Abstract
Immunodeficient mice engrafted with functional human cells and tissues, i.e., “humanized mice”, have become increasingly important as small pre-clinical animal models for the study of human diseases. Since the description of immunodeficient mice bearing mutations in the IL2 receptor common gamma chain (IL2rgnull) in the early 2000’s, investigators have been able to engraft murine recipients with human hematopoietic stem cells that develop into functional human immune systems. These mice can also be engrafted with human tissues such as islets, liver, skin, and most solid and hematologic cancers. Humanized mice are permitting significant progress in studies of human infectious disease, cancer, regenerative medicine, graft versus host disease, allergies, and immunity. Ultimately, use of humanized mice may lead to the implementation of truly “personalized” medicine in the clinic. This review discusses recent progress in the development and use of humanized mice, and highlights their utility for the study of human diseases.
Keywords: Humanized mice, infectious disease, cancer, regenerative medicine, allergy, autoimmunity, immunodeficient mice
INTRODUCTION
Animal models are used as surrogates of human biology due to the logistical and ethical restrictions of working with cell and tissue samples from human donors. Small animals such as mice and rats are widely used mammalian model systems due to their small size, ease of maintenance and handling, a short reproductive cycle, sharing of genomic and physiological properties with humans and ability to be readily manipulated genetically. Despite the vast amount of basic biology obtained from mouse studies, there are limitations to mouse models when investigating human biology. Several components of mouse biological systems are incongruent with those of humans, particularly their immune system (1). For example, there are many differences in innate immune molecules, including the lack of a functional TLR10 in mice and the expression of TLR11, TLR12, and TLR13 in mice that are not present in the human genome (1). Furthermore, many drugs and human pathogens are species-specific. The nature and pathogenesis of immune responses mounted against pathogens that can infect only human cells may markedly differ from that of murine infectious agents or human infectious agents that have been murine-adapted (2–6). These issues underscore the need for better small animal models that can more faithfully recapitulate human biological systems.
Humanized mice have begun to fill this gap and have become important pre-clinical tools for biomedical research. This is due to the continuous improvement of immunodeficient recipients used to generate humanized mice over the last 25 years [reviewed in (3–6)]. The key breakthrough was the development of immunodeficient mice bearing mutations in the IL2 receptor common gamma chain (IL2rgnull) in the early 2000’s. The common gamma chain constitutes an important component of receptors for IL2, IL4, IL7, IL9, IL15 and IL21 and is indispensable for high-affinity binding and signaling of these cytokines. When combined with either the protein kinase DNA activated catalytic polypeptide mutation (Prkdcscid or scid) or with recombination activating gene (Rag) 1 or 2 (Rag1null or Rag2null) mutations, these mice lack adaptive immunity and exhibit severe deficiencies of innate immunity, including absence of murine NK cells.
Three strains of immunodeficient IL2rgnull mice are widely used today: NOD.Cg-PrkdcscidIl2rgtm1Wjl (NSG), NODShi.Cg-PrkdcscidIl2rgtm1Sug (NOG), and C;129S4- Rag2tm1FlvIl2rgtm1Flv (commonly referred to as BALB/c-Rag2null IL2rgnull mice or BRG) mice [reviewed in (3–6)]. NOG mice have a truncated cytoplasmic domain of the gamma chain that binds cytokines but lacks the signaling domain while NSG and BRG mice completely lack the gamma chain. When engrafted with human cells, tissues and immune systems, the biological responses in humanized mice more faithfully recapitulate that seen in humans than in any previous models of humanized mice (3–6). A recent NIH workshop focusing on HIV research has highlighted many of the new emerging models of immunodeficient IL2rgnull mice that are being created to address some of the remaining limitations that are present in the basic models (7).
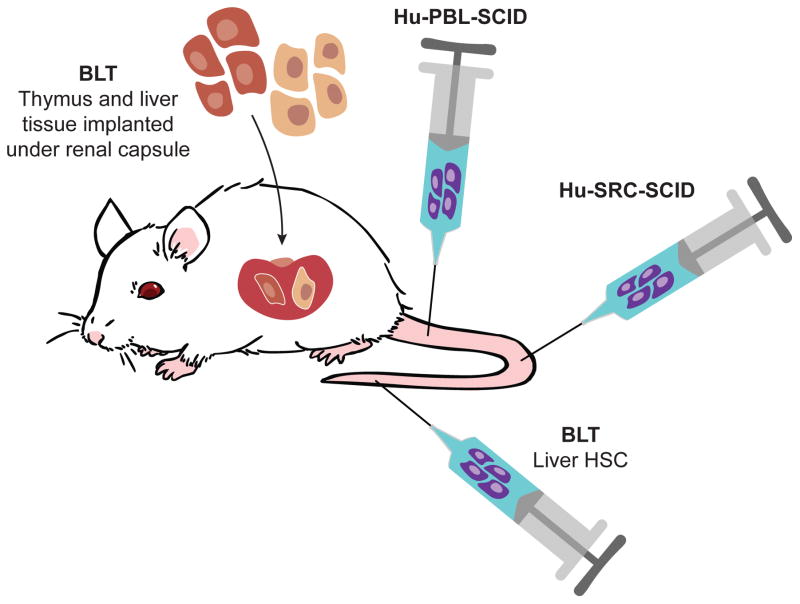
There are three general approaches of engrafting human immune systems into immunodeficient IL2rgnull mice (Figure 1). The first model, known as Hu-PBL-SCID, is created by injection of human peripheral blood leukocytes. This model results in rapid engraftment of human CD3+ T cells by the end of the first week. This model is excellent for studying human T cell function in vivo, but only has a short experimental window due to the development of lethal xenogeneic graft-versus-host disease (GVHD), usually within 4–8 weeks. However, this experimental window can be extended using NSG recipients lacking murine MHC class I or class II (8). The second model, known as Hu-SRC-SCID, encompasses the intravenous (IV) or intrafemoral injection of human SCID repopulating cells, i.e., human CD34+ HSCs derived from bone marrow (BM), umbilical cord blood (UBC), fetal liver or G-CSF-mobilized peripheral blood. This model supports engraftment of a complete human immune system. Although B cells, T cells, myeloid cells and antigen-presenting cells (APCs) are present in the peripheral hematopoietic tissues, granulocytes, platelets and red blood cells that are generated in the bone marrow are observed at only very low levels in the blood. In addition, the human T cells are educated in the mouse thymus and are thus H2, not HLA-restricted (9). Furthermore, the murine thymus appears to lack some human-specific factors necessary to fully mimic all aspects of human T cell development (10).
Figure 1. Model systems for engraftment of human immune systems into immunodeficient mice.
Hu-PBL-SCID; IV or IP injection of mature lymphoid populations including peripheral blood mononuclear cells, lymph node cells, and splenocytes. The advantage of this model is the easy access to clinically relevant samples, but engraftment is limited to predominately human CD3+ T cells. The disadvantage of this model is the development of GVHD leading to a short experimental window. Hu-SRC-SCID; IV or intra-femoral injection of human CD34+ HSCs derived from umbilical cord blood, bone marrow, fetal liver or G-CSF-mobilized peripheral blood stem cells. The advantage of this model is the development of a complete human immune system, but the human T cells are educated on the murine thymus and are H2-restricted. Poor human mucosal immune system development is also observed. BLT; engraftment of human fetal thymus and liver fragments under the renal capsule of the kidney and IV injection of human CD34+ HSCs from the autologous fetal liver. This is a more complete human immune system with improved mucosal immunity. The human T cells are educated on their autologous thymus and are HLA-restricted. The major disadvantage is the development of a wasting syndrome following long-term engraftment that is observed in most laboratories.
The third model is the bone marrow/liver/thymus “BLT” model that is established by transplantation of human fetal liver and thymus under the kidney capsule and IV injection of autologous fetal liver HSCs (11, 12). As in the Hu-SRC-SCID model, all lineages of human hematopoietic cells develop. However, BLT mice also develop a robust mucosal human immune system and the human T cells are educated in an autologous human thymus and are HLA-restricted. One of the main caveats of the BLT model is that, in most laboratories, the mice develop a wasting GVHD-like syndrome that limits the time window for experimentation (3–5). Each model has its advantages and limitations and therefore it is important for researchers to select a model appropriate for their specific biological questions.
While immunodeficient IL2rgnull mice are much improved in their ability to support the engraftment of human cells, tissues, and immune systems over that of previous models, continuous efforts to improve the models as well as create new models of human disease are underway in many laboratories worldwide. These modifications have been recently reviewed (3–5, 7). These improved humanized mouse models are now being used to study many human biological responses and diseases and are increasingly employed as preclinical tools for evaluation of drugs and for identifying underlying mechanisms in a broad array of diseases. In particular humanized mice are playing an increasing role in the study of human-specific infectious agents such as HIV and are widely used as pre-clinical models in cancer biology. Moreover, humanized mice are being increasingly utilized as translational models in many additional areas of biomedical research including regenerative medicine, transplantation, and immunity.
INFECTIOUS DISEASE IN HUMANIZED MICE
Humanized mice provide an opportunity to study species-specific agents that require human tissues for infection and replication and permit study of the developing human immune response. Engraftment of human immune cells into immunodeficient mice allows for the productive infection by many human-specific pathogens, but differences between mouse strains and engraftment methods make the choice of the optimal animal model dependent on the question being asked. Discussed below are some of the infectious pathogens that have been studied using humanized mice. Additional novel models of human infectious agents with more limited reports are outlined in Table 1.
Table 1.
Humanized mouse models of infectious disease
| Pathogen | Model | Infection route | Major findings | reference |
|---|---|---|---|---|
| Neisseria meningitides | SCID/Beige mice transplanted with human skin | Intravenously (IV) | N. meningitidis displays human specificity of adherence to human microvessels and causes vascular leakage, purpuric rashes and tissue necrosis. N. meningitides adhered to the human dermal microvessels in transplanted human skin and mediated vascular damage, inflammation and in some cases purpuric rash. | (145) |
| Herpes simplex virus type 2 | HSC-engrafted BRG mice |
intravaginal | Human T cells migrated into the iliac lymph node and vaginal tract and mounted a T cell, NK cell and antibody response to the virus and were able to reduce local viral replication | (146) |
| Human Herpes virus 6 (HHV6) | HSC-engrafted BRG mice |
HHV6A-infected CD34+HSC IP or cell free virus IP | HHV6A can infect the thymus, lymph nodes and bone marrow and infection leads to an increase in double-positive CD4+CD8+ T cells in blood and loss of CD3−CD4−CD8− thymus progenitors | (147) |
| Human Cytomegalovirus | HSC-engrafted NSG mice. |
HSC isolated from HCMV donors | Humanized mice created using G-CSF mobilized peripheral blood stem cells (PBSC) from CMV seropositive donors led to detection of CMV in the liver, spleen, and bone marrow of the recipients providing a model to study CMV transmission during PBSC transplantation | (148) |
| John Cunningham virus | NSG-BLT mice | JCV-specific IgM antibody responses, viral replication in both blood and urine; no CNS symptoms or infection suggesting factors required for productive JCV infection and induction of progressive multifocal leukoencephalopathy (PML) are absent in NSG-BLT mice | (149) | |
| Leishmania major virus | HSC-engrafted NSG mice |
foot pad injections | Infection of human macrophages and a human T cell response is observed but progresses to a systemic infection. Dose dependent footpad swelling, weight loss and mortality; CD4 and CD8 T cells infiltrate the foot pad | (150) |
| Varicella-zoster virus | CB17-scid mice with fetal human thymus/liver, sensory neurons, or skin transplants | Intraperitoneal | Human-specific pathogen that causes chickenpox and when reactivated in older individuals, causes shingles. Humanized mice have been used to study viral replication in human grafts and how the virus establishes latency. | For review, see (151) |
| Human T cell leukemia virus | NOG mice | Engraftment of CD133+ human stem cells | Productive infection for 4–5 months, rapid expansion of CD4+ T cells, and HTLV-1 specific immune responses were observed. | (152) |
| Nipah virus | NSG mice | Intragraft inocularion | Human lung xenograft model that was successfully infected with Nipah Virus, which replicated to high titers in the engrafted lung tissues. | (153) |
| Chlamydia | NSG BLT mice | Transcervicallly into the uterus | UV-killed Chlamydia complexed with synethic adjuvant particles induced a protective immune response. Vaccinated mice had CD4+ T cells producing IFNγ and had decreased bacterial burdens 4 days post rechallenge. | (154) |
Human immunodeficiency virus (HIV)
Prior to the development of CB17-scid mice engrafted with human immune systems in 1988, the only animal model available for the study of HIV was the chimpanzee (4). HIV is a retrovirus and the causative agent for AIDS. Since HIV infects human CD4 T cells, macrophages and dendritic cells, humanized mice provide an opportunity to study HIV in vivo and have been used to study HIV infection, disease progression, latency and virology [for review, see (13)]. Although some laboratories use the Hu-SRC-SCID model to study HIV, most laboratories use the BLT model due to the higher level of engraftment of the human mucosal system permitting vaginal and rectal transmission of HIV to be studied (13–17). These models have supported testing of numerous prophylactic drugs, anti-HIV antibodies, and cellular therapies for prevention of HIV replication and HIV clearance.
