Abstract
Objective
The aim of this study was to identify risk factors for early-onset GBS (EOGBS) disease in neonates of mothers with negative antenatal screening.
Study Design
We performed a retrospective cohort study of neonates born to mothers with negative antenatal GBS screening between 2002–2012. Our primary outcome was EOGBS infection. We used multivariable logistic regression to assess factors associated with EOGBS.
Results
EOGBS was confirmed in 492 of the 179,818 neonates that met the study inclusion criteria. Risk factors for EOGBS included black race (reference: white, odds ratio [OR] =1.81 [95% confidence interval; 1.43, 2.31]), maternal age <18 years (reference: >35 years, OR=2.63 [1.54, 4.51]), and maternal age 18–35 years (reference: >35 years, OR=1.94 [1.30, 2.88]).
Conclusion
Maternal age <18 years and black race were the strongest predictors of EOGBS. Further research investigating contributors to the discordance between screening results and neonatal outcomes in these populations is needed.
Keywords: GBS, Universal Screening, Health Disparities, infant, early-onset sepsis
INTRODUCTION
Despite widespread implementation of strategies to prevent Group B Streptococcus (GBS) vertical transmission from mothers to neonates,(1, 2) GBS infection persists as the leading cause of infectious morbidity and mortality for neonates in the United States.(2–4) Term neonates with early-onset GBS (EOGBS) have a case-fatality of 4–6%, and in premature neonates, mortality may be as high as 30%.(3, 5, 6) Given the potentially severe outcomes associated with infection, neonatal clinicians seek early identification and treatment of neonates with EOGBS to prevent disease progression and minimize disease burden.
Changing risk factors and demographics in the era of intrapartum antibiotic prophylaxis (IAP) for GBS challenge current strategies for prompt recognition and secondary prevention of GBS disease.(7, 8) For example, while maternal GBS colonization was historically the strongest risk factor for disease development,(9, 10) a recent study documented that up to 80% of cases of EOGBS in term neonates occur in neonates born to mothers with negative antenatal GBS screening (largely due to the success of universal screening and IAP).(4)
Neonates born to mothers with negative GBS antenatal testing are least likely to benefit from the Centers for Disease Control and Prevention (CDC) guidelines for living with intrapartum antibiotic prophylaxis (IAP), and thus may be the group for which improved risk stratification and early recognition of disease may be most important. The purpose of this study was to identify risk factors for EOGBS in neonates whose mothers had negative antenatal GBS screening in the era of universal screening and IAP.
SUBJECTS AND METHODS
Patient Selection
We identified a cohort of neonates born to mothers with negative antenatal GBS screening and admitted on the first day of life to 327 neonatal intensive care units (NICUs) managed by the Pediatrix Medical Group, and discharged from 2002–2012. We limited the study to these years to reflect the revised CDC guidelines published in 2002 that recommended universal screening and IAP. Data was collected prospectively from daily progress notes generated by clinicians, and then analyzed retrospectively. To prevent missing a positive culture from an outside facility not recorded in the database, we excluded neonates born at outside hospitals and transferred into one of the Pediatrix Medical Group’s NICUs.
Definitions
Mothers were defined as having negative antenatal GBS screening if a GBS culture was obtained with a negative result. Mothers for whom GBS cultures were not obtained or had unknown or positive GBS cultures were excluded (Figure 1). EOGBS was defined as isolation of GBS from blood, urine, or CSF cultures obtained in the first 3 days of life. Samples were processed in local clinical microbiology laboratories according to their local hospital standards. The first day of life was defined as day of life (DOL) 0. Prolonged rupture of membranes (PROM) was defined as rupture of membranes >18 hours, and the use of intrapartum antibiotics was defined as administration of any antibiotic to a mother at any time prior to delivery during her birth admission.
Figure 1.
