Abstract
Among perinatally HIV-infected (PHIV) and perinatally HIV-exposed, uninfected (PHEU) youth, we evaluated the contributions of home environment, psychosocial, and demographic factors and, among PHIV only, HIV disease severity and antiretroviral treatment (ART), to cognitive functioning (CF) and behavioral functioning (BF). A structural equation modeling (SEM) approach was utilized. Exploratory factor analysis was used to reduce predictor variables to major latent factors. SEMs were developed to measure associations between the latent factors and CF and BF outcomes. Participants included 231 PHIV and 151 PHEU youth (mean age = 10.9 years) enrolled in the PHACS adolescent master protocol. Youth and caregivers completed assessments of CF, BF, psychosocial factors and HIV health. Medical data were also collected. Clusters of predictors were identified, establishing four parsimonious SEMs: child-assessed and caregiver-assessed BF in PHIV and PHEU youth. Among both groups, higher caregiver-child stress predicted worse BF. Caregiver resources and two disease severity variables, late presenter and better past HIV health, were significant predictors of CF in PHIV youth. Higher youth CF was associated with better caregiver-reported BF in both groups. Caregiver resources predicted caregiver-reported BF in PHEU youth, which was mediated via youth CF. Among PHIV youth, better past HIV health and caregiver resources mediated the effects of CF on caregiver-assessed BF. Using SEMs, we found a deleterious impact of caregiver and child stress on BF in both groups and of HIV disease factors on the CF of PHIV youth, reinforcing the importance of early comprehensive intervention to reduce risks for impairment.
Keywords: Cognition, Behavior, HIV, Youth, Structural equation models, Antiretroviral therapy
Introduction
Despite advances in medical treatment in the era of highly active antiretroviral therapy (HAART), children and adolescents with perinatally acquired HIV infection (PHIV) and perinatal HIV exposure (PHEU) remain at-risk for impaired cognitive functioning (CF) and behavioral functioning (BF) [1, 2]; etiology of deficits are varied and complex. HIV-related cognitive dysfunction can manifest across a wide range of severity levels. Mild to moderate selective deficits, including decreased attention and concentration, reduced speed of information processing, executive dysfunction, and verbal memory impairment [3, 4], have been observed in both PHIV and PHEU youth. Global cognitive impairment has been noted among PHIV and PHEU, particularly among PHIV youth with CDC class C diagnosis (AIDS) and severe progressive encephalopathy [4]. Behavioral problems, which are more prevalent among PHIV and PHEU youth compared to the general population [5, 6], include externalizing and internalizing problems, as well as DSM-IV and DSM-V psychiatric diagnoses of depression, anxiety, and attention deficit hyperactivity disorder (ADHD) [5–14]. Behavioral problems may be exacerbated in the presence of cognitive impairment [15].
Prospective longitudinal investigations during the era of HAART have contributed to existing knowledge regarding the etiology and nature of cognitive and behavioral sequelae of HIV infection and exposure. Potential mechanisms by which HIV and/or HAART impact brain development and function include neuronal injury prior to HAART, neuronal injury secondary to inflammatory responses and neurotoxic viral effects of in utero antiretroviral exposure for PHIV and PHEU youth [1, 16]. Markers of HIV disease severity are associated with white matter microstructure changes and cognitive deficits among youth with PHIV [17].
Recent studies have identified the significant impact of psychosocial and contextual factors, such as family income, parental education, and stress, on neural plasticity and brain structure and function throughout development [18–20]. Among both PHIV and PHEU youth, poverty, limited family resources and highly stressful caregiving environments are not uncommon and may be implicated to varying degrees in the quality of cognitive and behavioral functioning and the presence and persistence of deficits or disorders [12, 21].
HIV disease-related factors, psychosocial factors and environmental characteristics have been evaluated simultaneously via multivariate modeling in an effort to identify their differential and combined impact on cognitive and behavioral outcomes. However, synthesis of findings has been difficult due to methodological differences, including the wide variation in participants and number and types of factors that were evaluated in statistical modeling. Few investigations have examined the complex interactions, multiple pathways and directionality of disease-related and psychosocial factors inherent to the longitudinal impact of HIV infection and HIV exposure on children and adolescents [2, 22]. Comparing developmental pathways of youth with PHIV and PHEU youth with in utero HIV/ARV exposure will potentially elucidate the unique contributions of HIV in the context of similar environmental risk factors and may contribute to our understanding of the service needs of youth with PHIV and a growing population of youth globally who are exposed to HIV but remain uninfected [2, 5, 6, 9, 14].
Using structural equation modeling (SEM), the purpose of this study was to evaluate the direct and indirect relationships of HIV disease, HAART therapies, home environment and psychosocial factors with both cognitive and behavioral functioning in youth with PHIV; we also evaluated the indirect effects of these factors on behavioral functioning among PHIV youth, as mediated by their effects on cognition. To more fully understand the risks associated with in utero HIV and ART exposure among PHEU youth, we examined the relationships of home environment and psychosocial factors with cognitive and behavioral functioning.
Methods
Participants
Data for these analyses were obtained from the adolescent master protocol (AMP) of the pediatric HIV/AIDS cohort study (PHACS), a prospective, longitudinal study examining the impact of perinatal HIV infection and antiretroviral (ARV) therapy on children and adolescents affected by HIV. PHIV and PHEU youth were enrolled in 15 AMP sites in urban areas of the United States including Puerto Rico. Eligibility criteria included: (1) perinatal HIV exposure, (2) age 7–<16 years at study entry, (3) English or Spanish speaking, and (4) among PHIV youth, engaged in medical care with available ARV history (see Table 1).
Table 1. Demographic and psychosocial factors by cohort for 382 youth enrolled in PHACS AMP study with perinatal HIV-infection or HIV-exposed but uninfected.