HIV Virology
HIV expresses nine proteins, five of which are essential for productive infection in culture; however, it was not until the utilization of humanized mouse models that the role for an additional four accessory proteins was determined (18). Viral infectivity factor (vif) was found to be essential for HIV replication in vivo. Viral protein u (vpu) promotes viral replication in vivo. HSC-engrafted NOG mice infected with vpu-deficient virus had reduced levels of viral replication with lower levels of free virus suggesting a role for vpu in virion release. In vivo negative factor (nef) was found to promote viral replication and pathogenicity. Patients infected with nef-deficient HIV-1 fail to develop AIDS. In NSG-BLT or NOD-scid BLT mice, nef-deficient virus led to slower viral kinetics and a reduced loss of CD4 T cells and thymocytes (18).
HIV immunology
Hu-PBL-SCID mice led to early understanding of HIV infection, replication and pathogenesis in vivo, while HSC-engrafted and BLT models recapitulated human disease. HSC-engrafted immunodeficient mice can be infected with either CCR5 or CXCX4-tropic HIV strains as indicated by serum viremia for prolonged periods (over a year) (14, 19). The BLT model has been useful in the study of HIV mutations resulting in escape from CD8 T cell responses (20). Humanized mouse models establish latent HIV reservoirs (20–22) and show CNS infection and neuropathology (23). In NOD-scid BLT mice, HIV-infected T cells were found to form syncytia blocking the migration of infected T cells out of the lymph node suggesting cell to cell transmission (26). This was confirmed directly using NSG-BLT mice to demonstrate that trans-infection contributes to retroviral spread in vivo, providing new mechanistic means that permits retrovirus to spread among cells and should lead to new approaches for blocking virus spreading (24). This novel infection mechanism is particularly important in interpreting the recent clinical trial that showed anti-retroviral therapy (ART) can reduce the transmission of HIV from infected females to their partners (25). The hypothesis that this was due to suppression of HIV in the cervicovaginal secretions was tested and validated in ART-suppressed HIV-infected NSG-BLT mice (26).
Therapeutic Interventions for HIV
HIV-infected humanized mice have been used to examine the impact of anti-retroviral therapy (ART), shRNA therapy, and immunotherapies such as immune modulation and T cell receptor (TCR) transduction. ART inhibits viral replication in HSC-engrafted, SCID-Hu and BLT humanized mouse models (14, 20, 22). However, after treatment and subsequent reduction of viral load, latently infected CD4 T cells are still detectable (14, 20) indicating ART did not fully eliminate the HIV reservoir.
In an effort to recapitulate the “Berlin patient” who was cured of HIV by a bone marrow transplant from a CCR5 deficient donor, several studies have targeted CCR5 expression in HSC prior to engraftment in mice. Holt et al. demonstrated a significant decrease in HIV copy numbers in vivo in NSG mice engrafted with human HSC in which CCR5 was knocked down. Targeting of CCR5 in HSC using zinc finger nuclease technology resulted in reduced loss of CD4 T cells and lowering of viral load (27). Myburgh et al used lentivirus technology to transduce human HSCs with a microRNA that down-regulated CCR5 and transplanted the transduced HSCs into NSG mice. These mice displayed a resistance to infection with a CCR5-tropic HIV strain as demonstrated by the persistence of human CD4+ T cells and decreased viral load (28). However, HIV rebounded in one mouse, presumably as a result of the emergence of a CXCR4-tropic strain. This recapitulated the clinical findings in a HIV+ patient that was transplanted with CCR5-null HSC but subsequently developed an active infection with a CXCR4 tropic variant that required re-initiation of ART therapy (29).
Additional approaches have been used to genetically modify human HSCs to prevent HIV infection in humanized mice. A dual-combination HIV lentivirus vector constructed to downregulate CCR5 was also found to be active against CXCR4-tropic viruses. This vector was used to transduce human HSCs used to establish NSG-BLT mice, and the subsequent ex vivo infection of splenocytes from these mice demonstrated resistance to infection with both CCR5− and CXCR4-tropic viruses. Furthermore, the NSG-BLT mice challenged with R5-tropic HIV-1 displayed significant protection of CD4 T cells and reduced viral loads (30).
HSCs have also been transduced with HIV-specific CD8 TCRs. Compared to control NSG-BLT mice, there was a reduction in HIV replication and improved survival of CD4 T cells and an expansion of SL9-specific effector CD8 T cells following engraftment with transduced HSCs (34). HSCs transduced to express anti-HIV polymeric IgA protected BLT mice against CD4 depletion after a vaginal HIV challenge (31). Using immunomodulatory therapy, treatment with an anti-PD-1 antibody led to CD8 T cell activation, a decrease in HIV plasma loads, and an increase in the T cells of HSC-engrafted (32) and BLT mice (33). Broadly neutralizing antibodies have also been shown to prevent HIV transmission in HSC-engrafted mice (34). Use of AAV to deliver antibodies to NSG-BLT mice protected against HIV infection when the virus was delivered intravenously or intravaginally (35). Clearly, these preclinical studies in HIV using humanized mice are providing a rapid pre-clinical platform for testing new drugs prior to entering clinical trials.
Dengue Virus
Dengue (DENV) is a flavivirus that is transmitted through mosquitoes and can induce life-threatening dengue hemorrhagic fever or dengue shock syndrome. There are currently no effective vaccines available and the lack of an appropriate mouse model for DENV infection has limited research. Both HSC-engrafted and BLT models of humanized mice support DENV infection with virus detectable in spleen, bone marrow and liver and the development of human disease-like symptoms, such as fever (36–41). Hu-SRC-SCID HLA-A2 transgenic NSG mice produced multiple A2-restricted DENV-2-specific responses (39). Similar to the Hu-SRC-SCID model in NSG mice, NSG-BLT mice developed both IgM anti-DENV antibodies and IFNγ-producing T cell responses (37, 38).
Of emerging interest is the possible use of humanized mice to study Zika virus (ZIKV). ZIKV is a flavivirus and is a close relative to DENV. Establishment of a humanized mouse model for DENV is ongoing, and it is likely that similar models can be applied for the study of ZIKV as research into this virus is being accelerated due to the WHO declaration of a worldwide response plan for dealing with the emerging ZIKV outbreak (42).
Epstein Barr Virus (EBV)
Epstein-Barr virus (EBV) infects 90% of humans worldwide and has an exclusive tropism for humans. The majority of infections are asymptomatic, however, infection can also result in a variety of diseases such as lymphoproliferative disorders (LPD), cancer (Burkitt’s lymphoma, Hodgkin lymphoma, nasopharngeal carcinoma and gastric carcinoma) and autoimmune diseases (rheumatoid arthritis and multiple sclerosis) (43). EBV infects human epithelial cells and B cells making the use of humanized mouse models essential for in vivo studies with this virus. EBV can transform infected cells, which in healthy individuals are attacked by CD8 T cells. If these transformed cells are not killed, LPD and cancer can develop. EBV-induced LPD and tumors develop in both the Hu-PBL-SCID and Hu-SRC-SCID models (44, 45).
In patients, EBV infection causes an expansion of T cells and inverts the CD4:CD8 T cell ratio (43). Depletion of CD8 T cells leads to increased viral titers and mortality suggesting CD8 T cell-mediated control of EBV viral load. Humanized mice have been reported to develop EBV-antigen specific T cell responses. Both HSC-engrafted and BLT mice developed HLA class I-dependent CD8 T cell responses (11, 46) and HLA-A2 transgenic HSC-engrafted mice developed an HLA-A2-restricted EBV epitope-specific responses (47). In addition to T cell responses, NK cell responses have also been found to play a role in controlling EBV-infection. Depletion of NK cells using antibody treatment of HSC-engrafted mice was associated with higher viral loads and augmented symptomatic lytic infection (48).
Influenza
Hu-SRC NOD-scid β2mnull mice engrafted with autologous T cells isolated from the donors of mobilized HSCs have been used to study the infection and immune response produced against the live-attenuated trivalent influenza vaccine (LAIV) (49). Infection caused an expansion of Flu-M1-specific CD8 T cells and clearly documented the importance of the myeloid compartment and human DC in the immune response. Macrophages are also important in early viral responses. In the BRG model, injection of human IL-3 and GM-CSF increased myeloid engraftment within the lung and led to increased innate responses to virus infection (50). Treatment of NSG Hu-SRC-SCID mice with M-CSF led to decreased viral transcript levels early after infection and was associated with a burst of inflammatory cytokines, such as IL-6 and TNF at 48 hours post infection (51).
KSHV
Kaposi’s sarcoma-associated herpesvirus or human herpes virus 8 is associated with primary effusion lymphoma (PEL) and multicentric Castleman’s disease. Studying disease induction and infection has been challenging prior to development of the NSG-BLT humanized mouse model. Oral infection of NSG-BLT mice led to persistent latent infection of macrophages and B cells within the spleen and viral dissemination into the skin, providing an animal model for the study of this virus (52).
Typhoid Fever/Salmonella
Salmonella enterica serovar Typhi is the causative agent for typhoid fever and is human specific. The majority of NSG Hu-SRC-SCID mice infected with S typhi succumbed to infection within 72 hours and displayed enhanced bacterial burden in liver over non-engrafted controls (53). While, BRG mice engrafted with HSCs showed increased bacterial loads in spleen and liver compared with non-engrafted controls, neither of these models recapitulated the mortality observed in HSC-engrafted NSG mice (54, 55).
Mycobacterium (tuberculosis)
Mycobacterium tuberculosis is the causative agent for tuberculosis, which predominantly attacks the lungs with hallmark granuloma development. Unfortunately, mouse models of tuberculosis usually fail to form granulomas, but HSC-engrafted NSG mice formed granuloma-like structures after infection with M. bovis (Bacillus Calmette-Guerin, BCG). Granuloma formation was CD4 T cell-dependent (56) and treatment with GM-CSF demonstrated increased macrophage differentiation and better control of BCG infection (51) providing a model to study the impact of macrophages in tuberculosis. NSG-BLT mice develop a progressive mycobacterial infection that disseminates from the lungs to the spleen after intranasal infection (57). HLA-A2 transgenic NSG-BLT mice infected with BCG exhibited lung lesions that contained macrophages as well as CD4 and CD8 T cells, but did not resemble true human granulomas (58).
Ebola Virus and Hantavirus
The recent Ebola virus outbreak highlighted the need to rapidly and efficiently test new therapeutics for this virus. However, the only initial animal model available was the non-human primate, which is limited by ethical considerations, cost and size. Recently there have been two reports of humanized mouse models of Ebola infection that recapitulated the disease observed in humans. In NSG-HLA-A2 Tg mice engrafted with HLA-A2 HSCs infected with wild-type Ebola virus, 100% of the mice with high engraftment of human cells died within 20 days exhibiting features associated with viremia, cell damage, liver steatosis, and hemorrhage (59). In NSG-BLT mice, infection with two different wild-type strains of Ebola virus led to histological changes in the liver and upregulation of cytokines and chemokines, symptoms consistent with Ebola hemorrhagic fever (60). These models are being used in Ebola research to identify the pathogenesis of the infection and to test new drugs designed to prevent disease. This also serves as an example of the potential of humanized mice to be used for the study of new emerging human-specific pathogens such as is occurring in the recent outbreak of Zika virus or other as yet unidentified emerging infectious agents.
Hantavirus infection can cause hemorrhagic fever with renal syndrome in humans. To create a humanized mouse model for the study of Hantavirus, NSG-HLA-A2 mice were engrafted with HLA-A2 matched cord blood HSCs (61). Infected mice experienced weight loss, pulmonary inflammation, and ~75% loss of human platelets (but no loss of mouse platelets), providing another humanized mouse model for the study of BSL4 level infectious agents.
Malaria
Natural Plasmodium infection involves both a hepatic phase and an erythrocytic (RBC) phase. Human liver chimeric mice can be utilized to study the liver phase and human blood chimeric mice can be used to study the blood phase (62, 63). HSC-engrafted mice have few circulating human RBC due to mouse macrophage-mediated phagocytosis making infection of P. falciparum limited unless daily RBC injections are administered (64). Complete P. falciparum replication has been possible using human liver chimeric Fahnull Rag2null IL2rgnull (FRG) NOD and thymidine kinase (TK) NOG mice engrafted with human hepatocytes and infused with human RBCs (65, 66). Humanized mice are now being used to study P. falciparum genetic traits and phenotypes associated with severity of disease (67) and for testing the efficacy of new therapeutics that block entry of the virus into hepatocytes (68).
Sepsis
Sepsis is a whole body inflammatory condition that can induce death through cytokine storm and apoptosis of lymphocytes (69, 70). Cecal ligation and puncture (CLP) in HSC-engrafted NSG mice led to increased inflammatory cytokines and lymphocyte apoptosis (70). In addition, decreased cellularity in the bone marrow and lower numbers of human HSCs were observed following CLP-induced sepsis or LPS administration (71). Using NSG-BLT mice, it was shown that silencing high-mobility group protein 1 (HMGB1) decreased cytokine production and cellular apoptosis leading to increased survival (72).