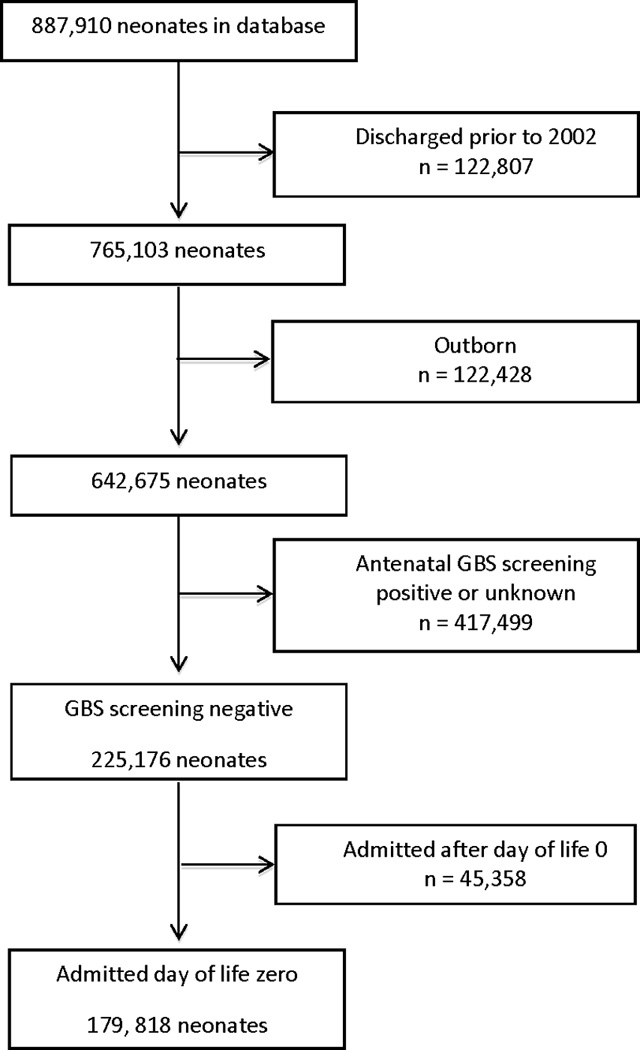
Study Population
Statistical Analysis
Univariable comparisons were performed with Fisher’s exact tests to evaluate the association of neonatal and maternal risk factors (including gestational age, sex, maternal race, PROM, delivery type, maternal antibiotic exposure, and maternal age) and EOGBS. We then used multivariable logistic regression and included all variables in the model. Using a conservative estimate of GBS incidence of 0.40 cases per 1,000 live births2, a sample size of at least 175,000 infants would allow for 10 events per variable.(11) We reported odds ratios with 95% confidence intervals, and two-tailed P-values <0.05 were considered statistically significant. We performed a sensitivity analysis in which we repeated our multivariable logistic regression analysis including neonates of mothers with either GBS negative or GBS unknown antenatal screening. We performed this sensitivity analysis with neonates of GBS unknown mothers because they represent a group of women that may not benefit from universal screening because they deliver prior to routine GBS screening at 35 weeks. Statistical analyses were performed using STATA 13.1 (College Station, TX). The Duke University Institutional Review Board provided permission to conduct this investigation.
RESULTS
A total of 179,818 neonates met the inclusion criteria (figure 1). Of these, 144,284 (80%) had a blood, urine, or CSF culture obtained on DOL 0, 1, or 2. EOGBS was confirmed in 492 neonates (incidence: 2.7 per 1000). Blood cultures were positive in 490 neonates, and 3 neonates had a positive CSF culture (1 neonate had positive cultures from both blood and CSF). No neonates had a positive urine culture. Positive cultures were collected on DOL 0 for 423 neonates, DOL 1 for 68 neonates, and DOL 2 for 1 neonate. Of the 492 neonates with EOGBS, 457 (93%) neonates were discharged to home, 29 (6%) were transferred to another facility, 2 (0.4%) were missing discharge data, and 4 (0.8%) did not survive. All 4 neonates who died were premature (gestational ages of 28 weeks (3 neonates) and 32 weeks (1 neonate)). Intrapartum antibiotics were given to 46,220 mothers, of which 27,543 (59.6%) delivered a neonate at <36 weeks gestational age, 18,497 (40.0%) at 36–41 weeks, 180 (0.4%) at ≥ 42 weeks gestational age. The median length of stay of the neonates with EOGBS that were discharged to home was 11 days (range: 6–153 days).
