Summary
Background
Plg-RKT is a novel integral membrane plasminogen receptor that binds plasminogen via a C-terminal lysine exposed on the cell surface and promotes plasminogen activation on the cell surface by both tissue plasminogen activator and urokinase plasminogen activator.
Objectives
To evaluate the role of Plg-RKT in vivo we generated Plg-RKT-/- mice using a homologous recombination technique.
Methods
We characterized the effect of Plg-RKT deletion on reproduction, viability, health, and spontaneous thrombosis and inflammation.
Results
Plg-RKT-/- mice were viable and fertile. Survival of Plg-RKT-/- mice and Plg-RKT+/+ littermates was not significantly different. However, quite strikingly, all pups of Plg-RKT-/- females died within 2 days of birth, consistent with a lactation defect in Plg-RKT-/- mothers. Additionally, there was a significant effect of Plg-RKT deficiency on growth rates of female, but not male mice. In experimental peritonitis studies, Plg-RKT-/- mice exhibited a marked defect in macrophage recruitment. As a contributing mechanism, the capacity of Plg-RKT-/- macrophages for plasminogen binding was markedly decreased.
Conclusions
These studies demonstrate that Plg-RKT is required for plasminogen binding and macrophage migration in vivo. In addition, Plg-RKT deficiency is not compatible with survival of the species, due to death of all offspring of Plg-RKT-/- females. This new mouse model will be important for future studies aimed at delineating the role of cell surface plasminogen activation in challenge and disease models in vivo.
Keywords: Inflammation, Peritonis, Plasminogen, Receptors, Cell Surface, Thioglycolates
Introduction
The activation of plasminogen to the broad spectrum serine protease, plasmin, is markedly enhanced when plasminogen is co-localized with plasminogen activators on cell surfaces [reviewed in [1]]. Plasmin remains bound to the cell surface where it is relatively protected from inactivation by α2-antiplasmin [reviewed in [1]]. Thus, a local nidus of cell membrane-associated broad spectrum proteolytic activity is achieved, a key feature of pathological processes in which plasmic degradation of extracellular matrices is required for efficient cell migration [reviewed in [2]].
Studies with plasminogen null (Plg-/-) mice have demonstrated a major physiologic role for plasminogen in functions consistent with a role for the interaction of plasminogen with cell surfaces. These include wound healing, tissue remodeling, tumor growth and dissemination, and inflammation [reviewed in [2]]. In particular, these studies have demonstrated a key role for cell surface binding of plasminogen via proteins exposing a C-terminal basic residue in the inflammatory cell recruitment response [3-5].
The plasminogen receptor, Plg-RKT, is a recently discovered integral membrane protein that binds plasminogen via a C-terminal lysine exposed on the cell surface [6,7]. Plg-RKT also binds tissue plasminogen activator (tPA) and promotes tPA-dependent plasminogen activation [6,8]. Furthermore, Plg-RKT is highly colocalized with the urokinase plasminogen activator (uPA) receptor (uPAR) [6,8] and promotes uPA-dependent plasminogen activation [9]. Notably, Plg-RKT is highly conserved with high interspecies homology, high identity, no gaps in the sequences and the presence of a C-terminal lysine for all 36 mammalian orthologs for which sequence information is available [see [7], Table 2 for alignment of 20 representative orthologs].
In order to assess the physiological function of Plg-RKT we disrupted the PLGRKT gene in mice, and characterized the effect of Plg-RKT deletion on reproduction, viability, health, spontaneous thrombosis, and inflammation.
Materials and Methods
Proteins
Human Glu-plasminogen was purified as described [10,11] and labeled with fluorescein isothiocyanate (FITC) using a FITC labeling kit (EMD, EMD Millipore, Darmstadt, Germany). Phycoerythrin labeled annexin V was from R&D Systems, Minneapolis, MN. Antibodies used were: polyclonal rabbit anti-casein (Abbiotec, San Diego, CA), anti-β-actin MAb (LI-COR, Lincoln, NE), and HRP-conjugated secondary antibody (Biosource, San Diego, CA) or appropriate fluoresceinated secondary antibodies (LI-COR, Lincoln, NE).
Mice
Plg-RKT-/- mice were generated as described in Fig.1 and backcrossed ten generations into the C57Bl/6J background.
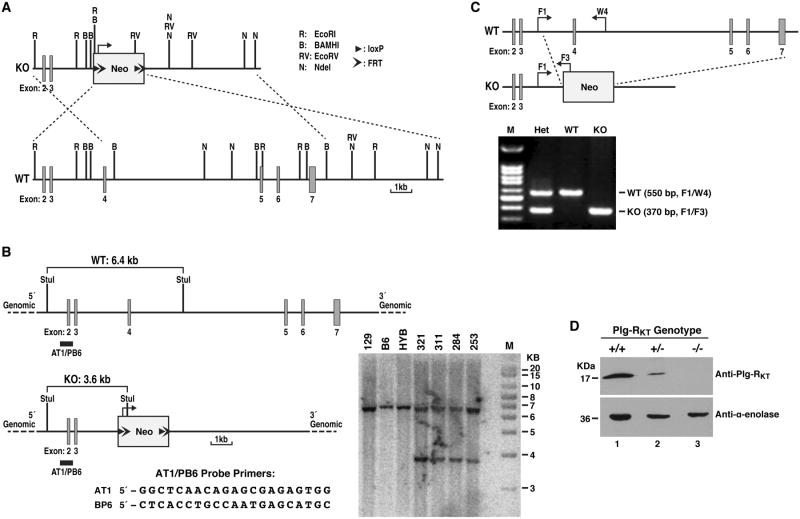
Figure 1. Generation and characterization of Plg-RKT deficient mice.