HUMAN-MURINE LIVER CHIMERIC MOUSE MODELS FOR INFECTIOUS DISEASE
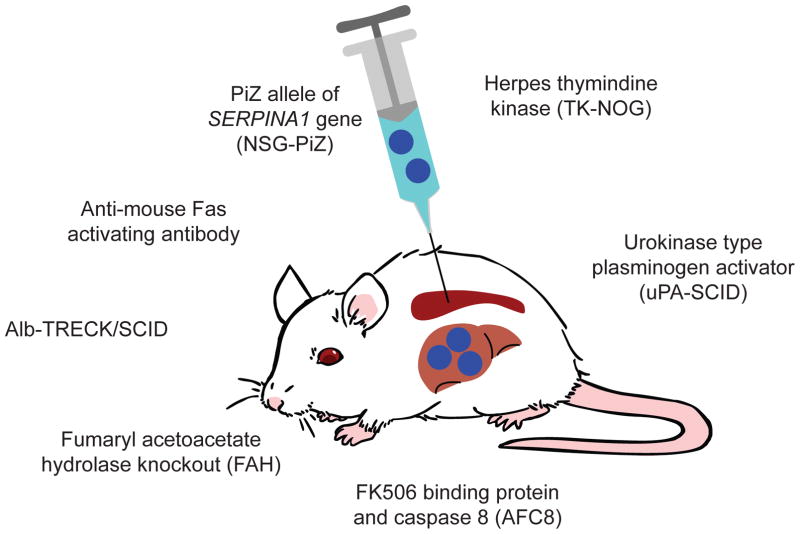
To study hepatotropic pathogens, human liver chimeras have been developed [Figure 2 and reviewed in (73, 74)]. In all models, the common theme is destruction of murine hepatocytes to permit space for engraftment of human hepatocytes. For example, a commonly used model is the targeting of fumaryl acetoacetate hydrolase (FAH). The benefit to this model is that mice can be treated with 2-(2-nitro-4-fluorome thylbenzoyl)-1,3-cyclohexanedione (NTBC) in the drinking water to prevent hepatocyte death. Alternatively, mice that express the herpes simplex virus type 1 thymindine kinase (TK) transgene can be treated with gancyclovir to induce hepatocyte injury. Rag2null IL2rgnull mice that express active caspase 8 fused with FK506 binding domain can be treated with FK506 to selectively kill mouse hepatocytes. A recent report suggests that NSG mice transgenically expressing a mutant allele of SERPINA1* (NSG-PiZ) crippled murine hepatocytes enabling human hepatocyte engraftment and in vivo targeting of the human hepatocytes by rAAV8 (75).
Figure 2. Murine models of human hepatocyte engraftment.
The engraftment of human hepatocytes into immunodeficient mice requires depletion of murine hepatocytes to create space for human hepatocytes. FAH: expression of fumaryl acetoacetate hydrolase leads to an accumulation of toxic tyrosine metabolic intermediates. Provision of NTBC in the drinking water prevents hepatocyte death until human hepatocytes are engrafted. TK-NOG: herpes simplex virus thymidine kinase transgene under the control of the Albumin promoter. Specific mouse hepatocyte depletion is induced by administration of gancyclovir, but male TK-NOG mice are sterile making breeding of this stock difficult. uPA-SCID: urokinase plasminogen activator gene under the control of the major urinary protein promoter. Over-expression of the uPA gene in an uncontrolled manner leads to high lethality of newborn pups that must be rapidly transplanted with human hepatocytes for their survival. NSG-PiZ: Transgenic expression of SERPINA1 leads to misfolding of human alpha-1 anti-trypsin and death of murine hepatocytes, providing a competitive advantage for human hepatocyte engraftment. AFC8: Expression of active caspase 8 fused with FK506 binding domain that when treated with FK506 selectively kills mouse hepatocytes. Alb-TRECK/SCID: Transgenic expression of the diphtheria toxin receptor under the control of the albumin promoter. Injection of diphtheria toxin selectively kills murine hepatocytes expressing the receptor. Anti-mouse Fas activating antibody: Injection of Jo2 Fas-activating antibody selectively kills murine hepatocytes creating space for human hepatocytes.
Hepatitis C Virus
Hepatitis C virus (HCV) initiates a chronic infection and progressive hepatic cirrhosis leading to hepatocellular carcinoma. To study HCV, SCID/Alb-uPA (76) and FahnullRag2nullIL2rgnull mice (77) have been used. Both models support HCV viral replication. More recent studies used the BRG strain expressing the FKBP-capase 8 fusion gene under the control of the albumin promoter (AFC8). Dimerization of the active Caspase 8 by AP20187 permits engraftment of human hepatocytes in combination with human HSCs. These mice could be productively infected with HCV, which led to human virus-specific T cell responses to HCV and hepatic fibrosis (78). However, HCV replication was observed in only ~50% of the mice highlighting the need for continual improvement of models to study HCV disease pathogenesis and immunity.
HCV entry into a cell is mediated by the tight junction protein claudin-1 and antibody blocking of this receptor inhibited viral replication and reduced the number of infected hepatocytes in human liver chimeric uPA-SCID mice (79). Blocking the co-receptor scavenger receptor class B type I also blocked HCV replication (80) even when the antibody was administered after HCV infection. Additionally, combination therapy of multiple HCV-specific broadly neutralizing antibodies by administration of AAV vectors expressing the antibodies reduced HCV replication and viral load in NRG-Fahnull mice engrafted with human hepatocytes (81). Furthermore, HCV-infected human liver engrafted SCID/beige mice expressing the urokinase plasminogen activator (uPA) gene under control of the major urinary protein (MUP) promoter (MUP-uPA/SCID/beige) mice provide unique opportunities for the study of tumorogenesis induced by HCV as about 25% of infected mice develop primary liver cancer (82).
Hepatitis B virus
Hepatitis B virus can cause cirrhosis and hepatocellular carcinoma. HBV is a noncytolytic virus and disease is immune mediated. Human liver chimeric uPA/SCID mice can become persistently infected with HBV and treatment with entecavir reduced HBV replication (83). This model may be useful in testing other therapeutic treatments for HBV. Alternatively, 75% of HLA-A2 transgenic NSG mice treated with anti-murine Fas antibody and transplanted with human liver and HSC developed persistent HBV infection out to 4 months with the development of liver fibrosis. Liver disease was associated with infiltration of M2-macrophages (84) providing a model to study the role of macrophages in liver disease progression during HBV infection.
Hepatitis D and E virus
Hepatitis D virus (HDV) is a defective virus that requires envelope proteins from hepatitis B virus to create infectious particles. Humanized liver chimeric SCID/beige mice expressing uPA driven by the albumin promoter were co-infected with HBV/HDV and viral replication for both viruses in the liver was detected at 3 weeks post infection and sustained to the end of the experiment (16 weeks) (85). Hepatitis E virus (HEV) is a major cause of acute hepatitis as well as chronic infection in immunocompromised individuals. A recent report indicated that human liver-engrafted UPA/SCID/Beige mice could be productively infected with HEV for up to 25 weeks, providing the first small animal model for the study of this virus (86).
HUMAN-MURINE LIVER CHIMERIC MOUSE MODELS FOR THE STUDY OF LIVER DISEASES AND DRUG METABOLISM
Many drugs are metabolized in the liver, but the metabolism of mouse and human liver are significantly different, making it difficult to use these small animal models for the study of drug pharmokinetics (87). A number of humanized mouse models have been developed that incorporate destruction of host hepatocytes followed by intrasplenic injection of human hepatocytes (Figure 2). For example, a new model of immunodeficient mice transgenically expresses the human heparin-binding epidermal growth factor-like receptor (diphtheria toxin receptor, DTR) under the control of the albumin promoter (Alb-TRECK/SCID). When injected with diphtheria toxin, the murine hepatocytes are rapidly destroyed and can be repopulated with human hepatocytes. These human liver chimeric mice metabolized ketoprofen and debrisoquine with similar characteristics to that observed in humans (88). Similarly, human liver chimeric TK-NOG mice have been used to study drug toxicity. Furosemide is toxic in mice, but can be used in humans due to species differences in its metabolism. The toxicity of Furosemide is reduced in the TK-NOG mice, similar to what is seen in humans (87). Human liver chimeric TK-NOG mice have also been used to model cholestatic liver toxicity induced by bosentan, which is not seen in control mice (89). Finally, Fahnull Rag2null IL2rgnull mice were engrafted with hepatocytes obtained from a donor with familial hypercholesterolaemia. The mice developed hypercholesterolaemia and human cholesteryl ester transfer protein as well as apolipoprotein A, which are not found in normal mice, were present in the serum. Replacement of the low-density lipoprotein receptor by AAV9-based gene therapy restored normal lipoprotein profiles, showing that this model could also be used to test gene therapy treatments for this disease (89).
TUMOR GROWTH AND CANCER IMMUNOLOGY IN HUMANIZED MICE
For more than half a century, xenografting patient-derived tumors into athymic nude mice and more recently into genetically modified, immunodeficient mice (4, 5) has been invaluable to researchers for improving treatments against many human cancers. Unfortunately, athymic mice retain murine B cells as well as an innate immune system, specifically NK cells that can hinder tumor growth and metastasis [reviewed in (90)]. Immunodeficient IL2rgnull mice lack NK cells and contain additional innate immune deficiencies that have enabled the successful engraftment of various human tumors including tumor cell lines and primary solid and hematological tumors (94).
Patient derived xenografts (PDX) engrafted into immunodeficient IL2rgnull mice initially maintain the original tumor heterogeneity and stroma (91, 92). However, PDX tumors upon secondary transfer rapidly lose tumor stroma that is replaced by mouse stromal cells (93). One of the contributors to non-autonomous heterogeneity in the tumor microenvironment is the presence of human tumor infiltrating immune cells (94). To that end, immunodeficient IL2rgnull mice allow the opportunity to study tumor-immune system interactions in vivo in mice engrafted with tumor cells and human immune system enabling researchers to interrogate how tumors interact with the immune system, mechanisms of tumor immune escape, and the mechanism and therapeutic potential of immune modulation (Figure 3).
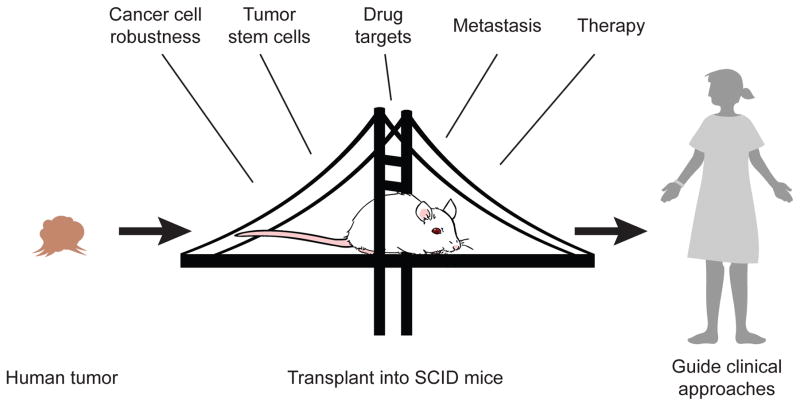
Figure 3. Humanized mice in cancer biology and therapy.
Humanized mice are used as preclinical models for investigation of cancer cell biology, identification of tumor stem cells, use as an in vivo platform for identifying and testing potential drug targets, investigation of mechanisms of tumor metastasis, and for evaluation of potential new therapeutics prior to their entry into the clinic.
Modeling human tumor-immune system interaction in humanized mice using tumor cell lines
In both PBL and HSC-engrafted mice human immune cells traffic and infiltrate the tumor microenvironment, similar to what is found in primary patient tumors, and provide a model to study tumor-immune system interactions.
In a Hu-SRC-SCID model of human breast cancer, neonatal NSG mice were concurrently injected with CD34+ HSCs and tumor cells (94, 95). T cells and NK cells were able to infiltrate the tumor microenvironment and tumor-bearing organs had higher CD4:CD8 T cells ratios, with a majority of T cells expressing a CD45RA−CD27+ memory phenotype (95). In the Hu-PBL-SCID model, even though a majority of T cells from both the spleen and the tumor had a memory phenotype (CD45RA−), splenic T cells included both central memory (CM) and effector memory (EM) cell populations, whereas a majority of TILs were EM. This differs with the TILs found in primary patient samples that are predominantly CM (96).
To improve myeloid cell development in BRG mice, Rongvaux et al. generated knockin mice that express human SIRPα, M-CSF, IL-3, GM-CSF and TPO (MISTRG) to promote human innate cell development. MISTRG mice engrafted with human fetal liver HSCs and subcutaneously injected with the human melanoma cell line Me290 showed an M2 macrophage infiltration into the tumor microenvironment similar to that observed in primary tumors (97). Tumor growth was increased in Hu-SRC-SCID MISTRG tumor-bearing mice when compared to Hu-SRC-SCID NSG tumor bearing mice suggesting macrophages/myeloid cells promote tumor growth (97).