The overall median gestational age was 37 weeks (range: 24–41) with median birth weight of 2800 g (range: 570–4586). In univariable analysis, neonates who developed EOGBS were more likely to be term with gestational age between 37–41 weeks (p <0.001), more likely to be delivered vaginally compared to cesarean section (p <0.001), more likely to have black maternal race (p <0.001), and less likely to have a mother that received intrapartum antibiotics (p <0.001) (Table 1). On multivariable analysis, statistically significant risk factors for GBS disease included black compared to white maternal race, maternal age <18 years compared to >35 years, maternal age 18–35 years compared to >35 years, lack of maternal intrapartum antibiotics, vaginal delivery compared to cesarean delivery, and gestational age of ≥42 weeks compared to ≤36 weeks (Table 2).
Table 1.
Characteristics of infants with and without early-onset Group B Streptococcus infection
| EOGBS Positive n= 492, (%) |
EOGBS Negative n=179,326, (%) |
||
|---|---|---|---|
| Birth weight, g | |||
| <750 | 4 (0.8) | 2152 (1) | |
| 750–1499 | 9 (2) | 11,926 (7) | |
| 1500–2499 | 23 (5) | 46,408 (26) | |
| 2500–3499 | 262 (53) | 77,396 (43) | |
| ≥3500 | 194 (39) | 41,338 (23) | |
| Gestational age, weeks | |||
| ≤ 26 | 4 (0.8) | 3442 (2) | |
| 27–29 | 5 (1) | 5591 (3) | |
| 30–36 | 39 (8) | 62,579 (35) | |
| 37–41 | 441 (90) | 106,921 (60) | |
| ≥42 | 2 (0.4) | 740 (0.4) | |
| Male | 272 (55) | 101,458 (57) | |
| Cesarean section | 166 (34) | 90,868 (52) | |
| PROM | 46 (10) | 19,724 (11) | |
| Intrapartum antibiotics | 62 (13) | 46,147 (26) | |
| Race/ethnicity | |||
| White | 220 (46) | 92,381 (54) | |
| Black | 103 (22) | 26,381 (15) | |
| Hispanic | 133 (28) | 41,733 (24) | |
| Other | 23 (5) | 10,708 (6) | |
| Maternal age, years | |||
| < 18 | 30 (6) | 6548 (4) | |
| 18–35 | 429 (88) | 150,294 (84) | |
| > 35 | 29 (6) | 21,803 (12) | |
EOGBS: Early-onset Group B Streptococcus; PROM: Prolonged rupture of membranes
Table 2.
Multivariable regression of predictors for early-onset Group B Streptococcus disease
| OR (95% CI) | |
|---|---|
| Gestational Age, weeks | |
| ≤ 36 | 0.24* (0.06, 0.98) |
| 37–41 | 1.42 (0.35, 5.69) |
| ≥42 | REF |
| Sex | |
| Male | 0.94 (0.78, 1.13) |
| Delivery Type | |
| C-section | 0.56** (0.46, 0.68) |
| Labor Details | |
| PROM | 1.38 (0.99, 1.91) |
| Intrapartum antibiotics | 0.54** (0.40, 0.71) |
| Race or ethnicity | |
| White | REF |
| Black | 1.81** (1.43, 2.31) |
| Hispanic | 1.24 (0.99, 1.55) |
| Maternal Age, years | |
| <18 | 2.63** (1.54, 4.51) |
| 18–35 | 1.94** (1.30, 2.88) |
| >35 | REF |
p-value<0.05,
p-value <0.001
OR: Odds Ratio; CI: Confidence interval; PROM: Prolonged rupture of membranes
In our sensitivity analysis including neonates born to mothers with GBS unknown status in addition to GBS negative status, there were 408,827 total neonates and 835 cases of EOGBS. In multivariable regression we found black race compared to white race and maternal age < 18 years compared to > 35 years were still significant predictors of EOGBS (OR 1.53 [95%CI 1.30, 1.84] and OR 1.91 [1.29, 2.83], respectively). Additionally, we found PROM to be a predictor (OR 1.65 [1.32, 2.08]) and male sex to be protective (OR 0.80 [0.69,0.92]) of EOGBS. In the sensitivity analysis, gestational age was not a predictor of EOGBS.