(A) Construction of a conventional targeting vector for Plg-RKT. An 18.90 kb region was first subcloned from a positively identified 129 BAC clone (RPCI-22: 24B9) using a homologous recombination-based technique. The targeting vector was designed such that the long homology arm extended 5.40 kb 3′ to the 3′ end of a pGK-gb2 loxP/FRT-flanked neomycin cassette. The short homology arm was located on the 5′ side of the neomycin cassette and was 3.08 kb long. An Nde I site was engineered into the neomycin cassette for Southern blot analysis. The targeting vector was confirmed by restriction analysis after each modification step and by sequencing using primers designed to read from the selection cassette into the 3′ end of the short homology arm and the 5′ end of the long homology arm, or from primers that anneal to the vector sequence, and read into the 5′ and 3′ ends of the BAC sub clone. The BAC was subcloned into a 2.4 kb (pSP72, Promega) backbone vector containing an ampicillin selection cassette for retransformation of the construct prior to electroporation. The total size of the targeting construct was 10.18 kb. The pGK-gb2 loxP/FRT-flanked neomycin cassette was inserted into the gene. Exons 4-7 were conventionally targeted and the neomycin cassette replaced 10.42 kb of this gene. The targeting construct was linearized using Not I prior to electroporation into ES cells. Restriction enzyme sites, LoxP and FRT sites are indicated. (B) Southern blotting strategy used to identify ES cells carrying the neomycin cassette. DNA was digested with StuI and hybridized with a probe targeted against the 5′ internal region. The expected sizes are indicated on the schematic. Clones 284, 311, 321 and 253 were confirmed as correctly targeted. DNA from C57Bl/6 (B6), 129/SvEv (129), and C57Bl/6 × 129/SvEv hybrid (HYB) mouse strains were used as wild type controls. Positive clones were injected into C57Bl/6 blastocysts and implanted into pseudopregnant C57Bl/6 females. (C) Polymerase chain reaction-based strategy for identification of Plg-RKT-/- (KO), Plg-RKT+/- (Het) and Plg-RKT+/+ (WT) mice. Mice were genotyped to confirm germline transmission using a polymerase chain reaction- based strategy. A common forward primer (F1, AAGAGGATGAGACCTGTGTCAGAG) in Plg-RKT in the intron between exons 3 and 4 and a reverse primer (W4, GAAGCCGAGCATGCGTAACCATGAAC) in Plg-RKT exon 4 yielded an amplicon of 550 bp for the wild type gene. The forward primer (F1) and the reverse primer (F3, GCATAAGCTTGGATCCGTTCTTCGGA) in the Neo resistance gene provided a 370 bp amplicon only for the targeted gene. Unless otherwise indicated, mice backcrossed 10 generations into C57Bl/6J were used in these studies and maintained in a Helicobacter free room. (D) Western blot analysis was carried out as described [9] on lysates of thioglycollate elicited macrophages from Plg-RKT-/-, Plg-RKT+/- and Plg-RKT+/+ mice using anti-Plg-RKT MAb [8,9]. Anti-α-enolase MAb [30] was used as loading control.
Experimental Peritonitis
Plg-RKT-/- and Plg-RKT+/+ sex matched mice (12 to 14 weeks of age) were injected intraperitoneally with 4% thioglycollate (Sigma-Aldrich, St. Louis, MO). At specific time points, mice were killed and peritoneal leukocytes were collected by peritoneal lavage. All animal experiments were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Quantitative flow cytometry was performed and binding parameters were analyzed as described [6]
Blood Cell Counts and Differentials
Blood cell counts and differentials were determined using a Vet ABC hematology analyzer (SCIL Animal Company, Gurnee, IL).
Hematological Analyses
Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT) were determined in duplicate with an ST4 semi-automated mechanical coagulation instrument (Diagnostica Stago, Parsippany, NJ).
Plasma plasminogen and fibrinogen levels were determined using Mouse Plasminogen Assay Kit (IMPLGKT-TOT) and Mouse Fibrinogen Antigen ELISA kit (MFBGNKT-1212), respectively, from Innovative Research, Inc, Novi, MI.
Immunohistochemistry and Histology
Harvested mouse tissues were fixed in 4% paraformaldehyde, paraffin processed, and embedded. The slides were either stained with hematoxylin & eosin or immunostained for the presence of fibrin(ogen) using goat polyclonal anti-fibrinogen (GAM/Fbg Batch #4951, Nordic Immunological Labs, Eindhoven, The Netherlands) as described [12].