NK cells and cytokine therapy
NK and NKT cells mediate tumor immune-surveillance and alterations in both number and function of NK and NKT cells have been associated with different tumor types (98). Efforts to stimulate NK and NKT cell anti-tumor activities have focused on the use of cytokine therapies.
HSC-engrafted NSG mice were injected with a human neuroblastoma cell line and ex vivo-expanded human NKT cells. NKT cells were found within the tumor microenvironment where they co-localized with tumor-associated macrophages (TAMs). CCL20 secreted by TAMs, however, inhibited survival and function of the NKT cells allowing tumor growth. By transducing NKT cells with IL15 prior to transfer Lie et al. showed decreased tumor growth due to increased NKT cell survival and demonstrated a role for IL15 cytokine therapy (99).
IL15 immune therapy was used to expand NK cell populations of Hu-SRC NSG mice transplanted with human breast cancer, leading to increased numbers of activated CD56+CD27− NK cells (95). Low dose IL15 treatment was also found to increase the survival and expansion of human NK cells in vivo and resulted in significantly less leukemia cell line engraftment and increased survival (100).
In addition to IL15, IL12 has also been investigated in humanized tumor-bearing mice as a method to stimulate the immune system to attack cancer cells. An antibody-IL12 fusion protein (NHS-IL12) that targets naked histones/DNA complexes found in necrotic tissue, such as tumors, was used in conjunction with IL7 (FcIL7) or antibody-complexed IL2 (IL2MAB602) in a Hu-SRC-SCID model of rhabdomyosarcoma (106). Treatment with NHS-IL12/IL2MAB206 reduced tumor growth and increased survival as well as eliminating tumors in 3 out of 4 mice over a long-term treatment. NHS-IL12/IL2MAB206 increased tumor infiltrates of T cells, NK cells, and macrophages as well as altering the phenotypes of these infiltrates. (101). Additionally, tumors in mice treated with NHS-IL12 featured a high percentage of senescent tumor cells and signs of myogenic differentiation that was more pronounced in mice treated with NHS-IL12/IL2MAB206.
Humanized Patient-Derived Xenograft (PDX) Tumor Models
While the previous section highlights the utility and potential of humanized mouse models for studying tumor and immune system interactions, the use of cell lines limits the applicability of these models. The next step forward is the generation of humanized patient-derived xenograft (PDX) tumor models that combine the heterogeneity lost in tumor cells lines with the more complete tumor microenvironment afforded in primary engrafted PDX tumors.
One potential pitfall of generating humanized mice for PDX studies is the unavailability of large numbers of autologous HSCs to generate cohorts for study. Recently, a method of expanding HSCs isolated from cord blood or G-CSF mobilized PBL by transduction with tat-MYC and tat-Bcl2 fusion proteins allowed for the in vitro proliferation of HSCs (102). NSG mice were engrafted with in vitro-expanded HSCs (called “XactMice”) and transplanted with autologous PDX samples from head and neck squamous cell carcinoma (HNSCC) patients. Tumors in XactMice were found to have human CD45+CD151+ cells and an increased density of lymphatic vessels suggesting immune cell and stromal cell components of the tumor microenvironment had been recreated in the mice.
T Cell Editing
One mechanism of targeting the immune system to improve anti-tumor immunity is to redirect T cell specificity through transgenic TCR or chimeric antigen receptor (CAR) engineered T cell therapy. CAR therapy is based on construction of a T cell receptor with specificity for an antigen independent of MHC restrictions, permitting the TCR to be redirected to any target of choice (103). Although T cell redirection therapy is being used in the clinic, humanized mouse models are being employed to optimize the efficacy and safety of TCR/CAR manipulation as well as to broaden the scope of cancer treatment
Adoptive cell therapy, the isolation and expansion of a patient’s own tumor-specific T cells for re-infusion, has been effective for renal cell carcinoma and melanoma (104) but has potential pitfalls for widespread use. The ability to isolate a potentially rare T cell population and the time needed for adequate expansion is not feasible for most patients. Mechanisms to optimize the transfer of transgenic (Tg) tumor-specific TCRs into T cells have been studied in humanized mouse models. One safety concern with Tg TCR therapy is the ability for the Tg TCR to mis-pair with the endogenous TCR to create off target specificity of the T cells increasing cytotoxicity. Provasi E et al., using zinc finger nucleases against the endogenous TCRα and TCRβ genes, demonstrated the ability to edit Tg T cells to express only Wilm’s Tumor-1 (WT-1) specific TCRs in a Hu-PBL-SCID model of WT-1+ leukemia. NSG mice receiving edited WT-1+ T cells developed neither leukemia or GVHD 50 days post leukemic infusion, while mice receiving un-edited WT-1+ T cells had all succumbed to GVHD and mice that did not receive PBMC all died of leukemia (105).
CARs against mesothelin for mesothelioma (106), ROR1 for mantle cell lymphoma (107), and CD44v6 for AML and multiple myeloma (108), have been generated and tested in humanized NSG mice. Additionally, to increase the efficiency of CAR signaling, co-stimulatory motifs have been built into CARs in tandem to the CD3ζ in the intracellular signaling domain. Humanized mice have been used to test and compare the function and efficiency of co-stimulatory domains such as CD27, ICOS, CD28, and 4-1BB (109, 110). CARs that incorporate co-stimulatory domains are more efficient in targeting tumors and this has been utilized to try to increase the safety of CAR therapy. Because the antigens commonly used in CAR therapy are not truly tumor specific, Kloss et al. generated a system in which the combination of two antigens was needed to trigger CAR signaling. A CAR molecule without a costimulation motif was generated that was specific for one antigen while a chimeric costimulatory receptor (CCR) molecule was generated with specificity for a second antigen (117). T cells transduced with both molecules would need to interact with cells displaying both antigens to trigger optimal signaling to induce a T cell response. In a Hu-PBL-SCID NSG model, a physiological relevant combination of the prostate tumor antigens of PSCA-CAR and PSMA-CCR demonstrated the ability to eradicate a tumor cell line that expressed both antigens and mount a response to PSCA+PSMA- tumors that eventually relapsed (111).
An additional modification aimed at the safety of CAR generation is the use of mRNA transfection as a mechanism of CAR generation as opposed to the use of viral transduction. As this method does not involve the integration of DNA into the genome, it takes away inherent risks of genomic editing. mRNA transduction has been used to generate anti-mesothelin CAR T cells in a Hu-PBL-SCID NSG model targeted to mesothelioma (106) and anti-CD20 CAR NK cells in a Hu-PBL-SCID NSG model targeted to Non-Hodgkins Lymphoma (112).
To address the heterogeneity in tumor antigen expression, a biotin binding immune receptor (BBIR) was generated extending the idea of CAR technology (113). BBIR extracellular avidin connects to an intracellular TCR signaling domain. Mice are then treated with a biotinylated antibody that will bind to a tumor associated antigen, such as EpCAM (119). In a Hu-PBL-SCID NSG model, BBIR humanized mice treated with bio-EpCAM had delayed tumor growth (113). Humanized mouse models will continue to be useful to optimize CAR-based approaches to increase their therapeutic potential.
Finally, to induce Wilms tumor 1 (WT-1)-specific human CD8 cytotoxic T cells from human HSCs, Najima et al transplanted WT-1 specific TCR transduced human HSCs into HLA class I matched transgenic NSG mice. WT-1 tetramer positive T cells differentiated in the thymus and splenic CTLs from these mice killed leukemia, target cells in an antigen-specific HLA-restricted manner (114)
Costimulatory Enhancement and Checkpoint Inhibitors
As demonstrated by its use as a costimulatory motif in the generation of higher efficiency CAR molecules, 4-1BB has the potential to increase anti-tumor T responses in vivo. An anti-4-1BB agonist mAb, PF-05082566 was tested against various cell line generated tumors in a modified Hu-PBL-SCID/Beige mouse model (115). Tumor cells were mixed with PBMCs and injected subcutaneously in SCID/Beige mice. Mice treated with PF-05082566 had growth inhibition of 40–60% of controls, suggesting that agonist immunomodulation has potential to affect tumor growth in humans (115).
Another mechanism of immunomodulation is the use of checkpoint inhibitors. Two inhibitors experiencing some clinical success are the cytotoxic T lymphocyte antigen 4 (CTLA-4) mAb, ipilimumab, which blocks this inhibitory pathway and activates T cells and an antagonist anti-programmed cell death-1 (anti PD-1) mAb, which blocks PD-1 interaction with its ligand programmed cell death-ligand 1 (PD-L1) (116). These blockages have been effective against melanoma but not all patients respond to CTLA-4 and anti-PD-1 therapy (117). Humanized mice engrafted with tumors are therefore being used to better understand how checkpoint blockade interacts with the immune system and also to test the efficacy and effects of immunomodulation agents. It was recently demonstrated that humanized mice could be used to analyze the pharmacodynamics and anti-tumor properties of an immunostimulatory mAb. Administration of urelumab (anti-hCD137 agonist) and nivolumab (anti-PD-1) significantly reduced tumor growth in Rag2null IL2rgnull mice injected with human colorectal HT-29 carcinoma cells and engrafted with allogeneic human PBMCs or in mice transplanted with patient-derived gastric carcinoma and engrafted with autologous PBMCs. The slowing of growth kinetics was coupled with an increase in IFNγ secreting human T cells and decreased numbers of human Tregs in the tumor xenografts that correlated with reduction of tumor burden (118).
One of the major concerns associated with using antibodies to target checkpoint inhibitors is the induction of a cytokine release syndrome. Screening these antibodies in rodent models and non-human primate may or may not reveal the response of a human immune system to the drug as highlighted by the TGN1412 (anti-CD28) clinical trial (119). To address this need, two humanized mouse models for the detection of a cytokine release syndrome were created. NSG or NRG-AB0 mice were engrafted with PBMC and injected with muromonab-CD3 (120), or TGN1412 (121). Muromonab-CD3 reproducibly induced acute clinical symptoms such as pilorection, hypomobility and hypothermia whereas TGN1412 injected mice engrafted with 65–100x106 PBL, rapidly lost body temperature and died within 2–6 hours. Extension of this approach to Hu-SRC-SCID and BLT models will permit innate immune cell reactions to antibodies directed against these cell populations to also be tested.
HUMANIZED MOUSE MODELS OF TRANSPLANTATION
Allogeneic rejection has been extensively studied in murine models, but immune-based therapeutics such as antibody-based biologics cannot be investigated in mice due to species-specific differences. Humanized mice have become an excellent preclinical model to study mechanisms underlying human immune responses and for the assessment of new therapies.
Human Allografts
Allograft rejection in humanized mice has been extensively studied for over two decades [for reviews, see (122–124)]. Historically the majority of studies were done in the Hu-PBL-SCID model but limited engraftment of human cell subsets other than CD3+ T cells can hinder rejection and results in variability between studies (122, 123). However, immunodeficient mice transplanted with human skin were used to investigate therapeutic drugs such as cyclosporine and rapamycin to begin to identify the role that IFNγ-producing T cells have in graft survival as well as the mechanisms by which Tregs modulate the human T cell allograft response (122–124).
Human Islet Allografts
Humanized mouse models allow for the study of the cellular mechanisms of rejection of human islets by allogeneic immune responses and provide a preclinical environment to test new therapeutics (122–124). Interestingly, HSC-engrafted NSG and BRG animals have shown contrasting rejection of allogeneic human islet grafts, documenting the importance of which model system is used for experimentation. Diabetic HSC-engrafted BRG mice did not reject human islets and had limited T cell infiltration (125). However, within 20 days 62% of hyperglycemic NRG-Akita (NOD-Rag1Tm1Mom IL2gnull Ins2Akita) mice that were HSC-engrafted reject their human islet grafts (126). By engrafting streptozotocin-treated diabetic NSG mice with a greater number of HSCs (2x105 compared to the 5x104 used in the previous study) 100% of NSG mice were able to reject islets within 17 days (127). Graft rejection was determined by hyperglycemia, immune cell infiltration and the loss of human insulin production. These differences in human islet allogeneic rejection may be due to differences in the host strain or in the numbers of human HSCs used for engraftment leading to differences in function of the human immune cells in these models.
Human Cardiac Allografts
Endothelial cells that line arterial transplants are strong promoters of immune responses and T cell activation. Opposed to the studies done with human islet and skin transplants, the vast majority of arterial graft experiments have been done in humanized SCID/beige mice. NSG mice transplanted with human arteries have a high prevalence of paralysis and death (128).
Arterial transplantation followed by allogeneic PBMC injection leads to human cell infiltration and morphological changes within the graft associated with rejection [reviewed in (124)]. Similar to skin and islet allografts the rejection is associated with IFNγ production by allospecific T cells, which mediates neointima, or scarring within the blood vessels of the graft. Blocking IFNγ, pretreatment of human artery with rapamycin reduced graft injury whereas blockade of PD-1 resulted in increased graft injury. Similarly, allografts produce TGFβ, and blockade of TGFβ increases graft injury. Other inflammatory cytokines expressed by the allografts have been found to induce graft injury. For example, blockade of either IL-6 or IL-1 signaling reduced graft injury. Tregs have also been found to block arterial graft rejection and prevent the development of arteriosclerosis in BRG mice transplanted with human aortic grafts and injected with PBMC (124).
