optimism that, with larger studies underway and additional datasets being made available, we should not be too far away from the dawn of some major breakthrough in this quest.
Disclosures
None.
Acknowledgments
R.C.W.M. acknowledges support from Research Grants Council Theme-based research scheme T12-402/13-N, the Focused Innovation Scheme, and VC One-Off Discretionary Fund of the Chinese University of Hong Kong.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related article, “The Genetic Landscape of Renal Complications in Type 1 Diabetes,” on pages 557–574.
References
- 1.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L: Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 370: 1514–1523, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Afkarian M, Zelnick LR, Hall YN, Heagerty PJ, Tuttle K, Weiss NS, de Boer IH: Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. JAMA 316: 602–610, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E, Eggers PW, Gillen D, Gipson D, Hailpern SM, Hall YN, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Kalantar-Zadeh K, Kovesdy CP, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, Nguyen DV, O'Hare AM, Plattner B, Pisoni R, Port FK, Rao P, Rhee CM, Sakhuja A, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, White S, Woodside K, Hirth RA: US Renal Data System 2015 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 61: S1–305, 2015
- 4.Chan JC, Lau ES, Luk AO, Cheung KK, Kong AP, Yu LW, Choi KC, Chow FC, Ozaki R, Brown N, Yang X, Bennett PH, Ma RC, So WY: Premature mortality and comorbidities in young-onset diabetes: A 7-year prospective analysis. Am J Med 127: 616–624, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Thomas MC, Cooper ME, Zimmet P: Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol 12: 73–81, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Plenge RM, Scolnick EM, Altshuler D: Validating therapeutic targets through human genetics. Nat Rev Drug Discov 12: 581–594, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Scott RA, Freitag DF, Li L, Chu AY, Surendran P, Young R, Grarup N, Stancáková A, Chen Y, Varga TV, Yaghootkar H, Luan J, Zhao JH, Willems SM, Wessel J, Wang S, Maruthur N, Michailidou K, Pirie A, van der Lee SJ, Gillson C, Al Olama AA, Amouyel P, Arriola L, Arveiler D, Aviles-Olmos I, Balkau B, Barricarte A, Barroso I, Garcia SB, Bis JC, Blankenberg S, Boehnke M, Boeing H, Boerwinkle E, Borecki IB, Bork-Jensen J, Bowden S, Caldas C, Caslake M, Cupples LA, Cruchaga C, Czajkowski J, den Hoed M, Dunn JA, Earl HM, Ehret GB, Ferrannini E, Ferrieres J, Foltynie T, Ford I, Forouhi NG, Gianfagna F, Gonzalez C, Grioni S, Hiller L, Jansson JH, Jørgensen ME, Jukema JW, Kaaks R, Kee F, Kerrison ND, Key TJ, Kontto J, Kote-Jarai Z, Kraja AT, Kuulasmaa K, Kuusisto J, Linneberg A, Liu C, Marenne G, Mohlke KL, Morris AP, Muir K, Müller-Nurasyid M, Munroe PB, Navarro C, Nielsen SF, Nilsson PM, Nordestgaard BG, Packard CJ, Palli D, Panico S, Peloso GM, Perola M, Peters A, Poole CJ, Quirós JR, Rolandsson O, Sacerdote C, Salomaa V, Sánchez MJ, Sattar N, Sharp SJ, Sims R, Slimani N, Smith JA, Thompson DJ, Trompet S, Tumino R, van der A DL, van der Schouw YT, Virtamo J, Walker M, Walter K, Abraham JE, Amundadottir LT, Aponte JL, Butterworth AS, Dupuis J, Easton DF, Eeles RA, Erdmann J, Franks PW, Frayling TM, Hansen T, Howson JM, Jørgensen T, Kooner J, Laakso M, Langenberg C, McCarthy MI, Pankow JS, Pedersen O, Riboli E, Rotter JI, Saleheen D, Samani NJ, Schunkert H, Vollenweider P, O’Rahilly S, Deloukas