Abstract
Sjögren’s syndrome is a common autoimmune disease that presents with sicca symptoms and extraglandular features. Sjögren’s syndrome is presumably as common as RA; yet it is poorly understood, underdiagnosed and undertreated. From the usual identity as an autoimmune exocrinopathy to its most recent designate as an autoimmune epithelitis – the journey of SS is complex. We herein review some of the most important milestones that have shed light on different aspects of pathogenesis of this enigmatic disease. This includes role of salivary gland epithelial cells, and their interaction with cells of innate and adaptive immune system. Non-immune factors acting in concert or in parallel with immune factors may also be important. The risk genes identified so far have only weak association, nevertheless advances in genetics have enhanced understanding of disease mechanisms. Role of epigenetic and environmental factors are also being explored. SS also has some unique features such as congenital heart block and high incidence of lymphoma; disease mechanisms accounting for these manifestations are also reviewed.
Keywords: Sjögren’s syndrome, etiology, pathogenesis, genetics, epigenetics, immunological factors, non-immunological factors
Introduction
Sjögren’s syndrome (SS) is a chronic autoimmune disease characterised by infiltration of exocrine glands by autoreactive lymphocytes resulting in glandular dysfunction and sicca symptoms. The disease manifests with protean extraglandular manifestations as well. However, SS is often under-diagnosed, either because the physician does not recognize the manifold features of this systemic condition or the patients may not feel it unnecessary to visit their doctor for dry eyes and mouth. Pathogenesis of this disease is obscure. Research on the crucial immune as well as nonimmune role of salivary gland epithelial cells (SGEC) added new dimensions to the understanding of its pathogenesis. Conventionally, both innate and adaptive immune responses are implicated in causation of SS, possibly triggered by viral infections and hormonal factors in a genetically susceptible host. Several multisystem extraglandular features of SS, on the other hand, have some unique underlying mechanisms. We present the most recent data on pathogenetic mechanisms behind presentations of SS.
Role of Salivary gland epithelial cell: Look like the innocent flower but be the serpent under’t
Salivary gland epithelial cells (SGEC), once believed to be passive onlookers, seem to be the nidus of pathogenetic events in SS. Initial intrinsic epithelial activation followed by altered glandular homeostasis precedes lymphocytic infiltration in SS. SGEC in SS, therefore, are active players in the inflammatory and autoimmune response according to recent data [1]. Once activated, these cells orchestrate innate and adaptive immune response as follows:
Proinflammatory molecules expressed by SGEC cause complex interactions
Increased expression of MHC Class II especially HLA-DR on SGEC predominantly in areas of lymphocytic infiltration, empowers them as antigen presenting cells to T cells [2,3].
Presence of co-stimulatory factors CD80, CD86 and CD40 on SGEC enhances interaction with immune cells leading to secretion of Th1 cytokines. This results in a feed forward loop, thereby further upregulating expression of HLA antigens, costimulatory molecules and adhesion molecules on SGEC [4]. Production of chemokines such as CXCL13, CCL17, CCL19, CCL21 and CCL22 by SGEC results in dendritic cell (DC) infiltration [5]. IFNγ, a Th1 cytokine, also induces production of chemokines like CXCL10 and CXCL9 that aid in homing of T cells into salivary gland from blood [6]. Another chemokine, CXCL13 is also produced by SGEC that directs B cell movement into salivary gland and further formation of lymphoid structures.
SGEC produce a variety of cytokines that are crucial for both innate and adaptive immune responses. This includesinterferons and other cytokines involved in Th1, Th17, T follicular helper cell response and B cell stimulation. Reports also suggest that mRNA expression of inflammatory cytokines IL-1, IL-6 and TNFα is increased several fold in SGEC of patients with SS as compared to controls [7]. In addition to IL-6, activated SGEC also secrete IL-7, IL-18 and IL-22, which are crucial cytokines for adaptive immune response and T cell activation [7–10]. Of these, IL-18 and IL-7 regulate the Th1 response [8,11]. High SGEC expression of IL-22 has also been reported in SS [10]. Interestingly, one of its crucial receptors, namely IL22-R1, is solely expressed by epithelial cells [12]. A recent study has demonstrated that SGEC by virtue of IL-6 and ICOSL expression could cause differentiation of CD4+ cells into follicular helper T cells(Tfh) [13]. SGEC also produce BAFF on being induced by interferons. BAFF has a crucial role in B cell maturation, class switching, survival and proliferation especially in advanced disease. The end result of the production of these proinflammatory molecules is immune cell interactions leading to salivary gland inflammation, lymphocyte proliferation and occasional ectopic germinal center formation as depicted in Figure 1.
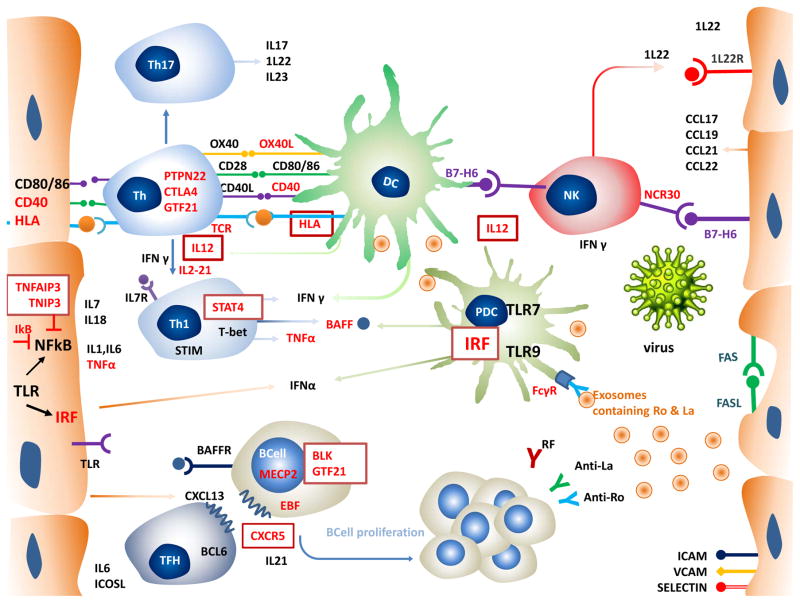
Figure 1. Role of SGEC and Immune cells in Pathogenesis of Sjogren’s Syndrome.
SGEC express a) MHC class II and costimulatory factors CD40 and CD80/86 that enhances interaction with T cells, and B7-H6, a ligand for NK cell receptor NCR30 b) chemokines CCL17,CCL19, CCL21, CCL22 that recruits T cells and dendritic cells; and CXCL13 that causes B cell homing, c)cytokines like TNFα, IL1, IL6, IL7,IL18,IL22, IFNα, BAFF d) IL33R e) adhesion molecules-ICAM,VCAM and selectin; and TLR3 f)FAS-FASL expression resulting in apoptosis g) SGEC also releases exosomes. Autoantigens Ro and La are contained in exosome bodies and apoptotic bodies.
Immune cells –pDC are most important sources of IFNα. pDCs are believed to be activated by virus or endogenous nucleic acid containing immune complexes bound to TLRS. DCs interact closely with T cells and NK cells. DC, T cells as well as NK cells produce IFN γ which contributes to the IFN signature which acts as link between innate and adaptive immunity. IFN signature mediates lymphocytic infiltration and lymphocyte activation and stimulate BAFF production. Th1 pathway and Th17 pathway and their cytokines are important players in pathogenesis. IL 12, 1l21, IFNγ drives stimulates Th1 specific transcription factors and results in stimulation of TH1 pathway and its cytokines that is IFN γ, TNF. Th 17 cells produce il-17 and IL22 that augment the inflammatory response
BAFF plays a crucial role in B cell maturation and proliferation. CXCL13 directs B cell homing into the salivary gland. CXCL13 binds to its receptor CXCR5 that is present on TFH cells and B cells, TFH and its cytokine IL21 induce B cell activation and formation of germinal centre. BAFF along with IFN also promotes autoantibody production by B cells. Immune complexes formed between antibodies and autoantigens further activates pDC enhancing and perpetuating inflammation.
SGEC - Salivary gland epithelial cell, MHC-Major histocompatibility complex, NK-Natural killer, NCR-Natural-cytotoxicity-triggering receptor 3, IFN-Interferon, BAFF- B-cell activating factor, ICAM-Intercellular adhesion molecule, VCAM- vascular cell adhesion molecule 1,DC-Dendritic cell, pDC-plasmacytoid dendritic cell,
Toll like receptors (TLR) signalling in SGEC may be an inciting event
TLRs, especially TLR3, are reported to be constitutively expressed in SGEC [14]. Though distribution of TLR3 is generally intracellular, experiments on SGEC cell lines and NZB/WF1 female mice have demonstrated its surface expression as well [14,15]. TLR signalling in the NZB/W F1 female mice was found to upregulatie IL-6, TNFα and IFNβ1 as well as type 1 IFN responsive genes. Salivary gland hypofunction, which ensued in these mice, was found to precede lymphocytic infiltration and could be reversed on discontinuing TLR stimulation [15].
TLR3 recognises viral dsRNA, endogenous dsRNA as well as the synthetic agonist, polyI:C, under experimental conditions. TLR3 ligation by polyI:C leads to upregulation of apoptotic proteins resulting in programmed cell death of SS-SGEC cell lines pretreated with RNA synthesis inhibitor [16]. Some investigators have proposed a similar mechanism, involving viral infection or endogenous nucleic acid, as inciting initial pathogenetic events in SS. TLR signalling in SGEC can also upregulate MHC, costimulatory molecules and adhesion molecules, in addition to causing increased apoptosis and cytokine production as mentioned above [14–16]. Cytokine production resulting from TLR3 ligation could be mediated by Interferon Regulatory Factor (IRF) and NFkB(nuclear factor kappa-light-chain-enhancer of activated B cells) pathways.
NFkB dysregulation in SGEC causes inflammatory cytokine production in SS
SGEC in SS may also have impaired function of IkB and TNFAIP3, the 2 major negative feedback regulators of NFkB; the implications are easily understood as NFkB is a master transcription factor involved in production of inflammatory cytokines. Mice models with homozygous knock-in mutated kB enhancers were found to have lymphocytic infiltration of exocrine glands, higher serum IL-1α, IL-17, and TNFα, anti-Ro and anti-La antibodies mimicking SS phenotype [17].
SGEC from SS patients are also reported to have lower TNFAIP3 expression as compared to controls and hence, higher NFkB levels [18]. Interestingly, GWAS has also implicated the gene (TNIP1) encoding TNFAIP3-interacting protein 1 in SS [19]. These studies provide supporting evidence for NFkB dysregulation in SS.
Apoptosis of SGEC release intracellular autoantigens-Ro and La
SGEC release intracellular antigens in apoptotic bodies and exosomes. Ro and La antigens, thus released, are presented by DC to T cells. Apoptosis, which is induced by prior autocrine mechanisms, is mediated by Fas-Fas Ligand pathway in SGEC [20,21]. Infiltrating Th1 cells secrete interferon gamma and tumor necrosis factor alpha which also induce Fas expression. Fas ligand is also present on NK cells and T cells in addition to SGEC. B cells can also induce apoptosis of SGEC acting via protein kinase C delta activation [22]. Unlike SGEC, lymphocytes, per se, are resistant to apoptosis due to their high expression of bcl2. Thus, apoptosis of SGEC may be enhanced by infiltrating immune cells and result in altered expression of autoantigens.
Interferons, by virtue of inducing apoptosis of SGECs, also trigger a vicious cycle. IFNα causes upregulation of Ro-52, which is a RNA binding protein and key autoantigen in SS [23]. This in turn could possibly stimulate increased autoantibody production. These anti-RNA antibodies bind to RNA in apoptotic bodies resulting in further IFNα production. In the only report to assess the relationship, a positive correlation between anti-Ro and anti-La titers with interferon gene signature was found [24].
Summary of the preceding paragraphs on SGEC imply that these apparently innocent cells indeed initiate the inflammatory process, amplify and sustain this response leading to chronic proliferation of T and B cells. The resulting autoimmune epithelitis is responsible not only for the glandular manifestation, but have also been implicated in periepithelial and extraglandular manifestations involving organs like the liver, kidneys and lungs. The well orchestrated immune response abetted by cytokines and chemokines results in a vicious cycle of inflammation and autoimmunity.
Dendritic Cells
Both plasmacytoid dendritic cells (pDCs) and classic dendritic cells(cDCs) have a role in SS pathogenesis. Plasmacytoid DC (pDCs) are present in the lymphocytic foci of salivary glands in patients with SS but not in healthy controls. The identity of pDC as the predominant IFNα producing cells was confirmed by the presence of pDC surface markers. Self nucleic acids released during apoptosis and viral nucleic acids are recognised by TLR 7 and TLR 9 of the pDCs resulting in interferon production. Classic dendritic cells (cDCs) infiltration also has been noted in salivary gland of SS [25]. These DCs cluster around epithelial cells, whereas follicular DCs are found in the periphery of lymphocytic infiltrates. Decreased peripheral blood levels of pDC and some cDCs could suggest increased migration of these cells from blood to salivary gland [26]. This process may be aided by expression of chemokines such as CXCL13, CCL17, CCL19, CCL21 and CCL22 in SGEC, while the receptors for these cytokines are expressed on DCs. cDCs can activate T cells to proliferate and produce proinflammatory cytokines.
Further, evidence for role of DCs in SS comes from 2 experimental mice models, namely ID3 knockout and DC immunoreceptor (Dcir) knockout mouse model [27,28]; SS-like sialadenitis develops in both. ID3 probably inhibits development of pDC, while Dcir is involved in maintaining tolerance.
Interferons engage SGEC in the orchestra of innate and adaptive immune response in SS- Adding fuel to the fire
Interferon signature in salivary gland as well as peripheral blood of patients with SS, akin to that seen in lupus, has been demonstrated by gene expression studies using microarray [24,29]. IFNα is produced from activated plasmacytoid dendritic cells (pDCs) in response to upregulated TLR7 and TLR9 and IL-12 [30]. INFγ, which also contributes substantially to IFN signature in SS, is produced by NK cells, DC and Th1 cells on being primed with IFNα [31]. These are scenarios mimicking innate responses to viral infections, thereby further supporting the possibility of a viral etiology in SS.
A recent study on CD14 positive monocytes also detected interferon type I signature in 55% of patients with SS as against 4.5% of healthy controls [32]. These SS patients with increased expression of interferon-regulated genes also had high disease activity, higher serological titres and BAFF gene expression as well.
Indeed, IFN act as the link between innate and adaptive immune responses in SS. Both IFNα and INFγ mediate lymphocytic infiltration of salivary glands with T and B cells, activate lymphocytes, induce expression of MHC and costimulatory molecules on SGEC, stimulate BAFF and autoantibody production as well as promote apoptosis.
