Summary
The impact of real-time electronic monitoring on antiretroviral therapy adherence warrants further study. We conducted an analysis of cohort participants that initially involved standard electronic adherence monitoring (EAM), followed by real-time EAM plus home visits for sustained ≥48-hour adherence interruptions. Immediately after switching between the two types of EAM, mean adherence among 112 participants increased from 84% to 93% and remained elevated for six months (p<0.001). Real-time EAM is a promising approach for improving adherence.
Keywords: HIV, adherence, real-time electronic adherence monitoring, antiretroviral therapy
Introduction
In electronic adherence monitoring (EAM), a device records each opening with a date-and-time stamp as a proxy for medication ingestion. Standard EAM devices store this data for later transfer to a computer; wireless devices are been increasingly used and transmit this data over cellular networks in real time[1].
Recent randomized trials have generally shown improvement in adherence when real-time EAM are coupled with text message reminders[2–4]; however, it is unclear how EAM monitoring itself or other types of associated interventions influence adherence behavior.
We present an ad hoc analysis of a cohort study of adults taking ART in Uganda that initially involved a standard EAM device, followed subsequently by a real-time EAM device plus home visits for sustained adherence interruptions. We assessed differences in overall adherence and sustained adherence interruptions between these two periods.
Methods
Participants were drawn from a observational cohort (NCT01596322)[5,6] in which ART adherence was monitored by standard EAM (medication event monitoring system [MEMS; WestRock, Switzerland]) from 2005–2011, followed by real-time EAM (Wisepill; Wisepill Technologies, South Africa) from 2011–2015. During real-time EAM, sustained (≥48-hour) interruptions triggered home visits to characterize the cause and assess HIV RNA levels (“real-time EAM plus follow-up”). Cohort enrollment occurred through 2012. Some participants were therefore monitored with both types of EAM; others were monitored only with real-time EAM.
We analyzed data from participants whose ART adherence was monitored for six months with standard EAM, and who were switched within one day to monitoring with real-time EAM plus follow-up for six additional months. We used regression modeling (linear, logistic, or Poisson) with fixed effects and robust standard errors to compare participant characteristics, weekly average adherence, and ≥48-hour adherence interruptions between the six-month periods. Next, we used least squares regression modeling to 1)project estimated standard EAM adherence per participant as if he/she had not switched to real-time EAM plus follow-up, and 2)compare projected and observed adherence during real-time EAM plus follow-up. We estimated the total difference between projected and observed adherence per participant, and tested the null hypothesis of no difference between the two variables, stratifying by tertiles of time on ART. We used generalized estimating equations to compare adherence data during real-time EAM plus follow-up for participants initiating ART versus participants who had six months of prior ART with standard EAM.
Ethical approval was received from Mbarara University of Science and Technology, the Uganda National Council for Science and Technology, Partners Healthcare, and the University of California San Francisco.
Results
One hundred twelve participants had standard EAM for six months, followed by six months of real-time EAM plus follow-up. Median age was 36 years, 68% were female, 82% were literate, and pre-ART CD4 count was 141 cells/ml (similar to the clinic from which participants were recruited)[7,8]. No change was seen in household size, household income, time to clinic, alcohol use[9], depression[10], social support[11], food insecurity[12], or ART regimen between the two monitoring periods (all p>0.05).
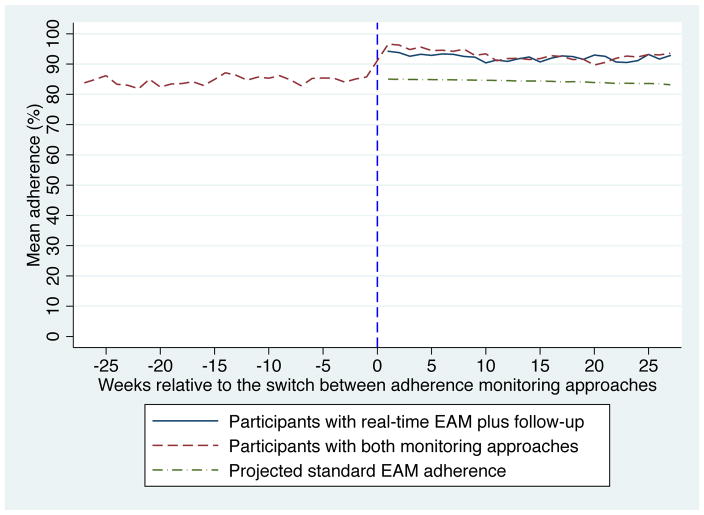
Immediately after switching from standard EAM to real-time EAM plus follow-up, mean adherence increased from 84% to 93% (Figure 1; p<0.001). The increase was similar for participants triggering home visits ≤30 versus >30 days after the device switch. When compared to projected average adherence with standard EAM and adjusting for time on ART, this difference persisted over six months. The mean number of ≥48-hour interruptions per six-month monitoring period decreased from 2.2 (SD 3.1) to 0.7 (SD 1.2) per participant after switching from standard EAM to real-time EAM plus follow-up. No difference was seen in viral suppression (6% versus 7%, p=0.48).