| Characteristica | HIV infection status | ||
|---|---|---|---|
|
| |||
| Perinatally HIV-infected (PHIV) (N = 231) | Perinatally HIV-exposed uninfected (PHEU) (N = 151) | Total (N = 382) | |
| Youth characteristics | |||
| Age at entry (yrs), mean (SD) | 11.3 (2.3) | 10.3 (2.2) | 10.9 (2.3) |
| Age at cognitive assessment (yrs), mean (SD) | 13.7 (2.0) | 13.0 (1.8) | 13.4 (1.9) |
| Age at behavioral assessment (yrs), mean (SD) | 15.3 (2.4) | 14.3 (2.2) | 14.9 (2.3) |
| Gender | |||
| Male | 106 (46 %) | 75 (50 %) | 181 (47 %) |
| Female | 125 (54 %) | 76 (50 %) | 201 (53 %) |
| Race/ethnicity | |||
| White non-hispanic | 14 (6 %) | 8 (5 %) | 22 (6 %) |
| Black non-hispanic | 154 (67 %) | 84 (56 %) | 238 (62 %) |
| Hispanic | 52 (23 %) | 52 (34 %) | 104 (27 %) |
| Other/not reported | 11 (5 %) | 7 (5 %) | 18 (5 %) |
| Cognitive functioning from WISC or WAIS, mean (SD) | |||
| Full scale IQ | 85.9 (15.7) | 88.2 (14.0) | 86.8 (15.1) |
| Verbal | 88.4 (15.4) | 87.9 (14.0) | 88.2 (14.9) |
| Processing speed | 88.6 (15.1) | 91.2 (13.7) | 89.6 (14.6) |
| Perceptual reasoning | 90.1 (15.1) | 92.8 (14.9) | 91.2 (15.0) |
| Working memory | 88.1 (14.9) | 90.7 (13.1) | 89.1 (14.3) |
| Behavioral functioning from BASC-2, mean (SD) | |||
| Child-reported T-scores | |||
| Personal adjustment | 51.8 (8.7) | 52.3 (8.0) | 52.0 (8.4) |
| Internalizing | 46.1 (8.9) | 45.7 (8.8) | 45.9 (8.9) |
| School problems | 48.2 (9.6) | 47.5 (9.6) | 47.9 (9.6) |
| Inattention/hyperactivity | 50.9 (11.6) | 50.6 (11.0) | 50.8 (11.4) |
| Caregiver-reported T-scores | |||
| Adaptability | 51.3 (10.7) | 47.8 (10.4) | 49.9 (10.7) |
| Internalizing | 47.7 (10.2) | 49.9 (12.2) | 48.5 (11.0) |
| Externalizing | 49.1 (10.7) | 51.9 (10.9) | 50.2 (10.8) |
| Caregiver characteristics | |||
| Caregiver WASI category | |||
| <70 | 8 (3 %) | 8 (5 %) | 16 (4 %) |
| 70– < 85 | 64 (28 %) | 47 (31 %) | 111 (29 %) |
| 85+ | 100 (43 %) | 53 (35 %) | 153 (40 %) |
| Not done | 59 (26 %) | 43 (28 %) | 102 (27 %) |
| Caregiver relationship | |||
| Single biological parent | 70 (30 %) | 88 (58 %) | 158 (41 %) |
| Both biological parents | 31 (13 %) | 33 (22 %) | 64 (17 %) |
| Other | 130 (56 %) | 30 (20 %) | 160 (42 %) |
| Marital status of caregiver | |||
| Married or widowed | 124 (53 %) | 55 (37 %) | 179 (47 %) |
| Separated/divorced | 36 (16 %) | 26 (17 %) | 62 (16 %) |
| Single, never married | 71 (31 %) | 70 (46 %) | 141 (37 %) |
| Parent-child relationship inventory (PCRI): any problem (support, involvement, communication, limit-setting) | 90 (40 %) | 57 (41 %) | 147 (40 %) |
| Caregiver is high school graduate | 175 (76 %) | 104 (69 %) | 279 (73 %) |
| Caregiver mental health problem | 58 (27 %) | 54 (38 %) | 112 (31 %) |
| Caregiver drug abuse | 5 (2 %) | 6 (4 %) | 11 (3 %) |
| # drugs used last 6 months (self-reported) | |||
| 0 | 132 (62 %) | 74 (51 %) | 206 (58 %) |
| 1 | 44 (21 %) | 36 (25 %) | 80 (22 %) |
| 2 or more | 37 (17 %) | 34 (24 %) | 71 (20 %) |
| Household characteristics | |||
| Annual household income <$20 K | |||
| Total # in household | 95 (43 %) | 99 (67 %) | 194 (52 %) |
| 1–2 | 67 (29 %) | 45 (30 %) | 112 (29 %) |
| 3–4 | 100 (43 %) | 74 (49 %) | 174 (46 %) |
| 5 or more | 64 (28 %) | 32 (21 %) | 96 (25 %) |
| # supported by household income | |||
| 1–2 | 35 (15 %) | 21 (14 %) | 56 (15 %) |
| 3–4 | 100 (43 %) | 69 (46 %) | 169 (44 %) |
| 5 or more | 96 (42 %) | 61 (40 %) | 157 (41 %) |
| # Stressful life events | |||
| 0 | 103 (45 %) | 56 (37 %) | 159 (42 %) |
| 1 | 55 (24 %) | 39 (26 %) | 94 (25 %) |
| 2–3 | 51 (22 %) | 39 (26 %) | 90 (24 %) |
| 4+ | 22 (10 %) | 17 (11 %) | 39 (10 %) |
| Life events checklist: # negative life events | |||
| 0 or less | 70 (31 %) | 32 (21 %) | 102 (27 %) |
| 1–2 | 78 (35 %) | 53 (35 %) | 131 (35 %) |
| 3–4 | 40 (18 %) | 27 (18 %) | 67 (18 %) |
| 5+ | 31 (14 %) | 37 (25 %) | 68 (18 %) |
SD standard deviation, BASC-2 behavioral assessment system for children, second edition, WISC-IV wechsler intelligence scales for children, WAIS-IV wechsler adult intelligence scales, WASI wechsler abbreviated scales of intelligence
Characteristics not available or not reported for some participants, including race/ethnicity (18), household income (12), caregiver mental health, drug abuse, and # drugs reported on the Client Diagnostic Questionnaire (25), # negative life events from the Life Events Checklist (8), and parent–child relationship inventory (18)
In order to be included in this analysis, AMP subjects were additionally required to have a valid assessment of CF at the 3 year AMP visit and a valid assessment of both child- and caregiver-reported BF within the subsequent 2 years. Each assessment is rated for validity by the centrally-trained, administering psychologist then reviewed by a quality assurance team who re-assesses appropriateness of data inclusion based on the specific research questions under study.
Procedures
AMP was approved by all sites' Institutional Review Boards and by the Harvard T.H. Chan School of Public Health. Parents, legal guardians or youth ≥18 years provided written informed consent; youth <18 years provided assent according to age and local institutional review board guidelines.
Data collection for AMP occurred at study entry and at 6 month (2007–2010) and annual (2010–2014) visits. These included physical exam, medical record review, and structured demographic interviews. Cognitive data were collected at year 3 study visit and behavioral evaluations were completed within 2 years of cognitive evaluation. Psychosocial and biomedical information from the entry visit and information regarding stressful life events from the one year visit were included in this analysis.
Measures
Predictor (exogenous) Measures
Psychosocial Factors
Psychosocial measures indicative of potential sources of familial stress and support were examined including the caregivers' relationship to the child (e.g., birth parent, adoptive parents) and marital status (e.g., married/widowed, separated/divorced, single/never married), the family's annual household income and number of family members supported by it, caregiver illicit drug use in the last 6 months, the caregiver's high school graduation status and IQ as assessed on the Wechsler Abbreviated Scale of Intelligence [23], the parent–child relationship as measured on the parent–child relationship inventory [24] and specific information related to all stressful events as assessed on the caregiver report of Quality of Life and negative life events as assessed by the child on the Life Events Checklist [25].
