Summary
Although conventional antimicrobial drugs have been viewed as miraculous cure-alls for the past 80 years, increasing antimicrobial drug resistance requires a major and rapid intervention. However, the development of novel but still conventional systemic antimicrobial agents, having only a single mode or site of action, will not alleviate the situation because it is probably only a matter of time until any such agents will also become ineffective. To continue to produce new agents based on this notion is unacceptable, and there is an increasing need for alternative approaches to the problem. By contrast, light-activated molecules called photoantimicrobials act locally via the in-situ production of highly reactive oxygen species, which simultaneously attack various biomolecular sites in the pathogenic target and therefore offer both multiple and variable sites of action. This non-specificity at the target circumvents conventional mechanisms of resistance and inhibits the development of resistance to the agents themselves. Photoantimicrobial therapy is safe and easy to implement and, unlike conventional agents, the activity spectrum of photoantimicrobials covers bacteria, fungi, viruses, and protozoa. However, clinical trials of these new, truly broad-spectrum, and minimally toxic agents have been few, and the funding for research and development is almost non-existent. Photoantimicrobials constitute one of the few ways forward through the morass of drug-resistant infectious disease and should be fully explored. In this Personal View, we raise awareness of the novel photoantimicrobial technologies that offer a viable alternative to conventional drugs in many relevant application fields, and could thus slow the pace of resistance development.
Introduction
In the past 4 years, reports and positional statements from governments and regional and global health authorities have finally acknowledged the seriousness of antimicrobial drug resistance.1, 2, 3, 4 Similar statements have been made and discussions undertaken very regularly—with plenty of well-reasoned arguments—among the scientific community during the past 25 years without any apparent acknowledgment from those wielding legislative powers. However, WHO, the US Centers for Disease Control and Prevention, the European Commission, G7 Summit, and national governments among industrialised countries have now spoken with one voice, calling for changes in clinical practice and the rapid development of new approaches for infection control, and encouraging basic research.1, 2, 3, 4
Unfortunately, this recent agreement does not mean that we can look forward to a happy future where infectious disease is concerned.2, 3, 4 There is a desire among the various strata of health-care providers— perhaps unstated, but there nonetheless—simply to have the failing antimicrobial drugs replaced with a brand new set. This desire is understandable given the ease of treatment associated with antibiotics when the microbes display no resistance, and a common belief remains that, in principle, we could return to the glory days of the antibiotic era as if nothing had changed.5 However, we know that microorganisms can rapidly develop resistance against a variety of costly antimicrobial agents.6 This problem largely derives from their single mode of action which, a large body of evidence suggests, is the reason that it is only a matter of time for microorganisms to develop ample resistance.7
An improved approach to avoid future development of resistance is to focus on compounds with modes of action that interact with multiple targets. The potentially new antibiotic teixobactin appears promising in this respect because it can attack alternative targets in the face of resistance; however, this antibiotic seems to be mainly active against only Gram-positive bacteria.8 Alternative safer and more efficient antimicrobial strategies are urgently needed—including those with efficacy against Gram-negative bacteria, mycobacteria, fungal, viral, and protozoal pathogens.9 In this Personal View, we contend that the use of light-activated molecules (photoantimicrobials)10 is a viable option that deserves to be fully explored. We discuss photoantimicrobials and their uses and prospects for adoption as mainstream clinical antimicrobials in the fight against conventional drug resistance.
Photoantimicrobials
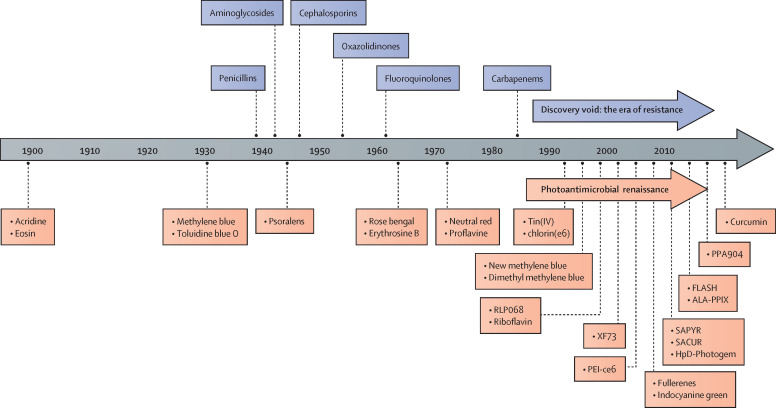
The original report11 concerning a photoantimicrobial effect was published in 1900, when Raab showed the inactivation of Paramecium caudatum when exposed to the dyes acridine or eosin and then illuminated with sunlight. Raab's supervisors, von Tappeiner and Jesionek, applied this approach to tumour cells and reported their results in 1905.12 Thus, both the antimicrobial and anticancer approaches of photodynamic therapy have been known for more than a century. Anticancer photodynamic therapy is now a clinical reality in hospitals and dermatology clinics worldwide, including for the treatment of actinic keratosis and basal cell carcinoma for more than 25 years,13 but antimicrobial photodynamic therapy is still largely unappreciated by clinicians and bodies responsible for health-care provision. Figure 1 shows that photoantimicrobial discovery has been more extensive than that of conventional agents since 1900, with a substantial increase since the beginning of the so-called era of resistance.
Figure 1.