Results and Discussion
Viability, Fertility, Growth and Health of Plg-RKT Deficient Mice
To study the role of Plg-RKT in murine health and reproduction, we generated a Plg-RKT null mouse strain using a homologous recombination-based technique as described in Fig. 1. Heterozygous mice were crossed and viable Plg-RKT+/-, Plg-RKT-/- and Plg-RKT+/+ offspring were born. Plg-RKT gene disruption was confirmed by PCR genotyping with specific primers (Fig. 1C) and Plg-RKT protein was not detected in Plg-RKT-/- cells (Fig. 1D).
Among 2,340 littermates resulting from heterozygous matings, 611 were Plg-RKT+/+ (26%), 1,136 were Plg-RKT+/- (49%) and 593 were Plg-RKT-/- (25%). This distribution was not significantly different (Chi-square test) from the expected Mendelian 1:2:1 ratios. Additionally, the distribution of genotypes among male and female mice was not significantly different from the expected Mendelian ratios. Thus, Plg-RKT+/+, Plg-RKT+/- and Plg-RKT-/- mice of both sexes were equally viable and there was no evidence of fetal loss of Plg-RKT-/- mice.
Male Plg-RKT-/- mice (age 10 weeks, n=5), mated for 12-19 weeks to female wild-type C57Bl/6J female mice (age 6 weeks, n=5) produced 3 to 6 litters each of 6.5±0.5 offspring per litter. These values were not significantly different from the breeding characteristics of Plg-RKT+/+ male littermates (age, 10 weeks, n=6) that produced 3 to 5 litters each of 6.7±0.7 offspring per litter (P=0.769).
Female Plg-RKT-/- mice (age 8 weeks, n=6), mated for 15-19 weeks to male wild-type C57Bl/6J male mice (age 8 weeks, n=6) produced 4 to 5 litters each of 5.1±0.5 offspring per litter. These values were not significantly different from the breeding characteristics of Plg-RKT+/+ female littermates that produced 3-8 litters of 5.2±0.8 offspring per litter (P=0.923). Notably, plasminogen deficiency does not affect ovulation, when follicular wall rupture and ovulation efficiency are analyzed directly [13]. Thus, our results exclude a role for Plg-RKT in regulating ovulation via other proteases.
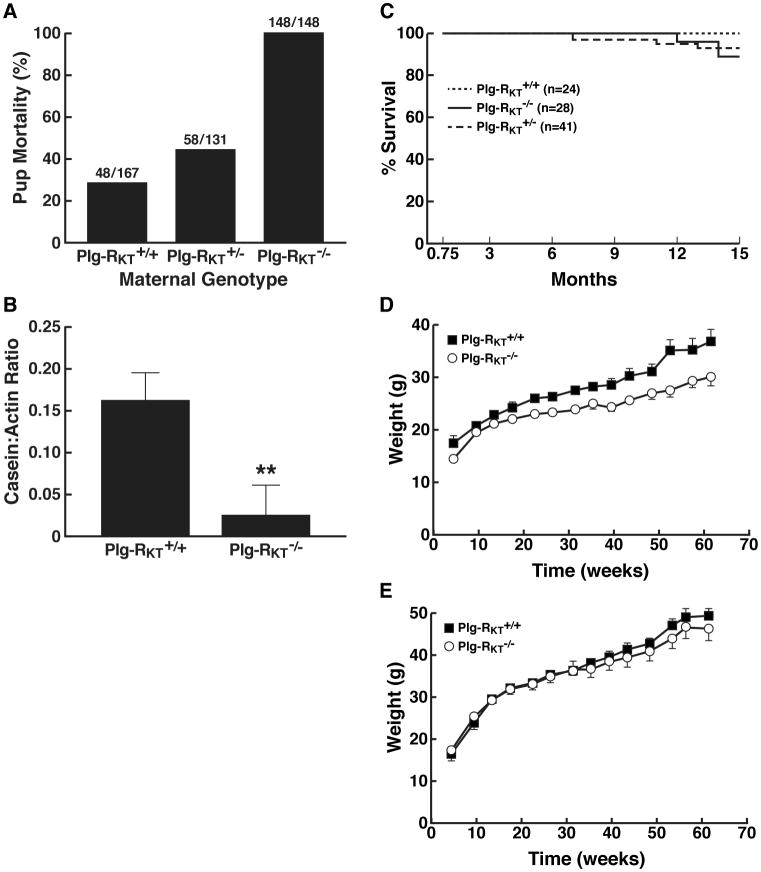
However, quite strikingly, no offspring of Plg-RKT-/- mothers survived to weaning (dying within 48 hours after birth) (Fig. 2A). No milk was observed in the stomachs of offspring of Plg-RKT-/- females. There was a significant maternal gene dosage effect on pup survival, with pups of Plg-RKT+/- female mice, having survival rates intermediate between offspring of Plg-RKT-/- and Plg-RKT+/+ female mice (Fig. 2A). These results are consistent with a requirement for Plg-RKT for lactational competence. A less severe lactation defect is present in Plg-/- mice [14,15]. Milk production (assessed by casein content) was severely and significantly decreased in mammary glands harvested 2 days postpartum from Plg-RKT-/- females (Fig. 2B). Notably, milk production by Plg-/- females is not affected at either 2 or 7 days postpartum [15]. Because milk production by secretory epithelium requires a correctly oriented extracellular matrix (ECM) [16-19], it is possible that Plg-RKT may regulate proteases in addition to plasmin that are involved in ECM remodeling in the mammary gland.