HIV Control (HIV Severity and Treatment)
Biomedical markers were examined to identify both past and current HIV disease status, including nadir and current CD4 %, peak and current HIV RNA plasma levels (viral loads, VL), HIV-related encephalopathy prior to entry, and prior AIDS defining condition based on center for disease control and prevention (CDC) class C criteria. Age of initiation of HAART, and specific components of the regimen (PI vs NNRTI based), were considered, as well as age at peak VL and nadir CD4 %.
Outcome (endogenous) Measures
Cognitive Functioning (CF)
The Wechsler Intelligence Scale for Children, Fourth Edition (25: WISC-IV) and the Wechsler Adult Intelligence Scale, Fourth Edition (26: WAIS-IV) were used as the primary endogenous indicators of current CF. The WAIS-IV and WISC-IV demonstrate high reliability and validity [26, 27] and are widely used for research and clinical practice with adolescents and children with HIV infection [28–32]. Each measure provides age-normed standard scores in four domains: verbal comprehension, perceptual reasoning, working memory, and processing speed (mean score = 100; SD = 15).
Behavioral Functioning (BF)
The Behavioral Assessment System for Children (33: BASC-2) was used as the primary BF outcome. The BASC-2 has demonstrated good reliability and validity [33] and has been used in both research and clinical practice for children and adolescents with HIV [5, 34–36]. The BASC-2 is comprised of four behavioral indices for the child version (youth self-report: YSR) and three behavioral indices of the adult version (parent rating scale: PRS) and includes information pertaining to internalizing and externalizing problems, school and behavior problems, and adaptive behavior. Raw scores attained are converted into age-normed standard scores for each index, with higher scores reflecting greater behavioral problems (mean = 50; SD = 10).
Statistical Analysis
We conducted an exploratory factor analysis (EFA) to reduce the large number of predictor variables to a smaller set of latent factors. We then built SEMs to measure associations between the latent factors representing meaningful clusters of predictors and the CF and BF outcomes. Exploratory factor analyses were performed separately for PHIV and PHEU youth. Pearson correlations were used for the factor analysis on CF and BF outcome variables (domain scores for CF and behavioral indices for BF). For the factor analyses on predictor variables, a mixed correlation matrix was created with Pearson correlations calculated between two continuous measures, polychoric correlations calculated between two ordinal measures, and polyserial correlations calculated for a continuous measure with an ordinal measure. All factor analyses used squared multiple correlations as prior communality estimates, the principal factor method of extraction, and an oblique varimax rotation. The number of factors retained was based on the eigenvalues, scree plot, and interpretability of the rotated factor patterns.
Based on the number and nature of latent variables identified in the factor analyses, four separate SEMs were then analyzed and included (1) child-assessed BF among PHIV youth, (2) caregiver-assessed BF among PHIV youth (3) child-assessed BF among PHEU youth, and (4) caregiver-assessed BF among PHEU youth. The structure of these SEMs is shown in Fig. 1. Factor loadings and standardized path coefficients with p-values < 0.05 were considered statistically significant. Overall SEM fit was assessed using absolute (standardized root mean square residual, SRMR), parsimony (root mean square error of approximation, RMSEA), and incremental (Bentler Comparative Fit Index, CFI) indices. Adequate fit is generally described as having a CFI greater than 0.9 and RMSEA less than 0.05. statistical analysis software (SAS) version 9.3 (SAS Institute, Inc., Cary, NC, USA) was used for all analyses.
Fig. 1.
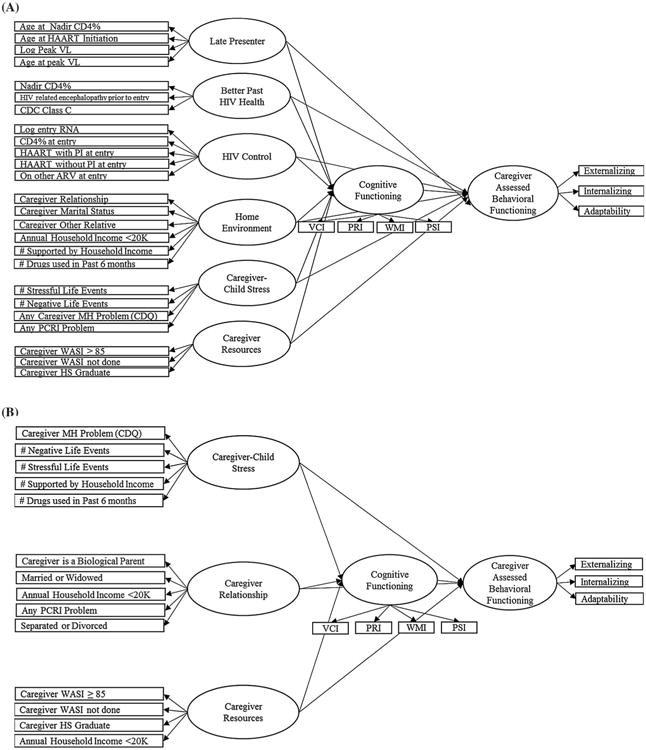
Conceptual models post-EFA. a PHIV caregiver-assessed BF Model, b PHEU caregiver-assessed BF model
Results
Study Population
Of the total 678 enrolled in AMP (see Table 1), 382 had complete data and met the stringent analyses requirements. A total of 296 subjects (43 %) could not be included in our analysis, consisting of 48 % of the 451 PHIV youth and 33 % of the PHEU youth. The majority of these (n = 182, 62 %) did not have a CF assessment at the year 3 visit, and an additional 100 youth had a CF assessment but did not have both a parent and child BF assessment completed (47 had neither, and 53 were lacking one or the other). Ten subjects had both the parent and child BASC completed, but it was not 2 years after the CF assessment. Finally, four subjects were confirmed to have an invalid assessment of either CF or BF after team review. A summary of the background demographic and psychosocial characteristics for the 382 eligible subjects is provided in Table 1, overall and by cohort. The subjects were on average 11 years old at entry (SD = 2.49), just over half female, and primarily Black, including those who self-identify as African–American, as well as other racial categories such as Black Caribbean. Those included in the analysis were younger on average than those not included, but did not differ in terms of other sociodemographic characteristics such as sex, race, caregiver makeup, ethnicity, parental education or family income. Table 2 provides a summary of HIV-related clinical and laboratory markers.