Timeline for conventional and photoantimicrobial discovery
RLP068=tetracationic Zn(II) phthalocyanine chloride. XF73=positively charged porphyrin. PEI-ce6=polyethyleneimine chlorin(e6) conjugate. SAPYR=perinapthenone derivative. SACUR=curcumin derivative. HpD-Photogem=haematoporphyrin derivative. FLASH=cationic riboflavin derivative. ALA-PPIX=5-aminolevulinic acid-induced protoporphyrin IX. PPA904=tetrabutyl derivative of methylene blue.
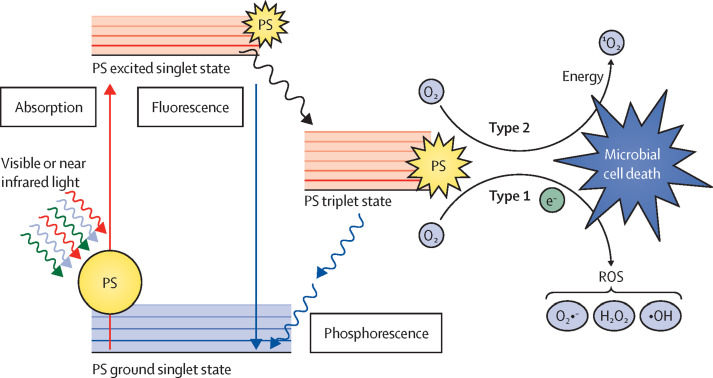
The basic principle of the photodynamic antimicrobial concept is the combination of visible or near infrared light, oxygen, and a photoantimicrobial that is able to absorb and transfer energy or electrons after light absorption to molecular oxygen for the generation of reactive oxygen species (figure 2 ). Reactive oxygen species, such as singlet oxygen, superoxide anions, and hydroxyl radicals, have a broad spectrum of activity and can destroy numerous molecular microbial targets (eg, proteins, lipids, and nucleic acids),9, 14 which makes it very unlikely that the microbes can develop resistance. It is worth emphasising that internalisation of the drug is not a prerequisite for cell kill, which further prevents the onset of resistance15 and has been observed in vitro via the repeated sublethal photosensitisation of bacteria. This mechanism of action neither selects for photoantimicrobial resistance nor does it alter sensitivity to conventional antibacterial drugs.16
Figure 2.
Mechanism of photoantimicrobial action
The generation of reactive oxygen species (ROS) can follow two alternative pathways after light activation by a given photosensitiser (PS). The PS can absorb a photon in the ground state, forming the excited singlet state. This state can undergo intersystem crossing to a longer-lived triplet state that might interact with oxygen by two mechanisms: in type 1, the generation of O2·−, ·OH, and H2O2 by electron transfer from the excited PS; in type 2, the triplet state of the PS can directly undergo energy exchange with triplet ground state oxygen, leading to the formation of excited 1O2. The generated ROS rapidly react with their environment depending on the localisation of the excited PS—eg, microorganism cell walls, lipid membranes, peptides, and nucleic acids. The PS returns to its initial state after this cycle, ready to absorb a new photon and generate additional ROS. O2·−=superoxide anions. ·OH=hydroxyl radical. H2O2=hydrogen peroxide. 1O2=singlet oxygen. e−=electron.
With respect to efflux, the major classes of antimicrobial photosensitisers have been tested extensively in vitro with an array of knockout and overexpressed bacterial efflux mutants. Only phenothiaziniums among the photosensitiser classes tested have been found to be efflux substrates.17 Despite this finding, the use of specific efflux pump inhibitors can reverse the reduced phenothiazinium phototoxicity due to efflux.18, 19
Efflux systems are central components for intrinsic antimicrobial resistance, but photoantimicrobial action requires three components (ie, drug, light, and oxygen): the phototoxic effect of light and subsequent production of reactive oxygen species constitute by default the mechanism of action, and are thus the primary target for resistance development. This mechanism of action provides photoantimicrobials with the unique competitive microbicidal advantage because the potential pathogen target, independently of whether it carries sophisticated efflux systems or not, must cope with two alternative assaults thus minimising substantially the possibility of resistance development.20
Additionally, efflux systems are transmembrane proteins, and it is well documented that proteins are highly susceptible to the photodynamic effect.21 Furthermore, potential resistance to the oxidative burst resulting from illumination might include enzymes such as superoxide dismutase and catalase, but these enzymes are ineffective against singlet oxygen and are, indeed, inactivated by it.22
Consequently, for example, Staphylococcus aureus or meticillin-resistant S aureus, Klebsiella pneumoniae (whether or not carbapenemase-positive) or Escherichia coli producing extended-spectrum β-lactamase and New Delhi metallo-β-lactamase-1 are each susceptible to cationic photoantimicrobials activated with harmless light (figure 2).
In addition to being active against both Gram-positive and Gram-negative bacteria, these photoantimicrobials are effective against fungal,23 viral,24 and protozoal targets,25 and exert their killing effects much more rapidly than conventional agents that might take hours or days to become effective, even against susceptible strains. This broad-spectrum activity would clearly be useful in the empirical treatment of emerging or undiagnosed infectious diseases.