NK Cells
Of late, NK cells have also been implicated in SS pathogenesis. The distribution of NK cells was similar to that of pDC in minor salivary glands and the former showed a positive correlation with the focus score in biopsy specimens as well. NK cells from SS patients expressed higher amount of the NK cell activating receptor, NCR3/NKp30. The ligand for this receptor, B7-H6 is found on DC and SGEC. NKp30-B7-H6 interaction is crucial for NK-DC and NK-SGEC cross talk. Upon binding SGEC via the ligand B7-H6, NK cells also release INFγ, which has key role in salivary gland dysfunction in SS as mentioned above [33].
T cells and B cells
The infiltrating lymphoid cells, predominantly T cells and also B cells, give rise to the characteristic histological finding in SS. Composition of salivary gland infiltrates vary from mild to severe lesions. T cells and interdigitating dendritic cells were found in mild lesions, whereas B cells and macrophages were detected in severe lesions, correlating strongly with focus score [34].
Th1 cells in SS
Traditionally, SS was considered to be Th1 predominant disease, though the balance between Th1 and Th2 is influenced by the stage of the disease [35]. In C57BL/6.NOD-Aec1Aec2(B6.NOD-Aec) mouse model, IL7 acting via upregulation of Th1 and IFNγ/CXCR3 accelerated the development of SS [36]. This effect was abrogated on blocking IL7Rα.
INFγ is mainly produced by Th1 cells; INFγ along with IL-12 causes differentiation of CD4+ T cells into Th1 lymphocytes. In this context, it is interesting to recall that IL-12A loci has been implicated in recent genome-wide association studies (GWAS) of SS [19]. Also, the development of SS and increased acinar cell apoptosis, hyposalivation and abnormal salivary protein expression seen in NOD mice was abolished in NOD.IFNγ−/− and NOD.IFNγR−/−, highlighting the role played by this cytokine in pre-immune phase as well [37].
Th17 cells in SS
The TH17 pathway has an important role in disease pathogenesis and formation of germinal centres in SS. In SS, increased IL-17 expression in blood as well as its mRNA and protein expression in MSG biopsies was demonstrated; these were found to correlate with focus score on histopathology [38]. Further evidence for a role of Th17 in SS was demonstrated by increased levels of IL-6 and IL-23 in plasma as well as MSG of patients with SS [39]. IL-22, a cytokine downstream of IL-23 in the Th17 pathway, was also found to be overexpressed in MSG of SS patients [10]. IL-22 acting along with IL-17 elicit an intense inflammatory response in SS [40]. Further, a recent study has demonstrated correlation of serum IL-22 levels with low salivary flow and serology (anti-Ro, anti-La, rheumatoid factor) in SS [41].
Higher serum and SG expression of IL-21, another Th17 related cytokine, was seen in SS patients; and, these measures of IL-21 correlated with serum immunoglobulin levels and lymphocytic infiltration, respectively. IL-21 is produced by T follicular helper cells, which have an important role in B cell activation and germinal centre formation. Taken together, the above studies reiterate the role of T cells, especially Th17, in pathogenesis of SS.
Follicular helper T cells
Tfh cells play a key role in lymphoid follicle formation and ectopic germinal centre formation in salivary gland in SS. As mentioned previously, SGEC induce differentiation of T helper cells to Tfh by secretion of IL6 and ICOS ligand. CXCR5 positive Tfh cells secrete IL21 that mediates B cell maturation, proliferation and germinal centre formation.
T reg cells
The increased frequency of Treg cells seen in MSG was inversely related to their presence in peripheral blood and this may represent an effort by Tregs to counteract the inflammation. But with increased inflammation and cytokine milieu favouring Th17, the balance is tipped in favour of Th17 cells. This could possibly explain why the Treg cells predominate in mild to intermediate lesions and subsequently plateau at lower levels in advanced disease [42]. Interestingly, low FOXp3 expression in SG has been shown to be a predictor of lymphoma in SS. Recently a new subset of Treg cells, low in CD25 but expressing glucocorticoid-induced TNF receptor-related protein, was found to be doubled in peripheral blood of SS with inactive disease, as compared to those with active SS as well as healthy controls [43].
The importance of T cells in SS is reiterated by the study of mice models in which the stromal interaction molecules STIM1 and STIM2 were knocked out [44]. These mice developed features of autoimmune exocrinopathy including antibody production against Ro and La. STIM1 and STIM2 are constituents of calcium release-activated calcium (CRAC) channels, which have key roles in T cell activation and signalling. Interestingly, STIM1 and STIM2 protein deficiency in T lymphocytes from peripheral blood as well as from salivary gland infiltrates of patients with SS is reported [44].
B cell
The characteristic immunological features of SS such as hypergammaglobulinemia, cryoglobulinemia, antibody positivity, germinal center formation and higher incidence of lymphoma are all evidence for B cell hyperactivity in SS. As mentioned previously, B cell infiltration increases with increase in severity of inflammation in the SG, and ectopic germinal centres are seen in the minor salivary gland biopsies [45] of up to a quarter of SS patients. Germinal centers are sites of increased apoptotic activity as well as antibody production. The presence of germinal centres is also a predisposing factor for lymphoma. Transitional Type II B cells and marginal zone like B cells, both of which have a role in autoantibody production, were found in the germinal centres [46].
Recent, albeit partial, success with B cell directed therapy in SS indicates that the role of B cell may extend beyond germinal centre formation and autoantibody production. Both peripheral blood and salivary gland B cell abnormalities have been described in SS. The distribution of peripheral blood B cell subsets can help to distinguish SS from other rheumatic diseases. Activated naive and CD27-memory B-cells cells are over-represented, whereas CD27+ memory B-cells are decreased in number in the peripheral blood of SS patients [47]. In healthy individuals, IgM-IgD+CD27-B-cells bind autoantigens and hence this expanded B cell subset is likely to be the source of autoantibodies in SS. Notably, SS patients with higher focus score were noted to have higher plasma cells in peripheral blood.
The chemokine CXC ligand 13 protein (CXCL13), also known as B-cell-attracting chemokine-1 or B-lymphocyte chemoattractant (BLC), is the key cytokine responsible for homing of B cells to the salivary gland. CXCL13 is secreted by follicular dendritic cells and stromal cells and also T cell subsets such as Tfh. The receptor for CXCL13 is CXCR5, which is located on B cells as well as Tfh. CXCL13 guides B cell entry into the follicles. In fact, lymphoid organisation seen in salivary gland biopsies of SS is attributed to CXCL13 together with CXCR5.
CXCL13 is poised to become a useful biomarker in SS. Experiments from mouse models reveal that CXCL13 reflects disease stage. Blockade of CXCL13 by a neutralising monoclonal antibody also abrogates inflammation in the salivary gland[48]. This concept was further supported by the finding of elevated salivary and serum levels of CXCL13 in patients with SS [48]. Notably, variants in CXCR5 have been implicated in recent GWAS of SS [19], supplying further evidence for the importance of CXCL13-CXCR5 interaction in SS.
Further, abnormalities of BAFF is implicated in B cell hyperactivity in SS. Induced by interferons, BAFF has a role in B cell maturation, class switching, survival and proliferation especially in advanced disease. These effects are more pronounced in autoreactive B cells. BAFF is produced by SGEC, DC, macrophages, activated T cells and also B cells [49]. Development of SS-like disease in BAFF- transgenic mice provides supporting evidence for BAFF in disease pathogenesis [50]. In addition, increased expression of BAFF has been observed in several autoimmune diseases including SS, and its level correlates well with autoantibody titres [51].
Autoantigens and Autoantibodies in SS
Ro-52, Ro-60 and La are well known autoantigens in SS
Ro-52 is ubiquitous in distribution, though predominantly present in immune cells like T cells. Ro-52 is generally found in cytoplasm and is transported into nucleus during stressful conditions and viral infection. Ro-52 also plays a crucial role in anti-viral and other innate responses, cellular proliferation and apoptosis. Ro-52 is induced by interferons during viral infection and forms a negative feedback loop [23]. This protein ubiquitinates and causes proteasomal degradation of IRF3, IRF7 and IRF8. By ubiquitinating IκB kinase β (IKKβ), Ro-52 also inhibits NFkB signalling [52–54].
Ro-52, also called TRIM21, is a member of TRIM (tripartite motif proteins) and consists of the following domains - RING, B-box, Coiled-coil and B30.2 (or PRYSPRY). These domains are responsible for the localisation and characteristic functions of Ro-52. For instance, the RING domain has E3 ubiquitin ligase activity that is responsible for ubiquitination. The coiled-coil domain is necessary for the protein’s cytoplasmic location [55]. The B30.2 (or PRYSPRY) domain has strong affinity for Fc portion of IgG, by virtue of which it binds to antibody coated virus particles and subsequently ubiquitinates and degrade them via proteasomal complex [56].
Ro-52 also causes increased apoptosis by inhibiting bcl2 production [57,58]. During apoptosis, autoantigens, including Ro-52, are transferred onto the surface of apoptotic blebs where these autoantigens may interact with the immune system in a way that leads to activation and eventually autoantibody formation.
Relevance of Ro-52
Overexpression of Ro-52 transcripts has been reported in PBMC of SS as compared to healthy controls [58]. Similar increased expression of Ro-52 has been reported in salivary ductal epithelium as well, and the level of expression tallied with the degree of inflammation [59]. Further evidence for Ro-52 in autoimmunity comes from two Ro-52 deficient mice models [54,60]. Though there were differences in phenotypes of these mice, both the mice models had elevated proinflammatory cytokines and concurred on the fact that Ro-52 was a negative regulator of inflammation. The mouse model developed by Espinosa, et al. developed severe skin manifestation and systemic autoimmunity when injured by using ear tags. These effects were abrogated when Ro-52 deficient mice was crossed with IL-23p19 deficient mice, underscoring the role played by Th17/IL-23 pathway in driving autoimmunity in Ro-52 deficient mice [60].
Both Ro-60 and La are RNA binding proteins. Ro-60 binds to small non-coding RNA called Y-RNAs that consist of approximately 100 nucleotides with an internal loop rich in pyrimidine. Base pairing of 5′ and 3′ ends forms a long stem [61]. A bulged helix within this stem forms the binding site for Ro-60. Ro-60 prevents Y-RNA from degradation and Y-RNA in turn maintains Ro-60 within the cytoplasm. Ro-60 also binds to misfolded small RNA variants and has a role in RNA quality control. As with Ro-52, Ro-60 also is transported into nucleus during stress and UV radiation. Ro-60 is believed to have a role in repair of intracellular damage following UV radiation [62]. Hence it is believed that Ro-60 may be instrumental in preventing autoimmunity by clearing abnormal RNA and preventing exposure of RNA to immune system.
La binding to pre-miRNA increases stability and prevents its degradation by nucleases [63]. La also mediates RNA interference and it has antiviral actions [64]. Three different transcripts of La have been identified in SS, though it is not clearly known if this variability accounts for La antigenicity [65].
Production of antibodies and their relevance
Increased Ro and La expression has been demonstrated in MSG of patients with SS as compared with controls [59,66]. Also those with higher La expression were detected to have higher anti-La antibody implicating antigen driven autoantibody response [67]. Ectopic germinal centres with local production of anti-Ro and anti-La antibody[68] as well as production of recombinant anti-Ro and anti-La monoclonals from MSG-infiltrating B cells[69] are further testimonial to the same. Anti-Ro is strongly associated with extraglandular manifestions in SS; however, pathogenecity of these antibodies and pathophysiology in different organ manifestation of SS have not been proven. Though, there seems to be a strong case for a pathogenic role of antibodies in congenital heart block (detailed below). There is evidence to suggest that anti-Ro and anti-La antibodies bind to Fcγ receptors and cause apoptosis in salivary gland cell line by activating caspase 3 [70]. Further, upregulation of caspase 8 mRNA was demonstrated in cultures of SGEC from MSG biopsies of patients with SS [71]. In human SGEC cultures, decreased fibulin-6 mRNA expression, an ECM component was seen after exposure to anti-Ro antibody. These cells also demonstrated cell detachment and death [72].
It was generally believed that antibodies against intracellular constituents could not enter the cell and hence are unlikely to be pathogenic unless the antigen is released extracellularly or expressed onto the cell surface. However, this tenet was challenged by experiments that demonstrated that antibodies to dsDNA and ribosomal P proteins could penetrate living cells and cause cellular dysfunction in living cells as well as in culture[73,74]. Also, antibodies to Ro and La gained access to salivary gland cells in A-253 cell line experiments via Fcγ receptors resulting in apoptosis[70]. The other antibodies believed to be pathogenic in SS are anti-M3 muscarinic acetylcholine receptor antibodies (described below in section on mechanisms of secretory dysfunction) and anti-carbonic anhydrase II antibodies. In SS patients, anti-carbonic anhydrase II antibodies have been detected in 12.5–20.8% and are believed to have a pathogenic role in renal tubular acidosis (RTA) [75,76]. Immunization of mice with human carbonic anhydrase II resulted in autoimmune sialadenitis and induction of anti-carbonic-anhydrase-II antibody in the same mice model resulted in urinary acidification defect [77,78].
Genetics
Twin studies
SS can be considered as a complex genetic disorder. However, there are little data concerning the heritability of SS and the relative genetic risk is unknown. Two groups have reported identical twins with SS, but the concordance of SS among twin pairs is unknown [79,80].
Familial aggregation
Familial clustering of different autoimmune diseases (30–35%) has been described in SS. The most frequently reported autoimmune disease among first degree relatives were autoimmune thyroid disease, SLE and RA. There are also reports of systemic sclerosis and multiple sclerosis amongst relatives of patients with SS [81,82].
HLA
Like in other autoimmune disease, both HLA and non-HLA genes have been associated with SS. HLA-DR and HLA-DQ have the strongest association with SS and this has been demonstrated in different populations including Caucasian, Japanese and Chinese populations, though variation in HLA alleles/haplotypes is seen across different ethnicities [83,84]. The HLA associations were found to be stronger in the subsets with anti-Ro and anti-La positivity [85–87]. Recently, negative epistatic interaction was revealed between HLADR3 and proinflammatory P2RX7 receptor polymorphisms [88]. Gain of function in the latter increases susceptibility to seropositive SS only in those lacking HLA DR3 risk alleles. P2RX7 receptor activation causes opening of a cationic channel that has a role in cell death.
A recent meta-analysis on HLA class II alleles, analysed 1166 SS patients and 6470 controls from 23 studies across the world. HLA DQA1*05:01, DQB1*02:01, and DRB1*03:01 alleles were found to be associated with increased disease risk with odds ratio of 3.41 (p<0.001), 1.85 (p=0.011) and 2.28 (p<0.001), respectively. Conversely, DQA1*02:01, DQA1*03:01 and DQB1*05:01 alleles were found to be protective [89]. Non-HLA genes implicated in SS are given in Table 1.
Table 1.
