Abstract
Vascular integrity is primarily determined by endothelial cell (EC) cytoskeletal structure that is differentially regulated by various stimuli. In this study, atomic force microscopy (AFM) was used to characterize structural and mechanical properties in the cytoskeleton of cultured human pulmonary artery EC (HPAEC) and human lung microvascular EC (HLMVEC) by determining elastic properties (Young’s modulus) in response to endogenous barrier protective agents sphingosine 1-phosphate (S1P) and hepatocyte growth factor (HGF), or the barrier disruptive molecule thrombin. Initial studies in unstimulated cells indicate higher baseline peripheral elastic modulus values in HPAEC (mean 2.9 KPa) than in HLMVEC (1.8 KPa). After 30 minutes of stimulation, S1P induced the highest Young’s modulus increase (6.1KPa) compared to the other barrier enhancing stimuli, HGF (5.8KPa) and the pharmaceutical agent and S1P analog FTY720 (4.1KPa). In contrast, the barrier disruptive agent thrombin decreased values from 2.5 KPa to 0.7 KPa depending on the cell type and treatment time. AFM topographical imaging supports these quantitative biophysical data regarding differential peripheral elastic properties in EC. Overall, these AFM studies provide novel insights into the biomechanical properties of human lung EC that regulate vascular barrier function and have potential applicability to pathophysiologic vascular leak syndromes such as acute lung injury.
Keywords: AFM, barrier regulation, endothelial cell, S1P, FTY720
INTRODUCTION
Acute lung injury syndromes are marked by critical vascular leak resulting from endothelial barrier compromise within the pulmonary vasculature. Improved understanding of the cellular mechanisms regulating endothelial cell (EC) permeability could lead to the development of novel therapeutic strategies for the treatment of pathophysiologic vascular leak. In this study we utilize the advantageous properties of atomic force microscopy (AFM) to characterize biomechanical responses of human lung EC, which act as a semipermeable barrier between the pulmonary spaces and vascular components to regulate tissue fluid homeostasis and inflammatory responses. When this barrier is disrupted during inflammation, fluids and macromolecules are released into the pulmonary air spaces. Pulmonary vascular barrier function is determined by an interplay of multiple forces – EC contractile forces, adhesive cell-cell and cell-matrix tethering forces that are linked through the EC actin cytoskeleton. [1] Although prior work by our group and others have described some biophysical aspects of EC cytoskeletal remodeling in response to barrier regulating agonists [2-5], the biomechanical effects of barrier regulatory agents on the strength and distribution of the EC cytoskeletal network remain incompletely defined.
The endothelial monolayer consists of two primary types of cell-cell contact complexes, adherens and tight junctions. These complexes in conjunction with the actin microfilaments provide the cell with mechanical stability and regulate extracellular signal transduction. [1] Focal adhesions further aid in barrier regulation by transducing signals between actin filaments and underlying cell-matrix interfaces. During inflammatory disease processes such as acute lung injury and sepsis, vascular leak contributes significantly to respiratory failure and is dependent on the barrier-regulatory properties of the lung endothelial cytoskeleton. Barrier-disruptive agents such as thrombin, VEGF, hydrogen peroxide and others cause stress fiber formation and accumulation of phosphorylated myosin light chains in the center of the cellular structure, resulting in actomyosin contraction, cell retraction and disruption of the EC monolayer. [1, 6, 7] Conversely, barrier-protective stimuli such as sphingosine 1-phosphate (S1P), hepatocyte growth factor (HGF), and shear stress increase accumulation of peripheral F-actin and phosphorylated myosin light chains, indicative of cytoskeletal changes at the outer edges of the cells that enhance cell-cell connections. [8, 9]
Live cell microscopy has been used to assess contractility in cell cultures [10], but the approaches have limited resolution for assessing the region of interest involving contractile force measurements. Because of these limitations, AFM techniques are used in the current study to measure subtle biomechanical variations in the peripheral EC regions in order to correlate structural cell shape changes with exposure to barrier-regulatory stimuli, advancing knowledge gained from prior work in this area. [2-5, 11] AFM is a form of scanning probe microscopy and an important tool in modern day nanotechnology. Its unique capabilities make it useful for studying biomechanical parameters of cells and tissues. A major advantage of AFM is the ability to measure cell properties without fixation, including in buffer solutions, in situ, and in vitro, if not in vivo. Unlike other imaging modalities, with AFM there are no restrictions on the sample preparation, the temperature conditions, and buffer composition (e.g., no requirement to be transparent). Also, biological sample measurements have high lateral resolution (molecular) of about 1 nm, whereas the vertical resolution can be even higher in the range of 0.1 nm with AFM studies. We therefore measured the local elastic modulus induced in human lung EC by multiple barrier-regulatory agonists--S1P, HGF, FTY720 and thrombin--which provide important biophysical correlations of these agonists. We also used AFM to biomechanically characterize the differential responses of human macrovascular (pulmonary artery) (HPAEC) and human (HLMVEC) lung EC to provide novel insights into the properties of cells from different regions in the pulmonary vasculature. Data were collected describing topographical and quantitative elastic modulus variations.