In practice, the major selectivity criterion of the photodynamic approach is the short incubation period of the photosensitiser before illumination (typically a few minutes), which favours localisation in or on microorganisms and minimises penetration into host tissue (observed only after several hours), in addition to physical control allowed by the local application of light.26 Rapid uptake by target cells relative to the host, combined with physically targeted illumination, provides distinct and selective therapeutic advantage to photoantimicrobials compared with conventional drugs for topical or local applications. This statement does not entirely apply to systemic infections that will be the next and more challenging research frontier for photoantimicrobials.
Photoantimicrobial action furthermore affects the expression of virulence factors (eg, protein toxins, proteases, α-haemolysin, sphingomyelinase, and lipo-polysaccharide), causing their degradation by chemical oxidation.27, 28 The effects of photoantimicrobials in destroying virulence factors is of extreme importance because they might be present during the infection process, secreted by the microorganism, but they can also be present in the absence of active infection, as in the case of endotoxins that can cause severe damage to the host.
Photoantimicrobials also disrupt many sophisticated phenotypic processes of multidrug resistance related to biofilm formation (extracellular slime, stationary bacterial growth, and architecture and viability of early and mature biofilms).29, 30 Additionally, photoantimicrobial action has been associated with upregulation of the key oxidative stress enzyme superoxide dismutase, and the induction of the bacterial heat shock proteins GroEL and Dnak, which cause refolding of denatured proteins and stabilise lipid membranes during stress.31
At the concentrations applied—typically in the micromolar range—photoantimicrobials are harmless to host tissue without light activation, and with light activation only in the millimolar range and at a longer time interval. Because of their chemical nature—a majority of the compounds are phenothiazinium salts, porphyrins, or phthalocyanines—the biocompatibility is high. This chemical property is also the case for natural product photosensitisers such as curcumin (approved as the food additive E100),32, 33 riboflavin (vitamin B2),34 and hypericin.10
Activation of photoantimicrobials is simple, with use of low-powered lasers, light-emitting diodes, or conventional (halogen) lamps. With fibre-optic technology, most regions of the anatomy are accessible. In many cases, loci of infection (ear nose and throat, gastrointestinal tract, lungs, urinary tract, or bowel) could be managed endoscopically, allowing local application both of the photoantimicrobial agent and light. Even for deep-seated infections a transcutaneous needle could deliver both photoantimicrobial agent and light via a fibre. Additionally, the integration of plastic optical fibres into textile structures enables uniform light distribution to complex geometries of the human anatomy.35 Development of light-emitting bandages and wearable light-emitting garments is also underway.35
It is worth remembering that the therapeutic use of light-activated pharmaceuticals was, until relatively recently, ubiquitous in dermatology departments for the treatment of psoriasis using psoralen ultraviolet A irradiation therapy. Ironically, this combination therapy is also photoantimicrobial.36
Toxicity
Photoantimicrobials are not generally toxic molecules and should not be confused with biocides. Because of the photocatalytic mode of action (a single photosensitiser molecule can generate as many as 10 000 molecules of singlet oxygen before it is destroyed), they kill microbes more rapidly and at much lower concentrations than biocides, and clinical management is simply achieved by controlling the dose of light administered. Furthermore, photoantimicrobials fulfil the general criteria for new interventions to address the antibiotic resistance crisis—namely, preventing infection and resistance, preserving available antibiotics, slowing resistance, and developing microbe-attacking treatments with diminished potential to drive resistance.7
Toxicity to the human host would—quite correctly—be a concern for any new antimicrobial agent, conventional or otherwise, but there are highly effective photoantimicrobials, such as gentian violet or methylene blue, that have been in clinical systemic use in people for more than a century with excellent safety records. For example, the systemic use of methylene blue has been extensively examined during its testing as a conventional antimalarial agent in sub-Saharan Africa. Systemic single human doses of methylene blue (10 mg/kg) have been used in conventional antimalarial trials.37 This dose converts to a maximum concentration of 2000 μmol/L, whereas, for example, the minimum photobactericidal concentration for this photoantimicrobial against in-vitro S aureus or E coli is in the region of 20 μmol/L.