Selected Non-HLA genes implicated in SS.
| Pathway | Gene | Gene Function | Reference |
|---|---|---|---|
| T cell function and signalling | |||
| PTPN22 | T-cell receptor and B-cell receptor signalling | [90] | |
| CTLA4 | negative regulator of the T cell function and thereby maintains peripheral tolerance | [91] | |
| GTF2IRD1-GTF2I | GTF2IRD1- Function not established GTF2I -general transcription factor IIi (TFII-I), important for transcription and signal transduction in T cells and B cells |
(92) | |
| STAT4 | Transcription factor regulating T-cell function | [19,92–97] | |
| TCRBV | T cell Receptor | [98] | |
| B cell function and signalling | |||
| BLK | Non-receptor tyrosine kinase involved in B-cell receptor signaling and B-cell development |
[19] | |
| FAM167A-BLK | FAM167A- not established | (93) | |
| EBF-1 | Transcription factor for B cell development | [93] | |
| BAFF | B-cell activating factor, crucial for B cell maturation | [99] | |
| Antigen presentation, processing and costimulation | |||
| Ro-52 | Autoantigen | [100,101] | |
| TAP2 | Antigen Processing | [102] | |
| CD40 | Costimulatory molecule on B cells, other antigen presenting cells | [93] | |
| CD14 | Pattern recognition receptor | [93] | |
| TNFSF4/OX40L | Cytokine expressed on multiple immune cells Binds to TNFRSF4 resulting in T-cell proliferation and cytokine production. |
[93] | |
| Cytokine, related factors and receptors | |||
| IL-10 | Anti-inflammatory | [103–105] | |
| IL-4 receptor alpha (IL-4Rα) | Cytokine of Th2 pathway | [106] | |
| IRF5(Interferon regulatory factor 5) | Transcription factor regulating IFN production | [19,92,107,108] | |
| IRF5-TNPO3 | Transcription factor regulating IFN production | [93] | |
| IL-12 A | Important cytokine of Th1 pathway | [19] | |
| TNF-α | Pro-inflammatory | [109] | |
| Chemokine Receptor | |||
| CXCR5 | Receptor for CXC chemokine ligand 13 Crucial for B cell homing and lymphoid follicle organisation |
[19] | |
| NFkB signalling | |||
| IkBα | Inhibitor of NFkB | [110] | |
| TNFAIP3 | Inhibitor of NFkB | [95] | |
| TNIP1 | Inhibitor of NFkB | [19] | |
| Miscellaneous | |||
| FCGR | Bind to Fc portion of IgG Copy number variation decreases clearance of immune complexes |
[111] | |
| MBL | Activates third pathway of complement | [112] | |
| NCR3 | NK-cell-activating receptor NK cell–specific receptor interaction with with B7-H6 on SGEC causes INFγ release |
[33] | |
| ApoE | Regulates inflammation | [113] | |
| Fas | Apoptosis | [114] | |
| MECP2 | DNA methylation-induced transcription silencing | [115] | |
| CHRM3 | Muscarinic receptor 3 | [116] | |
| GST | Glutathione S-transferase prevents oxidative damage | [117] | |
PTPN22- Protein tyrosine phosphatase, non-receptor type 22
CTLA4 -Cytotoxic T lymphocyte–associated antigen 4
GTF2IRD1-GTF2I- General transcription factor Iii/GTF2I repeat domain containing 1
STAT4 -Signal transducer and activator of transcription 4
TCRBV - T cell receptor b variable
BLK- B lymphocyte kinase
FAM167A-BLK Family with Sequence Similarity 167, Member A
EBF-1 Early B-cell factor 1
BAFF B-cell activating factor
TAP2 Transporters associated with antigen processing
TNFSF4/OX40L- Tumor necrosis factor (ligand) superfamily, member 4
IRF5 -Interferon regulatory factor 5
TNF-α Tumor necrosis factor-α
CXCR5 Chemokine (C-X-C motif) receptor 5
TNFAIP3 Tumor necrosis factor, alpha-induced protein 3
TNIP1-- TNFAIP3-interacting protein 1
FCGR Immunoglobulin gamma Fc region receptor II-b
MBL Mannose-binding lectin
NCR3-- Natural-cytotoxicity-triggering receptor 3
MECP2 Methyl CpG binding protein 2
CHRM3 Muscarinic receptor 3
GST-Glutathione-S-transferase
Genome-Wide Association Studies (GWAS)
Understanding of genetics in SS has advanced with the advent of GWAS. GWAS of patients of European descent included a discovery cohort of 424 SS cases and 2120 healthy controls and a replication cohort of 1194 SS cases and 2930 healthy controls. This GWAS, while reiterating previously described associations of HLA, IRF5, STAT4, and BLK genetic loci, also detected novel susceptible loci at IL-12A and CXCR5 regions [19]. GWAS from Han Chinese population identified GTF2IRD1-GTF2I as a new genetic risk factor for SS [95]. Association with TNFAIP3 in Han Chinese and TNIP3 in Caucasians implicate a role for NFkB signalling in SS as described above.
Epigenetics
As in other autoimmune diseases, the relevance of epigenetics in SS is just being appreciated. (Table 2)
Table 2.
Epigenetic modifications identified in SS.
| Gene/Target | Alteration in SS | Functional significance | Reference |
|---|---|---|---|
|
| |||
| MicroRNA | |||
|
| |||
| mir-17-92 | Downregulated in MSG | Lymphoproliferation | [118] |
|
| |||
| miR-574 | Downregulated in MSG | miR-768-3p increases, miR-574 decreases with increasing focus scores | |
|
| |||
| miR-768-3p | Upregulated in MSG | ||
|
| |||
| miR-146a/b | Upregulated in MSG | Promotes phagocytosis and negative regulator of TLR signalling | [119] |
|
| |||
| miR-146a/b | Upregulated in PBMC | ||
|
| |||
| miR-155 | Upregulated in PBMC | Increases Th1/Th17 subsets | |
|
| |||
| DNA methylation | |||
|
| |||
| CD70 | Hypomethylated | Overexpression of CD70 in pSS CD4+ Tcells | [120] |
|
| |||
| Global DNA methylation in SGEC, peripheral T cells and B cells | Demethylation in SGEC from SS but not in T and B cells Demethylation in SGEC may be due to PKC inhibition |
Decrease in DNA methyl transferase and increase in Gadd45-alpha expression ICAM1 overexpression |
[121] |
|
| |||
| FOXP3 | Hypermethylated | Decreased FOXP3expression | [122] |
|
| |||
| LTA | Hypomethylated | Promotes IFN production, lymphoid structure formation TCR zeta chain |
[123] |
| CD247 | T cell proliferation | ||
| TNFRSF25 | T- and B-cell receptor signalling | ||
| PTPRC | |||
| GSTM1 | Detoxification of electrophilic compounds | ||
| PDCD1 | Negative regulator of immune system | ||
|
| |||
| STAT1 IFI44L USP18 IFITM1 |
Hypomethylated | Interferon signature | |
|
| |||
| RUNX1 | Hypermethylated | Lymphoma risk | |
|
| |||
| SLC11A1 SLC11A2 SLC22A23 SLC25A25 SLC25A3 SLC25A33 SLC6A20 |
Hypomethylated | Solute carrier proteins-may be relevant for exocrine function, renal tubular acidosis | |
|
| |||
| SLC9A1 | Hypermethylated | ||
LTA -lymphotoxin α.
TNFRSF25
PTPRC -Protein tyrosine phosphatase, receptor type, C/CD45 antigen/leukocyte common antigen (LCA)
GSTM1- Glutathione S-transferase Mu 1
PDCD1- Programmed cell death protein 1
Environmental factors
Hypothetically, in a genetically predisposed individual, an environmental trigger initiates the process of autoimmunity. The environmental triggers are believed to be infectious agents, most likely a virus. The limited clonality of T cell receptor repertoire and B cell hypermutation are suggestive of an antigen driven response.
Viral trigger
Viral nucleic acid is usually recognized by TLRs and this leads to interferon production, apoptosis and upregulation of adhesion molecules. The possible candidate viruses are Epstein–Barr virus (EBV), cytomegalovirus (CMV), human herpes virus type 8 (HHV-8), human T-lymphotropic virus type 1 (HTLV-1), hepatitis C virus, and enterovirus.
EBV
EBV is an ubiquituous virus that evades human immune system and causes chronic infection. It is believed to induce autoimmunity and has been linked to multiple autoimmune diseases such as RA, SLE and MS [124,125]. EBV has a predilection for infecting B cells and causes chronic lymphoproliferation. High viral load of EBV have been found in saliva, salivary gland and lacrimal gland biopsies in SS as compared to controls indicating possibility of chronic infection of epithelium [126–129] as a trigger for autoimmunity. Also, a peptide on Epstein-Barr virus nuclear antigen-1 (EBNA-1) was found to cross react to an epitope on Ro-60, implying a role for molecular mimicry [130]. Fox, et al. also reported that presence of EBV in saliva and salivary gland correlated with diseases severity and extraglandular manifestations. However, results were not consistent and some studies showed no difference between presence of viral antigen in salivary gland of SS and healthy controls [131,132]. All the studies mentioned so far with the exception of study on a Chinese population, looked into EBV expression in salivary gland epithelium only and not lymphocytes, though B cell tropism of the virus is well known. The study on salivary glands in a Chinese population found EBV on both epithelial cells and lymphocytes by DNA in situ hybridization. But interestingly, EBV-encoded small RNAs (EBER) were detected in lymphocytes only and not in epithelial cells [127]. Role of EBV infection of B lymphocytes and a possible role of the virus in SS-associated B cell activation and lymphoproliferation can be appreciated from a recent study by Croia et al [133]. Latent EBV protein represented by LMP2A was expressed in CD20+B cell follicles of SS salivary gland. As in the Chinese study, EBER was detected in B cells of SS salivary gland, more so in those B cells with active germinal centres. BFRF1, an EBV protein expressed during lytic replication representative of EBV reactivation was detected exclusively in the perifollicular plasma cells of the ectopic lymphoid structures of SS salivary gland. Interestingly, BFRF1 was mostly localised to the autoreactive plasma cells expressing immunoreactivity for Ro-52. In another study, saliva from patients with SS was found to be endowed with the ability to activate EBV via mitogen activated protein kinase pathway [134]. Taken together, these studies provide some insight into a role for EBV in SS, though they do not imply causality.
HTLV-1
Evidence for role of HTLV1 in SS comes from both endemic as well as non-endemic regions. In Nagasaki prefecture of Japan, a region endemic for HTLV-1, 13 out of 20 patients with HTLV-I-associated myelopathy fulfilled European Community criteria for SS [135]. Antibodies to HTLV1 have also been detected in patients with SS in non-endemic regions like France [136]. Tax gene of the virus was detected in LSG samples; and further, HTLV-1 tax transgenic mice exhibited SS-like features of autoimmune exocrinopathy. Serology for HTLV1 and 2 were, however, negative in one pilot study on systemic autoimmune diseases including SS in Asian Indian patients [137].
Coxsackie
Coxsackie viral RNA was demonstrated in LSG biopsies by RT-PCR, however these results could not be replicated in a subsequent study [138,139]. Antibody to a homologous peptide of Coxsackie virus 2B protein was found to cross react with antibodies to major epitope of Ro60 kD autoantigen of SS [140].
Viral Trigger Summary
From the above evidence, a ubiquitous virus as a causative agent is likely, though at present conclusive evidence for a viral infection and the identity of such a virus remains elusive. More recently, in Italian patients, there has been some suggestion of involvement of Chlamydophila psittaci in SS pathogenesis as well as associated lymphomagenesis [141].
Sex Hormones
Putative role for hormonal factors in disease pathogenesis is hypothesized based on the increased disease incidence of SS in women around the perimenopausal age and skewed sex ratio. Both estrogen and androgens appear to protect glandular epithelial cells from apoptosis [142,143]. Experiments on salivary gland cell lines demonstrated that defective androgen influence could result in impaired extracellular matrix remodelling and acinar atrophy in SS patients as compared to healthy controls [144]. Ovariectomy has been shown to cause SS-like disease in mice models [145]. Also, aromatase knock out mice, which is representative of estrogen deficient state, developed B cell hyperplasia, salivary gland infiltration and anti-fodrin antibodies mimicking SS; the effects were aggravated when the mice were fed phytoestrogen-free diet [146].
Effect of ovariectomy or menopause may be less pronounced in humans as, unlike mice, humans possess an adrenal reticular zone that produces dehydroepiandrosterone (DHEA). In circulation, DHEA exists in the sulphated form of DHEA-S. Based on local tissue requirements, DHEA-S is metabolised by aromatase into sex steroids in the peripheral organs including salivary gland [147]. Interestingly, both serum and salivary levels of DHEA, testosterone and dihydrotestosterone in SS were found to be low as compared to healthy controls [148–150]. Impaired processing of DHEA-S in salivary gland of patients with SS could account for low salivary levels of DHEA [151]. Low serum levels, on the other hand, could result from adrenal insufficiency or hypothalamopituitary axis dysfunction. Notably antibodies to 21-hydroxylase was detected in 17.5% of patients with SS and were found to correlate with salivary gland expression of IFNα and BAFF. These patients had subnormal adrenal response to ACTH, though no frank insufficiency was seen [152]. In addition, there is some suggestion of impaired hypothalamopituitary gonadal axis, prolactin and thyroid abnormalities in SS [153]. Such abnormalities may be the result of chronic illness. In fact, sex hormones measured at the onset of illness in SLE patients do not differ from controls [154]. Thus, similar to the situation of a viral trigger, cause and effect is not established for sex hormone abnormalities in SS. Other potential explanations for the female bias of the disease such as skewed X chromosome inactivation, acquired X monosomy or X chromosome dose effect have not been studied. However, we have found that 47,XXY is found in excess among men with SS, while 47,XXX is found in excess among women with SS (Harris V, Danda D, Kurien BT, Scofield RH, unpublished data).
Pathogenesis of features characteristic and unique to SS
Exocrine dysfunction and sicca symptoms
Dysfunction of lacrimal and salivary glands is the characteristic feature of SS. Traditionally, it has been believed to occur due to glandular damage resulting from lymphocytic infiltration, apoptosis and cytotoxic cell death mediated by granzyme and Fas-Fas ligand interactions [20,155]. However, apoptosis has been found to be unusual in MSG biopsy of patients with SS. Also, there is disparity between lymphocytic infiltration and secretory dysfunction such that sicca symptoms are out of proportion to what could be explained by glandular destruction alone. The fact that secretagogues stimulate secretion of these glands indicates that glandular tissue is potentially functional [156]. Production of saliva is a complex process involving the acinar cells and salivary exocytosis. The structure of acinar cell as well as the secretory apparatus can be affected in SS due to altered expression and function of proteins involved.
The cause of secretory dysfunction is probably multifactorial. The different mechanisms involved potentially include
Altered glandular homeostasis -
Inflammatory –Lymphocytic infiltration, Cytokines, Anti-M3 muscarinic receptor antibody.