Figure 2. Viability, Growth and Health of Plg-RKT Deficient Mice.
(A) Pup mortality as a function of maternal genotype. Ratios for each group are shown above the respective bars. §2=174.660, df=2, P=1.18 × 10-38. Loss of offspring of wild type females (28.7%) was similar to the loss of offspring in the C57Bl/6J breeding colony at The Scripps Research Institute (152/577=26.3%). (B). To assess milk production, mammary glands (harvested 2 days postpartum) were lysed and western blotted with rabbit polyclonal anti-casein IgG and with anti-actin MAb as a loading control. Casein: actin ratios were quantified by scanning gels in the LI-COR system. Bars show mean ± S.E.M. **P=0.004, n=4. (C) Survival data are shown for a cohort of offspring of heterozygous matings: 24 Plg-RKT+/+ mice, 28 Plg-RKT-/- mice and 41 Plg-RKT+/- mice. Survival curves show mice that survived to be weaned and genotyped at 0.75 months. Survival curves for Plg-RKT-/-, Plg-RKT+/-, and Plg-RKT+/+ mice were not significantly different §2=2.428, df=2, P=0.297 by Log-rank (Mantel-Cox) test and there was no significant trend (P=0.318, Log-rank test for trend). (D,E). Mice were weighed weekly. (D) For females (n=9 Plg-RKT-/- mice and 13 Plg-RKT+/+ mice) repeated measures analysis of variance showed a significant effect for time (P<0.001), for genotype (P=0.008) and a significant time X genotype interaction (P=0.008). (E) For males (n=15 Plg-RKT-/- mice and 11 Plg-RKT+/+ mice) there was a significant effect for time (P<.001), no effect for genotype (P=0.545) or for time X genotype interaction (P=0.444).
Survival curves for Plg-RKT-/-, Plg-RKT+/-, and Plg-RKT+/+ offspring of heterozygous matings (showing plots for mice that survived to be weaned) were not significantly different [P=0.297, Log-rank (Mantel Cox) test] and there was no significant trend (P=0.318, Log-rank test for trend) (Fig. 2C). No consistencies in causes of death were apparent for the Plg-RKT-/- and Plg-RKT+/- in these cohorts. Furthermore, all Plg-RKT-/- mice in our cohort of 28 mice survived for at least 12 months.
Total body weights were determined weekly for 60 weeks (beginning at 4.5 weeks). Plg-RKT deficiency significantly affected the growth rate of female mice (Fig. 2D) but there was no significant effect on growth rates of male mice (Fig. 2E). Effects of Plg-RKT deletion on growth rates of female mice may be due to effects on mammary gland growth and development. Plg-/- mice of both sexes exhibit decreased growth rates compared with wild type littermates [20,21].
Spontaneous dermatitis developed by 4 months and was observed 4-fold more frequently in Plg-RKT-/- mice [16/838 (1.9%)] compared with Plg-RKT+/+ littermates [6/1164 (0.5%)] and 2-fold more frequently in Plg-RKT+/- mice [(22/1884 (1.2%)] compared with Plg-RKT+/+ littermates (X2=8.497, df=2, P=0.014). Extensive non-healing skin ulcerations are also observed in mice doubly deficient for tPA and uPA [22], consistent with a requirement for plasminogen activation for maintenance of skin health.
Macroscopic and Microscopic Organ and Tissue Analyses
Upon dissection, gross examination, and examination of H&E-stained tissues including liver, lung, brain, heart muscle, skeletal muscle, lymph nodes, thymus, spleen, stomach, small intestine, large intestine, colon, cecum, rectum, pancreas, adrenal, kidney, uterus, ovaries, vas deferens, testes, epididymis, (collected from 12 Plg-RKT+/+ and 12 Plg-RKT-/- littermates sacrificed at 8-9 months), no differences were detected between the genotypes. Furthermore, Plg-RKT deficiency did not have a significant effect on either spontaneous fibrin deposition or spontaneous inflammation. In contrast, Plg-/- mice survive to adulthood but exhibit severe spontaneous thrombotic lesions in multiple organs [23,24]. This results in retarded postnatal growth after weaning [20], severe wasting and high mortality [23,24]. Notably, removal of fibrinogen eliminates these effects of plasminogen deficiency on survival, growth and health [25]. Thus, surveillance against spontaneous thrombosis may be receptor independent or, alternatively, may be accomplished by redundant functions of additional plasminogen receptors. Notably, mice null for components of the annexin A2 heterotetramer (a phosphatidyl-serine-linked cell surface plasminogen binding profibrinolytic complex) also do not exhibit extensive fibrin-dependent organ damage and there is no reported effect of A2 heterotetramer deletion on murine health and survival [21,26].