It should also be noted that local, rather than systemic, administration is proposed. Moreover, healing of the tissue is known to be good after photodynamic therapy,38 making photoantimicrobials a suitable choice for wounds or other traumatic infections (ie, acute bacterial skin and skin structure infections). An additional consideration is that in the treatment of many localised infections by photoantimicrobials, the photosensitiser is locally administered to the infected area but the red light that is then delivered diffuses and scatters well beyond the actual area of the infection. This red light can have a substantial beneficial effect in stimulating healing and repair in the surrounding tissue by the process known as photobiomodulation or low-level laser therapy.39
Other areas
The photodynamic approach is also applicable in veterinary medicine—a required addition to the activity spectrum to enable future conservation of conventional antimicrobials.40 For example, treatment of caseous lymphadenitis abscesses in sheep with photoantimicrobials was effective, and healing time was much shorter than the described conventional approaches.41 Similarly, photoantimicrobials are suitable for the removal of (typically bacterial or fungal) contaminants during food production42 including use on production surfaces,43 which is important given that foodborne and waterborne diarrhoeal diseases kill about 2·2 million people worldwide per year.44 Environmental applications of the photodynamic antimicrobial procedure range from management of plant pathogens,45 control of aquatic pathogens in fish farming,46 to inactivation of multidrug-resistant bacteria in hospital wastewaters.47 Furthermore, the use of photoantimicrobial materials can furnish light-activated surfaces that might be used to strengthen environmental infection control in the health-care setting, or be used in devices such as indwelling catheters or prostheses.48, 49
Although many instrumentation platforms and detection methods have been developed and commissioned as countermeasures to biological warfare agents, the threat and use of biological agents in bioterrorist attacks still remains a leading cause of global concern.50 Bacillus spp spores are susceptible to photoinactivation by phenothiazinium dyes (methylene blue or toluidine blue O) and low doses of red light, indicating applicability in anthrax spore decontamination.51 Nevertheless, the majority of biological warfare agents are selected for their respiratory mode of transmission such as melioidosis (Burkholderia pseudomallei), glanders (Burkholderia mallei), tularaemia (Francisella tularensis), or pneumonic plague (Yersinia pestis). Airborne pathogens constitute a challenge to photoactive drug and light delivery which is an active field of investigation.52
Current situation
A major factor in any 21st century anti-infectious disease programme is the influence of globalisation—ie, the ease of transcontinental movement and increasing numbers of population migration. Resistance development in an established population can be modelled and resources duly planned, but the problem of mobile populations disseminating infectious diseases previously unseen or with novel resistance patterns is potentially disastrous.6 Examples include the outbreak of severe acute respiratory syndrome (SARS) caused by a coronavirus in the early 2000s,53 or the global spread of Acinetobacter baumannii infections in civilian hospitals dealing with wounded military personnel.54, 55 Concerns also exist that drug-resistant tuberculosis could easily spread from its enclaves in the former Soviet Union and Africa to become a global problem.56
The control of emerging infectious diseases is often complicated by the fact that antibiotics are used indiscriminately, and are often self-administered by the affected population.5 Consequently, microbial strains might easily acquire resistance or have lowered susceptibility to approved prescribed drugs. Additionally, there is the potential for transmission of resistant phenotypes to other species among the indigenous microbiota.57 However, since photoantimicrobials are truly antimicrobial, they are expected to be effective against emerging or unknown pathogens, or those with new resistant traits, as well as established, conventionally susceptible or conventionally resistant species.
Because systemic microbial diseases are the most severe in terms of morbidity and mortality, the search for systemically administered therapies must be continued. However, in many situations, an effective alternative local approach could be advantageous—eg, in patient decolonisation, or the treatment of burn wounds or diabetic foot ulcers, thus avoiding the use of valuable systemic drugs that could then be conserved for critical care. Also, a rapidly acting photoantimicrobial intervention might help to prevent a localised infection becoming systemic. Localised infections are important in the clinic because a substantial percentage of health-care-associated infections originate from surgical wounds, many of which are located in the skin and soft tissues and can eventually cause systemic infections by invasion of the bloodstream. These considerations point to an urgent need for antimicrobials that work on multiple targets and on a local basis. The use of photoantimicrobials meets such requirements.
Clinical photoantimicrobials
If the pharmaceutical industry had produced a con-ventional agent with a broad range of activity similar to that exhibited by cationic photoantimicrobials, this agent would have been lauded as a game-changing breakthrough, particularly at the time when large pharmaceutical companies are substantially reducing their presence in the field of antimicrobials.58 However, the impressive performance of this light-activated technology is being widely ignored, despite around a quarter of a century of encouraging experimental and clinical studies, and countless attempts at bringing the new approach to the attention of health-care providers. No other reported antimicrobial approach has the potential to produce a similar change in concept in the way in which we address diseases caused by microbial infections.
Such an approach is clearly more appropriate when there is a shortage of conventional clinical agents and a worsening of antimicrobial resistance, as is the case with tuberculosis. In addition to presentations for which there is resistance to one of the standard drugs (usually isoniazid), there are increasing cases of multiple and extensively drug resistance, often associated with HIV comorbidity. Both multidrug resistant and extensively drug resistant mycobacteria have been shown to be susceptible to photoantimicrobials in vitro.59
Thus, despite overwhelming evidence, clinical trials have been few (table ). Only three photoantimicrobial agents (methylene blue, toluidine blue O, and indocyanine green) have so far received clinical approval, but only in dentistry as an adjuvant approach.70 Unsurprisingly, the combinatorial use of photoantimicrobials with conventional drugs has received considerable attention.71, 72, 73
Table.
