Abstract
Purpose
The authors investigated how different variants of clear speech affect segmental and suprasegmental acoustic measures of speech in speakers with Parkinson's disease and a healthy control group.
Method
A total of 14 participants with Parkinson's disease and 14 control participants served as speakers. Each speaker produced 18 different sentences selected from the Sentence Intelligibility Test (Yorkston & Beukelman, 1996). All speakers produced stimuli in 4 speaking conditions (habitual, clear, overenunciate, and hearing impaired). Segmental acoustic measures included vowel space area and first moment (M1) coefficient difference measures for consonant pairs. Second formant slope of diphthongs and measures of vowel and fricative durations were also obtained. Suprasegmental measures included fundamental frequency, sound pressure level, and articulation rate.
Results
For the majority of adjustments, all variants of clear speech instruction differed from the habitual condition. The overenunciate condition elicited the greatest magnitude of change for segmental measures (vowel space area, vowel durations) and the slowest articulation rates. The hearing impaired condition elicited the greatest fricative durations and suprasegmental adjustments (fundamental frequency, sound pressure level).
Conclusions
Findings have implications for a model of speech production for healthy speakers as well as for speakers with dysarthria. Findings also suggest that particular clear speech instructions may target distinct speech subsystems.
Clear speech is a strategy talkers use to maximize the likelihood of being understood. Speakers naturally might increase speech clarity in a noisy environment or when trying to repair communication breakdowns (Uchanski, 2005). A clear speaking style has also been recommended as a behavioral therapy technique for speakers with a variety of dysarthrias and neurological diagnoses, including Parkinson's disease (PD; Duffy, 2005; Hustad & Weismer, 2007). However, the instructions used to elicit clear speech vary widely across studies. In our previous research with young healthy adult speakers, three variants of clear speech instruction—including “Overenunciate each word,” “Speak to someone with a hearing impairment,” and “Speak clearly”—differentially affected the magnitude of acoustic adjustments and, in turn, positively correlated to the amount of clear speech intelligibility benefit (Lam & Tjaden, 2013a, 2013b; Lam, Tjaden, & Wilding, 2012). In the current study, we extend this line of inquiry to speakers with PD and a group of age- and sex-matched controls.
Investigating the underlying acoustic changes that accompany clear speech variants in speakers with PD is important for optimizing clear speech training programs (e.g., Park, Theodoros, Finch, & Cardell, 2014). Furthermore, improved understanding of the acoustic basis of dysarthria can inform therapy decisions (Duffy, 2013) as well as provide insight into a model of dysarthria (Duffy, 2005; Kain, Amano-Kusumoto, & Hosom, 2008; Mengistu & Rudzicz, 2011). This work also has implications for Lindblom's (1990) hypo–hyperarticulate theory of typical speech production. The hypo–hyperarticulate theory describes speech production on a continuum wherein hypoarticulation, or conversational speech, represents one end of the continuum and hyperarticulation, or clear speech, represents the other end. In our previous work, we found that young healthy speakers produced a gradient of acoustic adjustments, similar to those described in Lindblom's hypo–hyperarticulate theory, in response to three variants of clear speech instruction (clear, hearing impaired, and overenunciate). Although the hypo–hyperarticulate theory is frequently discussed in the context of neurologically typical speech production, it is unclear whether the hypo–hyperarticulate theory can be extended to speakers with neurological impairment. In this manner, the current study provides insight into the application of Lindblom's hypo–hyperarticulate theory beyond that of neurologically healthy speakers.
Acoustic Characteristics of PD
Speakers with PD often present with hypokinetic dysarthria that is characterized perceptually by a decreased vocal intensity, articulatory imprecision, diminished prosodic modulation, and a breathy or harsh voice (Darley, Aronson, & Brown, 1969). More recent acoustic studies have helped characterize the nature of the speech impairment in PD. For example, relative to healthy controls, speakers with PD produce more centralized vowel space areas (VSAs; Liu, Tsao, & Kuhl, 2005; Tjaden, Lam, & Wilding, 2013; Turner, Tjaden, & Weismer, 1995; Weismer, Jeng, Laures, Kent, & Kent, 2001), less spectrally distinct consonants (Tjaden & Wilding, 2004), weakened or less precise stop closures (Ackermann & Ziegler, 1991), and shallower second formant (F2) slopes, indicating slowed changes in vocal tract adjustments (Kent & Adams, 1989; Kim, Kent, & Weismer, 2011; Walsh & Smith, 2011). At the suprasegmental level, speakers with PD tend to pause more often (Torp & Hammen, 2000; but see Goberman & Elmer, 2005), utilize an increased fundamental frequency (F0; Canter, 1963; Goberman, Coelho, & Robb, 2002), and exhibit decreased variability in F0 (Canter, 1963, 1965; Flint, Black, Campbell-Taylor, Gailey, & Levinton, 1992; Skodda, Visser, & Schlegel, 2011a). Some studies have also reported reduced sound pressure levels (SPLs; Fox & Ramig, 1997; Skodda, Visser, & Schlegel, 2011b; Tjaden et al., 2013; Walsh & Smith, 2011). However, other studies have reported no differences in mean SPL for speakers with PD relative to healthy controls (Canter, 1963; Sadagopan & Huber, 2007; Tjaden & Wilding, 2004). Last, a variety of studies have reported rate abnormalities in PD (Canter, 1963, 1965; Flint et al., 1992; Hammen & Yorkston, 1996; Ludlow & Bassich, 1983; Metter & Hanson, 1986; Solomon & Hixon, 1993; Skodda & Schlegel, 2008), although other studies reported no differences in rate relative to healthy controls (Ackermann & Ziegler, 1991; Caligiuri, 1989; Goberman et al., 2002). It is interesting to note that speakers with PD can compensate for some of the disease-related changes in production using speech clarity techniques, resulting in a reduced articulation rate, increased F0 variability, increased VSA, and increased SPL (Dromey, 2000; Goberman & Elmer, 2005; Tjaden et al., 2013; Tjaden, Richards, Kuo, Wilding, & Sussman, 2014; Tjaden, Sussman, & Wilding, 2014). Thus, as elaborated in the following section, clear speech appears to hold promise as a therapy technique for addressing the speech impairment in PD. First, however, it is useful to consider briefly the sizeable clear speech literature for neurologically healthy talkers.
Clear Speech Acoustic Adjustments
A variety of segmental and suprasegmental changes have been reported in the literature on typical clear speech. For example, clear speech studies have reported increased VSAs (Bradlow, Kraus, & Hayes, 2003; Ferguson & Kewley-Port, 2002, 2007; Johnson, Flemming, & Wright, 1993; Lam et al., 2012; Moon & Lindblom, 1994; Picheny, Durlach, & Braida, 1986; but see Krause & Braida, 2004) and increased rates of spectral change in clearly produced monophthongs, diphthongs, and liquid transitions relative to conversational speech (Ferguson & Kewley-Port, 2007; Ferguson & Quené, 2014; Lam et al., 2012; Moon & Lindblom, 1994; Tjaden et al., 2014; Wouters & Macon, 2002; but see Tasko & Greilick, 2010). Clear speech effects have also been studied in consonants produced by healthy speakers, wherein clearly produced fricatives tend to be longer in duration (Maniwa, Jongman, & Wade, 2009). Spectral moment analyses also have shown that clear speech tends to elicit more anterior places of articulation, as indicated by higher measures of spectral mean (Maniwa et al., 2009). A clear speaking style is also associated with suprasegmental changes such as increased vocal intensities (Lam et al., 2012; Moon & Lindblom, 1994; Picheny, Durlach, & Braida, 1985), reduced speaking rates (Bradlow et al., 2003; Lam et al., 2012; Picheny, Durlach, & Braida, 1989; but see Krause & Braida, 2002, 2004), increased mean and variation in F0 (Bradlow et al., 2003), and increased pause durations and pause frequency (Picheny et al., 1985).
Many of the same segmental and suprasegmental acoustic adjustments produced by healthy speakers have also been reported in the few published clear speech studies of dysarthria (Goberman & Elmer, 2005; Tjaden et al., 2013; Tjaden et al., 2014; Whitfield & Goberman, 2014). In these studies, the majority of speakers with PD increased VSA in clear relative to conversational speech. A clear speech technique can also help speakers with PD produce increased vocal intensity, increased spectral change, higher mean F0, greater F0 variation, and slower articulation rates (Dromey, 2000; Goberman & Elmer, 2005; Tjaden et al., 2014; Tjaden et al., 2014). Clear speech therefore appears to be a feasible therapeutic strategy for addressing speech impairment secondary to PD. As discussed in the following section, however, the instructions used to elicit clear speech vary widely across dysarthria studies and in studies of neurologically typical speech.
Clear Speech Instructions
Common instructions for eliciting clear speech include “Speak clearly” (Ferguson, 2004; Ferguson & Kewley-Port, 2007), “Hyperarticulate” (Dromey, 2000; Moon & Lindblom, 1994), “Speak to someone with a hearing impairment” (Bradlow et al., 2003), “Speak with nonnative speakers” (Smiljanic & Bradlow, 2008), and “Speak to someone who has difficulty understanding you” (Goberman & Elmer, 2005; Rosen et al., 2011). Other studies have elicited clear speech through an imitation task or training paradigm (Beukelman, Fager, Ullman, Hanson, & Logemann, 2002; Krause & Braida, 2004), by providing a grade or reward system (Perkell, Zandipour, Matthies, & Lane, 2002), or by asking for a repetition (Maniwa et al., 2009; Oviatt, MacEachern, & Levow, 1998). Hazan and Baker (2011) elicited clear speech by simulating a hearing impairment during a joint problem-solving task between two people. Thus, the speaker without impairment would be using a strategy similar to “speaking to someone with a hearing impairment” in order to communicate to the partner with the simulated hearing loss. Furthermore, some studies have elicited clear speech using a combination of the instructions listed above (Searl & Evitts, 2013; Tjaden et al., 2013). To date, studies directly comparing clear speech variants have been conducted only in young healthy adults.
In a study of clear speech instruction in young healthy adult speakers, Lam et al. (2012) reported that although similar types of acoustic adjustments were associated with variants of clear instruction, the magnitude of adjustment differed as a function of instruction. Measures such as VSA, vowel spectral change, segment durations, and articulation rate were associated with the greatest acoustic change when speakers were instructed to “overenunciate each word” (overenunciate condition), followed by moderate change when speakers were told to “speak to someone with a hearing impairment” (hearing impaired condition) and “speak clearly” (clear condition). SPL, however, was slightly higher in the hearing impaired condition relative to the habitual condition, followed by the overenunciate condition.