Table 2. HIV and ARV-related characteristics for youth with perinatal HIV infection.
| Characteristic | Total perinatally HIV-infected (N = 231) | |
|---|---|---|
| Measures of past disease severity | ||
| Nadir CD4 %, median (IQR) | 19 (12, 25) | |
| Age at nadir CD4 %, yrs, median (IQR) | 3.40 (1.50, 8.10) | |
| Peak HIV RNA viral load (log10 copies/mL), median (IQR) | 5.67 (5.10, 5.88) | |
| Age at peak viral load, yrs, median (IQR) | 1.80 (0.40, 4.70) | |
| CDC Class C (AIDS-defining diagnosis), N (%) | 56 (24 %) | |
| HIV-related encephalopathy prior to entry, N (%) | 19 (8 %) | |
| Age at HAART initiationa (yrs), median (IQR) | 2.20 (0.60, 4.90) | |
| Measures of disease severity at study entry | ||
| Entry CD4 %, median (IQR) | 34 (28, 39) | |
| Entry RNA viral loada (log10 copies/mL), median (IQR) | 2.34 (1.70, 2.63) | |
| Entry HIV RNA <400 copies/mL | 171 (75 %) | |
| ARV regimen at entry, N (%) | HAART with PI | 162 (70 %) |
| HAART without PI | 38 (16 %) | |
| Non-HAART ARV | 19 (8 %) | |
| Not on ARV | 12 (5 %) | |
ARV antiretroviral, CDC centers for disease control and prevention, IQR interquartile range (25th percentile, 75th percentile), HAART highly active antiretroviral therapy (defined as at least 3 ARV drugs from at least 2 drug classes), PI protease inhibitor
2 participants had no available viral load measures within 6 months prior to study entry; age at HAART initiation was not available for 3 participants
Exploratory Factor Analysis (EFA): CF and BF Outcome (Endogenous) Measures
All domains (subscales) of CF assessed on both the WISC-IV and WAIS-IV exhibited moderate correlations with each other (r = 0.36–0.66) across the PHEU, PHIV groups and overall sample. The eigenvalues and scree plot from the EFA clearly indicated that a single factor (“Cognitive Functioning”) could be retained.
Exploratory Factor Analysis (EFA): Predictor (Exogenous) Variables
Among PHIV youth, results of the EFA supported a 3-factor model for the disease severity variables, including “better past HIV health” (higher nadir CD4 %, no HIV related encephalopathy at entry, no CDC Class C diagnosis), “HIV Control (HAART with PI at study entry, HAART without PI at study entry, other ARV at study entry, lower CD4 % at entry, higher log entry RNA) and “late presenter” (age at HAART initiation, age at nadir CD4 %, age at peak VL, log peak VL). The EFA also identified three factors among the psychosocial variables: “Home Environment” (caregiver relationship to child, caregiver marital status, annual household income <$20,000, number of drugs used by caregiver in last 6 months, number supported by household income), “caregiver-child stress” (any problem as assessed on the PCRI, caregiver mental health problem as assessed on the CDQ, number of negative life events, number of stressful life events) and “caregiver resources” (intellectual and educational resources, including caregiver IQ and high school graduation status).
Among the PHEU youth, results of the EFA supported a 3-factor model: “caregiver relationship” (any problem as assessed on the PCRI, caregiver is biological parent, annual household income <$20,000, caregiver married or widowed, caregiver separated or divorced), “caregiver-child stress” (caregiver mental health problem as assessed on the CDQ, number of stressful life events, number of negative life events, number of drugs used in the last 6 months, number supported by household income), and “caregiver resources” (caregiver WASI >/equal to 85, caregiver WASI not completed, annual household income <$20,000 (double loading) and caregiver high school graduation status). The loading factors for all EFAs are summarized in Supplemental Tables 1 (PHIV) and 2 (PHEU).
SEMs for PHIV Youth
Child-reported BF model
Significant positive associations were observed for Better Past HIV Health and Caregiver Resources with higher CF (standardized coefficient = 0.40, Z = 4.45, p < 0.001 and standardized coefficient = 0.33, Z = 4.55, p < 0.001, respectively; Table 3), and a negative association observed between late presenters and CF (standardized coefficient = −0.19, Z = −2.28, p < 0.05). caregiver-child stress was the only significant predictor of child-reported BF, suggesting that higher levels of stress were associated with higher scores (worse) on BF (standardized coefficient = 0.51, Z = 3.45, p < 0.001).
Table 3. Structural equation model (SEM) direct path coefficients for perinatally HIV-infected (PHIV) and perinatally HIV-exposed uninfected (PHEU) youth in PHACS AMP study.
| Model and fit statistics | Latent predictor | Cognitive functioning coefficient (SE), Z, p value | Behavioral functioning coefficient (SE), Z, p-value |
|---|---|---|---|
| SEM models in PHIV youth, N = 231 | |||
| Model A. child-assessed behavioral functioning SRMR = 0.0814, RMSEA = 0.0800 BCF Index = 0.6127 |
Cognitive functioning | – | −0.20 (0.107), Z = −1.89, 0.06 |
| Late presenter | −0.19 (0.084), Z = −2.28, 0.02 | 0.11 (0.099), Z = 1.07, 0.28 | |
| Better past HIV health | 0.40 (0.089), Z = 4.45, < 0.001 | 0.16 (0.122), Z = 1.33, 0.18 | |
| HIV Control | 0.05 (0.098), Z = 0.54, 0.59 | 0.04 (0.112), Z = 0.38, 0.70 | |
| Home environment | 0.05 (0.100), Z = 0.47, 0.64 | −0.12 (0.116), Z = −1.01, 0.31 | |
| Caregiver-Child stress | 0.20 (0.127), Z = 1.56, 0.12 | 0.51 (0.147), Z = 3.45, < 0.001 | |
| Caregiver resources | 0.33 (0.072), Z = 4.55, < 0.001 | 0.02 (0.084), Z = 0.25, 0.80 | |
| Model B. Caregiver-assessed behavioral functioning SRMR = 0.0818, RMSEA = 0.0826 BCF Index = 0.6412 |
Cognitive functioning | – | −0.29 (0.109), Z = −2.68, 0.007 |
| Late presenter | −0.19 (0.084), Z = −2.27, 0.02 | 0.03 (0.103), Z = 0.27, 0.78 | |
| Better past HIV health | 0.39 (0.089), Z = 4.42, < 0.001 | 0.24 (0.125), Z = 1.92, 0.06 | |
| HIV Control | 0.05 (0.098), Z = 0.50, 0.61 | 0.04 (0.114), Z = 0.39, 0.70 | |
| Home environment | 0.06 (0.102), Z = 0.54, 0.59 | −0.20 (0.123), Z = −1.63, 0.10 | |
| Caregiver-Child stress | 0.18 (0.128), Z = 1.42, 0.16 | 0.50 (0.153), Z = 3.25, 0.001 | |
| Caregiver resources | 0.34 (0.072), Z = 4.68, < 0.001 | 0.15 (0.090), Z = 1.69, 0.09 | |
| SEM models in PHEU youth, N = 151 | |||
| Model C. Child-assessed behavioral functioning SRMR = 0.0809 RMSEA = 0.0634 BCF Index = 0.8282 |
Cognitive functioning | – | −0.13 (0.124), Z = −1.06, 0.29 |
| Caregiver Stress | 0.15 (0.120), Z = 1.25, 0.21 | 0.40 (0.124), Z = 3.21, 0.001 | |
| Caregiver relationship | 0.32 (0.107), Z = 3.00, 0.003 | 0.11 (0.133), Z = 0.80, 0.42 | |
| Caregiver resources | 0.37 (0.100), Z = 3.76, < 0.001 | −0.01 (0.116), Z = −0.05, 0.96 | |
| Model D. Caregiver-assessed behavioral functioning SRMR = 0.0831 RMSEA = 0.0751 BCF Index = 0.7898 |
Cognitive functioning | – | −0.54 (0.116), Z = −4.61, < 0.001 |
| Caregiver Stress | 0.12 (0.118), Z = 1.03, 0.30 | 0.58 (0.111), Z = 5.28, < 0.001 | |
| Caregiver relationship | 0.32 (0.107), Z = 2.95, 0.003 | 0.21 (0.127), Z = 1.67, 0.10 | |
| Caregiver resources | 0.38 (0.098), Z = 3.90, < 0.001 | 0.25 (0.114), Z = 2.23, 0.03 |
SE standard error, SRMR standardized root mean square residual, RMSEA root mean square error of approximation, BCF index bentler comparative fit index, = is the R2 for the behavioral functioning factor
Caregiver-Reported BF Model
Very similar associations were observed: positive associations of better past HIV health and caregiver resources with higher CF and a negative association of late presenters with CF. Caregiver-child stress was again found to be associated with higher (worse) scores for caregiver-reported BF (standardized coefficient = 0.50, Z = 3.25, p < 0.01). A negative association (standardized coefficient = −0.29, Z = −2.68, p < 0.01) was found between CF and caregiver-reported but not child-reported BF.