DISCUSSION
We conducted a large retrospective cohort study to determine risk factors for EOGBS in the era of universal IAP for GBS. We included only neonates of mothers with negative antenatal GBS screens to investigate risk factors for EOGBS in this population, for which limited clinical guidance exists. Risk stratification in this population may be the most critical as these neonates are least likely to benefit from universal screening and IAP.(12)
In this study, maternal demographics, including maternal age and race/ethnicity, were the strongest predictors of EOGBS disease. These factors remained significant predictors of EOGBS in our sensitivity analysis that included neonates of both GBS negative and GBS unknown mothers. Consistent with prior studies, maternal age <18 years and black maternal race were significant risk factors for EOGBS.(2, 10, 13–15) The incidence of EOGBS in mothers <18 years was more than three times that in mothers >35 years (4.6 versus 1.3 per 1000 neonates, respectively). Additionally, neonates born to black or Hispanic mothers had a higher incidence of disease compared with white neonates (3.9 and 3.2 versus 2.4 per 1000, respectively). Maternal demographic factors were stronger predictors of disease than delivery characteristics including PROM.
We did not investigate the cause of differences in risk of EOGBS observed by different demographic groups. However, the incidence of GBS disease has not decreased for black neonates to the same extent as white neonates since the introduction of universal screening.(2, 3) While the overall incidence of GBS decreased from 0.52 per 1000 during 2000–2003 to 0.31 per 1000 during 2003–2006, the incidence in black neonates increased during these same time periods from 0.53 to 0.86 per 1000.(1, 2, 6) Provider non-compliance with current guidelines has been hypothesized to contribute to differences in the incidence of EOGBS according to race and maternal age.(16) However, in large observational studies, screening rates have been high among women of all races and have not been shown to be a significant contributor toward racial disparities in neonatal GBS.(1) In our study, differential screening cannot be attributed as a cause of unequal rates of EOGBS by race or age as all women included were screened, and tested negative. One possible explanation of our study findings is that young and non-white mothers had higher rates of GBS colonization and acquisition late in pregnancy, after GBS screening, which increased their neonate’s risk of exposure and invasive disease.
GBS vaginal or rectal colonization during pregnancy can be transient, intermittent, or persistent, and may contribute to false negative screening and EOGBS disease in neonates of mothers with negative screening.(17, 18) Prospective studies of pregnant women demonstrate that approximately 9% of women who test negative at their antenatal screening between 35–37 weeks will have a positive test result if re-cultured shortly after delivery.(19) The change in GBS status late in pregnancy may be due to acquisition of GBS late in pregnancy or inadequate sampling and false negative results during the antenatal screening. Differential rates of GBS acquisition and clearance by race, ethnicity, and age may contribute to disparities in neonatal EOGBS.
Epidemiologic studies of pregnant and non-pregnant women have suggested higher rates of GBS acquisition (and colonization) in black women compared to white women.(20–22) Indeed, a longitudinal study of non-pregnant women found that African-American women had a 50% increased risk of acquiring vaginal GBS colonization within 4–12 months of enrollment compared with Caucasian women(20). In the era of universal screening and IAP, increased rates of GBS colonization in black compared to white women should not translate into higher rates of EOGBS in black neonates, given that colonized women should test positive on antenatal screening and receive IAP. However, higher rates of GBS acquisition in black compared to white women may contribute to the racial disparities in EOGBS given that black women who have negative antenatal testing may be more likely to become colonized prior to delivery, and will not receive IAP. Further studies are needed to delineate if the higher representation of falsely-screened young and black women in our study are due to different rates of acquisition versus inadequate sampling and disparities in obstetrical care. Intrapartum polymerase chain reaction for GBS has been shown to be highly sensitive and specific, (23–26) and could increase sensitivity for detecting GBS that is acquired after typical prenatal screening.