MATERIALS AND METHODS
Cell culture
Human lung microvascular endothelial cells (HLMVEC) (#CC-2527), human pulmonary artery endothelial cells (HPAEC) (#CC-2530), and EGM2-MV media (#CC-3202) were purchased from Lonza (Walkersville, MD). EC were cultured in EGM2-MV media with 5% fetal bovine serum (Sigma-Aldrich, St. Louis, MO) in a 5% CO2 humidified 37° C incubator.
For AFM topographical imaging, cells were plated on 25 mm gelatin coated coverslips and treated with the respective agonist after starvation for 3 hours in EBM-2 containing 0.1% FBS. The cells were then fixed in 3.7% para-formaldehyde in PBS for 10 min and washed with PBS three times before measurements. For AFM elasticity measurements, cells in their fifth and sixth passage were plated in a six well plate on 22 × 22 microscope cover glasses (Fisherbrand, Pittsburgh, PA) coated with 0.02% gelatin. Cells attained 100% confluency within 24 hours.
AFM operation
Two AFM setups were employed in this study: Nanoscope III SPM (Plainview, NY) and Novascan Synergy ESPM 3D (Ames, IA). Colloidal HYDRA2R-50N probes were purchased from AppNano (Santa Clara, CA) for topographical imaging. These probes have rectangular narrow cantilevers for contact mode and tapping mode applications. The colloidal tip has a spring constant of 0.084N/m, which is ideal for imaging biological samples. Novascan (Ames, IA) specific PT.GS tips were used that have a 5.0 μm borosilicate glass particle attached at the cantilever tip with a spring constant of 0.12N/m for force-distance measurements. [12, 13] The tips were cleaned with alcohol and compressed air after each data acquisition.
Microindentation was measured in live cells using a probe supported by a flexible cantilever, and hence microcantilever deflection is measured instead of direct force measurement. [14] The force-displacement relation is collected and analyzed with the Hertz model [15, 16]. The Hertz model describes the indentation of an infinitely hard spherical structure, which is the probe on the elastic sample surface with normal force. The elastic modulus is the slope of the withdrawal portion of the force-displacement curve that is plotted based on this equation:
where F is the applied load, ν is Poisson’s ratio for the sample, Rtip is the radius of curvature of the AFM probe, δ = (s0-s) is the indentation depth, s0 is the point of zero indentation, s is the position at the indentation depth, Esurface is the local Young’s elastic modulus. Adhesion force is taken to be zero in the Hertz model. [15, 16]
Nanoscope III was primarily used to obtain images of cytoskeletal fibers in control and agonist treated cells. Cells fixed on a circular cover slip were attached to a magnetic holder (sample holder) with an adhesive. Laser adjustment over the tip in the x-y direction was performed in conjunction with the lateral movements of the tip located over the sample.
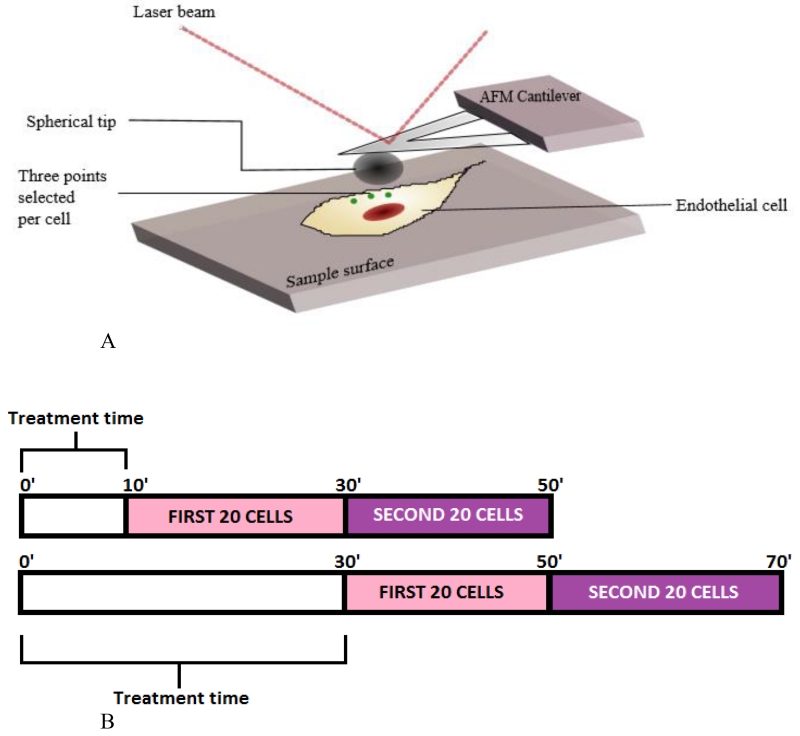
Novascan was employed primarily for determining Young’s moduli of cells after agonist treatment. Cells were plated on 22 × 22 mm microscope cover glasses and secured into the AFM sample chamber in Hank’s buffered salt solution 1× (HBSS) (Gibco, Carlsbad, CA). The cantilever descended towards the cell at a velocity of ~2 mm/s until a trigger force of 3 nN was reached, and then retracted. [17] Force curves were recorded by probing each cell of the sample at three different locations close to the cell periphery (Figure 1A). This procedure was repeated for up to 40 cells in a single sample to provide reproducible results. We compared three barrier enhancing and one barrier disrupting agonist versus control cells: - S1P (Sigma-Aldrich, Saint Louis, MO), HGF (PeproTech, Rocky Hill, NJ), FTY720 (generously provided by Novartis, Basel, Switzerland), and thrombin (Sigma-Aldrich). Each agonist was measured beginning at a 10 minute or 30 minute treatment time after stimulation (Figure 1B). The following concentrations were used: S1P and FTY720 (1μM), HGF (25 ng/mL), and thrombin (10 mg/mL). Both cell types (HLMVEC and HPAEC) were exposed to each of the agonists in order to assess their differential responses. Results were analyzed with software developed from Visual Basic. The final result is the Young’s modulus value obtained from the Hertz model.