HUMANIZED MOUSE MODELS OF AUTOIMMUNITY
Due to the differences in the immune system of mice and humans (1) spontaneous models of autoimmunity that arise in mice do not fully recapitulate the conditions seen in humans. Additionally, these differences make therapeutic development more difficult in murine models. Adoptive transfer of PBMCs from individuals with specific autoimmune conditions into CB17-scid or NOD-scid mice has led to the ability to isolate antigen-specific autoantibodies for multiple conditions yet none of these models resulted in lymphocytic infiltration or destruction of the target cell type; likely due to the low and variable engraftment of these mouse strains with human immune cells. The generation of NSG and NRG mouse strains have increased in the engraftment potential and led to the ability to study autoimmune disorders in a more physiological way.
Type 1 Diabetes
Type 1 diabetes (T1D) is an autoimmune condition in which the immune system attacks the insulin producing β cells in the pancreas leading to an inability to regulate blood glucose levels. However, the numerous species-specific differences in both the immune system and islets of rodents and human have limited the ability to translate the findings in the rodent models of T1D to the clinic.
The introduction of autoantigen-expanded HLA-DR4-restricted CD4 T cells from T1D diabetic donors resulted in islet infiltration by CD4 T cells in NSG-DR4 transgenic mice (129). Additionally human CD8 T cells transduced with TCR specific to IGRP265-273 and engrafted into NSG-HHD (HLA-A2 Tg) mice led to T cell infiltration of pancreatic islets (130). To further elucidate the role of autoimmune CD8 T cells in the destruction of β cells, autoreactive IGRP-specific CD8 T cell clones from a T1D donor engrafted into NSG-HLA-A2 transgenic mice displayed intra-islet infiltration of IGRP CD8+ T cells and some islet destruction (131). While all of these models were able to induce some degree of insulitis, there was no development of overt diabetes.
Coxsackie virus has long been suspected to be one of the inciting triggers for development of T1D. To begin to investigate possible mechanisms by which Coxsackie infection could trigger T1D, human islets were infected in vitro with Coxsackie virus, and it was demonstrated that the virus could directly infect human β cells, a mechanism of how the virus could detrimentally affect human β cells not previously recognized (132). When NSG mice were transplanted with human islets and infected with Coxsackie virus, almost half of the mice reverted to a hyperglycemic state and a human-specific gene expression of profiles in grafts from infected mice showed the induction of interferon signature genes, suggesting that the infected human islets may have a contributing role in their ultimate destruction by an autoimmune response (132).
The next steps towards creation of a humanized mouse model of T1D could involve emerging iPS technology. In this system, iPS cells would be generated from T1D donors, and then differentiated into iPS-derived β cells (which has been achieved (133), HSCs, and thymic epithelium (ongoing work in a number of laboratories). Reconstituting humanized mice with these three critical populations, all from T1D donors, could recapitulate the disease process and provide a model for the study of T1D pathogenesis. Of interest using this approach is the question of immunogenicity of the iPS-derived β cells. NSG-BLT mice engrafted with iPS cells that formed teratomas demonstrated that some, but not all autologous differentiated tissues induced an immunogeneic reaction (134). This suggests that humanized mice may be a model to identify the differentiated autologous tissues that will be tolerated or will induce an immune response as iPS-derived tissues enter the clinic.
Systemic Lupus Erythematosus (SLE)
SLE is a highly variable autoimmune disorder that attacks the heart, lungs, liver, kidneys, skin, nervous system, joints and blood vessels making diagnosis difficult in some individuals. There is currently no cure and hallmarks of the disease include anti-nuclear antibodies and immune complexes in the kidneys.
PBMCs from SLE donors were injected into BRG mice with engraftment varying between 20%–80% (135). This model recapitulated many features of SLE, including skewed CD4 to CD8 T cell ratio, proteinuria, IgG immune complexes in the kidney, as well as variable levels of anti-dsDNA and anti-ANA antibodies. Mice engrafted from one specific donor who had high anti-phospholipid antibodies presented with up to 3 times more anti-cardiolipin antibodies than mice engrafted with other SLE or healthy donors. This model may be useful to analyze the contribution of different immune cell types to SLE pathogenesis and for testing therapeutics.
Recently, the expression of the DNA-binding protein ARID3a in HSCs has been linked to the severity of SLE in the hu-SRC-SCID model. Mice engrafted with HSCs expressing high amounts of ARID3a were more likely to produce anti-ANAs than mice engrafted with healthy donor or ARID3a low-expressing cells (136). This work supports further analysis of ARID3a expression in the pathogenesis of SLE and the potential utility of humanized mice in identifying mechanisms important in SLE pathogenesis.
Arthritis
The association of EBV infection with rheumatoid arthritis has been exploited to induce erosive arthritis in HSC-engrafted NOG mice (137). EBV infected HSC-engrafted mice developed arthritis in their knee and ankle joints, characterized by edema, synovial proliferation, pannus formation, and immune cell infiltration as seen in human rheumatoid arthritis. However, EBV replication was not found in the affected joints, but was present in the nearby bone marrow. An induced humanized mouse model of inflammatory arthritis has been generated by injecting complete Freund’s adjuvant (CFA) into joints of HSC-engrafted NSG mice. Injected joints exhibited human immune cell localization, edema, and bone damage (138). A drug used for rheumatoid arthritis, Etanercept, attenuated the arthritis symptoms.
Chronic Inflammation (Ulcerative Colitis/Atopic Dermatitis/Vasculitis)
Oxazolone-induced colitis is used in mouse models to study inflammatory bowel diseases (IBDs). To broaden the use of this tool, oxazolone was used in the Hu-PBL-SCID model to create a model of ulcerative colitis (UC) (139). PBMCs from UC and atopic dermatitis (AD) patients were engrafted into NSG mice. No inflammatory symptoms were observed unless these mice were also sensitized and then challenged with oxazolone. In UC or AD PBMC-engrafted NSG mice, but not healthy donors, ethanol controls also developed UC symptoms indicating that the immune cells from afflicted patients lowered the threshold needed to induce inflammation. This model will be of use to study T cell migration to sites of inflammation and therapeutics targeting lymphocyte infiltration in UC (139).
Oxazolone administered to the skin of PBMC-engrafted NSG mice from AD donors developed keratosis and hyperplasia, characterized by infiltration of human CD45+ leukocytes to the dermis and epidermis and an increase of IgE in serum. As noted above, when engrafted with AD donor PBMCs but not healthy donor cells, treatment with ethanol vehicle alone was enough to induce AD, showing that the AD T cells are primed to react regardless of the stimulus provided (139).
Allergic Inflammation
Gut inflammation was recently assessed in a Hu-PBL-SCID NSG model where PBMCs from donors with known allergies were used for engraftment. Mice injected with PBMCs from allergic donors then challenged with specific allergens developed intestinal wall thickening and leukocyte infiltration that was mediated by human IgE production. Blocking IgE with the anti-IgE antibody omalizumab reduced gut inflammation. Co-transfer of allergic PBMC and the patient’s own Tregs blocked IgE production (140). Additionally, ex vivo-activated Tregs expressing high levels of glycoprotein A repetitions predominant (GARP) protein further reduced gut inflammation. Blocking GARP resulted in the return of gut inflammation symptoms. This study showed the utility of the hu-PBMC-SCID model to investigate the role of Tregs in allergic disease and the ability to assess therapeutics (140).
Similarly, NSG mice engrafted with PBMCs from donors allergic to birch pollen when challenged developed airway inflammation (141). Airway inflammation was driven by CD4 T cells, and treatment with soluble recombinant HIV-1 gp120, a high affinity CD4 ligand known to activate Tregs prior to allergenic challenge protected mice (141).
Allergic Anaphylaxis
Mast cells are important mediators of allergic diseases. One limitation of the NSG humanized mouse models (PBL, SRC, and BLT) is the limited development of the myeloid lineages (4). To overcome this limitation, when NOG mice transgenically expressing human IL-3 and GM-CSF are engrafted with HSCs, granulocytes, basophils and mast cells develop (142). Sensitization with anti-NP-IgE and challenge with NP-BSA induced a local inflammatory response as well as a passive cutaneous anaphylaxis (PCA) reaction. When mice were sensitized with sera from patients that were allergic to Japanese cedar pollen and then challenged with antigen, they developed a robust PCA reaction.
A second model has been developed that exhibits increased numbers of human mast cell engraftment. NSG mice expressing human transgenes for SCF, GM-CSF, and IL-3 were used to generate NSG-SGM3 BLT mice (143). The human mast cells in the NSG-SGM3 BLT mice were functionally and phenotypically similar to primary human mast cells. Sensitization with chimeric human (ch)IgE-anti-NP mAb induced a PCA reaction upon NP-BSA challenge in NSG-SGM3 BLT mice but not NSG BLT mice, which develop few human mast cells, providing support for the PCA reaction being human mast cell-mediated. Additionally, NSG-SGM3 BLT mice were able to undergo a passive systemic anaphylaxis (PSA) response, the first to be reported in humanized mice. NSG-SGM3 BLT mice sensitized by IV injection of chIgE-anti-NP mAb and then challenged intravenously with NP-BSA exhibited PSA reactions ranging from mild to fatal (143). The ability of this model to develop functional human mast cells will allow it to be used to study the role of mast cells in contexts other than allergic responses such as immune responses to infections. Additionally, these mice can be used to generate large numbers of human mast cells to use for in vitro experiments.
Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome
In an effort to overcome one limitation of an incomplete adaptive immune response in NSG humanized mouse models, Goettel et al. developed an NSG strain lacking mouse MHC class II and expressing human HLA class II DR-1 molecule (termed NSGAB0 DR-1 mice). When engrafted with healthy HSCs, NSGAB0 DR-1 mice displayed increased T cell and monocyte engraftment (144). NSGAB0 DR-1 mice engrafted with CD34+ HSCs from an IPEX patient led to defects in the development of FOXP3+ Tregs, decreased weight, and increased mortality within 8 weeks of engraftment. The IPEX HSC-engrafted NSGAB0 DR-1 mice were characterized by splenomegaly and exhibited hCD45+ cells infiltration into the lung, liver, and colon as well as increased autoantibody production (152).
REMAINING LIMITATIONS AND FUTURE DIRECTIONS
There are several limitations associated with humanized mouse models that are currently being addressed (Table 2). The residual murine innate immune system continues to impede human cell engraftment. To address this, various knockout strains are being created to further reduce murine innate immunity including approaches to cripple granulocyte and macrophage function. Following human HSC engraftment, differentiation and maturation of human immune cells are impaired due to species-specific differences between human and mouse cytokines. To address this, many human cytokines are being provided transgenically or using knockin approaches. One potential problem with the transgenic approach is the interference of the murine cytokine, which may bind but not signal, the human cell target. Therefore, many of the transgenic mice created expressing human molecules will likely need to also target the murine cytokine gene counterpart. A continuing limitation is the poorly organized secondary lymphoid structures that likely contribute to limitations in humoral responses, including impaired class switching and affinity maturation following immunization. To address this, approaches such as attempts to enhance lymphoid tissue inducer cell development without permitting signaling through the IL2rg receptor are underway. Several modifications have also been made to the existing strains by knocking out mouse MHC class I and class II genes to reduce the development of GVHD when human PBMC are engrafted as their primary xenotarget is the mouse MHC. The overall result is an increasing array of improved humanized mice with higher human immune cell engraftment levels and enhanced functionality that are becoming available to the scientific community [reviewed in (3, 4, 7)].
Table 2.
Remaining challenges and opportunities to address these challenges
| Challenge | Opportunity |
|---|---|
| Many mouse cytokines do not fully support human lymphoid and myeloid development | Generate human cytokine transgenic and knockout mice, delivery of cytokines using viral vectors |
| Human mature T cell engraftment results in xenogeneic GVHD | Recipient MHC class I/II knockouts |
| Thymus education of human T cells occurs in the context of mouse MHC molecules resulting in H2-restricted human T cells | HLA class I and II transgenic mice in Hu-SRC-SCID model or use BLT model |
| Sensitivity to genotoxic drugs and radiation therapy | Use of Rag1null or Rag2null mice rather than Prkdcscid mice |
| Absence of hemolytic complement in immunodeficient mice on a NOD background due to lack of functional C5 | Replace defective NOD Hc gene with functional C5 complement gene |
| Remaining host innate immunity | Further genomic editing to knockout genes contributing to innate immunity |
| Impaired humoral immune responses | Provide human growth factors needed for optimal T cell, B cell, and APC development and function Devise approaches for optimizing lymph node development and lymphoid architecture in IL2rgnull mice |
| Distinguish human from mouse stroma in recipients of human tissue grafts | eGFP transgenic mice |
SUMMARY POINTS.
Humanized mice are being increasingly used as preclinical models in multiple biological fields including infectious diseases, immunology, cancer, regenerative medicine, hematology, and autoimmunity.