1. Altered glandular homeostasis in the preimmune and non-immune phase
There is evidence to suggest that altered glandular homeostasis could be the initiating event, prior to onset of inflammation, leading to secretory dysfunction. Altered homeostasis could also trigger autoimmune response and inflammation contributing to tissue damage and further dysfunction. Both patients and mouse models have been observed to have exocrine dysfunction preceding evidence of inflammation. NOD mice are a spontaneous model for SS and are believed to be representative of the complex pathogenesis of disease in humans. Experiments on NOD mice give insight into early glandular changes in SS. These mice have been shown to have a preimmune phase followed by an autoimmune phase [157]. Rosignoli, et al. reported decreased nitric oxide synthase (NOS) production and impaired vasocactive intestinal polypeptide (VIP) signalling in parotid and submandibular salivary gland of 12 week old NOD mice preceding inflammation [158]. These neurosecretory abnormalities correlated with decreased glandular function. Further, acinar tissue loss in NOD-SCID mice in the absence of inflammation highlights the role played by non-immune factors in secretory dysfunction [159]. NOD-SCID mice had alteration in salivary protein profile such as increased protease activity, which could account for acinar tissue loss and secretory dysfunction [160]. Increased protease activity and increased parotid secretory protein l could also result in the production of abnormal protein with increased immunogenicity [161].
Interestingly, changes in NOD mice are not only seen just prior to onset of inflammation but as early as neonatal period. The mice predisposed to autoimmune exocrinopathy had abnormal organogenesis of submandibular gland, decreased acinar cell proliferation, increased matrix metalloproteinase activity and increased apoptosis [162]. Hence it can be conceived that these aberrations provide fertile soil for induction of autoimmunity, in addition to causing secretory dysfunction.
Maintenance of polarity of acinar cell is crucial for normal secretory function. The structure of acinar cell can be affected in SS due to altered expression and function of proteins involved. The acinar cells of patients with SS secrete matrix metalloproteinase in excess of the tissue inhibitor of metalloproteinase resulting in destruction of basal lamina. These changes did not correlate with inflammation [163]. Also, in SS patients with disrupted basal lamina, altered levels of a6b4 integrin were detected [164]. This adhesion receptor is important in maintaining cell-cell interactions; and, therefore, crucial in acinar cell survival. Changes in components of acinar proteins such as ezrin, actin cytoskeleton, and tight junction proteins as well as disrupted microvilli have been described in patients with SS. Tight junction proteins include occludin, ZO-1, claudins 1, 3 and 4. The first two were downregulated whereas claudin 1 and claudin 4 were upregulated in SS patients. Claudin 3 was redistributed to basolateral membrane from apical region as compared to controls. While these changes could be induced by TNF and IFNγ, the change in claudin distribution was found to be independent of inflammation [165]. Thus, structural integrity of acinar cells may be crucial to the pathogenesis of SS.
Secretory apparatus involved in trafficking and membrane fusion has also been found to be impaired in SS. Rab3D, a member of Ras-related small G protein family has a crucial role in exocytosis. Alteration and redistribution of Rab3D could cause change in quality and quantity of saliva [166]. While low Rab3D inversely correlated with glandular function in SS patients as measured by scintigraphy, no relation with focus score was seen. SNARE proteins- STX4, SNAP-23 and VAMP8 were found to be redistributed to basolateral membrane in acinar cells of patients with SS [167]. There is also evidence to suggest that distribution of aquaporin-5 in salivary gland acini is altered in SS with more basal expression than acinar expression and that this could contribute to hyposalivation. Abnormal aquaporin distribution was seen even in those lacking inflammation [168]. Like in NOD mice, it is possible that altered epithelial cell organisation that is critical to exocrine secretion could lead to loss of tolerance and chronic autoimmunity in human SS.
Calcium signalling is an integral part of any secretory process. In SS, impairment of this signalling may occur due to high nitric oxide (NO) exposure [169,170]. High NO exposure has been related to salivary hyposecretion [171]. The role of calcium signalling is also exemplified by development of SS-lke phenotype in STIM 1 and STIM 2 deficient models described previously [44].
Further, increased oxidative damage has also been described in SS [172,173]. This was not dependent on lymphocytic infiltration. Increased oxidative stress induced production of antioxidant thioredoxin, levels of which inversely correlated with salivary secretion [173].
2. Immune Phase and inflammation
Lymphocytic infiltration could result in secretory dysfunction by disrupting glandular architecture, cytokine production or autoantibody formation. Th1, Th2 and Th17 cytokines are all implicated in exocrine dysfunction [37,174,175]. These cytokines act by aggravating lymphocytic infiltration and potentiation of inflammation, but they also have other functions for instance role of IL-4 in antibody production and role of TNF in disrupting tight junctions [165,176]. As mentioned earlier INFγ has an important role in pre-immune and immune phase as well as exemplified by findings in NOD.IFNγ −/−and NOD.IFNγR−/− mice [37]. The inflammatory environment induces the release of matrix metalloproteinases that cause destruction of extracellular matrix and could interfere with polarity of cell.
Cytokines especially IL-1 and TNFα could also inhibit release of acetylcholine from nerve terminals and also blunt response of muscarinic ACh receptor s (mAChRs) to neurotransmitter again resulting in glandular dysfunction [177]. Increased levels of cholinesterases have been demonstrated in salivary glands and saliva of mice models and patients, respectively. These could degrade Ach and lead to decreased availability of this neurotransmitter [178,179].
As mentioned previously aquaporins facilitate movement of water and studies, though inconsistent have shown redistribution of aquaporin 5 in salivary glands of patients with SS [180,181]. Recently, this has been found to correlate with the degree of inflammation seen in MSG biopsy of animal models of SS [182].
Autoantibodies against muscarinic acetylcholine receptor M3 (M3R) have been described in patients with SS. Wide variation in frequency of these antibodies in literature (9–97%) have been described [183–185]. Functional assays have demonstrated that antibodies to M3R, especially that against the 2nd extracellular domain, could promote inflammation by activating phospholipase A2 and result in decrease salivary secretion by inhibiting carbachol and cevimeline induced Ca2+ release, inhibiting NOS and cGMP production and trafficking of aquaporin-5 [183,186,187].
Mucins are also an important constituent of saliva and impaired secretion and change in quality of mucin contributes to perception of dryness in SS. Hyposulfation of a mucin called MUC5B occurs due to inflammation and subsequent decreased sulfotransferase activity in the Golgi complex of acinar cells [188]. Sulfation results in water retaining capacity and hyposulfation in labial salivary gland has been found to correlate with inflammation, antibodies and xerostomia in patients with SS [189].
Congenital Heart Block
Congenital heart block (CHB) is a clinical manifestation of neonatal lupus that is a passively acquired autoimmune condition related to the presence of maternal antibodies to Ro/SSA and/or La (SS-B) rather than the maternal diagnosis of SS, or any other disease. Strictly speaking, this condition is not limited to mothers with SS and the mother may be asymptomatic or have SLE. In addition to heart block, the fetal endocardium and myocardium are also involved and such involvement portends poor prognosis. The pathogenetic mechanisms are far from clear and have been recently reviewed [190,191]. There are two main hypotheses in vogue:
The apoptotic hypothesis states that the maternal anti-Ro60 antibodies bind to Ro-60 found in apoptotic blebs of the fetal cardiac system initiating an inflammatory reaction mediated by macrophages culminating in fibrosis. This has been substantiated by autopsy studies on fetal heart with CHB/myocarditis demonstrating apoptosis, immunoglobulin deposition, macrophages, fibrosis and microcalcification not only in the conduction system, but also in the myocardium and endocardium [192]. Impaired physiologic apoptosis and phagocytosis stimulates plasmin pathway via urokinase plasminogen activator (uPA)/uPA receptor upregulation leading on to TGFβ release and fibrosis [193]. Endothelin-1 secereted by macrophages has also been implicated in fibrosis [194].
The other hypopthesis is based on molecular mimicry and cross reactivity. Anti-Ro-52 antibodies cross-react with the cardiac membrane proteins and interfere with signalling. The candidate proteins against which cross-reactivity has been identified are serotoninergic 5-HT 4 receptor and L type calcium channels (LTCC)[195,196]. The potential role for LTCC in SS is further reiterated by data from experiments on LTCC knock out and transgenic pups of mothers passively immunized with anti-Ro and anti-La antibodies [197]. The LTCC knock out mice developed heart block and while in the transgenic pups, the effect of maternal antibodies was abrogated to some extent.
Recently, anti-Ro-52 monoclonal antibodies specific for amino acid 200–239, also called p200 on being injected into pregnant rat was found to induce CHB in the offspring [198]. Further, the same authors also demonstrated that anti-Ro-52 p200 antibodies could interfere with calcium signalling of cardiac myocytes which signified a functional role for these antibodies in pathogenesis of CHB.
The risk of fetus developing CHB in a mother with anti-Ro antibodies and previously unaffected fetus is 2%. After one affected child, the recurrence risk is 12–20% in subsequent pregnancies. Thus, mechanisms beyond maternal antibodies are involved in the pathogenesis. Maternal and fetal genetic make-up, maternal age as well as time of conception and role for vitamin D deficiency are being investigated [199,200]. For instance, genetic polymorphisms of TNFα and TGFβ have been implicated, in addition to other genes related to inflammation and apoptosis identified at loci 6p21 and 21q22 in a recently conducted GWAS study [201].
Lymphoma
With a Standardised Incidence Ratio (SIR) of 18.8% for lymphoma in a recent metaanalysis, SS has the highest prevalence of lymphoma among all autoimmune diseases [202]. This complication occurs at a median of 7.5 years after SS diagnosis [203]. Marginal zone lymphomas, which may be nodal or extranodal (MALT lymphoma), are the most common followed by high grade diffuse large B cell lymphoma (DLBCL) in most [204–206] but not all cohorts [207,208]. Persistent enlargement of parotid glands, splenomegaly, lymphadenopathy, cutaneous vasculitis, neuropathy, mixed monoclonal cryoglobulinemia, CD4 T-lymphocytopenia and low C4 levels are listed risk factors for lymphomagenesis [204,209].
Ectopic GC described earlier is probably the starting point of lymphomagenesis in SS. It is likely that this ectopic GC is representative of the widespread immunological aberration and lymphoproliferation and may be representative of similar process throughout the body in those at high risk of lymphoma. The journey from ectopic GC to lymphoma is a multi-step complex process orchestrated by chemokines, cytokines in the background of viral infection and genetic susceptibility. Several characteristics of ectopic GC may lead to breakdown of tolerance resulting in production of autoreactive B cells. This includes marginal zone like phenotype and absence of encapsulation of GC resulting in constant exposure to antigens, cytokines and immune cells. Further the presence of activation-induced cytidine deaminase (AICDA) that catalyses somatic hypermutation also results in loss of tolerance. [45,210]. Aided by T cells, these autoreactive B cells proliferate resulting in oligoclonal and monoclonal antibody production. Neoplastic B cell clones from SS-associated lymphoma commonly produce rheumatoid factor [211]. Chronic antigenic stimulation by virus or autoantigen and cytokines could initiate and sustain these clones with restricted use of IGH and kappa (IGK) gene repertoires.
Variants in several genes have been described in SS-associated lymphoma. These include BAFF, FMS-like tyrosine kinase 3 ligand and TNFAIP3[212–214]. The chromosomal translocation t(11;18) has been described in a small subset of SS-associated MALT lymphomas not expressing RF. MALT lymphomas acquire further genetic aberrations, for instance, inactivation of tumor suppressor gene p53 and p15 and p16 genes and subsequently transform into high grade lymphoma [215].
Summary
While it is unlikely that a single etiological agent, gene or a unified mechanism could explain various facets of this protean disease, recent developments have provided interesting insights regarding pathogenesis. SGEC, cells of innate and adaptive immune system fuelled by interferons and other cytokines trigger and maintain chronic immune activation and vicious cycle of autoimmunity in a host with genetic susceptibility. The resultant autoimmune epithelitis affects exocrine glands as well as various other organs. In addition to immune mechanisms, several non-immune factors may be involved in pathogenesis. The role of immune and non-immune factors is summarised in Figure 2.
Figure 2.
Interplay of immune and non-immune factors in pathophysiology of SS
EC - Epithelial cell, DC - Dendritic cell, CHB - Congenital Heart Block.
Identity of etiological agent still seems elusive though there is some evidence for role of EBV in lymphoproliferation. The GWAS and other gene studies highlights the role played by interferon pathway, NFkB pathway, Th subsets and B cell stimulation with words of caution, in view of low odds ratios of these genetic associations. This may argue for a greater role for environmental and epigenetic factors in pathogenesis. Better understanding of etiopathogenesis is expected to evolve novel therapeutics, preventive measures and even a possible cure for this enigmatic disease in the long run.