FIGURE 1.
(A) Diagrammatic representation of the AFM setup and cellular locations where AFM measurements were performed. Three individual measurements were taken per cell in the peripheral regions as indicated (green dots). (B) Diagrammatic representation of the timeframe of Elastic modulus measurements for each condition. 40 cells were analyzed per condition, starting at either 10 (top) or 30 minutes (bottom) after addition of individual agonists at time 0. Approximately one minute is required for data collection from each cell. As a result, the first 20 cells in the “10 minute group” represent data collected from 10-30 minutes, while the second set of 20 cells represent data collected from 30-50 minutes in this group.
Statistical Analysis
Data are presented as means of the respective groups with standard error bars. Statistical significance was determined through t-test hypothesis testing represented as a p-value assuming unequal variance of data sets. This statistical test was used to explicitly demonstrate if the control and treatment cases are significantly different based on the Young’s modulus. Statistical significance was defined as p < 0.05 assuming a two tailed distribution.
RESULTS
AFM studies were divided into two parts- a) topographical imaging of human lung EC in response to various agonists as recorded with the Nanoscope III; and b) determination of cell elasticities (Young’s modulus) under these conditions.
Topographical Imaging
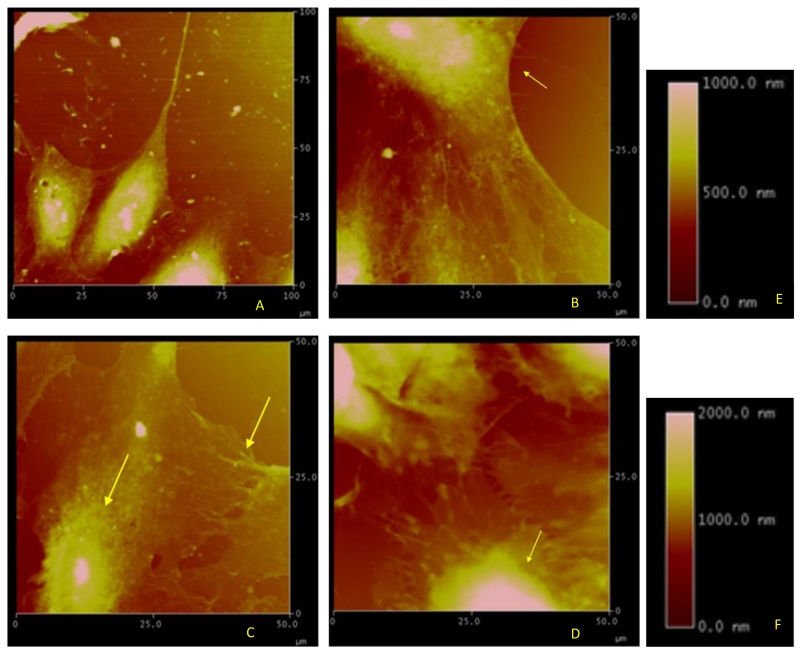
To optimize topographical imaging of fixed human lung EC, AFM scan size was maintained at an area of 100 μm2 using a tetrahedral tip with a 6 nm radius of curvature and a scan rate of 1.001 Hz, with an average scan time of 4.3 minutes per image. Before stimulation, both HPAEC and HLMVEC exhibited height ranges from 1000 nm at the center of the cell (nucleus) to approximately 500 nm towards the periphery. Figure 2A demonstrates a representative distribution of cell topography in unstimulated, fixed cells. In our previous AFM study we reported height measurements ranging from 3150 nm at the nucleus to 2200 nm in the cytoplasm in unstimulated, fixed HPAEC [11]. It is likely that the differences in height measured in the two studies are due to variations in cell preparation, fixation, AFM technique, and the height thresholding limit on the Nanoscope III.
FIGURE 2.
Topographic images of fixed HPAEC obtained with the Nanoscope and displaying the corresponding height information on a scale of 0 to 2000 nm. (A) Control HPAEC. (B) S1P-stimulated HPAEC (10 min) displaying a more uniform distribution of elastic fibers towards the periphery (indicated by the arrow) and a slightly heightened nucleus (around 1000 nm). (C) HGF-stimulated HPAEC (10 min) displaying characteristics similar to those after S1P (arrows) along with junction formation enhanced by cortical ring formation. (D) Thrombin-stimulated HPAEC (10 min) displaying visible rounding and extreme height increase at the nucleus (indicated by the arrow) with a maximum of 2000 nm. (E) represents the height scale for A-C. (F) represents the height scale for D. Representative images from multiple experiments (5-10 per condition) are shown.