Photoantimicrobials in clinical trials
| Chemical class | Wavelength | Spectrum of activity | Pathogen or type of infection treated | Clinical trial registration (used to treat, last updated) | |
|---|---|---|---|---|---|
| Methylene blue | Phenothiazinium | Red 660 nm | Broad | MRSA surgical site, chronic sinusitis, periodontitis, halitosis,60 oral candidiasis, oral mucositis (phase 3), severe sepsis and septic shock (phase 3), and onychomycosis61 | NCT02555501 (mucositis, 2015), NCT01854619 (sinusitis, 2013 ongoing), NCT02007993 (halitosis, 2014 ongoing), NCT02407379 and NCT01535690 (periodontitis, 2015 and 2012), and NCT01981460 (skin pathogens and blood infections, 2013) |
| Toluidine blue O | Phenothiazinium | Red 660 nm | Broad | Wounds, burns, diabetic ulcers,62 periodontitis63 (phase 2), and carious dentin lesion (phase 1) | NCT02479958 (carious dentin, 2015) and NCT01330082 (periodontitis, 2011 ongoing) |
| PPA904 | Phenothiazinium | Red 660 nm | Broad | MRSA, Pseudomonas aeruginosa, chronically non-healing streptococcal wounds,64, 65 and periodontitis | NCT00825760 (leg ulcers, 2013) |
| RLP068 | Phthalocyanine | Far red 670–780 nm | Broad | MRSA skin abrasion, and diabetic foot ulcers | EudraCT Number: 2010-019598-13 |
| ALA-PPIX | Porphyrin | Red 630 nm | Narrow | Propionibacterium acne and acne vulgaris,66 chronic skin ulcers, and Pseudomonas aeruginosa | NCT00706433 and NCT01689935 (acne, 2011 and 2013) |
| Indocyanine green | Indocyanine | Near infrared 810 nm | Narrow | Propionibacterium acne, acne vulgaris,67 and periodontitis | NCT02043340 (periodontitis, 2014) |
| Curcumin | Curcuminoid | Blue 420 nm | Narrow | Oral disinfection (phase 1), and oral mucositis (phase 1 or 2) | NCT02152475 (oral disinfection, 2014) and NCT02337192 (oral decontamination agent and mucositis infections, 2015 ongoing) |
| Riboflavin | Flavin | Blue 360 nm | N/A | Infectious keratitis68 | NCT01739673 (keratitis, 2015) |
| PEI-ce6 | Chlorin | Red 660 nm | Broad | Endodontic infection69 | IRB approved trial in São Paulo, Brazil |
All clinical trial phases are completed unless otherwise stated as ongoing. MRSA=meticillin-resistant Staphylococcus aureus. PPA904=tetrabutyl derviative of methylene blue. RLP068=tetracationic Zn(II) phthalocyanine chloride. ALA-PPIX=5-aminolevulinic acid-induced protoporphyrin IX. N/A=not applicable. PEI-ce6=polyethyleneimine chlorin(e6) conjugate. IRB=institutional review board.
Path forward
In terms of infection control, the photodynamic community has, so far, consisted of mainly various academic groups working towards the introduction of clinical photoantimicrobials without a consensus regarding the microbial target or the disease indication. With the continuing shortage of support and scarcity of grant funding—certainly in comparison with conventional anti-infective research—we provide a consensus and a statement of intent in this Personal View. We firmly believe that the photodynamic antimicrobial approach offers enormous potential savings, both in terms of the conservation of essential conventional drugs and for the rapid treatment of initial localised infection, thus requiring less exposure of the microbiome to the selective pressure exerted by conventional antimicrobials.
US$2 billion of forward funding for antimicrobial resistance programmes has been suggested by the 2014 UK O'Neill Report,74 whereas the 2016 US figure is $1·2 billion.75 It would be tragic if all that happens, should such funding be forthcoming, is that the current situation is maintained and funding goes solely to conventional antimicrobial development. The key worldwide health organisations have spoken of the requirement for novel alternative approaches: now they should be brave enough to use them.
Contributors
All authors contributed equally to the manuscript.
Declaration of interests
We declare no competing interests.
References
- 1.ECDC Surveillance report. Annual epidemiological report. Antimicrobial resistance and healthcare-associated infections. 2014. http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-annual-epidemiological-report.pdf (accessed Oct 15, 2015).
- 2.ECDC Surveillance report. Annual epidemiological report. Reporting on 2010 surveillance data and 2011 epidemic intelligence data. 2012. http://ecdc.europa.eu/en/publications/Publications/Annual-Epidemiological-Report-2012.pdf (accessed Oct 15, 2015).
- 3.WHO Antimicrobial resistance. Global report on surveillance. 2014. http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf?ua=1 (accessed Oct 15, 2015).
- 4.European Commission Commission staff working document. Progress report on the action plan against the rising threats from antimicrobial resistance. 2015. http://ec.europa.eu/health/antimicrobial_resistance/docs/2015_amr_progress_report_en.pdf (accessed Oct 16, 2015).