P, Danesh J, Goodarzi MO, Kathiresan S, Meigs JB, Ehm MG, Wareham NJ, Waterworth DM; CVD50 consortium; GERAD_EC Consortium; Neurology Working Group of the Cohorts for Heart; Aging Research in Genomic Epidemiology (CHARGE); Alzheimer’s Disease Genetics Consortium; Pancreatic Cancer Cohort Consortium; European Prospective Investigation into Cancer and Nutrition–Cardiovascular Disease (EPIC-CVD); EPIC-InterAct; CHARGE consortium; CHD Exome+ Consortium; CARDIOGRAM Exome Consortium: A genomic approach to therapeutic target validation identifies a glucose-lowering GLP1R variant protective for coronary heart disease. Sci Transl Med 8: 341ra76, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuchsberger C, Flannick J, Teslovich TM, Mahajan A, Agarwala V, Gaulton KJ, Ma C, Fontanillas P, Moutsianas L, McCarthy DJ, Rivas MA, Perry JR, Sim X, Blackwell TW, Robertson NR, Rayner NW, Cingolani P, Locke AE, Fernandez Tajes J, Highland HM, Dupuis J, Chines PS, Lindgren CM, Hartl C, Jackson AU, Chen H, Huyghe JR, van de Bunt M, Pearson RD, Kumar A, Müller-Nurasyid M, Grarup N, Stringham HM, Gamazon ER, Lee J, Chen Y, Scott RA, Below JE, Chen P, Huang J, Go MJ, Stitzel ML, Pasko D, Parker SC, Varga TV, Green T, Beer NL, Day-Williams AG, Ferreira T, Fingerlin T, Horikoshi M, Hu C, Huh I, Ikram MK, Kim BJ, Kim Y, Kim YJ, Kwon MS, Lee J, Lee S, Lin KH, Maxwell TJ, Nagai Y, Wang X, Welch RP, Yoon J, Zhang W, Barzilai N, Voight BF, Han BG, Jenkinson CP, Kuulasmaa T, Kuusisto J, Manning A, Ng MC, Palmer ND, Balkau B, Stancáková A, Abboud HE, Boeing H, Giedraitis V, Prabhakaran D, Gottesman O, Scott J, Carey J, Kwan P, Grant G, Smith JD, Neale BM, Purcell S, Butterworth AS, Howson JM, Lee HM, Lu Y, Kwak SH, Zhao W, Danesh J, Lam VK, Park KS, Saleheen D, So WY, Tam CH, Afzal U, Aguilar D, Arya R, Aung T, Chan E, Navarro C, Cheng CY, Palli D, Correa A, Curran JE, Rybin D, Farook VS, Fowler SP, Freedman BI, Griswold M, Hale DE, Hicks PJ, Khor CC, Kumar S, Lehne B, Thuillier D, Lim WY, Liu J, van der Schouw YT, Loh M, Musani SK, Puppala S, Scott WR, Yengo L, Tan ST, Taylor HA Jr., Thameem F, Wilson G Sr., Wong TY, Njølstad PR, Levy JC, Mangino M, Bonnycastle LL, Schwarzmayr T, Fadista J, Surdulescu GL, Herder C, Groves CJ, Wieland T, Bork-Jensen J, Brandslund I, Christensen C, Koistinen HA, Doney AS, Kinnunen L, Esko T, Farmer AJ, Hakaste L, Hodgkiss D, Kravic J, Lyssenko V, Hollensted M, Jørgensen ME, Jørgensen T, Ladenvall C, Justesen JM, Käräjämäki A, Kriebel J, Rathmann W, Lannfelt L, Lauritzen T, Narisu N, Linneberg A, Melander O, Milani L, Neville M, Orho-Melander M, Qi L, Qi Q, Roden M, Rolandsson O, Swift A, Rosengren AH, Stirrups K, Wood AR, Mihailov E, Blancher C, Carneiro MO, Maguire J, Poplin R, Shakir K, Fennell T, DePristo M, Hrabé de Angelis M, Deloukas P, Gjesing AP, Jun G, Nilsson P, Murphy J, Onofrio R, Thorand B, Hansen T, Meisinger C, Hu FB, Isomaa B, Karpe F, Liang L, Peters A, Huth C, O’Rahilly SP, Palmer CN, Pedersen O, Rauramaa R, Tuomilehto J, Salomaa V, Watanabe RM, Syvänen AC, Bergman RN, Bharadwaj D, Bottinger EP, Cho YS, Chandak GR, Chan JC, Chia KS, Daly MJ, Ebrahim SB, Langenberg C, Elliott P, Jablonski KA, Lehman DM, Jia W, Ma RC, Pollin TI, Sandhu M, Tandon N, Froguel P, Barroso I, Teo YY, Zeggini E, Loos RJ, Small KS, Ried JS, DeFronzo RA, Grallert H, Glaser B, Metspalu A, Wareham NJ, Walker M, Banks E, Gieger C, Ingelsson E, Im HK, Illig T, Franks PW, Buck G, Trakalo J, Buck D, Prokopenko I, Mägi R, Lind L, Farjoun Y, Owen KR, Gloyn AL, Strauch K, Tuomi T, Kooner JS, Lee JY, Park