S1P is a barrier enhancing agent that induces rearrangement of the actin cytoskeletal fibers towards the periphery that serves to strengthen the cortical ring and prevent fluid leakage. [8, 18] In Figure 2B, S1P-stimulated HPAEC are magnified to a 50 μm2 scale with the same height ranges as in the control cells. Actin fibers show interaction among neighboring cells with increased paracellular connections. These cytoskeletal changes correlate with the dose dependent barrier-enhancing effect of S1P in EC. [8]
HGF induced a similar response, with the magnified image demonstrating formation of paracellular connections along with actin fiber arrangement along the cell periphery (Figure 2C). The peripheral regions are consistent with the observation that HGF-treated EC display a strong augmentation of the cortical actin ring and cytoplasmic actin stress fiber dissolution.[19] Height scales up to 1 μm were observed.
Thrombin is a barrier disruptive agonist that produces vascular leak. [1] Thrombin induces formation of F-actin stress fibers in the cytoplasm of the EC, which is reflected in these studies by the heightened response displayed at the centers of the cells (Figure 2D). Note that the height scale has been increased to 2 μm because of the increased rearrangement of filaments towards the nucleus, consistent with contraction and a barrier disruptive effect on the EC. Cells also display an obvious rounding, consistent with earlier studies [20]. Additional EC were treated with S1P following initial thrombin stimulation. These cells displayed the barrier-promoting effects of S1P and reversed the barrier disruption caused by thrombin (data not shown).
AFM Elasticity Measurements
Live unstimulated cells were next compared to agonist-treated EC to study variations in Young’s modulus over time as a measurement of biophysical properties important for structural function. Figure 1 depicts the regions of the cells that were recorded in order to determine the effects of these agonists on cell elasticity in the cytoplasm and periphery. Stimulated EC were then assessed over two overlapping 40 minute intervals, with the first starting 10 minutes after agonist stimulation (i.e., 10 minute to 50 minute time gap), and the second starting 30 minutes after agonist stimulation (i.e., 30 minute to 70 minute time gap) (with an average of 1 minute per cell and measurements made in sets of either 20 or 40 cells) (Figure 1B). Thus, measurements beginning at the 10 minute time point reflect analyses obtained during the 10-50 minute interval, while measurements beginning at the 30 minute time point reflect analyses during the 30-70 minute interval. All conditions were compared in both HPAEC and HLMVEC to characterize the differential elasticity effects of these agonists on subpopulations of EC from different vascular regions within the lung.
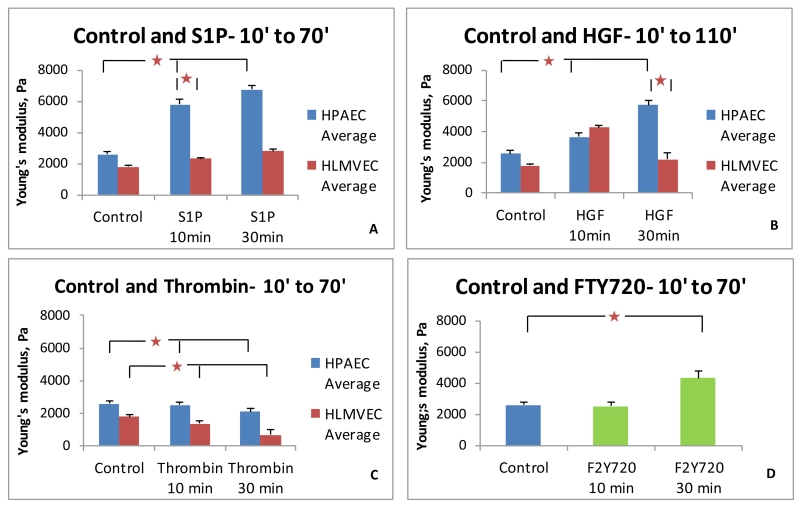
Comparison of control and S1P-treated HPAEC and HLMVEC are shown in Figure 3A. Young’s moduli displayed by HLMVEC are numerically lower under all conditions as compared to that of HPAEC. S1P-treated HPAEC demonstrate a steady increase in modulus values, with a difference of almost 1000 Pa between S1P-treated HPAEC and HLMVEC. While HPAEC demonstrate an increase in elastic modulus of 3.2 KPa and 4.2 KPa over the average value of 2.6 KPa recorded for control cells, HLMVEC exhibit significantly smaller increases of 0.6 KPa and 1 KPa only. Significant differences (p < 0.05) exist between control and S1P-stimulated HPAEC, and between HPAEC and HLMVEC after S1P, but not between the control and S1P-stimulated HLMVEC. HGF-stimulated HPAEC demonstrate Young’s moduli variations of a lower magnitude than after S1P (Figure 3B). HLMVEC display higher Young’s modulus after HGF for the 10-50 minute interval as compared to the 30-70 minute interval. Significant differences are observed in both HPAEC and HLMVEC after HGF compared to control cells, but the patterns are different as HLMVEC peaks earlier than HPAEC. However, thrombin stimulation demonstrated a similar pattern in both HPAEC and HLMVEC (Figure 3C). In both cell types thrombin decreased the peripheral elastic modulus as compared to the control. Absolute peripheral elasticities are much lower than those observed in S1P and HGF- stimulated cells. Though both HPAEC and HLMVEC exhibit statistically significant declines within their groups after thrombin, the differences between HPAEC and HLMVEC groups are not consistently significant. The pharmaceutical agent and S1P analog, FTY720, increased the Young’s modulus in HPAEC only at the later time point (Figure 3D), consistent with its previously described delayed onset of barrier enhancement relative to S1P. [21]
FIGURE 3.