A follow-up study by Lam and Tjaden (2013b) further showed that clear speech variants produced a gradient of intelligibility benefits wherein the overenunciate condition produced the largest clear speech benefits, followed by the hearing impaired and clear conditions. Acoustic variables likely explaining these intelligibility variations are suggested in a variety of studies. For example, segmental metrics such as static and dynamic vowel measures, consonant spectral moments, and consonant distinctiveness measures have been reported as predictors of intelligibility (Amano-Kusumoto, Holsom, Kain, & Aronoff, 2014; Fogerty, 2013; Fogerty & Humes, 2010; Kay, 2012; Kim et al., 2011; Lansford & Liss, 2014; Maniwa, Jongman, & Wade, 2008; Owren & Cardillo, 2006; Tjaden & Wilding, 2004; Turner et al., 1995; Weismer, Martin, Kent, & Kent, 1992). In addition, suprasegmental measures of F0 and global timing measures have shown to be strong predictors of intelligibility (Bradlow, Torretta, & Pisoni, 1996; Bunton, Kent, Kent, & Duffy, 2001; Kim et al., 2011; Laures & Weismer, 1999).
In summary, a clear speech style elicits a variety of acoustic adjustments at both the segmental and suprasegmental levels. Not only are these adjustments observed in healthy controls, but several dysarthria studies have indicated that clear speech is a promising therapeutic technique for addressing the speech impairment in PD. Across studies, a variety of instructions have been used to elicit a clear speaking style, and it is unknown how variants of clear speech instruction affect the acoustic signal in speakers with PD. This knowledge is essential for optimizing therapeutic use of a clear speaking style and would enhance the scientific evidence base for dysarthria treatment. Therefore, in the current study we investigated how different variants of clear speech affect segmental and suprasegmental acoustic measures in speakers with PD.
Method
Participants
A total of 28 participants were recruited for the study, including 14 speakers with PD (nine men, five women) and 14 age- and sex-matched healthy control speakers. The ratio of men to women is in line with research indicating that PD is diagnosed approximately 1.5 times more often in men than in women (Wooten, Currie, Bovbjerg, Lee, & Patrie, 2004). Participant age ranged from 55 to 81 years, with a mean of 68.3 years (SD = 6.7) and 67.8 years (SD = 6.5) for the PD and control groups, respectively. All speakers underwent a bilateral audiological screening at the University at Buffalo's Speech and Hearing Clinic prior to speech recording. Hearing thresholds were obtained bilaterally at octave frequencies between 250 and 8000 Hz. Screening results were provided to each speaker as an indication of their overall hearing status but did not exclude speakers from participating (see also Sussman & Tjaden, 2012). Ten of the 14 speakers in each group had thresholds of 40 dB or better in at least one ear at 1, 2, and 4 kHz (Darling & Huber, 2011; Weinstein & Ventry, 1983). Thus, the same proportion of speakers in both groups had some degree of hearing loss, which is not atypical for individuals with an average age of more than 65 years. No participant wore a hearing aid or cochlear implant, and all participants were able to follow verbal instructions.
Inclusionary Criteria
Speakers were judged to speak Standard American English as a first language and were recruited from the Western New York region. Speakers were paid a modest fee for participating. All speakers had achieved at least a high school diploma and reported adequate vision for reading. All participants must have achieved a 26 or better on the Mini Mental State Examination (Molloy, 1999). Similar criteria have also been reported in the dysarthria literature (Bunton & Keintz, 2008; De Letter et al., 2010; Sussman & Tjaden, 2012). Control speakers reported no history of speech, language, or hearing pathology. Speakers with PD reported no history of neurological impairment other than PD, and any speech therapy received postdiagnosis was documented but did not exclude participants from the current study. Five speakers with PD reported receiving the Lee Silverman Voice Treatment (LSVT). Four of these individuals had completed the treatment program more than 2 years prior to the current study, and one speaker had completed the treatment more than 1 year prior to the current study. At the time of data collection, the speaker who had most recently completed LSVT and one other speaker with a history of LSVT were participating in weekly group therapy sessions practicing an increased vocal loudness. All speakers were required to report no history of neurosurgical treatments (i.e., deep brain stimulation) and no cochlear implantation and were required to have two unaided ears.
To document baseline intelligibility and speech severity, perceptual testing was completed by three speech-language pathologists (SLPs). All SLPs had at least 3 years of experience with dysarthria. For each speaker, 11 sentences were generated randomly using the Computerized Sentence Intelligibility Test (SIT; Yorkston & Beukelman, 1996). Perceptual testing took place over a 2-hr session, and all testing was completed in a quiet room via binaural headphones (MDRV300, Sony, Tokyo, Japan). Presentation of speech stimuli was blocked by speaker, and each SLP had a different random ordering of speakers. For every speaker, the intelligibility task was completed first followed by the speech severity task.
Procedures for the intelligibility task paralleled those of the SIT (Yorkston & Beukelman, 1996). Listeners were presented with sentences one at a time and asked to type out the words they heard. Following the intelligibility task, listeners then completed the speech severity task. Procedures and instructions for the severity task were adapted from Sussman and Tjaden (2012). For this task, the same 11 sentences from the transcription task were played continuously while listeners were asked to judge overall severity, “paying attention to voice quality, resonance, articulatory precision, speech rhythm, prosody, and naturalness … with out focusing on how understandable or intelligible the person is.” After all sentences were presented, listeners were prompted to make a single judgment of overall severity using a computerized visual analog scale. SLPs were presented with a vertical line 150 mm long and asked to click anywhere along the line, ranging from no impairment at the bottom of the scale to severely impaired at the top. Ratings were converted using custom software (MMscript; Johnson, 2010) to a scale of 0.0 to 1.0, where 0.0 represents no impairment and scores closer to 1.0 represent severe impairment.
Mean SIT scores for the PD and control groups were 98.7% (SD = 2.4%) and 97.9% (SD = 1.4%), respectively. Mean scaled severity scores could range from 0.0 to 1.0, where 0.0 represents no impairment and 1.0 represent severe impairment. On average, the PD group (0.27; SD = 0.19) was rated as more impaired than the control group (0.17; SD = 0.16). Comparison of speaker pairs (e.g., PD 1 vs. control 1) revealed that for all but three pairs of speakers, the speaker with PD was always rated as more impaired than the control speaker. Although SIT scores and scaled severity might suggest that speakers with PD in the current study did not have dysarthria, a majority of speakers reported developing speech difficulties after being diagnosed with PD. In addition, one third of the speakers in the PD group reported having had speech therapy postdiagnosis. Therefore, despite the lack of substantial differences in SIT or speech severity, speakers with PD who participated in the current study are representative of the clinical population that may pursue speech therapy.
Data Collection
Data collection occurred over two sessions. During the first session, the patient history, cognitive screening, audiological screening, and clinical speech sample were completed. During the second visit, speech recordings of experimental stimuli were collected. Each session was between 60 and 90 min in length. Sessions 1 and 2 were separated by at least 1 hr and were held no more than 5 days apart. In an attempt to control for potential medication effects, recording sessions for speakers with PD were scheduled 1 hr after taking antiparkinsonian medications.
Speakers were seated in a sound-treated booth in front of a computer screen. All speech stimuli were presented one at a time using PowerPoint (Microsoft, Redmond, WA). Speakers were recorded using an over-the-ear Isomax condenser microphone (E6IOP5L2; Countryman, Menlo Park, CA). A mouth-to-microphone distance of 6 cm was maintained throughout the recording session. Audio samples were recorded using a MobilePre USB preamp (M-Audio, Cumberland, RI) and digitized to a computer at a sampling rate of 22 kHz using TF32 (Milenkovic, 2005) and Praat (Boersma & Weenink, 2014). Two speakers were recorded in TF32; due to software complications, the remainder of the recordings were completed in Praat. A 1000-Hz calibration tone of known intensity was recorded prior to each recording session for the purpose of computing SPL measures from the acoustic signal. All acoustic analyses were completed using TF32 (Milenkovic, 2005).
Experimental Speech Stimuli
For each speaker within a group, experimental stimuli consisted of 18 different sentences, ranging from five to 12 words selected from the SIT (Yorkston & Beukelman, 1996). Fourteen different sentence sets were constructed for the 28 speakers. Therefore, each age- and sex-matched speaker pair (e.g., PD 1 and control 1) produced the same sentence set. Each sentence set included three to five occurrences of the monophthongs /i/, /u/, /ӕ/, /ɑ/, /ɪ/, /ʌ/, /ɛ/, and /ʊ/ and two to four occurrences of the diphthongs /ɑɪ/ and /eɪ/, produced in the stressed syllable of content words. In addition, each sentence set included at least three occurrences of the consonants /t/, /k/, /s/, and /ʃ/ in word-initial position. All sentence stimuli were randomized for presentation within each speaking condition.
Experimental stimuli were recorded in four speaking conditions: habitual, clear, hearing impaired, and overenunciate. The habitual condition was always recorded first. For the habitual condition, speakers were asked to read the sentences aloud. Six different orderings of the nonhabitual conditions (clear, hearing impaired, and overenunciate) were randomized and blocked across speakers. In the clear condition, speakers were instructed to “say the following sentences while speaking clearly.” For the hearing impaired condition, speakers were asked to “say the following sentences while speaking to someone with a hearing impairment,” and for the overenunciate condition, speakers were asked to “say the following sentences while overenunciating each word.” Written instructions for each condition were presented both visually and verbally once at the beginning and once midway throughout recording for each condition. Speakers were engaged in informal conversation or provided a break between conditions to minimize carryover effects.
Acoustic Measures
Prior to the acoustic analysis, the experimenter was blinded to the identity of each speaking condition to control for any experimenter bias during the acoustic analysis.
Segmental Vowel Measures
Linear predictive coding–generated formant trajectories for the first formant (F1) and F2 were computed in TF32 (Milenkovic, 2002) for each vowel nucleus. Formant tracks were manually corrected, and data files were extracted from TF32 and imported into Microsoft Excel for data reduction.