T, Donnelly P, Morris AD, Hattersley AT, Bowden DW, Collins FS, Atzmon G, Chambers JC, Spector TD, Laakso M, Strom TM, Bell GI, Blangero J, Duggirala R, Tai ES, McVean G, Hanis CL, Wilson JG, Seielstad M, Frayling TM, Meigs JB, Cox NJ, Sladek R, Lander ES, Gabriel S, Burtt NP, Mohlke KL, Meitinger T, Groop L, Abecasis G, Florez JC, Scott LJ, Morris AP, Kang HM, Boehnke M, Altshuler D, McCarthy MI: The genetic architecture of type 2 diabetes. Nature 536: 41–47, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rich SS: Diabetes: Still a geneticist’s nightmare. Nature 536: 37–38, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Ma RC: Genetics of cardiovascular and renal complications in diabetes. J Diabetes Investig 7: 139–154, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mooyaart AL, Valk EJ, van Es LA, Bruijn JA, de Heer E, Freedman BI, Dekkers OM, Baelde HJ: Genetic associations in diabetic nephropathy: A meta-analysis. Diabetologia 54: 544–553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas MC, Brownlee M, Susztak K, Sharma K, Jandeleit-Dahm KA, Zoungas S, Rossing P, Groop PH, Cooper ME: Diabetic kidney disease. Nat Rev Dis Primers 1: 15018, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandholm N, Van Zuydam N, Ahlqvist E, Juliusdottir T, Deshmukh HA, Rayner NW, Di Camillo B, Forsblom C, Fadista J, Ziemek D, Salem RM, Hiraki LT, Pezzolesi M, Trégouët D, Dahlström E, Valo E, Oskolkov N, Ladenvall C, Marcovecchio ML, Cooper J, Sambo F, Malovini A, Manfrini M, McKnight AJ, Lajer M, Harjutsalo V, Gordin D, Parkkonen M, Tuomilehto J, Lyssenko V, McKeigue PM, Rich SS, Brosnan MJ, Fauman E, Bellazzi R, Rossing P, Hadjadj S, Krolewski A, Paterson AD, Florez JC, Hirschhorn JN, Maxwell AP, Dunger D, Cobelli C, Colhoun HM, Groop L, McCarthy MI, Groop PH; FinnDiane Study Group; DCCT/EDIC Study Group; GENIE Consortium; SUMMIT Consortium: The genetic landscape of renal complications in type 1 diabetes. J Am Soc Nephrol 28: 557–574, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandholm N, Salem RM, McKnight AJ, Brennan EP, Forsblom C, Isakova T, McKay GJ, Williams WW, Sadlier DM, Mäkinen VP, Swan EJ, Palmer C, Boright AP, Ahlqvist E, Deshmukh HA, Keller BJ, Huang H, Ahola AJ, Fagerholm E, Gordin D, Harjutsalo V, He B, Heikkilä O, Hietala K, Kytö J, Lahermo P, Lehto M, Lithovius R, Osterholm AM, Parkkonen M, Pitkäniemi J, Rosengård-Bärlund M, Saraheimo M, Sarti C, Söderlund J, Soro-Paavonen A, Syreeni A, Thorn LM, Tikkanen H, Tolonen N, Tryggvason K, Tuomilehto J, Wadén J, Gill GV, Prior S, Guiducci C, Mirel DB, Taylor A, Hosseini SM, Parving HH, Rossing P, Tarnow L, Ladenvall C, Alhenc-Gelas F, Lefebvre P, Rigalleau V, Roussel R, Tregouet DA, Maestroni A, Maestroni S, Falhammar H, Gu T, Möllsten A, Cimponeriu D, Ioana M, Mota M, Mota E, Serafinceanu C, Stavarachi M, Hanson RL, Nelson RG, Kretzler M, Colhoun HM, Panduru NM, Gu HF, Brismar K, Zerbini G, Hadjadj S, Marre M, Groop L, Lajer M, Bull SB, Waggott D, Paterson AD, Savage DA, Bain SC, Martin F, Hirschhorn JN, Godson C, Florez JC, Groop PH, Maxwell AP; DCCT/EDIC Research Group: New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet 8: e1002921, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forbes JM, Cooper ME: Mechanisms of diabetic complications. Physiol Rev 93: 137–188, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Jiang G, Hu C, Tam CH, Lau ES, Wang Y, Luk AO, Yang X, Kong AP, Ho JS, Lam VK, Lee HM, Wang J, Zhang R, Tsui SK, Ng MC, Szeto CC, Jia W, Fan X, So WY, Chan JC, Ma RC: Genetic and