Comparison of the peripheral Elastic modulus values for HPAEC and HLMVEC in response to barrier-regulatory agonists. (A) S1P produces an increase in Young’s modulus in both cell types, but the magnitude is lower in HLMVEC. (B) HGF stimulation demonstrated similar trends as S1P for HPAEC but at a lower absolute magnitude. HLMVEC demonstrated an earlier peak in magnitude compared to S1P. (C) Thrombin decreases peripheral elastic modulus values in both cell types. (D) FTY720 increases the elastic modulus in HPAEC in the 30 minute group. (* p < 0.05 between indicated conditions. 40 cells per condition).
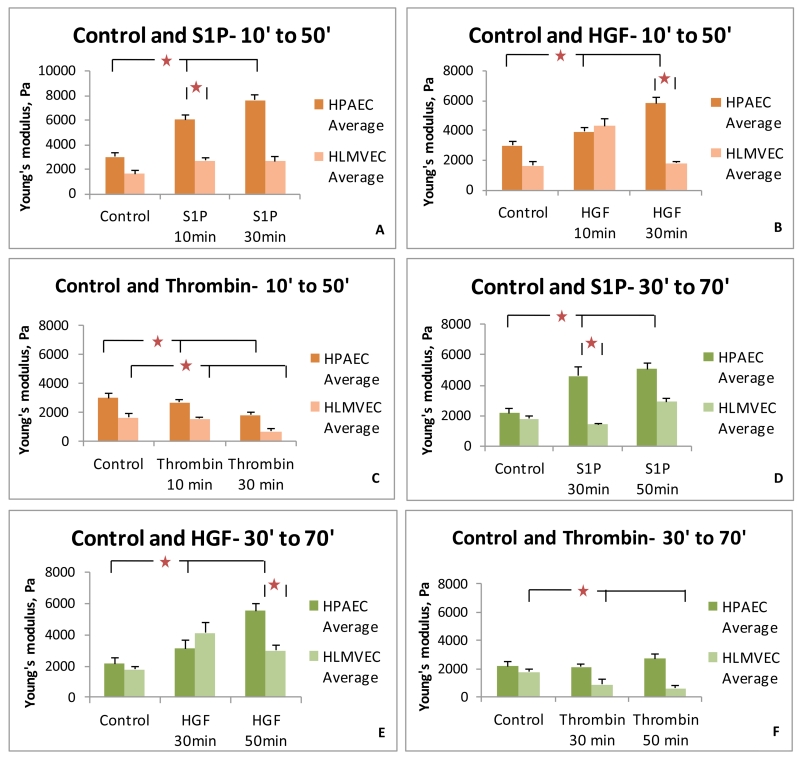
To more closely examine the time course effects of these agonists, subgroup data analysis was performed for the first 20 cells measured immediately after treatment (Figure 4A-C). These data demonstrate trends similar to those of the complete data shown in Figure 3, thus providing further evidence that these observations are valid for the time periods studied. We next analyzed the data obtained from the second set of 20 cells obtained from each condition (Figure 4D-F). Again, the trends are relatively similar to the complete data set except for HPAEC stimulated with thrombin. No decline in elasticity was observed in the second set of 20 cells for this condition.
FIGURE 4.
Subgroup comparison of the peripheral Elastic modulus values for HPAEC and HLMVEC in response to barrier-regulatory agonists. (A-C) Young’s modulus data are shown for the first 20 cells collected under each condition (representing data obtained 10-30 minutes or 30-50 minutes after stimulation). (D-E) Young’s modulus data are shown for the second 20 cells collected under each condition (representing data obtained 30-50 minutes or 50-70 minutes after stimulation). Similar trends are observed in these subgroups as seen in the complete data set shown in Figure 4, supporting the reproducible validity of this approach. (* p < 0.05 between indicated conditions)
DISCUSSION
The primary goal of these AFM studies was to determine the differential elasticity effects of barrier-modifying agonists on two types of human lung endothelial cells, macrovascular HPAEC and microvascular HLMVEC. The hypothesis is that functional effects of S1P, HGF, FTY720, and thrombin on EC cytoskeletal structure correlate with elastic modulus values and the known barrier-altering consequences of these agents. The observations derived from each agent are summarized and discussed as follows.