Footnotes
Conflict of Interest:
Authors declare no conflict of interest
References
- 1.Manoussakis MN, Kapsogeorgou EK. The role of intrinsic epithelial activation in the pathogenesis of Sjögren’s syndrome. J Autoimmun. 2010;35(3):219–24. doi: 10.1016/j.jaut.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Lindahl G, Hedfors E, Klareskog L, Forsum U. Epithelial HLA-DR expression and T lymphocyte subsets in salivary glands in Sjögren’s syndrome. Clin Exp Immunol. 1985;61(3):475–82. [PMC free article] [PubMed] [Google Scholar]
- 3.Moutsopoulos HM, Hooks JJ, Chan CC, Dalavanga YA, Skopouli FN, Detrick B. HLA-DR expression by labial minor salivary gland tissues in Sjögren’s syndrome. Ann Rheum Dis. 1986;45(8):677–83. doi: 10.1136/ard.45.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsunawaki S, Nakamura S, Ohyama Y, et al. Possible function of salivary gland epithelial cells as nonprofessional antigen-presenting cells in the development of Sjögren’s syndrome. J Rheumatol. 2002;29(9):1884–96. [PubMed] [Google Scholar]
- 5.Xanthou G, Polihronis M, Tzioufas AG, Paikos S, Sideras P, Moutsopoulos HM. “Lymphoid” chemokine messenger RNA expression by epithelial cells in the chronic inflammatory lesion of the salivary glands of Sjögren’s syndrome patients: possible participation in lymphoid structure formation. Arthritis Rheum. 2001;44(2):408–18. doi: 10.1002/1529-0131(200102)44:2<408::AID-ANR60>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa N, Ping L, Zhenjun L, Takada Y, Sugai S. Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjögren’s syndrome. Arthritis Rheum. 2002;46(10):2730–41. doi: 10.1002/art.10577. [DOI] [PubMed] [Google Scholar]
- 7.Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjögren’s syndrome. J Immunol. 1994;152(11):5532–9. [PubMed] [Google Scholar]
- 8.Jin J-O, Shinohara Y, Yu Q. Innate immune signaling induces interleukin-7 production from salivary gland cells and accelerates the development of primary Sjögren’s syndrome in a mouse model. PloS One. 2013;8(10):e77605. doi: 10.1371/journal.pone.0077605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bombardieri M, Barone F, Pittoni V, et al. Increased circulating levels and salivary gland expression of interleukin-18 in patients with Sjögren’s syndrome: relationship with autoantibody production and lymphoid organization of the periductal inflammatory infiltrate. Arthritis Res Ther. 2004;6(5):R447–56. doi: 10.1186/ar1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciccia F, Guggino G, Rizzo A, et al. Potential involvement of IL-22 and IL-22-producing cells in the inflamed salivary glands of patients with Sjogren’s syndrome. Ann Rheum Dis. 2012;71(2):295–301. doi: 10.1136/ard.2011.154013. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Tsutsui H, Yoshimoto T, et al. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8(3):383–90. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 12.Sonnenberg GF, Fouser LA, Artis D. Functional biology of the IL-22-IL-22R pathway in regulating immunity and inflammation at barrier surfaces. Adv Immunol. 2010;107:1–29. doi: 10.1016/B978-0-12-381300-8.00001-0. [DOI] [PubMed] [Google Scholar]
- 13.Gong Y-Z, Nititham J, Taylor K, et al. Differentiation of follicular helper T cells by salivary gland epithelial cells in primary Sjögren’s syndrome. J Autoimmun. 2014;51:57–66. doi: 10.1016/j.jaut.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Spachidou MP, Bourazopoulou E, Maratheftis CI, et al. Expression of functional Toll-like receptors by salivary gland epithelial cells: increased mRNA expression in cells derived from patients with primary Sjögren’s syndrome. Clin Exp Immunol. 2007;147(3):497–503. doi: 10.1111/j.1365-2249.2006.03311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deshmukh US, Nandula SR, Thimmalapura P-R, Scindia YM, Bagavant H. Activation of innate immune responses through Toll-like receptor 3 causes a rapid loss of salivary gland function. J Oral Pathol Med. 2009;38(1):42–7. doi: 10.1111/j.1600-0714.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manoussakis MN, Spachidou MP, Maratheftis CI. Salivary epithelial cells from Sjogren’s syndrome patients are highly sensitive to anoikis induced by TLR-3 ligation. J Autoimmun. 2010;35(3):212–8. doi: 10.1016/j.jaut.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Peng B, Ling J, Lee AJ, et al. Defective feedback regulation of NF-kappaB underlies Sjogren’s syndrome in mice with mutated kappaB enhancers of the IkappaBalpha promoter. Proc Natl Acad Sci U S A. 2010;107(34):15193–8. doi: 10.1073/pnas.1005533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sisto M, Lisi S, Lofrumento DD, Ingravallo G, Maiorano E, D’Amore M. A failure of TNFAIP3 negative regulation maintains sustained NF-κB activation in Sjögren’s syndrome. Histochem Cell Biol. 2011;135(6):615–25. doi: 10.1007/s00418-011-0821-3. [DOI] [PubMed] [Google Scholar]
- 19.Lessard CJ, Li H, Adrianto I, et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren’s syndrome. Nat Genet. 2013;45(11):1284–92. doi: 10.1038/ng.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumura R, Umemiya K, Kagami M, et al. Glandular and extraglandular expression of the Fas-Fas ligand and apoptosis in patients with Sjögren’s syndrome. Clin Exp Rheumatol. 1998;16(5):561–8. [PubMed] [Google Scholar]
- 21.Matsumura R, Umemiya K, Goto T, et al. Interferon gamma and tumor necrosis factor alpha induce Fas expression and anti-Fas mediated apoptosis in a salivary ductal cell line. Clin Exp Rheumatol. 2000;18(3):311–8. [PubMed] [Google Scholar]
- 22.Varin M-M, Guerrier T, Devauchelle-Pensec V, Jamin C, Youinou P, Pers J-O. In Sjögren’s syndrome, B lymphocytes induce epithelial cells of salivary glands into apoptosis through protein kinase C delta activation. Autoimmun Rev. 2012;11(4):252–8. doi: 10.1016/j.autrev.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Strandberg L, Ambrosi A, Espinosa A, et al. Interferon-alpha induces up-regulation and nuclear translocation of the Ro52 autoantigen as detected by a panel of novel Ro52-specific monoclonal antibodies. J Clin Immunol. 2008;28(3):220–31. doi: 10.1007/s10875-007-9157-0. [DOI] [PubMed] [Google Scholar]
- 24.Emamian ES, Leon JM, Lessard CJ, et al. Peripheral blood gene expression profiling in Sjögren’s syndrome. Genes Immun. 2009;10(4):285–96. doi: 10.1038/gene.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Blokland SC, van Helden-Meeuwsen CG, Wierenga-Wolf AF, et al. Two different types of sialoadenitis in the NOD- and MRL/lpr mouse models for Sjögren’s syndrome: a differential role for dendritic cells in the initiation of sialoadenitis? Lab Invest. 2000;80(4):575–85. doi: 10.1038/labinvest.3780062. [DOI] [PubMed] [Google Scholar]
- 26.Vogelsang P, Brun JG, Oijordsbakken G, Skarstein K, Jonsson R, Appel S. Levels of plasmacytoid dendritic cells and type-2 myeloid dendritic cells are reduced in peripheral blood of patients with primary Sjogren’s syndrome. Ann Rheum Dis. 2010;69(6):1235–8. doi: 10.1136/ard.2009.118158. [DOI] [PubMed] [Google Scholar]
- 27.Guo Z, Li H, Han M, Xu T, Wu X, Zhuang Y. Modeling Sjögren’s syndrome with Id3 conditional knockout mice. Immunol Lett. 2011;135(1–2):34–42. doi: 10.1016/j.imlet.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujikado N, Saijo S, Yonezawa T, et al. Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat Med. 2008;14(2):176–80. doi: 10.1038/nm1697. [DOI] [PubMed] [Google Scholar]
- 29.Hjelmervik TOR, Petersen K, Jonassen I, Jonsson R, Bolstad AI. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjögren’s syndrome patients from healthy control subjects. Arthritis Rheum. 2005;52(5):1534–44. doi: 10.1002/art.21006. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y-J. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 31.Gottenberg J-E, Cagnard N, Lucchesi C, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren’s syndrome. Proc Natl Acad Sci U S A. 2006;103(8):2770–5. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brkic Z, Maria NI, van Helden-Meeuwsen CG, et al. Prevalence of interferon type I signature in CD14 monocytes of patients with Sjogren’s syndrome and association with disease activity and BAFF gene expression. Ann Rheum Dis. 2013;72(5):728–35. doi: 10.1136/annrheumdis-2012-201381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rusakiewicz S, Nocturne G, Lazure T, et al. NCR3/NKp30 contributes to pathogenesis in primary Sjogren’s syndrome. Sci Transl Med. 2013;5(195):195ra96. doi: 10.1126/scitranslmed.3005727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjögren’s syndrome. J Autoimmun. 2010;34(4):400–7. doi: 10.1016/j.jaut.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Mitsias DI, Tzioufas AG, Veiopoulou C, et al. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren’s syndrome. Clin Exp Immunol. 2002;128(3):562–8. doi: 10.1046/j.1365-2249.2002.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin J-O, Kawai T, Cha S, Yu Q. Interleukin-7 enhances the Th1 response to promote the development of Sjögren’s syndrome-like autoimmune exocrinopathy in mice. Arthritis Rheum. 2013;65(8):2132–42. doi: 10.1002/art.38007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cha S, Brayer J, Gao J, et al. A dual role for interferon-gamma in the pathogenesis of Sjogren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand J Immunol. 2004;60(6):552–65. doi: 10.1111/j.0300-9475.2004.01508.x. [DOI] [PubMed] [Google Scholar]
- 38.Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with Sjögren’s syndrome immunopathogenesis. Am J Pathol. 2009;175(3):1167–77. doi: 10.2353/ajpath.2009.090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren’s syndrome: findings in humans and mice. Arthritis Rheum. 2008;58(3):734–43. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010;207(6):1293–305. doi: 10.1084/jem.20092054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavoie TN, Stewart CM, Berg KM, Li Y, Nguyen CQ. Expression of interleukin-22 in Sjögren’s syndrome: significant correlation with disease parameters. Scand J Immunol. 2011;74(4):377–82. doi: 10.1111/j.1365-3083.2011.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarigul M, Yazisiz V, Bassorgun CI, et al. The numbers of Foxp3 + Treg cells are positively correlated with higher grade of infiltration at the salivary glands in primary Sjogren’s syndrome. Lupus. 2010;19(2):138–45. doi: 10.1177/0961203309348234. [DOI] [PubMed] [Google Scholar]
- 43.Alunno A, Petrillo MG, Nocentini G, et al. Characterization of a new regulatory CD4+ T cell subset in primary Sjögren’s syndrome. Rheumatology (Oxford) 2013;52(8):1387–96. doi: 10.1093/rheumatology/ket179. [DOI] [PubMed] [Google Scholar]
- 44.Cheng KT, Alevizos I, Liu X, et al. STIM1 and STIM2 protein deficiency in T lymphocytes underlies development of the exocrine gland autoimmune disease, Sjogren’s syndrome. Proc Natl Acad Sci U S A. 2012;109(36):14544–9. doi: 10.1073/pnas.1207354109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theander E, Vasaitis L, Baecklund E, et al. Lymphoid organisation in labial salivary gland biopsies is a possible predictor for the development of malignant lymphoma in primary Sjögren’s syndrome. Ann Rheum Dis. 2011;70(8):1363–8. doi: 10.1136/ard.2010.144782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daridon C, Pers J-O, Devauchelle V, et al. Identification of transitional type II B cells in the salivary glands of patients with Sjögren’s syndrome. Arthritis Rheum. 2006;54(7):2280–8. doi: 10.1002/art.21936. [DOI] [PubMed] [Google Scholar]
- 47.Binard A, Le Pottier L, Devauchelle-Pensec V, Saraux A, Youinou P, Pers J-O. Is the blood B-cell subset profile diagnostic for Sjogren syndrome? Ann Rheum Dis. 2009;68(9):1447–52. doi: 10.1136/ard.2008.096172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kramer JM, Klimatcheva E, Rothstein TL. CXCL13 is elevated in Sjögren’s syndrome in mice and humans and is implicated in disease pathogenesis. J Leukoc Biol. 2013;94(5):1079–89. doi: 10.1189/jlb.0113036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ittah M, Miceli-Richard C, Eric Gottenberg J, et al. B cell-activating factor of the tumor necrosis factor family (BAFF) is expressed under stimulation by interferon in salivary gland epithelial cells in primary Sjögren’s syndrome. Arthritis Res Ther. 2006;8(2):R51. doi: 10.1186/ar1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackay F, Woodcock SA, Lawton P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190(11):1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pers J-O, Daridon C, Devauchelle V, et al. BAFF overexpression is associated with autoantibody production in autoimmune diseases. Ann N Y Acad Sci. 2005;1050:34–9. doi: 10.1196/annals.1313.004. [DOI] [PubMed] [Google Scholar]
- 52.Higgs R, Ní Gabhann J, Ben Larbi N, Breen EP, Fitzgerald KA, Jefferies CA. The E3 ubiquitin ligase Ro52 negatively regulates IFN-beta production post-pathogen recognition by polyubiquitin-mediated degradation of IRF3. J Immunol. 2008;181(3):1780–6. doi: 10.4049/jimmunol.181.3.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Higgs R, Lazzari E, Wynne C, et al. Self protection from anti-viral responses--Ro52 promotes degradation of the transcription factor IRF7 downstream of the viral Toll-Like receptors. PloS One. 2010;5(7):e11776. doi: 10.1371/journal.pone.0011776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshimi R, Chang T-H, Wang H, Atsumi T, Morse HC, Ozato K. Gene disruption study reveals a nonredundant role for TRIM21/Ro52 in NF-kappaB-dependent cytokine expression in fibroblasts. J Immunol. 2009;182(12):7527–38. doi: 10.4049/jimmunol.0804121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oke V, Wahren-Herlenius M. The immunobiology of Ro52 (TRIM21) in autoimmunity: a critical review. J Autoimmun. 2012;39(1–2):77–82. doi: 10.1016/j.jaut.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 56.Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21) Proc Natl Acad Sci U S A. 2010;107(46):19985–90. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jauharoh SNA, Saegusa J, Sugimoto T, et al. SS-A/Ro52 promotes apoptosis by regulating Bcl-2 production. Biochem Biophys Res Commun. 2012;417(1):582–7. doi: 10.1016/j.bbrc.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 58.Espinosa A, Zhou W, Ek M, et al. The Sjogren’s syndrome-associated autoantigen Ro52 is an E3 ligase that regulates proliferation and cell death. J Immunol. 2006;176(10):6277–85. doi: 10.4049/jimmunol.176.10.6277. [DOI] [PubMed] [Google Scholar]