Development of immunodeficient IL2rgnull mice in the early 2000’s was a major breakthrough permitting the study of functional human cells, tissues and tumors in vivo in a small animal model
Immunodeficient IL2rgnull mice engrafted with human peripheral blood leukocytes permit the study of mature human T cells in vivo
Immunodeficient IL2rgnull mice engrafted with human hematopoietic stem cells generate functional human immune systems
Most primary human tumors can be engrafted into immunodeficient IL2rgnull mice permitting the in vivo study of human tumor biology and provides a platform for the in vivo assessment of drugs on patient-derived xenograft (PDX) tumors
Immunodeficient IL2rgnull mice can be engrafted with PDX tumors and complete human immune systems permitting the investigation of human tumor/immune interactions in vivo
Immunodeficient IL2rgnull mice engrafted with human hematopoietic stem cells have permitted the development of small animal models for the study of human-specific infectious agents and are the only small animal models for agents such as HIV and Salmonella typhi
FUTURE ISSUES.
Further reduction of host innate immunity is required for optimal engraftment of functional human cells, tissues, and immune systems and expression of human molecules such as HLA alleles matched to the donor human cells are needed for appropriate human immune development and function
Approaches to permit development of lymphoid architecture such as lymph nodes with germinal centers in immunodeficient IL2rgnull mice engrafted with human hematopoietic stem cells are required for development of a fully functional human immune response
Continued need to identify and replace human-specific factors that are absent in mice but are needed for optimal human cell differentiation and function
Validation of the utility of the PDX and PDX/immune models for faithfully recapitulating the interactions of tumors and immune systems in patients
Approaches to decrease or prevent the development of graft-versus-host disease that occurs in many of the human immune engrafted models
Acknowledgments
We acknowledge the excellent work by a number of investigators in this field that could not be cited due to space limitations. This work was supported in part by NIH grants UC4 DK104218, OD018259 and CA034196 and by grants from the Maine Cancer Foundation and the Raymond and Beverly Sackler Foundation.
Footnotes
DISCLOSURE STATEMENT
Michael Brehm and Dale Greiner receive research support from and are consultants for The Jackson Laboratory. Michael Brehm, Dale Greiner, and Leonard Shultz are consultants for Allakos, Inc.
LITERATURE CITED
- 1.Zschaler J, Schlorke D, Arnhold J. Differences in innate immune response between man and mouse. Crit Rev Immunol. 2014;34:433–54. [PubMed] [Google Scholar]
- 2.Ai M, Curran MA. Immune checkpoint combinations from mouse to man. Cancer Immunol Immunother. 2015;64:885–92. doi: 10.1007/s00262-014-1650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brehm MA, Bortell R, Verma M, Shultz LD, Greiner DL. Humanized Mice in Translational Immunology. In: Tan SL, editor. Translational Immunology: Mechanisms and Pharmacological Approaches. Elsevier; 2016. pp. 285–326. [Google Scholar]
- 4.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12:786–98. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–30. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 6.Theocharides AP, Rongvaux A, Fritsch K, Flavell RA, Manz MG. Humanized hemato-lymphoid system mice. Haematologica. 2016;101:5–19. doi: 10.3324/haematol.2014.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akkina R, Allam A, Balazs AB, Blankson JN, Burnett JC, et al. Improvements and Limitations of Humanized Mouse Models for HIV Research: NIH/NIAID “Meet the Experts” 2015 Workshop Summary. AIDS Res Hum Retroviruses. 2016;32:109–19. doi: 10.1089/aid.2015.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King MA, Covassin L, Brehm MA, Racki W, Pearson T, et al. Hu-PBL-NOD-scid IL2rgnull mouse model of xenogeneic graft-versus-host-like disease and the role of host MHC. Clinical & Experimental Immunology. 2009;157:104–18. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe Y, Takahashi T, Okajima A, Shiokawa M, Ishii N, et al. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/gammac(null) (NOG) mice (hu-HSC NOG mice) Int Immunol. 2009;21:843–58. doi: 10.1093/intimm/dxp050. [DOI] [PubMed] [Google Scholar]
- 10.Halkias J, Yen B, Taylor KT, Reinhartz O, Winoto A, et al. Conserved and divergent aspects of human T-cell development and migration in humanized mice. Immunol Cell Biol. 2015;93:716–26. doi: 10.1038/icb.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12:1316–22. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 12.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–92. doi: 10.1182/blood-2005-11-4388. Ref 11 and 12 describe the development of the BLT model, the most robust human immune system engraftment model available. [DOI] [PubMed] [Google Scholar]
- 13.Policicchio BB, Pandrea I, Apetrei C. Animal Models for HIV Cure Research. Front Immunol. 2016;7:12. doi: 10.3389/fimmu.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denton PW, Sogaard OS, Tolstrup M. Using animal models to overcome temporal, spatial and combinatorial challenges in HIV persistence research. J Transl Med. 2016;14:44. doi: 10.1186/s12967-016-0807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veselinovic M, Charlins P, Akkina R. Modeling HIV-1 Mucosal Transmission and Prevention in Humanized Mice. Methods Mol Biol. 2016;1354:203–20. doi: 10.1007/978-1-4939-3046-3_14. [DOI] [PubMed] [Google Scholar]
- 16.Gaska JM, Ploss A. Study of viral pathogenesis in humanized mice. Curr Opin Virol. 2015;11:14–20. doi: 10.1016/j.coviro.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karpel ME, Boutwell CL, Allen TM. BLT humanized mice as a small animal model of HIV infection. Curr Opin Virol. 2015;13:75–80. doi: 10.1016/j.coviro.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada E, Yoshikawa R, Nakano Y, Misawa N, Koyanagi Y, Sato K. Impacts of humanized mouse models on the investigation of HIV-1 infection: illuminating the roles of viral accessory proteins in vivo. Viruses. 2015;7:1373–90. doi: 10.3390/v7031373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akkina R. New generation humanized mice for virus research: comparative aspects and future prospects. Virology. 2013;435:14–28. doi: 10.1016/j.virol.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choudhary SK, Archin NM, Cheema M, Dahl NP, Garcia JV, Margolis DM. Latent HIV-1 infection of resting CD4 T cells in the humanized Rag2/gammac/mouse. J Virol. 2012;86:114–20. doi: 10.1128/JVI.05590-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honeycutt JB, Wahl A, Archin N, Choudhary S, Margolis D, Garcia JV. HIV-1 infection, response to treatment and establishment of viral latency in a novel humanized T cell-only mouse (TOM) model. Retrovirology. 2013;10:121. doi: 10.1186/1742-4690-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsden MD, Kovochich M, Suree N, Shimizu S, Mehta R, et al. HIV latency in the humanized BLT mouse. J Virol. 2012;86:339–47. doi: 10.1128/JVI.06366-11. Ref 2021 and 22 describe humanized mouse latency models for HIV, a critical tool needed for identification of treatments that can eliminate this reservoir of HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honeycutt JB, Sheridan PA, Matsushima GK, Garcia JV. Humanized mouse models for HIV-1 infection of the CNS. J Neurovirol. 2015;21:301–9. doi: 10.1007/s13365-014-0299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sewald X, Ladinsky MS, Uchil PD, Beloor J, Pi R, et al. Retroviruses use CD169-mediated trans-infection of permissive lymphocytes to establish infection. Science. 2015;350:563–7. doi: 10.1126/science.aab2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olesen R, Swanson MD, Kovarova M, Nochi T, Chateau M, et al. ART influences HIV persistence in the female reproductive tract and cervicovaginal secretions. J Clin Invest. 2016 doi: 10.1172/JCI64212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt N, Wang J, Kim K, Friedman G, Wang X, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28:839–47. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myburgh R, Ivic S, Pepper MS, Gers-Huber G, Li D, et al. Lentivector Knockdown of CCR5 in Hematopoietic Stem and Progenitor Cells Confers Functional and Persistent HIV-1 Resistance in Humanized Mice. J Virol. 2015;89:6761–72. doi: 10.1128/JVI.00277-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kordelas L, Verheyen J, Beelen DW, Horn PA, Heinold A, et al. Shift of HIV tropism in stem-cell transplantation with CCR5 Delta32 mutation. N Engl J Med. 2014;371:880–2. doi: 10.1056/NEJMc1405805. [DOI] [PubMed] [Google Scholar]
- 30.Burke BP, Levin BR, Zhang J, Sahakyan A, Boyer J, et al. Engineering Cellular Resistance to HIV-1 Infection In Vivo Using a Dual Therapeutic Lentiviral Vector. Mol Ther Nucleic Acids. 2015;4:e236. doi: 10.1038/mtna.2015.10. [DOI] [PubMed] [Google Scholar]
- 31.Hur EM, Patel SN, Shimizu S, Rao DS, Gnanapragasam PN, et al. Inhibitory effect of HIV-specific neutralizing IgA on mucosal transmission of HIV in humanized mice. Blood. 2012;120:4571–82. doi: 10.1182/blood-2012-04-422303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer BE, Neff CP, Lecureux J, Ehler A, Dsouza M, et al. In vivo blockade of the PD-1 receptor suppresses HIV-1 viral loads and improves CD4+ T cell levels in humanized mice. J Immunol. 2013;190:211–9. doi: 10.4049/jimmunol.1201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seung E, Dudek TE, Allen TM, Freeman GJ, Luster AD, Tager AM. PD-1 blockade in chronically HIV-1-infected humanized mice suppresses viral loads. PLoS ONE. 2013;8:e77780. doi: 10.1371/journal.pone.0077780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halper-Stromberg A, Lu CL, Klein F, Horwitz JA, Bournazos S, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158:989–99. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481:81–4. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox J, Mota J, Sukupolvi-Petty S, Diamond MS, Rico-Hesse R. Mosquito bite delivery of dengue virus enhances immunogenicity and pathogenesis in humanized mice. J Virol. 2012;86:7637–49. doi: 10.1128/JVI.00534-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frias-Staheli N, Dorner M, Marukian S, Billerbeck E, Labitt RN, et al. Utility of humanized BLT mice for analysis of dengue virus infection and antiviral drug testing. J Virol. 2014;88:2205–18. doi: 10.1128/JVI.03085-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaiswal S, Pazoles P, Woda M, Shultz LD, Greiner DL, et al. Enhanced humoral and HLA-A2-restricted dengue virus-specific T-cell responses in humanized BLT NSG mice. Immunol. 2012;136:334–43. doi: 10.1111/j.1365-2567.2012.03585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaiswal S, Pearson T, Friberg H, Shultz LD, Greiner DL, et al. Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2rgammanull mice. PLoS ONE. 2009;4:e7251. doi: 10.1371/journal.pone.0007251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaiswal S, Smith K, Ramirez A, Woda M, Pazoles P, et al. Dengue virus infection induces broadly cross-reactive human IgM antibodies that recognize intact virions in humanized BLT-NSG mice. Exp Biol Med (Maywood) 2015;240:67–78. doi: 10.1177/1535370214546273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuruvilla JG, Troyer RM, Devi S, Akkina R. Dengue virus infection and immune response in humanized RAG2(−/−)gamma(c)(−/−) (RAG-hu) mice. Virology. 2007;369:143–52. doi: 10.1016/j.virol.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 42.McCarthy M. WHO sets out $56m Zika virus response plan. BMJ. 2016;352:i1042. doi: 10.1136/bmj.i1042. [DOI] [PubMed] [Google Scholar]
- 43.Taylor GS, Long HM, Brooks JM, Rickinson AB, Hislop AD. The immunology of Epstein-Barr virus-induced disease. Annu Rev Immunol. 2015;33:787–821. doi: 10.1146/annurev-immunol-032414-112326. [DOI] [PubMed] [Google Scholar]
- 44.Fujiwara S, Imadome K, Takei M. Modeling EBV infection and pathogenesis in new-generation humanized mice. Exp Mol Med. 2015;47:e135. doi: 10.1038/emm.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gujer C, Chatterjee B, Landtwing V, Raykova A, McHugh D, Munz C. Animal models of Epstein Barr virus infection. Curr Opin Virol. 2015;13:6–10. doi: 10.1016/j.coviro.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Strowig T, Gurer C, Ploss A, Liu YF, Arrey F, et al. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J Exp Med. 2009;206:1423–34. doi: 10.1084/jem.20081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, et al. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci U S A. 2010;107:13022–7. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chijioke O, Muller A, Feederle R, Barros MH, Krieg C, et al. Human natural killer cells prevent infectious mononucleosis features by targeting lytic Epstein-Barr virus infection. Cell Rep. 2013;5:1489–98. doi: 10.1016/j.celrep.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu CI, Becker C, Wang Y, Marches F, Helft J, et al. Human CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF-beta. Immunity. 2013;38:818–30. doi: 10.1016/j.immuni.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willinger T, Rongvaux A, Takizawa H, Yancopoulos GD, Valenzuela DM, et al. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proc Natl Acad Sci U S A. 2011;108:2390–5. doi: 10.1073/pnas.1019682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Chen Q, Zheng D, Yin L, Chionh YH, et al. Induction of functional human macrophages from bone marrow promonocytes by M-CSF in humanized mice. J Immunol. 2013;191:3192–9. doi: 10.4049/jimmunol.1300742. [DOI] [PubMed] [Google Scholar]