S1P
Our results demonstrate that S1P potently increases the peripheral elastic modulus in HPAEC, beginning within 10 minutes and persisting throughout the time studied (at least 70 minutes). Interestingly, the effect of S1P on HLMVEC was much less pronounced and did not reach statistical significance (Figure 3A), suggesting that the barrier-promoting effects of S1P may be less pronounced in the microvasculature of the lung. The biophysical properties of this important lung EC subtype had not been explored in detail in prior work. [2-4, 11] These results add to previous reports of multiple phenotypic differences between macro- and microvascular lung EC. [22] S1P causes rapid cortical actomyosin redistribution in the EC cytoskeleton [8, 23], which correlates with the height response from the AFM images. Published studies indicate that this redistribution is associated with Rac protein activity that mediates the downstream actions of S1P and translocates cortactin (F-actin binding protein) towards the cell periphery followed by creation of focal adhesion complexes. [8, 18, 24] As a result, we elected to characterize AFM elasticity measurements at the cell periphery during S1P stimulation. Our results demonstrate increased elastic modulus at the periphery concurrent with the actin arrangement in the periphery regions. [11, 23, 25] S1P induces a decrease in height and effective cell flattening, thus measuring a maximum height of 1 μm at the nucleus in the AFM images. The differences in responses elicited within HPAEC and HLMVEC are not completely understood as discussed below.
FTY720
FTY720 is a pharmaceutical analog of S1P and an FDA-approved medication for multiple sclerosis [26, 27] that has the potential for clinical use to modulate endothelial barrier function. It induces less cortical actin formation and has a delayed onset of action relative to S1P, usually requiring a minimum of 30 minutes to elicit endothelial barrier enhancement [21]. The Young’s modulus results obtained here are consistent with these prior observations of FTY720 (Figure 3D). The 10 minute treatment group did not display any change in elasticity compared to controls, but the 30 minute group demonstrated a 1.7 KPa increase in Young’s modulus, consistent with a delayed onset of action relative to S1P.
HGF
HGF has barrier enhancing effects that are similar but less potent than those induced by S1P. [28] HGF augments the cortical actin ring and leads to the dissolution of cytoplasmic actin stress fibers. However, in the current study, HLMVEC elasticity measurements demonstrate a different trend after HGF as compared to S1P (Figure 3B). The HGF effects peak earlier, as the 10 minute treatment time demonstrates a higher elasticity value as compared to the 30 minute treatment. In contrast, HPAEC demonstrate an increase in elasticity that continues to increase throughout the time period studied (until 70 minutes), similar to the S1P pattern. In HLMVEC, longer exposure to HGF (greater than 30 minutes) may reduce its barrier-enhancing effects as has been observed with FTY720 and some of its analogs. [26, 29] However, HGF is capable of reversing the barrier disruptive effects of thrombin and therefore remains a potent barrier-enhancing agent [19], and our data suggest that it may be more effective in lung microvasculature (HLMVEC) than S1P (Figure 3A).
Thrombin
AFM topographic analysis indicates variations in deposition of cellular stress fibers with a maximum height of 2 μm after barrier-disrupting thrombin, as opposed to 1 μm observed for the barrier-enhancing stimuli tested (Figure 2). Prior research has measured nucleus heights of up to 5 μm with thrombin treatment [11, 30] but the AFM used for this current study had a height threshold, so it is possible the EC heights were even greater. Topographic analysis also indicates visible rounding of cells and actin rearrangement into stress fibers after thrombin. Prior AFM studies of EC displayed an increase in cell height with actin depolymerization, which is an immediate effect of thrombin and other barrier-disrupting agonists. [1, 31] This finding contributes to the altered peripheral structure after thrombin, leading to lower elastic modulus values in this subcellular location. Our data indicate that thrombin decreases peripheral elasticity in a similar time course pattern in both HPAEC and HLMVEC, although the absolute Young’s modulus is lower in HLMVEC (Figure 3C). These findings suggest that there may be a magnitude differential in the level of barrier dysfunction and vascular leak induced in HLMVEC compared to HPAEC by thrombin.
Macro- vs. Microvascular Lung Endothelial Cells
Interestingly, HLMVEC displayed lower numerical elastic modulus values than HPAEC for all conditions studied. These results strongly suggest differential mechanical properties of the two types of human lung endothelial cells. Multiple potential causes could be responsible for this varied response – for example, increased drug dosage may be required to elicit a higher response with S1P and thrombin, receptors may be expressed at different levels, or existence of slight variations in the stimulation pathways at the molecular scale. [32] Future work will explore these important phenotypic differences in human lung EC.
AFM Advantages and Limitations
As noted in the introduction, AFM is well-suited to provide novel biophysical insights in cellular studies because it has the ability to measure cell properties in the nano- to micrometer range without fixation, unlike electron microscopy. There are no restrictions on the sample preparation, the temperature conditions, and buffer composition. In addition, it allows for high lateral resolution of about 1 nm, with even higher vertical resolution in the range of 0.1 nm. Thus, AFM allows for the longitudinal measurement of dynamic biophysical properties in living cells at the nano level in near real-time.
It also is important to discuss several potential limitations of these studies. First, the probe used in AFM imaging of biological samples usually has a smooth tip, and as a consequence, the image represents the interaction of the probe with the sample and not necessarily the exact topography. Tip contamination is another major problem faced when imaging biological samples. This hampers the sensitivity and resonance frequency of the tip, causing distorted images and irregular recording of force. The tip should be cleaned before and after every use to minimize deposition of salts from the buffer.