- 5.Berendonk T, Manaia CM, Merlin C. Tackling antibiotic resistance: the environmental framework. Nat Rev Microbiol. 2015;13:310–317. doi: 10.1038/nrmicro3439. [DOI] [PubMed] [Google Scholar]
- 6.Hwang T, Gibbs KA, Podolsky SH, Linder JA. Antimicrobial stewardship and public knowledge of antibiotics. Lancet Infect Dis. 2015;15:1000–1001. doi: 10.1016/S1473-3099(15)00235-2. [DOI] [PubMed] [Google Scholar]
- 7.Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368:299–302. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling LL, Schneider T, Peoples AJ. A new antibiotic kills pathogens without detectable resistance. Nature. 2015;517:455–459. doi: 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamblin M, Hassan T. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin R, Hamblin MR. Antimicrobial photosensitizers: drug discovery under the spotlight. Curr Med Chem. 2015;22:2159–2185. doi: 10.2174/0929867322666150319120134. [DOI] [PubMed] [Google Scholar]
- 11.Raab O. Über die Wirkung fluoreszcierender Stoffe aus Infusorien. Z Biol. 1900;39:524. (in German). [Google Scholar]
- 12.Jesionek A, von Tappeiner H. Zur behandlung der hautcarcinome mit fluorescierenden stoffen. Arch Klin Med. 1905;82:223. (in German). [Google Scholar]
- 13.Agostinis P, Berg K, Cengel KA. Photodynamic therapy of cancer: an update for clinicians. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vatansever F, de Melo WC, Avci P. Antimicrobial strategies centered around reactive oxygen species—bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol Rev. 2013;37:955–989. doi: 10.1111/1574-6976.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wainwright M, Crossley KB. Photosensitising agents—circumventing resistance and breaking down biofilms: a review. Int Biodeterior Biodegradation. 2004;53:119–126. [Google Scholar]
- 16.Lauro F, Pretto P, Covolo L, Jori G, Bertoloni G. Photoinactivation of bacterial strains involved in periodontal diseases sensitized by porphycene–polylysine conjugates. Photochem Photobiol Sci. 2002;1:468–470. doi: 10.1039/b200977c. [DOI] [PubMed] [Google Scholar]
- 17.Tegos G, Hamblin MR. Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob Agents Chemother. 2006;50:196–203. doi: 10.1128/AAC.50.1.196-203.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tegos G, Masago K, Aziz F, Higginbotham A, Stermitz FR, Hamblin MR. Inhibitors of bacterial multidrug efflux pumps potentiate antimicrobial photoinactivation. Antimicrob Agents Chemother. 2008;52:3202–3209. doi: 10.1128/AAC.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prates R, Kato IT, Ribeiro MS, Tegos GP, Hamblin MR. Influence of multidrug efflux systems on methylene blue-mediated photodynamic inactivation of Candida albicans. J Antimicrob Chemother. 2011;66:1525–1532. doi: 10.1093/jac/dkr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vera D, Haynes MK, Ball AR. Strategies to potentiate antimicrobial photoinactivation by overcoming resistant phenotypes. Photochem Photobiol. 2012;88:499–511. doi: 10.1111/j.1751-1097.2012.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alves E, Faustino MA, Neves MG, Cunha A, Tome J, Almeida A. An insight on bacterial cellular targets of photodynamic inactivation. Future Med Chem. 2014;6:141–164. doi: 10.4155/fmc.13.211. [DOI] [PubMed] [Google Scholar]
- 22.Cieplik F, Späth A, Regensburger J. Photodynamic biofilm inactivation by SAPYR—an exclusive singlet oxygen photosensitizer. Free Radic Biol Med. 2013;65:477–487. doi: 10.1016/j.freeradbiomed.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 23.Dai T, Fuchs BB, Coleman JJ. Concepts and principles of photodynamic therapy as an alternative antifungal discovery platform. Front Microbiol. 2012;3:120. doi: 10.3389/fmicb.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee I, Douaisi MP, Mondal D, Kane RS. Light-activated nanotube-porphyrin conjugates as effective antiviral agents. Nanotechnology. 2012;23:105101. doi: 10.1088/0957-4484/23/10/105101. [DOI] [PubMed] [Google Scholar]
- 25.Akilov O, Kosaka S, O'Riordan K. The role of photosensitizer molecular charge and structure on the efficacy of photodynamic therapy against Leishmania parasites. Chem Biol. 2006;13:839–847. doi: 10.1016/j.chembiol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Maisch T, Bosl C, Szeimies RM, Love B, Abels C. Determination of the antibacterial efficacy of a new porphyrin-based photosensitizer against MRSA ex vivo. Photochem Photobiol Sci. 2007;6:545–551. doi: 10.1039/b614770d. [DOI] [PubMed] [Google Scholar]
- 27.Kömerik N, Wilson M, Poole S. The effect of photodynamic action on two virulence factors of Gram-negative bacteria. Photochem Photobiol. 2000;72:676–680. doi: 10.1562/0031-8655(2000)072<0676:teopao>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Tubby S, Wilson M, Nair SP. Inactivation of staphylococcal virulence factors using a light-activated antimicrobial agent. BMC Microbiol. 2009;9:211. doi: 10.1186/1471-2180-9-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Melo W, Avci P, de Oliveira MN. Photodynamic inactivation of biofilm: taking a lightly colored approach to stubborn infection. Expert Rev Anti Infect Ther. 2013;11:669–693. doi: 10.1586/14787210.2013.811861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cieplik F, Tabenski L, Buchalla W, Maisch T. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front Microbiol. 2014;12:405. doi: 10.3389/fmicb.2014.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakonieczna J, Michta E, Rybicka M, Grinholc M, Gwizdek-Wiśniewska A, Bielawski KP. Superoxide dismutase is upregulated in Staphylococcus aureus following protoporphyrin-mediated photodynamic inactivation and does not directly influence the response to photodynamic treatment. BMC Microbiol. 2010;10:323. doi: 10.1186/1471-2180-10-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahl T, Bilski P, Reszka KJ, Chignell CF. Photocytotoxicity of curcumin. Photochem Photobiol. 1994;59:290–294. doi: 10.1111/j.1751-1097.1994.tb05036.x. [DOI] [PubMed] [Google Scholar]