- 52.Wang LX, Kang G, Kumar P, Lu W, Li Y, et al. Humanized-BLT mouse model of Kaposi’s sarcoma-associated herpesvirus infection. Proc Natl Acad Sci U S A. 2014;111:3146–51. doi: 10.1073/pnas.1318175111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Libby SJ, Brehm MA, Greiner DL, Shultz LD, McClelland M, et al. Humanized nonobese diabetic-scid IL2rgammanull mice are susceptible to lethal Salmonella Typhi infection. Proc Natl Acad Sci U S A. 2010;107:15589–94. doi: 10.1073/pnas.1005566107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Firoz MM, Pek EA, Chenoweth MJ, Ashkar AA. Humanized mice are susceptible to Salmonella typhi infection. Cell Mol Immunol. 2011;8:83–7. doi: 10.1038/cmi.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song J, Willinger T, Rongvaux A, Eynon EE, Stevens S, et al. A mouse model for the human pathogen Salmonella typhi. Cell Host Microbe. 2010;8:369–76. doi: 10.1016/j.chom.2010.09.003. Ref 53, 54, and 55 are the first animal models for Salmonella typhi infection that have been described. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heuts F, Gavier-Widen D, Carow B, Juarez J, Wigzell H, Rottenberg ME. CD4+ cell-dependent granuloma formation in humanized mice infected with mycobacteria. Proc Natl Acad Sci U S A. 2013;110:6482–7. doi: 10.1073/pnas.1219985110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calderon VE, Valbuena G, Goez Y, Judy BM, Huante MB, et al. A humanized mouse model of tuberculosis. PLoS ONE. 2013;8:e63331. doi: 10.1371/journal.pone.0063331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee J, Brehm MA, Greiner D, Shultz LD, Kornfeld H. Engrafted human cells generate adaptive immune responses to Mycobacterium bovis BCG infection in humanized mice. BMC Immunol. 2013;14:53. doi: 10.1186/1471-2172-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ludtke A, Oestereich L, Ruibal P, Wurr S, Pallasch E, et al. Ebola virus disease in mice with transplanted human hematopoietic stem cells. J Virol. 2015;89:4700–4. doi: 10.1128/JVI.03546-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bird BH, Spengler JR, Chakrabarti AK, Khristova ML, Sealy TK, et al. Humanized Mouse Model of Ebola Virus Disease Mimics the Immune Responses in Human Disease. J Infect Dis. 2015;213:703–11. doi: 10.1093/infdis/jiv538. Ref 59 and 60 describe the development of highly needed small animal models for Ebola virus that will permit rapid evaluation of therapies for Ebola infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobak L, Raftery MJ, Voigt S, Kuhl AA, Kilic E, et al. Hantavirus-induced pathogenesis in mice with a humanized immune system. J Gen Virol. 2015;96:1258–63. doi: 10.1099/vir.0.000087. [DOI] [PubMed] [Google Scholar]
- 62.Siu E, Ploss A. Modeling malaria in humanized mice: opportunities and challenges. Ann N Y Acad Sci. 2015;1342:29–36. doi: 10.1111/nyas.12618. [DOI] [PubMed] [Google Scholar]
- 63.Good MF, Hawkes MT, Yanow SK. Humanized Mouse Models to Study Cell-Mediated Immune Responses to Liver-Stage Malaria Vaccines. Trends Parasitol. 2015;31:583–94. doi: 10.1016/j.pt.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 64.Jimenez-Diaz MB, Mulet T, Viera S, Gomez V, Garuti H, et al. Improved murine model of malaria using Plasmodium falciparum competent strains and non-myelodepleted NOD-scid IL2Rgammanull mice engrafted with human erythrocytes. Antimicrob Agents Chemother. 2009;53:4533–6. doi: 10.1128/AAC.00519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soulard V, Bosson-Vanga H, Lorthiois A, Roucher C, Franetich JF, et al. Plasmodium falciparum full life cycle and Plasmodium ovale liver stages in humanized mice. Nat Commun. 2015;6:7690. doi: 10.1038/ncomms8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vaughan AM, Mikolajczak SA, Wilson EM, Grompe M, Kaushansky A, et al. Complete Plasmodium falciparum liver-stage development in liver-chimeric mice. J Clin Invest. 2012;122:3618–28. doi: 10.1172/JCI62684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vaughan AM, Pinapati RS, Cheeseman IH, Camargo N, Fishbaugher M, et al. Plasmodium falciparum genetic crosses in a humanized mouse model. Nat Methods. 2015;12:631–3. doi: 10.1038/nmeth.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foquet L, Meuleman P, Hermsen CC, Sauerwein R, Leroux-Roels G. Assessment of Parasite Liver-Stage Burden in Human-Liver Chimeric Mice. Methods Mol Biol. 2015;1325:59–68. doi: 10.1007/978-1-4939-2815-6_5. [DOI] [PubMed] [Google Scholar]
- 69.Ernst W, Zimara N, Hanses F, Mannel DN, Seelbach-Gobel B, Wege AK. Humanized mice, a new model to study the influence of drug treatment on neonatal sepsis. Infect Immun. 2013;81:1520–31. doi: 10.1128/IAI.01235-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Unsinger J, McDonough JS, Shultz LD, Ferguson TA, Hotchkiss RS. Sepsis-induced human lymphocyte apoptosis and cytokine production in “humanized” mice. J Leukoc Biol. 2009;86:219–27. doi: 10.1189/jlb.1008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skirecki T, Kawiak J, Machaj E, Pojda Z, Wasilewska D, et al. Early severe impairment of hematopoietic stem and progenitor cells from the bone marrow caused by CLP sepsis and endotoxemia in a humanized mice model. Stem Cell Res Ther. 2015;6:142. doi: 10.1186/s13287-015-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ye C, Choi JG, Abraham S, Wu H, Diaz D, et al. Human macrophage and dendritic cell-specific silencing of high-mobility group protein B1 ameliorates sepsis in a humanized mouse model. Proc Natl Acad Sci U S A. 2012;109:21052–7. doi: 10.1073/pnas.1216195109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng L, Li F, Bility MT, Murphy CM, Su L. Modeling hepatitis B virus infection, immunopathology and therapy in mice. Antiviral Res. 2015;121:1–8. doi: 10.1016/j.antiviral.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.von SM, Ding Q, Ploss A. Visualizing hepatitis C virus infection in humanized mice. J Immunol Methods. 2014;410:50–9. doi: 10.1016/j.jim.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li S, Ling C, Zhong L, Li M, Su Q, et al. Efficient and Targeted Transduction of Nonhuman Primate Liver With Systemically Delivered Optimized AAV3B Vectors. Mol Ther. 2015 doi: 10.1038/mt.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, et al. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–33. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- 77.Bissig KD, Wieland SF, Tran P, Isogawa M, Le TT, et al. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J Clin Invest. 2010;120:924–30. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Washburn ML, Bility MT, Zhang L, Kovalev GI, Buntzman A, et al. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology. 2011;140:1334–44. doi: 10.1053/j.gastro.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mailly L, Xiao F, Lupberger J, Wilson GK, Aubert P, et al. Clearance of persistent hepatitis C virus infection in humanized mice using a claudin-1-targeting monoclonal antibody. Nat Biotechnol. 2015;33:549–54. doi: 10.1038/nbt.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vercauteren K, Van Den EN, Mesalam AA, Belouzard S, Catanese MT, et al. Successful anti-scavenger receptor class B type I (SR-BI) monoclonal antibody therapy in humanized mice after challenge with HCV variants with in vitro resistance to SR-BI-targeting agents. Hepatology. 2014;60:1508–18. doi: 10.1002/hep.27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Jong YP, Dorner M, Mommersteeg MC, Xiao JW, Balazs AB, et al. Broadly neutralizing antibodies abrogate established hepatitis C virus infection. Sci Transl Med. 2014;6:254ra129. doi: 10.1126/scitranslmed.3009512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Z, Wu N, Tesfaye A, Feinstone S, Kumar A. HCV infection-associated hepatocellular carcinoma in humanized mice. Infect Agent Cancer. 2015;10:24. doi: 10.1186/s13027-015-0018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Allweiss L, Volz T, Lutgehetmann M, Giersch K, Bornscheuer T, et al. Immune cell responses are not required to induce substantial hepatitis B virus antigen decline during pegylated interferon-alpha administration. J Hepatol. 2014;60:500–7. doi: 10.1016/j.jhep.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 84.Bility MT, Cheng L, Zhang Z, Luan Y, Li F, et al. Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog. 2014;10:e1004032. doi: 10.1371/journal.ppat.1004032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Giersch K, Allweiss L, Volz T, Helbig M, Bierwolf J, et al. Hepatitis Delta co-infection in humanized mice leads to pronounced induction of innate immune responses in comparison to HBV mono-infection. J Hepatol. 2015;63:346–53. doi: 10.1016/j.jhep.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 86.Allweiss L, Gass S, Giersch K, Groth A, Kah J, et al. Human liver chimeric mice as a new model of chronic hepatitis E virus infection and preclinical drug evaluation. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 87.Xu D, Michie SA, Zheng M, Takeda S, Wu M, Peltz G. Humanized thymidine kinase-NOG mice can be used to identify drugs that cause animal-specific hepatotoxicity: a case study with furosemide. J Pharmacol Exp Ther. 2015;354:73–8. doi: 10.1124/jpet.115.224493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang RR, Zheng YW, Li B, Tsuchida T, Ueno Y, et al. Human hepatic stem cells transplanted into a fulminant hepatic failure Alb-TRECK/SCID mouse model exhibit liver reconstitution and drug metabolism capabilities. Stem Cell Res Ther. 2015;6:49. doi: 10.1186/s13287-015-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu D, Wu M, Nishimura S, Nishimura T, Michie SA, et al. Chimeric TK-NOG mice: a predictive model for cholestatic human liver toxicity. J Pharmacol Exp Ther. 2015;352:274–80. doi: 10.1124/jpet.114.220798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shultz LD, Goodwin N, Ishikawa F, Hosur V, Lyons BL, Greiner DL. Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb Protoc. 2014;2014:694–708. doi: 10.1101/pdb.top073585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shankavaram UT, Bredel M, Burgan WE, Carter D, Tofilon P, Camphausen K. Molecular profiling indicates orthotopic xenograft of glioma cell lines simulate a subclass of human glioblastoma. J Cell Mol Med. 2012;16:545–54. doi: 10.1111/j.1582-4934.2011.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosfjord E, Lucas J, Li G, Gerber HP. Advances in patient-derived tumor xenografts: from target identification to predicting clinical response rates in oncology. Biochem Pharmacol. 2014;91:135–43. doi: 10.1016/j.bcp.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 93.Maykel J, Liu JH, Li H, Shultz LD, Greiner DL, Houghton J. NOD-scidIl2rg (tm1Wjl) and NOD-Rag1 (null) Il2rg (tm1Wjl): a model for stromal cell-tumor cell interaction for human colon cancer. Dig Dis Sci. 2014;59:1169–79. doi: 10.1007/s10620-014-3168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cassidy JW, Caldas C, Bruna A. Maintaining Tumor Heterogeneity in Patient-Derived Tumor Xenografts. Cancer Res. 2015;75:2963–8. doi: 10.1158/0008-5472.CAN-15-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wege AK, Ernst W, Eckl J, Frankenberger B, Vollmann-Zwerenz A, et al. Humanized tumor mice-A new model to study and manipulate the immune response in advanced cancer therapy. Int J Cancer. 2011;129:2194–206. doi: 10.1002/ijc.26159. [DOI] [PubMed] [Google Scholar]
- 96.Roth MD, Harui A. Human tumor infiltrating lymphocytes cooperatively regulate prostate tumor growth in a humanized mouse model. J Immunother Cancer. 2015;3:12. doi: 10.1186/s40425-015-0056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, et al. Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol. 2014;32:364–72. doi: 10.1038/nbt.2858. Ref 97 describes a humanized mouse model that permits study of the interaction of the human innate immune system with tumors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rezvani K, Rouce RH. The Application of Natural Killer Cell Immunotherapy for the Treatment of Cancer. Front Immunol. 2015;6:578. doi: 10.3389/fimmu.2015.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu D, Song L, Wei J, Courtney AN, Gao X, et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J Clin Invest. 2012;122:2221–33. doi: 10.1172/JCI59535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cany J, van der Waart AB, Tordoir M, Franssen GM, Hangalapura BN, et al. Natural killer cells generated from cord blood hematopoietic progenitor cells efficiently target bone marrow-residing human leukemia cells in NOD/SCID/IL2Rg(null) mice. PLoS ONE. 2013;8:e64384. doi: 10.1371/journal.pone.0064384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schilbach K, Alkhaled M, Welker C, Eckert F, Blank G, et al. Cancer-targeted IL-12 controls human rhabdomyosarcoma by senescence induction and myogenic differentiation. Oncoimmunology. 2015;4:e1014760. doi: 10.1080/2162402X.2015.1014760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morton JJ, Bird G, Keysar SB, Astling DP, Lyons TR, et al. XactMice: humanizing mouse bone marrow enables microenvironment reconstitution in a patient-derived xenograft model of head and neck cancer. Oncogene. 2016;35:290–300. doi: 10.1038/onc.2015.94. Ref 102 describes a potential breakthrough for ex vivo expansion of human HSCs for engraftment into immunodeficient mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Varga J, Lopatin M, Boden G. Hypoglycemia due to antiinsulin receptor antibodies in systemic lupus erythematosus. J Rheumatol. 1990;17:1226–9. [PubMed] [Google Scholar]