Hematologic Analyses of Plg-RKT-/- Mice
There was no effect of Plg-RKT deletion on general hematological parameters with regard to platelet counts, leukocyte counts, erythrocyte counts, hemoglobin and hematocrit or on specific coagulation and fibrinolytic parameters (Table 1). Notably, higher plasma fibrinogen concentrations are present in both Plg-/- mice [24,27] and mice expressing plasminogen with a plasmin-inactivating site mutation [27], which has been attributed to an acute-phase response in the spontaneous disease resulting from the absence of active plasmin [27].
Table 1. Hematologic parameters of Plg-RKT-/- and Plg-RKT+/+ Mice.
| Plg-RKT -/- | Plg-RKT+/+ | Two Way ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Male | Female | Male | Female | Genotype | Gender | Genotype*Gender | ||||
|
| ||||||||||
| F | P | F | P | F | P | |||||
|
|
||||||||||
| Platelets/μl | 581,987 ± 15,189 (13) | 986,545 ± 73,087 (11) | 617,603 ± 154,339 (15) | 938,938 ± 44,731 (16) | 0 | 9.358 | 9.358 | 0.004 | 0.123 | 0.727 |
| WBC/μl | 2,008 ± 176 (13) | 1,345 ± 159 (11) | 1,573 ± 111 (15) | 1,438 ± 110 (16) | 1.532 | 0.222 | 8.325 | 0.006 | 3.622 | 0.063 |
| Lym/μl | 777 ± 82 (13) | 593 ± 61 (11) | 693 ± 67 (15) | 626 ± 48 (16) | 0.151 | 0.699 | 3.643 | 0.062 | 0.792 | 0.378 |
| Gra/μl | 919 ± 175 (13) | 559 ± 86 (11) | 611 ± 64 (15) | 581 ± 53 (16) | 1.959 | 0.168 | 3.647 | 0.062 | 2.601 | 0.113 |
| Eos/μl | 66 ± 4 (13) | 65 ± 6 (11) | 69 ± 4 (15) | 72 ± 9 (14) | 1.116 | 0.296 | 0.069 | 0.793 | 0.111 | 0.740 |
| Mono/μl | 313 ± 28 (13) | 193 ± 23 (11) | 253 ± 30 (15) | 230 ± 22 (16) | 0.172 | 0.680 | 6.963 | 0.011 | 3.288 | 0.076 |
| Hemoglobin g/dl | 13.9 ± 0.2 (14) | 14.0 ± 0.3 (11) | 13.8 ± 0.1 (15) | 14 ± 0.2 (15) | 0.17 | 0.844 | 0.168 | 0.683 | 0.408 | 0.666 |
| HCT, % | 41 ± 1 (14) | 42 ± 1 (11) | 41 ± 1 (15) | 41 ± 1 (16) | 0.082 | 0.922 | 0.373 | 0.543 | 0.071 | 0.932 |
| APTT (sec) | 31.9 ± 1.1 (7) | 33.5 ± 3.4 (4) | 29.4 ± 1.8 (7) | 35.4 ± 2.2 (5) | 0.03 | 0.863 | 4.251 | 0.053 | 1.38 | 0.255 |
| PT (sec) | 11.3 ± 0.1 (7) | 11.6 ± 0.6 (5) | 11.3 ± 0.1 (7) | 11.6 ± 0.2 (5) | 0.002 | 0.968 | 0.855 | 0.366 | 0.007 | 0.968 |
| Fibrinogen mg/ml | 2.02 ± 0.31 (18) | 1.98 ± 0.22 (13) | 1.43 ± 0.14 (13) | 1.94 ± 0.31 (10) | 1.239 | 0.271 | 0.702 | 0.406 | 0.953 | 0.334 |
| Plasminogen μg/ml | 50.9 ± 5.6 (18) | 81.4 ± 3.6 (13) | 51.9 ± 0.5 (13) | 80.0 ± 12.9 (9) | 0.001 | 0.980 | 9.453 | 0.003 | 0.017 | 0.897 |
Data presented are the mean ±S.E.M. with the number of mice analyzed shown in parentheses. Data were analyzed by two way ANOVA. Significant P values are bolded.
There was no genotype effect of Plg-RKT deletion on plasma plasminogen levels (Table 1). However, there was a gender effect on circulating plasminogen, with males having significantly lower plasminogen concentrations (P=0.003). Notably, female sex is also associated with higher plasminogen levels in humans [28].
There was no effect of Plg-RKT deletion on general blood chemistry values (glucose, blood urea nitrogen, creatinine, electrolytes, and liver function panel (data not shown).