Measures of VSA were included as an index of vowel segmental integrity (Turner et al., 1995; Weismer, Laures, Jeng, Kent, & Kent, 2000). Previous research has suggested that changes in clear speech differentially affect tense and lax VSA (Chen, 1980; Lam et al., 2012; Picheny et al., 1985). Therefore, tense and lax VSAs were calculated separately from each of the four vowels, /i, u, ɑ, ӕ/ and /ɪ, ʊ, ʌ, ɛ/, respectively. Onsets and offsets were operationally defined as the first and last glottal pulse of the vocalic nucleus, respectively, as indicated by energy in both F1 and F2 (Tjaden, Rivera, Wilding, & Turner, 2005; Turner et al., 1995). For each speaker and condition, midpoint formant frequencies for F1 and F2 were averaged across all tokens of each vowel. The four tense or four lax vowels were used to form respective quadrilaterals. VSA was calculated using Heron's formula: (√(s(s − a)(s − b))s − c)), where s = (a + b + c)/2.
Slope measures were calculated for two diphthongs (/ɑɪ/ and /eɪ/) using criteria approximating the 20 Hz/20 ms rule (Tjaden et al., 2014; Weismer, Kent, Hodge, & Martin, 1988). For each speaker, condition, and diphthong, slopes were averaged across all tokens to obtain a single measure of mean F2 slope.
Segmental characteristics of stops and fricatives were quantified using M1 coefficient difference measures. M1 coefficients correspond to the mean frequency of the spectrum between 0 and 11 kHz. M1 difference measures were included to provide an objective measure of consonant distinctiveness for two consonant pairs: /t-k/ and /s-ʃ/. Following procedures from Maniwa et al. (2009), M1 coefficients for fricatives were obtained at 25%, 50%, and 75% of the fricative duration. For every speaker, condition, and fricative, M1 coefficients were then averaged across three time points. All audio files were fast Fourier transformed and pre-emphasized. A 20-ms hamming window was centered over each of the three time points. Fricative onsets were defined as the onset of frication indicated by aperiodic noise in the waveform and an increase of energy in the spectrogram. Likewise, offsets were defined as the point where intensity reached a minimum prior to the onset of vowel periodicity. Criteria were selected on the basis of procedures in Maniwa et al. (2009). Stop onsets were defined as the left edge of the first stop burst release, and offsets were defined as the point where aspiration intensity reached a minimum prior to the onset of vowel periodicity. For stops, M1 coefficients were obtained for a 20-ms interval starting at the left edge of the release burst (Tjaden & Wilding, 2004). For every speaker, condition, and stop consonant (t, k), the mean of M1 was averaged across productions. Average M1 values were used to calculate M1 difference measures. For each speaker and condition, difference measures were calculated from average M1 values for the consonant pairs /t-k/ and /s-ʃ/.
Measures of Global Timing and Segmental Timing
Articulation rate was included to reflect rate of speech produced per unit time, excluding pauses. Standard acoustic criteria were used to identify onsets and offsets of each utterance. Total utterance durations were calculated by subtracting times at offset and onset. Run durations were calculated by subtracting total utterance duration from any interword pauses of 200 ms or greater. Articulation rate (syllables/second) was calculated by dividing the total number of syllables by run duration. Mean articulation rate was calculated for each speaker and condition by averaging across sentences.
Two measures of segmental timing (vowel duration and consonant duration) were included in the current study. Operationally defined criteria (as described above) were used in identifying onsets and offsets for vowels (Tjaden et al., 2005; Turner et al., 1995). Vowel segment durations were averaged separately across tense (/i/, /u/, /ӕ/, and /ɑ/) and lax (/ɪ/, /ʌ/, /ʊ/, and /ɛ/) vowels for a given speaker and condition. Onsets and offsets described above for fricatives were used to calculate segment duration (in milliseconds). Fricative segment durations (in milliseconds) were averaged for each speaker as a function of fricative and condition.
Suprasegmental Measures
Two measures of F0 (mean and interquartile range; IQR) were included to capture prosodic characteristics of clear speech. F0 time histories for each utterance were inspected and hand corrected on the basis of inspection of the waveform. F0 measurements were made for all vocalic segments where a full glottal pulse could be identified. For each speaker and condition, utterance-level measures of mean F0 and F0 IQR were averaged across all sentences.
SPL was used to index vocal intensity. Using TF32, the root-mean-square voltage was converted into dB SPL using each speaker's calibration tone (Tjaden et al., 2005). For each speaker and condition, a measure of average intensity and intensity variation (standard deviation) was calculated by averaging measures across sentences.
Data Analysis
Descriptive statistics in the form of means and standard deviations were calculated for all dependent variables. All statistical analyses were completed using SPSS Statistics 22 (Windows version; IBM, Armonk, NY). A one-way repeated measures analysis of covariance was used to investigate differences for each acoustic measure as a function of condition and group. The between-subjects factor of group consisted of two levels: PD and control. The within-subject repeated measure of condition consisted of four factors: habitual, clear, hearing impaired, and overenunciate. An alpha level of .05 was used for all omnibus testing. Following a significant main effect of condition or significant Group × Condition interaction, Bonferroni-corrected post hoc analyses were completed. To control for sex differences in various dependent measures and unequal numbers of men and women, sex was included as a covariate in all analyses. Similar procedures have been used previously (Tjaden & Wilding, 2004, 2011).
Intrarater reliability and interrater reliability were completed for 10% of the data. Three speakers (one PD, two controls) were randomly selected for use in determining reliability. Absolute measurement errors and Pearson product–moment correlations were used to index reliability.
Intrarater Reliability
The correlations between the first and second sets of vowel segment, consonant segment, and run duration measures were .97 (mean absolute difference = 0.007 s, SD = 0.015 s), .99 (mean absolute difference = 0.005 s, SD = 0.006 s), and .99 (mean absolute difference = 0.03 s, SD = 0.11 s), respectively. The correlations between the first and second sets of midpoint F1 and F2 values for monophthongs and F2 slope measures were .99 (mean absolute difference = 22.2 Hz, SD = 61.1 Hz) and .93 (mean absolute difference = 0.63 Hz/ms, SD = 0.87 Hz/ms), respectively. The correlation between the first and second sets of M1 values was .99 (mean absolute difference = 0.11 Hz, SD = 0.18 Hz). The correlation between the first and second sets of SPL measures (mean and SPL standard deviation) was .99 (mean absolute difference = 1.0 dB, SD = 2.0 dB). Last, the correlation between the first and second sets of F0 measures (mean and F0 IQR) was .98 (mean absolute difference = 2.9 Hz, SD = 3.8 Hz).
Interrater Reliability
The second author, who was not involved in performing any of the original acoustic measures, performed the interrater reliability. The correlations between the first and second sets of vowel segment, consonant segment, and run durations were .97 (mean absolute difference = 0.007 s, SD = 0.013 s), .99 (mean absolute difference = 0.006 s, SD = 0.007 s), and .99 (mean absolute difference = 0.04 s, SD = 0.17 s), respectively. The correlations between the first and second sets of midpoint F1 and F2 values for monophthongs and F2 slope measures were .99 (mean absolute difference = 24.7 Hz, SD = 63.1 Hz) and .95 (mean absolute difference = 0.61 Hz/ms, SD = 0.72 Hz/ms), respectively. The correlation between the first and second sets of M1 values was .98 (mean absolute difference = 0.23 Hz, SD = 0.53 Hz). The correlation between the first and second sets of SPL measures (mean and SPL standard deviation) was .99 (mean absolute difference = 0.41 dB, SD = 1.1 dB). Last, the correlation between the first and second sets of F0 measures (mean and F0 IQR) was .98 (mean absolute difference = 3.8 Hz, SD = 4.7 Hz).
Results
Table 1 summarizes the results for parametric statistics of all segmental and suprasegmental measures of interest. Significant main effects (p < .05) are indicated by an asterisk.
Table 1.
Analysis of covariance results for each dependent variable.
| Dependent measure | Group | Condition | Group × Condition |
|---|---|---|---|
| Tense vowel space area | * | * | — |
| Lax vowel space area | — | * | — |
| Tense vowel duration | — | * | — |
| Lax vowel duration | — | * | — |
| F2 slope (ɑɪ) | — | — | — |
| F2 slope (eɪ) | — | — | — |
| /s/ duration | * | * | — |
| /ʃ/ duration | * | * | — |
| M1 difference stops | * | — | * |
| M1 difference fricatives | — | — | — |
| F0 mean | — | * | — |
| F0 IQR | — | * | — |
| Mean SPL | — | * | — |
| SPL standard deviation | * | — | — |
| Articulation rate | * | * | — |
Note. A dash indicates a nonsignificant effect. F2 = second formant; M1 = first moment; IQR = interquartile range; SPL = sound pressure level.
p < .05.
Segmental Measures
Statistical analyses indicated a significant effect of group for tense VSA, F(1, 25) = 5.53, p = .03, ηp 2 = .18, but not for lax VSA, F(1, 25) = 1.17, p = .29, ηp 2 = .05. On average, controls (318138 Hz2) produced significantly larger tense VSAs compared with speakers with PD (253869 Hz2). Although not statistically significant, a similar trend was observed for lax VSA (controls: 83457 Hz2; PD: 73853 Hz2). A significant effect of condition was also observed for both tense VSA, F(3, 25) = 17.33, p < .001, ηp 2 = .41, and lax VSA, F(3, 25) = 16.94, p < .001, ηp 2 = .65. Figure 1 illustrates that for both lax and tense vowels, the habitual condition was associated with the smallest VSAs, followed by the clear, hearing impaired, and overenunciate conditions. Post hoc analyses of tense VSA indicated that all pairs of conditions were significantly different (p < .001), with the exception of the hearing impaired versus clear conditions (p = .24). Post hoc analyses for lax VSA were similar, such that all pairwise comparisons were significantly different (p < .001), with the exception of the hearing impaired versus clear (p = .06) and hearing impaired versus overenunciate (p = .43) conditions. The Group × Condition interaction was not significant for either tense VSA, F(1, 25) = 0.813, p = .49, ηp 2 = .03, or lax VSA, F(1, 25) = 0.541, p = .66, ηp 2 = .02.
Figure 1.
Means and standard deviations (error bars) for vowel space area as a function of group (black = control, CON; gray = Parkinson's disease, PD), condition, and sex. Lax vowel space area is shown in the upper two panels, and tense vowel space area is shown in the lower two panels.