Figure 1.
Comparison of adherence during monitoring with standard EAM and real-time EAM plus follow-up.
Two hundred fifty-five participants initiated ART with real-time EAM plus follow-up. We found no difference in average adherence for the first six months of follow-up in these participants compared to the first six months of real-time EAM plus follow-up in the 112 participants who had prior experience with standard EAM (92% versus 93%; p=0.35); the mean number of ≥48-hour adherence interruptions per participant was significantly higher for those initiating ART with real-time EAM plus follow-up (1.9 [SD 2.8] versus 0.7 [SD 1.2]; p<0.001).
Discussion
Compared to standard EAM, real-time EAM plus home visits for sustained interruptions was associated with increased average adherence and fewer adherence interruptions—both of which are associated with viral suppression[13,14] and reduced immune activation[15]. No differences in common factors affecting adherence were seen between the two monitoring periods.
Adherence with real-time EAM plus follow-up was high regardless of prior experience with standard EAM, suggesting that a real-time approach may effectively promote adherence during early and chronic treatment. Sustained adherence interruptions during real-time EAM with follow-up were more frequent for those initiating ART compared to those with prior ART experience, possibly reflecting initial challenges in establishing high adherence habits[16].
Our findings strengthen growing evidence that real-time EAM with follow-up triggered by incomplete adherence is an effective intervention. One mechanism may be provision of support precisely when needed. Follow-up visits were not designed as interventions; however, participants likely perceived support from research staff. Given the resource intensity of home visits, cellular phone follow-up may be more feasible, especially if adherence challenges are frequent. Additionally, real-time monitoring itself can convey a sense of support[17]. Indeed, the similarity in increased adherence when comparing study participants who triggered home visits early versus later after the device switch suggests the change in monitoring, not the follow-up, may be responsible for the effect. The impact of anticipated follow-up, including phlebotomy for HIV RNA assessment, however, cannot be excluded.
This analysis has limitations. First, it is ad hoc and compares different adherence measurement devices; differences in technology and/or acceptability may have influenced the measurements. Second, we assume no other confounding changes occurred concomitantly with the device switch and trends in these other factors were stable throughout the observation period. Third, overall high adherence reduced the ability to show a difference in viral suppression between the monitoring periods. Additionally, we did not directly compare standard versus real-time adherence monitoring and cannot estimate relative Hawthorne effects[18].
In conclusion, this analysis provides support for the effectiveness of real-time EAM with follow-up as an ART adherence intervention.
Acknowledgments
Funding: R01MH054907, P30AI027783
The authors would like to thank the participants in the Uganda AIDS Rural Treatment Outcomes Study, as well as all study staff.