SEMs for PHEU Youth
Child-Reported BF Model
Caregiver-child stress was associated with child-assessed BF (standardized coefficient = 0.40, Z = 3.21, p < 0.01) indicating that higher levels of stress were associated with higher (or worse) BF scores (or worse BF). Among PHEU youth, both caregiver relationship and caregiver resources were positively associated with CF (standardized coefficient = 0.32, Z = 3.00, p < 0.01, and standardized coefficient = 0.37 Z = 3.76, p < 0.001 respectively; Table 3).
Caregiver-Reported BF Model
For caregiver-reported BF, caregiver relationship and caregiver resources were positively associated with CF, and caregiver-child stress showed a stronger association with BF (standardized coefficient = 0.58, Z = 5.28, p < 0.001) than observed with child-report. In addition, Caregiver Resources was associated with higher (worse) BF scores (standardized coefficient = 0.25; Z = 2.23, p < 0.05). Similar to PHIV youth, a negative association was observed between CF and caregiver-reported BF in PHEU youth (standardized coefficient = −0.54; Z = −4.61, p < 0.001).
Direct, Indirect and Total Effects on Behavioral Functioning
No indirect effects of the latent predictors were observed for child-reported BF among PHIV youth (see Table 4). Only caregiver-child stress had a significant direct effect on BF, which contributed to the significant total effect for this predictor (total effect = 0.47, Z = 3.29, p < 0.01). For caregiver-assessed BF, caregiver-child stress again had a direct effect on BF which contributed to the significant total effect for this latent predictor (total effect = 0.44, Z = 3.03, p < 0.01). In addition, better past HIV health and caregiver resources exhibited significant indirect effects on caregiver-reported BF (indirect effect = −0.12, Z = −2.05, p < 0.05, and indirect effect = -0.10, Z = −2.28, p < 0.05, respectively; Table 4).
Table 4. Direct, indirect and total effects on behavioral functioning from full structural equation models (SEMs) for perinatally HIV-infected (PHIV) and perinatally HIV-exposed uninfected (PHEU) youth in PHACS AMP study.
| Model | Latent predictor | Behavioral functioning coefficient (SE), Z, p-value | ||
|---|---|---|---|---|
|
| ||||
| Direct | Indirect | Total | ||
| SEM Models in PHIV+ youth, N = 231 | ||||
| Model A. Child-assessed behavioral functioning | Late presenter | 0.11 (0.099), Z = 1.07, 0.28 | 0.04 (0.027), Z = 1.44, 0.15 | 0.15 (0.095), Z = 1.51, 0.13 |
| Better past HIV health | 0.16 (0.122), Z = 1.34, 0.18 | −0.08 (0.050), Z = −1.59, 0.11 | 0.08 (0.105), Z = 0.79, 0.43 | |
| HIV control | 0.04 (0.112), Z = 0.38, 0.70 | −0.01 (0.021), Z = −0.51, 0.61 | 0.03 (0.111), Z = 0.29, 0.77 | |
| Home environment | −0.12 (0.116), Z = −1.01, 0.31 | −0.01 (0.020), Z = −0.48, 0.63 | −0.13 (0.115), Z = −1.10, 0.27 | |
| Caregiver-child stress | 0.51 (0.147), Z = 3.45, 0.001 | −0.04 (0.039), Z = −1.02, 0.31 | 0.47 (0.139), Z = 3.29, 0.001 | |
| Caregiver resources | 0.02 (0.084), Z = 0.25, 0.80 | −0.07 (0.038), Z = −1.75, 0.08 | −0.05 (0.076), Z = −0.60, 0.55 | |
| Model B. Caregiver-assessed behavioral functioning | Late presenter | 0.03 (0.103), Z = 0.27, 0.78 | 0.06 (0.033), Z = 1.68, 0.09 | 0.08 (0.098), Z = 0.86, 0.39 |
| Better past HIV health | 0.24 (0.125), Z = 1.92, 0.06 | −0.12 (0.056), Z = −2.05, 0.04 | 0.12 (0.107), Z = 1.16, 0.25 | |
| HIV control | 0.04 (0.114), Z = 0.39, 0.70 | −0.01 (0.029), Z = −0.49, 0.62 | 0.03 (0.113), Z = 0.26, 0.79 | |
| Home environment | −0.20 (0.123), Z = −1.63, 0.10 | −0.02 (0.029), Z = −0.56, 0.58 | −0.22 (0.122), Z = −1.78, 0.08 | |
| Caregiver-child stress | 0.50 (0.153), Z = 3.25, 0.001 | −0.05 (0.049), Z = −1.09, 0.28 | 0.44 (0.146), Z = 3.03, 0.002 | |
| Caregiver resources | 0.15 (0.090), Z = 1.69, 0.09 | −0.10 (0.043), Z = −2.28, 0.02 | 0.05 (0.080), Z = 0.67, 0.50 | |
| SEM models in PHEU youth, N = 151 | ||||
| Model C. Child-assessed behavioral functioning | Caregiver relationship | 0.11 (0.133), Z = 0.80, 0.42 | −0.04 (0.044), Z = −0.96, 0.34 | 0.06 (0.120), Z = 0.54, 0.59 |
| Caregiver resources | −0.01 (0.116), Z = −0.05, 0.96 | −0.05 (0.048), Z = −1.03, 0.30 | −0.05 (0.103), Z = −0.53, 0.60 | |
| Caregiver Stress | 0.40 (0.124), Z = 3.21, 0.001 | −0.02 (0.027), Z = −0.73, 0.47 | 0.38 (0.121), Z = 3.13, 0.002 | |
| Model D. Caregiver-assessed behavioral functioning | Caregiver relationship | 0.21 (0.127), Z = 1.67, 0.10 | −0.17 (0.075), Z = −2.24, 0.02 | 0.04 (0.118), Z = 0.36, 0.72 |
| Caregiver resources | 0.25 (0.114), Z = 2.23, 0.03 | −0.20 (0.073), Z = −2.79, 0.005 | 0.05 (0.101), Z = 0.50, 0.62 | |
| Caregiver Stress | 0.58 (0.111), Z = 5.28, < 0.001 | −0.07 (0.070), Z = −0.93, 0.35 | 0.52 (0.111), Z = 4.70, < 0.001 | |
Among PHEU youth, no indirect effects of the latent predictors on child-reported BF were observed (see Table 4). However, caregiver resources and caregiver relationship both had significant indirect effects on caregiver-reported BF (indirect effect = −0.20, Z = −2.79, p < 0.01, indirect effect = −0.17, Z = −2.24, p < 0.05, respectively; Table 4), although the total effects were not significant for these two factors.