- 104.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Provasi E, Genovese P, Lombardo A, Magnani Z, Liu PQ, et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat Med. 2012;18:807–15. doi: 10.1038/nm.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–61. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hudecek M, Lupo-Stanghellini MT, Kosasih PL, Sommermeyer D, Jensen MC, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res. 2013;19:3153–64. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Casucci M, Nicolis di RB, Falcone L, Camisa B, Norelli M, et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. 2013;122:3461–72. doi: 10.1182/blood-2013-04-493361. [DOI] [PubMed] [Google Scholar]
- 109.Song DG, Powell DJ. Pro-survival signaling via CD27 costimulation drives effective CAR T-cell therapy. Oncoimmunology. 2012;1:547–9. doi: 10.4161/onci.19458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guedan S, Chen X, Madar A, Carpenito C, McGettigan SE, et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124:1070–80. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71–5. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chu Y, Hochberg J, Yahr A, Ayello J, van d V, et al. + Aggressive B-Cell Non-Hodgkin Lymphoma by Anti-CD20 CAR mRNA Modified Expanded Natural Killer Cells in vitro and in NSG Mice. Cancer Immunol Res. 2014 doi: 10.1158/2326-6066.CIR-14-0114. [DOI] [PubMed] [Google Scholar]
- 113.Urbanska K, Lanitis E, Poussin M, Lynn RC, Gavin BP, et al. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res. 2012;72:1844–52. doi: 10.1158/0008-5472.CAN-11-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Najima Y, Tomizawa-Murasawa M, Saito Y, Watanabe T, Ono R, et al. Induction of WT1-specific human CD8+ T cells from human HSCs in HLA class I Tg NOD/SCID/IL2rgKO mice. Blood. 2016;127:722–34. doi: 10.1182/blood-2014-10-604777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fisher TS, Kamperschroer C, Oliphant T, Love VA, Lira PD, et al. Targeting of 4-1BB by monoclonal antibody PF-05082566 enhances T-cell function and promotes anti-tumor activity. Cancer Immunol Immunother. 2012;61:1721–33. doi: 10.1007/s00262-012-1237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mullard A. New checkpoint inhibitors ride the immunotherapy tsunami. Nat Rev Drug Discov. 2013;12:489–92. doi: 10.1038/nrd4066. [DOI] [PubMed] [Google Scholar]
- 117.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sanmamed MF, Rodriguez I, Schalper KA, Onate C, Azpilikueta A, et al. Nivolumab and Urelumab Enhance Antitumor Activity of Human T Lymphocytes Engrafted in Rag2−/−IL2Rgammanull Immunodeficient Mice. Cancer Res. 2015;75:3466–78. doi: 10.1158/0008-5472.CAN-14-3510. [DOI] [PubMed] [Google Scholar]
- 119.Horvath C, Andrews L, Baumann A, Black L, Blanset D, et al. Storm forecasting: additional lessons from the CD28 superagonist TGN1412 trial. Nat Rev Immunol. 2012;12:740. doi: 10.1038/nri3192-c1. [DOI] [PubMed] [Google Scholar]
- 120.Brady JL, Harrison LC, Goodman DJ, Cowan PJ, Hawthorne WJ, et al. Preclinical screening for acute toxicity of therapeutic monoclonal antibodies in a hu-SCID model. Clin Transl Immunology. 2014;3:e29. doi: 10.1038/cti.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weissmuller S, Kronhart S, Kreuz D, Schnierle B, Kalinke U, et al. TGN1412 Induces Lymphopenia and Human Cytokine Release in a Humanized Mouse Model. PLoS ONE. 2016;11:e0149093. doi: 10.1371/journal.pone.0149093. Ref 120 and 121 described humanized mouse models for evaluation of a cytokine release syndrome that is critical for evaluating new drugs prior to their advance into the clinic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brehm MA, Shultz LD. Human allograft rejection in humanized mice: a historical perspective. Cell Mol Immunol. 2012 doi: 10.1038/cmi.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hogenes M, Huibers M, Kroone C, de WR. Humanized mouse models in transplantation research. Transplant Rev (Orlando) 2014;28:103–10. doi: 10.1016/j.trre.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 124.Kenney LL, Shultz LD, Greiner DL, Brehm MA. Humanized Mouse Models for Transplant Immunology. Am J Transplant. 2016;16:389–97. doi: 10.1111/ajt.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jacobson S, Heuts F, Juarez J, Hultcrantz M, Korsgren O, et al. Alloreactivity but failure to reject human islet transplants by humanized Balb/c/Rag2gc mice. Scand J Immunol. 2010;71:83–90. doi: 10.1111/j.1365-3083.2009.02356.x. [DOI] [PubMed] [Google Scholar]
- 126.Brehm MA, Bortell R, DiIorio P, Leif J, Laning J, et al. Human immune system development and rejection of human islet allografts in spontaneously diabetic NOD-Rag1null IL2rgnull Ins2Akita mice. Diabetes. 2010;59:2265–70. doi: 10.2337/db10-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xiao F, Ma L, Zhao M, Huang G, Mirenda V, et al. Ex vivo expanded human regulatory T cells delay islet allograft rejection via inhibiting islet-derived monocyte chemoattractant protein-1 production in CD34+ stem cells-reconstituted NOD-scid IL2rgammanull mice. PLoS ONE. 2014;9:e90387. doi: 10.1371/journal.pone.0090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kirkiles-Smith NC, Harding MJ, Shepherd BR, Fader SA, Yi T, et al. Development of a humanized mouse model to study the role of macrophages in allograft injury. Transplantation. 2009;87:189–97. doi: 10.1097/TP.0b013e318192e05d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Viehmann Milam AA, Maher SE, Gibson JA, Lebastchi J, Wen L, et al. A humanized mouse model of autoimmune insulitis. Diabetes. 2014;63:1712–24. doi: 10.2337/db13-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Babad J, Mukherjee G, Follenzi A, Ali R, Roep BO, et al. Generation of beta cell-specific human cytotoxic T cells by lentiviral transduction and their survival in immunodeficient human leucocyte antigen-transgenic mice. Clin Exp Immunol. 2015;179:398–413. doi: 10.1111/cei.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Unger WW, Pearson T, Abreu JR, Laban S, van der Slik AR, et al. Islet-Specific CTL Cloned from a Type 1 Diabetes Patient Cause Beta-Cell Destruction after Engraftment into HLA-A2 Transgenic NOD/SCID/IL2RG Null Mice. PLoS ONE. 2012;7:e49213. doi: 10.1371/journal.pone.0049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gallagher GR, Brehm MA, Finberg RW, Barton BA, Shultz LD, et al. Viral infection of engrafted human islets leads to diabetes. Diabetes. 2015;64:1358–69. doi: 10.2337/db14-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pagliuca FW, Millman JR, Gurtler M, Segel M, Van DA, et al. Generation of Functional Human Pancreatic beta Cells In Vitro. Cell. 2014;159:428–39. doi: 10.1016/j.cell.2014.09.040. ref 133 describes the first in vitro maturation of human pluripotent stem cells into fully functional beta cells and their ability to regulate glucose homeostasis in hyperglycemic immunodeficient mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhao T, Zhang ZN, Westenskow PD, Todorova D, Hu Z, et al. Humanized Mice Reveal Differential Immunogenicity of Cells Derived from Autologous Induced Pluripotent Stem Cells. Cell Stem Cell. 2015;17:353–9. doi: 10.1016/j.stem.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Andrade D, Redecha PB, Vukelic M, Qing X, Perino G, et al. Engraftment of peripheral blood mononuclear cells from systemic lupus erythematosus and antiphospholipid syndrome patient donors into BALB-RAG-2−/− IL-2Rgamma−/− mice: a promising model for studying human disease. Arthritis Rheum. 2011;63:2764–73. doi: 10.1002/art.30424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ratliff ML, Ward JM, Merrill JT, James JA, Webb CF. Differential expression of the transcription factor ARID3a in lupus patient hematopoietic progenitor cells. J Immunol. 2015;194:940–9. doi: 10.4049/jimmunol.1401941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kuwana Y, Takei M, Yajima M, Imadome K, Inomata H, et al. Epstein-Barr virus induces erosive arthritis in humanized mice. PLoS ONE. 2011;6:e26630. doi: 10.1371/journal.pone.0026630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Misharin AV, Haines GK, III, Rose S, Gierut AK, Hotchkiss RS, Perlman H. Development of a new humanized mouse model to study acute inflammatory arthritis. J Transl Med. 2012;10:190. doi: 10.1186/1479-5876-10-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nolte T, Zadeh-Khorasani M, Safarov O, Rueff F, Gulberg V, et al. Oxazolone and ethanol induce colitis in non-obese diabetic-severe combined immunodeficiency interleukin-2Rgamma(null) mice engrafted with human peripheral blood mononuclear cells. Clin Exp Immunol. 2013;172:349–62. doi: 10.1111/cei.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Eschborn M, Weigmann B, Reissig S, Waisman A, Saloga J, Bellinghausen I. Activated glycoprotein A repetitions predominant (GARP)-expressing regulatory T cells inhibit allergen-induced intestinal inflammation in humanized mice. J Allergy Clin Immunol. 2015;136:159–68. doi: 10.1016/j.jaci.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 141.Martin H, Reuter S, Dehzad N, Heinz A, Bellinghausen I, et al. CD4-mediated regulatory T-cell activation inhibits the development of disease in a humanized mouse model of allergic airway disease. J Allergy Clin Immunol. 2012;129:521–8. 528. doi: 10.1016/j.jaci.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 142.Ito R, Takahashi T, Katano I, Kawai K, Kamisako T, et al. Establishment of a human allergy model using human IL-3/GM-CSF-transgenic NOG mice. J Immunol. 2013;191:2890–9. doi: 10.4049/jimmunol.1203543. [DOI] [PubMed] [Google Scholar]
- 143.Bryce PJ, Falahati R, Kenney L, Leung J, Bebbington C, et al. Humanized mouse model of mast cell-mediated passive cutaneous anaphylaxis and passive systemic anaphylaxis. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.01.049. In press. Ref 142 and 143 describe the first humanized mouse models for human anaphylaxis responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goettel JA, Biswas S, Lexmond WS, Yeste A, Passerini L, et al. Fatal autoimmunity in mice reconstituted with human hematopoietic stem cells encoding defective FOXP3. Blood. 2015;125:3886–95. doi: 10.1182/blood-2014-12-618363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Melican K, Aubey F, Dumenil G. Humanized mouse model to study bacterial infections targeting the microvasculature. J Vis Exp. 2014 doi: 10.3791/51134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kwant-Mitchell A, Ashkar AA, Rosenthal KL. Mucosal innate and adaptive immune responses against herpes simplex virus type 2 in a humanized mouse model. J Virol. 2009;83:10664–76. doi: 10.1128/JVI.02584-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tanner A, Carlson SA, Nukui M, Murphy EA, Berges BK. Human herpesvirus 6A infection and immunopathogenesis in humanized Rag2(−)/(−) gammac(−)/(−) mice. J Virol. 2013;87:12020–8. doi: 10.1128/JVI.01556-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hakki M, Goldman DC, Streblow DN, Hamlin KL, Krekylwich CN, et al. HCMV infection of humanized mice after transplantation of G-CSF-mobilized peripheral blood stem cells from HCMV-seropositive donors. Biol Blood Marrow Transplant. 2014;20:132–5. doi: 10.1016/j.bbmt.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tan CS, Broge TA, Jr, Seung E, Vrbanac V, Viscidi R, et al. Detection of JC virus-specific immune responses in a novel humanized mouse model. PLoS ONE. 2013;8:e64313. doi: 10.1371/journal.pone.0064313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wege AK, Florian C, Ernst W, Zimara N, Schleicher U, et al. Leishmania major infection in humanized mice induces systemic infection and provokes a nonprotective human immune response. PLoS Negl Trop Dis. 2012;6:e1741. doi: 10.1371/journal.pntd.0001741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Arvin AM, Moffat JF, Sommer M, Oliver S, Che X, et al. Varicella-zoster virus T cell tropism and the pathogenesis of skin infection. Curr Top Microbiol Immunol. 2010;342:189–209. doi: 10.1007/82_2010_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tezuka K, Xun R, Tei M, Ueno T, Tanaka M, et al. An animal model of adult T-cell leukemia: humanized mice with HTLV-1-specific immunity. Blood. 2014;123:346–55. doi: 10.1182/blood-2013-06-508861. [DOI] [PubMed] [Google Scholar]
- 153.Valbuena G, Halliday H, Borisevich V, Goez Y, Rockx B. A human lung xenograft mouse model of Nipah virus infection. PLoS Pathog. 2014;10:e1004063. doi: 10.1371/journal.ppat.1004063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, et al. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science. 2015;348:aaa8205. doi: 10.1126/science.aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]