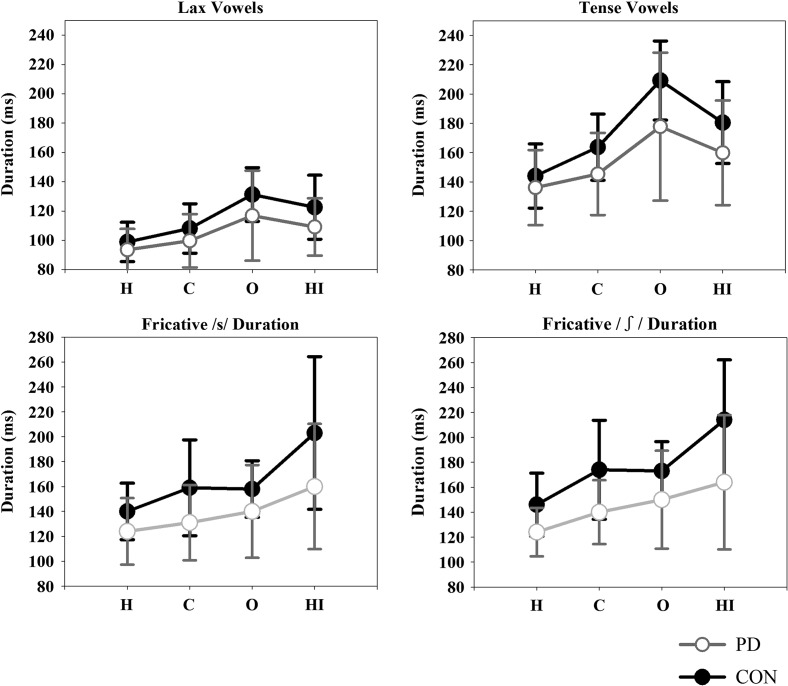
For both tense and lax vowel duration, the main effect of group was not significant; tense: F(1, 25) = 4.13, p = .05, ηp 2 = .14; lax: F(1, 25) = 2.77, p = .11, ηp 2 = .10. There was a significant effect of condition for both tense vowels, F(3, 25) = 32.65, p < .001, ηp 2 = .57, and lax vowels, F(3, 25) = 17.436, p < .001, ηp 2 = .70. Follow-up comparisons indicated that for tense vowels, all pairwise comparisons were significantly different (p < .05). Similar results were found for lax vowels, with the exception of the hearing impaired versus overenunciate (p = .52) comparison. The top panel of Figure 2 illustrates that on average, both tense and lax vowels were shortest in the habitual condition followed by the clear, hearing impaired, and overenunciate conditions. Last, the Group × Condition interaction was not significant for either tense vowel durations, F(3, 25) = 1.765, p = .16, ηp 2 = .07, or lax vowel durations, F(3, 25) = 0.800, p = .50, ηp 2 = .03.
Figure 2.
Means and standard deviations (error bars) for segment durations as a function of group (black = control, CON; gray = Parkinson's disease, PD) and condition. Vowel durations (lax and tense) and fricative durations (/s/ and /ʃ/) are shown in the upper and lower panels, respectively. H = habitual; C = clear; O = overenunciate; HI = hearing impaired.
For both fricatives, statistical analyses indicated a significant effect of group, /s/: F(1, 25) = 4.61, p = .04, ηp 2 = .16; /ʃ/: F(1, 25) = 8.43, p = .008, ηp 2 = .25. On average, control speakers produced longer durations (/s/: 164.8 ms; /ʃ/: 176.8 ms) compared with speakers with PD (/s/: 138.8 ms; /ʃ/: 144.5 ms). There was also a significant effect of condition for /s/, F(3, 25) = 13.39, p < .001, ηp 2 = .35, and /ʃ/, F(3, 25) = 19.13, p < .001, ηp 2 = .43. On average, fricatives were longest in the hearing impaired condition, followed by the overenunciate, clear, and habitual conditions. The bottom panel of Figure 2 shows that this pattern held for each group. Post hoc comparisons for /s/ revealed that all pairwise comparisons were significant with the exception of habitual versus clear and clear versus overenunciate (p > .05). For /ʃ/, all pairwise comparisons were significant with the exception of clear versus overenunciate (p > .05). Last, for both fricatives, there was no Group × Condition interaction; /s/: F(3, 25) = 1.60, p = .20, ηp 2 = .06; /ʃ/: F(3, 25) = 2.06, p = .11, ηp 2 = .08.
For F2 slope of diphthongs, statistical analyses revealed no significant main effects of condition; /ɑɪ/: F(3, 25) = 2.53, p = .06, ηp 2 = .09; /eɪ/: F(3, 25) = 1.78, p = .16, ηp 2 = .07. Analysis also revealed no significant main effects of group, /ɑɪ/: F(1, 25) = 0.16, p = .70, ηp 2 = .006; /eɪ/: F(1, 25) = 0.006, p = .94, ηp 2 = .00, or Group × Condition interaction, /ɑɪ/: F(3, 25) = 0.56, p = .65, ηp 2 = .02; /eɪ/: F(3, 25) = 1.00, p = .39, ηp 2 = .04.
For M1 difference measures, there was a significant effect of group for /t-k/, F(1, 25) = 5.99, p = .02, ηp 2 = .19, but not for /s-ʃ/, F(1, 25) = 0.19, p = .67, ηp 2 = .01. On average, controls (1.91 kHz) produced significantly larger M1 difference measures for /t-k/, indicating greater spectral distinctiveness, compared with speakers with PD (1.25 kHz). Statistical analyses indicated no main effect of condition for either consonant pair, /t-k/: F(3, 25) = 0.48, p = .70, ηp 2 = .02; /s-ʃ/: F(3, 25) = 2.29, p = .09, ηp 2 = .08.
Last, there was a significant Group × Condition interaction for /t-k/, F(3, 25) = 3.55, p = .02, ηp 2 = .12, but not for /s-ʃ/, F(3, 25) = 0.35, p = .79, ηp 2 = .01. Figure 3 illustrates that control speakers maximized /t-k/ distinctiveness in the hearing impaired and clear conditions, whereas speakers with PD tended to maximize distinctiveness measures in the overenunciate condition.
Figure 3.
Mean and standard deviations (error bars) for first moment (M1) difference measures for consonant pairs /t-k/ and /s-ʃ/ as a function of group (black = control, CON; gray = Parkinson's disease, PD) and condition. H = habitual; C = clear; O = overenunciate; HI = hearing impaired.
Suprasegmental Measures
For F0 mean, there was no significant main effect of group, F(1, 25) = 0.02, p = .90, ηp 2 = .00, but the main effect of condition was significant, F(3, 25) = 5.61, p = .002, ηp 2 = .18. All pairwise comparisons for F0 mean were significant, with the exception of overenunciate versus hearing impaired (p = .19). Shown in Figure 4, F0 mean was greatest in the hearing impaired condition followed by the overenunciate, clear, and habitual conditions. The Group × Condition interaction was not significant for F0 mean, F(3, 25) = 2.40, p = .09, ηp 2 = .09.
Figure 4.
Means and standard deviations (error bars) for fundamental frequency (F0) and sound pressure level (dB SPL) as a function of group (black = control, CON; gray = Parkinson's disease, PD) and condition. Data for mean F0 and F0 interquartile range (IQR) are shown in the upper panels, and data for mean SPL and SPL standard deviation (SD) are shown in the lower panel. H = habitual; C = clear; O = overenunciate; HI = hearing impaired.
For F0 IQR, the main effect of group was not significant, F(1, 25) = 0.13, p = .72, ηp 2 = .00, but a significant effect of condition was observed, F(3, 25) = 4.34, p = .01, ηp 2 = .15. Post hoc analyses indicated that all nonhabitual conditions were significantly different from the habitual condition (p < .001). However, no significant differences for F0 IQR were observed between the nonhabitual conditions (p > .05). Figure 4 illustrates that on average, F0 IQR was greatest in the hearing impaired condition, followed by overenunciate, clear, and habitual conditions. Last, a significant Group × Condition interaction was not observed for F0 IQR, F(3, 25) = 1.22, p = .31, ηp 2 = .05.
For mean SPL, statistical analyses indicated no effect of group, F(1, 25) = 0.02, p = .89, ηp 2 = .00, but a significant effect of condition, F(3, 25) = 7.33, p < .001, ηp 2 = .23. Shown in the bottom panel of Figure 4, the hearing impaired condition tended to be associated with the largest mean SPL, followed by the overenunciate condition and then the clear condition. Post hoc analyses indicated that the habitual condition was different from the overenunciate and hearing impaired conditions (p < .001) and that the clear condition was different from the overenunciate and hearing impaired conditions (p < .001). No differences were observed between habitual and clear conditions (p > .05) or between overenunciate and hearing impaired conditions (p > .05). Last, the Group × Condition interaction was not significant for mean SPL, F(3, 25) = 0.09, p = .97, ηp 2 = .00.
For SPL standard deviation, there was a significant main effect of group, F(1, 25) = 11.26, p = .003, ηp 2 = .31, and condition, F(3, 25) = 12.25, p < .001, ηp 2 = .33, but no Group × Condition interaction, F(3, 25) = 1.50, p = .22, ηp 2 = .06. SPL standard deviation was larger for the control group (10.5 dB) compared with the PD group (9.6 dB). All pairwise comparisons of condition were significant, with the exception of hearing impaired versus overenunciate (p > .05). Shown in Figure 4, the overenunciate condition elicited the greatest SPL variation, followed by the hearing impaired condition and then the clear condition.
There was a significant effect of group for articulation rate, F(1, 25) = 11.78, p = .002, ηp 2 = .32. On average, the control speakers produced slower articulation rates (3.51 syllables/s) compared with speakers with PD (4.40 syllables/s). There was also a main effect of condition, F(3, 25) = 30.73, p < .001, ηp 2 = .55. As shown in Figure 5, the overenunciate condition elicited the slowest articulation rates, followed by the hearing impaired, clear, and habitual conditions. Post hoc comparisons revealed that all pairwise comparisons were significant, with the exception of clear versus hearing impaired (p = .57). The Group × Condition interaction for articulation rate was not significant, F(3, 25) = 1.35, p = 0.27, ηp 2 = .05.
Figure 5.
Means and standard deviations (error bars) for articulation rate (syllables/second) as a function of group (black = control, CON; gray = Parkinson's disease, PD) and condition. H = habitual; C = clear; O = overenunciate; HI = hearing impaired.