Model fit
Based on standard fit statistics for overall model fit in SEMs, we did not observe a strong fit for either caregiver-reported or child-reported BF models in both study populations (PHIV and PHEU; see Table 3), reflecting the small number of factors associated with BF.
Discussion
The present study is one of the first to statistically explore the differential impact of home environment, psychosocial factors, and cognitive functioning on the behavioral functioning of youth infected with or affected by HIV, using structural equation modeling to identify the best combination of concomitant factors that contribute to cognitive and behavioral functioning. The inclusion of PHEU youth within this study allowed us to examine and compare the psychosocial and socio-demographic variables that uniquely contribute to their behavioral and cognitive profiles. Previous research regarding PHIV and PHEU youth has noted that both groups may be born into similar vulnerable communities but may not share the same opportunities and access to comprehensive medical and mental health care.
For PHIV youth, we were additionally interested in the role of HIV disease severity and treatment history and the individual and combined influences on both cognitive and behavioral functioning. Our results indicate that caregiver and family characteristics and histories of stressful life events predicted worse behavioral functioning scores among youth with PHIV and PHEU, aligning with theoretically driven hypotheses and earlier studies regarding the deleterious impact of stressful circumstances on cognitive and behavioral risk among the general population and HIV-exposed youth [2, 37, 38]. Although a causal relationship cannot be established, our results suggest that youth whose families experience multiple personal and psychosocial stressors are at higher risk for CF and BF problems, regardless of their HIV infection status.
Among youth with PHIV, two HIV- disease severity variables, late presenter and better past HIV health, were significant predictors of cognitive functioning, supporting previous observations regarding consequences of earlier severe HIV disease progression and the importance of early access to effective ART [30, 39]. These findings also align with current research regarding the function of age of presentation and its association with both clinical presentation of the disease and later CF [16]. Interestingly, in the caregiver-reported BF models for both PHIV and PHEU youth, higher child CF was associated with significantly lower (better) caregiver-reported BF, supporting resilience theories that identify intelligence as a protective asset that is linked with more positive outcomes among youth in general and among those with chronic illness, such as HIV [40].
The findings in the current study have important implications for clinical practice. In both PHIV and PHEU models, caregiver resources, including caregiver cognitive function, high school graduation status and family income (PHEU only), was an important predictor of both CF and BF (caregiver-reports only), as observed in earlier studies [41–43]. Consideration of caregiver characteristics may clarify appropriate resource options for children, caregivers and families, particularly in the context of multiple stressful life experiences. Collaborative child, caregiver, and family care, including appropriate educational, psychological and social support, may reduce long-term cognitive and behavioral risk for perinatally HIV-exposed children, regardless of the child's HIV status. This is clearly important for those PHIV youth who experienced early severe HIV disease, and may be at increased risk for cognitive impairment, and more vulnerable to the adverse effects of family/psychosocial risk. For those youth who experienced early severe HIV disease, ongoing monitoring of cognitive deficits, as well as other important family stressors is important for optimal remediation effects. Our data also suggest it is necessary for PHEU youth and their families who may have less consistent access to ongoing comprehensive medical care and community resources.
The present study extends current understanding of the roles of psychosocial and disease severity factors on CF and BF among HIV-affected youth, but is not without limitations. The strict inclusion criteria as well as requirements of longitudinal study participation may have increased the possibility of sampling bias and limited generalizability of findings. Although SEM was initially termed “causal modeling” [44], the ability to infer an explicitly causal relationship between the exogenous and endogenous variables remains controversial [45]. In addition, model fit statistics (e.g. SRMR, RMSEA, and Bentler Comparative Fit Index) for the final models were not ideal. However, the goal of this investigation was to examine the effects of HIV-related and psychosocial characteristics on BF and to evaluate whether CF mediated these relationships rather than to test every potential predictor within a single regression model or develop the best predictive model for BF. Utilizing other statistical methodologies such as hierarchical linear modeling (HLM), future studies should examine caregiver and stress-related factors, their continuity, and their longitudinal impact on both cognitive and behavioral functioning to better describe developmental trajectories of youth affected by HIV.
It is important to note that while the goal of the following paper was to allow a comprehensive assessment of multiple domains and their complex inter-relationships with both CF and BF, the variables included in this study are by no means exhaustive. Behavioral functioning during dynamic periods of child and adolescent development is inherently variable and difficult to predict among those at heightened risk, particularly due to factors such as puberty and its particular impact on disease course and both behavioral and psychosocial functioning. Recent investigations have begun to explore the role of puberty in HIV intervention, including its role in treatment adherence [46], first sexual activity and disclosure [47], identity development and adolescent-oriented mental health care [48] and disease-related factors, such as age associated metabolic complications [49] and renal dysfunction [50] which further add to the complicated task of statistically capturing behavioral and cognitive functioning and its impact on disease outcomes during this developmental period.
We were able to identify several significant associations and also detected pathways which were seemingly less important for BF. The results confirm the importance of acknowledging the influence and ongoing interactions of caregiver and psychosocial characteristics, home environment, and for those with HIV, early HIV health history, on the pathways to later developmental outcomes. In addition, the present study supports not only the utilization of SEM as a useful approach to understand complex interrelationships among psychosocial factors, cognition and behavioral outcomes of youth affected by HIV, but also the use of exploratory factor analytic strategies to “fine-tune” and create a more parsimonious model prior to SEM. Going forward, the key factors that served as potential predictors may be utilized to establish assessment, prevention, and treatment methodologies that acknowledge the powerful impact of individual and family stress, caregiver and youth resources, and both past disease severity and timing of disease presentation in order to establish risk profiles and culturally appropriate, evidence-informed services for those in our community who remain most vulnerable to HIV.