clinical variables identify predictors for chronic kidney disease in type 2 diabetes. Kidney Int 89: 411–420, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Gaede P, Vedel P, Parving H-H, Pedersen O: Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: The Steno type 2 randomised study. Lancet 353: 617–622, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Look AHEAD Research Group: Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: A secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2: 801–809, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan JC, So WY, Yeung CY, Ko GT, Lau IT, Tsang MW, Lau KP, Siu SC, Li JK, Yeung VT, Leung WY, Tong PC; SURE Study Group: Effects of structured versus usual care on renal endpoint in type 2 diabetes: The SURE study: A randomized multicenter translational study. Diabetes Care 32: 977–982, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawicki PT, Didjurgeit U, Mühlhauser I, Bender R, Heinemann L, Berger M: Smoking is associated with progression of diabetic nephropathy. Diabetes Care 17: 126–131, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Feodoroff M, Harjutsalo V, Forsblom C, Thorn L, Wadén J, Tolonen N, Lithovius R, Groop PH: Smoking and progression of diabetic nephropathy in patients with type 1 diabetes. Acta Diabetol 53: 525–533, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Chuahirun T, Wesson DE: Cigarette smoking predicts faster progression of type 2 established diabetic nephropathy despite ACE inhibition. Am J Kidney Dis 39: 376–382, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Chan KH, Huang YT, Meng Q, Wu C, Reiner A, Sobel EM, Tinker L, Lusis AJ, Yang X, Liu S: Shared molecular pathways and gene networks for cardiovascular disease and type 2 diabetes mellitus in women across diverse ethnicities. Circ Cardiovasc Genet 7: 911–919, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Ma RC, Tutino GE, Lillycrop KA, Hanson MA, Tam WH: Maternal diabetes, gestational diabetes and the role of epigenetics in their long term effects on offspring. Prog Biophys Mol Biol 118: 55–68, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Cooper ME, El-Osta A: Epigenetics: Mechanisms and implications for diabetic complications. Circ Res 107: 1403–1413, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Todd J, Salem RM, Sandholm N, Valo EA, Hiraki LT, Liao CD, Pezzolesi MG, Smiles A, Onengut-Gumuscu G, Chen WM, McGurnaghan S, McKeigue PM, McKnight AJ, Maxwell AP, Colhoun HM, Krolewski AS, Paterson AD, Rich SS, Hirschhorn JN, Florez JC: Novel genetic determinants of diabetic kidney disease. Diabetes 65[Suppl 1]: A100, 2016 [Google Scholar]
- 27.Accelerating Medicines Partnership (AMP): Type 2 Diabetes Knowledge Portal, 2016. Available at: http://www.type2diabetesgenetics.org/. Accessed September 16, 2016
- 28.InterConnect: Global Data for Diabetes and Obesity Research, 2016. Available at: http://www.interconnect-diabetes.eu/. Accessed September 16, 2016
- 29.GTEx Consortium: Human genomics. The genotype-tissue expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 348: 648–660, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Applied Systems Biology Core: University of Michigan O'Brien Renal Center, Neprhoseq, 2016. Available at: https://www.nephroseq.org/resource/login.html. Accessed September 16, 2016
- 31.Darshi M, Van Espen B, Sharma K: Metabolomics in diabetic kidney disease: Unraveling the biochemistry of a silent killer. Am J Nephrol 44: 92–103, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]