Footnotes
Conflicts of interest: None
References
- 1.Haberer JE, Kahane J, Kigozi I, Emenyonu N, Hunt P, Martin J, et al. Real-time adherence monitoring for HIV antiretroviral therapy. AIDS Behav. 2010;14(6):1340–1346. doi: 10.1007/s10461-010-9799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabin LL, Bachman DeSilva M, Gill CJ, Zhong L, Vian T, Xie W, et al. Improving adherence to antiretroviral therapy with triggered real-time text message reminders: the China adherence through technology study. J Acquir Immune Defic Syndr. 2015;69(5):551–559. doi: 10.1097/QAI.0000000000000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orrell C, Cohen K, Mauff K, Bangsberg DR, Maartens G, Wood R. A randomized controlled trial of real-time electronic adherence monitoring with text message dosing reminders in people starting first-line antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;70(5):495–502. doi: 10.1097/QAI.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 4.Haberer JE, Musiimenta A, Atukunda EC, Musinguzi N, Wyatt MA, Ware NC, et al. Short message service (SMS) reminders and real-time adherence monitoring improve antiretroviral therapy adherence in rural Uganda. AIDS. 2016;30(8):1295–1300. doi: 10.1097/QAD.0000000000001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adakun SA, Siedner MJ, Muzoora C, Haberer JE, Tsai AC, Hunt PW, et al. Higher baseline CD4 cell count predicts treatment interruptions and persistent viremia in patients initiating ARVs in rural Uganda. J Acquir Immune Defic Syndr. 2013;62(3):317–321. doi: 10.1097/QAI.0b013e3182800daf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musinguzi N, Mocello RA, Boum Y, 2nd, Hunt PW, Martin JN, Haberer JE, et al. Duration of viral suppression and risk of rebound viremia with first-line antiretroviral therapy in rural uganda. AIDS Behav. 2016 doi: 10.1007/s10461-016-1447-1. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kigozi IM, Dobkin LM, Martin JN, Geng EH, Muyindike W, Emenyonu NI, et al. Late-disease stage at presentation to an HIV clinic in the era of free antiretroviral therapy in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2009 Oct 1;52(2):280–9. doi: 10.1097/QAI.0b013e3181ab6eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geng EH, Bwana MB, Muyindike W, Glidden DV, Bangsberg DR, Neilands TB, et al. Failure to initiate antiretroviral therapy, loss to follow-up and mortality among HIV-infected patients during the pre-ART period in Uganda. J Acquir Immune Defic Syndr. 2013 Jun 1;63(2):e64–71. doi: 10.1097/QAI.0b013e31828af5a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C):an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 10.Bolton P, Wilk CM, Ndogoni L. Assessment of depression prevalence in rural Uganda using symptom and function criteria. Soc Psychiatry Psychiatr Epidemiol. 2004;39(6):442–447. doi: 10.1007/s00127-004-0763-3. [DOI] [PubMed] [Google Scholar]
- 11.Tsai AC, Weiser SD, Steward WT, Mukiibi NF, Kawuma A, Kembabazi A, et al. Evidence for the reliability and validity of the internalized AIDS-related stigma scale in rural Uganda. AIDS Behav. 2013 Jan;17(1):427–33. doi: 10.1007/s10461-012-0281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai AC, Bangsberg DR, Frongillo EA, Hunt PW, Muzoora C, Martin JN, et al. Food insecurity, depression and the modifying role of social support among people living with HIV/AIDS in rural Uganda. Soc Sci Med. 2012 Jun;74(12):2012–9. doi: 10.1016/j.socscimed.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nachega, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor-based HIV therapy and virologic outcomes. Ann Intern Med. 2007 Apr 17;146(8):564–73. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 14.Haberer JE, Musinguzi N, Boum Y, 2nd, Siedner MJ, Mocello AR, Hunt PW, et al. Duration of antiretroviral therapy adherence interruption is associated with risk of virologic rebound as determined by real-time adherence monitoring in rural Uganda. J Acquir Immune Defic Syndr. 2015;70(4):386–392. doi: 10.1097/QAI.0000000000000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castillo-Mancilla J, Brown TT, Erlandson KM, Palella FJ, Jr, Gardner EM, Macatangay BJ, et al. Suboptimal cART adherence is associated with higher levels of inflammation despite HIV suppression. Clin Infect Dis. 2016 Sep 22; doi: 10.1093/cid/ciw650. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson IB, Bangsberg DR, Shen J, Simoni JM, Reynolds NR, Goggin K, et al. Heterogeneity among studies in rates of decline of ART adherence over time: Results from the MACH14 study. J Acquir Immune Defic Syndr. 2013;64(5):448–454. doi: 10.1097/QAI.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ware NC, Pisarski EE, Tam M, Wyatt MA, Atukunda E, Musiimenta A, et al. The Meanings in the messages: how SMS reminders and real-time adherence monitoring improve antiretroviral therapy adherence in rural Uganda. AIDS. 2016;30(8):1287–1294. doi: 10.1097/QAD.0000000000001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monahan T, Fisher JA. Benefits of “observer effects”: lessons from the field. Qual Res. 2010;10(3):357–376. doi: 10.1177/1468794110362874. [DOI] [PMC free article] [PubMed] [Google Scholar]