GBS persistence, acquisition, or loss, and thus risk of change in colonization from screening to delivery may also be correlated to the pathogenic serotype with which a woman is colonized.(27, 28) For example, GBS serotype III has a longer median duration of colonization (around 6 weeks), is more likely to persist throughout a pregnancy, and is the most common invasive serotype in neonates less than 7 days of age.(28, 29) Neonatal risk for invasive disease is also likely dependent on the maternal and neonatal immune responses to the specific serotype with which the mother is colonized.(30)
Despite a high rate of neonatal exposure to maternal GBS colonization (approximately 25–30%), the incidence of EOGBS is relatively low (approximately 1% of those exposed) and may depend on maternal transfer of serotype-specific antibodies and neonatal host-organism interactions.(2) Supporting this theory, several studies have demonstrated an association between the risk of developing invasive EOGBS disease in the neonate and maternal anti-capsular GBS antibody levels.(31–33) Demographic differences in serotype distribution, antibody prevalence, and immunologic factors may all contribute to differences in EOGBS by race, ethnicity, and age.(27) Although large cross-sectional studies show no difference in serotype distribution by race,(34, 35) maternal age has been associated with lower levels of anti-GBS antibodies.(36) In theory, women are exposed to GBS and develop antibodies against different serotypes over time. Because young mothers may have less lifetime exposure to build serotype-specific antibodies, the higher incidence of EOGBS in neonates of young mothers may be due to lower levels of protective antibodies.(37, 38)
Our study strengths include the large sample size and diverse population with data obtained after the revised 2002 CDC guidelines recommending universal screening and IAP. The dataset did not include information on neonatal and maternal clinical presentation such as vital signs or physical exam. This hindered the ability to evaluate risk factors for EOGBS based on labor and delivery hospital course, such as known risk factors of maternal fever or chorioamnionitis, as well as neonatal presentation. We also could not determine the timing or type of intrapartum antibiotic administration, and clinical details regarding indication for antibiotics in mothers of term infants with negative antenatal GBS testing were not available in the dataset. Additionally, information on timing of screening was not available in the dataset and thus we could not control for timing of screening. Given the transient nature of GBS colonization, if young or black women were screened earlier in their pregnancy this might impact the negative predictive value of the screening. Lastly, we were unable to control for another recognized risk factor for EOGBS, history of GBS in a sibling, as this was not available in the database. The incorporation of these results into clinical practice should be interpreted with caution, as this sample is not reflective of all births during this time period and only included neonates admitted to a neonatal intensive care unit.
Future investigations are needed to further identify contributors to the excess burden of EOGBS disease among young, non-white mothers, and to determine the cause of increased false negative screening in these populations. Prospective studies are needed to determine if the increased discordance between screening results and neonatal disease is due to inadequate sampling and disparities in obstetric care versus increased rates of acquisition in these groups of women. Although improving access to screening may aid in narrowing this gap, our study suggests that disease incidence is higher even among young black women who undergo screening and have negative results. Prospective studies are also needed that investigate potential etiologies contributing to higher false-negative rates or changes in GBS colonization status in these populations as well as GBS serotype and antibody distribution in a diverse group of pregnant women. Lastly, intrapartum polymerase chain reaction to detect maternal GBS colonization should be further evaluated as a screening tool that may better identify mothers who should receive IAP.
Among mothers with negative antenatal GBS screening in our sample, demographic characteristics such as age <18 years and black race are the strongest predictors of their neonates developing EOGBS. Further research investigating contributors to false negative tests or changes in colonization status in these populations is needed to guide interventions to reduce health disparities in patients with EOGBS.
Acknowledgments
Sources of Support
This work was funded under the Best Pharmaceuticals for Children Act under the guidance of the National Institute of Child Health and Human Development (NICHD) contract HHSN275201000003I for the Pediatric Trials Network, as well as NICHD grant 1R25HD076475, and National Institutes of Health grant 5R01HD057956-05.