Discussion
Clear speech variants differentially affected segmental and suprasegmental acoustic measures. That is, for VSA, vowel duration, and articulation rate, the overenunciate condition elicited the greatest acoustic adjustments, followed by the hearing impaired condition and then the clear condition. A different pattern emerged for suprasegmental measures. For mean SPL, SPL standard deviation, F0 IQR, and F0 mean, the hearing impaired condition tended to elicit the greatest magnitude of change, followed by the overenunciate and clear conditions. A similar pattern was also observed for fricative durations. We first discuss condition effects and their implications and then consider group differences.
Condition Effects: Segmental Measures
Consistent with earlier clear speech studies (Bradlow et al., 2003; Ferguson & Kewley-Port, 2002, 2007; Goberman & Elmer, 2005; Johnson et al., 1993; Lam et al., 2012; Moon & Lindblom, 1994; Picheny et al., 1986; Tjaden et al., 2013; Tjaden et al., 2014; Whitfield & Goberman, 2014), all nonhabitual conditions elicited greater tense and lax VSAs for all speakers. Similar to Lam et al. (2012), the greatest VSAs were produced in the overenunciate condition, followed by the hearing impaired and clear conditions. Although researchers have suggested that other vowel metrics such as vowel articulation index (VAI) and formant centralization ratio (FCR) are shown to be more sensitive to articulatory impairment in dysarthria (Sapir, Ramig, Spielman, & Fox, 2010; Skodda et al., 2011b), recent work by Lansford and Liss (2014) showed that VSA (80% accuracy) outperformed FCR (70% accuracy) in classifying speakers with dysarthria, despite the fact that the two metrics were highly correlated. Moreover, it has been suggested that speech production measures used to evaluate therapeutic effects should be able to index functional speech abilities (i.e., intelligibility; Weismer, Yunusova, & Bunton, 2012). Previous studies have shown that VSA changes are related to changes in intelligibility (Kim et al., 2011; Lam & Tjaden, 2013b; Turner et al., 1995). However, the relationship between centralization metrics (e.g., FCR and VAI) and intelligibility has yet to be studied. Last, unlike FCR and VAI, changes in VSA have been well documented in the clear speech and dysarthria literature, thereby providing considerable data for comparison across studies (Bradlow et al., 2003; Ferguson & Kewley-Port, 2002, 2007; Goberman & Elmer, 2005; Johnson et al., 1993; Krause & Braida, 2004; Lam et al., 2012; Moon & Lindblom, 1994; Picheny et al., 1986; Tjaden et al., 2013; Tjaden et al., 2014).
Contrary to previous clear speech studies (Ferguson & Kewley-Port, 2007; Ferguson & Quené, 2014; Lam et al., 2012; Moon & Lindblom, 1994; Tjaden et al., 2014; Wouters & Macon, 2002; but see Tasko & Grelick, 2010), F2 slope was not affected by any of the nonhabitual conditions. Methodological factors may explain why findings from the current study are different from those reported in previous clear speech studies. In previous clear speech studies, a variety of measures and vowel segments (monophthongs or diphthongs) were used to capture dynamic characteristics of vowels. For example, some studies used a composite measure of F1 and F2 to describe dynamic spectral characteristics in monophthongs (Ferguson & Kewley-Port, 2007; Ferguson & Quené, 2014; Lam et al., 2012) and diphthongs (Tasko & Grelick, 2010), whereas Wouters and Macon (2002) fit linear regression lines to formant trajectories for the first three formants of monophthongs and diphthongs. This variation in measure and segment type makes it difficult to compare results across clear speech studies.
The context of the diphthongs selected for the current study also may have been a factor. In previous studies of F2 slope, all speakers tended to produce the same speech stimuli, and therefore all diphthongs had the exact same surrounding segments. In the current study, however, speakers produced different sets of sentences; therefore, diphthong productions and their surrounding segments were different. It is possible, then, that changes in F2 slope in diphthongs presented in the current study were less prevalent or neutralized when averaged across different diphthong productions. Nonetheless, it should be noted that upon further inspection of speaker data from previous studies, a clear speaking style has been shown to elicit shallower or unchanged slopes in F2 for healthy controls (Moon & Lindblom, 1994; Tjaden et al., 2014)—a result similar to that reported in the current study.
Condition Effects: Suprasegmental Measures
Clear speech adjustments have also been reported for suprasegemental measures such as F0 and SPL. Consistent with these previous studies (Bradlow et al., 2003; Goberman & Elmer, 2005; Tjaden et al., 2014), F0 mean and F0 IQR did in fact increase in all nonhabitual conditions relative to habitual. Among nonhabitual conditions, both measures of F0 tended to be maximized in the hearing impaired condition, followed by the overenunciate condition and then the clear condition. Sentence-level F0 range has further been proposed to be of perceptual importance, whereby narrower ranges have been associated with a decrease in intelligibility (Binns & Culling, 2007; Bunton, Kent, Kent, & Rosenbek, 2000; Laures & Bunton, 2003; Laures & Weismer, 1999; Miller, Schlauch, & Watson, 2010; Mori, Kobayashi, Kasuya, Kobayashi, & Hirose, 2005; Spitzer, Liss, & Mattys, 2007; Watson & Schlauch, 2008). Thus, further investigating the acoustic–perceptual relationship between F0 and intelligibility is of importance in identifying the clear speech variants that best maximize intelligibility.
In addition to F0, increases in mean SPL have been reported in previous clear speech studies (Dromey, 2000; Moon & Lindblom, 1994; Lam et al., 2012; Picheny et al., 1985). Consistent with the literature, mean SPL changes observed in the current study ranged anywhere from 1 to 5 dB, with the greatest mean SPL adjustments in the hearing impaired condition. Likewise, SPL standard deviation changes were also maximied in the hearing impaired condition. In general, for suprasegmental measures such as F0 and SPL, the hearing impaired instructions best elicited maximal acoustic adjustment.
Last, adjustments in articulation rate were consistent with previous clear speech studies (Bradlow et al., 2003; Lam et al., 2012; Picheny et al., 1989; but see Krause & Braida, 2002, 2004) such that the nonhabitual conditions elicited slower articulation rates compared with the habitual condition. The slowest rates were observed in the overenunciate condition, followed by the hearing impaired condition. In addition, increased vowel and fricative segment durations contributed to the decrease in rate. Similar increases in segment duration have also been noted in other studies of clear speech (Lam et al., 2012; Maniwa et al., 2009; Tjaden et al., 2013). It is interesting to note that vowel durations were maximized in the overenunciate condition, but fricative durations were maximized in the hearing impaired condition.
Implications
Both speakers with PD and healthy controls demonstrated that they can voluntarily create a gradient of clear speech acoustic adjustment. Thus, findings support extension of Lindblom's hypo–hyperarticulate theory to the PD population and indicate the relevance of hypo–hyperarticulate constructs beyond neurologically typical speech. The fact that there were very few interactions of condition with group further suggests that speakers with PD with mild speech impairment have the capacity to effect the same types and magnitudes of acoustic adjustment as controls in response to specific clear speech instructions. We also now know how variants of clear speech differentially affect segmental and suprasegmental acoustic measures in PD. Thus, different clear speech instructions could be used to help guide speakers in producing targeted clear speech acoustic adjustments. This might imply that different types of instruction could be used to address specific acoustic deficits for speakers with PD. For example, instructions to “overenunciate each word” elicit greater VSA expansion and maximize slow rates of speech, whereas instructions to “speak to someone with a hearing impairment” elicit the greatest prosodic adjustments with moderate changes in rate.
Results also have implications for clear speech research. Across studies, a variety of instructions have been used to elicit a clear speaking style, and the clear speech acoustic adjustments reported vary from study to study. Findings from the current study have shown that for both healthy controls and speakers with PD, the magnitude of clear speech adjustments is affected by instruction. Therefore, the variation in instruction should be considered when comparing results across studies.
Few group differences were observed across all acoustic measures. For segmental measures, groups differed only in tense VSA and M1 differences for /t-k/. Similar to previous studies (Liu et al., 2005; Tjaden et al., 2013; Turner et al., 1995; Weismer et al., 2001), speakers with PD produced smaller articulatory working spaces, as indicated by smaller tense VSAs compared with the control group; however, no group differences were observed for lax VSA. Moreover, patterns of centralizing stop consonants, such as in Tjaden and Wilding (2004), were also observed for /t-k/ differences in speakers with PD from the current study. Inspection of speaker data revealed that speakers with PD produced more centralized /t/ and /k/ productions, as indicated by lower M1 values for /t/ and higher M1 values for /k/, compared with healthy controls. Last, although previous studies reported shallower slope measures for speakers with PD relative to healthy controls (Tjaden & Wilding, 2004; Tjaden et al., 2014), no group differences were observed for the current study.
Likewise, only a few group differences were observed for suprasegmental measures. Contrary to previous studies (Canter, 1963; Fox & Ramig, 1997; Goberman et al., 2002; Skodda et al., 2011a; Tjaden et al., 2013; Walsh & Smith, 2011; but see Canter, 1963; Sadagopan & Huber, 2007; Tjaden & Wilding, 2004), mean SPL and mean F0 did not differ across groups in the current study. However, speakers with PD did produce less variation in sentence-level SPL compared with controls, as indexed by SPL standard deviation. Speakers with PD in the current study also produced faster articulation rates compared with healthy controls—a result similar to that reported in previous studies (Canter, 1965; Flint et al., 1992; Hammen & Yorkston, 1989; Metter & Hanson, 1986; Skodda & Schlegel, 2008; Solomon & Hixon, 1993). Last, contributing to the faster articulation rates and consistent with McRae, Tjaden, and Schoonings (2002), fricative durations were shorter for the PD group compared with the control group.
Although few group differences in acoustic measures were observed, group trends for each measure were consistent with those reported in the PD literature such that within each condition, the PD group always tended to produce smaller VSAs, reduced variability in SPL and F0, faster rates of speech, and shorter segment durations compared with the control group (see Figures 1 –5). These acoustic differences are further supported by baseline perceptual measures, wherein SIT scores and speech severity ratings were always poorer for speakers with PD relative to their age- and sex-matched control speakers. We recognize that the acoustic and baseline perceptual measures do not represent substantial group differences and that it would be premature to generalize findings to speakers with more severe involvement. However, as mentioned previously, the majority of speakers with PD reported speech changes postdiagnosis and/or having received speech therapy postdiagnosis. Thus, the systematic study of a variety of speakers with PD, regardless of the degree of perceived dysarthria, should be considered in future studies (see also Anand & Stepp, 2015).