- 59.Aqrawi LA, Kvarnström M, Brokstad KA, Jonsson R, Skarstein K, Wahren-Herlenius M. Ductal epithelial expression of Ro52 correlates with inflammation in salivary glands of patients with primary Sjögren’s syndrome. Clin Exp Immunol. 2014;177(1):244–52. doi: 10.1111/cei.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Espinosa A, Dardalhon V, Brauner S, et al. Loss of the lupus autoantigen Ro52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23-Th17 pathway. J Exp Med. 2009;206(8):1661–71. doi: 10.1084/jem.20090585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verhagen APM, Pruijn GJM. Are the Ro RNP-associated Y RNAs concealing microRNAs? Y RNA-derived miRNAs may be involved in autoimmunity. BioEssays. 2011;33(9):674–82. doi: 10.1002/bies.201100048. [DOI] [PubMed] [Google Scholar]
- 62.Chen X, Smith JD, Shi H, Yang DD, Flavell RA, Wolin SL. The Ro autoantigen binds misfolded U2 small nuclear RNAs and assists mammalian cell survival after UV irradiation. Curr Biol CB. 2003;13(24):2206–11. doi: 10.1016/j.cub.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 63.Liang C, Xiong K, Szulwach KE, et al. Sjogren syndrome antigen B (SSB)/La promotes global microRNA expression by binding microRNA precursors through stem-loop recognition. J Biol Chem. 2013;288(1):723–36. doi: 10.1074/jbc.M112.401323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y, Tan H, Tian H, Liang C, Chen S, Liu Q. Autoantigen La promotes efficient RNAi, antiviral response, and transposon silencing by facilitating multiple-turnover RISC catalysis. Mol Cell. 2011;44(3):502–8. doi: 10.1016/j.molcel.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grölz D, Bachmann M. The nuclear autoantigen La/SS-associated antigen B: one gene, three functional mRNAs. Biochem J. 1997;323(Pt 1):151–8. doi: 10.1042/bj3230151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barcellos KS, Nonogaki S, Enokihara MM, Teixeira MS, Andrade LE. Differential expression of Ro/SSA 60 kDa and La/SSB, but not Ro/SSA 52 kDa, mRNA and protein in minor salivary glands from patients with primary Sjögren’s syndrome. J Rheumatol. 2007;34(6):1283–92. [PubMed] [Google Scholar]
- 67.Tzioufas AG, Hantoumi I, Polihronis M, Xanthou G, Moutsopoulos HM. Autoantibodies to La/SSB in patients with primary Sjögren’s syndrome (pSS) are associated with upregulation of La/SSB mRNA in minor salivary gland biopsies (MSGs) J Autoimmun. 1999;13(4):429–34. doi: 10.1006/jaut.1999.0333. [DOI] [PubMed] [Google Scholar]
- 68.Salomonsson S, Jonsson MV, Skarstein K, et al. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren’s syndrome. Arthritis Rheum. 2003;48(11):3187–201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- 69.Maier-Moore JS, Koelsch KA, Smith K, et al. Antibody-secreting cell specificity in labial salivary glands reflects clinical presentation and serology in sjögren’s syndrome patients. Arthritis Rheumatol. 2014;66(12):3445–56. doi: 10.1002/art.38872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lisi S, Sisto M, Soleti R, et al. Fcgamma receptors mediate internalization of anti-Ro and anti-La autoantibodies from Sjögren’s syndrome and apoptosis in human salivary gland cell line A-253. J Oral Pathol. 2007;36(9):511–23. doi: 10.1111/j.1600-0714.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- 71.Lisi S, Sisto M, Lofrumento D, et al. Regulation of mRNA caspase-8 levels by anti-nuclear autoantibodies. Clin Exp Med. 2010;10(3):199–203. doi: 10.1007/s10238-009-0087-4. [DOI] [PubMed] [Google Scholar]
- 72.Lisi S, D’Amore M, Scagliusi P, Mitolo V, Sisto M. Anti-Ro/SSA autoantibody-mediated regulation of extracellular matrix fibulins in human epithelial cells of the salivary gland. Scand J Rheumatol. 2009;38(3):198–206. doi: 10.1080/03009740802520722. [DOI] [PubMed] [Google Scholar]
- 73.Reichlin M. Cellular dysfunction induced by penetration of autoantibodies into living cells: cellular damage and dysfunction mediated by antibodies to dsDNA and ribosomal P proteins. J Autoimmun. 1998;11(5):557–61. doi: 10.1006/jaut.1998.0219. [DOI] [PubMed] [Google Scholar]
- 74.Sun KH, Tang SJ, Lin ML, Wang YS, Sun GH, Liu WT. Monoclonal antibodies against human ribosomal P proteins penetrate into living cells and cause apoptosis of Jurkat T cells in culture. Rheumatol (Oxford) 2001;40(7):750–6. doi: 10.1093/rheumatology/40.7.750. [DOI] [PubMed] [Google Scholar]
- 75.Kyriakidis NC, Kapsogeorgou EK, Tzioufas AG. A comprehensive review of autoantibodies in primary Sjögren’s syndrome: Clinical phenotypes and regulatory mechanisms. J Autoimmun. 2014;51C:67–74. doi: 10.1016/j.jaut.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 76.Takemoto F, Hoshino J, Sawa N, et al. Autoantibodies against carbonic anhydrase II are increased in renal tubular acidosis associated with Sjogren syndrome. Am J Med. 2005;118(2):181–4. doi: 10.1016/j.amjmed.2004.07.049. [DOI] [PubMed] [Google Scholar]
- 77.Nishimori I, Bratanova T, Toshkov I, et al. Induction of experimental autoimmune sialoadenitis by immunization of PL/J mice with carbonic anhydrase II. J Immunol. 1995;154(9):4865–73. [PubMed] [Google Scholar]
- 78.Takemoto F, Katori H, Sawa N, et al. Induction of anti-carbonic-anhydrase-II antibody causes renal tubular acidosis in a mouse model of Sjogren’s syndrome. Nephron Physiol. 2007;106(4):63–8. doi: 10.1159/000104873. [DOI] [PubMed] [Google Scholar]
- 79.Scofield RH, Kurien BT, Reichlin M. Immunologically restricted and inhibitory anti-Ro/SSA in monozygotic twins. Lupus. 1997;6(4):395–8. doi: 10.1177/096120339700600409. [DOI] [PubMed] [Google Scholar]
- 80.Bolstad AI, Haga HJ, Wassmuth R, Jonsson R. Monozygotic twins with primary Sjögren’s syndrome. J Rheumatol. 2000;27(9):2264–6. [PubMed] [Google Scholar]
- 81.Reveille JD, Wilson RW, Provost TT, Bias WB, Arnett FC. Primary Sjögren’s syndrome and other autoimmune diseases in families. Prevalence and immunogenetic studies in six kindreds. Ann Intern Med. 1984;101(6):748–56. doi: 10.7326/0003-4819-101-6-748. [DOI] [PubMed] [Google Scholar]
- 82.Anaya J-M, Tobon GJ, Vega P, Castiblanco J. Autoimmune disease aggregation in families with primary Sjögren’s syndrome. J Rheumatol. 2006;33(11):2227–34. [PubMed] [Google Scholar]
- 83.Kang HI, Fei HM, Saito I, Sawada S, Chen SL, Yi D, et al. Comparison of HLA class II genes in Caucasoid, Chinese, and Japanese patients with primary Sjögren’s syndrome. J Immunol. 1993;150(8 Pt 1):3615–23. [PubMed] [Google Scholar]
- 84.Miyagawa S, Shinohara K, Nakajima M, et al. Polymorphisms of HLA class II genes and autoimmune responses to Ro/SS-A-La/SS-B among Japanese subjects. Arthritis Rheum. 1998;41(5):927–34. doi: 10.1002/1529-0131(199805)41:5<927::AID-ART21>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 85.Scofield RH, Frank MB, Neas BR, et al. Cooperative association of T cell beta receptor and HLA-DQ alleles in the production of anti-Ro in systemic lupus erythematosus. Clin Immunol Immunopathol. 1994;72(3):335–41. doi: 10.1006/clin.1994.1150. [DOI] [PubMed] [Google Scholar]
- 86.Rischmueller M, Lester S, Chen Z, et al. HLA class II phenotype controls diversification of the autoantibody response in primary Sjögren’s syndrome (pSS) Clin Exp Immunol. 1998;111(2):365–71. doi: 10.1046/j.1365-2249.1998.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harley JB, Reichlin M, Arnett FC, Alexander EL, Bias WB, Provost TT. Gene interaction at HLA-DQ enhances autoantibody production in primary Sjögren’s syndrome. Science. 1986;232(4754):1145–7. doi: 10.1126/science.3458307. [DOI] [PubMed] [Google Scholar]
- 88.Lester S, Stokes L, Skarratt KK, et al. Epistasis with HLA DR3 implicates the P2X7 receptor in the pathogenesis of primary Sjögren’s syndrome. Arthritis Res Ther. 2013;15(4):R71. doi: 10.1186/ar4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cruz-Tapias P, Rojas-Villarraga A, Maier-Moore S, Anaya J-M. HLA and Sjögren’s syndrome susceptibility. A meta-analysis of worldwide studies. Autoimmun Rev. 2012;11(4):281–7. doi: 10.1016/j.autrev.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 90.Gomez LM, Anaya J-M, Gonzalez CI, et al. PTPN22 C1858T polymorphism in Colombian patients with autoimmune diseases. Genes Immun. 2005;6(7):628–31. doi: 10.1038/sj.gene.6364261. [DOI] [PubMed] [Google Scholar]
- 91.Downie-Doyle S, Bayat N, Rischmueller M, Lester S. Influence of CTLA4 haplotypes on susceptibility and some extraglandular manifestations in primary Sjögren’s syndrome. Arthritis Rheum. 2006;54(8):2434–40. doi: 10.1002/art.22004. [DOI] [PubMed] [Google Scholar]
- 92.Nordmark G, Kristjansdottir G, Theander E, et al. Additive effects of the major risk alleles of IRF5 and STAT4 in primary Sjögren’s syndrome. Genes Immun. 2009;10(1):68–76. doi: 10.1038/gene.2008.94. [DOI] [PubMed] [Google Scholar]
- 93.Nordmark G, Kristjansdottir G, Theander E, et al. Association of EBF1, FAM167A(C8orf13)-BLK and TNFSF4 gene variants with primary Sjögren’s syndrome. Genes Immun. 2011;12(2):100–9. doi: 10.1038/gene.2010.44. [DOI] [PubMed] [Google Scholar]
- 94.Korman BD, Alba MI, Le JM, et al. Variant form of STAT4 is associated with primary Sjögren’s syndrome. Genes Immun. 2008;9(3):267–70. doi: 10.1038/gene.2008.1. [DOI] [PubMed] [Google Scholar]
- 95.Li Y, Zhang K, Chen H, Sun F, Xu J, Wu Z, et al. A genome-wide association study in Han Chinese identifies a susceptibility locus for primary Sjögren’s syndrome at 7q11.23. Nat Genet. 2013 Nov;45(11):1361–5. doi: 10.1038/ng.2779. [DOI] [PubMed] [Google Scholar]
- 96.Gestermann N, Mekinian A, Comets E, et al. STAT4 is a confirmed genetic risk factor for Sjögren’s syndrome and could be involved in type 1 interferon pathway signaling. Genes Immun. 2010;11(5):432–8. doi: 10.1038/gene.2010.29. [DOI] [PubMed] [Google Scholar]
- 97.Palomino-Morales RJ, Diaz-Gallo L-M, Witte T, Anaya J-M, Martín J. Influence of STAT4 polymorphism in primary Sjögren’s syndrome. J Rheumatol. 2010;37(5):1016–9. doi: 10.3899/jrheum.091007. [DOI] [PubMed] [Google Scholar]
- 98.Lawson CA, Donaldson IJ, Bowman SJ, et al. Analysis of the insertion/deletion related polymorphism within T cell antigen receptor beta variable genes in primary Sjögren’s syndrome. Ann Rheum Dis. 2005;64(3):468–70. doi: 10.1136/ard.2003.012823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nossent JC, Lester S, Zahra D, Mackay CR, Rischmueller M. Polymorphism in the 5′ regulatory region of the B-lymphocyte activating factor gene is associated with the Ro/La autoantibody response and serum BAFF levels in primary Sjogren’s syndrome. Rheumatol (Oxford) 2008;47(9):1311–6. doi: 10.1093/rheumatology/ken246. [DOI] [PubMed] [Google Scholar]
- 100.Imanishi T, Morinobu A, Hayashi N, et al. A novel polymorphism of the SSA1 gene is associated with anti-SS-A/Ro52 autoantibody in Japanese patients with primary Sjögren’s syndrome. Clin Exp Rheumatol. 2005;23(4):521–4. [PubMed] [Google Scholar]
- 101.Nakken B, Jonsson R, Bolstad AI. Polymorphisms of the Ro52 gene associated with anti-Ro 52-kd autoantibodies in patients with primary Sjögren’s syndrome. Arthritis Rheum. 2001;44(3):638–46. doi: 10.1002/1529-0131(200103)44:3<638::AID-ANR112>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 102.Kumagai S, Kanagawa S, Morinobu A, et al. Association of a new allele of the TAP2 gene, TAP2*Bky2 (Val577), with susceptibility to Sjögren’s syndrome. Arthritis Rheum. 1997;40(9):1685–92. doi: 10.1002/art.1780400919. [DOI] [PubMed] [Google Scholar]
- 103.Hulkkonen J, Pertovaara M, Antonen J, Lahdenpohja N, Pasternack A, Hurme M. Genetic association between interleukin-10 promoter region polymorphisms and primary Sjögren’s syndrome. Arthritis Rheum. 2001;44(1):176–9. doi: 10.1002/1529-0131(200101)44:1<176::AID-ANR23>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 104.Font J, García-Carrasco M, Ramos-Casals M, et al. The role of interleukin-10 promoter polymorphisms in the clinical expression of primary Sjögren’s syndrome. Rheumatol (Oxford) 2002;41(9):1025–30. doi: 10.1093/rheumatology/41.9.1025. [DOI] [PubMed] [Google Scholar]
- 105.Qin B, Wang J, Liang Y, Yang Z, Zhong R. The association between TNF-α, IL-10 gene polymorphisms and primary Sjögren’s syndrome: a meta-analysis and systemic review. PloS One. 2013;8(5):e63401. doi: 10.1371/journal.pone.0063401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Youn J, Hwang SH, Cho CS, et al. Association of the interleukin-4 receptor alpha variant Q576R with Th1/Th2 imbalance in connective tissue disease. Immunogenetics. 2000;51(8–9):743–6. doi: 10.1007/s002510000196. [DOI] [PubMed] [Google Scholar]
- 107.Miceli-Richard C, Comets E, Loiseau P, Puechal X, Hachulla E, Mariette X. Association of an IRF5 gene functional polymorphism with Sjögren’s syndrome. Arthritis Rheum. 2007;56(12):3989–94. doi: 10.1002/art.23142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miceli-Richard C, Gestermann N, Ittah M, et al. The CGGGG insertion/deletion polymorphism of the IRF5 promoter is a strong risk factor for primary Sjögren’s syndrome. Arthritis Rheum. 2009;60(7):1991–7. doi: 10.1002/art.24662. [DOI] [PubMed] [Google Scholar]
- 109.Gottenberg J-E, Busson M, Loiseau P, et al. Association of transforming growth factor beta1 and tumor necrosis factor alpha polymorphisms with anti-SSB/La antibody secretion in patients with primary Sjögren’s syndrome. Arthritis Rheum. 2004;50(2):570–80. doi: 10.1002/art.20060. [DOI] [PubMed] [Google Scholar]