Supplementary Material
Acknowledgments
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. We would like to extend a special thanks to Pim Brouwers, Associate Director of Infant, Child & Adolescent Research at the National Institutes of Health for his guidance and expertise. We also extend our gratitude to both Laurie Dooley and Danish Siddiqui for their support regarding data organization and management. The following institutions, clinical site investigators and staff participated in conducting PHACS AMP in 2014, in alphabetical order: Ann & Robert H. Lurie Children's Hospital of Chicago: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynnette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Mahboobullah Baig, Anna Cintron; Children's Diagnostic & Treatment Center: Ana Puga, Sandra Navarro, Patricia Garvie, James Blood; Children's Hospital, Boston: Sandra Burchett, Nancy Karthas, Betsy Kammerer; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Molly Nozyce; Rutgers - New Jersey Medical School: Arry Dieudonne, Linda Bettica, Susan Adubato; St. Christopher's Hospital for Children: Janet Chen, Maria Garcia Bulkley, Latreaca Ivey, Mitzie Grant; St. Jude Children's Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University Health Sciences Center: Margarita Silio, Medea Jones, Patricia Sirois; University of California, San Diego: Stephen Spector, Kim Norris, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Alisa Katai, Jennifer Dunn, Suzanne Paul; University of Miami: Gwendolyn Scott, Patricia Bryan, Elizabeth Willen.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10461-016-1508-5) contains supplementary material, which is available to authorized users.
Compliance with Ethical Standards: Conflict of Interest Katrina Hermetet-Lindsay, Katharine Correia, Paige Williams, Renee Smith, Kathleen Malee, Claude Mellins, and Richard Rutstein declare that they have no conflict of interest to report.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Smith R, Wilkins M. Perinatally acquired HIV infection: long-term neuropsychological consequences and challenges ahead. Child Neuropsychol. 2014;21:1–35. doi: 10.1080/09297049.2014.898744. [DOI] [PubMed] [Google Scholar]
- 2.Mellins CA, Malee KM. Understanding the mental health of youth living with perinatal HIV infection: lessons learned and current challenges. J Int AIDS Soc. 2013;16:1–19. doi: 10.7448/IAS.16.1.18593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koekkoek S, de Sonneville LM, Wolfs TF, Licht R, Geelen SP. Neurocognitive function profile in HIV-infected school-age children. Eur J Paediatr Neurol. 2008;12(4):290–7. doi: 10.1016/j.ejpn.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Nichols S, Brummel SS, Smith RA, et al. Executive functioning in children and adolescents with perinatal HIV infection. [Accessed 9 Feb 2013];PIDJ. 2015 34:969–975. doi: 10.1097/INF.0000000000000809. http://www.medscape.com/viewarticle/470017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malee KM, Tassiopoulos K, Huo Y, et al. Mental health functioning among children and adolescents with perinatal HIV infection and perinatal HIV exposure. AIDS Care. 2011;23(12):1533–44. doi: 10.1080/09540121.2011.575120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mellins CA, Tassiopoulos K, Malee K, et al. for the Pediatric HIV-AIDS Cohort Study. Behavioral health risks in perinatally HIV-exposed youth: co-occurrence of sexual and drug use behavior, mental health problems, and nonadherence to antiretroviral treatment. AIDS Patient Care STDs. 2011;25(7):413–22. doi: 10.1089/apc.2011.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kacanek D, Angelidou K, Williams PL, et al. the P1055 Study Team. Psychiatric symptoms and antiretroviral nonadherence in US youth with perinatal HIV: a longitudinal study. AIDS. 2015;29(10):1227–37. doi: 10.1097/QAD.0000000000000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGuire JL, Kempen JH, Localio R, Ellenberg JH, Douglas SD. Immune markers predictive of neuropsychiatric symptoms in HIV-infected youth. Clin Vaccine Immunol. 2015;22(1):27–36. doi: 10.1128/CVI.00463-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadow KD, Angelidou K, Chernoff M, et al. Longitudinal study of emerging mental health concerns in youth perinatally infected with HIV and peer comparisons. J Dev Behav Pediatr. 2012;33:456–68. doi: 10.1097/DBP.0b013e31825b8482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salama C, Morris M, Armistead L, et al. Depressive and conduct disorder symptoms in youth living with HIV: the independent and interactive roles of coping and neuropsychological functioning. AIDs Care. 2013;25(2):160–8. doi: 10.1080/09540121.2012.687815. [DOI] [PubMed] [Google Scholar]
- 11.Sirois PA, Aaron L, Montepiedra G, et al. Stimulant medications and cognition, behavior and quality of life in children and youth with HIV. Pediatr Infect Dis J. 2016;35(1):e12–8. doi: 10.1097/INF.0000000000000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mutumba M, Bauermeister JA, Elkington KS, et al. Changes in mental health symptoms among perinatally HIV-infected and HIV-exposed but uninfected urban youths. J Adolesc Health. 2016;58(4):460–6. doi: 10.1016/j.jadohealth.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nachman S, Chernoff M, Williams P, Hodge J, Heston J, Gadow KD. Human immunodeficiency virus disease severity, psychiatric symptoms, and functional outcomes in perinatally infected youth. Arch Pediatr Adolesc Med. 2012;166:528–35. doi: 10.1001/archpediatrics.2011.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gadow KD, Chernoff M, Williams PL, et al. Co-occuring psychiatric symptoms in children perinatally infected with HIV and peer comparison sample. J Dev Behav Pediatr. 2010;31(2):116–28. doi: 10.1097/DBP.0b013e3181cdaa20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laughton B, Cornell M, Boivin M, Van Rie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J Int AIDS Soc. 2013;16:18603. doi: 10.7448/IAS.16.1.18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowell C, Huo Y, Tassiopoulos K, et al. for the PACTG 219C Study Team and the Pediatric HIV/AIDS Cohort Study. Early viral suppression improves neurocognitive outcomes in HIV-infected children. AIDS. 2015;29:295–304. doi: 10.1097/QAD.0000000000000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uban K, Herting M, Williams P, et al. White matter microstructure among youth with perinatally acquired HIV is associated with disease severity. AIDS. 2015;29:1035–44. doi: 10.1097/QAD.0000000000000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hackman DA, Gallop R, Evans GW, Farah MJ. Socioeconomic status and executive function: developmental trajectories and mediation. Dev Science. 2015;18(5):686–702. doi: 10.1111/desc.12246. [DOI] [PubMed] [Google Scholar]