Conflict of Interest
D.K.B. Jr. receives support from the National Institutes of Health (NIH) (award 2K24HD058735-06, National Center for Advancing Translational Sciences award UL1TR001117, National Institute of Child Health and Human Development contract HHSN275201000003I, and National Institute of Allergy and Infectious Diseases contract HHSN272201500006I); he also receives research support from Cempra Pharmaceuticals (subaward to HHSO100201300009C) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
P.B.S. receives salary support for research from the National Institute of Child Health and Human Development (HHSN275201000003I and 1R21HD080606-01A1) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp).
R.G.G. receives salary support for research from the National Institutes of Health training grants (5T32HD043728-10 and 5T32HD043029-13).
REFERENCES
- 1.Van Dyke MK, Phares CR, Lynfield R, Thomas AR, Arnold KE, Craig AS, et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med. 2009 Jun 18;360(25):2626–2636. doi: 10.1056/NEJMoa0806820. [DOI] [PubMed] [Google Scholar]
- 2.Trends in perinatal group B streptococcal disease - United States, 2000–2006. MMWR Morb Mortal Wkly Rep. 2009 Feb 13;58(5):109–112. [PubMed] [Google Scholar]
- 3.Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA. 2008 May 7;299(17):2056–2065. doi: 10.1001/jama.299.17.2056. [DOI] [PubMed] [Google Scholar]
- 4.Stoll BJ, Hansen NI, Sanchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011 May;127(5):817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heath PT, Balfour G, Weisner AM, Efstratiou A, Lamagni TL, Tighe H, et al. Group B streptococcal disease in UK and Irish infants younger than 90 days. Lancet. 2004 Jan 24;363(9405):292–294. doi: 10.1016/S0140-6736(03)15389-5. [DOI] [PubMed] [Google Scholar]
- 6.Koenig JM, Keenan WJ. Group B streptococcus and early-onset sepsis in the era of maternal prophylaxis. Pediatr Clin North Am. 2009 Jun;56(3):689–708. doi: 10.1016/j.pcl.2009.04.003. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puopolo KM, Madoff LC, Eichenwald EC. Early-onset group B streptococcal disease in the era of maternal screening. Pediatrics. 2005 May;115(5):1240–1246. doi: 10.1542/peds.2004-2275. [DOI] [PubMed] [Google Scholar]
- 8.Pinto NM, Soskolne EI, Pearlman MD, Faix RG. Neonatal early-onset group B streptococcal disease in the era of intrapartum chemoprophylaxis: residual problems. Journal of perinatology : official journal of the California Perinatal Association. 2003 Jun;23(4):265–271. doi: 10.1038/sj.jp.7210899. [DOI] [PubMed] [Google Scholar]
- 9.Boyer KM, Gotoff SP. Strategies for chemoprophylaxis of GBS early-onset infections. Antibiot Chemother. 1985;35:267–280. doi: 10.1159/000410380. [DOI] [PubMed] [Google Scholar]
- 10.Benitz WE, Gould JB, Druzin ML. Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics. 1999 Jun;103(6):e77. doi: 10.1542/peds.103.6.e77. [DOI] [PubMed] [Google Scholar]
- 11.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996 Dec;49(12):1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 12.Verani JR, Schrag SJ. Group B streptococcal disease in infants: progress in prevention and continued challenges. Clinics in perinatology. 2010 Jun;37(2):375–392. doi: 10.1016/j.clp.2010.02.002. [Review] [DOI] [PubMed] [Google Scholar]
- 13.Schrag SJ, Zell ER, Lynfield R, Roome A, Arnold KE, Craig AS, et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N Engl J Med. 2002 Jul 25;347(4):233–239. doi: 10.1056/NEJMoa020205. [DOI] [PubMed] [Google Scholar]