Plg-RKT Null Mice Exhibit Deficient Macrophage Recruitment
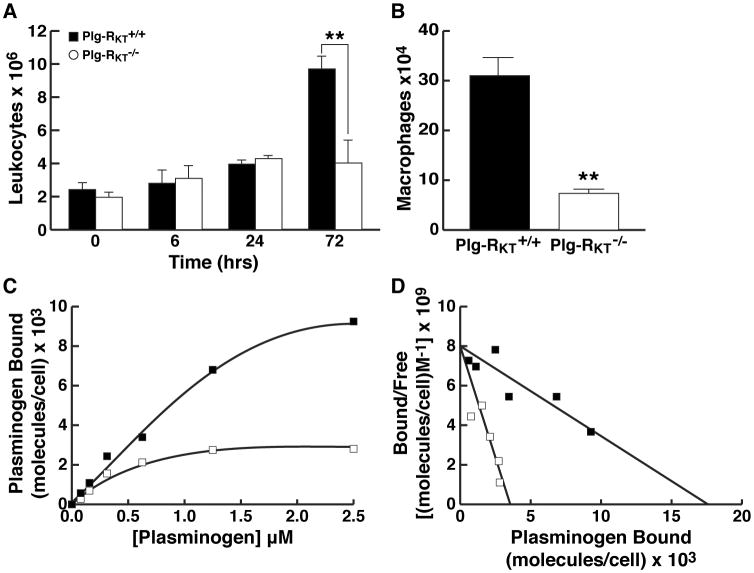
Because Plg-RKT is a plasminogen binding protein with a C-terminal lysine, we compared thioglycollate-elicited leukocyte recruitment in Plg-RKT-/- mice compared to wild type littermate controls. Leukocyte recruitment at 72 hr was substantially decreased in Plg-RKT-/- mice compared with Plg-RKT+/+ littermates (Fig. 3A).
Figure 3. Plg-RKT Null Mice Exhibit Deficient Macrophage Recruitment and Plasminogen Binding.
(A) Plg-RKT-/- and Plg-RKT+/+ sex matched mice (12 to 14 weeks of age) were injected intraperitoneally with 4% thioglycollate. At specific time points, mice were killed and peritoneal leukocytes were collected by peritoneal lavage with PBS (7 ml). Then 5 ml were centrifuged and resuspended in 50 μl PBS and counted. **P<0.01, by unpaired t test, n=5 Plg-RKT-/- mice and 6 Plg-RKT+/+ mice. Protein concentrations in peritoneal exudates were 1.17 ±0.03 and 0.115 ± 0.015 μg/ml for Plg-RKT+/+ mice and Plg-RKT-/- mice, respectively. P=0.0001, consistent with differences in cellular content. (B) Macrophages were isolated by plating leukocytes onto tissue culture dishes and harvesting adherent cells after 72 hr. **P<0.01, by unpaired t test, n= n=5 Plg-RKT-/- mice and 6 Plg-RKT+/+ mice. (C) Specific binding isotherms for the interaction of plasminogen with Plg-RKT+/+ and Plg-RKT-/- macrophages. Plg-RKT-/- and Plg-RKT+/+ mice (3 of each genotype) were injected separately with thioglycollate. Peritoneal leukocytes were harvested 72 hr later and cells from each genotype were pooled. Quantitative flow cytometric equilibrium binding of FITC-Glu-plasminogen to peritoneal leukocytes was analyzed using beads impregnated with FITC as described [6]. Macrophages were identified by their distinct forward scatter/side scatter properties and annexin V was used to gate for cells that were not apoptotic (i.e., did not express phosphatidyl serine, which binds annexin V, on their surfaces). Specific binding of FITC-plasminogen to the cells was calculated by subtracting binding in the presence of 0.2 M EACA (non-specific binding) from total binding. Quantitative flow cytometric equilibrium binding of FITC-plasminogen to cells was analyzed as described. [6] (D) Scatchard plots of data shown in panel C were determined using the single site binding equation [LR]=([L]Bmax)/([L]+Kd). Binding parameters were determined using the program, NLREG, version 6.5. Data are representative of 3 experiments. ■ = Plg-RKT+/+ and □ = Plg-RKT-/- macrophages.
Because monocyte recruitment peaks at 72 hr in this model [3], we further purified macrophages from the recruited peritoneal leukocyte population by adherence. Macrophage recruitment in Plg-RKT-/- null mice was markedly decreased (by 76%) compared with wild type littermate controls (Fig. 3B). The reduction in levels of recruited macrophages was not due to decreased levels of resident peritoneal cells in Plg-RKT-/- mice (Fig. 3A, see 0 time point). The time course of the effect on leukocyte recruitment was similar to that reported for Plg-/- mice [3].
Plg-RKT Null Macrophages Exhibit a Marked Deficiency in Plasminogen Binding Capacity
Because macrophage recruitment in response to thioglycollate requires plasminogen binding [4], we assessed plasminogen binding to Plg-RKT-/- macrophages. Plasminogen binding to thioglycollate elicited macrophages was evaluated using quantitative fluorescence activated cell sorting as described [6]. Macrophages were identified by their distinct forward scatter/side scatter properties and annexin V was used to gate for cells that were not apoptotic [i.e., did not express phosphatidyl serine, which binds annexin V, on their surfaces]. Specific binding of FITC-plasminogen to the cells was calculated by subtracting binding in the presence of 0.2 M EACA (non-specific binding) from total binding.