- 110.Ou T-T, Lin C-H, Lin Y-C, et al. IkappaBalpha promoter polymorphisms in patients with primary Sjögren’s syndrome. J Clin Immunol. 2008;28(5):440–4. doi: 10.1007/s10875-008-9212-5. [DOI] [PubMed] [Google Scholar]
- 111.Mamtani M, Anaya J-M, He W, Ahuja SK. Association of copy number variation in the FCGR3B gene with risk of autoimmune diseases. Genes Immun. 2010;11(2):155–60. doi: 10.1038/gene.2009.71. [DOI] [PubMed] [Google Scholar]
- 112.Wang ZY, Morinobu A, Kanagawa S, Kumagai S. Polymorphisms of the mannose binding lectin gene in patients with Sjögren’s syndrome. Ann Rheum Dis. 2001;60(5):483–6. doi: 10.1136/ard.60.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pertovaara M, Lehtimäki T, Rontu R, Antonen J, Pasternack A, Hurme M. Presence of apolipoprotein E epsilon4 allele predisposes to early onset of primary Sjogren’s syndrome. Rheumatol (Oxford) 2004;43(12):1484–7. doi: 10.1093/rheumatology/keh383. [DOI] [PubMed] [Google Scholar]
- 114.Bolstad AI, Wargelius A, Nakken B, Haga HJ, Jonsson R. Fas and Fas ligand gene polymorphisms in primary Sjögren’s syndrome. J Rheumatol. 2000;27(10):2397–405. [PubMed] [Google Scholar]
- 115.Cobb BL, Fei Y, Jonsson R, et al. Genetic association between methyl-CpG binding protein 2 (MECP2) and primary Sjogren’s syndrome. Ann Rheum Dis. 2010;69(9):1731–2. doi: 10.1136/ard.2009.122903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Appel S, Le Hellard S, Bruland O, et al. Potential association of muscarinic receptor 3 gene variants with primary Sjogren’s syndrome. Ann Rheum Dis. 2011;70(7):1327–9. doi: 10.1136/ard.2010.138966. [DOI] [PubMed] [Google Scholar]
- 117.Morinobu A, Kanagawa S, Koshiba M, Sugai S, Kumagai S. Association of the glutathione S-transferase M1 homozygous null genotype with susceptibility to Sjögren’s syndrome in Japanese individuals. Arthritis Rheum. 1999;42(12):2612–5. doi: 10.1002/1529-0131(199912)42:12<2612::AID-ANR15>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 118.Alevizos I, Alexander S, Turner RJ, Illei GG. MicroRNA expression profiles as biomarkers of minor salivary gland inflammation and dysfunction in Sjögren’s syndrome. Arthritis Rheum. 2011;63(2):535–44. doi: 10.1002/art.30131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pauley KM, Stewart CM, Gauna AE, et al. Altered miR-146a expression in Sjögren’s syndrome and its functional role in innate immunity. Eur J Immunol. 2011;41(7):2029–39. doi: 10.1002/eji.201040757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yin H, Zhao M, Wu X, et al. Hypomethylation and overexpression of CD70 (TNFSF7) in CD4+ T cells of patients with primary Sjögren’s syndrome. J Dermatol Sci. 2010;59(3):198–203. doi: 10.1016/j.jdermsci.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 121.Thabet Y, Le Dantec C, Ghedira I, et al. Epigenetic dysregulation in salivary glands from patients with primary Sjögren’s syndrome may be ascribed to infiltrating B cells. J Autoimmun. 2013;41:175–81. doi: 10.1016/j.jaut.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 122.Yu X, Liang G, Yin H, et al. DNA hypermethylation leads to lower FOXP3 expression in CD4+ T cells of patients with primary Sjögren’s syndrome. Clin Immunol. 2013;148(2):254–7. doi: 10.1016/j.clim.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 123.Altorok N, Coit P, Hughes T, et al. Genome-wide DNA methylation patterns in naive CD4+ T cells from patients with primary Sjögren’s syndrome. Arthritis Rheumatol. 2014;66(3):731–9. doi: 10.1002/art.38264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Toussirot E, Roudier J. Epstein-Barr virus in autoimmune diseases. Best Pract Res Clin Rheumatol. 2008;22(5):883–96. doi: 10.1016/j.berh.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 125.Ascherio A, Munger KL. Epstein-barr virus infection and multiple sclerosis: a review. J Neuroimmune Pharmacol. 2010;5(3):271–7. doi: 10.1007/s11481-010-9201-3. [DOI] [PubMed] [Google Scholar]
- 126.Fox RI, Pearson G, Vaughan JH. Detection of Epstein-Barr virus-associated antigens and DNA in salivary gland biopsies from patients with Sjogren’s syndrome. J Immunol. 1986;137(10):3162–8. [PubMed] [Google Scholar]
- 127.Wen S, Shimizu N, Yoshiyama H, Mizugaki Y, Shinozaki F, Takada K. Association of Epstein-Barr virus (EBV) with Sjögren’s syndrome: differential EBV expression between epithelial cells and lymphocytes in salivary glands. Am J Pathol. 1996;149(5):1511–7. [PMC free article] [PubMed] [Google Scholar]
- 128.Pflugfelder SC, Crouse CA, Monroy D, Yen M, Rowe M, Atherton SS. Epstein-Barr virus and the lacrimal gland pathology of Sjögren’s syndrome. Am J Pathol. 1993;143(1):49–64. [PMC free article] [PubMed] [Google Scholar]
- 129.Mariette X, Gozlan J, Clerc D, Bisson M, Morinet F. Detection of Epstein-Barr virus DNA by in situ hybridization and polymerase chain reaction in salivary gland biopsy specimens from patients with Sjögren’s syndrome. Am J Med. 1991;90(3):286–94. [PubMed] [Google Scholar]
- 130.McClain MT, Heinlen LD, Dennis GJ, Roebuck J, Harley JB, James JA. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nat Med. 2005;11(1):85–9. doi: 10.1038/nm1167. [DOI] [PubMed] [Google Scholar]
- 131.DiGiuseppe JA, Wu TC, Corio RL. Analysis of Epstein-Barr virus-encoded small RNA 1 expression in benign lymphoepithelial salivary gland lesions. Mod Pathol. 1994;7(5):555–9. [PubMed] [Google Scholar]
- 132.Venables PJ, Teo CG, Baboonian C, Griffin BE, Hughes RA. Persistence of Epstein-Barr virus in salivary gland biopsies from healthy individuals and patients with Sjögren’s syndrome. Clin Exp Immunol. 1989;75(3):359–64. [PMC free article] [PubMed] [Google Scholar]
- 133.Croia C, Astorri E, Murray-Brown W, et al. Implication of Epstein-Barr virus infection in disease-specific autoreactive B cell activation in ectopic lymphoid structures of Sjogren’s syndrome. Arthritis Rheumatol. 2014;66(9):2545–57. doi: 10.1002/art.38726. [DOI] [PubMed] [Google Scholar]
- 134.Nagata Y, Inoue H, Yamada K, et al. Activation of Epstein-Barr virus by saliva from Sjogren’s syndrome patients. Immunology. 2004;111(2):223–9. doi: 10.1111/j.0019-2805.2003.01795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eguchi K, Matsuoka N, Ida H, et al. Primary Sjögren’s syndrome with antibodies to HTLV-I: clinical and laboratory features. Ann Rheum Dis. 1992;51(6):769–76. doi: 10.1136/ard.51.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Coulderc LJ, Desgranges C, Coste J, Caubarrere I, Clauvel JP. Antibodies to HTLV-I in Sjögren’s syndrome. Lancet. 1995;345(8941):72. [PubMed] [Google Scholar]
- 137.Danda D, Ramalingam S, Kannagai R, Jayaprakash K, Thomas K, Babu PG. Absence of antibody to HTLV-1 in sera of Asian Indian patients with systemic autoimmune rheumatic diseases. Trop Doct. 2004;34(2):126. doi: 10.1177/004947550403400235. [DOI] [PubMed] [Google Scholar]
- 138.Triantafyllopoulou A, Tapinos N, Moutsopoulos HM. Evidence for coxsackievirus infection in primary Sjögren’s syndrome. Arthritis Rheum. 2004;50(9):2897–902. doi: 10.1002/art.20463. [DOI] [PubMed] [Google Scholar]
- 139.Gottenberg J-E, Pallier C, Ittah M, et al. Failure to confirm coxsackievirus infection in primary Sjögren’s syndrome. Arthritis Rheum. 2006;54(6):2026–8. doi: 10.1002/art.21906. [DOI] [PubMed] [Google Scholar]
- 140.Stathopoulou EA, Routsias JG, Stea EA, Moutsopoulos HM, Tzioufas AG. Cross-reaction between antibodies to the major epitope of Ro60 kD autoantigen and a homologous peptide of Coxsackie virus 2B protein. Clin Exp Immunol. 2005;141(1):148–54. doi: 10.1111/j.1365-2249.2005.02812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fabris M, Dolcetti R, Pasini E, et al. High prevalence of Chlamydophila psittaci subclinical infection in Italian patients with Sjögren’s syndrome and parotid gland marginal zone B-cell lymphoma of MALT-type. Clin Exp Rheumatol. 2014;32(1):61–5. [PubMed] [Google Scholar]
- 142.Azzarolo AM, Wood RL, Mircheff AK, et al. Androgen influence on lacrimal gland apoptosis, necrosis, and lymphocytic infiltration. Invest Ophthalmol Vis Sci. 1999;40(3):592–602. [PubMed] [Google Scholar]
- 143.Azzarolo AM, Eihausen H, Schechter J. Estrogen prevention of lacrimal gland cell death and lymphocytic infiltration. Exp Eye Res. 2003;77(3):347–54. doi: 10.1016/s0014-4835(03)00120-9. [DOI] [PubMed] [Google Scholar]
- 144.Porola P, Laine M, Virtanen I, Pöllänen R, Przybyla BD, Konttinen YT. Androgens and integrins in salivary glands in Sjogren’s syndrome. J Rheumatol. 2010;37(6):1181–7. doi: 10.3899/jrheum.091354. [DOI] [PubMed] [Google Scholar]
- 145.Ishimaru N, Arakaki R, Watanabe M, Kobayashi M, Miyazaki K, Hayashi Y. Development of autoimmune exocrinopathy resembling Sjögren’s syndrome in estrogen-deficient mice of healthy background. Am J Pathol. 2003;163(4):1481–90. doi: 10.1016/S0002-9440(10)63505-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shim G-J, Warner M, Kim H-J, et al. Aromatase-deficient mice spontaneously develop a lymphoproliferative autoimmune disease resembling Sjogren’s syndrome. Proc Natl Acad Sci U S A. 2004;101(34):12628–33. doi: 10.1073/pnas.0405099101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Konttinen YT, Fuellen G, Bing Y, et al. Sex steroids in Sjögren’s syndrome. J Autoimmun. 2012;39(1–2):49–56. doi: 10.1016/j.jaut.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 148.Laine M, Porola P, Udby L, et al. Low salivary dehydroepiandrosterone and androgen-regulated cysteine-rich secretory protein 3 levels in Sjögren’s syndrome. Arthritis Rheum. 2007;56(8):2575–84. doi: 10.1002/art.22828. [DOI] [PubMed] [Google Scholar]
- 149.Valtysdóttir ST, Wide L, Hällgren R. Low serum dehydroepiandrosterone sulfate in women with primary Sjögren’s syndrome as an isolated sign of impaired HPA axis function. J Rheumatol. 2001;28(6):1259–65. [PubMed] [Google Scholar]
- 150.Porola P, Virkki L, Przybyla BD, et al. Androgen deficiency and defective intracrine processing of dehydroepiandrosterone in salivary glands in Sjögren’s syndrome. J Rheumatol. 2008;35(11):2229–35. doi: 10.3899/jrheum.080220. [DOI] [PubMed] [Google Scholar]
- 151.Spaan M, Porola P, Laine M, Rozman B, Azuma M, Konttinen YT. Healthy human salivary glands contain a DHEA-sulphate processing intracrine machinery, which is deranged in primary Sjögren’s syndrome. J Cell Mol Med. 2009;13(7):1261–70. doi: 10.1111/j.1582-4934.2009.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mavragani CP, Schini M, Gravani F, Kaltsas G, Moutsopoulos HM. Brief report: adrenal autoimmunity in primary Sjögren’s syndrome. Arthritis Rheum. 2012;64(12):4066–71. doi: 10.1002/art.34679. [DOI] [PubMed] [Google Scholar]
- 153.Mavragani CP, Fragoulis GE, Moutsopoulos HM. Endocrine alterations in primary Sjogren’s syndrome: an overview. J Autoimmun. 2012;39(4):354–8. doi: 10.1016/j.jaut.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 154.Chang DM, Chang CC, Kuo SY, Chu SJ, Chang ML. Hormonal profiles and immunological studies of male lupus in Taiwan. Clin Rheumatol. 1999;18(2):158–62. doi: 10.1007/s100670050075. [DOI] [PubMed] [Google Scholar]
- 155.Alpert S, Kang HI, Weissman I, Fox RI. Expression of granzyme A in salivary gland biopsies from patients with primary Sjögren’s syndrome. Arthritis Rheum. 1994;37(7):1046–54. doi: 10.1002/art.1780370710. [DOI] [PubMed] [Google Scholar]
- 156.Dawson LJ, Fox PC, Smith PM. Sjogrens syndrome--the non-apoptotic model of glandular hypofunction. Rheumatol (Oxford) 2006;45(7):792–8. doi: 10.1093/rheumatology/kel067. [DOI] [PubMed] [Google Scholar]
- 157.Cha S, Peck AB, Humphreys-Beher MG. Progress in understanding autoimmune exocrinopathy using the non-obese diabetic mouse: an update. Crit Rev Oral Biol Med. 2002;13(1):5–16. doi: 10.1177/154411130201300103. [DOI] [PubMed] [Google Scholar]
- 158.Rosignoli F, Roca V, Meiss R, Leceta J, Gomariz RP, Perez Leiros C. Defective signalling in salivary glands precedes the autoimmune response in the non-obese diabetic mouse model of sialadenitis. Clin Exp Immunol. 2005;142(3):411–8. doi: 10.1111/j.1365-2249.2005.02930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kong L, Robinson CP, Peck AB, et al. Inappropriate apoptosis of salivary and lacrimal gland epithelium of immunodeficient NOD-scid mice. Clin Exp Rheumatol. 1998;16(6):675–81. [PubMed] [Google Scholar]
- 160.Robinson CP, Yamachika S, Alford CE, et al. Elevated levels of cysteine protease activity in saliva and salivary glands of the nonobese diabetic (NOD) mouse model for Sjögren syndrome. Proc Natl Acad Sci U S A. 1997;94(11):5767–71. doi: 10.1073/pnas.94.11.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Humphreys-Beher MG, Yamachika S, Yamamoto H, et al. Salivary gland changes in the NOD mouse model for Sjögren’s syndrome: is there a non-immune genetic trigger? Eur J Morphol. 1998;36(Suppl):247–51. [PubMed] [Google Scholar]
- 162.Cha S, van Blockland SC, Versnel MA, et al. Abnormal organogenesis in salivary gland development may initiate adult onset of autoimmune exocrinopathy. Exp Clin Immunogenet. 2001;18(3):143–60. doi: 10.1159/000049194. [DOI] [PubMed] [Google Scholar]
- 163.Pérez P, Kwon Y-J, Alliende C, et al. Increased acinar damage of salivary glands of patients with Sjögren’s syndrome is paralleled by simultaneous imbalance of matrix metalloproteinase 3/tissue inhibitor of metalloproteinases 1 and matrix metalloproteinase 9/tissue inhibitor of metalloproteinases 1 ratios. Arthritis Rheum. 2005;52(9):2751–60. doi: 10.1002/art.21265. [DOI] [PubMed] [Google Scholar]
- 164.Velozo J, Aguilera S, Alliende C, et al. Severe alterations in expression and localisation of {alpha}6{beta}4 integrin in salivary gland acini from patients with Sjogren syndrome. Ann Rheum Dis. 2009;68(6):991–6. doi: 10.1136/ard.2008.089607. [DOI] [PubMed] [Google Scholar]