- 33.Winter S, Tortik N, Kubin A, Krammer B, Plaetzer K. Back to the roots: photodynamic inactivation of bacteria based on water-soluble curcumin bound to polyvinylpyrrolidone as a photosensitizer. Photochem Photobiol Sci. 2013;12:1795–1802. doi: 10.1039/c3pp50095k. [DOI] [PubMed] [Google Scholar]
- 34.Maisch T, Eichner A, Späth A. Fast and effective photodynamic inactivation of multiresistant bacteria by cationic riboflavin derivatives. PLoS One. 2014;9:e111792. doi: 10.1371/journal.pone.0111792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cochrane C, Mordon SR, Lesage JC, Koncar V. New design of textile light diffusers for photodynamic therapy. Mater Sci Eng C Mater Biol Appl. 2013;33:1170–1175. doi: 10.1016/j.msec.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimura M, Namura S, Akamatsu H, Horio T. Antimicrobial effects of phototherapy and photochemotherapy in vivo and in vitro. Br J Dermatol. 1996;135:528–532. [PubMed] [Google Scholar]
- 37.Sahu K, Sharma M, Bansal H, Dube A, Gupta PK. Topical photodynamic treatment with poly-L-lysine-chlorin p6 conjugate improves wound healing by reducing hyperinflammatory response in Pseudomonas aeruginosa-infected wounds of mice. Lasers Med Sci. 2013;28:465–471. doi: 10.1007/s10103-012-1083-6. [DOI] [PubMed] [Google Scholar]
- 38.Avci P, Gupta A, Sadasivam M. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41–52. [PMC free article] [PubMed] [Google Scholar]
- 39.Sellera F, Sabino CP, Ribeiro MS. In vitro photoinactivation of bovine mastitis related pathogens. Photodiagnosis Photodyn Ther. 2016;13:276–281. doi: 10.1016/j.pdpdt.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Selleraa F, Gargano RG, Libera AM. Antimicrobial photodynamic therapy for caseous lymphadenitis abscesses in sheep: report of ten cases. Photodiagnosis Photodyn Ther. 2015;13:120–202. doi: 10.1016/j.pdpdt.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Tortik N, Spaeth A, Plaetzer K. Photodynamic decontamination of foodstuff from Staphylococcus aureus based on novel formulations of curcumin. Photochem Photobiol Sci. 2014;13:1402–1409. doi: 10.1039/c4pp00123k. [DOI] [PubMed] [Google Scholar]
- 42.Meissner P, Mandi G, Coulibaly B. Methylene blue for malaria in Africa: results from a dose-finding study in combination with chloroquine. Malar J. 2006;5:84. doi: 10.1186/1475-2875-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kairyte K, Lapinskas S, Gudelis V, Luksiene Z. Effective inactivation of food pathogens Listeria monocytogenes and Salmonella enterica by combined treatment of hypericin-based photosensitization and high power pulsed light. J Appl Microbiol. 2013;112:1144–1151. doi: 10.1111/j.1365-2672.2012.05296.x. [DOI] [PubMed] [Google Scholar]
- 44.WHO Sixty-third World Health Assembly. Resolutions and decisions annexes. 2010. http://apps.who.int/gb/ebwha/pdf_files/WHA63-REC1/WHA63_REC1-en.pdf (accessed Sept 10, 2016).
- 45.de Menezes H, Rodrigues GB, Teixeira Sde P. In vitro photodynamic inactivation of plant-pathogenic fungi Colletotrichum acutatum and Colletotrichum gloeosporioides with novel phenothiazinium photosensitizers. Appl Environ Microbiol. 2014;80:1623–1632. doi: 10.1128/AEM.02788-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arrojado C, Pereira C, Tome JP. Applicability of photodynamic antimicrobial chemotherapy as an alternative to inactivate fish pathogenic bacteria in aquaculture systems. Photochem Photobiol Sci. 2011;10:1691–1700. doi: 10.1039/c1pp05129f. [DOI] [PubMed] [Google Scholar]
- 47.Almeida J, Tome JP, Neves MG. Photodynamic inactivation of multidrug-resistant bacteria in hospital wastewaters: influence of residual antibiotics. Photochem Photobiol Sci. 2014;13:626–633. doi: 10.1039/c3pp50195g. [DOI] [PubMed] [Google Scholar]
- 48.McCoy CP, O'Neil EJ, Cowley JF. Photodynamic antimicrobial polymers for infection control. PLoS One. 2014;9:e108500. doi: 10.1371/journal.pone.0108500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Felgenträger A, Maisch T, Späth A, Schröder JA, Bäumler W. Singlet oxygen generation in porphyrin-doped polymeric surface coating enables antimicrobial effects on Staphylococcus aureus. Phys Chem Chem Phys. 2014;16:20598–20607. doi: 10.1039/c4cp02439g. [DOI] [PubMed] [Google Scholar]