Limitations and Future Directions
Five speakers with PD completed the LSVT program, with four of these speakers completing treatment more than 2 years prior to the current study. Two of the five speakers who had received LSVT also were participating in weekly group therapy, where they practiced using increased vocal loudness. Although LSVT has been shown to be effective immediately posttreatment (Ramig, Countryman, Thompson, & Horii, 1995; Ramig, Sapir, Fox, & Countryman, 2001; Sapir, Spielman, Ramig, Story, & Fox, 2007), the effects of LSVT have not been demonstrated beyond 2 years (Halpern et al., 2012; Ramig et al., 1995). At 6 months and 2 years post–LSVT, researchers have reported increases in SPL ranging from 1.5 to 3.0 dB (Halpern et al., 2012; Ramig et al., 1995; Ramig, Sapir, Fox, & Countryman, 2001). Descriptive differences in formant frequencies for /i/ and /u/ also have been reported post–LSVT in speakers with PD, but the long-term effects of LSVT on segmental articulation are unknown (Sapir et al., 2007). In general, neither long-term effects of dysarthria therapy nor generalization of trained skills related to treatment are well understood. On the basis of outcomes in related studies of PD including individuals with a history of LSVT (e.g., Tjaden et al., 2013) and the fact that most speakers had completed LSVT more than 2 years prior to the current study, it seems unlikely that LSVT history affected results. In addition, none of the clear speech variants explicitly directed speakers to modify vocal intensity.
The fact that some speakers did not pass a liberal hearing screening also deserves comment. Although four speakers from each group failed to meet the Weinstein and Ventry (1983) standard, this suggests that any effects of hearing loss on results were likely similar for the two groups. Both PD and hearing loss are associated with aging. In this manner, speakers in the current study are broadly representative of the aging population. Disentangling the separate effects of neurological disease and hearing loss on speech production in PD is an important topic for future studies, particularly given deficits in auditory perception in PD (see Troche, Troche, Berkowitz, Grossman, & Reilly, 2012).
The clear speech techniques studied were stimulation exercises, and findings cannot be generalized to a training paradigm. Further research is needed to investigate the impact of instruction when using a clear speech training program. Previous studies have reported that different clear speech instructions also vary in the amount of clear speech benefit or intelligibility increase in young healthy adults (Lam & Tjaden, 2013a). It is unknown whether this relationship holds for speakers with PD. Therefore, future research should also investigate how different clear speech instructions affect intelligibility and explore the acoustic–perceptual relationship in clear speech for speakers with PD. As previously noted, speakers with PD were not severely impaired, as indexed by SIT scores and scaled severity ratings. Therefore, results cannot be generalized to all individuals with PD.
Conclusions
Overall, speakers with PD as well as healthy controls were able to produce clear speech adjustments across the three nonhabitual conditions for many segmental and suprasegmental measures. Similar to previous studies (Goberman & Elmer, 2005; Tjaden et al., 2013; Tjaden et al., 2014), results show that clear speech adjustments are attainable by speakers with PD. Results further demonstrate that the magnitude of clear speech acoustic adjustment varied as a function of instruction for both speaker groups. In general, VSA, vowel segment durations, and articulation rate were maximized in the overenunciate condition, whereas fricative durations, F0, and SPL tended to be maximized in the hearing impaired condition.
Except for F2 slope, results are consistent with the notion that the overenunciate condition tended to maximize changes at the articulatory level (Lam et al., 2012). Lam et al. (2012) further suggested that the instructions in the hearing impaired condition might be associated with suprasegmental adjustments at the respiratory–phonatory level. Therefore, it is possible that the instructions to “overenunciate” might direct a speaker's attention to articulation, whereas the instructions to “speak to someone with a hearing impairment” might direct a speaker's attention to the respiratory–phonatory mechanism. It has been proposed that increased effort at the respiratory–phonatory level during loud speech not only increases SPL but also increases mean F0 (Dromey & Ramig, 1998; Ramig et al., 1995; Watson & Hughes, 2006). Given this relationship, it is possible that an increase in SPL and mean F0 can be interpreted as an increase in respiratory–phonatory effort. Although instructions in the current study were not specifically chosen to target loud speech, increases in mean SPL and F0 variability in the nonhabitual conditions are consistent with the notion of increased effort at the respiratory–phonatory level. To date, however, no studies have objectively studied the relationship between various acoustic measures and the construct of effort, nor is there a widely accepted objective method for quantifying effort in the speech production mechanism. Therefore, future studies exploring this relationship would further understanding of the construct of effort at various levels of the speech mechanism.
Acknowledgment
This research was conducted as part of the first author's doctoral dissertation, completed at the State University of New York at Buffalo, and was supported by the Mark Diamond Research Fund of the Graduate Student Association at the University at Buffalo and by National Institutes of Health Grant R01DC004689 (second author).
Funding Statement
This research was conducted as part of the first author's doctoral dissertation, completed at the State University of New York at Buffalo, and was supported by the Mark Diamond Research Fund of the Graduate Student Association at the University at Buffalo and by National Institutes of Health Grant R01DC004689 (second author).
References
- Ackermann H., & Ziegler W. (1991). Articulatory deficits in parkinsonian dysarthria: An acoustic analysis. Journal of Neurology, Neurosurgery & Psychiatry, 54, 1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano-Kusumoto A., Hosom J. P., Kain A., & Aronoff J. M. (2014). Determining the relevance of different aspects of formant contours to intelligibility. Speech Communication, 59, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S., & Stepp C. E. (2015). Listener perception of monopitch, naturalness, and intelligibility for speakers with Parkinson's disease. Journal of Speech, Language, and Hearing Research, 58, 1134–1144. doi:10.1044/2015_jslhr-s-14-0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beukelman D. R., Fager S., Ullman C., Hanson E., & Logemann J. (2002). The impact of speech supplementation and clear speech on the intelligibility and speaking rate of people with traumatic brain injury. Journal of Medical Speech-Language Pathology, 10, 237–242. [Google Scholar]
- Binns C., & Culling J. F. (2007). The role of fundamental frequency contours in the perception of speech against interfering speech. The Journal of the Acoustical Society of America, 122, 1765–1776. [DOI] [PubMed] [Google Scholar]
- Boersma P., & Weenink D. (2014). Praat: Doing Phonetics by Computer (Version 5.4) [Computer program]. Retrieved from http://www.praat.org/
- Bradlow A. R., Kraus N., & Hayes E. (2003). Speaking clearly for children with learning disabilities: Sentence perception in noise. Journal of Speech, Language, and Hearing Research, 46, 80–97. [DOI] [PubMed] [Google Scholar]
- Bradlow A. R., Torretta G. M., & Pisoni D. B. (1996). Intelligibility of normal speech I: Global and fine-grained acoustic-phonetic talker characteristics. Speech Communication, 20, 255–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunton K., & Keintz C. K. (2008). The use of a dual-task paradigm for assessing speech intelligibility in clients with Parkinson disease. Journal of Medical Speech-Language Pathology, 16, 141–155. [PMC free article] [PubMed] [Google Scholar]
- Bunton K., Kent R. D., Kent J. F., & Duffy J. R. (2001). The effects of flattening fundamental frequency contours on sentence intelligibility in speakers with dysarthria. Clinical Linguistics & Phonetics, 15(3), 181–193. [Google Scholar]
- Bunton K., Kent R., Kent J., & Rosenbek J. (2000). Perceptuo-acoustic assessment of prosodic impairment in dysarthria. Clinical Linguistics & Phonetics, 14, 13–24. [DOI] [PubMed] [Google Scholar]
- Caligiuri M. P. (1989). The influence of speaking rate on articulatory hypokinesia in parkinsonian dysarthria. Brain and Language, 36, 493–502. [DOI] [PubMed] [Google Scholar]
- Canter G. J. (1963). Speech characteristics of patients with Parkinson's disease: I. Intensity, pitch, and duration. Journal of Speech and Hearing Disorders, 28, 221–229. [DOI] [PubMed] [Google Scholar]
- Canter G. J. (1965). Speech characteristics of patients with Parkinson's disease: III. Articulation, diadochokinesis, and over-all speech adequacy. Journal of Speech and Hearing Disorders, 30, 217–224. [DOI] [PubMed] [Google Scholar]
- Chen F. R. (1980). Acoustic characteristics and intelligibility of clear and conversational speech (Unpublished master's project). Massachusetts Institute of Technology, Cambridge. [Google Scholar]
- Darley F. L., Aronson A. E., & Brown J. R. (1969). Differential diagnostic patterns of dysarthria. Journal of Speech and Hearing Disorders, 12, 246–269. [DOI] [PubMed] [Google Scholar]
- Darling M., & Huber J. E. (2011). Changes to articulatory kinematics in response to loudness cues in individuals with Parkinson's disease. Journal of Speech and Hearing Disorders, 54, 1247–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Letter M., Van Borsel J., Boon P., De Bodt M., Dhooge I., & Santens P. (2010). Sequential changes in motor speech across a levodopa cycle in advanced Parkinson's disease. International Journal of Speech-Language Pathology, 12, 405–413. [DOI] [PubMed] [Google Scholar]
- Dromey C. (2000). Articulatory kinematics in patients with Parkinson disease using different speech treatment approaches. Journal of Medical Speech-Language Pathology, 8, 155–161. [Google Scholar]