Other important potential limitations and/or sources of error in this specific work are as follows: (a) Effects of agonists could be affected by sample preparation technique and AFM measurement procedure. [18] (b) Individual cells sampled by AFM may express multiple types of phenotypic variability. (c) Earlier studies with multiple tips used for AFM studies on the same sample have produced varied results in spite of accurate calibration. [20, 33] (d) Lung EC are motile and may respond to interactions with the AFM tips to make live cell imaging complicated. (e) It is difficult to monitor the exact time scales of rapid agonist/antagonist action on the cells under the AFM. [11] However, our subgroup analyses indicate that AFM elasticity measurements of lung EC responses to barrier-altering stimuli obtained over 40 minute time frames (Figure 3) accurately reflect occurrences over shorter intervals (Figure 4), suggesting that AFM speed is sufficient for collecting biophysical data from multiple cells in a monolayer under these conditions.
Supplementary Material
HIGHLIGHTS.
AFM dynamically measures cellular biophysical properties in live endothelial cells
Macrovascular human lung EC exhibit different biophysical responses than microvascular
Agonists that promote EC barrier function increase peripheral elastic properties
Provide insights into potential therapeutic modulation of human lung EC
ACKNOWLEDGMENTS
We thank Dr. Shan Sun, University of Illinois at Chicago for her essential expertise with the AFM. We also thank the Nanotechnology Core Facility (NCF), University of Illinois for permission to use their facility. This work was supported by: NIH Funding; PO1 58064 [PI Garcia], R56 HL088144 [Dudek].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Dudek SM, Garcia JGN. Cytoskeletal regulation of pulmonary vascular permeability. Journal of Applied Physiology. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- [2].Birukova AA, Arce FT, Moldobaeva NBS, Dudek SM, Garcia JGN, Lal R, Birukov KG. Endothelial permeability is controlled by spatially defined cytoskeletal mechanics: AFM force mapping of pulmonary endothelial monolayer. Nanomedicine. 2009 doi: 10.1016/j.nano.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang X, Bleher R, Brown ME, Garcia JGN, Dudek SM, Shekhawat GS, Dravid VP. Nano-Biomechanical Study of Spatio-Temporal Cytoskeleton Rearrangements that Determine Subcellular Mechanical Properties and Endothelial Permeability. Scientific Reports. 2015;5:11097. doi: 10.1038/srep11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Terán Arce F, Meckes B, Camp SM, Garcia JGN, Dudek SM, Lal R. Heterogeneous elastic response of human lung microvascular endothelial cells to barrier modulating stimuli. Nanomedicine : nanotechnology, biology, and medicine. 2013;9:875–884. doi: 10.1016/j.nano.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].O’Callaghan R, Job KM, Dull RO, Hlady V. Stiffness and heterogeneity of the pulmonary endothelial glycocalyx measured by atomic force microscopy. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2011;301:L353–L360. doi: 10.1152/ajplung.00342.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Birukova AA, Smurova K, Birukov KG, Usatyuk P, Liu F, Kaibuchi K, Ricks-Cord A, Natarajan V, Alieva I, Garcia JG, Verin AD. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: role of Rho-dependent mechanisms. J Cell Physiol. 2004;201:55–70. doi: 10.1002/jcp.20055. [DOI] [PubMed] [Google Scholar]
- [7].Zhao Y, Davis HW. Hydrogen peroxide-induced cytoskeletal rearrangement in cultured pulmonary endothelial cells. J Cell Physiol. 1998;174:370–379. doi: 10.1002/(SICI)1097-4652(199803)174:3<370::AID-JCP11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- [8].Garcia JGN, L F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. Journal of Clinical Investigation. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Birukov KG, Birukova AA, Dudek SM, Verin AD, Crow MT, Zhan X, DePaola N, Garcia JG. Shear stress-mediated cytoskeletal remodeling and cortactin translocation in pulmonary endothelial cells. Am J Respir Cell Mol Biol. 2002;26:453–464. doi: 10.1165/ajrcmb.26.4.4725. [DOI] [PubMed] [Google Scholar]
- [10].Bogatcheva NV, Verin AD, Wang P, Birukova AA, Birukov KG, Mirzopoyazova T, Adyshev DM, Chiang ET, Crow MT, Garcia JG. Phorbol esters increase MLC phosphorylation and actin remodeling in bovine lung endothelium without increased contraction. Am J Physiol Lung Cell Mol Physiol. 2003;285:L415–426. doi: 10.1152/ajplung.00364.2001. [DOI] [PubMed] [Google Scholar]