Specific binding isotherms for the interaction of FITC-plasminogen with viable, non-apoptotic, Plg-RKT+/+ and Plg-RKT-/- macrophages were saturable (Fig. 3C). Apparent Kd values of 2.2±0.5 μM and 0.4±0.1 μM and Bmax values of=17,547±2,065 molecules/cell and 3,452±291 molecules/cell were calculated for Plg-RKT+/+ (R2=0.993) and Plg-RKT-/- (R2=0.967) macrophages, respectively (Fig. 3D). Thus, the capacity of Plg-RKT-/- macrophages for plasminogen was only 20% of that of Plg-RKT+/+ macrophages. This is consistent with the established requirement for binding of plasminogen to the cell surface via C-terminal basic residues as a contributing mechanism in inflammatory cell recruitment in response to sterile peritonitis induced by thioglycollate [3-5], and demonstrates a major role for Plg-RKT in this process.
In summary, Plg-RKT-/- mice mimic some, but not all, spontaneous phenotypes exhibited by Plg-/- mice [29], consistent with a role for this plasminogen receptor in specific physiological and pathological functions. Notably, although Plg-RKT deficiency was compatible with survival of individual mice, Plg-RKT deficiency is not compatible with survival of the species due to death of all offspring of Plg-RKT-/- females. This new mouse model will be important for future studies aimed at delineating the role of cell surface plasminogen activation in challenge and disease models in vivo.
Essentials.
Plg-RKT is a novel integral membrane plasminogen receptor
The functions of of Plg-RKT in vivo are not known
Plg-RKT is a key player in macrophage recruitment in the inflammatory response in vivo
Plg-RKT deficiency is not compatible with survival of the species
Acknowledgments
This work was supported by the National Institutes of Health (grants HL 081046 to L.A.M. and CA166473 to B.M.M. and L.A.M. and 2P30CA023100, NIH/NCI to N.M.V.) and by Merit Review Award #5I01BX002026 from the U.S. Department of Veterans Affairs (to R.J.P.).
Footnotes
Authors state no conflicts of interests.
Addendum: L. A. Miles and R. J. Parmer designed research studies, analyzed data and wrote the manuscript; N. Baik, S. Lighvani, S. Khaldoyanidid and H. Bai conducted experiments, acquired data and analyzed data; N. M. Varki and B. M. Mueller analyzed data.
References
- 1.Miles LA, Parmer RJ. Plasminogen receptors: the first quarter century. Semin Thromb Hemost. 2013 Jun;39:329–37. doi: 10.1055/s-0033-1334483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miles LA, Lighvani S, Baik N, Parmer CM, Khaldoyanidi S, Mueller BM, Parmer RJ. New insights into the role of Plg-RKT in macrophage recruitment. Int Rev Cell Mol Biol. 2014;309:259–302. doi: 10.1016/B978-0-12-800255-1.00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ploplis VA, French EL, Carmeliet P, Collen D, Plow EF. Plasminogen deficiency differentially affects recruitment of inflammatory cell populations in mice. Blood. 1998;91:2005–9. [PubMed] [Google Scholar]
- 4.Swaisgood CM, Schmitt D, Eaton D, Plow EF. In vivo regulation of plasminogen function by plasma carboxypeptidase B. J Clin Invest. 2002 Nov;110:1275–82. doi: 10.1172/JCI15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong Y, Hart E, Shchurin A, Hoover-Plow J. Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest. 2008 Sep;118:3012–24. doi: 10.1172/JCI32750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andronicos NM, Chen EI, Baik N, Bai H, Parmer CM, Kiosses WB, Kamps MP, Yates JR, III, Parmer RJ, Miles LA. Proteomics-based discovery of a novel, structurally unique, and developmentally regulated plasminogen receptor, Plg-RKT, a major regulator of cell surface plasminogen activation. Blood. 2010 Feb 18;115:1319–30. doi: 10.1182/blood-2008-11-188938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miles LA, Andronicos NM, Chen EI, Baik N, Bai H, Parmer CM, Lighvani S, Nangia S, Kiosses WB, Kamps MP, Yates JR, III, Parmer RJ. Identification of the novel plasminogen receptor, Plg-RKT. In: Man TK, Flores RJ, editors. Proteomics/Book 1: Human Diseases and Protein Functions. Intech; 2012. pp. 219–38. [Google Scholar]