- Dromey C., & Ramig L. O. (1998). Intentional changes in sound pressure level and rate: Their impact on measures of respiration, phonation, and articulation. Journal of Speech, Language, and Hearing Research, 41, 1003–1018. [DOI] [PubMed] [Google Scholar]
- Duffy J. R. (2005). Motor speech disorders: Substrates, differential diagnosis, and management (2nd ed.). New York, NY: Mosby. [Google Scholar]
- Duffy J. R. (2013). Motor speech disorders: Substrates, differential diagnosis, and management (3rd ed.). St. Louis, MO: Mosby. [Google Scholar]
- Ferguson S. H. (2004). Talker differences in clear and conversational speech: Vowel intelligibility for normal-hearing listeners. The Journal of the Acoustical Society of America, 116(4, Pt. 1), 2365–2373. [DOI] [PubMed] [Google Scholar]
- Ferguson S. H., & Kewley-Port D. (2002). Vowel intelligibility in clear and conversational speech for normal-hearing and hearing-impaired listeners. The Journal of the Acoustical Society of America, 112, 259–271. [DOI] [PubMed] [Google Scholar]
- Ferguson S. H., & Kewley-Port D. (2007). Talker differences in clear and conversational speech: Acoustic characteristics of vowels. Journal of Speech, Language, and Hearing Research, 50, 1241–1255. [DOI] [PubMed] [Google Scholar]
- Ferguson S. H., & Quené H. (2014). Acoustic correlates of vowel intelligibility in clear and conversational speech for young normal-hearing and elderly hearing-impaired listeners. The Journal of the Acoustical Society of America, 135, 3570–3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A. J., Black S. E., Campbell-Taylor I., Gailey G. F., & Levinton C. (1992). Acoustic analysis in the differentiation of Parkinson's disease and major depression. Journal of Psycholinguistic Research, 21, 383–389. [DOI] [PubMed] [Google Scholar]
- Fogerty D. (2013). Acoustic predictors of intelligibility for segmentally interrupted speech: Temporal envelope, voicing, and duration. Journal of Speech, Language, and Hearing Research, 56, 1402–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogerty D., & Humes L. E. (2010). Perceptual contributions to monosyllabic word intelligibility: Segmental, lexical, and noise replacement factors. The Journal of the Acoustical Society of America, 128, 3114–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. M., & Ramig L. O. (1997). Vocal sound pressure level and self-perception of speech and voice in men and women with idiopathic Parkinson disease. American Journal of Speech-Language Pathology, 6, 85–94. [Google Scholar]
- Goberman A., Coelho C., & Robb M. (2002). Phonatory characteristics of Parkinsonian speech before and after morning medication: The ON and OFF states. Journal of Communication Disorders, 35, 217–239. [DOI] [PubMed] [Google Scholar]
- Goberman A., & Elmer L. (2005). Acoustic analysis of clear versus conversational speech in individuals with Parkinson disease. Journal of Communication Disorders, 38, 215–230. [DOI] [PubMed] [Google Scholar]
- Halpern A. E., Ramig L. O., Matos C. E., Petska-Cable J. A., Spielman J. L., Pogoda J. M., … McFarland D. H. (2012). Innovative technology for the assisted delivery of intensive voice treatment (LSVT LOUD) for Parkinson disease. American Journal of Speech-Language Pathology, 21, 354–367. [DOI] [PubMed] [Google Scholar]
- Hammen V. L., & Yorkston K. T. (1996). Speech and pause characteristics following speech rate reduction in hypokinetic dysarthria. Communication Disorders, 29, 429–445. [DOI] [PubMed] [Google Scholar]
- Hazan V., & Baker R. (2011). Acoustic-phonetic characteristics of speech produced with communicative intent to counter adverse listening conditions. The Journal of the Acoustical Society of America, 130, 2139–2152. [DOI] [PubMed] [Google Scholar]
- Hustad K. C., & Weismer G. (2007). Interventions to improve intelligibility and communicative success for speakers with dysarthria. In Weismer G. (Ed.), Motor speech disorders (pp. 217–228). San Diego, CA: Plural. [Google Scholar]
- Johnson K. (2010). MMscript [Computer software]. Buffalo, NY: Department of Communicative Disorders and Sciences, University at Buffalo. [Google Scholar]
- Johnson K., Flemming E., & Wright R. (1993). The hyperspace effect: Phonetic targets are hyperarticulated. Linguistic Society of America, 69, 505–528. [Google Scholar]
- Kain A., Amano-Kusumoto A., & Hosom J. P. (2008). Hybridizing conversational and clear speech to determine the degree of contribution of acoustic features to intelligibility. The Journal of the Acoustical Society of America, 124, 2308–2319. [DOI] [PubMed] [Google Scholar]
- Kay T. (2012). Spectral analysis of stop consonants in individuals with dysarthria secondary to stroke (Unpublished master's thesis). Louisiana State University, Baton Rouge. [Google Scholar]
- Kent R. D., & Adams S. G. (1989). The concept and measurement of coordination in speech disorders. In Wallace S. A. (Ed.), Perspectives on the coordination of movement (pp. 415–449). North Holland, the Netherlands: Elsevier. [Google Scholar]
- Kim Y., Kent R., & Weismer G. (2011). An acoustic study of the relationships among neurologic disease, dysarthria type and severity of dysarthria. Journal of Speech, Language, and Hearing Research, 54, 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J., & Braida L. (2002). Investigating alternative forms of clear speech: The effects of speaking rate and speaking mode on intelligibility. The Journal of the Acoustical Society of America, 112, 2165–2172. [DOI] [PubMed] [Google Scholar]
- Krause J., & Braida L. (2004). Acoustic properties of naturally produced clear speech at normal speaking rates. The Journal of the Acoustical Society of America, 115, 362–378. [DOI] [PubMed] [Google Scholar]
- Lam J., & Tjaden K. (2013a). Intelligibility of clear speech: Effect of instruction. Journal of Speech, Language, and Hearing Research, 56, 1429–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J., & Tjaden K. (2013b). Acoustic-perceptual relationships in variants of clear speech. Folia Phoniatrica et Logopaedica, 65, 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J., Tjaden K., & Wilding G. (2012). Acoustics of clear speech: Effect of instruction. Journal of Speech, Language, and Hearing Research, 55, 1807–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansford K. L., & Liss J. M. (2014). Vowel acoustics in dysarthria: Speech disorder diagnosis and classification. Journal of Speech, Language, and Hearing Research, 57, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laures J. S., & Bunton K. (2003). Perceptual effects of a flattened fundamental frequency at the sentence level under different listening conditions. Journal of Communication Disorders, 36(6), 449–464. [DOI] [PubMed] [Google Scholar]
- Laures J. S., & Weismer G. (1999). The effects of flattened fundamental frequency on intelligibility at the sentence level. Journal of Speech, Language, and Hearing Research, 42, 1148–1156. [DOI] [PubMed] [Google Scholar]
- Lindblom B. (1990). Explaining phonetic variation: A sketch of the H & H Theory. In Hardcastle W. J., & Marchal A. (Eds.), Speech production and speech modeling (pp. 403–439). Dordrecht, the Netherlands: Kluwer Academic. [Google Scholar]
- Liu H.-M., Tsao F.-M., & Kuhl P. K. (2005). The effect of reduced vowel working space on speech intelligibility in Mandarin-speaking young adults with cerebral palsy. The Journal of the Acoustical Society of America, 117, 3879–3889. [DOI] [PubMed] [Google Scholar]
- Ludlow C. L., & Bassich C. J. (1983). Relationships between perceptual ratings and acoustic measures of hypokinetic speech. In McNeil M. R., Rosenbek J. C., & Aronson A. E. (Eds.), Dysarthria of speech: Physiology, acoustics, linguistics, management (pp. 163–196). San Diego, CA: College Hill Press. [Google Scholar]
- Maniwa K., Jongman A., & Wade T. (2008). Perception of clear fricatives by normal-hearing and simulated hearing-impaired listeners. The Journal of the Acoustical Society of America, 123, 1114–1125. [DOI] [PubMed] [Google Scholar]
- Maniwa K., Jongman A., & Wade T. (2009). Acoustic characteristics of clearly spoken English fricatives. The Journal of the Acoustical Society of America, 125, 3962–3973. [DOI] [PubMed] [Google Scholar]
- McRae P. A., Tjaden K., & Schoonings B. (2002). Acoustic and perceptual consequences of articulatory rate change in Parkinson disease. Journal of Speech, Language, and Hearing Research, 45, 35–50. [DOI] [PubMed] [Google Scholar]
- Mengistu K. T., & Rudzicz F. (2011, May). Adapting acoustic and lexical models to dysarthric speech. In Proceedings of the IEEE International Conference on Acoustics, Speech and Signal Processing (pp. 4924–4927). New York, NY: The Institute of Electrical and Electronics Engineers. [Google Scholar]
- Metter E. J., & Hanson W. R. (1986). Clinical and acoustical variability in hypokinetic dysarthria. Journal of Communication Disorders, 19, 347–366. [DOI] [PubMed] [Google Scholar]
- Milenkovic P. (2002). TF32 [Computer software]. Madison: Department of Electrical and Computer Engineering, University of Wisconsin-Madison. [Google Scholar]
- Milenkovic P. (2005). TF32 [Computer software]. Madison, WI: Department of Electrical and Computer Engineering, University of Wisconsin–Madison. [Google Scholar]
- Miller S. E., Schlauch R. S., & Watson P. J. (2010). The effects of fundamental frequency contour manipulations on speech intelligibility in background noise. The Journal of the Acoustical Society of America, 128, 435–443. [DOI] [PubMed] [Google Scholar]
- Molloy D. D. W. (1999). Standardized Mini-Mental State Examination. Troy, NY: Newgrange Press. [Google Scholar]
- Moon S., & Lindblom B. (1994). Interactions between duration, context, and speaking style in English stressed vowels. The Journal of the Acoustical Society of America, 96, 40–55. [Google Scholar]
- Mori K., Kobayashi Y., Kasuya H., Kobayashi N., & Hirose H. (2005). Evaluation of fundamental frequency (F0) characteristics of speech in dysarthrias: A comparative study. Acoustical Science and Technology, 26, 540–543. [Google Scholar]
- Oviatt S., MacEachern M., & Levow G. A. (1998). Predicting hyperarticulate speech during human-computer error resolution. Speech Communication, 24, 87–110. [Google Scholar]
- Owren M. J., & Cardillo G. C. (2006). The relative roles of vowels and consonants in discriminating talker identity versus word meaning. The Journal of the Acoustical Society of America, 119, 1727–1739. [DOI] [PubMed] [Google Scholar]