- 165.Ewert P, Aguilera S, Alliende C, et al. Disruption of tight junction structure in salivary glands from Sjögren’s syndrome patients is linked to proinflammatory cytokine exposure. Arthritis Rheum. 2010;62(5):1280–9. doi: 10.1002/art.27362. [DOI] [PubMed] [Google Scholar]
- 166.Bahamondes V, Albornoz A, Aguilera S, et al. Changes in Rab3D expression and distribution in the acini of Sjögren’s syndrome patients are associated with loss of cell polarity and secretory dysfunction. Arthritis Rheum. 2011;63(10):3126–35. doi: 10.1002/art.30500. [DOI] [PubMed] [Google Scholar]
- 167.Barrera M-J, Sánchez M, Aguilera S, et al. Aberrant localization of fusion receptors involved in regulated exocytosis in salivary glands of Sjögren’s syndrome patients is linked to ectopic mucin secretion. J Autoimmun. 2012;39(1–2):83–92. doi: 10.1016/j.jaut.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 168.Delporte C, Steinfeld S. Distribution and roles of aquaporins in salivary glands. Biochim Biophys Acta. 2006;1758(8):1061–70. doi: 10.1016/j.bbamem.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 169.Dawson LJ, Field EA, Harmer AR, Smith PM. Acetylcholine-evoked calcium mobilization and ion channel activation in human labial gland acinar cells from patients with primary Sjögren’s syndrome. Clin Exp Immunol. 2001;124(3):480–5. doi: 10.1046/j.1365-2249.2001.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Konttinen YT, Platts LA, Tuominen S, et al. Role of nitric oxide in Sjögren’s syndrome. Arthritis Rheum. 1997;40(5):875–83. doi: 10.1002/art.1780400515. [DOI] [PubMed] [Google Scholar]
- 171.Caulfield VL, Balmer C, Dawson LJ, Smith PM. A role for nitric oxide-mediated glandular hypofunction in a non-apoptotic model for Sjogren’s syndrome. Rheumatol (Oxford) 2009 Jul;48(7):727–33. doi: 10.1093/rheumatology/kep100. [DOI] [PubMed] [Google Scholar]
- 172.Norheim KB, Jonsson G, Harboe E, Hanasand M, Gøransson L, Omdal R. Oxidative stress, as measured by protein oxidation, is increased in primary Sjøgren’s syndrome. Free Radic Res. 2012;46(2):141–6. doi: 10.3109/10715762.2011.645206. [DOI] [PubMed] [Google Scholar]
- 173.Kurimoto C, Kawano S, Tsuji G, Hatachi S, Jikimoto T, Sugiyama D, et al. Thioredoxin may exert a protective effect against tissue damage caused by oxidative stress in salivary glands of patients with Sjögren’s syndrome. J Rheumatol. 2007;34(10):2035–43. [PubMed] [Google Scholar]
- 174.Gao J, Killedar S, Cornelius JG, Nguyen C, Cha S, Peck AB. Sjögren’s syndrome in the NOD mouse model is an interleukin-4 time-dependent, antibody isotype-specific autoimmune disease. J Autoimmun. 2006;26(2):90–103. doi: 10.1016/j.jaut.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 175.Nguyen CQ, Yin H, Lee BH, Carcamo WC, Chiorini JA, Peck AB. Pathogenic effect of interleukin-17A in induction of Sjögren’s syndrome-like disease using adenovirus-mediated gene transfer. Arthritis Res Ther. 2010;12(6):R220. doi: 10.1186/ar3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nguyen CQ, Gao J, Kim H, Saban DR, Cornelius JG, Peck AB. IL-4-STAT6 signal transduction-dependent induction of the clinical phase of Sjögren’s syndrome-like disease of the nonobese diabetic mouse. J Immunol. 2007;179(1):382–90. doi: 10.4049/jimmunol.179.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fox RI, Stern M. Sjögren’s syndrome: mechanisms of pathogenesis involve interaction of immune and neurosecretory systems. Scand J Rheumatol Suppl. 2002;116:3–13. [PubMed] [Google Scholar]
- 178.Dawson LJ, Christmas SE, Smith PM. An investigation of interactions between the immune system and stimulus-secretion coupling in mouse submandibular acinar cells. A possible mechanism to account for reduced salivary flow rates associated with the onset of Sjögren’s syndrome. Rheumatol (Oxford) 2000;39(11):1226–33. doi: 10.1093/rheumatology/39.11.1226. [DOI] [PubMed] [Google Scholar]
- 179.Dawson LJ, Caulfield VL, Stanbury JB, Field AE, Christmas SE, Smith PM. Hydroxychloroquine therapy in patients with primary Sjögren’s syndrome may improve salivary gland hypofunction by inhibition of glandular cholinesterase. Rheumatol (Oxford) 2005;44(4):449–55. doi: 10.1093/rheumatology/keh506. [DOI] [PubMed] [Google Scholar]
- 180.Beroukas D, Hiscock J, Jonsson R, Waterman SA, Gordon TP. Subcellular distribution of aquaporin 5 in salivary glands in primary Sjögren’s syndrome. Lancet. 2001;358(9296):1875–6. doi: 10.1016/S0140-6736(01)06900-8. [DOI] [PubMed] [Google Scholar]
- 181.Steinfeld S, Cogan E, King LS, Agre P, Kiss R, Delporte C. Abnormal distribution of aquaporin-5 water channel protein in salivary glands from Sjögren’s syndrome patients. Lab Invest. 2001;81(2):143–8. doi: 10.1038/labinvest.3780221. [DOI] [PubMed] [Google Scholar]
- 182.Soyfoo MS, Konno A, Bolaky N, et al. Link between inflammation and aquaporin-5 distribution in submandibular gland in Sjögren’s syndrome? Oral Dis. 2012;18(6):568–74. doi: 10.1111/j.1601-0825.2012.01909.x. [DOI] [PubMed] [Google Scholar]
- 183.Sumida T, Tsuboi H, Iizuka M, Asashima H, Matsumoto I. Anti-M3 muscarinic acetylcholine receptor antibodies in patients with Sjögren’s syndrome. Mod Rheumatol. 2013;23(5):841–5. doi: 10.1007/s10165-012-0788-5. [DOI] [PubMed] [Google Scholar]
- 184.Gao J, Cha S, Jonsson R, Opalko J, Peck AB. Detection of anti-type 3 muscarinic acetylcholine receptor autoantibodies in the sera of Sjögren’s syndrome patients by use of a transfected cell line assay. Arthritis Rheum. 2004;50(8):2615–21. doi: 10.1002/art.20371. [DOI] [PubMed] [Google Scholar]
- 185.Roescher N, Kingman A, Shirota Y, Chiorini JA, Illei GG. Peptide-based ELISAs are not sensitive and specific enough to detect muscarinic receptor type 3 autoantibodies in serum from patients with Sjogren’s syndrome. Ann Rheum Dis. 2011;70(1):235–6. doi: 10.1136/ard.2010.129049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Reina S, Sterin-Borda L, Passafaro D, Borda E. Anti-M(3) muscarinic cholinergic autoantibodies from patients with primary Sjögren’s syndrome trigger production of matrix metalloproteinase-3 (MMP-3) and prostaglandin E(2) (PGE(2)) from the submandibular glands. Arch Oral Biol. 2011;56(5):413–20. doi: 10.1016/j.archoralbio.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 187.Dawson LJ, Stanbury J, Venn N, Hasdimir B, Rogers SN, Smith PM. Antimuscarinic antibodies in primary Sjögren’s syndrome reversibly inhibit the mechanism of fluid secretion by human submandibular salivary acinar cells. Arthritis Rheum. 2006;54(4):1165–73. doi: 10.1002/art.21764. [DOI] [PubMed] [Google Scholar]
- 188.Alliende C, Kwon Y-J, Brito M, et al. Reduced sulfation of muc5b is linked to xerostomia in patients with Sjögren syndrome. Ann Rheum Dis. 2008;67(10):1480–7. doi: 10.1136/ard.2007.078246. [DOI] [PubMed] [Google Scholar]
- 189.Castro I, Aguilera S, Brockhausen I, et al. Decreased salivary sulphotransferase activity correlated with inflammation and autoimmunity parameters in Sjogren’s syndrome patients. Rheumatol (Oxford) 2012;51(3):482–90. doi: 10.1093/rheumatology/ker351. [DOI] [PubMed] [Google Scholar]
- 190.Ambrosi A, Wahren-Herlenius M. Congenital heart block: evidence for a pathogenic role of maternal autoantibodies. Arthritis Res Ther. 2012;14(2):208. doi: 10.1186/ar3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ambrosi A, Sonesson S-E, Wahren-Herlenius M. Molecular mechanisms of congenital heart block. Exp Cell Res. 2014;325(1):2–9. doi: 10.1016/j.yexcr.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 192.Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50(1):173–82. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 193.Briassouli P, Rifkin D, Clancy RM, Buyon JP. Binding of anti-SSA antibodies to apoptotic fetal cardiocytes stimulates urokinase plasminogen activator (uPA)/uPA receptor-dependent activation of TGF-β and potentiates fibrosis. J Immunol. 2011;187(10):5392–401. doi: 10.4049/jimmunol.1101288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Alvarez D, Briassouli P, Clancy RM, et al. A novel role of endothelin-1 in linking Toll-like receptor 7-mediated inflammation to fibrosis in congenital heart block. J Biol Chem. 2011;286(35):30444–54. doi: 10.1074/jbc.M111.263657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Eftekhari P, Sallé L, Lezoualc’h F, et al. Anti-SSA/Ro52 autoantibodies blocking the cardiac 5-HT4 serotoninergic receptor could explain neonatal lupus congenital heart block. Eur J Immunol. 2000;30(10):2782–90. doi: 10.1002/1521-4141(200010)30:10<2782::AID-IMMU2782>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 196.Qu Y, Xiao GQ, Chen L, Boutjdir M. Autoantibodies from mothers of children with congenital heart block downregulate cardiac L-type Ca channels. J Mol Cell Cardiol. 2001;33(6):1153–63. doi: 10.1006/jmcc.2001.1379. [DOI] [PubMed] [Google Scholar]
- 197.Karnabi E, Qu Y, Mancarella S, Boutjdir M. Rescue and worsening of congenital heart block-associated electrocardiographic abnormalities in two transgenic mice. J Cardiovasc Electrophysiol. 2011;22(8):922–30. doi: 10.1111/j.1540-8167.2011.02032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ambrosi A, Dzikaite V, Park J, et al. Anti-Ro52 monoclonal antibodies specific for amino acid 200–239, but not other Ro52 epitopes, induce congenital heart block in a rat model. Ann Rheum Dis. 2012;71(3):448–54. doi: 10.1136/annrheumdis-2011-200414. [DOI] [PubMed] [Google Scholar]
- 199.Strandberg LS, Ambrosi A, Jagodic M, et al. Maternal MHC regulates generation of pathogenic antibodies and fetal MHC-encoded genes determine susceptibility in congenital heart block. J Immunol. 2010;185(6):3574–82. doi: 10.4049/jimmunol.1001396. [DOI] [PubMed] [Google Scholar]
- 200.Ambrosi A, Salomonsson S, Eliasson H, et al. Development of heart block in children of SSA/SSB-autoantibody-positive women is associated with maternal age and displays a season-of-birth pattern. Ann Rheum Dis. 2012;71(3):334–40. doi: 10.1136/annrheumdis-2011-200207. [DOI] [PubMed] [Google Scholar]
- 201.Clancy RM, Marion MC, Kaufman KM, et al. Identification of candidate loci at 6p21 and 21q22 in a genome-wide association study of cardiac manifestations of neonatal lupus. Arthritis Rheum. 2010;62(11):3415–24. doi: 10.1002/art.27658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. 2005;165(20):2337–44. doi: 10.1001/archinte.165.20.2337. [DOI] [PubMed] [Google Scholar]
- 203.Voulgarelis M, Dafni UG, Isenberg DA, Moutsopoulos HM. Malignant lymphoma in primary Sjögren’s syndrome: a multicenter, retrospective, clinical study by the European Concerted Action on Sjögren’s Syndrome. Arthritis Rheum. 1999;42(8):1765–72. doi: 10.1002/1529-0131(199908)42:8<1765::AID-ANR28>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 204.Baimpa E, Dahabreh IJ, Voulgarelis M, Moutsopoulos HM. Hematologic manifestations and predictors of lymphoma development in primary Sjögren syndrome: clinical and pathophysiologic aspects. Medicine (Baltimore) 2009;88(5):284–93. doi: 10.1097/MD.0b013e3181b76ab5. [DOI] [PubMed] [Google Scholar]
- 205.Voulgarelis M, Ziakas PD, Papageorgiou A, Baimpa E, Tzioufas AG, Moutsopoulos HM. Prognosis and outcome of non-Hodgkin lymphoma in primary Sjögren syndrome. Medicine (Baltimore) 2012;91(1):1–9. doi: 10.1097/MD.0b013e31824125e4. [DOI] [PubMed] [Google Scholar]
- 206.Royer B, Cazals-Hatem D, Sibilia J, et al. Lymphomas in patients with Sjogren’s syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood. 1997;90(2):766–75. [PubMed] [Google Scholar]
- 207.Theander E, Henriksson G, Ljungberg O, Mandl T, Manthorpe R, Jacobsson LTH. Lymphoma and other malignancies in primary Sjögren’s syndrome: a cohort study on cancer incidence and lymphoma predictors. Ann Rheum Dis. 2006;65(6):796–803. doi: 10.1136/ard.2005.041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Solans-Laqué R, López-Hernandez A, Bosch-Gil JA, Palacios A, Campillo M, Vilardell-Tarres M. Risk, predictors, and clinical characteristics of lymphoma development in primary Sjögren’s syndrome. Semin Arthritis Rheum. 2011;41(3):415–23. doi: 10.1016/j.semarthrit.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 209.Ioannidis JPA, Vassiliou VA, Moutsopoulos HM. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjögren’s syndrome. Arthritis Rheum. 2002;46(3):741–7. doi: 10.1002/art.10221. [DOI] [PubMed] [Google Scholar]
- 210.Routsias JG, Goules JD, Charalampakis G, Tzima S, Papageorgiou A, Voulgarelis M. Malignant lymphoma in primary Sjögren’s syndrome: an update on the pathogenesis and treatment. Semin Arthritis Rheum. 2013;43(2):178–86. doi: 10.1016/j.semarthrit.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 211.Martin T, Weber JC, Levallois H, et al. Salivary gland lymphomas in patients with Sjögren’s syndrome may frequently develop from rheumatoid factor B cells. Arthritis Rheum. 2000;43(4):908–16. doi: 10.1002/1529-0131(200004)43:4<908::AID-ANR24>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 212.Nezos A, Papageorgiou A, Fragoulis G, et al. B-cell activating factor genetic variants in lymphomagenesis associated with primary Sjogren’s syndrome. J Autoimmun. 2014;51:89–98. doi: 10.1016/j.jaut.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 213.Tobón GJ, Saraux A, Gottenberg J-E, et al. Role of Fms-like tyrosine kinase 3 ligand as a potential biologic marker of lymphoma in primary Sjögren’s syndrome. Arthritis Rheum. 2013;65(12):3218–27. doi: 10.1002/art.38129. [DOI] [PubMed] [Google Scholar]
- 214.Nocturne G, Boudaoud S, Miceli-Richard C, et al. Germline and somatic genetic variations of TNFAIP3 in lymphoma complicating primary Sjogren’s syndrome. Blood. 2013;122(25):4068–76. doi: 10.1182/blood-2013-05-503383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tapinos NI, Polihronis M, Moutsopoulos HM. Lymphoma development in Sjögren’s syndrome: novel p53 mutations. Arthritis Rheum. 1999;42(7):1466–72. doi: 10.1002/1529-0131(199907)42:7<1466::AID-ANR21>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]