- 19.McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci. 2010;1186:190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noble KG, Houston SM, Brito NH, et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18:773–8. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochhauser CJ, Gaur S, Marone R, Lewis M. The impact of environmental risk factors on HIV-associated cognitive decline in children. AIDS Care. 2008;20:692–9. doi: 10.1080/09540120701693982. [DOI] [PubMed] [Google Scholar]
- 22.Bryan A, Schmiege SJ, Broaddus MR. Mediational analysis in HIV/AIDS research: estimating multivariate path analytic models in a structural equation modeling framework. AIDS Behav. 2008;11:365–83. doi: 10.1007/s10461-006-9150-2. [DOI] [PubMed] [Google Scholar]
- 23.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: Psychological Corporation; 1999. [Google Scholar]
- 24.Gerard AB. Parent-child relationship inventory. Los Angeles: Western psychological services; 1994. [Google Scholar]
- 25.Johnson JH, McCutcheon SM. Assessing life stress in older children and adolescents: preliminary findings with the Life Events Checklist. Stress Anxiety. 1980;7:111–25. [Google Scholar]
- 26.Wechsler D. Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV) San Antonio: The Psychological Corporation; 2003. [Google Scholar]
- 27.Wechsler D. Wechsler Adult Intelligence Scale-Fourth Edition (WAIS–IV) San Antonio: Psychological Corporation; 2008. [Google Scholar]
- 28.Garvie PA, Zeldow B, Malee K, et al. Discordance of cognitive and academic achievement outcomes in youth with perinatal HIV exposure. Pediatr Infect Dis J. 2014;33(9):e232–8. doi: 10.1097/INF.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen S, Ter Stege JA, Geurtsen GJ, et al. Poorer cognitive performance in perinatally HIV-infected children as compared to healthy socioeconomically matched controls. Clin Infect Dis. 2015;60(7):1111–9. doi: 10.1093/cid/ciu1144. [DOI] [PubMed] [Google Scholar]
- 30.Puthanakit T, Ananworanich J, Vonthanak S, et al. Cognitive function and neurodevelopmental outcomes in HIV-infected children older than 1 year of age randomized to early versus deferred antiretroviral therapy. Pediatr Infect Dis J. 2013;32:501–8. doi: 10.1097/INF.0b013e31827fb19d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerr SJ, Puthanakit T, Vibol U, et al. Neurodevelopmental outcomes in HIV-exposed-uninfected children versus those not exposed to HIV. AIDS Care. 2014;26(11):1327–35. doi: 10.1080/09540121.2014.920949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapetanovic S, Griner R, Zeldow B, et al. for the pediatric HIV AIDS cohort study. biomarkers and neurodevelopment in perinatally HIV-infected or exposed youth: a structural equation model analysis. AIDS. 2014;28:355–64. doi: 10.1097/QAD.0000000000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds CR, Kamphaus RW. Behavior Assessment for Children, (BASC-2) Circle Pines: American Guidance service; 2004. [Google Scholar]
- 34.Alperen J, Brummel S, Tassiopoulos K, et al. Prevalence of and risk factors for substance use among perinatally HIV-infected and perinatally exposed but uninfected youth. J Adolesc Health. 2014;54(3):341–9. doi: 10.1016/j.jadohealth.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chenneville T, Sibille K, Lujan-Zilbermann J, Rodriguez C, Brown M, Emmanuel P. Medical decisional capacity among children with HIV. AIDS Care. 2010;22:1359–66. doi: 10.1080/09540121003758499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elliott-DeSorbo DK, Martin S, Wolters PL. Stressful life events and their relationship to psychological and medical functioning in children and adolescents with HIV infection. J Acquir Immune Defic Syndr. 2009;52:364–78. doi: 10.1097/QAI.0b013e3181b73568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sameroff AJ, Rosenblum KL. Psychosocial constraints on the development of resilience. Ann N Y Acad Sci. 2006;1094:116–24. doi: 10.1196/annals.1376.010. [DOI] [PubMed] [Google Scholar]
- 38.Smith R, Malee K, Leighty R, et al. Effects of perinatal HIV infection and associated risk factors on cognitive development among young children. Pediatrics. 2006;117:851–62. doi: 10.1542/peds.2005-0804. [DOI] [PubMed] [Google Scholar]
- 39.Smith R, Chernoff M, Williams P, et al. Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr Infect Dis J. 2012;31:592–8. doi: 10.1097/INF.0b013e318253844b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker SP, Wachs TD, Grantham-McGregor S, et al. Inequality in early childhood: risk and protective factors for early child development. Lancet. 2011;378(9799):1325–38. doi: 10.1016/S0140-6736(11)60555-2. [DOI] [PubMed] [Google Scholar]
- 41.Abrams EJ, Wiener J, Carter R, et al. Maternal health factors and early pediatric antiretroviral therapy influence the rate of perinatal HIV-1 disease progression in children. AIDS. 2003;17:867–77. doi: 10.1097/00002030-200304110-00012. [DOI] [PubMed] [Google Scholar]
- 42.Coscia JM, Christensen BK, Henry RR, Wallston K, Radcliffe J, Rutstein R. Effects of home environment, socioeconomic status, and health status on cognitive functioning in children with HIV-1 infection. J Pediatr Psychol. 2001;26:321–9. doi: 10.1093/jpepsy/26.6.321. [DOI] [PubMed] [Google Scholar]
- 43.Stein A, Desmond C, Garbarino J, et al. Predicting long-term outcomes for children affected by HIV and AIDS: Perspectives from the scientific study of children's development. AIDS. 2014;28:S261–8. doi: 10.1097/QAD.0000000000000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright S. Correlation and causation. J Agric Res. 1921;20:557–85. [Google Scholar]
- 45.Bollen KA, Pearl J. Eight myths about causality and structural equation models In Handbook of causal analysis for social research. Springer; Netherlands: 2013. pp. 301–328. [Google Scholar]
- 46.Agwu AL, Fairlie L. Antiretroviral treatment, management challenges and outcomes in perinatally HIV-infected adolescents. J Int AIDS Soc. 2013;16(1):18579. doi: 10.7448/IAS.16.1.18579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fairlie L, Sipambo N, Fick C, Moultrie H. Focus on adolescents with HIV and AIDS. S Afr Med J. 2015;104(12):897–903. [Google Scholar]
- 48.Folayan MO, Odetoyinbo M, Brown B, Harrison A. Addressing the socio-development needs of adolescents living with HIV/AIDS in Nigeria: a call for action. Afr J Reprod Health. 2015;18(3):93–101. [PMC free article] [PubMed] [Google Scholar]
- 49.Barlow-Mosha L, Ross Eckard A, McComsey GA, Musoke P. Metabolic complications and treatment of perinatally HIV-infected children and adolescents. J Int AIDS Soc. 2013;16(1):18600. doi: 10.7448/IAS.16.1.18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fortuny C, Deyá-Martínez Á, Chiappini E, Galli L, De Martino M, Noguera-Julian A. Metabolic and renal adverse effects of antiretroviral therapy in HIV-infected children and adolescents. Pediatr Infect Dis J. 2015;34(5):S36–43. doi: 10.1097/INF.0000000000000663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