- 50.Tegos G. Biodefense: trends and challenges in combating biological warfare agents. Virulence. 2013;4:740–744. doi: 10.4161/viru.27170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demidova T, Hamblin MR. Photodynamic inactivation of Bacillus spores, mediated by phenothiazinium dyes. Appl Environ Microbiol. 2005;71:6918–6925. doi: 10.1128/AEM.71.11.6918-6925.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cassidy C, Tunney MM, Magee ND. Drug and light delivery strategies for photodynamic antimicrobial chemotherapy (PACT) of pulmonary pathogens: a pilot study. Photodiagnosis Photodyn Ther. 2011;8:1–6. doi: 10.1016/j.pdpdt.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Peiris JS, Guan Y, Yuen KY. Severe acute respiratory syndrome. Nat Med. 2004;10(suppl 12):S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abbott A. Medics braced for fresh superbug. Nature. 2005;436:758. doi: 10.1038/436758a. [DOI] [PubMed] [Google Scholar]
- 55.Dijkshoorn L, Neme A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 56.LoBue P, Sizemore C, Castro KG. Plan to combat extensively drug-resistant tuberculosis: recommendations of the Federal Tuberculosis Task Force. MMWR Recomm Rep. 2009;58:1–43. [PubMed] [Google Scholar]
- 57.Buffie C, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol. 2013;13:790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.May M. Drug development: time for teamwork. Nature. 2014;509:S4–S5. doi: 10.1038/509S4a. [DOI] [PubMed] [Google Scholar]
- 59.Sung N, Back S, Jung J. Inactivation of multidrug resistant (MDR)-and extensively drug resistant (XDR)-Mycobacterium tuberculosis by photodynamic therapy. Photodiag Photodyn Ther. 2013;10:694–702. doi: 10.1016/j.pdpdt.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 60.Lopes R, de Godoy CH, Deana AM. Photodynamic therapy as a novel treatment for halitosis in adolescents: study protocol for a randomized controlled trial. Trials. 2014;14:443. doi: 10.1186/1745-6215-15-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Figueiredo SLW, Souza SV, Botelho AC. Randomized controlled trial comparing photodynamic therapy based on methylene blue dye and fluconazole for toenail onychomycosis. Dermatol Ther. 2014;27:43–47. doi: 10.1111/dth.12042. [DOI] [PubMed] [Google Scholar]
- 62.Tardivo J, Adami F, Correa JA, Pinhal MA, Baptista MS. A clinical trial testing the efficacy of PDT in preventing amputation in diabetic patients. Photodiagnosis Photodyn Ther. 2014;11:342–350. doi: 10.1016/j.pdpdt.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 63.Neugebauer J, Jozsa M, Kubler A. Antimicrobial photodynamic therapy for prevention of alveolar ostitis and post-extraction pain. Mund Kiefer Gesichtschir. 2004;8:350–355. doi: 10.1007/s10006-004-0572-6. [DOI] [PubMed] [Google Scholar]
- 64.Brown S. Clinical antimicrobial photodynamic therapy: phase II studies in chronic wounds. J Natl Compr Canc Netw. 2012;10(suppl 2):S80–S83. doi: 10.6004/jnccn.2012.0182. [DOI] [PubMed] [Google Scholar]
- 65.Morley S, Griffiths J, Philips G. Phase IIa randomized, placebo-controlled study of antimicrobial photodynamic therapy in bacterially colonized, chronic leg ulcers and diabetic foot ulcers: a new approach to antimicrobialtherapy. Br J Dermatol. 2013;168:617–624. doi: 10.1111/bjd.12098. [DOI] [PubMed] [Google Scholar]
- 66.Barolet D, Boucher A. Radiant near infrared light emitting Diode exposure as skin preparation to enhance photodynamic therapy inflammatory type acne treatment outcome. Lasers Surg Med. 2010;42:171–178. doi: 10.1002/lsm.20886. [DOI] [PubMed] [Google Scholar]
- 67.Sakamoto FH, Torezan L, Anderson RR. Photodynamic therapy for acne vulgaris: a critical review from basics to clinical practice: part II. Understanding parameters for acne treatment with photodynamic therapy. J Am Acad Dermatol. 2010;63:195–211. doi: 10.1016/j.jaad.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 68.Said D, Elalfy MS, Gatzioufas Z. Collagen cross-linking with photoactivated riboflavin (PACK-CXL) for the treatment of advanced infectious keratitis with corneal melting. Ophthalmology. 2014;121:1377–1382. doi: 10.1016/j.ophtha.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 69.Garcez AS, Nunez SC, Hamblin MR, Ribeiro MS. Antimicrobial effects of photodynamic therapy on patients with necrotic pulps and periapical lesion. J Endod. 2008;34:138–142. doi: 10.1016/j.joen.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parker S. The use of diffuse laser photonic energy and indocyanine green photosensitiser as an adjunct to periodontal therapy. Br Dent J. 2013;215:167–171. doi: 10.1038/sj.bdj.2013.790. [DOI] [PubMed] [Google Scholar]
- 71.Arciola CR, Montanaro L, Costerton JW. New trends in diagnosis and control strategies for implant infections. Int J Artif Organs. 2011;34:727–736. doi: 10.5301/IJAO.2011.8784. [DOI] [PubMed] [Google Scholar]
- 72.Chibebe Junior J, Fuchs BB, Sabino CP. Photodynamic and antibiotic therapy impair the pathogenesis of Enterococcus faecium in a whole animal insect model. PLoS One. 2013;8:e55926. doi: 10.1371/journal.pone.0055926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanaka M, Mroz P, Dai T. Linezolid and vancomycin decrease the therapeutic effect of methylene blue-photodynamic therapy in a mouse model of MRSA bacterial arthritis. Photochem Photobiol. 2013;89:679–688. doi: 10.1111/php.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wellcome Trust and UK Department of Health Review on antimicrobial resistance. Tackling drug-resistant infections globally. 2014. http://amr-review.org/ (accessed Oct 16, 2015).
- 75.US CDC National strategy for the combating antibiotic-resistant bacteria. 2014. http://www.cdc.gov/drugresistance/pdf/carb_national_strategy.pdf (accessed Oct 16, 2015).