- [11].Arce FT, Whitlock JL, Birukova AA, Birukov KG, Arnsdorf MF, Lal R, Garcia JGN, Dudek SM. Regulation of the Micromechanical Properties of Pulmonary Endothelium by S1P and Thrombin: Role of Cortactin. Biophysical Journal. 2008;95:886–894. doi: 10.1529/biophysj.107.127167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Alcaraz J, Buscemi L, Grabulosa M, Trepat X, Fabry B, Farre R, Navajas D. Microrheology of human lung epithelial cells measured by atomic force microscopy. Biophys.J. 2003;84:2071–2079. doi: 10.1016/S0006-3495(03)75014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rotsch C, Radmacher M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. Biophys. J. 2000;78:520–535. doi: 10.1016/S0006-3495(00)76614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Titushkin I, Cho M. Regulation of Cell Cytoskeleton and Membrane Mechanics by Electric Field: Role of Linker Proteins. Biophysical Journal. 2009;96:717–728. doi: 10.1016/j.bpj.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Costa KD, Yin FP. Analysis of Indentation: Implications for Measuring Mechanical Properties With Atomic Force Microscopy. ASME. J Biomech Eng. 1999;121(5):462–471. doi: 10.1115/1.2835074. [DOI] [PubMed] [Google Scholar]
- [16].Radmacher M. Measuring the elastic properties of living cells by the atomic force microscope. Methods Cell Biol. 2002;68:67–90. doi: 10.1016/s0091-679x(02)68005-7. [DOI] [PubMed] [Google Scholar]
- [17].Titushkin I, Cho M. Modulation of Cellular Mechanics during Osteogenic Differentiation of Human Mesenchymal Stem Cells. Biophysical Journal. 2007;93:3693–3702. doi: 10.1529/biophysj.107.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang LW, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvascular Research. 2009;77:39–45. doi: 10.1016/j.mvr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Birukova AA, Aleekseva E, Mikaelyan A, Birukov KG. HGF attenuates thrombin-induced endothelial permeability by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. The FASEB Journal. 2007;21:2776–2786. doi: 10.1096/fj.06-7660com. [DOI] [PubMed] [Google Scholar]
- [20].Tatyana GK, Maria NS, Nicolai IY, Sergey AC, Renat IZ. Atomic force microscopy probing of cell elasticity. Micron. 2007;38:824–833. doi: 10.1016/j.micron.2007.06.011. [DOI] [PubMed] [Google Scholar]
- [21].Dudek SM, Camp SM, Chiang ET, Singleton PA, Usatyuk PV, Zhao Y, Natarajan V, Garcia JGN. Pulmonary endothelial cell barrier enhancement by FTY720 does not require the S1P1 receptor. Cellular Signalling. 2007;19:1754–1764. doi: 10.1016/j.cellsig.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvascular Research. 2004;67:139–151. doi: 10.1016/j.mvr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- [23].McVerry BJ, Garcia JGN. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cellular Signalling. 2005;17:131–139. doi: 10.1016/j.cellsig.2004.08.006. [DOI] [PubMed] [Google Scholar]
- [24].Dudek SM, Jacobson JR, Chiang ET, Birukov KG, Wang P, Zhan X, Garcia JGN. Pulmonary endothelial cell barrier enhancement by sphingosine 1-phosphate: roles for cortactin and myosin light chain kinase. J Biol Chem. 2004;279:24692–24700. doi: 10.1074/jbc.M313969200. [DOI] [PubMed] [Google Scholar]
- [25].Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha’afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- [26].Camp SM, Bittman R, Chiang ET, Moreno-Vinasco L, Mirzapoiazova T, Sammani S, Lu X, Sun C, Harbeck M, Roe M, Natarajan V, Garcia JG, Dudek SM. Synthetic analogs of FTY720 [2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol] differentially regulate pulmonary vascular permeability in vivo and in vitro. Journal of Pharmacology and Experimental Therapeutics. 2009;331:54–64. doi: 10.1124/jpet.109.153544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pelletier D, Hafler DA. Fingolimod for multiple sclerosis. N Engl J Med. 2012;366:339–347. doi: 10.1056/NEJMct1101691. [DOI] [PubMed] [Google Scholar]
- [28].Liu F, Schaphorst KL, Verin AD, Jacobs K, Birukova A, Day RM, Bogatcheva N, Bottaro DP, Garcia JGN. Hepatocyte growth factor enhances endothelial cell barrier function and cortical cytoskeletal rearrangement: potential role of glycogen synthase kinase-3β. The FASEB Journal. 2002;16:950–962. doi: 10.1096/fj.01-0870com. [DOI] [PubMed] [Google Scholar]
- [29].Wang L, Chiang ET, Simmons JT, Garcia JGN, Dudek SM. FTY720-induced human pulmonary endothelial barrier enhancement is mediated by c-Abl. European Respiratory Journal. 2011;38:78–88. doi: 10.1183/09031936.00047810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Michal JW, Naoki K, Tetsuya T, Guoping C. Monitoring of mechanical properties of serially passaged bovine articular chondrocytes by atomic force microscopy. Micron. 2009;40:870–875. doi: 10.1016/j.micron.2009.06.002. [DOI] [PubMed] [Google Scholar]
- [31].Hiesgen R, Haiber J. Measurement Methods | Structural Properties: Atomic Force Microscopy. Encyclopedia of Electrochemical Power Sources. 2009:696–717. [Google Scholar]
- [32].Chiara D, Paola B. Sphingosine 1-phosphate regulates cytoskeleton dynamics: Implications in its biological response. Biochimica et Biophysica Acta. 2006;1758:2037–2048. doi: 10.1016/j.bbamem.2006.06.015. [DOI] [PubMed] [Google Scholar]
- [33].Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvascular Research. 2009;77:53–63. doi: 10.1016/j.mvr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.