- 14.Puopolo KM, Draper D, Wi S, Newman TB, Zupancic J, Lieberman E, et al. Estimating the probability of neonatal early-onset infection on the basis of maternal risk factors. Pediatrics. 2011 Nov;128(5):e1155–e1163. doi: 10.1542/peds.2010-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuchat A, Oxtoby M, Cochi S, Sikes RK, Hightower A, Plikaytis B, et al. Population-based risk factors for neonatal group B streptococcal disease: results of a cohort study in metropolitan Atlanta. The Journal of infectious diseases. 1990 Sep;162(3):672–677. doi: 10.1093/infdis/162.3.672. [DOI] [PubMed] [Google Scholar]
- 16.Cardenas V, Davis RL, Hasselquist MB, Zavitkovsky A, Schuchat A. Barriers to implementing the group B streptococcal prevention guidelines. Birth. 2002 Dec;29(4):285–290. doi: 10.1046/j.1523-536x.2002.00203.x. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 17.Lewin EB, Amstey MS. Natural history of group B streptococcus colonization and its therapy during pregnancy. American journal of obstetrics and gynecology. 1981 Mar 1;139(5):512–515. doi: 10.1016/0002-9378(81)90509-3. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 18.Hoogkamp-Korstanje JA, Gerards LJ, Cats BP. Maternal carriage and neonatal acquisition of group B streptococci. The Journal of infectious diseases. 1982 Jun;145(6):800–803. doi: 10.1093/infdis/145.6.800. [DOI] [PubMed] [Google Scholar]
- 19.Berardi A, Rossi C, Creti R, China M, Gherardi G, Venturelli C, et al. Group B streptococcal colonization in 160 mother-baby pairs: a prospective cohort study. The Journal of pediatrics. 2013 Oct;163(4):1099 e1–104 e1. doi: 10.1016/j.jpeds.2013.05.064. [Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 20.Meyn LA, Moore DM, Hillier SL, Krohn MA. Association of sexual activity with colonization and vaginal acquisition of group B Streptococcus in nonpregnant women. American journal of epidemiology. 2002 May 15;155(10):949–957. doi: 10.1093/aje/155.10.949. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 21.Newton ER, Butler MC, Shain RN. Sexual behavior and vaginal colonization by group B streptococcus among minority women. Obstetrics and gynecology. 1996 Oct;88(4 Pt 1):577–582. doi: 10.1016/0029-7844(96)00264-5. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 22.Stapleton RD, Kahn JM, Evans LE, Critchlow CW, Gardella CM. Risk factors for group B streptococcal genitourinary tract colonization in pregnant women. Obstetrics and gynecology. 2005 Dec;106(6):1246–1252. doi: 10.1097/01.AOG.0000187893.52488.4b. [Research Support, N.I.H., Extramural] [DOI] [PubMed] [Google Scholar]
- 23.Honest H, Sharma S, Khan KS. Rapid tests for group B Streptococcus colonization in laboring women: a systematic review. Pediatrics. 2006 Apr;117(4):1055–1066. doi: 10.1542/peds.2005-1114. [Meta-Analysis Research Support, Non-U.S. Gov't Review] [DOI] [PubMed] [Google Scholar]
- 24.Goodman JR, Berg RL, Gribble RK, Meier PR, Fee SC, Mitchell PD. Longitudinal study of group B streptococcus carriage in pregnancy. Infectious diseases in obstetrics and gynecology. 1997;5(3):237–243. doi: 10.1155/S1064744997000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Helali N, Giovangrandi Y, Guyot K, Chevet K, Gutmann L, Durand-Zaleski I. Cost and effectiveness of intrapartum group B streptococcus polymerase chain reaction screening for term deliveries. Obstetrics and gynecology. 2012 Apr;119(4):822–829. doi: 10.1097/AOG.0b013e31824b1461. [Comparative Study] [DOI] [PubMed] [Google Scholar]
- 26.Edwards RK, Novak-Weekley SM, Koty PP, Davis T, Leeds LJ, Jordan JA. Rapid group B streptococci screening using a real-time polymerase chain reaction assay. Obstetrics and gynecology. 2008 Jun;111(6):1335–1341. doi: 10.1097/AOG.0b013e31817710ee. [Comparative Study Multicenter Study Research Support, Non-U.S. Gov't] [DOI] [PubMed] [Google Scholar]