- Park S., Theodoros D., Finch E., & Cardell E. (2014, February). Be clear: A new intensive speech treatment for adults with non-progressive dysarthria. Poster presented at the Biennial Conference on Motor Speech, Sarasota, FL. [Google Scholar]
- Perkell J. S., Zandipour M., Matthies M. L., & Lane H. (2002). Economy of effort in different speaking conditions. I. A preliminary study of intersubject differences and modeling issues. The Journal of the Acoustical Society of America, 112, 1627–1641. [DOI] [PubMed] [Google Scholar]
- Picheny M. A., Durlach N. I., & Braida L. D. (1985). Speaking clearly for the hard of hearing: I. Intelligibility differences between clear and conversational speech. Journal of Speech and Hearing Research, 28, 96–103. [DOI] [PubMed] [Google Scholar]
- Picheny M. A., Durlach N. I., & Braida L. D. (1986). Speaking clearly for the hard of hearing: II. Acoustic characteristics of clear and conversational speech. Journal of Speech and Hearing Research, 29, 434–445. [DOI] [PubMed] [Google Scholar]
- Picheny M. A., Durlach N. I., & Braida L. D. (1989). Speaking clearly for the hard of hearing III: An attempt to determine the contribution of speaking rate to differences in intelligibility between clear and conversational speech. Journal of Speech & Hearing Research, 32, 600–603. [PubMed] [Google Scholar]
- Ramig L. O., Countryman S., Thompson L. L., & Horii Y. (1995). Comparison of two forms of intensive speech treatment for Parkinson disease. Journal of Speech, Language, and Hearing Research, 38, 1232–1251. [DOI] [PubMed] [Google Scholar]
- Ramig L. O., Sapir S., Countryman S., Pawlas A. A., O'Brien C., Hoehn M., & Thompson L. L. (2001). Intensive voice treatment (LSVT) for patients with Parkinson's disease: A 2 year follow up. Journal of Neurology, Neurosurgery & Psychiatry, 71, 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramig L. O., Sapir S., Fox C., & Countryman S. (2001). Changes in vocal loudness following intensive voice treatment (LSVT) in individuals with Parkinson's disease: A comparison with untreated patients and normal age‐matched controls. Movement Disorders, 16, 79–83. [DOI] [PubMed] [Google Scholar]
- Rosen K. M., Folker J. E., Murdoch B. E., Vogel A. P., Cahill L. M., Delatycki M. B., & Corben L. A. (2011). Measures of spectral change and their application to habitual, slow, and clear speaking modes. International Journal of Speech-Language Pathology, 13, 165–173. [DOI] [PubMed] [Google Scholar]
- Sadagopan N., & Huber J. E. (2007). Effects of loudness cues on respiration in individuals with Parkinson's disease. Movement Disorders, 22, 651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir S., Ramig L. O., Spielman J. L., & Fox C. (2010). Formant centralization ratio: A proposal for a new acoustic measure of dysarthric speech. Journal of Speech, Language, and Hearing Research, 53, 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir S., Spielman J. L., Ramig L. O., Story B. H., & Fox C. (2007). Effects of intensive voice treatment (the Lee Silverman Voice Treatment [LSVT]) on vowel articulation in dysarthric individuals with idiopathic Parkinson disease: Acoustic and perceptual findings. Journal of Speech, Language, and Hearing Research, 50, 899–912. [DOI] [PubMed] [Google Scholar]
- Searl J., & Evitts P. (2013). Tongue-palate contact pressure, oral air pressure and acoustics of clear speech. Journal of Speech, Language, and Hearing Research, 56, 826–839. [DOI] [PubMed] [Google Scholar]
- Skodda S., & Schlegel U. (2008). Speech rate and rhythm in Parkinson's disease. Movement Disorders, 23, 985–992. [DOI] [PubMed] [Google Scholar]
- Skodda S., Visser W., & Schlegel U. (2011a). Gender-related patterns of dysprosody in Parkinson disease and correlation between speech variables and motor symptoms. Journal of Voice, 25, 76–82. [DOI] [PubMed] [Google Scholar]
- Skodda S., Visser W., & Schlegel U. (2011b). Vowel articulation in Parkinson's disease. Journal of Voice, 25, 467–472. [DOI] [PubMed] [Google Scholar]
- Smiljanic R., & Bradlow A. R. (2008). Temporal organization of English clear and conversational speech. The Journal of the Acoustical Society of America, 124, 3171–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon N. P., & Hixon T. J. (1993). Speech breathing in Parkinson's disease. Journal of Speech, Language, and Hearing Research, 36, 294–310. [DOI] [PubMed] [Google Scholar]
- Spitzer S., Liss J. M., & Mattys S. L. (2007). Acoustic cues to lexical segmentation: A study of resynthesized speech. The Journal of the Acoustical Society of America, 122, 3678–3687. [DOI] [PubMed] [Google Scholar]
- Sussman J. E., & Tjaden K. (2012). Perceptual measures of speech from individuals with Parkinson's disease and multiple sclerosis: Intelligibility and beyond. Journal of Speech, Language, and Hearing Research, 55, 1208–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasko S. M., & Greilick K. (2010). Acoustic and articulatory features of diphthong production: A speech clarity study. Journal of Speech, Language, and Hearing Research, 53, 84–99. [DOI] [PubMed] [Google Scholar]
- Tjaden K., Kain A., & Lam J. (2014). Hybridizing conversational and clear speech to investigate the source of increased intelligibility in Parkinson's disease. Journal of Speech, Language, and Hearing Research, 57, 1191–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden K., Lam J., & Wilding G. (2013). Vowel acoustics in Parkinson's disease and multiple sclerosis: Comparison of clear, loud and slow speaking conditions. Journal of Speech, Language, and Hearing Research, 56, 1485–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden K., Richards E., Kuo C., Wilding G., & Sussman J. (2014). The relationship of F2 slope to intelligibility and vocal intensity in Parkinson's disease. Folia Phoniatrica et Logopaedica, 65, 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden K., Rivera D., Wilding G., & Turner G. S. (2005). Characteristics of the lax vowel space in dysarthria. Journal of Speech, Language, and Hearing Research, 48, 554–566. [DOI] [PubMed] [Google Scholar]
- Tjaden K., Sussman J. E., & Wilding G. E. (2014). Impact of clear, loud, and slow speech on scaled intelligibility and speech severity in Parkinson's disease and multiple sclerosis. Journal of Speech, Language, and Hearing Research, 57, 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjaden K., & Wilding G. E. (2004). Rate and loudness manipulations in dysarthria: Acoustic and perceptual findings. Journal of Speech, Language, and Hearing Research, 47, 766–783. [DOI] [PubMed] [Google Scholar]
- Tjaden K., & Wilding G. (2011). Speech and pause characteristics associated with voluntary rate reduction in Parkinson's disease and multiple sclerosis. Journal of Communication Disorders, 44(6), 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torp J. N., & Hammen V. L. (2000). Perception of Parkinsonian speech rate. Journal of Medical Speech-Language Pathology, 8(4), 323–329. [Google Scholar]
- Troche J., Troche M. S., Berkowitz R., Grossman M., & Reilly J. (2012). Tone discrimination as a window into acoustic perceptual deficits in Parkinson's disease. American Journal of Speech-Language Pathology, 21, 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G. S., Tjaden K., & Weismer G. (1995). The influence of speaking rate on vowel space and speech intelligibility for individuals with amyotrophic lateral sclerosis. Journal of Speech and Hearing Research, 38, 1001–1013. [DOI] [PubMed] [Google Scholar]
- Uchanski R. M. (2005). Clear speech. In Pisoni D. B. & Remez R. E. (Eds.), The handbook of speech perception (pp. 207–235). Hoboken, NJ: Wiley. [Google Scholar]
- Walsh B., & Smith A. (2011). Linguistic complexity, speech production, and comprehension in Parkinson's disease: Behavioral and physiological indices. Journal of Speech, Language, and Hearing Research, 54, 787–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P. J., & Hughes D. (2006). The relationship of vocal loudness manipulation to prosodic F0 and durational variables in healthy adults. Journal of Speech, Language, and Hearing Research, 49, 636–644. [DOI] [PubMed] [Google Scholar]
- Watson P. J., & Schlauch R. S. (2008). The effect of fundamental frequency on the intelligibility of speech with flattened intonation contours. American Journal of Speech-Language Pathology, 17, 348–355. [DOI] [PubMed] [Google Scholar]
- Weinstein B. E., & Ventry I. M. (1983). Audiometric correlates of the hearing handicap inventory for the elderly. Journal of Speech and Hearing Disorders, 48, 379–384. [DOI] [PubMed] [Google Scholar]
- Weismer G., Jeng J. Y., Laures J. S., Kent R. D., & Kent J. F. (2001). Acoustic and intelligibility characteristics of sentence production in neurogenic speech disorders. Folia Phoniatrica et Logopaedica, 53(1), 1–18. [DOI] [PubMed] [Google Scholar]
- Weismer G., Kent R. D., Hodge M., & Martin R. (1988). The acoustic signature for intelligibility test words. The Journal of the Acoustical Society of America, 84, 1281–1291. [DOI] [PubMed] [Google Scholar]
- Weismer G., Laures J. S., Jeng J. Y., Kent R. D., & Kent J. F. (2000). Effect of speaking rate manipulations on acoustic and perceptual aspects of the dysarthria in amyotrophic lateral sclerosis. Folia Phoniatrica et Logopaedica, 52, 201–219. [DOI] [PubMed] [Google Scholar]
- Weismer G., Martin R., Kent R. D., & Kent J. F. (1992). Formant trajectory characteristics of males with amyotrophic lateral sclerosis. The Journal of the Acoustical Society of America, 91, 1085–1098. [DOI] [PubMed] [Google Scholar]
- Weismer G., Yunusova Y., & Bunton K. (2012). Measures to evaluate the effects of DBS on speech production. Journal of Neurolinguistics, 25, 74–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield J. A., & Goberman A. M. (2014). Articulatory-acoustic vowel space: Application to clear speech in individuals with Parkinson's disease. Journal of Communication Disorders, 51, 19–28. [DOI] [PubMed] [Google Scholar]
- Wooten G. F., Currie L. J., Bovbjerg V. E., Lee J. K., & Patrie J. (2004). Are men at greater risk of Parkinson's disease than women? Journal of Neurology, Neurosurgery & Psychiatry, 75, 637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouters J., & Macon M. W. (2002). Effects of prosodic factors on spectral dynamics. I. Analysis. The Journal of the Acoustical Society of America, 111(1, Pt. 1), 417–427. [DOI] [PubMed] [Google Scholar]
- Yorkston K. M., & Beukelman D. R. (1996). Sentence Intelligibility Test. Lincoln, NE: Tice Technologies. [Google Scholar]