- 8.Bai H, Baik N, Kiosses WB, Krajewski S, Miles LA, Parmer RJ. The novel plasminogen receptor, plasminogen receptor(KT) (Plg-R(KT)), regulates catecholamine release. J Biol Chem. 2011 Sep 23;286:33125–33. doi: 10.1074/jbc.M111.218693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lighvani S, Baik N, Diggs JE, Khaldoyanidi S, Parmer RJ, Miles LA. Regulation of macrophage migration by a novel plasminogen receptor Plg-R KT. Blood. 2011 Nov 17;118:5622–30. doi: 10.1182/blood-2011-03-344242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deutsch DG, Mertz ET. Plasminogen: Purification from human plasma by affinity chromatography. Science. 1970;170:1995–6. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- 11.Parmer RJ, Mahata M, Gong Y, Mahata S, Jiang Q, O'Connor DT, Xi XP, Miles LA. Processing of chromogranin A by plasmin provides a novel mechanism for regulating catecholamine secretion. J Clin Invest. 2000;106:907–15. doi: 10.1172/JCI7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta KK, Donahue DL, Sandoval-Cooper MJ, Castellino FJ, Ploplis VA. Abrogation of plasminogen activator inhibitor-1-vitronectin interaction ameliorates acute kidney injury in murine endotoxemia. PLoS One. 2015;10:e0120728. doi: 10.1371/journal.pone.0120728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ny A, Leonardsson G, Hagglund AC, Hagglof P, Ploplis VA, Carmeliet P, Ny T. Ovulation in plasminogen-deficient mice. Endocrinology. 1999 Nov;140:5030–5. doi: 10.1210/endo.140.11.7113. [DOI] [PubMed] [Google Scholar]
- 14.Lund LR, Bjorn SF, Sternlicht MD, Nielsen BS, Solberg H, Usher PA, Osterby R, Christensen IJ, Stephens RW, Bugge TH, Dano K, Werb Z. Lactational competence and involution of the mouse mammary gland require plasminogen. Development. 2000 Oct;127:4481–92. doi: 10.1242/dev.127.20.4481. [DOI] [PubMed] [Google Scholar]
- 15.Green KA, Nielsen BS, Castellino FJ, Romer J, Lund LR. Lack of plasminogen leads to milk stasis and premature mammary gland involution during lactation. Dev Biol. 2006 Nov 1;299:164–75. doi: 10.1016/j.ydbio.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991 Dec;115:1383–95. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, Bissell MJ. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995 May;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–3. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pullan S, Wilson J, Metcalfe A, Edwards GM, Goberdhan N, Tilly J, Hickman JA, Dive C, Streuli CH. Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J Cell Sci. 1996 Mar;109(Pt 3):631–42. doi: 10.1242/jcs.109.3.631. [DOI] [PubMed] [Google Scholar]
- 20.Hoover-Plow J, Wang N, Ploplis VA. Growth and behavioral development in plasminogen gene-targeted mice. Growth, Development and Aging. 1999;63:13–32. [PubMed] [Google Scholar]
- 21.Ling Q, Jacovina AT, Deora A, Febbraio M, Simantov R, Silverstein RL, Hempstead B, Mark WH, Hajjar KA. Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J Clin Invest. 2004 Jan;113:38–48. doi: 10.1172/JCI200419684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmeliet P, Schoonjans L, Kieckens L, Ream B, Degen J, Bronson R, De Vos R, Van den Oord JJ, Collen D, Mulligan RC. Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368:419–24. doi: 10.1038/368419a0. [DOI] [PubMed] [Google Scholar]
- 23.Bugge TH, Flick MJ, Daugherty CC, Degen JL. Plasminogen deficiency causes severe thrombosis but is compatible with development and reproducton. Genes and Development. 1995;9:794–807. doi: 10.1101/gad.9.7.794. [DOI] [PubMed] [Google Scholar]
- 24.Ploplis VA, Carmeliet P, Vazirzadeh S, Van VI, Moons L, Plow EF, Collen D. Effects of disruption of the plasminogen gene on thrombosis, growth, and health in mice. Circulation. 1995 Nov 1;92:2585–93. doi: 10.1161/01.cir.92.9.2585. [DOI] [PubMed] [Google Scholar]
- 25.Bugge TH, Kombrinck KW, Flick MJ, Daugherty CC, Danton MJS, Degen JL. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–19. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- 26.Surette AP, Madureira PA, Phipps KD, Miller VA, Svenningsson P, Waisman DM. Regulation of fibrinolysis by S100A10 in vivo. Blood. 2011 Sep 15;118:3172–81. doi: 10.1182/blood-2011-05-353482. [DOI] [PubMed] [Google Scholar]
- 27.Iwaki T, Malinverno C, Smith D, Xu Z, Liang Z, Ploplis VA, Castellino FJ. The generation and characterization of mice expressing a plasmin-inactivating active site mutation. J Thromb Haemost. 2010 Oct;8:2341–4. doi: 10.1111/j.1538-7836.2010.03995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Q, Ozel AB, Ramdas S, McGee B, Khoriaty R, Siemieniak D, Li HD, Guan Y, Brody LC, Mills JL, Molloy AM, Ginsburg D, Li JZ, Desch KC. Genetic variants in PLG, LPA, SIGLEC 14 as well as smoking contribute to plasma plasminogen levels. Blood. 2014 Nov 13;124:3155–64. doi: 10.1182/blood-2014-03-560086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuo O, Lijnen HR, Ueshima S, Kojima S, Smyth SS. A guide to murine fibrinolytic factor structure, function, assays, and genetic alterations. J Thromb Haemost. 2007 Apr;5:680–9. doi: 10.1111/j.1538-7836.2007.02409.x. [DOI] [PubMed] [Google Scholar]
- 30.Redlitz A, Fowler BJ, Plow EF, Miles LA. The role of an enolase-related molecule in plasminogen binding to cells. Eur J Biochem. 1995;227:407–15. doi: 10.1111/j.1432-1033.1995.tb20403.x. [DOI] [PubMed] [Google Scholar]