- 27.Manning SD, Lewis MA, Springman AC, Lehotzky E, Whittam TS, Davies HD. Genotypic diversity and serotype distribution of group B streptococcus isolated from women before and after delivery. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008 Jun 15;46(12):1829–1837. doi: 10.1086/588296. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwatra G, Adrian PV, Shiri T, Buchmann EJ, Cutland CL, Madhi SA. Serotype-specific acquisition and loss of group B streptococcus recto-vaginal colonization in late pregnancy. PloS one. 2014;9(6):e98778. doi: 10.1371/journal.pone.0098778. [Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madzivhandila M, Adrian PV, Cutland CL, Kuwanda L, Schrag SJ, Madhi SA. Serotype distribution and invasive potential of group B streptococcus isolates causing disease in infants and colonizing maternal-newborn dyads. PloS one. 2011;6(3):e17861. doi: 10.1371/journal.pone.0017861. [Research Support, Non-U.S. Gov't] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker CJ, Carey VJ, Rench MA, Edwards MS, Hillier SL, Kasper DL, et al. Maternal antibody at delivery protects neonates from early onset group B streptococcal disease. The Journal of infectious diseases. 2014 Mar 1;209(5):781–788. doi: 10.1093/infdis/jit549. [Multicenter Study Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker CJ, Kasper DL. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976 Apr 1;294(14):753–756. doi: 10.1056/NEJM197604012941404. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 32.Lin FY, Philips JB, 3rd, Azimi PH, Weisman LE, Clark P, Rhoads GG, et al. Level of maternal antibody required to protect neonates against early-onset disease caused by group B Streptococcus type Ia: a multicenter, seroepidemiology study. The Journal of infectious diseases. 2001 Oct 15;184(8):1022–1028. doi: 10.1086/323350. [Multicenter Study Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 33.Lin FY, Weisman LE, Azimi PH, Philips JB, 3rd, Clark P, Regan J, et al. Level of maternal IgG anti-group B streptococcus type III antibody correlated with protection of neonates against early-onset disease caused by this pathogen. The Journal of infectious diseases. 2004 Sep 1;190(5):928–934. doi: 10.1086/422756. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 34.Zaleznik DF, Rench MA, Hillier S, Krohn MA, Platt R, Lee ML, et al. Invasive disease due to group B Streptococcus in pregnant women and neonates from diverse population groups. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000 Feb;30(2):276–281. doi: 10.1086/313665. [Multicenter Study Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 35.Harrison LH, Elliott JA, Dwyer DM, Libonati JP, Ferrieri P, Billmann L, et al. Serotype distribution of invasive group B streptococcal isolates in Maryland: implications for vaccine formulation. Maryland Emerging Infections Program. The Journal of infectious diseases. 1998 Apr;177(4):998–1002. doi: 10.1086/515260. [Research Support, Non-U.S. Gov't Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 36.Campbell JR, Hillier SL, Krohn MA, Ferrieri P, Zaleznik DF, Baker CJ. Group B streptococcal colonization and serotype-specific immunity in pregnant women at delivery. Obstetrics and gynecology. 2000 Oct;96(4):498–503. doi: 10.1016/s0029-7844(00)00977-7. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 37.Baker CJ, Edwards MS, Kasper DL. Role of antibody to native type III polysaccharide of group B Streptococcus in infant infection. Pediatrics. 1981 Oct;68(4):544–549. [Research Support, U.S. Gov't, P.H.S.] [PubMed] [Google Scholar]
- 38.Vogel LC, Boyer KM, Gadzala CA, Gotoff SP. Prevalence of type-specific group B streptococcal antibody in pregnant women. The Journal of pediatrics. 1980 Jun;96(6):1047–1051. doi: 10.1016/s0022-3476(80)80639-1. [Research Support, U.S. Gov't, P.H.S.] [DOI] [PubMed] [Google Scholar]


